Abstract
Due to the fact that the treatment of breast cancer depends significantly on the molecular markers present in the cancer, including estrogen receptor (+), progesterone receptor (+) or erbB2 receptor (+), further investigation targeting triple-negative breast cancer (TNBC) subtypes may assist in elucidating the mechanisms of recurrence of TNBC and enable the identification of novel therapeutic strategies for patients with TNBC. The aim of the present study was to compare the gene expression profiles between TNBC samples that were identified as having recurrent and non-recurrent statuses. Between June 2011 and May 2012, a total of 30 patients with TNBC were examined using a follow-up period of at least 5 years. Their clinicopathological information was retrospectively reviewed and they were classified with a status either of recurrence [n=15 stage II (9), IIIA (2), IIIC (4)] or non-recurrence [n=15 stage II (6), IIIA (1), IIIC (8)]. The total RNA from tissue samples obtained from the recurrent and non-recurrent TNBC patients were used to performed oligonucleotide microarray analysis. The dataset was analyzed using GeneSpring software and validated using reverse transcription-quantitative polymerase chain reaction. Principal component analysis demonstrated that there was a marked difference in the gene expression distribution between the stage IIIc recurrent samples and early stage (stages IIa, IIb and IIIa) recurrent samples. In early stage recurrence, the significant pathway-associated upregulated genes were matrix metalloproteinases (MMPs) and genes associated with cancer cell migration (CDH2) and cell adhesion/motility (KRAS, CDC42, RAC1, ICAM and SRGAP2). By contrast, during stage IIIc recurrence, the significant pathway-associated upregulated genes in the recurrent samples were WNT signaling genes, including WNT 4 and WNT 16. It was concluded that there were markedly different distributions and gene expression profiles between stage IIIc recurrent TNBC tumors and early stage (IIa, IIb, IIIa) recurrent TNBC tumors, which provides important information for the development of effective treatment strategies for TNBC.
Keywords: breast cancer, triple-negative, subtype, gene expression, microarray
Introduction
Breast cancer is the most common type of invasive cancer in females worldwide (1,2). In addition to conventional prognostic factors, including tumor size, lymph node status, estrogen receptor (ER)/progesterone receptor (PR) status, erbB2 receptor (HER2) status, several other biological markers, including Ki67, tau and topo II, have been investigated in order to correlate their status with the prognosis of patients with breast cancer (3,4). Due to a lack of specific receptors, triple-negative (ER−, PR−, HER2−) breast cancer (TNBC) exhibits marked resistance to chemotherapy, hormone therapy and targeted therapy (5–7). The subtype comprises ~15% of all cases of breast cancer and results in tumors, which are typically larger in size and higher in grade, compared with other types of breast cancer.
The identification of intrinsic subtypes of breast cancer has provided a number of novel therapeutic targets and enabled the design of novel clinical trials (8). Due to the lack of specific therapeutic targets with TNBC, previous studies have aimed to identify novel molecular markers, including phosphoinositide 3-kinase, epidermal growth factor receptor (EGFR), cell-cycle regulatory proteins, heat shock proteins, epigenetic pathways and androgen receptors, and clinical trials have been performed (8,9). In this context, the loss of androgen receptor expression has been found to predict early recurrence in triple-negative and basal-like breast cancer (10). However, the therapeutic outcomes have not reached initial expectations.
Previous genome-wide association investigations have identified genetic variants, which are associated with an increased risk of developing breast cancer. Furthermore, gene expression profile analyses have provided multi-gene signatures associated with TNBC carcinogenesis (11,12). Although investigations are increasingly focussing on the recurrence (5,6,13) and treatment (7) of TNBC, the molecular profiles of paired specimens obtained from patients with and without subsequent recurrence require further investigation. Therefore, the aim of the present study was to investigate and compare the gene expression profiles obtained from patients with TNBC, with and without recurrent status.
Patients and methods
Human TNBC tissue samples
In the present study, 30 patients, who had been diagnosed with TNBC based on pathological confirmation (ER <1%; PR <1%; HER2, not amplified) and who had been followed up for ≥5 years were recruited between June 2011 and May 2012. The tumor stage of these patients was determined using the American Joint Committee on Cancer TNM system (version 6; Springer, Inc., New York, NY, USA). The clinicopathological information was retrospectively reviewed and identified as either recurrent [n=15, stage II (9), IIIA (2), IIIC (4)] or non-recurrent [n=15, stage II (6), IIIA (1), IIIC (8)] at the end of the investigation period (2011–2012). Subsequent to obtaining informed consent from the patients, tumor specimens were obtained during surgery and were stored in liquid nitrogen under the regulations of Taipei Veterans General Hospital (Taipei, China). The present study was approved by the institutional review board of the hospital (2011-06-001GCF; Taipei, China) for microarray gene expression profiling analysis. The tumor tissues were divided into two groups; the recurrent group, defined as the patients being having a recurrence status years later, and the non-recurrent group, defined as those without recurrence at the end of the investigation.
Isolation of RNA from tumor tissues and oligonucleotide microarray analysis
Total RNA was isolated using a modified single-step guanidinium thiocyanate method (TRIzol reagent; cat. no. T-9424; Sigma-Aldrich, St. Louis, MO, USA). The concentration of total RNA required for oligonucleotide micro-array analysis was ≥0.6 g/l with a quality ratio ≥1.8 for absorbance at 260/280 nm and 260/230 nm using a NanoDrop 1000 (Thermo Fisher Scientific, Pittsburgh, PA, USA). Suitable RNA samples were then sent to Genome Research Center, National Yang-Ming University (Taiwan, China), for analysis with the human Affymetrix expression set, Hu-133 2.0 (Affymetrix, Inc., Santa Clara, CA, USA). The dataset obtained was analyzed following normalization against the global signal. The relative expression levels for each gene were obtained using GeneSpring software (GeneSpring GX 11.5; Agilent Technologies, Inc., Santa Clara, CA, USA) and further pathway analysis using Ingenuity Pathway Analysis software (IPA; Qiagen, Redwood City, CA, USA).
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Following extraction of tumor total RNA, complementary DNA (cDNA) was created using a First Strand cDNA Synthesis kit (Invitrogen Life Technologies, CA, USA). TaqMan® Gene Expression Assays (Invitrogen Life Technologies) were then used to validate the differential expression at the mRNA level of various identified genes, such as MMP9 and SERPINE1 set in the recurrent and non-recurrent TNBC samples. The TaqMan system is supported by a well established primer database, which significantly reduces experimental failure due to inappropriate primer design (Invitrogen Life Technologies). All samples were analyzed in triplicate.
Statistical analysis
Analysis of the microarray dataset was divided into three steps. Firstly, the differentially expressed genes were identified using Student's t-test and a repeated measures one-way analysis of variance. The purpose of this step was to identify a gene set associated with recurrent TNBC and with the various cancer grades. Secondly, clustering analysis was performed to validate the performance of the identified gene set, through separation of the different cancer groups, namely recurrent, vs. non-recurrent TNBCs and grade 1, vs. grade 2, vs. grade 3. The purpose of this step was to reduce the number of gene sets originally obtained and to obtain gene sets, which were optimal for cancer subtype classification. The final step was gene set annotation using GeneSpring software and IPA. Gene, Gene Ontology and pathway information were integrated in order to evaluate the biomedical importance of the identified gene sets.
Results
Clinical and pathological information, which was obtained by retrospective review completed at the end of 2012, resulted in two groups of patients, which were divided into those with subsequent recurrence [n=15, stage II (9), IIIA (2), IIIC (4)] and those without recurrence [n=15, stage II (6), IIIA (1), IIIC (8)], as shown in Fig. 1.
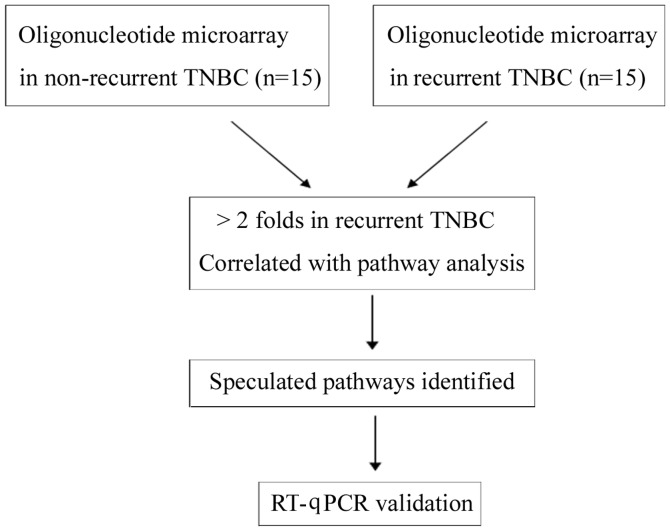
Figure 1.
Experimental protocols. TNBC, triple-negative breast cancer; RT-qPXR, reverse transcription-quantitative polymerase chain reaction.
Hierarchical clustering analysis of 30 patients with triple-negative breast cancer
A microarray dataset of the 30 TNBC samples was collected and a total 54,675 genes were eligible for analysis. Gene expression data were normalized against normal tissue controls using quantile normalization. The molecular characteristics of the TNBC were investigated at the mRNA level, according to previously described criteria (8). Hierarchical clustering analysis of the 30 TNBC samples, of which 15 were non-recurrent and 15 were recurrent, was performed to identify genes that were upregulated by ≥2-fold. The results demonstrated no significant clustering among the non-recurrent and recurrent samples (data not shown).
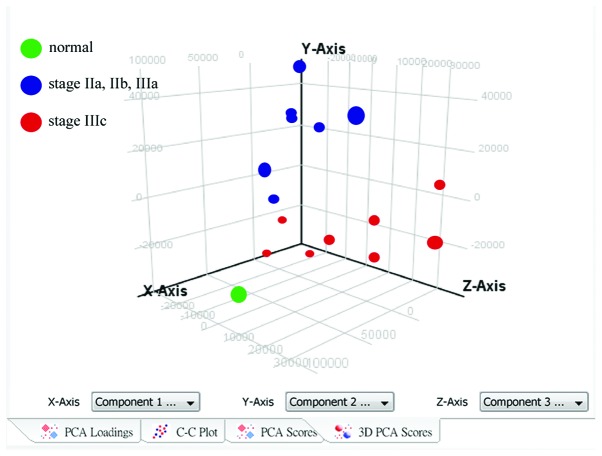
Principal component analysis of 15 patients with recurrent triple-negative breast cancer
To investigate the level of heterogeneity within the recurrent tumor samples, principal component analysis was performed and the results demonstrated a marked difference in distribution between the stage IIIc recurrent samples and the early stage (stage IIa, IIb, IIIa) recurrent samples (Fig. 2). Thedr results suggested that the gene expression profiles of the stage IIIc TNBC tumors were different from those of the early stage TNBC tumors, and they were also different from those of the stage IV, metastatic tumors (data not shown).
Figure 2.
Principal component analysis of 15 recurrent triple-negative breast cancer samples. To investigate heterogeneity in the recurrent tumor samples, principal component analysis was performed with GeneSpring software. The results revealed that there was a markedly different distribution between the stage IIIc samples and the stage IIa, IIb and IIIa recurrent samples.
A number of significant pathways involving specific genes were either upregulated or downregulated during the early stages of recurrent TNBC. For validation of the microarray information, RT-qPCR was performed on selected genes, including MMP9 (Fig. 3A) and SERPINE1 (Fig. 3B).
Figure 3.
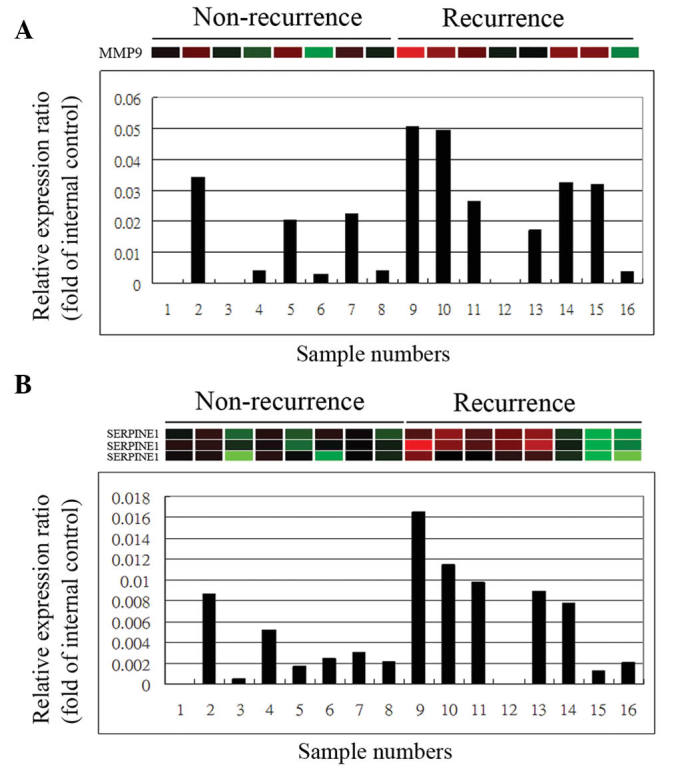
Reverse transcription-quantitative polymerase chain reaction for validation of the gene expression profiles. Subsequent to extracting total RNA from the tumors, complementary DNA was created using a First Strand cDNA Synthesis kit. Gene expression assays were then used to validate the differential mRNA expression levels of various identified genes, including (A) MMP9 and (B) SERPINE1 in the recurrent and non-recurrent triple-negative breast cancer samples. MMP9, matrix metalloproteinase 9.
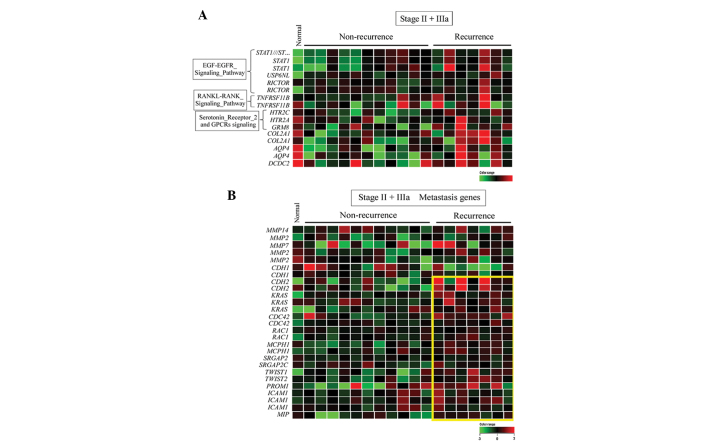
Using bioinformatics software analysis, the 30 TNBC samples were further stratified into four subgroups depending on stage, classified as early stage (IIa, IIb, IIIa) or stage IIIc, and recurrence, classified as non-recurrent or recurrent. In early stage recurrence, the significant pathways with upregulated genes in the recurrence samples included the osteoblast and ossification pathway, the serotonin receptor 2 pathway, various prostate cancer genes and the epidermal growth factor (EGF)-EGFR pathway (Fig. 4A; Table I); while the significant pathways with downregulated genes included the actin cytoskeleton pathway, the G protein pathway, the inflammatory response pathway and the insulin synthesis pathway (Table II).
Figure 4.
Heat map representation of (A) pathway- and (B) metastasis-associated upregulated genes in early stage recurrent triple-negative breast cancer. Each row represents a gene and each column represents a patient. Early stage is defined as stages IIa, IIb and IIIa. Red indicates upregulation, green indicates downregulation and black indicates no change. The yellow box indicates genes that are upregulated in tumors with subsequent recurrence compared to those without. The expression ratios ranged between −3 and 3 on a log scale.
Table I.
Significant pathways associated with upregulated genes in early astage recurrent triple-negative breast cancer.
| Pathway | P-value |
|---|---|
| Hs_Osteoblast_Signaling_WP322_53892 | 0.00118489 |
| Hs_Endochondral_Ossification_WP474_45241 | 0.00172066 |
| Hs_Serotonin_Receptor_2_and_ELK-SRF-GATA4_signaling_WP732_49572 | 0.00175804 |
| Hs_GPCRs,_Other_WP117_45343 | 0.00466699 |
| Hs_Spinal_Cord_Injury_WP2431_56064 | 0.00606436 |
| Hs_Monoamine_GPCRs_WP58_48221 | 0.00656731 |
| Hs_Integrated_Cancer_pathway_WP1971_44858 | 0.00777973 |
| Hs_Prostate_Cancer_WP2263_54728 | 0.00807849 |
| Hs_Serotonin_Receptor_2_and_STAT3_Signaling_WP733_45035 | 0.01460303 |
| Hs_EGF-EGFR_Signaling_Pathway_WP437_47973 | 0.01537961 |
| Hs_RANKL-RANK_Signaling_Pathway_WP2018_48442 | 0.01752249 |
| Hs_MAP_kinase_cascade_WP1844_44888 | 0.01822052 |
| Hs_Myometrial_Relaxation_and_Contraction_Pathways_WP289_45373 | 0.01972625 |
| Hs_SIDS_Susceptibility_Pathways_WP706_55364 | 0.02248320 |
| Hs_Oncostatin_M_Signaling_Pathway_WP2374_54418 | 0.02260932 |
| Hs_Glial_Cell_Differentiation_WP2276_53125 | 0.02541592 |
| Hs_Heme_Biosynthesis_WP561_45350 | 0.03255886 |
| Hs_Apoptosis_Modulation_and_Signaling_WP1772_44957 | 0.03513467 |
| Hs_APC-C-mediated_degradation_of_cell_cycle_proteins_WP1782_44955 | 0.03611077 |
Early stage was defined as stages IIa, IIb and IIIa.
Table II.
Significant pathways associated with downregulated genes in early stagea recurrent triple-negative breast cancer.
| Pathway | P-value |
|---|---|
| Hs_Calcium_Regulation_in_the_Cardiac_Cell_WP536_44983 | 7.37E-05 |
| Hs_Biogenic_Amine_Synthesis_WP550_44978 | 3.17E-04 |
| Hs_Regulation_of_Actin_Cytoskeleton_WP51_49262 | 3.96E-04 |
| Hs_Regulation_of_beta-cell_development_WP1897_45053 | 5.57E-04 |
| Hs_Myometrial_Relaxation_and_Contraction_Pathways_WP289_45373 | 5.90E-04 |
| Hs_G_Protein_Signaling_Pathways_WP35_45294 | 0.00159171 |
| Hs_Neural_Crest_Differentiation_WP2064_47071 | 0.00229661 |
| Hs_Hypothetical_Network_for_Drug_Addiction_WP666_45365 | 0.00281034 |
| Hs_Benzo(a)pyrene_metabolism_WP696_44980 | 0.00286947 |
| Hs_Inflammatory_Response_Pathway_WP453_41201 | 0.00308023 |
| Hs_Selenium_Pathway_WP15_56489 | 0.00656218 |
| Hs_Osteoblast_Signaling_WP322_53892 | 0.00703770 |
| Hs_Insulin_Synthesis_and_Processing_WP1830_44856 | 0.00703770 |
| Hs_Tryptophan_metabolism_WP465_45056 | 0.00858096 |
| Hs_Estrogen_signaling_pathway_WP712_48214 | 0.01417347 |
| Hs_SIDS_Susceptibility_Pathways_WP706_55364 | 0.01731102 |
| Hs_EPO_Receptor_Signaling_WP581_41162 | 0.02338902 |
| Hs_miRs_in_Muscle_Cell_Differentiation_WP2012_45377 | 0.03248199 |
| Hs_Metabolism_of_amino_acids_and_derivatives_WP1847_52373 | 0.03248199 |
| Hs_Endothelin_WP2197_56443 | 0.03644705 |
Early stage was defined as stage IIa, IIb, IIIa.
Metastasis-associated gene expression in early stage recurrent TNBC
Since metastasis is a mechanism associated with tumor recurrence in TNBC, a subset of overexpressed genes were identified in tumor samples obtained from the patients with early stage (IIa, IIb or IIIa) TNBC, who had undergone subsequent tumor recurrence. These included genes encoding MMPs, genes involved in cancer cell migration (CDH2) and genes involved in cell adhesion/motility (KRAS, CDC42, RAC1, ICAM and SRGAP2) (Fig. 4B). Notably, genes associated with tumor stemness and angiogenesis were not overexpressed in these tumor samples.
Significant pathways in stage IIIc recurrent TNBC associated with upregulated and downregulated genes
In contrast to the observations for early recurrence, the significant pathways involving upregulated genes present in stage IIIc recurrence samples included the WNT signaling pathway, glycogen metabolism pathway, integrated pancreatic cancer pathway, mitogen-activated protein kinase (MAPK) cascade pathway, brain-derived neurotrophic factor (BDNF) signaling pathway and prostaglandin synthesis pathway (Table III), while the significant pathways involving downregulated genes in the stage IIIc recurrence samples included the AMPK signaling pathway, interleukin (IL)-2 signaling pathway and BDNF signaling pathway (Table IV).
Table III.
Significant pathways associated with upregulated genes in stage IIIc recurrent triple-negative breast cancer.
| Pathway | P-value |
|---|---|
| Hs_Factors_involved_in_megakaryocyte_development_and_platelet_production_WP1815_42038 | 8.41E-05 |
| Hs_Wnt_Signaling_Pathway_and_Pluripotency_WP399_54212 | 0.00149959 |
| Hs_Wnt_Signaling_Pathway_WP428_45008 | 0.00162369 |
| Hs_Nuclear_receptors_in_lipid_metabolism_and_toxicity_WP299_45336 | 0.00262814 |
| Hs_Glycogen_Metabolism_WP500_45329 | 0.00337628 |
| Hs_G13_Signaling_Pathway_WP524_45304 | 0.00365162 |
| Hs_Integrated_Pancreatic_Cancer_Pathway_WP2256_54274 | 0.00683842 |
| Hs_Integrated_Pancreatic_Cancer_Pathway_WP2377_56677 | 0.00683842 |
| Hs_G_Protein_Signaling_Pathways_WP35_45294 | 0.00755248 |
| Hs_Calcium_Regulation_in_the_Cardiac_Cell_WP536_44983 | 0.00855703 |
| Hs_Synaptic_Vesicle_Pathway_WP2267_56444 | 0.00898066 |
| Hs_Eicosanoid_Synthesis_WP167_45234 | 0.01199396 |
| Hs_Muscle_contraction_WP1864_44927 | 0.01665074 |
| Hs_MAPK_Cascade_WP422_44889 | 0.02439614 |
| Hs_Prostaglandin_Synthesis_and_Regulation_WP98_45273 | 0.02763810 |
| Hs_BDNF_signaling_pathway_WP2380_54595 | 0.03042269 |
| Hs_Endothelin_WP2197_56443 | 0.03104245 |
| Hs_Vitamin_A_and_carotenoid_metabolism_WP716_52902 | 0.04216790 |
| Hs_DNA_damage_response_(only_ATM_dependent)_WP710_46091 | 0.04453868 |
Table IV.
Significant pathways associated with downregulated genes in stage IIIc recurrent triple-negative breast cancer.
| Pathway | P-value |
|---|---|
| Hs_Inflammatory_Response_Pathway_WP453_41201 | 2.37E-04 |
| Hs_Synaptic_Vesicle_Pathway_WP2267_56444 | 9.45E-04 |
| Hs_GPCRs,_Class_C_Metabotropic_glutamate,_pheromone_WP501_45341 | 0.00142081 |
| Hs_AMPK_signaling_WP1403_44950 | 0.00217159 |
| Hs_Transport_of_inorganic_cations-anions_and_amino_acids-oligopeptides_WP1936_45061Hs_Binding_of_RNA_by_Insulin-like_Growth_Factor-2_mRNA_Binding_Proteins_f(IGF2BPs-IMPs-VICKZs)_WP1789_44977 | 0.00530772 0.01120121 |
| Hs_IL-2_Signaling_pathway_WP49_48400 | 0.01140687 |
| Hs_BDNF_signaling_pathway_WP2380_54595 | 0.01594213 |
| Hs_Regulation_of_Actin_Cytoskeleton_WP51_49262 | 0.01748728 |
| Hs_Calcium_Regulation_in_the_Cardiac_Cell_WP536_44983 | 0.01878150 |
| Hs_GPCR_downstream_signaling_WP1824_42047 | 0.02959318 |
| Hs_Arrhythmogenic_right_ventricular_cardiomyopathy_WP2118_47057 | 0.03163952 |
| Hs_Focal_Adhesion_WP306_45270 | 0.03231586 |
| Hs_Peroxisomal_lipid_metabolism_WP1878_45246 | 0.03323053 |
| Hs_RalA_downstream_regulated_genes_WP2290_53118 | 0.03323053 |
| Hs_Integrated_Pancreatic_Cancer_Pathway_WP2256_54274 | 0.03932082 |
| Hs_Integrated_Pancreatic_Cancer_Pathway_WP2377_56677 | 0.03932082 |
| Hs_BMP_signalling_and_regulation_WP1425_44981 | 0.04406138 |
| Hs_Neurotransmitter_Release_Cycle_WP1871_42089 | 0.04406138 |
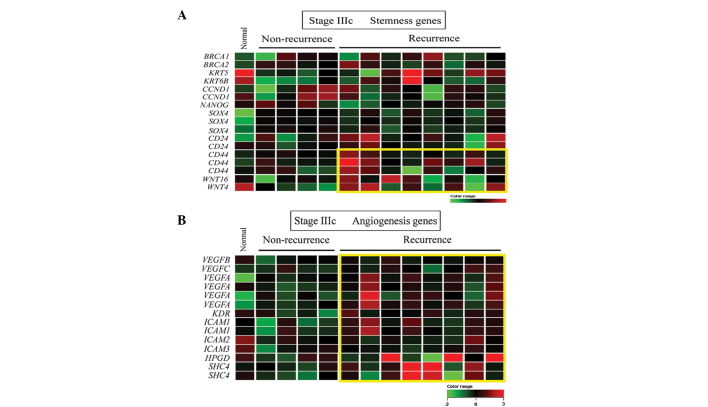
Stemness-associated gene expression in stage IIIc recurrent TNBC
Since cancer stemness involves self-renewal ability and aggressive behavior, it was not unexpected that a subset of overexpressed stemness genes, including CD44, WNT 4 and WNT 16, were identified in tumor samples obtained from patients with late stage (IIIc) TNBC and subsequent recurrence (Fig. 5A). In addition, a gene set associated with angiogenesis (Fig. 5B), but not metastasis, was to be overexpressed in these tumor samples.
Figure 5.
Heat map representation of (A) stemness-associated and (B) angiogenesis-associated upregulated genes in stage IIIc recurrent triple-negative breast cancer. Each row represents a gene and each column represents a patient. Red indicates upregulatio, green indicates downregulation and black indicates no change. The yellow box indicates genes that are upregulated in tumors with subsequent recurrence compared to those without. The expression ratios ranged between −3 and 3 on a log scale.
Functional pathway analysis
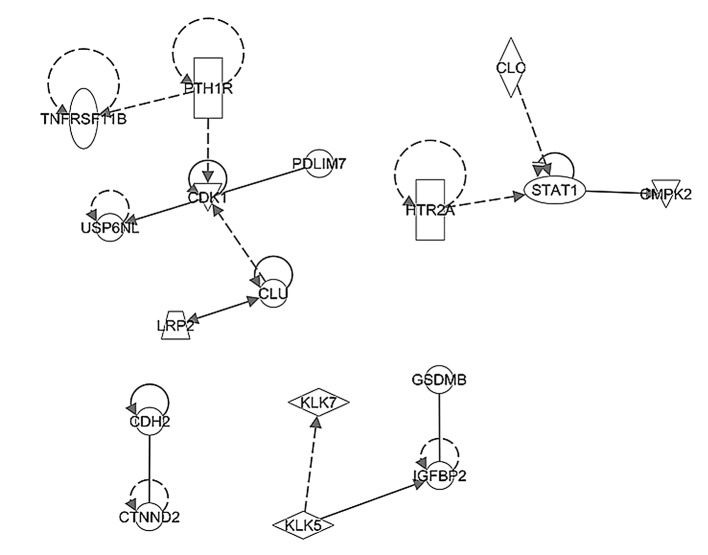
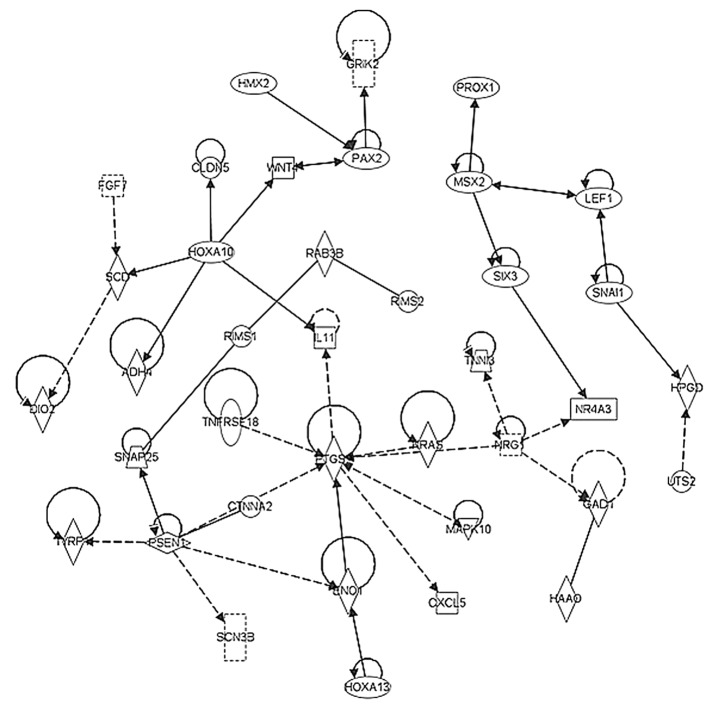
In order to elucidate the possible networks involving the pathways identified in the present study, the above-mentioned upregulated and downregulated genes in the early stage and stage IIIc tumor samples were investigated using ingenuity pathway analysis. The network shown in Fig 6 represents the significant pathway network incorporating CDK1 and STAT1, which regulated cell growth in the early stage TNBC tumor samples. By contrast, Fig. 7 shows the significant pathway network incorporating PTGS, RAS and WNT, which promoted angiogenesis and stemness in the late stage TNBC tumors.
Figure 6.
Pathway network analyses of upregulated genes from early stage (stages II and IIIa) TNBC. Arrow direction indicates a functional association between an upstream regulator and a downstream element. The results demonstrated that there were significant pathway networks involving CDK1 and STAT1, regulating cell growth of early stage TNBC tumors. TNBC, triple-negative breast cancer.
Figure 7.
Pathway network analyses on upregulated genes from late stage (stage IIIc) TNBC. Arrow direction indicates a functional association between an upstream regulator and a downstream element. The results demonstrated that there were significant pathway networks involving PTGS, RAS and WNT, promoting angiogenesis and stemness of late stage TNBC tumors. TNBC, triple-negative breast cancer.
Discussion
It has been suggested that TNBC is not a complete proxy for basal-like breast cancer. Although an appreciation of the significance of basal-like breast cancers predates gene-expression investigations by a number of years, this term was not in widespread use until later (14,15). Several of the most well-known genes associated with basal-like breast cancer, including keratin 5 and keratin 17, do not differ significantly different between caucasian and Asian populations (16). This observation is contradictory to the fact that the epidemiology and prognosis of breast cancer between different ethnicities is generally reported to be different (17). There is no internationally accepted definition of these tumors. However, the majority of basal-like cancers are also TNBCs, and the majority of cases of TNBC (~80%) are also cases of basal-like breast cancer; therefore, it has been suggested that the triple-negative phenotype and the basal-like phenotype are effectively synonymous (15,16). However, clinical, microarray and immunohistochemical observations have demonstrated that this is not the case (18). The present study enrolled TNBC cases with ER (<1%), PR (<1%) and non amplified HER2. Furthermore, in the present study, the gene expression levels of basal-like breast cancer specific genes, including keratin 5 and keratin 17, were not uniquely upregulated, which was in agreement with those previously reported in Asian TNBC patients (16), indicating that these types of cancer do not possess basal-like breast cancer characteristics.
Compared with previous study designs that used tumor and non-tumor samples for TNBC analysis, the present study was the first, to the best of out knowledge, to focus on the analysis of paired recurrent, vs. non-recurrent TNBC samples. Therefore, the design of the present study was likely to identify potentially important biomarkers and therapeutic targets for the treatment and prevention of TNBC recurrence. It was hypothesized that the TNBC population is heterogeneous and that various subgroups will exhibit different, and possibly specific, expression signatures. The identification of such signatures offers potential perspective into the individualized treatment protocols that are available. These signaling pathways are also likely to be involved in the upregulation of metastatic and/or cancer cell self-renewal genes, leading to higher levels of metastatic activity and resulting in the recurrence of TNBC.
TNBC is a highly diverse type of cancer, and subtyping of TNBC tumors is necessary in order to identify appropriate molecular-based therapies. Evolving technologies are permitting increasing quantities of molecular data to be obtained from tumor tissues, which enable the development of more personalized treatment strategies (19). In this context, whether all basal-like cancers are enriched with cancer stem cells or whether they have a disproportionately high content of cells undergoing epithelial-to-mesenchymal transition (EMT) remains to be elucidated (20). In the present study, which focused on the molecular mechanisms in paired specimens obtained from patients with and without recurrence, the results suggested that the significant pathways involving upregulated genes that are present in stage IIIc recurrent samples included the WNT, glycogen metabolism, integrated pancreatic cancer, MAPK cascade, BDNF and prostaglandin signaling pathways. In addition, the presence of overexpressed stemness genes, including CD44, WNT 4 and WNT 16, were also identified. By contrast, a different subset of overexpressed genes, which included genes encoding MMPs, and genes involved in the cancer cell migration (CDH2) pathway, cell adhesion or motility (KRAS, CDC42, RAC1, ICAM and SRGAP2) and EMT (TWIST1), were identified in the tumor samples obtained from patients with early (stage IIa, IIb, IIIa) tumors. These observations may be useful for biomarker selection, drug discovery and clinical trial design, all of which may assist in the identification of appropriate targeted therapies for patients with TNBC (13).
Kuo et al (13) reported that deregulated genes within the transforming growth factor (TGF)-β signaling pathway were markedly involved in the distant recurrence of TNBC (13). The overexpression of TGF-β1 has been observed to be mediated by two upstream regulators, tumor necrosis factor and IL-1β, which are known mediators of the immune/inflammatory response; furthermore, TGF-β1 itself is crucial to the regulation of T cell-mediated immunity (21). In the present study, similar deregulation of the inflammatory response pathway and the IL-2 signaling pathway were observed in the samples from patients with stage IIIc TNBC recurrence. Taken together, these findings suggested that the distant metastatic invasion of TNBC may be induced by immune/inflammatory deregulation.
According to the St Gallen consensus for chemotherapy guidelines (22), all triple-negative patients are recommended to receive adjuvant systemic chemotherapy in combination of anthracycline-based regimens with taxanes, however, this approach often results in serious side effects in patients. A number of pathway-targeted agents, including EGFR inhibitors, DNA repair pathway inhibitors and anti-angiogenic agents, have been used in clinical trials as targeted therapies for TNBC (7,23). These may be used, alongside traditional chemotherapy treatments, to treat triple-negative patients with an unfavorable prognosis. The gene profiling in the current study may provide a prognostic predictor and, thus may become a clinically useful tool for the identification of triple-negative patients who are at low risk of recurrence. The subsequent provision of moderate doses of combined regimens, or the anthracycline-based regimens alone, in these patients can be offered to reduce patient side effects. Among the stage IIIc recurrence group, the prostaglandin synthesis and regulation signaling pathway exhibited significant alterations in expression. COX-2, an inducible form of cyclooxygenase, is the rate limiting step in the production of prostaglandins, which has been suggested to be involved in long-term inflammation and the promotion of cancer growth. Therefore, the results of the present study suggest that this pathway is likely to be important in the late stages of tumor growth and metastasis.
Acknowledgments
The present study was supported, in part, by the Division of Experimental Surgery of the Department of Surgery, Taipei Veterans General Hospital (Taiwan, China; grant no. 101DHA0100010) and the Ministry of Health and Welfare (Center of Excellence for Cancer Research at Taipei Veterans General Hospital Phase II; M OHW103-TD-B-111-02).
References
- 1.Lin YS, Jen YM, Wang BB, Lee JC, Kang BH. Epidemiology of oral cavity cancer in taiwan with emphasis on the role of betel nut chewing. ORL J Otorhinolaryngol Relat Spec. 2005;67:230–236. doi: 10.1159/000089214. [DOI] [PubMed] [Google Scholar]
- 2.DeSantis C, Siegel R, Bandi P, Jemal A. Breast cancer statistics, 2011. CA Cancer J Clin. 2011;61:409–418. doi: 10.3322/caac.20134. [DOI] [PubMed] [Google Scholar]
- 3.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 4.Gradishar WJ. Tamoxifen-what next? Oncologist. 2004;9:378–384. doi: 10.1634/theoncologist.9-4-378. [DOI] [PubMed] [Google Scholar]
- 5.Yamamoto Y, Iwase H. Clinicopathological features and treatment strategy for triple-negative breast cancer. Int J Clin Oncol. 2010;15:341–351. doi: 10.1007/s10147-010-0106-1. [DOI] [PubMed] [Google Scholar]
- 6.Podo F, Buydens LM, Degani H, Hilhorst R, Klipp E, Gribbestad IS, Van Huffel S, van Laarhoven HW, Luts J, Monleon D, et al. Triple-negative breast cancer: Present challenges and new perspectives. Mol Oncol. 2010;4:209–229. doi: 10.1016/j.molonc.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O'Shaughnessy J, Osborne C, Pippen JE, Yoffe M, Patt D, Rocha C, Koo IC, Sherman BM, Bradley C. Iniparib plus chemotherapy in metastatic triple-negative breast cancer. N Engl J Med. 2011;364:205–214. doi: 10.1056/NEJMoa1011418. [DOI] [PubMed] [Google Scholar]
- 8.Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 9.Mohamed A, Krajewski K, Cakar B, Ma CX. Targeted therapy for breast cancer. Am J Pathol. 2013;183:1096–1112. doi: 10.1016/j.ajpath.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 10.Thike AA, Yong-Zheng Chong L, Cheok PY, Li HH, Wai-Cheong Yip G, Huat Bay B, Tse GM, Iqbal J, Tan PH. Loss of androgen receptor expression predicts early recurrence in triple-negative and basal-like breast cancer. Mod Pathol. 2014;27:352–360. doi: 10.1038/modpathol.2013.145. [DOI] [PubMed] [Google Scholar]
- 11.Chen LH, Kuo WH, Tsai MH, Chen PC, Hsiao CK, Chuang EY, Chang LY, Hsieh FJ, Lai LC, Chang KJ. Identification of prognostic genes for recurrent risk prediction in triple negative breast cancer patients in Taiwan. PLoS One. 2011;6:e28222. doi: 10.1371/journal.pone.0028222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hicks C, Kumar R, Pannuti A, Backus K, Brown A, Monico J, Miele L. An integrative genomics approach for associating GWAS information with triple-negative breast cancer. Cancer Inform. 2013;12:1–20. doi: 10.4137/CIN.S10413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuo WH, Chang YY, Lai LC, Tsai MH, Hsiao CK, Chang KJ, Chuang EY. Molecular characteristics and metastasis predictor genes of triple-negative breast cancer: A clinical study of triple-negative breast carcinomas. PLoS One. 2012;7:e45831. doi: 10.1371/journal.pone.0045831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sotiriou C, Pusztai L. Gene-expression signatures in breast cancer. N Engl J Med. 2009;360:790–800. doi: 10.1056/NEJMra0801289. [DOI] [PubMed] [Google Scholar]
- 15.Kreike B, van Kouwenhove M, Horlings H, Weigelt B, Peterse H, Bartelink H, van de Vijver MJ. Gene expression profiling and histopathological characterization of triple-negative/basal-like breast carcinomas. Breast Cancer Res. 2007;9:R65. doi: 10.1186/bcr1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peppercorn J, Perou CM, Carey LA. Molecular subtypes in breast cancer evaluation and management: divide and conquer. Cancer Invest. 2008;26:1–10. doi: 10.1080/07357900701784238. [DOI] [PubMed] [Google Scholar]
- 17.Kurebayashi J, Moriya T, Ishida T, Hirakawa H, Kurosumi M, Akiyama F, Kinoshita T, Takei H, Takahashi K, Ikeda M. The prevalence of intrinsic subtypes and prognosis in breast cancer patients of different races. Breast. 2007;16(Suppl 2):S72–S77. doi: 10.1016/j.breast.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 18.Weigelt B, Baehner FL, Reis-Filho JS. The contribution of gene expression profiling to breast cancer classification, prognostication and prediction: A retrospective of the last decade. J Pathol. 2010;220:263–280. doi: 10.1002/path.2648. [DOI] [PubMed] [Google Scholar]
- 19.Rodenhiser DI, Andrews JD, Vandenberg TA, Chambers AF. Gene signatures of breast cancer progression and metastasis. Breast Cancer Res. 2011;13:201. doi: 10.1186/bcr2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zavadil J, Böttinger EP. TGF-beta and epithelial-to-mesenchymal transitions. Oncogene. 2005;24:5764–5774. doi: 10.1038/sj.onc.1208927. [DOI] [PubMed] [Google Scholar]
- 21.Liu Y, Teige I, Birnir B, Issazadeh-Navikas S. Neuron-mediated generation of regulatory T cells from encephalitogenic T cells suppresses EAE. Nat Med. 2006;12:518–525. doi: 10.1038/nm1402. [DOI] [PubMed] [Google Scholar]
- 22.Untch M, Gerber B, Harbeck N, et al. 13th st Gallen international breast cancer conference 2013: primary therapy of early breast cancer evidence, controversies, consensus - opinion of a german team of experts (zurich 2013) Breast Care (Basel) 2013;8:221–229. doi: 10.1159/000351692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hudis CA, Gianni L. Triple-negative breast cancer: An unmet medical need. Oncologist. 2011;16(Suppl 1):1–11. doi: 10.1634/theoncologist.2011-S1-01. [DOI] [PubMed] [Google Scholar]