Abstract
Bone marrow-derived mesenchymal stem cells (BM-MSCs) are considered to be a potential therapy for end-stage liver disease. However, the therapeutic mechanism of BM-MSCs remains unclear. The aim of the current study was to investigate the role of paracrine signaling in BM-MSCs in liver cirrhosis in vitro. Human BM-MSCs and hepatic stellate cells (HSCs) were cultured using a vertical double cell co-culture system. Groups were divided into HSCs alone (control group) and the co-culture system of BM-MSCs with HSCs (experimental group). HSC morphology was observed by inverted phase contrast microscopy. The proliferative capacity of HSCs was measured with the MTT assay and flow cytometry. Hoechst staining was performed to examine the apoptosis of HSCs. Transforming growth factor (TGF)-β1 and Smad7 mRNA expression were detected by reverse transcription-quantitative polymerase chain reaction and western blotting. BM-MSCs did not inhibit the proliferation of HSCs at 24 h, however significantly inhibited the proliferation of HSCs at 48 and 72 h. BM-MSCs additionally induced the apoptosis of HSCs at 48 h. The concentration of TGF-β1 in the supernatant at 24 h and 48 h in the co-cultured system was observed to be significantly lower than in the control group (P<0.05). The level of TGF-β1 mRNA in the experimental group at 48 h was significantly lower than the control group, however Smad7 mRNA levels were significantly greater than in the control group. Additionally, TGF-β1 protein levels were significantly lower than in the control group, however levels of Smad7 were greater than the control group. It was concluded that BM-MSCs are able to inhibit the proliferation and promote the apoptosis of HSCs. In addition, the mechanism may be associated with inhibition of the TGF-β1/Smad pathway in HSCs.
Keywords: bone marrow-derived mesenchymal stem cells, hepatic stellate cells, transforming growth factor β1, Smad7, proliferation, apoptosis
Introduction
End-stage liver disease is associated with clear patient mortality (1), and orthotopic liver transplantation is the last resort for its treatment. However, due to factors including the shortage of donors, immune rejection and a high cost, the clinical use of orthotopic liver transplantation is restricted (2). Therefore, alternative treatment strategies are urgently required. The activation of hepatic stellate cells (HSCs) and subsequent secretion of large amounts of collagen is considered to be the core mechanism of liver fibrosis (3,4). Bone marrow-derived mesenchymal stem cells (BM-MSCs) possess a wide range of clinical applications in the field of cell therapy and tissue engineering due to their high plasticity, low immunogenicity, rapid amplification rate in vitro and stable genetic background (5).
Liver fibrosis is an early stage of liver cirrhosis, therefore reversing liver fibrosis holds clear clinical potential. An optimal treatment would prevent the development of liver fibrosis, in addition to inhibiting the formation of scar tissue in the liver, in order to stabilize and improve liver function (6). Previous studies have suggested that BM-MSCs may serve a role in the treatment of liver fibrosis (7–9). A previous study identified that the supernatant of cultured BM-MSCs is able to inhibit the expression of matrix metalloproteinase-2 (MMP-2) and tissue inhibitor of matrix metalloproteinase-1 (TIMP-1) in HSCs (10). These results indirectly confirmed that BM-MSCs are able to influence the proliferation of HSCs via the exocrine mechanism. However, it remains unclear which cytokines are secreted by BM-MSCs and which signal transduction pathways are used in HSCs. The current study aimed to further elucidate whether BM-MSCs are able to inhibit the proliferation of HSCs, then further explore alterations in transforming growth factor (TGF)-β1 during co-culture of BM-MSCs with HSCs.
Materials and methods
Materials
The human HSC cell line (LX2) was donated by Professor Scott L. Friedman from Icahn School of Medicine at Mount Sinai (New York City, NY, USA). Low glucose-Dulbecco's modified Eagle's medium (L-DMEM) and fetal bovine serum (FBS) (GE Healthcate Life-Sciences, Logan, UT, USA); MTT reagent cartridge (Sigma-Aldrich, St. Louis, MO, USA); the TGF-β1 ELISA reagent cartridge (R&D Systems, Inc., Minneapolis, MN, USA); rabbit anti-human TGF-β1 (1:500 dilution; cat. no. BS1361) and rabbit anti-human β-actin (1:5,000 dilution; cat. no. AP0733) polyclonal antibodies (Bioworld Technology, Inc., St. Louis Park, MN, USA), monoclonal mouse anti-human Smad7 antibody (cat. no. ab55493; Abcam, Pak Shek Kok, Hong Kong); horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG polyclonal antibody (1:5,000; cat. no. ab6741; Bioworld Technology, Inc.); RNA extraction reagent RNAiao Plus (Takara Bio, Inc., Otsu, Japan); PrimeScript® RT-PCR kit (Takara Bio, Inc.); enhanced chemiluminescence (ECL) light kit (Thermo Fisher Scientific, Inc., Waltham, MA, USA); polyvinylidene difluoride (PVDF) membrane (Merck Millipore, Darmstadt, Germany); semipermeable Transwell insert film (Corning Incorporated, Corning, NY, USA); Hoechst dye (Sigma-Aldrich); bicinchoninic acid (BCA) protein concentration assay kit (Beyotime Institute of Biotechnology, Shanghai, China); protein lysate (Beyotime Institute of Biotechnology).
Culture of human BM-MSCs and HSCs
Human BM-MSCs and HSCs were cultured in L-DMEM containing 10% FBS at 37°C in a 5% CO2 incubator. Cells were inoculated with a density of 1×105 cells/ml in culture flasks with a base area of 25 cm2. When cells were 80–90% confluent, they were washed with phosphate-buffered saline (PBS), digested with 0.25% trypsin (Thermo Fisher Scientific, Inc.) and subcultured. The growth and morphological characteristics of BM-MSCs and HSCs (third passage) were examined using an inverted phase contrast microscope (AE21; Motic China Group Co., Ltd., Xiamen, China). The growth curve of the cells cultured for 1–9 days was evaluated by MTT assay.
Cell co-culture
The upper and lower double-cell co-culture system was established as previously described (11,12). Cells were cultured in a 6-well plastic cell plate with 2 ml BM-MSCs (10×105 cells/ml) in the upper semi-permeable membrane (Transwell insert) and with 2 ml HSCs (1×105 cells/ml) in the lower membrane layer. Cells were divided into two experimental groups: i) Control group, HSCs were cultured alone (upper, L-DMEM media only); and ii) experimental group, BM-MSCs and HSCs were co-cultured. The morphological alterations were examined using an inverted phase contrast microscope at 24 and 48 h subsequent to incubation.
MTT assay to detect the HSC proliferation inhibition rate
Following co-culture of the cells for 24, 48 and 72 h, 400 µl MTT solution (5 g/l) was added to each well. Cells were incubated for 4 h, and the culture medium was discarded. A total of 2 ml dimethyl sulfoxide (Sigma-Aldrich) was added to each well and the wells were agitated for 10 min in the dark. Once the crystals had fully dissolved, cells were transferred to 96-well plates, each well containing 150 µl and each group consisting of 10 wells. The absorbance value of each well was measured at 490 nm in an enzyme-linked immunosorbent monitor (Thermo Fisher Scientific, Inc.) and recorded as the A value. The cell growth inhibition rate was calculated as follows: (1 - A value of experimental group / control group A value) ×100%.
Flow cytometry to detect apoptosis
Following co-culture for 24 or 48 h, the culture medium was discarded. Cells were washed with PBS and digested with 0.25% trypsin (excluding EDTA). Cells were resuspended in PBS and centrifuged for 5 min at 800 × g. The supernatant was discarded and the cells were divided evenly. A total of 5 µl annexin V-fluorescein isothiocyanate (FITC) (Beckman Coulter, Inc., Brea, CA, USA) was added into each tube, which were then vortexed prior to incubation in the dark at room temperature for 15 min. A total of 5 µl propidium iodide (PI) dye was added to each tube prior to detection for 5 min. Prior to detection, 200 µl 1X binding buffer was added into the tubes.
Hoechst staining of apoptotic cell bodies
Subsequent to co-culture for 24 and 48 h, the culture medium was removed and 2 ml 4% formaldehyde was added into each well. Cells were fixed for 15 min at room temperature and were washed twice with PBS, for 5 min each time. HSCs were stained with Hoechst dye for 6–9 min in the dark at room temperature. Apoptotic cell bodies in HSCs were examined using a fluorescence microscope (BX431-46; Olympus Corporation, Tokyo, Japan).
ELISA for TGF-β1 levels
Following co-culture for 24 and 48 h, the supernatants from the upper and lower Transwell chambers were collected and centrifuged at 750 x g for 20 min. TGF-β1 concentration was measured in accordance with the ELISA kit (R&D Systems, Inc.) instructions.
Total RNA extraction of HSCs and reverse transcription-quantitative polymerase chain reaction detection
HSCs were collected and counted at 48 h in each group. The primer sequences used were as follows: β-actin, upstream sequence TGGCACCCAGCACAATGAA and downstream sequence CTAAGTCATAGTCCGCCTAGAAGCA; TGF-β1, upstream sequence TGGAAACCCACAACGAAATC and downstream sequence GTATTTCTGGTACAGCTCCA; Smad7, upstream sequence TCTGCGAACTAGAGTCTCCC and downstream sequence ACGCACCAGTGTGACCGATC. The total RNA was extracted using RNAiso Plus reagent and RNA integrity and concentration were measured in the spectrophotometer (Tecan Group Ltd., Männedorf, Switzerland). A total of 500 ng RNA was used to synthesize 10 µl cDNA using the PrimeScript® RT-PCR kit. The reaction system volume was 10 µl. The reaction conditions were as follows: 37°C for 15 min then 85°C for 5 sec. Genes were amplified using a LightCycler amplification system (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The reaction system volume was 20 µl. The reaction conditions were as follows: 95°C for 30 sec (20°C/sec) for 1 cycle, then at 95°C for 5 sec (20°C/sec) and 60°C for 20 sec (20°C/sec) for 40 cycles. TGF-β1, Smad7 mRNA expression levels were detected. The data were stated as 2−∆∆Ct.
HSCs total protein extraction and western blot analysis
Following co-culture for 24 and 48 h, cells were lysed and total protein extracted from HSCs. Protein content was measured using the BCA protein concentration determination kit. The sample quantity was 30 µg. Proteins were separated by SDS-PAGE (10% gel; Bio-Rad Laboratories, Inc.), transferred to a PVDF membrane, blocked with Tris-buffered saline and Tween 20 and 5% skimmed milk and incubated with anti-human TGF-β1 (1:500) at 4°C overnight. The membrane was incubated with the HRP-conjugated secondary antibody, then developed using ECL. Data were analyzed using Image J 3.0 (Bio-Rad Laboratories, Inc.). The gray ratio of TGF-β1 proteins and β-actin represented the relative level of the target protein. Smad7 protein was detected as mentioned above.
Statistical analysis
SPSS software, version 18.0 (SPSS, Inc., Chicago, IL, USA) was used for statistical analysis. The count data were analyzed using the Chi-square test, measurement data were subjected to Student's t-test. Experimental data are expressed as mean ± standard deviation. P<0.05 was considered to indicate a statistically significant difference.
Results
Culture of BM-MSCs and HSCs
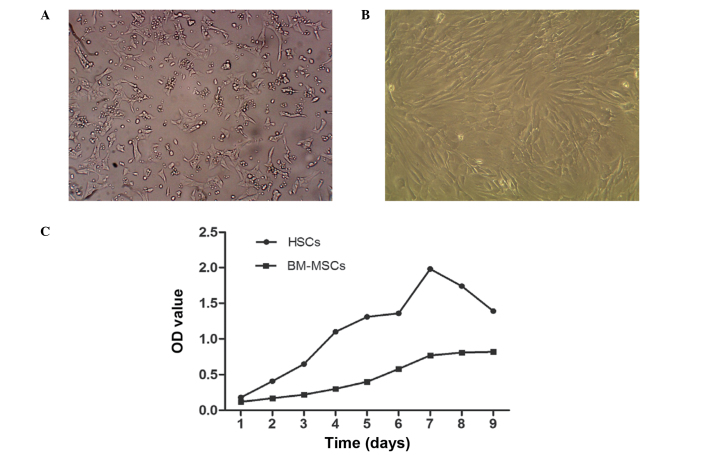
HSCs were observed to be adherent and oval or spindle-shaped, and cytoplasmic lipid droplets appeared following inoculation for 2–3 h, with certain cells beginning to stretch out during this time. Subsequent to culture of HSCs for 2–3 days, cells were 80–90% confluent and could be sub-cultured. Lipid droplets in the cytoplasm gradually reduced or disappeared, and cells displayed pseudopodia. HSCs exhibiting rapid growth and proliferation when passaged for 3–4 generations were used for the experiments (Fig. 1A). Cultured BM-MSCs were observed to be round or oval and were of varying sizes following inoculation. Subsequent to the media being replaced, cell adherence increased and the cells became uniform in shape, tightly packed, multi-fusiform and began to merge into a sheet. When cells were digested by trypsin, they became round, began to adhere, gradually restoring their long spindle morphology over 2–3 h. Subsequent to culture for 72 h, BM-MSCs were highly confluent, of uniform shape and fused with typical swirling growth (Fig. 1B). The third passage cells were for transplantation. The growth curve of HSCs and BM-MSC demonstrated that HSCs grew faster than BM-MSCs (Fig. 1C).
Figure 1.
The in vitro growth of human BM-MSCs and HSCs. (A) The morphology of HSCs subsequent to being passaged for 72 h (the 3rd generation; magnification, ×100); (B) the morphology of human HSCs subsequent to being passaged for 24 h (the 3rd generation; magnification, ×100); (C) the growth curve of HSCs and BM-MSCs. BM-MSCs, bone marrow-derived mesenchymal stem cells; HSCs, hepatic stellate cells; OD, optical density.
Inhibitory effect of BM-MSC on HSCs
Hoechst staining revealed the number of apoptotic bodies in the experimental group was significantly increased compared with the control group (Fig. 2A–D). The inhibitory effect of BM-MSCs on HSCs was detected by MTT assay following culture of the cells for 24, 48 and 72 h. The absorbance values of the control group at 24, 48 and 72 h (at 490 nm) were 0.149±0.012, 0.405±0.007 and 3.146±0.033, respectively. The absorbance values of the experimental group were 0.149±0.160, 0.358±0.007 and 0.986±0.020, respectively. There was no significant difference between the control and experimental group at 24 h (P=1.000), however the absorbance values at 48 and 72 h between the two groups were significantly different (P<0.001). BM-MSCs inhibited the proliferation of HSCs, with the inhibitory rates of BM-MSCs on HSCs at 24, 48 and 72 h observed to be 0, 11.5 and 68.7%, respectively. BM-MSCs did not inhibit the proliferation of HSCs at 24 h, however significantly inhibited the proliferation of HSCs in a time-dependent manner at 48 and 72 h (Fig. 2E).
Figure 2.
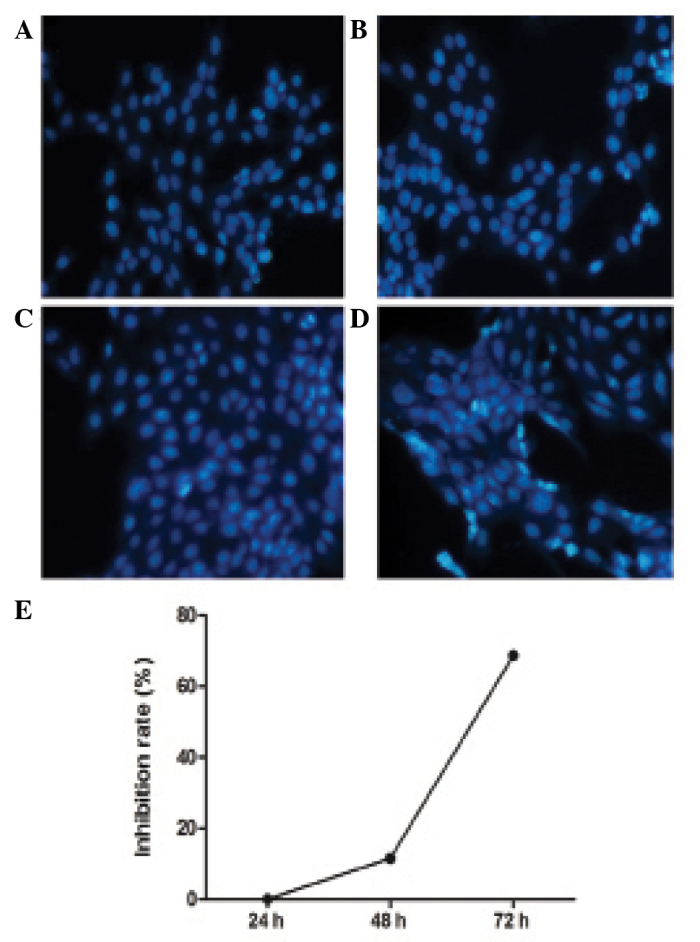
Apoptosis and inhibition of proliferation of HSCs caused by BM-MSCs. (A) Control group at 24 h; (B) experimental group at 24 h; (C) control group at 48 h; (D) experimental group at 48 h); (E) the proliferation rate of HSCs by BM-MSCs. BM-MSCs, bone marrow-derived mesenchymal stem cells; HSCs, hepatic stellate cells.
Apoptotic effect of BM-MSCs on HSCs
Following co-culture of BM-MSCs with HSCs for 24 and 48 h, cells were double stained with annexin V-FITC and PI to detect the rate of apoptosis of HSCs using flow cytometry (Fig. 3A–D). The rate of apoptosis was not identified to significantly differ between the control and experimental groups at 24 h (2.08% for control group and 1.00% for experimental group). However, the rate of apoptosis was significantly increased in the experimental group at 48 h (7.51% for control group and 10.28% for experimental group) (Fig. 3E).
Figure 3.
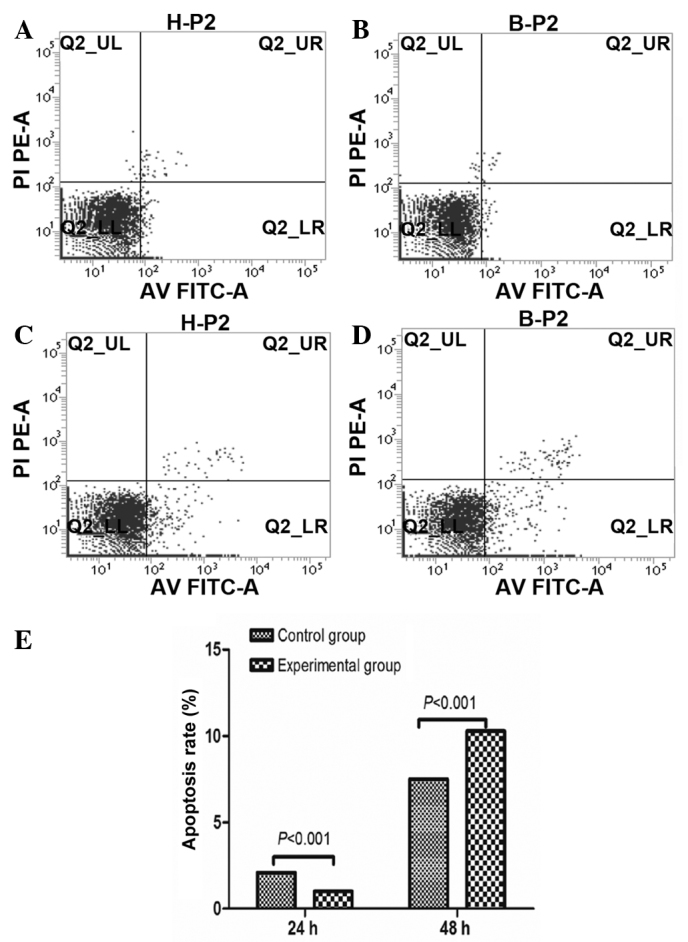
The apoptotic rate of HSCs caused by BM-MSCs. (A) Apoptosis rate of control group at 24 h; (B) apoptotic rate of experimental group at 24 h; (C) apoptotic rate of control group at 48 h; (D) apoptotic rate of experimental group at 48 h; (E) the apoptotic effect of BM-MSCs on HSCs at 24 and 48 h. BM-MSCs, bone marrow-derived mesenchymal stem cells; HSCs, hepatic stellate cells; PI, propidium iodide; AV FITC; annexin V fluorescein isothiocyanate.
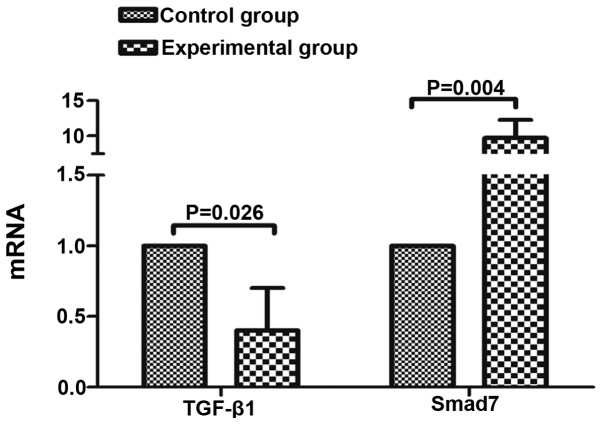
Expression of TGF-β1 and SMAD7 mRNA
The TGF-β1 and Smad7 mRNA expression levels in the control and experimental groups were as follows: Control group, 1.00±0.00 and 1.00±0.00, respectively; experimental group, 0.401±0.301 and 9.697±2.591, respectively. Following co-culture for 48 h, TGF-β1 mRNA in the experimental group was significantly lower than in the control group, and Smad7 mRNA was significantly higher than that in the control group (P<0.05; Fig. 4).
Figure 4.
The mRNA expression of TGF-β1 and Smad7. TGF-β1, transforming growth factor β1.
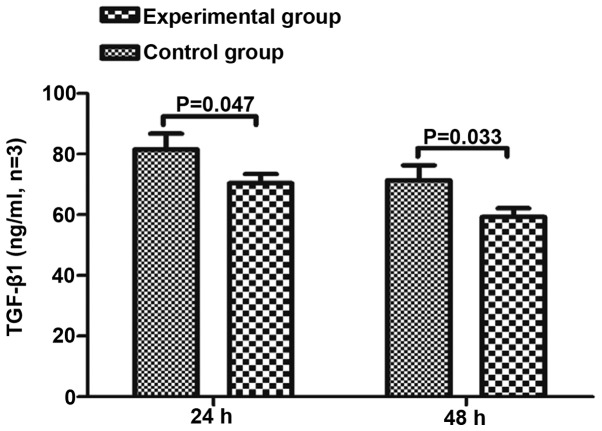
Concentration of TGF-β1 in the supernatant
To clarify whether BM-MSCs impact the secretion of TGF-β1 from HSCs, the TGF-β1 concentration in the supernatant was measured in the BM-MSCs and HSCs co-culture group, BM-MSCs group and the HSCs group at 24 and 48 h. The concentration of TGF-β1 in the BM-MSCs single culture group was below the ELISA kits minimum detection value, thus could not be measured. The concentration of TGF-β1 in the co-culture group was significantly lower than in the HSC single culture group at 24 and 48 h (P<0.05; Fig. 5).
Figure 5.
Impact of BM-MSCs on secretion of TGF-β1 from HSCs. BM-MSCs, bone marrow-derived mesenchymal stem cells; TGF-β1, transforming growth factor β1; HSCs, hepatic stellate cells.
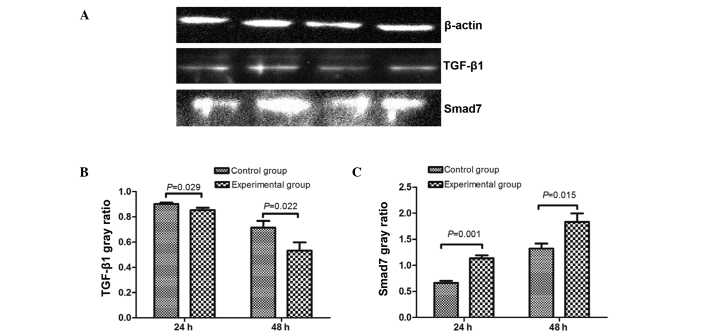
Expression of TGF-β1 and Smad7 protein
The expression of TGF-β1 and Smad7 protein was measured in the control group and experimental group at 24 and 48 h, and levels were observed to be significantly different between the groups (Fig. 6). Expression of TGF-β1 protein in the experimental group was lower than in the control group at 24 and 48 h (24 h control group, 0.902±0.012; 24 h experimental group, 0.854±0.018; 48 h control group, 0.715±0.053; 48 h experimental group, 0.532±0.066), and Smad7 protein levels were higher than that of the control group (24 h control group, 0.661±0.043; 24 h experimental group, 1.134±0.059; 48 h control group, 1.322±0.095; 48 h experimental group, 1.834±0.161).
Figure 6.
The expression of TGF-β1 and Smad7 protein in the control and experimental groups. (A) TGF-β1 and Smad7 expression. From left to right: Control group at 24 h, experimental group at 24 h, control group at 48 h and the experimental group at 48 h. Quantification of the protein expression levels of (B) TGF-β1 and (C) Smad7. TGF-β1, transforming growth factor β1.
Discussion
Liver fibrosis is an early stage in the development of liver cirrhosis. In-depth study of the mechanisms controlling the development of liver fibrosis may aid in reducing the occurrence of liver fibrosis (13), thereby preventing the progression of liver cirrhosis and improving quality of life for patients. Previous studies have confirmed that HSCs serve a key role in the development of liver fibrosis (14,15). Following the activation of HSCs by a variety of stimulating factors, they are able to proliferate, secrete excessive TIMP-1 and inhibit MMP-1 activity, leading to an imbalance between collagen deposition and degradation (16). TGF-β1 is a key factor for the promotion of HSCs activation and expression of the extracellular matrix (17).
In the present study, a co-culture system was established in which BM-MSCs cannot pass through the Transwell insert while cytokines are able to pass freely. BM-MSCs were seeded in the upper layer and HSCs in the lower layer, and the proliferative and apoptotic effects of BM-MSCs on HSCs were observed. The results of the MTT assay suggested that BM-MSCs did not significantly inhibit the proliferation of HSCs at 24 h. However, BM-MSCs significantly inhibited the proliferation of HSCs when they were co-cultured for 48 and 72 h. Additionally, BM-MSCs did not promote apoptosis of HSCs at 24 h, however induced significant apoptosis at 48 h. The number of apoptotic cell bodies in the experimental group was significantly increased compared with the control group. Therefore, it was concluded that BM-MSCs inhibit proliferation and induce apoptosis in HSCs. The non-contact co-culture method excluded the contact inhibition effect of BM-MSC on HSCs. These data suggest that BM-MSCs may secrete cytokines that pass through the Transwell insert mesh and inhibit proliferation and promote apoptosis of HSCs. The present study demonstrated that HSCs synthesise and secrete a reduced quantity of TGF-β1 and greater Smad7 following co-culture with BM-MSCs. These data further confirm that BM-MSCs inhibit the proliferation and induce apoptosis of HSCs through the secretion of certain cytokines involved in the TGFβ/Smad signaling pathway. Further experiments are required to identify the cytokines secreted by BM-MSCs.
In conclusion, the current study indicated that BM-MSC may serve a role in inhibiting the proliferation of HSCs and promoting apoptosis of HSCs through paracrine signaling, suggesting that infusion of the BM-MSC culture supernatant may be used to clinically treat liver fibrosis patients. However, in the present study TGF-β1 and Smad7 mRNA and protein expression levels were observed to be altered following co-culture for 24 h. However, from the results at 24 h, the alterations of TGF-β1 and Smad7 did not appear to affect cell proliferation and apoptosis during this time. This suggests that the effect of alterations in fibrogenic factors and fibrosis inhibitors on the mRNA and protein levels in HSCs co-cultured for 24 h may not be large enough to inhibit proliferation and promote apoptosis of HSCs. Following 48 h of co-culture, alterations at the gene and protein levels were observed, in addition to inhibition of HSC proliferation, and an increase in the number of apoptotic cells. In the early stages of the experiment, three observation time points were used, 24, 48 and 72 h, however from the MTT assay it was established that co-culture of BM-MSCs and HSCs for 72 h significantly inhibited the proliferation of HSCs. Additionally, the density of HSCs in the control group culture was observed to impact upon levels of apoptosis, with the number of adherent cells unable to meet the requirements of the follow-up experiments. Therefore, two observation time points, 24 and 48 h, were used in the follow-up experiment.
There are several limitations of the current study. The experiments used cultured BM-MSCs and HSCs cell lines in vitro, which cannot accurately reflect the effect of BM-MSCs in vivo. In addition, the results did not account for the influence of the inoculation on cell concentration. Therefore, future studies should address these limitations.
In summary, the present study identified that BM-MSCs are able to inhibit the proliferation of HSCs and promote their apoptosis, and the mechanism may be associated with inhibition of the TGF-β1/Smad pathway in HSCs.
References
- 1.Mutimer DJ, Lok A. Management of HBV- and HCV-induced end stage liver disease. Gut. 2012;61(Suppl 1):i59–i67. doi: 10.1136/gutjnl-2012-302076. [DOI] [PubMed] [Google Scholar]
- 2.Olson JC, Wendon JA, Kramer DJ, Arroyo V, Jalan R, Garcia-Tsao G, Kamath PS. Intensive care of the patient with cirrhosis. Hepatology. 2011;54:1864–1872. doi: 10.1002/hep.24622. [DOI] [PubMed] [Google Scholar]
- 3.Friedman SL. Hepatic stellate cells: Protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008;88:125–172. doi: 10.1152/physrev.00013.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kisseleva T, Brenner DA. Role of hepatic stellate cells in fibrogenesis and the reversal of fibrosis. J Gastroenterol Hepatol. 2007;22(Suppl 1):S73–S78. doi: 10.1111/j.1440-1746.2006.04658.x. [DOI] [PubMed] [Google Scholar]
- 5.Sancho-Bru P, Najimi M, Caruso M, Pauwelyn K, Cantz T, Forbes S, Roskams T, Ott M, Gehling U, Sokal E, et al. Stem and progenitor cells for liver repopulation: Can we standardise the process from bench to bedside? Gut. 2009;58:594–603. doi: 10.1136/gut.2008.171116. [DOI] [PubMed] [Google Scholar]
- 6.Mormone E, George J, Nieto N. Molecular pathogenesis of hepatic fibrosis and current therapeutic approaches. Chem Biol Interact. 2011;193:225–231. doi: 10.1016/j.cbi.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rabani V, Shahsavani M, Gharavi M, Piryaei A, Azhdari Z, Baharvand H. Mesenchymal stem cell infusion therapy in a carbon tetrachloride-induced liver fibrosis model affects matrix metalloproteinase expression. Cell Biol Int. 2010;34:601–605. doi: 10.1042/CBI20090386. [DOI] [PubMed] [Google Scholar]
- 8.Mohamadnejad M, Alimoghaddam K, Bagheri M, Ashrafi M, Abdollahzadeh L, Akhlaghpoor S, Bashtar M, Ghavamzadeh A, Malekzadeh R. Randomized placebo-controlled trial of mesenchymal stem cell transplantation in decompensated cirrhosis. Liver Int. 2013;33:1490–1496. doi: 10.1111/liv.12228. [DOI] [PubMed] [Google Scholar]
- 9.Nunes de Carvalho S, Helal-Neto E, de Andrade DC, Costa Cortez EA, Thole AA, Barja-Fidalgo C, de Carvalho L. Bone marrow mononuclear cell transplantation increases metalloproteinase-9 and 13 and decreases tissue inhibitors of metalloproteinase-1 and 2 expression in the liver of cholestatic rats. Cells Tissues Organs. 2013;198:139–148. doi: 10.1159/000353215. [DOI] [PubMed] [Google Scholar]
- 10.Wang S, Zhang LT, Chen H. Effect of human bone marrow mesenchymal stem cells culture supernatant on related enzyme expression in hepatic stellate cells. J Clin Rehab Tissue Eng Res. 2012;16:8374–8379. [Google Scholar]
- 11.Parekkadan B, van Poll D, Megeed Z, Kobayashi N, Tilles AW, Berthiaume F, Yarmush ML. Immunomodulation of activated hepatic stellate cells by mesenchymal stem cells. Biochem Biophys Res Commun. 2007;363:247–252. doi: 10.1016/j.bbrc.2007.05.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shi L, Li G, Wang J, Sun B, Yang L, Wang G, Wang D, Mu L, Chen H, Jin L, et al. Bone marrow stromal cells control the growth of hepatic stellate cells in vitro. Dig Dis Sci. 2008;53:2969–2974. doi: 10.1007/s10620-008-0227-9. [DOI] [PubMed] [Google Scholar]
- 13.Friedman SL. Hepatic fibrosis - overview. Toxicology. 2008;254:120–129. doi: 10.1016/j.tox.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 14.Friedman SL. Mechanisms of disease: Mechanisms of hepatic fibrosis and therapeutic implications. Nat Clin Pract Gastroenterol Hepatol. 2004;1:98–105. doi: 10.1038/ncpgasthep0055. [DOI] [PubMed] [Google Scholar]
- 15.Friedman SL. Seminars in medicine of the Beth Israel Hospital, Boston. The cellular basis of hepatic fibrosis. Mechanisms and treatment strategies. N Engl J Med. 1993;328:1828–1835. doi: 10.1056/NEJM199306243282508. [DOI] [PubMed] [Google Scholar]
- 16.Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655–1669. doi: 10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li JH, Huang XR, Zhu HJ, Johnson R, Lan HY. Role of TGF-beta signaling in extracellular matrix production under high glucose conditions. Kidney Int. 2003;63:2010–2019. doi: 10.1046/j.1523-1755.2003.00016.x. [DOI] [PubMed] [Google Scholar]