Abstract
Bone remodeling is a vital physiological process of healthy bone tissue in humans. It is characterized by the formation of bone by osteoblasts and its resorption by osteoclasts, and the bone resorbed by osteoclasts is replaced through the differentiation and activity of osteoblasts. Imbalances in this vital process lead to pathological conditions, including osteoporosis. Intermedin (IMD) as a newly discovered peptide in the calcitonin (CT) family of peptides, which shares similar functions with CT, calcitonin gene-related peptide and amylin in bone resorption. However, the mechanism underlying its effect remains to be elucidated. This was investigated in the present study using the osteoblastic MC3T3-E1 cell line, which was treated with different doses of IMD (0, 1, 10 and 100 nM). Cell proliferation, apoptosis and the expression of receptor activator of NF-κB ligand (RANKL), osteoprotegerin (OPG) and macrophage colony-stimulating factor (M-CSF) were measured following treatment using multiple detection techniques, including an MTT assay, flow cytometry, reverse transcription-quantitative polymerase chain reaction and western blot analysis. The resulting data demonstrated that IMD significantly inhibited the apoptosis of MC3T3-E1 cells induced by serum-free culture and dexamethasone, however, no significant effects on MC3T3-E1 cell proliferation were observed. IMD had additional functions on the MC3T3-E1 cells, including inhibition of the expression of RANKL and M-CSF, and promotion of the expression of OPG. Previous studies have also demonstrated that RANKL and M-CSF are two vital factor produced by osteoblasts to promote the maturation and differentiation of osteoclasts, and it has been reported that IMD can inhibit the osteoclast formation stimulated by RANKL and M-CSF. Together with these findings, the present study concluded that IMD reduces bone resorption by inhibiting osteoblast apoptosis, decreasing the RANKL/OPG ratio and the expression of M-CSF, and inhibiting osteoclast maturation and differentiation.
Keywords: intermedin, osteoblast, macrophage colony-stimulating factor, nuclear factor-κB ligand/osteoprotegerin, bone resorption
Introduction
Intermedin (IMD), also known as adrenomedullin 2 (ADM2), is a newly discovered member of the calcitonin family peptides, which was independently identified by two groups in 2004 (1,2). IMD is expressed in a variety of organ systems, including the gastrointestinal tract, lungs, cardiovascular system, hypothalamus, pituitary gland, adrenal gland and placenta (2–6). Similar to other CT family members, including calcitonin gene-related peptide (CGRP) and AMD, IMD exerts its actions through receptor complexes comprising calcitonin receptor-like receptor (CRLR) and one of three receptor activity modifying proteins, (RAMP1, RAMP2 and RAMP3) (1,7), and stimulate the formation of cyclic AMP.
A series of physiological and pharmacological functions of IMD have been reported. IMD protects the mammalian vasculature, myocardium and kidney from acute ischemia-reperfusion injury, chronic oxidative stress and pressure-loading (5,8). IMD also inhibits apoptosis, attenuates maladaptive tissue remodelling and preserves cardiac and renal functions (9,10). Previously, it was reported that bone appears to be a common target for all the peptides of the calcitonin family, although the specific bone effects of the peptides vary (11). The administration of calcitonin produces rapid lowering of serum calcium levels, predominantly through the inhibition of bone resorption by osteoclasts (12). In vitro investigations and a number of animal experimental models have demonstrated that amylin and CGRP are also effective in inhibiting osteoclast activity and bone resorption (12). Susanne et al identified that IMD exhibits significant inhibitory effects on mouse calvarial bone matrix degradation stimulated by PTH and osteoclastogenesis (13), and IMD receptors, RAMPd and CRLP are expressed in osteoblasts (14). However, whether IMD can affect osteoblasts and is involved in bone resorption remains to be elucidated.
In the present study, the effects of IMD on the proliferation and apoptosis of osteoblasts were investigated. Subsequently, whether IMD is involved in osteoblastic and osteoclastic activity was determined by assessing molecules, which are closely associated with the processes, including receptor activator of NF-κB ligand (RANKL), osteoprotegerin (OPG) and macrophage colony-stimulating factor (M-CSF). The MC3T3-E1 osteoblast cell line was used as a cell model in vitro. In addition, the present study investigated the possible mechanism underlying the inhibitory effects of IMD on bone resorption.
Materials and methods
Reagents
Intermedin/Adrenomedullin-2 (IMD) was purchased from Phoenix Biotech Company. MTT was obtained from Sigma-Aldrich (St. Louis, MO, USA) and Annexin V-Fluorescein isothiocyanate (FITC)/propidium iodide (PI) kit from BD Biosciences (San Jose, CA, USA). The caspase-3 activity assay kit was purchased from Promega Corporation (Madison, WI, USA). Alkaline phosphatase (ALP) detection buffer was purchase from Sigma-Aldrich and the Micro BCA protein assay kit was obtained from Thermo Fisher Scientific (Waltham, MA, USA). All RNA primers and the PrimeScript RT-PCR kit were obtained from Takara, Bio, Inc. (Otsu, Japan). TRIzol reagent was obtained from Gibco Life Technologies (Carlsbad, CA, USA). Anti-OPG, RANKL, M-CSF and caspase-3 antibodies were purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA) The MC3T3-E1 mouse osteoblastic cell line was obtained from American Type Culture Collection (Manassas, VA, USA).
Mouse MC3T3-E1 cell culture
The MC3T3-E1 cells were cultured in minimum essential medium (MEM) containing 10% fetal bovine serum (FBS; Gibco Life Technologies), 100 U/ml penicillin, 100 mg/ml streptomycin (Beyotime Institute of Biotechnology, Haimen, China) and 50 mg/ml of ascorbic acid (Sigma-Aldrich). The cells were maintained in a humidified, 95% air, 5% CO2 atmosphere at 37°C. The medium was replaced every 3 days, and the cells were subcultured using 0.05% trypsin with 0.01% EDTA (Beyotime Institute of Biotechnology).
Cell proliferation assay
The MC3T3-E1 cells, at a density of 3×103 cells/well, were seeded into 96-well plates, cultured for 24 h at 37°C and then treated with different concentrations of IMD (1, 10 or 100 nM) for 48 h. Cell viability was detected by measuring the absorbance of each well at 490 nm using a Bio-Rad 680 microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA) and the experiment was performed five times. The proliferation rate was calculated from the optical density (OD) value and was used to determine the effect of IMD on MC3T3-E1 proliferation.
Osteoblast differentiation
The MC3T3-E1 cells (3×105) were cultured in MEM containing 10% FBS, 50 mg/ml ascorbic acid and 10 mM β-glycerophosphate (Sigma-Aldrich) treated with IMD (1, 10 or 100 nM) at 37°C. At differentiation days 7 and 14, the activity of ALP was measured. Briefly, the cells were washed twice with PBS and then lysed. The lysates were incubated in ALP detection buffer for 30 min at 37°C and were monitored at 405 nm. Total protein was detected using a Micro BCA protein assay kit and read at 562 nm. The relative activity of ALP was normalized to the total protein content of the sample (405/562 nm). In addition, the mRNA levels of osteoblast differentiation markers, OCN and BSP were measured, and Alizarin red staining (Sigma-Aldrich) was performed on differentiation days 14 and 21. For Alizarin red staining, the medium was removed, and the cell layer was rinsed with PBS and fixed in 70% ethanol for 1 h at −20°C. The cell layer was washed with deionized water and then the fixed cells were stained with 40 mM Alizarin red S (pH 4.2) for 10 min at room temperature.
Cell apoptosis analysis
The apoptosis of the MCT3-E1 cells was quantified using flow cytometry. The cells (3×105) were cultured at 37°C in serum-free medium or 10−7 M dexamethasone (DEX; Sigma-Aldrich) for 24 h in the absence or presence of 1–100 nM IMD. Treatment with 1% FBS was used to determine the basal levels of apoptosis. Briefly, following treatment with IMD for 48 h at 37°C, the cells were freshly harvested and washed with PBS. Subsequently 150 µl chilled Annexin V binding buffer was added, followed by 5 µl Annexin V-FITC and incubation for 15 min. During the final 5 min, 5 µl PI was added and further incubated for 10 min. Following staining, 350 µl Annexin V binding buffer was added and the cells were analyzed using a BD FACSCalibur (BD Biosciences).
Caspase-3 activity was determined by measuring the cleavage of the Ac-DEVD-pNA chromogenic caspase substrate, which is a caspase-3 substrate. The OD value at 405 nm was determined to reflect the quantity of caspase-3. The specific caspase-3 activity, normalized to that of the total proteins of the cell, was then expressed as the fold change from the baseline caspase-3 activity of the control cells.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) analysis
The MC3T3-E1 cells were cultured for 48 h in the presence of 1–100 nM IMD. Following treatment, the mRNA of cells was extracted using TRizol reagent, and cDNA was synthesized using the PrimeScript RT-PCR kit. The quantity of samples used in the RT-qPCR were as follows: SYBR Green Mastermix 10 µl, cDNA 2 µl, forward primer (10 µM) 1 µl, reverse primer (10 µM) 1 µl and H2O 5 µl. The sequences of the primers of RANKL, OPG, M-CSF, osteocalcin (OCN), bone sialoprotein (BSP) and the housekeeping β-actin gene are presented in Table I. qPCR was performed on a RotorGene real-time DNA amplification system using the following cycling protocol: 95°C denaturation for 10 min, followed by 40 cycles of 95°C (10 sec), 60°C annealing (20 sec) and 72°C extension (30 sec). The 2−ΔΔCT method was used to determine the gene expression levels (15).
Table I.
Primer sequences used in reverse transcription-quantitative polymerase chain reaction analysis in the present study.
| Gene | Primer | Primer sequence (5′-3′) |
|---|---|---|
| OPG | Forward | TGAGAGAACGAGAAAGACCTGC |
| Reverse | CGGATTGAACCTGATTCCCTAT | |
| RANKL | Forward | TCCTGAGACTCCATGAAAACGCAG |
| Reverse | GCCACATCCAACCATGAGCCTTC | |
| M-CSF | Forward | TTTTCCTGGGCATTGTGGTCT |
| Reverse | AGGAGGTTCAGGGCTTCTTTG | |
| OCN | Forward | GAGGGCAATAAGGTAGTGAA |
| Reverse | CATAGATGCGTTTGTAGGC | |
| BSP | Forward | CAGGGAGGCAGTGACTCTTC |
| Reverse | AGTGTGGAAAGTGTGGCGTT | |
| β-actin | Forward | CTGTGCCCATCTACGAGGGCTAT |
| Reverse | TTTGATGTCACGCACGATTTCC |
OPG, osteoprotegerin; RANKL, receptor activator of NF-κB ligand; M-CSF, macrophage colony-stimulating factor; OCN, osteocalcin; BSP, bone sialoprotein.
Western blot analysis
Following treatment with either serum-free medium or 10−7 M DEX in the presence or absence of 1–100 nM IMD for 24 h, the MC3T3-E1 cells were lysed in lysis buffer, containing 150 mM TrisHCl (pH 7.4), 150 mM NaCl, 0.1% SDS, 1% Triton X-100, 1% Nonidet P-40, protease inhibitor, 1 M NaF, 1 M b-glycerophosphate, 0.5 M Na3VO4, 1 M DTT, 1% sodium deoxycholate and 5 mM EDTA. Following 30 min incubation at 4°C, the lysates were sonicated and centrifuged at 12,000×g for 10 min at 4°C, and heated for 5 min at 95°C. The samples were resolved on a SDS-PAGE gel and transferred onto a nitrocellulose membrane (EMD Millipore, Billerica, MA, USA). Immunoblotting was then performed using antibodies against rabbit polyclonal RANKL (cat. no. SC-9073), rabbit polyclonal OPG (cat. no. SC-11383), rabbit polyclonal M-CSF (cat. no. SC-13103), rabbit polyclonal caspase-3 (cat. no. SC-7148) and mouse monoclonal β-actin (cat. no. SC-8342). Following 1 h incubation at 25°C with 1:2,000 goat anti-rabbit secondary antibodies, the blots were visualized using enhanced chemiluminescence (7Sea Biotech, Shanghai, China) and quantified using a Gel-Pro-Analyzer 4.0 (Media Cybernetics, Inc., Rockville, MD, USA).
Statistical analysis
Data are presented as the mean ± standard deviation, and all experiments were performed at least three times with similar results. Comparisons were made using a one-way analysis of variance and an unpaired t-test. Statistical analysis was performed using SPSS version 17.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Effects of IMD on the proliferation and differentiation of MC3T3-E1 cells
Following treatment with different doses of IMD (0, 10 or 100 nM) for 48 h, the effects of IMD on the growth of MC3T3-E1 cells was determined using MTT assays (Fig. 1). The data revealed no significant enhancement in the proliferation of MC3T3-E1 cells exposed to IMD, compared with the control group (P>0.05). In addition, no significant effects on ALP activity, Alizarin red staining or the mRNA expression levels of OCN or BSP were observed (P>0.05; Fig. 2). Therefore, IMD had no effect on the proliferation or differentiation of MC3T3-E1 cells, and was unable to promote bone formation (data not shown).
Figure 1.
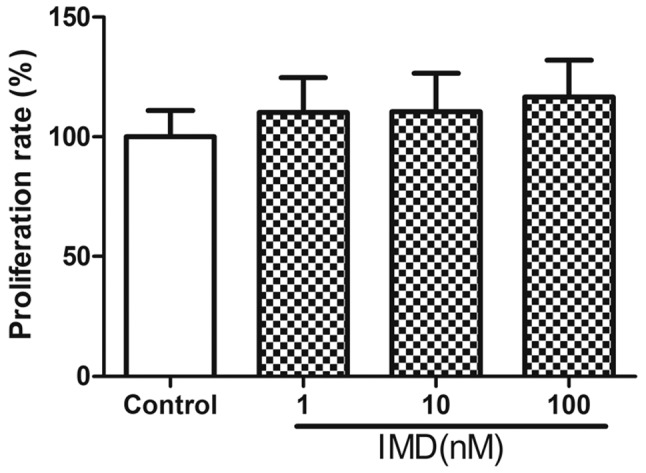
Effect of IMD on MC3T3-E1 cell proliferation was examined using an MTT assay. Cells were incubated with IMD (1–100 nM) for 48 h. No significant differences were observed, compared with the control group. Data are presented as the mean ± standard deviation. IMD, intermedin.
Figure 2.
Effect of IMD on MC3T3-E1 cell differentiation. (A) Relative ALP activity. No differences were observed between the 100 nM IMD group and the control group at days 7 or 14 following culture in osteoblastic differentiation medium. (B) Relative expression levels of osteogenic markers on days 14 and 21. No differences were observed in the mRNA levels of OCN and BSP. (C) Alizarin red staining of mineralized MC3T3-E1 cells on days 14 and 21 following osteogenic differentiation. No significant effect was observed. Data are presented as the mean ± standard deviation. IMD, intermedin; ALP, alkaline phosphatase; OCN, osteocalcin; BSP, bone sialoprotein.
Effects of IMD on the apoptosis of MC3T3-E1 cells
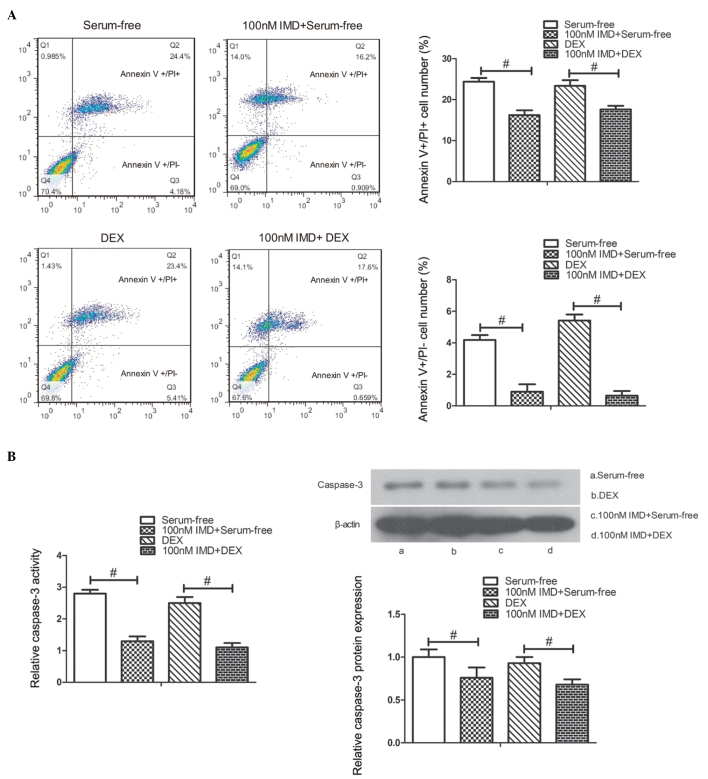
To examine the role of IMD on the apoptosis of MC3T3-E1 cells, the cells were cultured in serum-free medium or 10−7 M DEX with IMD (1–100 nM) for 24 h, and apoptotic progression was detected using flow cytometric analysis. FITC-conjugated annexin V was used to stain the early apoptotic biomarker, phosphoserine, at the cell surface, whereas PI was used to stain later apoptotic and necrotic cells. As shown in Fig. 3A, the number of PI-positive and annexin V-positive cells significantly decreased in the cells treated with 100 nM IMD, compared with those in serum-free medium or 10−7 M DEX alone (P<0.01).
Figure 3.
Effect of IMD on MC3T3-E1 cell apoptosis. (A) Flow cytometric analysis. Cell apoptosis was induced by incubation in serum-free medium or 10−7 M DEX. Following treatment with IMD (1–100 nM), the number of PI-positive (Q2 quadrant) and annexin V-positive (Q3 quadrant) cells were measured. A significant decrease in apoptosis was observed in the 100 nM IMD, compared with the serum-free or 10−7 M DEX cultured cells. (B) Caspase-3 activity and protein expression were determined. Caspase-3 activity and protein expression levels were significantly decreased in the 100 nM IMD group, compared with the untreated culture group at 24 h. #P<0.01. Data are presented as the mean ± standard deviation. IMD, intermedin; DEX, dexamethasone; PI, propidium iodide.
As caspase-3 is important in the process of apoptosis, caspase-3 activity and protein expression were measured to assess apoptosis in the present study. To evaluate the effect of IMD on caspase-3 activity and protein expression, the MC3T3-E1 cells cultured in serum-free medium or 10−7 M DEX were treated with different doses of IMD between 1 and 100 nM for 24 h. The results indicated that IMD significantly decreased caspase-3 activity and protein expression at a concentration of 100 nM, compared with the untreated cells at 24 h (P<0.01; Fig. 3B). These findings suggested that IMD inhibited the apoptosis of MC3T3-E1 cells (data not shown).
Effects of IMD on ratio of RANKL/OPG
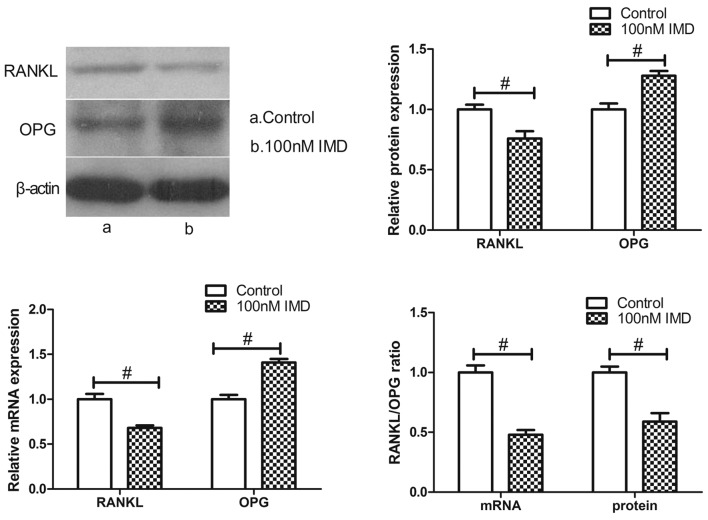
The expression ratio of RANKL/OPG, which are synthesized by osteoblasts, represent bone resorption. To verify the expression of RANKL and OPG in the MC3T3-E1 cells treated with IMD, the present study performed RT-qPCR and western blot analysis. The results revealed that the expression of RANKL at the mRNA and protein levels were markedly downregulated by various doses of IMD, whereas the levels of OPG were significantly upregulated (Fig. 4). IMD at 100 nM significantly decreased the mRNA and protein expression levels of RANKL (P<0.01), and upregulated the mRNA and protein expression levels of OPG (P<0.01). Therefore, IMD significantly improved the imbalance in the RANKL/OPG ratio (P<0.01), which is likely to induce an inhibitory effect on bone resorption.
Figure 4.
Effect of IMD on the expression ratio of RANKL/OPG. The MC3T3-E1 cells were treated with IMD (1–100 nM) for 48 h. The results demonstrated that IMD significantly suppressed the expression of RANKL and enhanced the expression of OPG, compared with the control. Therefore, IMD downregulated the ratio of RANKL/OPG. #P<0.01. Data are presented as the mean ± standard deviation. IMD, intermedin; RANKL, receptor activator of NF-κB ligand; OPG, osteoprotegerin.
Effects of IMD on the expression of M-CSF
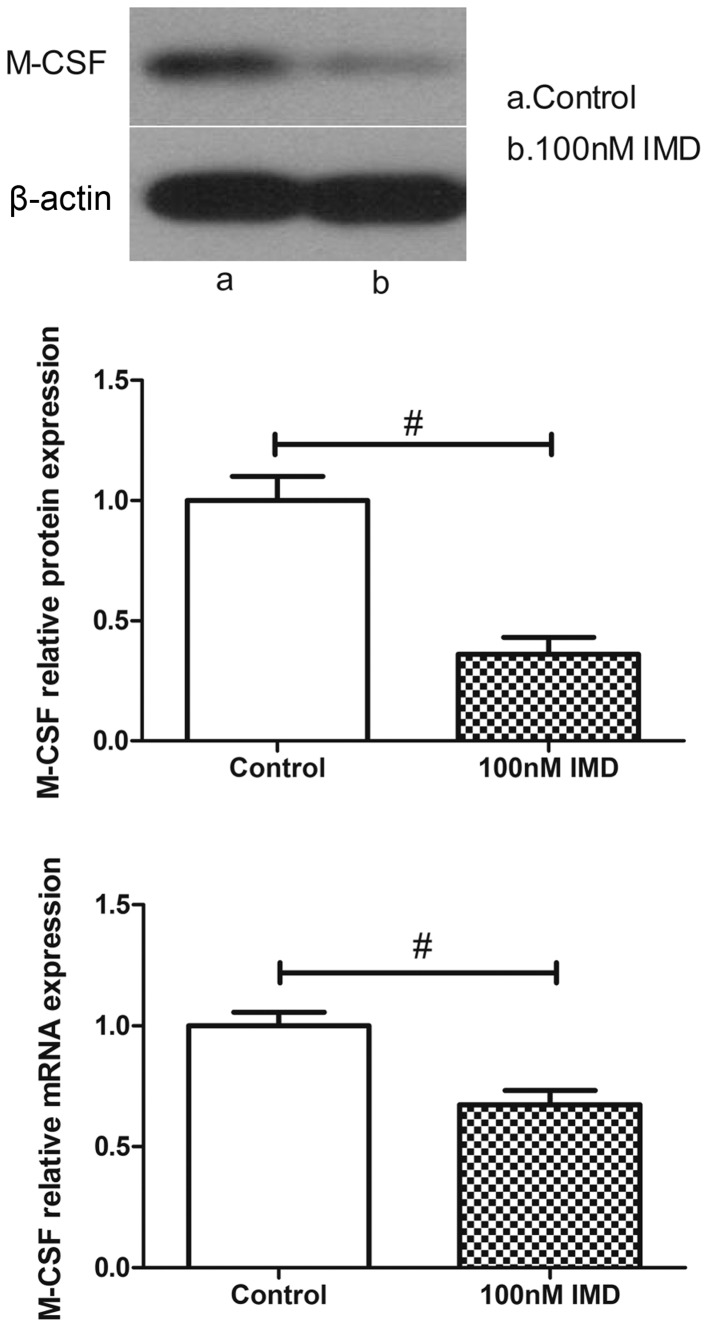
M-CSF is another osteoblast-mediated activation marker of bone resorption. As shown in Fig. 5, the expression levels of M-CSF were measured using RT-qPCR and western blot analysis. In the untreated group, the MC3T3-E1 cells expressed a basal level of M-CSF. By contrast, the level of M-CSF in the MC3T3-E1 cells was significantly altered following IMD treatment. The data demonstrated that IMD significantly downregulated the mRNA and protein expression levels of M-CSF (P<0.01), which suggested suppression of the osteoclast-mediated bone resorption by IMD.
Figure 5.
Effect of IMD on the expression of M-CSF. The mRNA and protein expression levels of M-CSF in the MC3T3-E1 cells were measured following treatment with (1–100 nM) IMD for 48 h. Treatment with 100 nM IMD significantly decreased the mRNA and protein expression levels of M-CSF. #P<0.01. Data are presented as the mean ± standard deviation. IMD, inter-medin; M-CSF, macrophage colony-stimulating factor.
Discussion
Osteoporosis is a common bone disease characterized by a reduction in bone mineral density and an increase in fracture risk, which affects quality of life (16). Bone is a dynamic tissue that constantly undergoes a process of renewal and repair (17) termed 'bone remodeling', in which a balance of bone formation by osteoblasts and resorption by osteoclasts continues throughout life (18,19). This balance involves the coordinated regulation and interaction of two cell types, osteoclasts and osteoblasts (17,20). Either a decrease in osteoblastic activity or an increase in osteoclastic activity in the bone can lead to imbalance by accelerating bone resorption (21).
Osteoblasts, which are derived from mesenchymal stem cells (MSCs), are responsible for the production of bone extracellular matrix and are able to mineralize into bone matrix (22,23). ALP, OCN and BSP have been reported to be involved in the molecular events of osteoblast differentiation (24,25). Using MTT analysis, the present study demonstrated that IMD had no effect on the proliferation of the MC3T3-E1 osteoblast lineage. In addition, IMD treatment had no effect on ALP activity, Alizarin red staining or the mRNA expression levels of OCN and BSP, suggesting that IMD had no effect on the differentiation of osteoblasts. Notably, the results of the Annexin V/PI flow cytometry revealed that IMD significantly inhibited the apoptosis of the MC3T3-E1 cells. The present study subsequently measured caspase-3 activity and protein expression levels and, in accordance with the above findings, IMD markedly attenuated caspase-3 activity and decreased protein expression levels. These results indicated that IMD suppressed the apoptosis induced by serum-free medium or DEX in the MC3T3-E1 cells.
Osteoclasts originate from the monocyte/macrophage hematopoietic lineages and are responsible for bone resorption (26,27). Previous studies have confirmed that osteoblasts can modulate osteoclast function by secreting RANKL, OPG and M-CSF (28–30). The OPG/RANKL/M-CSF system is fundamental for regulation between osteoblasts and osteoclasts (31). The expression of RANKL by osteoblast cells, is essential for osteoclast differentiation and maturity via its receptor, RANK, located on the osteoclast membrane (32) and M-CSF, which binds to its receptor, c-Fms, and appears to be necessary for osteoclast development (33,34). OPG, produced by osteoblastic cells, inhibits osteoclast differentiation through its binding to RANKL (34). Thus, the RANKL/OPG ratio and the expression of M-CSF determine osteoclast activity in addition to bone resorption (35). The present study is the first, to the best of our knowledge, to provide insight into the effects of IMD on the imbalance of RANKL/OPG ratio and the expression of M-CSF. The data obtained in the present study demonstrated that IMD downregulated the ratio of RANKL/OPG and the expression of M-CSF at the mRNA and protein levels, suggesting that IMD is a novel negative regulator of OC function and resists bone resorption.
In conclusion, the results of the presents study demonstrated that IMD decreased apoptosis and also decreased the RANKL/OPG ratio and the expression of M-CSF in MC3T3-E1 cells. These results provide insight into the molecule mechanism underlying the inhibitory effects of IMD on bone resorption.
Acknowledgments
This study was supported by the National Science Foundation of China (grant no. 81370981).
References
- 1.Roh J, Chang CL, Bhalla A, Klein C, Hsu SY. Intermedin is a calcitonin/calcitonin gene-related peptide family peptide acting through the calcitonin receptor-like receptor/receptor activity-modifying protein receptor complexes. J Biol Chem. 2004;279:7264–7274. doi: 10.1074/jbc.M305332200. [DOI] [PubMed] [Google Scholar]
- 2.Takei Y, Inoue K, Ogoshi M, Kawahara T, Bannai H, Miyano S. Identification of novel adrenomedullin in mammals: A potent cardiovascular and renal regulator. FEBS Lett. 2004;556:53–58. doi: 10.1016/S0014-5793(03)01368-1. [DOI] [PubMed] [Google Scholar]
- 3.Chauhan M, Balakrishnan M, Yallampalli U, Endsley J, Hankins GD, Theiler R, Yallampalli C. Adrenomedullin 2/intermedin regulates HLA-G in human trophoblasts. Biol Reprod. 2011;85:1232–1239. doi: 10.1095/biolreprod.110.086835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takahashi K, Kikuchi K, Maruyama Y, Urabe T, Nakajima K, Sasano H, Imai Y, Murakami O, Totsune K. Immunocytochemical localization of adrenomedullin 2/intermedin-like immunore-activity in human hypothalamus, heart and kidney. Peptides. 2006;27:1383–1389. doi: 10.1016/j.peptides.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 5.Takahashi K, Morimoto R, Hirose T, Satoh F, Totsune K. Adrenomedullin 2/intermedin in the hypothalamo-pituitary-adrenal axis. J Mol Neurosci. 2011;43:182–192. doi: 10.1007/s12031-010-9413-2. [DOI] [PubMed] [Google Scholar]
- 6.Chauhan M, Yallampalli U, Dong YL, Hankins GD, Yallampalli C. Expression of adrenomedullin 2 (ADM2)/intermedin (IMD) in human placenta: Role in trophoblast invasion and migration. Biol Reprod. 2009;81:777–783. doi: 10.1095/biolreprod.108.074419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McLatchie LM, Fraser NJ, Main MJ, Wise A, Brown J, Thompson N, Solari R, Lee MG, Foord SM. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature. 1998;393:333–339. doi: 10.1038/30666. [DOI] [PubMed] [Google Scholar]
- 8.Pan CS, Yang JH, Cai DY, Zhao J, Gerns H, Yang J, Chang JK, Tang CS, Qi YF. Cardiovascular effects of newly discovered peptide intermedin/adrenomedullin 2. Peptides. 2005;26:1640–1646. doi: 10.1016/j.peptides.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 9.Fujisawa Y, Nagai Y, Miyatake A, Takei Y, Miura K, Shoukouji T, Nishiyama A, Kimura S, Abe Y. Renal effects of a new member of adrenomedullin family, adrenomedullin2, in rats. Eur J Pharmacol. 2004;497:75–80. doi: 10.1016/j.ejphar.2004.06.039. [DOI] [PubMed] [Google Scholar]
- 10.Holmes D, Campbell M, Harbinson M, Bell D. Protective effects of intermedin on cardiovascular, pulmonary and renal diseases: Comparison with adrenomedullin and CGRP. Curr Protein Pept Sci. 2013;14:294–329. doi: 10.2174/13892037113149990049. [DOI] [PubMed] [Google Scholar]
- 11.Granholm S, Lundberg P, Lerner UH. Expression of the calcitonin receptor, calcitonin receptor-like receptor, and receptor activity modifying proteins during osteoclast differentiation. J Cell Biochem. 2008;104:920–933. doi: 10.1002/jcb.21674. [DOI] [PubMed] [Google Scholar]
- 12.Naot D, Cornish J. The role of peptides and receptors of the calcitonin family in the regulation of bone metabolism. Bone. 2008;43:813–818. doi: 10.1016/j.bone.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 13.Granholm S, Henning P, Lerner UH. Comparisons between the effects of calcitonin receptor-stimulating peptide and intermedin and other peptides in the calcitonin family on bone resorption and osteoclastogenesis. J Cell Biochem. 2011;112:3300–3312. doi: 10.1002/jcb.23256. [DOI] [PubMed] [Google Scholar]
- 14.Uzan B, de Vernejoul MC, Cressent M. RAMPs and CRLR expressions in osteoblastic cells after dexamethasone treatment. Biochem Biophys Res Commun. 2004;321:802–808. doi: 10.1016/j.bbrc.2004.07.037. [DOI] [PubMed] [Google Scholar]
- 15.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 16.Hsu WL, Chen CY, Tsauo JY, Yang RS. Balance control in elderly people with osteoporosis. J Formos Med Assoc. 2014;113:334–339. doi: 10.1016/j.jfma.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 17.Manolagas SC, Jilka RL. Bone marrow, cytokines and bone remodeling. Emerging insights into the pathophysiology of osteoporosis. N Engl J Med. 1995;332:305–311. doi: 10.1056/NEJM199502023320506. [DOI] [PubMed] [Google Scholar]
- 18.Brunner M, Jurdic P, Tuckerman JP, Block MR, Bouvard D. New insights into adhesion signaling in bone formation. Int Rev Cell Mol Biol. 2013;305:1–68. doi: 10.1016/B978-0-12-407695-2.00001-9. [DOI] [PubMed] [Google Scholar]
- 19.Roodman GD. Advances in bone biology: The osteoclast. Endocr Rev. 1996;17:308–332. doi: 10.1210/edrv-17-4-308. [DOI] [PubMed] [Google Scholar]
- 20.Roux S, Orcel P. Bone loss. Factors that regulate osteoclast differentiation: An update. Arthritis Res. 2000;2:451–456. doi: 10.1186/ar127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reid IR. Anti-resorptive therapies for osteoporosis. Semin Cell Dev Biol. 2008;19:473–478. doi: 10.1016/j.semcdb.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 22.Long F. Building strong bones: Molecular regulation of the osteoblast lineage. Nat Rev Mol Cell Biol. 2011;13:27–38. doi: 10.1038/nrm3254. [DOI] [PubMed] [Google Scholar]
- 23.Matsumura S, Hiranuma H, Deguchi A, Maeda T, Jikko A, Fuchihata H. Changes in phenotypic expression of osteoblasts after X irradiation. Radiat Res. 1998;149:463–471. doi: 10.2307/3579786. [DOI] [PubMed] [Google Scholar]
- 24.Matsuguchi T, Chiba N, Bandow K, Kakimoto K, Masuda A, Ohnishi T. JNK activity is essential for Atf4 expression and late-stage osteoblast differentiation. J Bone Miner Res. 2009;24:398–410. doi: 10.1359/jbmr.081107. [DOI] [PubMed] [Google Scholar]
- 25.Kim HK, Cho SG, Kim JH, Doan TK, Hu QS, Ulhaq R, Song EK, Yoon TR. Mevinolin enhances osteogenic genes (ALP, type I collagen and osteocalcin), CD44, CD47 and CD51 expression during osteogenic differentiation. Life Sci. 2009;84:290–295. doi: 10.1016/j.lfs.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 26.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 27.Zhao Q, Shao J, Chen W, Li YP. Osteoclast differentiation and gene regulation. Front Biosci. 2007;12:2519–2529. doi: 10.2741/2252. [DOI] [PubMed] [Google Scholar]
- 28.Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Lüthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, et al. Osteoprotegerin: A novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–319. doi: 10.1016/S0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 29.Martin T, Gooi JH, Sims NA. Molecular mechanisms in coupling of bone formation to resorption. Crit Rev Eukaryot Gene Expr. 2009;19:73–88. doi: 10.1615/CritRevEukarGeneExpr.v19.i1.40. [DOI] [PubMed] [Google Scholar]
- 30.Khosla S. Minireview: The OPG/RANKL/RANK system. Endocrinology. 2001;142:5050–5055. doi: 10.1210/endo.142.12.8536. [DOI] [PubMed] [Google Scholar]
- 31.Kaji H, Kanatani M, Sugimoto T, Chihara K. Statins modulate the levels of osteoprotegerin/receptor activator of NFkappaB ligand mRNA in mouse bone-cell cultures. Horm Metab Res. 2005;37:589–592. doi: 10.1055/s-2005-870538. [DOI] [PubMed] [Google Scholar]
- 32.Giuliani N, Colla S, Sala R, Moroni M, Lazzaretti M, La Monica S, Bonomini S, Hojden M, Sammarelli G, Barillè S, et al. Human myeloma cells stimulate the receptor activator of nuclear factor-kappa B ligand (RANKL) in T lymphocytes: A potential role in multiple myeloma bone disease. Blood. 2002;100:4615–4621. doi: 10.1182/blood-2002-04-1121. [DOI] [PubMed] [Google Scholar]
- 33.Udagawa N, Takahashi N, Akatsu T, Tanaka H, Sasaki T, Nishihara T, Koga T, Martin TJ, Suda T. Origin of osteoclasts: Mature monocytes and macrophages are capable of differentiating into osteoclasts under a suitable microenvironment prepared by bone marrow-derived stromal cells. Proc Natl Acad Sci USA. 1990;87:7260–7264. doi: 10.1073/pnas.87.18.7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jimi E, Akiyama S, Tsurukai T, Okahashi N, Kobayashi K, Udagawa N, Nishihara T, Takahashi N, Suda T. Osteoclast differentiation factor acts as a multifunctional regulator in murine osteoclast differentiation and function. J Immunol. 1999;163:434–442. [PubMed] [Google Scholar]
- 35.Abdallah BM, Stilgren LS, Nissen N, Kassem M, Jorgensen HR, Abrahamsen B. Increased RANKL/OPG mRNA ratio in iliac bone biopsies from women with hip fractures. Calcif Tissue Int. 2005;76:90–97. doi: 10.1007/s00223-004-0074-4. [DOI] [PubMed] [Google Scholar]