Abstract
The present study aimed to investigate the effects of treatment with thymosin α1 (TA1) or interferon α (IFNα) following the establishment of severe acute pancreatitis (SAP) in rats. A total of 144 Sprague-Dawley rats were randomly divided into four groups. The rats in all four groups were celiotomized, and the rats in the control group were administered with an intravenous injection of saline. The three other groups were administered with 5% 1 ml/kg sodium taurocholate via the cholangiopancreatic duct. SAP group rats were administered with an intravenous injection of saline; TA1 group rats received 26.7 µg/kg TA1; and interferon α (INFα) group rats received 4.0×105 U/kg IFNα. The rats were anesthetized and blood samples were collected from the animals 3, 12 and 24 h after surgery. The levels of T cell subsets, serum enzyme indicators, cytokines and procalcitonin (PCT) were measured. The general conditions of the rats were observed until sacrifice, and pancreatic and lung tissue samples were sampled for hematoxylin and eosin staining and histological scoring. The expression levels of aspartate transaminase, lactate dehydrogenase, α-amylase (AMY), P-type-amylase, lipase, PCT, tumor-necrosis factor α, interleukin (IL)-4, IL-5, and IL-18 in the TA1 and IFNα-treated rats were significantly lower, compared with those of the SAP rats within the first 24 h of model establishment (P<0.05). The TA1 and IFNα-treated rats exhibited significantly increased levels of CD3+, CD4+ and CD8+ T cells, and an increased ratio of CD4+/CD8+ cells, compared with SAP rats. Histological analysis revealed that the TA1 and IFNα-treated rats exhibited significantly ameliorated pancreas and lung damage, and mortality rates were reduced from 50.0% (6/12) to 25.0% (3/12) and 33.3% (4/12), respectively. The immunomodulatory agents TA1 and IFNα reduced acute inflammation, decreasing cell damage and enhancing immune function and survival rates in the SAP rats.
Keywords: severe acute pancreatitis, thymosin α1, interferon α, rat, immune response
Introduction
Severe acute pancreatitis (SAP) is a common acute abdominal disease with rapid progression and high mortality rates of ~20.8–36% (1,2), due to the lack of effective treatments for SAP, its progression is often associated with local or systemic inflammatory responses, and current treatment strategies focus on ameliorating inflammatory cytokine activation (3,4).
During the progression of SAP-induced systemic inflammatory response syndrome, the release of high levels of inflammatory mediators triggers the synthesis and release of anti-inflammatory cytokines. Anti-inflammatory cytokines inhibit pathogenic inflammation, however, they also precipitate the systemic suppression of immune functions, particularly cellular immunity directed against exogenous pathogens, resulting in the development of compensatory anti-inflammatory response syndrome (5). The precise association between pro- and anti-inflammatory cytokine responses is difficult to determine, however, numerous clinical studies have suggested that SAP progression is closely associated with immune dysfunction (4).
With the significance of immune function in the pathophysiology of SAP being increasingly emphasized, immune intervention has become an important aspect of SAP treatment. The multifunctional cytokines, interferon α (IFNα) and thymosin α1 (TA1), have been used in the treatment of chronic cancer, immune deficiencies and viral pancreatitis (6,7). TA1 is a polypeptide hormone with multiple bioactivities, including the induction of T cell differentiation and maturation, promotion of cytokine production and enhancement of the B cell antibody reaction (6,8). IFNα is an endogenous immune regulator and antiviral agent, which has an important regulatory role in T cell activation and cytokine release (9). However, the effects of TA1 and IFNα on SAP remain to be elucidated. The present study aimed to investigate the effects of TA1 and IFNα on cellular immune functions in a rat model of SAP, and to establish an experimental basis for the further administration of TA1 and IFNα in SAP.
Materials and methods
Animals and treatment
All experiments were performed with the consent of the University Animal Care and Usage Committee of the Experimental Animals Center of Xi'an Jiaotong University (Xi'an, China). A total of 144 Sprague-Dawley rats (aged 8–10 weeks, weighing 200–250 g) were provided by the Medical Experimental Animal Center of Xi'an Jiaotong University. The rats were fed and housed at 23±3°C, 40–50% humidity, with a 12 h light/dark cycle and <60 dB interior noise, were provided with free access to food and water.
The rats were randomly divided into four groups (n=36/group). Animals were anesthetized by intraperitoneal injection of and 10% chloral hydrate (320 mg/kg body weight). All rats were celiotomized and, following pulling the pancreas out of the body re-inserting it, the surgical incision was closed. The control rats were administrated with an intravenous injection of saline 0.5 h following the surgical procedure. The three other groups of rats were administered with 5% 1 ml/kg sodium taurocholate (Sigma-Aldrich, St. Louis, MO, USA) via the cholangiopancreatic duct for 5 min, and the SAP group rats were administered with saline by intravenous injection 0.5 h later. The TA1 group rats received 26.7 µg/kg TA1 (American Science and Engineering, Inc., Billerica, MA, USA) in the saline infusion. The INFα group rats received 4.0×105 U/kg IFNα (Shanghai Senxiong Biotech Industry Co., Ltd., Shanghai, China) in the saline infusion. The rats were then anesthetized and blood samples (2 ml) were collected from the inferior vena cava 3, 12 and 24 h following surgery. Samples of the pancreatic and lung tissue were collected at 2, 3, 12 and 24 h post-surgery by resection of the intact pancreas and left middle lung tissue following celiotomy, and the samples were stained with hematoxylin and eosin (HE; GefanBio Co. Ltd, Shanghai, China). After 24 h, the mortality and survival rates were recorded for a further 24 h. The general conditions of the rats were also observed, including mental status, physical activity, water consumption and hair smoothness.
The present study was approved by the ethics committee of the First Affiliated Hospital of Medical School, Xi'an Jiaotong University (Xi'an, China).
T cell subpopulation detection
The lymphocytes were separated from the heparinized venous blood by density gradient centrifugation (500 x g for 20 min at 4°C) using Ficoll-Hypaque (GE Healthcare Bio-Sciences, Pittsburg, PA, USA), resuspended in phosphate-buffered saline (PBS) and incubated with fluorescein isothiocyanate-conjugated anti-CD3 (cat no. sc-20047 monoclonal mouse anti-human; 1/1,000 dilution), phycoerythrin-conjugated anti-CD4 (cat no. sc-19642; monoclonal rat anti-mouse; 1/1,000 dilution) or allophycocyanin-conjugated anti-CD8 (cat no. sc-18913; monoclonal rat anti-mouse; 1/1,000 dilution) (all from Santa Cruz Biotechnology, Inc., Dallas, TX, USA) at 4°C for 15 min. Appropriately conjugated isotype-matched antibodies were used as controls. Populations of fluorescent cells (1×104/sample) were measured using a CyFlow® ML flow cytometer (Sysmex Europe GmbH, Norderstedt, Germany) following two washes with PBS.
Serum enzyme assay
Anticoagulant-free blood (5 ml) was centrifuged (1,700 x g, 18°C, 4 min), and the serum was collected for the measurement of the levels of aspartate transaminase (AST), lactate dehydrogenase (LDH), α-amylase (AMY), lipase (LPS) and P-type amylase (P-AMY) using an Olympus AU5400 automatic biochemical analyzer (Olympus Corporation, Tokyo, Japan).
Cytokine and procalcitonin (PCT) assay
The serum expression levels of tumor necrosis factor α (TNFα), interleukin (IL)-4, IL-5, IL-6, IL-18 and PCT were detected by ELISA using a commercial BD OptEIATM ELISA kit (BD Biosciences, San Jose, CA, USA), according to the manufacturer's instructions. The sensitivities for TNFα and the ILs were 15 and 5 pg/ml, respectively.
Histological examination
The tail of the pancreas and the left superior lobe of the lung were fixed with 5% paraformaldehyde (Dingguo Changsheng Biotechnology Co. Ltd, Beijing, China), dehydrated and embedded in paraffin wax (GefanBio Co. Ltd). Sections (3 mm thick) were cut, dewaxed and stained with HE for histological examination using a BX-5D1TF microscope (Olympus, Tokyo, Japan). The pathological scores of pancreatitis (10) and lung tissue samples (11) were evaluated. For the pancreatic tissue samples, a scale of 0–4 was used for interstitial edema and hemorrhage, inflammatory cell infiltration and acinar cell vacuolization and necrosis, consistent with the improved scoring method, described by Sharif et al (10). For the lung tissue samples, a scale of 0–3 was used for interstitial edema and hemorrhage, and inflammatory cell infiltration, consistent with a method previously described by Chooklin (11). The assessment was performed by two experienced pathologists in a blinded-manner. Disagreements in scoring were resolved by discussion.
Statistical analysis
Statistical analysis was performed using SPSS 13.0 (SPSS, Inc., Chicago, IL, USA). The data are presented as the mean ± standard deviation. Statistical differences were evaluated using one-way analysis of variance and a least significant difference test. Spearman's linear correlation analysis was used for correlation analysis. Survival rates were compared between groups using Kaplan-Meier survival curves and a Log-rank test. P<0.05 was considered to indicate a statistically significant difference.
Results
Expression levels of serum enzymes in the different treatment groups
Circulating expression levels of AMY, LPS and P-AMY were significantly higher in the rats in the SAP group, compared with the rats in the control group. The highest expression levels observed ~12 h following celiotomy and remained elevated for a further 12 h (P<0.05, Fig. 1A) indicating pancreatic damage. Elevated expression levels of AST and LDH were also observed 3, 12 and 24 h following SAP induction (P<0.05; Fig. 1B), These results suggested that multiple organ damage was induced by SAP.
Figure 1.
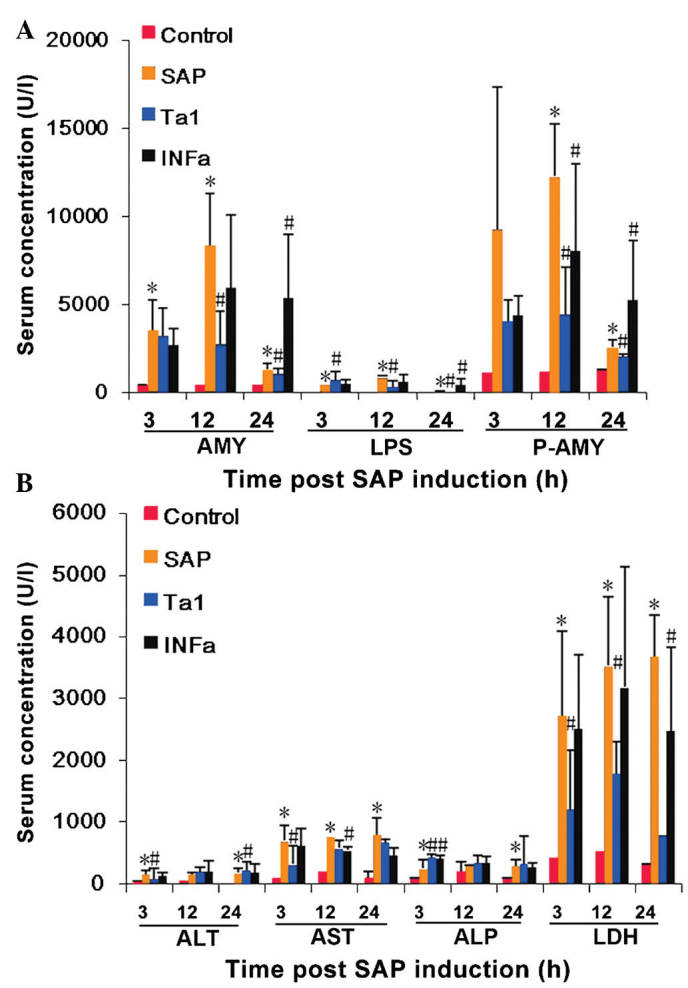
Detection of organ damage in rats following SAP induction and TA1 or INFα treatment. A total of 144 Sprague-Dawley rats, randomly divided into four groups (n=36), were celiotomized and three groups were administered with sodium taurocholate to induce SAP. These rats were then administered saline, TA1 or INFα, and blood samples were collected over the following 24 h. (A) Serum levels of ALT, AST, ALP, and LHD were measured using an automatic biochemical analyzer. (B) Serum levels of LPS, AMY and P-AMY were analyzed using ELISA. The data are presented as the mean ± standard deviation.*P<0.05, vs. control group; #P<0.05, vs. SAP group. SAP, severe acute pancreatitis; TA1, thymosin α1; IFNα, interferon α; ALT, alanine aminotransferase; AST, aspartate transaminase; ALP, alkaline phosphatase; LDH, lactate dehydrogenase; LPS, lipase; AMY, α-amylase; P-AMY, P-type-amylase.
Although the concentration levels of AMY, LPS and P-AMY in the TA1 group rats were higher than those of the control group rats, they were significantly lower, compared with those of the SAP group 3, 12 and 24 h post-SAP induction (P<0.05; Fig. 1A). The expression levels of circulating AMY, LPS and P-AMY were also lower in the INFα group 12 h following SAP induction (P<0.05; Fig. 2).
Figure 2.
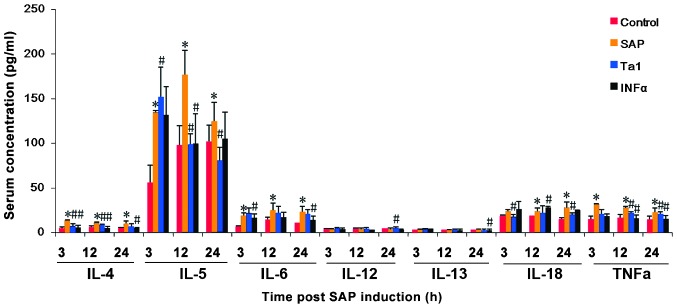
Expression levels circulating cytokines in rats following SAP and subsequent Ta1 of INFα treatment. Blood samples were collected over the following 24 h. Serum cytokine levels were measured using ELISA. The data are presented as the mean ± standard deviation. *P<0.05, vs. control group; #P<0.05, vs. SAP group. IL, interleukin; SAP, severe acute pancreatitis; TA1, thymosin α1; IFNα, interferon α.
Levels of serum cytokines following celiotomy
The circulating levels of TNFα, IL-4, IL-5, IL-6 and IL-18 were significantly higher in the SAP group rats, compared with the control group rats 3, 12 and 24 h following the indiction of SAP (P<0.05; Fig. 3). In the rats treated with TA1 or INFα, the levels of TNFα, IL-4, IL-5 and IL-18 were significantly lower, compared with those in the SAP group (P<0.05, Fig. 2).
Figure 3.
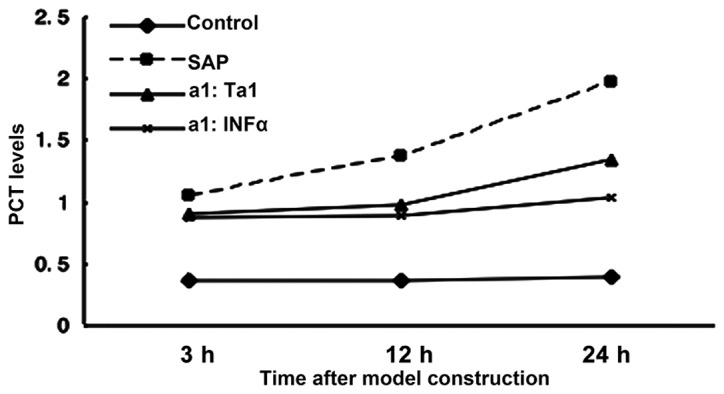
Circulating levels of PCT in rats following SAP and subsequent Ta1 of INFα treatment. Blood samples were collected over the following 24 h. The levels of PCT were measured using ELISA. PCT, procaltitonin; SAP, severe acute pancreatitis; TA1, thymosin α1; IFNα, interferon α. P>0.05 for SAP group vs. Ta1 group at 3 h. P<0.05 for Ta1 group vs. SAP group at 12 and 24 h.
Expression levels of serum PCT in the different treatment groups
At 24 h post-SAP, the circulating levels of PCT increased in the SAP group rats, whereas the expression levels of PCT in the rats, which were not administered with sodium taurocholate remained stable. Circulating levels of PCT were significantly decreased in the rats treated with TA1 or IFNα at 12 and 24 h post celiotomy (P>0.05; Fig. 4).
Figure 4.
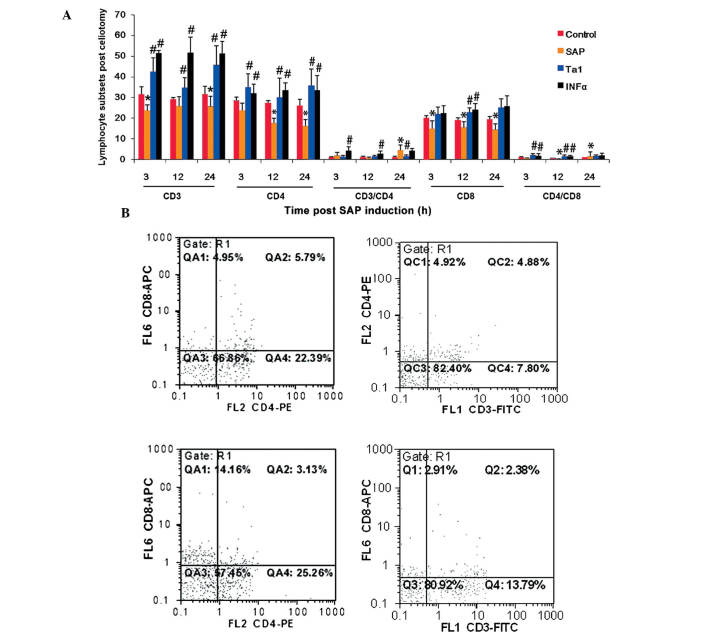
(A) Lymphocyte subpopulation in rats following SAP and subsequent Ta1 of INFα treatment. Lymphocytes were purified from the peripheral blood and labeled for CD3, CD4 and CD8 surface markers. The data are presented as the mean ± standard deviation. *P<0.05, vs. control group; #P<0.05, vs. SAP group. The populations of lymphocytes were analyzed using flow cytometry. (B) Representative results of lymphocyte subpopulations analysis using flow cytometry. SAP, severe acute pancreatitis; TA1, thymosin α1; IFNα, interferon α; PE, phycoerythrin; FITC, fluorescein isothiocyanate; ACP, allophycocyanine.
Lymphocyte subsets in the peripheral blood of the different treatment groups
T cell subsets were significantly affected by the establishment of SAP. Compared with the control animals, the proportion of T lymphocytes expressing CD3 and CD8 decreased 3 h post-celiotomy, the proportion of T lymphocytes expressing CD4 and CD8 decreased 12 h post-celiotomy, and the proportion of T lymphocytes expressing CD3, CD4 and CD8 decreased 24 h post-celiotomy (Fig. 4). The animals treated with TA1 exhibited higher levels of CD3+ and CD4+ (3, 12 and 24 h), CD8+ (12 h), and CD4+/CD8+ (3 and 12 h) T lymphocytes (Fig. 4). The animals administered with INFα exhibited higher levels of CD3+ and CD4+ (3 h, 12 h, 24 h), CD8+ (24 h) and CD4+/CD8+ (3 h, 12 h) T lymphocytes (Fig. 4).
Histological observation of rat pancreatic and lung tissue samples
Pancreatic and lung tissue samples were removed from the experimental animals 3, 12 and 24 h post-SAP to evaluate tissue damage. As shown in Fig. 5A, which shows representative images of the tissue samples at 12 h, the pancreatic samples removed from the control rats exhibited intact pancreatic structure with normal alveoli, with no lobular necrosis, hemorrhage or inflammatory cell infiltration. In the pancreatic tissue samples of the SAP rats, marked pancreatic edema, lobular damage, inflammatory cell infiltration, acinar necrosis and hemorrhage were observed. Compared with the SAP group, the rats administered with TA1 or INFα exhibited alleviated symptoms at the corresponding time-points.
Figure 5.

Pathological changes in the pancreas and lung 12 h post-SAP and Ta1 or INFα treatment. Pancreatic and lung tissue samples were harvested at different time-points post-treatment and following hematoxylin and eosin staining, tissue damage was evaluated. (A) Representative histological images of tissues 12 h post-treatment. Marked pancreatic edema, lobular damage, inflammatory cell infiltration, acinar necrosis and hemorrhage were observed in the SAP group. TA1 or INFα-treated rat tissue samples exhibited alleviated symptoms at the corresponding time-points. SAP lung samples exhibited pulmonary edema, scattered bleeding, alveolar wall rupture, marginal pleural effusion, interstitial hyperemia and edema, with significant widening and neutrophil infiltration in the alveolar and interstitial lung. TA1 and INFα-treated rats exhibited alleviated symptoms at the corresponding time-points. (B) Damage was quantified and compared between groups. Data are presented as the mean ± standard deviation. *P<0.05, vs. control group; #P<0.05, vs. SAP group. SAP, severe acute pancreatitis; TA1, thymosin α1; IFNα, interferon α.
Histological analysis of the lung tissue samples obtained from the SAP rats revealed pulmonary edema, scattered bleeding, alveolar wall rupture, marginal pleural effusion, interstitial hyperemia and edema, and marked widening and neutrophil infiltration of the alveoli and interstitial lung. The TA1 and INFα-treated rats exhibited markedly alleviated symptoms at the corresponding time-points (Fig. 5A).
Pathological scoring (Fig. 5B) revealed that damage to the pancreas and lung had been induced 3 h following SAP induction, and thes symptoms were aggravated with time. Tissue damage was significantly ameliorated following the administration of TA1 and INFα in the pancreas and lung 12 and 24 h post-model induction, compared with the SAP group.
Correlation between pathological scores and levels of cytokines and PCT
At 24 h post-model establishment, the histological scoring of the pancreatic damage in the SAP rats was positively correlated with the level of lung damage and the serum levels of TNFα, IL-4, IL-5, IL-18 and IL-6 (coefficients of 0.879, 0.857, 0.803, 0.788, 0.696 and 0.574, respectively; P<0.05; Table I). The histological scoring of the lung damage in the SAP rats was also positively correlated with the serum levels of TNFα, IL-4 and IL-5, with correlation coefficients of 0.763, 0.669 and 0.589, respectively (P<0.05; Table I). The circulating levels of PCT were also positively correlated with the levels of lung (r=0.789; P<0.05) and pancreatic damage (r=0.824, P<0.05; Table I).
Table I.
Correlation between pathological score and levels of cytokines and PCT at 24 h post-SAP establishment.
| Factor | Pancreatic damage (r-value) | Lung damage (r-value) | TNFα (r-value) | IL-4 (r-value) | IL-5 (r-value) | IL-18 (r-value) | IL-6 (r-value) |
|---|---|---|---|---|---|---|---|
| Pancreatic damage | 0.879a | 0.857a | 0.803a | 0.788a | 0.696a | 0.574a | |
| Lung damage | 0.879a | 0.763a | 0.669a | 0.589a | |||
| PCT | 0.789a | 0.824a |
P<0.05. PCT, procalcitonin; TNFα, tumor necrosis factor α; IL, interleukin; r, correlation coefficient.
Mortality and survival following celiotomy
Rats administered with TA1 or INFα survived for significantly longer periods of time, compared with the rats in the SAP group (P<0.05; Fig. 6).
Figure 6.
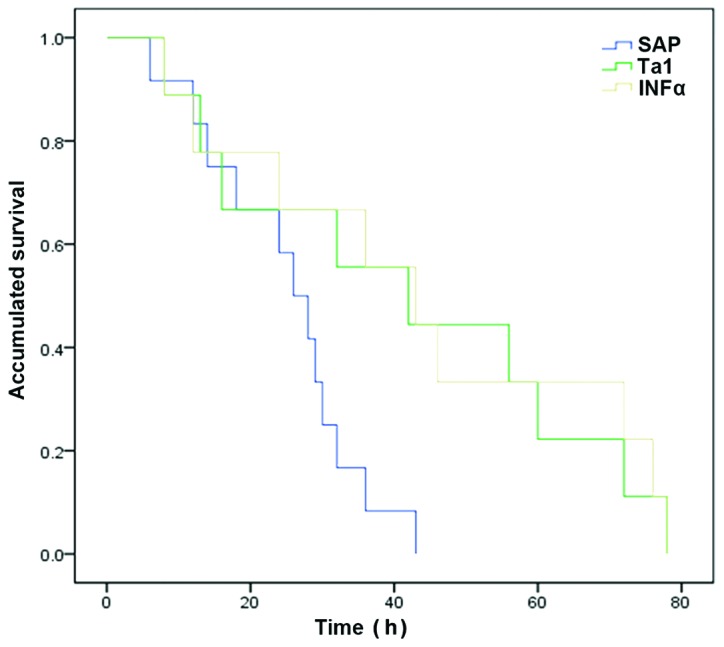
Kaplan-Meier survival curves of the rats in the different treatment groups. SAP, severe acute pancreatitis; TA1, thymosin α1; IFNα, interferon α.
Discussion
The present study aimed to investigate the effects of TA1 and IFNα on cellular immune functions in a rat model of SAP, and to establish an experimental basis for the administration of TA1 and IFNα in the treatment of SAP. The results demonstrated that administration of either TA1 or IFNα decreased the expression levels of AST, LDH, AMY, p-AMY, LPS, PCT and inflammatory cytokines, following the establishment of SAP. Administration of either TA1 or IFNα also resulted in a significantly higher proportion of CD3+, CD4+ and CD8+ T cells in the circulating blood, and histological examination of the pancreas and lung revealed a reduction in damage. Administration of either TA1 or IFNα also extended the survival rates of the rats in this SAP model, suggesting that TA1 and IFNα reduced the inflammatory reaction, decreased cell damage and enhanced immune function in the SAP rats.
T helper (Th)1/Th2 cell-expressed cytokines and tissue damage levels were used as indicators of immune function, and thus the efficacy of TA1 and IFNα in the treatment of SAP. A significant correlation was observed between serum levels of PCT and lung damage in the SAP rats, with improvement in following TA1 and IFNα treatment. These findings indicated that TA1 and IFNα enhanced T cell function by elevating the percentage of CD3+ and CD4+ T cells, and the CD4+/CD8+ ratio, and by inhibiting the release of inflammatory cytokines.
The pancreatic enzyme activation-induced self-digestion of pancreatic tissues can result in acute pancreatitis (AP), however, the mechanisms underlying the development of AP remain to be fully elucidated. A previous study suggested that in the early phases of AP, macrophages, neutrophils and the complement system activated by local lesions can trigger the release of high levels of proinflammatory cytokines and vasoactive substances, which then lead to a cascade reaction and excessive systemic inflammatory responses, resulting in the development of SAP (12). Therefore, the release of proinflammatory cytokines is considered to be a major contributor to the uncontrolled systemic inflammation detected during AP progression. In the present study, the levels of enzymatic indicators and inflammatory cytokines in SAP rats increased with the severity of SAP, however, the rats treated with TA1 and INFα exhibited significantly decreased enzyme levels, suggesting reduced damage to the pancreas and other organs. The association between cytokine levels and AP progression was first suggested in the 1990s (13), and subsequent experiments and clinical trials suggested the involvement of TNFα and IL-18 in the activation of immune disorder during SAP (14). Previous studies also identified a correlation between circulating levels of IL-4, TNFα, IL-8, IL-18 and IL-6, and the severity of SAP and the development of complications (15–17). These findings are consistent with the progression of SAP in the rat model of the present study.
TNFα is predominantly produced by mononuclear macrophages, and is the first inflammatory cytokine to increase in the early phase of AP. TNFα induces the apoptosis of pancreatic acinar cells (18); the activation of cytokines, including IL-6 and IL-8; and the production of vasoactive substances, including platelet-activating factors, oxygen-free radicals and endothelial cell adhesion molecules (19). Therefore, TNFα can induce local and systemic tissue damage. The circulating levels of serum IL-6 increase following the surge of TNFα, perhaps indicating IL-6 induction by TNFα (20). In the present study, circulating levels of IL-6 were maintained at relatively high levels in the acute phase of SAP. High levels of IL-6 can directly damage endothelial cells, facilitate immune adhesion and micro-thrombosis formation, inhibit endothelial repair, and increase vascular permeability to cause tissue damage (21).
IL-18 can induce the transcription of IFNγ in primary human CD4+ T cells, and also independently induces the secretion of IFNγ from natural killer cells (22). In synergy with mitogen or anti-CD3 monoclonal antibody, IL-18 induces the production of granulocyte-macrophage colony-stimulating factor from human peripheral blood mononuclear cells or enriched T cells, in a dose-dependent manner (23). The present study demonstrated that the serum expression levels of TNFα, IL-6 and IL-8 in the SAP rats were significantly higher, compared to those of the control, and this elevation was positively correlated with the severity of pancreatic and lung damage, indicating that TNFα, IL-6 and IL-18 are associated with AP progression, and may represent important indicators of SAP severity.
PCT functions to amplify inflammatory responses, and to regulate the activation of various cytokines. Serum expression levels of PCT have been demonstrated to increase more substantially in infected pancreatic necrotic tissues than in aseptic necrotic tissues (24), suggesting that serum PCT may be a valuable indicator of AP development, progression and infected pancreatic necrosis. In the present study, no significant change in PCT levels were observed within the first 3 h of SAP establishment, however PCT levels gradually increased over the following 24 h. The administration of TA1 and INFα reduced circulating levels of PCT from 12 h post-SAP.
Previous studies have demonstrated that, when abnormal immune function occurs in early SAP, the levels of circulating T lymphocytes are also reduced, and T cell subset abnormalities are observed. In the middle and late phases of SAP, however, excessive immune responses precipitate immunosuppression (25). In the present study, the percentage of CD3+, CD4+ and CD8+ T cells, and the CD4+/CD8+, ratio were all reduced following the establishment of SAP. The percentage of CD4+ T cells and the CD4+/CD8+ ratio may partially reflect cell-mediated immune function in patients with SAP (26). CD4+ T cells secrete IL-2 to induce the secretion of IL-1 from mononuclear macrophages, and CD4+ T cells also stimulate stationary T lymphocytes to express IL-2 receptors, which then bind to IL-2 to trigger various immune responses and to enhance DNA synthesis in lymphocytes (27). Therefore, decreased CD4+ T cell counts impair immune function and increase susceptibility to infection.
In the present study, as SAP progressed, pathological changes were observed in the pancreas and lung. Hemorrhage, necrosis and inflammatory cell infiltration were observed in pancreatic lesions, whereas lung edema and neutrophil infiltration were observed in the lungs. The present study identified a direct correlation between lung damage and inflammatory mediators, and cytokines were detected in the pancreatic and extra-pancreatic tissue samples. This indicated that inflammatory cytokines are only involved in the development of SAP, but they are also key in the progression of SAP local lesions to systemic disease, and may be directly involved in the development of early multiple organ dysfunction syndrome during SAP.
The use of immune agents in the early treatment of SAP may alleviate lymphocyte damage and protect immune function to various extents. However, previous inflammatory mediators intervention in clinical treatment of SAP achieved no significant efficacy, suggesting that blunt suppression of inflammation may instead aggravate immune disorders (28). Therefore, the use of immunomodualatory agents, including TA1 and IFNα may contribute significantly to alleviating inflammatory responses, reducing cell damage, decreasing enzymatic abnormalities and improving immune disorders, thus reducing secondary infections. Although the results of immunomodulation in animal models do not directly translate to clinical settings, the clinical application of immunomodulation therapy in SAP treatment merits further investigation.
Acknowledgments
The present study was supported by the International Science and Technology Cooperation and Exchange Program of Shaanxi Province (grant no. 2015KW-045, XW).
References
- 1.Otsuki M, Takeda K, Matsuno S, Kihara Y, Koizumi M, Hirota M, Ito T, Kataoka K, Kitagawa M, Inui K, Takeyama Y. Criteria for the diagnosis and severity stratification of acute pancreatitis. World J Gastroenterol. 2013;19:5798–5805. doi: 10.3748/wjg.v19.i35.5798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schepers NJ, Besselink MG, van Santvoort HC, Bakker OJ, Bruno MJ, Dutch Pancreatitis Study Group Early management of acute pancreatitis. Best Pract Res Clin Gastroenterol. 2013;27:727–743. doi: 10.1016/j.bpg.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 3.Panek J, Karcz D, Pieton R, Zasada J, Tusinski M, Dolecki M, Winiarski M. Blood serum levels of proinflammatory cytokines in patients with different degrees of biliary pancreatitis. Can J Gastroenterol. 2006;20:645–648. doi: 10.1155/2006/372892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kylanpaa ML, Repo H, Puolakkainen PA. Inflammation and immunosuppression in severe acute pancreatitis. World J Gastroenterol. 2010;16:2867–2872. doi: 10.3748/wjg.v16.i23.2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bone RC. Sir Isaac Newton, sepsis, SIRS and CARS. Crit Care Med. 1996;24:1125–1128. doi: 10.1097/00003246-199607000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Garaci E, Pica F, Sinibaldi-Vallebona P, Pierimarchi P, Mastino A, Matteucci C, Rasi G. Thymosin alpha (1) in combination with cytokines and chemotherapy for the treatment of cancer. Int Immunopharmacol. 2003;3:1145–1150. doi: 10.1016/S1567-5769(03)00053-5. [DOI] [PubMed] [Google Scholar]
- 7.Aman MJ, Tretter T, Eisenbeis I, Bug G, Decker T, Aulitzky WE, Tilg H, Huber C, Peschel C. Interferon-alpha stimulates production of interleukin-10 in activated CD4+ T cells and monocytes. Blood. 1996;87:4731–4736. [PubMed] [Google Scholar]
- 8.Romani L, Bistoni F, Montagnoli C, Gaziano R, Bozza S, Bonifazi P, Zelante T, Moretti S, Rasi G, Garaci E, Puccetti P. Thymosin alpha1: An endogenous regulator of inflammation, immunity and tolerance. Ann N Y Acad Sci. 2007;1112:326–338. doi: 10.1196/annals.1415.002. [DOI] [PubMed] [Google Scholar]
- 9.Pestka S, Krause CD, Walter MR. Interferons, interferon-like cytokines, and their receptors. Immunol Rev. 2004;202:8–32. doi: 10.1111/j.0105-2896.2004.00204.x. [DOI] [PubMed] [Google Scholar]
- 10.Sharif S, Broman M, Babcock T, Ong E, Jho D, Rudnicki M, Helton WS, Espat NJ. A priori dietary omega-3 lipid supplementation results in local pancreatic macrophage and pulmonary inflammatory response attenuation in a model of experimental acute edematous pancreatitis (AEP) JPEN J Parenter Enteral Nutr. 2006;30:271–276. doi: 10.1177/0148607106030004271. [DOI] [PubMed] [Google Scholar]
- 11.Chooklin S. Pathogenic aspects of pulmonary complications in acute pancreatitis patients. Hepatobiliary Pancreat Dis Int. 2009;8:186–192. [PubMed] [Google Scholar]
- 12.de Beaux AC, Ross JA, Maingay JP, Fearon KC, Carter DC. Proinflammatory cytokine release by peripheral blood mono-nuclear cells from patients with acute pancreatitis. Br J Surg. 1996;83:1071–1075. doi: 10.1002/bjs.1800830811. [DOI] [PubMed] [Google Scholar]
- 13.Curley PJ. Endotoxin, cellular immune dysfunction and acute pancreatitis. Ann R Coll Surg Engl. 1996;78:531–535. [PMC free article] [PubMed] [Google Scholar]
- 14.Ueda T, Takeyama Y, Yasuda T, Matsumura N, Sawa H, Nakajima T, Ajiki T, Fujino Y, Suzuki Y, Kuroda Y. Significant elevation of serum interleukin-18 levels in patients with acute pancreatitis. J Gastroenterol. 2006;41:158–165. doi: 10.1007/s00535-005-1735-4. [DOI] [PubMed] [Google Scholar]
- 15.Kaya E, Dervisoglu A, Polat C. Evaluation of diagnostic findings and scoring systems in outcome prediction in acute pancreatitis. World J Gastroenterol. 2007;13:3090–3094. doi: 10.3748/wjg.v13.i22.3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Denham W, Fink G, Yang J, Ulrich P, Tracey K, Norman J. Small molecule inhibition of tumor necrosis factor gene processing during acute pancreatitis prevents cytokine cascade progression and attenuates pancreatitis severity. Am Surg. 1997;63:1045–1049. discussion 1049–1050. [PubMed] [Google Scholar]
- 17.Stimac D, Fisić E, Milić S, Bilić-Zulle L, Perić R. Prognostic values of IL-6, IL-8 and IL-10 in acute pancreatitis. J Clin Gastroenterol. 2006;40:209–212. doi: 10.1097/00004836-200603000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Karne S, Gorelick FS. Etiopathogenesis of acute pancreatitis. Surg Clin North Am. 1999;79:699–710. doi: 10.1016/S0039-6109(05)70036-0. [DOI] [PubMed] [Google Scholar]
- 19.Springer TA. Adhesion receptors of the immune system. Nature. 1990;346:425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- 20.Jiang CF, Shiau YC, Ng KW, Tan SW. Serum interleukin-6, tumor necrosis factor alpha and C-reactive protein in early prediction of severity of acute pancreatitis. J Chin Med Assoc. 2004;67:442–446. [PubMed] [Google Scholar]
- 21.Zhao X, Andersson R, Wang X, Dib M, Wang X. Acute pancreatitis-associated lung injury: Pathophysiological mechanisms and potential future therapies. Scand J Gastroenterol. 2002;37:1351–1358. doi: 10.1080/003655202762671206. [DOI] [PubMed] [Google Scholar]
- 22.Barbulescu K, Becker C, Schlaak JF, Schmitt E, Meyer zum Buschenfelde KH, Neurath MF. IL-12 and IL-18 differentially regulate the transcriptional activity of the human IFN-gamma promoter in primary CD4+ T lymphocytes. J Immunol. 1998;160:3642–3647. [PubMed] [Google Scholar]
- 23.Matsumoto S, Tsuji-Takayama K, Aizawa Y, Koide K, Takeuchi M, Ohta T, Kurimoto M. Interleukin-18 activates NF-kappaB in murine T helper type 1 cells. Biochem Biophys Res Commun. 1997;234:454–457. doi: 10.1006/bbrc.1997.6665. [DOI] [PubMed] [Google Scholar]
- 24.Rau BM, Kemppainen EA, Gumbs AA, Büchler MW, Wegscheider K, Bassi C, Puolakkainen PA, Beger HG. Early assessment of pancreatic infections and overall prognosis in severe acute pancreatitis by procalcitonin (PCT): A prospective international multicenter study. Ann Surg. 2007;245:745–754. doi: 10.1097/01.sla.0000252443.22360.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li J, Yang WJ, Huang LM, Tang CW. Immunomodulatory therapies for acute pancreatitis. World J Gastroenterol. 2014;20:16935–16947. doi: 10.3748/wjg.v20.i45.16935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li XM, Chen JZ, Zhang NY. Association of TCM-bionzheng with Tlymphrocyte subsets and thyroidism in patients with diabetes mellitus. J Fourth Mil Med Univ. 2000;2:223–234. In Chinese. [Google Scholar]
- 27.Watson J, Mochizuki D. Interleukin 2: A class of T cell growth factors. Immunol Rev. 1980;51:257–278. doi: 10.1111/j.1600-065X.1980.tb00324.x. [DOI] [PubMed] [Google Scholar]
- 28.Li J, Yang WJ, Huang LM, Tang CW. Immunomodulatory therapies for acute pancreatitis. World J Gastroenterol. 2014;20:16935–16947. doi: 10.3748/wjg.v20.i45.16935. [DOI] [PMC free article] [PubMed] [Google Scholar]