Abstract
In cardiomyocytes, Ca2+ plays a central role in governing both contraction and signaling events that regulate gene expression. Current evidence indicates that discrimination between these two critical functions is achieved by segregating Ca2+ within subcellular microdomains: transcription is regulated by Ca2+ release within nuclear microdomains, and excitation–contraction coupling is regulated by cytosolic Ca2+. Accordingly, a variety of agonists that control cardiomyocyte gene expression, such as endothelin-1, angiotensin-II or insulin-like growth factor-1, share the feature of triggering nuclear Ca2+ signals. However, signaling pathways coupling surface receptor activation to nuclear Ca2+ release, and the phenotypic responses to such signals, differ between agonists. According to earlier hypotheses, the selective control of nuclear Ca2+ signals by activation of plasma membrane receptors relies on the strategic localization of inositol trisphosphate receptors at the nuclear envelope. There, they mediate Ca2+ release from perinuclear Ca2+ stores upon binding of inositol trisphosphate generated in the cytosol, which diffuses into the nucleus. More recently, identification of such receptors at nuclear membranes or perinuclear sarcolemmal invaginations has uncovered novel mechanisms whereby agonists control nuclear Ca2+ release. In this review, we discuss mechanisms for the selective control of nuclear Ca2+ signals with special focus on emerging models of agonist receptor activation.
Keywords: Nuclear Ca2+, Cardiomyocyte, Insulin-like growth factor-1, Endothelin-1, Angiotensin II, Sarcolemmal receptor
1. Introduction
Calcium homeostasis is regulated by the combined action of a variety of channels, transporters, and binding proteins which allow cells to increase or decrease intracellular Ca2+ concentration on demand [1]. Ca2+-releasing events, or Ca2+ transients, occur when Ca2+ channels embedded within either the plasma membrane or in select internal membranes open, allowing Ca2+ to move down its electrochemical gradient from either external sources or intracellular Ca2+ stores, flooding the cytosolic compartment. Cytosolic Ca2+ increases a remarkable 50-fold this way with each heart beat (0.1μM in diastole to ≈5μM in systole). Then, Ca2+ is rapidly removed from the cytosol by Na+–Ca2+ exchangers and ATP-dependent transporters that pump Ca2+ out of the cell or back into intracellular stores [2]. This Ca2+ cycle defines the Ca2+ transient, whereas repeated Ca2+ cycles comprise a Ca2+ oscillation [3,4].
Ca2+ oscillations can be tuned in frequency, amplitude, and duration, providing a biological signal with limitless possible combinations for encoding information [5]. Cardiac contraction provides an excellent example of the importance of Ca2+ oscillations, and the need to maintain them under fine control [6]. Under normal conditions, the human heart beats once every second, therefore, each cardiomyocyte undergoes a full, coordinated Ca2+ cycle nearly 60 times per minute [2]. Many biological inputs ultimately exert control over heart rate by impacting various components governing Ca2+ oscillation.
Although Ca2+ oscillations are central to driving cardiomyocyte contraction, non-contractile Ca2+-dependent signaling has emerged as an important regulatory mechanism of both transcriptional control and structural remodeling in the heart. In a wonderfully intricate manner, Ca2+ manages to regulate these processes independent of the whole-cell Ca2+ oscillations that drive contraction. Ca2+-mediated changes in gene expression often occur in response to agonist binding to receptors at the plasma membrane, or sarcolemma [7,8]. This mechanism allows cells to reprogram their gene expression profiles to meet ever-changing cardiac demand. Ca2+-mediated signaling can also influence transcriptional control of cardiomyocyte development [9], differentiation [10], survival [11], hypertrophic growth [12,13], metabolism [14] and cell death [11]. At present, we are only beginning to understand how a cardiomyocyte decodes a Ca2+ signal to alter gene expression without interfering with, or being controlled by, the essential and ongoing process of contraction [15]. A growing body of evidence indicates that such discrimination is accomplished by triggering local Ca2+ release in segregated subcellular compartments (cytosol versus nucleus) or specific sub-regions of these compartments, generating microdomains of localized Ca2+-signaling events. In this review, we focus on mechanisms currently proposed to explain such selective control of nuclear Ca2+ signals.
2. Cytosolic source of nuclear Ca2+ signals
Although it is currently controversial whether the initiation of nuclear Ca2+ signals derives from cytosolic Ca2+ entry into the nucleus, or generated by the nuclear release of Ca2+, there is evidence that both mechanisms exist in cardiomyocytes (as summarized in Fig. 1). Indeed, several studies in cardiomyocytes and other cell types suggest that elevations nuclear Ca2+ are the direct consequence of changes in cytosolic Ca2+ [16–18]. On the other hand, it has also been shown in different cardiac muscle cells that changes in nuclear Ca2+ can be regulated independent of cytosolic Ca2+ and derive from Ca2+ released inside, or in close proximity to, the nucleus [18–20].
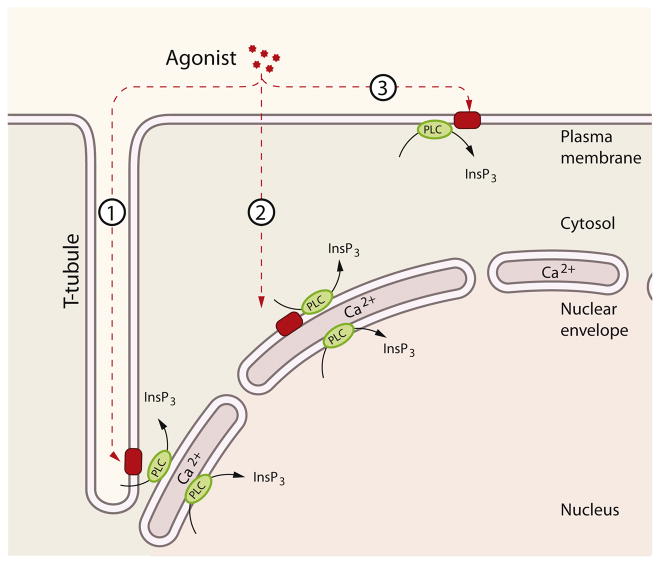
Fig. 1.
Nuclear and cytosolic pathways controlling nuclear Ca2+ homeostasis in cardiomyocytes. Different pathways can lead to nuclear Ca2+ signals. Pathway 1: InsP3 is generated by PLC either in the plasma membrane or in peri/intra-nuclear compartments. Pathway 2: InsP3 can diffuse in and out of the nucleus. Pathway 3: InsP3 from cytosolic or nuclear origin triggers Ca2+ release inside and/or outside the nucleus. Pathway 4: Ca2+ triggers Ca2+ release from RyRs in the cytoplasm or in the nucleus of cardiomyocytes (nuclear RyR is present only in neonatal cardiac myocytes). Pathway 5: Ca2+ diffusion from the cytosol can also generate nuclear Ca2+ transients and vice versa.
2.1. Ca2+ diffusion through the nuclear pore complex
It was initially held that primary access for Ca2+ to the nuclear compartment occurred through passive diffusion of cytosolic Ca2+ through nuclear pores connecting the nucleus and the cytoplasm. This is a reasonable hypothesis given that the nuclear pore complex, a multiprotein structure integral to the nuclear envelope (NE), has an approximate diameter of 8nm although this was estimated in isolated Xenopus oocyte nuclei [21]. Although Ca2+ has an ionic radius of only 0.99°Å, its hydrophobicity in solution gives it an effective diameter of 12Å per hydrated ion (or 1.2nm). This would allow unlimited traffic of Ca2+ ions between the cytoplasm and nucleus as postulated by pioneer studies in amphibian and insect cells [22,23].
The concept of passive diffusion of Ca2+ into the nucleus from a cytoplasmic source is supported in cardiomyocytes by several lines of evidence, such as the observations of synchronous elevations in nuclear and cytoplasmic Ca2+ [16]. Indeed, the cytosolic Ca2+ wave propagated during cardiomyocyte contraction can invade the nucleoplasm via diffusion [16], favored by the lower Ca2+ buffer capacity of the nucleus [24]. In this model, the NE functions as a barrier and the nuclear pores provide the entryway for regulated diffusion of high cytosolic Ca2+, and this has also been observed in mouse neuroblastoma cells [25].
Like any gate, the nuclear pore is subject to regulation. Diffusion through nuclear pores is complex and regulated by several mechanisms, including passive diffusion of small molecules (up to 10kDa), Ca2+-regulated transport of intermediate molecules (10–70kDa) and also involves active transport for larger molecules [26]. Ca2+ itself can influence diffusion through nuclear pores, and Ca2+ store depletion decreases diffusion of intermediate but not small size molecules or ions [26–28]. Consistent with this, atomic force microscopy demonstrates that the nuclear pore complex is a dynamic structure capable of responding to changes in intracellular Ca2+ [29]. Indeed, some hormones that increase cytosolic Ca2+ levels also increase permeability of the nuclear pore complex [30]. Thus, regulation of nuclear pore function is complex, rendering it capable of either increasing or decreasing the traffic of Ca2+ and other middle size molecules into and out of the nucleus. The origin of a Ca2+ signal and its proximity to the nucleus can also be important factors influencing regulated diffusion of cytosolic Ca2+ into the nucleus [16,31,32].
2.2. Whole-cell Ca2+ oscillations
Propagation of cytosolic Ca2+ oscillations into the nucleus has also been reported in different cell types. In adrenal chromaffin and pituitary cells, both high and low frequency Ca2+ oscillations invade the nucleus, and interestingly high frequency oscillations are more able to do so than those of low frequency [33]. It is not clear whether in cardiomyocytes cytosolic Ca2+ diffusion into the nucleus during cardiac contraction is a function of the frequency and amplitude of cytosolic Ca2+ fluctuations, however, it has been proposed that during cardiomyocyte hypertrophy the nuclear machinery can decode distinct Ca2+ oscillatory patterns and respond specifically [34]. Distinct patterns of Ca2+ oscillations can alter the threshold of activation for Ca2+ as a second messenger by preferentially activating or inactivating distinct Ca2+-dependent compartments, resulting in directed, downstream changes in gene expression or cell function [35–38]. This may be of particular importance during inotropic and chronotropic responses where oscillatory patterns of Ca2+ handling are quickly shaped in response to hormonal stimulation.
3. Perinuclear sources of nuclear Ca2+ signals
A second hypothesis for the generation of nuclear Ca2+ signals has been proposed based on the ability of the nuclear envelope itself to function as a perinuclear Ca2+ store, capable of releasing Ca2+ into the nuclear matrix in response to stimulation [39]. This concept is supported by the identification of specific perinuclear structures that generate, modulate, and shape nuclear Ca2+ signals.
3.1. Perinuclear endoplasmic reticulum
Rough ER is distinguished by the abundance of attached protein-synthesizing ribosomes and is the type of ER most closely associated with the NE. Recent studies have explored the importance of a perinuclear subset of rough ER in the regulation of nucleoplasmic Ca2+. Over-expression of calsequestrin-2 targeted to the perinuclear rough ER promotes amplified RyR2-mediated nuclear Ca2+ transients in both adult and neonatal cardiomyocytes [40], suggesting that perinuclear rough ER acts as a source, in addition to the NE, for nucleoplasmic Ca2+ transients. For instance, in atrial cardiomyocytes, cytosolic Ca2+ sparks must be close to the nucleus (within 5μm) to contribute to a nuclear Ca2+ signal [41], demonstrating the importance of localized events in the perinuclear endoplasmic reticulum for nuclear Ca2+ signaling.
3.2. The nuclear envelope
Two membranes comprise the NE: the outer nuclear membrane (ONM), which is a structure continuous with the endoplasmic reticulum (ER), and the inner nuclear membrane (INM), which is in direct contact with the nuclear matrix. The perinuclear space (PS) is the space between the ONM and INM [42]. The NE of cardiomyocytes harbors Ca2+, which can generate Ca2+ signals independent of cytosolic Ca2+ [26,39,43]. Fluorescence recovery after photobleaching of Fluo-5N in intact adult cardiomyocytes demonstrates that the lumen of the sarcoplasmic reticulum (SR) and the PS are interconnected, enabling rapid diffusion of intraluminal Ca2+ ions and limiting the generation of spatial gradients during Ca2+ release events [20]. In other words, Ca2+ inside the NE is connected to cytoplasmic Ca2+ stores and fully available for rapid signaling, only requiring nuclear Ca2+ channels to trigger release.
The ONM of embryonic chicken cardiomyocytes harbors the sarco/endoplasmic reticulum Ca2+-ATPase 2 (SERCA2), which actively transports Ca2+ from the cytosol surrounding the nucleus into the PS [44]. Although Ca2+-ATPases have not been identified in the INM, there is a Na+/Ca2+ exchanger complex in the IMM, suggesting a mechanism for maintaining Ca2+ homeostasis in the nucleoplasm through Na+/Ca2+ exchange and allowing restoration of resting Ca2+ levels by transfer of Ca2+ to the NE or PS [45,46]. Thus, in addition to the intraluminal equilibrium of Ca2+ stores between the PS and the SR, the NE participates in active uptake of Ca2+ from both inside and outside the nucleus, thereby exhibiting Ca2+ storage capabilities similar to the ER. Buffering of cytoplasmic Ca2+ by binding proteins and organelles in the cytoplasm can also directly and indirectly influence nuclear Ca2+ levels.
3.3. The nucleoplasmic reticulum
In 2003, a model, derived from SKHep1 epithelial cells, of the dynamic interconnections between ER and NE membranes was extended to structures inside the nuclear matrix, comprising a nucleoplasmic reticulum (NR). The NR is a continuous reticular network that invaginates the INM of the NE toward the nuclear matrix [47]. Subsequently, these nuclear structures were reported in neonatal and adult cardiomyocytes [48], as well as in many other mammalian and plant cells [49]. Interestingly, this discrete Ca2+ rich structure can regulate Ca2+ signals in specific subregions of the nucleus, as Ca2+ releasing channels are localized to specific regions of the NR, forming nucleoplasmic Ca2+-release hotspots.
Invaginations of both the ONM and INM into the nuclear matrix have been described in many cell types [49]. The lumen of these structures, or “NE tunnels” as they have been called, contains both ER and mitochondrial markers [50,51], indicating that they bring cytoplasmic elements and organelles in close physical proximity to the nuclear matrix. As a result, they comprise intranuclear signaling microdomains that can segregate, generate, or amplify Ca2+ release from the NE, possibly in response to T-tubule invaginations.
3.4. Perinuclear mitochondria
Mitochondria can also shape the dynamics of nuclear Ca2+ [50,52] by acting as a perinuclear buffer, thereby influencing the magnitude and duration of nuclear Ca2+ signals. Mitochondria are often found in close proximity to the ER, particularly in the perinuclear region, and can rapidly uptake Ca2+ released from InsP3-receptors, buffering the impact of InsP3-mediated cytoplasmic Ca2+ release [53]. During E–C coupling this same process of mitochondrial Ca2+ uptake through the VDAC/mitochondrial Ca2+ uniporter (MCU) complex can buffer the amplitude of cytosolic Ca2+ transients, theoretically limiting the passive diffusion of cytosolic Ca2+ into the nucleus [54].
All the above-mentioned sources of Ca2+ can contribute or shape nuclear Ca2+, but despite the multiple sources of nuclear Ca2+ signals, whether the origin is nuclear or perinuclear, remains in dispute. A recent report may help reconcile these two models. In situ calibration of Ca2+ concentrations in the nucleus and cytosol of murine adult cardiomyocytes reveals that nuclear Ca2+ transients evoked by electrical stimulation consist of at least two separate components, a passive component of Ca2+ diffusion from the cytosol to the nucleus through nuclear pores and an active component of Ca2+ release via InsP3 receptors [18]. Thus, nuclear Ca2+ transients in adult cardiomyocytes arising from electrical stimulation are both dependent and independent of cytosolic Ca2+. Together, these studies unveil the complex regulation of cardiomyocyte nuclear Ca2+ signals through combined actions of highly structured nuclear and perinuclear sub-compartments.
4. Nucleus-initiated nuclear Ca2+ release
Despite advances in our understanding of the nuclear Ca2+ store, the question remains whether these specialized structures can be activated independent of cytosolic Ca2+ release. For instance, does targeted stimulation by a hormone act directly on the nuclear Ca2+ release machinery to trigger Ca2+ release?
4.1. Nucleus-restricted molecular tools
To address this question, targeted molecular tools were engineered such that their mode of action is restricted to a specific subcellular compartment. The high-capacity, low-affinity Ca2+ buffer protein, parvalbumin (PV) was targeted to either the nucleus or the cytosol by addition of a peptide signal that directs the protein to a specific subcellular location [55]. These fusion proteins were used in HepG2 cells to reveal that nuclear Ca2+ buffering suppresses ATP induction of nuclear Ca2+ signals without affecting the rise in cytosolic Ca2+. By contrast, cytosol-targeted PV accomplished the opposite, blocking cytosolic Ca2+ transients but sparing nuclear transients. In the same study, EGF-mediated activation of the transcription factor Elk-1 was abolished specifically by buffering nuclear Ca2+, indicating that, in this context, nucleus-restricted Ca2+ increases drive the regulation of gene expression [56].
When this technology was applied to cardiomyocytes, global E–C coupling-related Ca2+ transients were largely decreased by buffering cytosolic Ca2+, as expected. However, buffering nuclear Ca2+ had the surprising effect of decreasing the amplitude of Ca2+ oscillations while increasing both their frequency and action potential duration [48]. Together, these findings suggest that a componcient of nuclear Ca2+ release helps shape global Ca2+ transients during the cardiac contraction cycle, likely via a nucleus-delimited E–C coupling process.
4.2. Nuclear Ca2+ contribution to E–C coupling
The concept that nuclear Ca2+ is an important component of E–C coupling was first suggested by structural studies demonstrating the existence of perinuclear T-tubules and nuclear Ca2+ microdomains along with RyR2 [48] and L-type Ca2+ release channels [57,58]. Amplification of cytosolic Ca2+ signals by this nuclear E–C coupling mechanism may occur through passive diffusion of Ca2+ from the nucleus to the cytosol through nuclear pores, and is favored by the nuclear-to-cytosolic Ca2+ gradient and relatively lower Ca2+ buffering capacity of the nucleus [18].
4.3. Nuclear Ca2+ signals independent of E–C coupling
Although nuclear Ca2+ signals coordinated with cardiac E–C coupling exhibit features both dependent and independent of changes in cytosolic Ca2+, activation of nuclear Ca2+ in response to certain stimuli can occur entirely independent of cytosolic Ca2+. For example, this has been demonstrated for insulin-like growth factor-1 (IGF-1) receptor signaling [59]. Treating neonatal cardiomyocytes with IGF-1 initiates an InsP3-dependent nuclear Ca2+ transient that precedes the cytosolic transient [59]. Blocking the nuclear Ca2+ transient with targeted PV abrogated both the nuclear and cytosolic responses, whereas cytosol-localized PV blocked only the cytosolic response, indicating that the signal originates in the nucleus [58]. This cytosol-independent nuclear Ca2+ response is required for activation of the transcription factor MEF-2C, a mediator of IGF-1-induced cardiomyocyte hypertrophy [58]. Using a similar approach, a nucleus-restricted InsP3 buffer inhibited the hypertrophic response of neonatal cardiomyocytes to either IGF-1 or ET-1 [60]. A different study showed that phenylephrine-stimulated cardiomyocytes showed an increased frequency of nuclear Ca2+ sparks leading to nuclear Ca2+ transients [61], which is also in favor of the notion that both InsP3- and Ca2+-dependent signaling occur within the nucleus in response to hypertrophic agonists to regulate cardiac transcription, and this signaling is not dependent on cytosolic Ca2+ release.
Thus, current evidence suggests that, depending on whether a nuclear Ca2+ transient arises during an E–C coupling cycle or in response to activation of a receptor on the plasma membrane, the elevation in nuclear Ca2+ can be either partially dependent or fully independent of cytosolic Ca2+. Considering that cardiac contraction is a continuous, episodic process that itself can be shaped by extracellular stimuli, the details of the interplay between nuclear and cytoplasmic Ca2+ signaling are likely to be complex. With the exception of extreme situations, such as cardioplegic arrest during surgery, receptor activation in cardiomyocytes must always occur simultaneously with E–C coupling. Therefore, Ca2+ signals arising from nuclear stores are constantly influenced by regulatory signals that diffuse from the cytosol. How nuclear Ca2+ stores detect, decode, and respond appropriately to all the possible inputs delivered at a given time remains an important and unanswered question.
5. Ca2+ channels mediating nuclear Ca2+ transients
Cardiomyocytes harbor three classes of ion channels that mediate the rapid mobilization of Ca2+ ions from intracellular stores: inositol 1,4,5-trisphosphate receptors (InsP3Rs), ryanodine receptors (RyRs) [10] and NAADP-sensitive endolysosomal Ca2+ channels [62]. While two pore channels (TPCs) have been proposed as the NAADP-sensitive Ca2+ channels at endolysosomal stores [63], the best-characterized channels that release Ca2+ from intracellular stores are InsP3Rs and RyRs. Both of the latter families form high-conductance, low-selectivity cation channels by association of four high-molecular mass protein monomers in combination with a large number of accessory proteins [64–68]. The InsP3R family consists of at least three distinct gene products, encoding monomers of molecular mass around 300°kDa, with three conserved and characterized domains (i.e. binding, regulatory/coupling, and channel pore). All three of these proteins bind their endogenous agonist InsP3 and share approximately 70% amino acid identity with one another [64,65]. There is general agreement that the type-2 InsP3R is the predominant InsP3R isoform in cardiomyocytes [69,70], although its expression levels are nearly 100-fold lower than that of RyRs, the primary release channels during E–C coupling [71]. Thus, InsP3Rs are not the primary source of Ca2+ release. That said, InsP3R localization in cardiomyocytes is concentrated in the NE where it is found at the ONM, INM, and at the nucleoplasmic reticulum [48,72–74]. Accordingly, the introduction of InsP3 into the nucleus has been shown to evoke Ca2+ release from the NE [75–77]. Release of Ca2+ from the NE can be inhibited by microinjection of heparin (an InsP3R antagonist) into the nucleus, [76] or by an antibody against the InsP3R [77,78]. InsP3 triggers rapid nuclear Ca2+ signals in permeabilized atrial cardiomyocytes that can be blocked by InsP3Rinhibition, but not by inhibition of RyR [74]. In isolated cardiomyocyte nuclei, InsP3 triggers increases in nucleoplasmic Ca2+ and decreases in the PS [74]. InsP3 increases the frequency of perinuclear Ca2+ sparks in permeabilized neonatal cardiomyocytes [61]. InsP3 also induces prominent monophasic nuclear Ca2+ transients in immortalized HL-1 cells, where InsP3R type-1 is localized to the perinuclear region [79]. Thus, it is not the relative abundance of InsP3Rs, but rather their strategic localization to nuclear membranes, that renders these Ca2+ channels relevant for gating nuclear Ca2+ signals in cardiomyocytes.
Release of Ca2+ via the RyR has been implicated in the regulation of nuclear Ca2+ signals, as suggested in the proposed mechanism 4 from Fig. 1, but this has been restricted to neonatal cardiomyocytes, whereas evidence for nuclear localization of RyRs in adult cardiomyocytes has not been clearly established. Hypothetically, this could be a difference lost with differentiation, due to the importance of E-C coupling in promoting an adult cardiomyocyte phenotype. Similarly in a different myocyte cell, photo-release of caged Ca2+ directly within the nucleus of C2C12 myoblasts triggers localized Ca2+-induced Ca2+ release (CICR) in the nucleus which could be suppressed by the RyR inhibitor dantrolene [80]. RyR1 was found to localize to intranuclear extensions of the NE in C2C12 myoblasts. RyR2 was also found to localize to the perinuclear region of embryonic chick cardiomyocytes [44], and the nucleoplasmic reticulum of neonatal rat cardiomyocytes [48], providing structural evidence that nuclear RyRs control locally-restricted nuclear CICR in neonatal cells. Together, these studies unveil selective regulation of nuclear Ca2+ signals by specific InsP3R and RyR isoforms localized to nuclear membranes, where they mediate Ca2+ release from perinuclear Ca2+ stores by the combined action of the second messengers InsP3 and Ca2+. These mechanisms could play important roles in the establishment of the adult cardiac phenotype, and also may be involved in pathological conditions where neonatal gene programs are activated.
6. Diffusion of cytosolic second messengers into the nucleus
Activation of nuclear Ca2+ channels can occur via diffusion into the nucleus of second messengers initially generated in the cytoplasm or via second messengers generated on-site. Several second messengers traverse the nuclear pore to promote nuclear Ca2+ release [76,81].
6.1. Diffusion of Ca2+
Ca2+ itself is an excellent candidate for regulating the initiation and maintenance of nuclear Ca2+ signals. Analogous to signaling events in the cytosol, an increase in nucleoplasmic Ca2+ facilitates Ca2+ release from InsP3Rs [82,83]. Indeed, various isoforms of InsP3R are either activated or inhibited by high Ca2+ concentrations [64,65,68,84,85]. It is possible to envision a mechanism of nuclear Ca2+ regulation of CICR similar to that of cytoplasmic CICR, where an increase in nuclear Ca2+ serves as a positive modulator, initiating, amplifying and ultimately terminating the signal at NE Ca2+-release hotspots [18,24,86]. This positive feedback between nuclear Ca2+ and nuclear Ca2+ release channels would then operate as an amplifying mechanism to direct nucleoplasmic Ca2+-mediated signaling.
6.2. Diffusion of InsP3
Evidence exists for diffusion of InsP3 from the cytosol to the nucleus, as illustrated in Fig. 1. FRET-based InsP3 biosensors have revealed that either stimulation with ET-1 or delivery of InsP3 directly into the cytosol induces diffusion of InsP3 into the nucleus, with delayed kinetics relative to cytosolic concentration [87]. Diffusion of InsP3 into the nucleus initiates a CaM/CaMK II signaling pathway ultimately controlling HDAC5 phosphorylation and nuclear export [73]. These findings confirm the ability of cytosolic InsP3 to diffuse into the nucleus and are consistent with the documented timing of nuclear Ca2+ transients in response to extracellular ET-1. This is illustrated in mechanism 2 from Fig. 1. However, diffusion of InsP3 from the cytosol is not sufficient to explain the rate of nuclear Ca2+ responses which exceed that of InsP3 diffusion. InsP3 generated at the plasma membrane has a limited capacity for traversing large distances in the cell (20–50μm) [88,89]. The cytosolic diffusion rate of InsP3 is 15μm/s [89] and is compatible with the kinetics of the nuclear Ca2+ signal in response to ET-1. As with Ca2+, it is unclear how nuclear InsP3Rs discriminate between InsP3 generated in the cytoplasm versus InsP3 generated in the nucleus. Rates of InsP3 degradation are likely an important factor. InsP3 is rapidly hydrolyzed to inactive forms, such as InsP2 [90]. Therefore, cytoplasm-localized InsP3Rs encounter the bulk of InsP3 generated in the cytoplasm, whereas, the concentration of cytoplasmic InsP3 at the nucleus is relatively small. This argument hints at the ability to generate InsP3 inside the nucleus, near its site of action, as will be discussed below.
7. Generation of second messenger inside the nucleus
Increasing evidence indicates that nuclear Ca2+ signals can be initiated by second messengers generated in and/or around the nucleus. The nucleus of many cell types is equipped with a toolkit for inositol lipid metabolism [91], as nuclear membranes harbor enzymatic systems for the production and degradation of InsP3: phospholipases [92,93], PIP2 [94] and kinases [91,95]. However, and importantly, these have mainly been identified in cell types different than cardiomyocytes. Evidence for a nuclear phosphoinositide cycle in cardiomyocytes has indeed been more elusive. In particular, nuclear PLCε has been detected in neonatal cardiomyocytes, and its knock down in the nucleus inhibited the hypertrophy of neonatal cardiomyocytes induced by ET-1, isoproterenol or norepinephrine [96]. PLCβ3 and PLCγ have been observed in the nucleus in confocal studies in cardiomyocytes leading to nuclear Ca2+ signals, but further subcellular fractionation experiments are necessary to confirm such observations, specifically as the localization of these enzymes in perinuclear microdomains could account for the effects on nuclear Ca2+ [58], as proposed in mechanism 1 from Fig. 2. One study did suggest that PLCβ1 was localized to the nucleus in adult cardiomyocytes [97], although others have questioned the antibody used in this study (Santa Cruz, G12 PLCβ antibody). Conversely, Zhang et al. [98] suggested that the substrate PIP2 is not at the nuclear membrane in neonatal cardiomyocytes, rather that PLCε is at the nucleus. However, this localization was not related to nuclear InsP3 generation, but instead to the production of DAG from PI4P for controlling PKD phosphorylation and nuclear import [96]. Disruption of nuclear localization of PLCε did not affect nuclear Ca2+ transients induced by ET-1, while PLCβ knock down did affect it [98], in agreement with the notion that perinuclear PLCβ is necessary for nuclear Ca2+ regulation [58]. Other studies, however, have shown that PLCβ isoforms also adopt nuclear localization. Nuclear PLCβ1 have been reported in adult cardiomyocytes [97] whereas nuclear PLCβ3 and PLCγ have been shown in neonatal cardiomyocytes [58]. Moreover, stimulation of neonatal cardiomyocytes with IGF-1 induces a fast and transient increase in intracellular InsP3 levels [59]. This effect is reversed by preincubating cells with the Gαi blocker pertussis toxin [59] or by over-expressing the Gβγ scavenger peptide βARKct [58], indicating that a G protein-sensitive PLC isoform, likely PLCβ, is involved in this response. Subcellular analysis of PLC activation by means of a fluorescent reporter demonstrates that PIP2 hydrolysis occurs exclusively in the perinuclear region upon IGF-1 stimulation, without affecting the peripheral PIP2 pool [58], although the dynamic range for the fluorescent PIP2 reporter in the nuclear region seems to be lower than the one observed in the plasma membrane [98], which may reflect local differences in phosphoinositide composition and availability. Thus, different nuclear PLC isoforms may contribute differently on nuclear Ca2+ regulation or nuclear lipid signaling in cardiomyocytes.
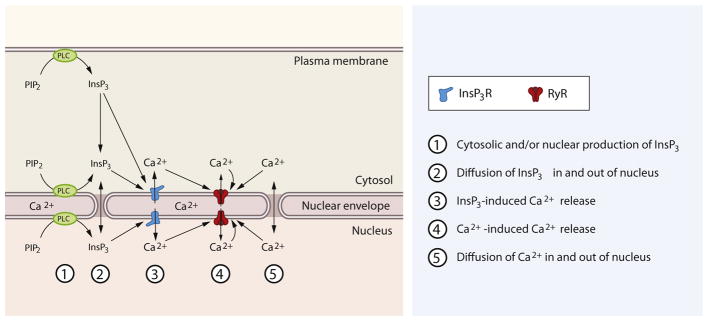
Fig. 2.
An integrated view of receptor-initiated nuclear Ca2+ signals in cardiomyocyte. Different agonists can activate receptors located in the sarcolemma (1 and 3) or intracellular receptors (2). Pathway 1: Perinuclear receptors located in deep T-tubules can lead to PLC-dependent InsP3 generation in peri/nuclear compartments. Pathway 2: Intracellular receptors can be activated by lipophilic ligands (steroid hormones) or endocytosed ligands, leading to PLC-dependent InsP3 generation in peri/nuclear compartments. Pathway 3: Receptors located in peripheral sarcolemma lead to generation of InsP3 and its diffusion into the nucleus triggers nuclear Ca2+ signals.
A central unanswered question is how nuclear PLC isoforms are activated by an extracellular ligand. In cancer cells, ERK-mediated kinases activate nuclear PLCβ [99]. However, the kinetics of this MAPK cascade are slower (15–30min) than the fast nuclear response to IGF-1 of cardiomyocytes (<15s). This suggests that IGF-1 increases nuclear PLC activity via a different mechanism. Moreover, evidence indicates that not all agonists use the same signaling mechanisms to provoke a nuclear Ca2+ transient [100].
8. Working models of receptor-initiated nuclear Ca2+ release
As mentioned above, different extracellular ligands trigger a range of signaling mechanisms to achieve specific regulation of nuclear Ca2+ release p[100], as shown in Fig. 2. For example, a number of agonists induce nuclear Ca2+ release in cardiomyocytes. Endothelin-1 (ET-1) is perhaps the best studied and therefore much of our understanding of nuclear Ca2+ signaling has been built upon mechanisms triggered by ET-1 [19,73,101]. However, other agonists, such as IGF-1 [59], insulin [102], angiotensin II (Ang II) [103], ATP [53], extracellular Ca2+ [104], and testosterone [105], also mobilize cardiomyocyte nuclear Ca2+, although a full understanding of underlying mechanisms remains elusive.
8.1. Activation of sarcolemmal receptors
ET-1 receptor A (ETA) signals via the classic model for ligand activation of cell membrane receptors [100]. Binding of ET-1 to its cognate receptor in the cardiomyocyte plasma membrane triggers a dose-dependent increase in nuclear Ca2+ via Gαq-mediated activation of PLC, which triggers hydrolysis of sarcolemmal PIP2 to generate InsP3 [19,73]. InsP3 generated in the cytosol diffuses into the nucleus and activates perinuclear Ca2+ release through type-2 InsP3 receptors, as shown in pathway 3 from Fig. 2. In this model, the selective regulation of nuclear Ca2+ is achieved by the strategic localization of InsP3Rs in nuclear membranes. Two physically segregated signaling hubs can be identified: one at the plasma membrane, formed by the ET-1/ET-1 receptor complex–Gq–PLCβ–PIP2 generating InsP3, which then diffuses through nuclear pores to the second hub, comprising the nuclear InsP3R and the perinuclear Ca2+ store. This chain of events is consistent with the kinetics of ET-1-elicited nuclear Ca2+ transients, i.e. ranging between 30 and 60 s. It remains an intriguing question why the second messenger, InsP3, is produced in excess far from the target site for its effect. One possibility is that by filtering the InsP3 signal through cytosolic elements that facilitate or limit InsP3 diffusion into the nucleus, the signal can be tuned to facilitate an appropriate response. Importantly, ET-1 receptor localization is not restricted to the sarcolemma but can also be detected in intracellular membranes, especially in the vicinity of the NE.
8.2. Perinuclear receptors
This working model is designed to explain the rapid effects of IGF-1 on nuclear Ca2+ release. A key component of this model is that a significant fraction of plasma membrane IGF-1R in cardiomyocytes is localized to perinuclear plasma membrane invaginations, or perinuclear T-tubules. This brings the surface receptor complex into close proximity of NE Ca2+ stores and provides direct access for extracellular ligands to the juxtanuclear receptor, thereby facilitating a localized nuclear response [58]. In this near-nuclear receptor model [100], plasma membrane IGF-1 receptors are directly apposed to the NE where they stimulate local production of InsP3 mediated by Gαi-dependent activation of perinuclear PLC. InsP3 then enters the nucleus to activate InsP3Rs and release Ca2+ into the nucleoplasm [59]. This perinuclear Ca2+ signaling microdomain provides spatial insulation of nuclear Ca2+ signals from large cytosolic Ca2+ fluctuations [57].
This model is characterized by a single signaling hub, as all components share close proximity to the nucleus, thus avoiding the rate-limiting process of long-distance InsP3 diffusion and degradation. This likely contributes to the fact that cardiomyocytes display the fastest nuclear Ca2+ response to IGF-1 of any cell type described to date, with a nuclear peak of only 2–6 s. This model is also potentially more efficient, because the total amount of InsP3 necessary for triggering a nuclear Ca2+ response is significantly smaller than that required in the classic model. In summary, this model connects surface receptor activation directly with the nuclear Ca2+ store through a specialized and highly structured perinuclear Ca2+ microdomain, which is independent and physically separated from the cytosolic Ca2+ machinery.
8.3. Nuclear membrane receptors
A third working model situates a fraction of classical plasma membrane receptors (Ang II, ET-1 receptors or α1-adrenergic receptors) and their downstream signaling elements intracellularly, at nuclear membranes [106]. Activation of GPCRs located in nuclear membranes sets in motion a signaling cascade involving G protein–PLC–PIP2 hydrolysis to produce InsP3 directly in the nucleoplasm promoting a very rapid InsP3 receptor release of nuclear Ca2+ from NE stores [107]. Similar to the near-nucleus receptor model, a single signaling hub can be identified in this model, as all the signaling elements are recruited to act together in close proximity in the nucleus. However, an important difference between the two models is the way in which ligands gain access to their cognate receptors. Indeed, it is a matter of debate whether the nuclear-delimited receptors are activated by the same ligands as their plasma membrane counterparts or by alternative ligands made available through intracrine mechanisms [106]. Mechanisms of ligand action may be receptor-specific, as will be discussed next.
8.3.1. Angiotensin II receptors
Functional Ang II receptors (AT1 and AT2) have been detected in the cardiomyocyte nuclear membrane [106]. Other components of the renin–angiotensin system, such as angiotensinogen, angiotensin converting enzyme (ACE) and renin are also expressed in adult cardiomyocytes and found to act intracellularly [108]. Thus, cardiomyocytes possess the potential for an entirely cell-autonomous renin–angiotensin axis. In addition, cardiomyocytes can take up and activate circulating prorenin [109]. Functional ACE has been detected in the nuclei of neonatal cardiomyocytes, suggesting that this can be a site of Ang II production [110]. Activation of AT1 receptors in nuclei isolated from cardiomyocytes triggers increases in nucleoplasmic Ca2+ via release from InsP3 receptors [107]. This intracellular Ang II-mediated Ca2+ transient is required for downstream activation of transcription. Together, these facts provide evidence for an intracrine pathway of intracellular Ang II production which can activate Ca2+-dependent transcriptional events involved in the expression of specific cardiac genes through the modulation of InsP3 levels and nuclear Ca2+ release.
8.3.2. ET-1 receptors
In contrast to type A ET receptors (ETA) that localize to the sarcolemma (see Section 8.1), ETB receptors localize to nuclear membranes of adult cardiomyocytes [111]. This localization is not the consequence of surface receptor internalization, as demonstrated by the absence of N-glycosylated ETB receptor in nuclear protein fractions [112]. Moreover, endocytosed ETB receptor is destined for lysosomal degradation and does not traffic to the NE, suggesting that a significant pool of ETB receptor is targeted directly to nuclear membranes following biosynthesis [112]. Photo-release of caged ET-1 induces InsP3-dependent nucleoplasmic Ca2+ increases that can be selectively blocked by intracellular delivery of an ETB antagonist, but not by an extracellular exposure to the same drug [112]. It is well established that ET-1 can be produced, stored, and secreted by cardiomyocytes under basal or stimulated conditions [113], suggesting that nuclear ETB can be activated by intracellular ET-1, comprising an intracrine system of nuclear Ca2+ release control.
8.3.3. α1-Adrenergic receptors
Evidence is also available for the nuclear localization and signaling of α1-adrenergic receptors in cardiomyocytes. α1-Adrenergic receptors localize to the nucleus of adult mouse cardiomyocytes and colocalize with nuclear Gαq and PLCβ1 [97]. Endogenous nuclear α1-adrenergic receptor signaling complex can be activated locally by extracellular cathecolamines since cardiomyocytes uptake catecholamines through a norephinephrine-uptake mechanism. Activation of nuclear-delimited α1-adrenergic receptors leads to receptor oligomerization, ERK phosphorylation [114], nuclear PKCδ activation and phosphorylation of cardiac TnI, which in turn increase myocyte contractility [115]. Although nuclear α1-adrenergic receptor signaling has been extensively demonstrated in cardiomyocytes, the exact involvement of nuclear receptor signaling on nuclear Ca2+ homeostasis remains unknown and future studies are needed to clarify the link between these 2 pathways.
9. Conclusion
Nuclear Ca2+ signals provide a strategic mechanism which allows cardiomyocytes to segregate the control of Ca2+-dependent transcription from cytosolic Ca2+ transients mediating E–C coupling [15]. Although different models have been proposed to explain the selective control of nuclear Ca2+ release in response to hormone stimulation [100], current evidence suggests that cardiomyocytes harbor a variety of mechanisms to activate and regulate nuclear Ca2+ release (Fig. 1). Furthermore, organization and signaling through the same receptor type may be different when localized to the sarcolemma versus the nuclear environment (Fig. 2).
There are many fascinating and unresolved questions. First, it is intriguing that cardiomyocytes are equipped with multiple mechanisms for controlling a common Ca2+-dependent transcriptional response. As discussed earlier, it may be that the diversity of mechanisms, all culminating in nuclear Ca2+ release, provides a physiological filter that encodes information regarding both the cellular environment and the nature of the specific agonist. Shaping the magnitude and temporal dynamics of nuclear Ca2+ release could impact the constellation of genes affected and the duration of the transcriptional or translational response. This is certainly plausible considering that agonists that use different mechanisms to control nuclear Ca2+ release also differ in their phenotypic effects. For example, Ang II and ET-1 generally mediate the expression of maladaptive genes and pathological cardiac hypertrophy, whereas IGF-1 mediates adaptive physiological cardiac hypertrophy [116]. In addition, many of these extracellular ligands activate other pathways different than Ca2+ signals, and their compartmentation in different subcellular locations may be a key for achieving selective responses.
Second, when a single agonist promotes nuclear Ca2+ release via multiple pathways, how is this decoded by the nucleus? As an example, ET-1 can initiate a signal at a sarcolemmal receptor and/or at a nuclear-membrane receptor. Decoding these signals by the nuclear machinery may lead to divergent phenotypic responses, or be integrated to give rise to a specified phenotype. Such a response could be further tuned to the initiating signal depending upon the balance of flux between the mechanisms. Third, it is not known whether there is cross-talk among the various mechanisms controlling nuclear Ca2+ or how the resulting nuclear Ca2+ signals are coordinated with other important pathways activated by the same ligand or receptor.
Finally, current knowledge regarding mechanisms activating nuclear Ca2+ signals and their downstream consequences has been limited to experimental models, primarily in vitro. Applying this knowledge in the context of human heart disease may provide new therapeutic tools that harness mechanisms through which cardiomyocytes decode and respond to agonist-activated nuclear Ca2+ signals.
Thus, the conceptual framework of nuclear Ca2+ signals is in rapid flux, with exciting new insights emerging. Further elucidation of underlying mechanisms is necessary to fully define the complex roles of Ca2+ in the governance of contractile function and intracellular signaling in the heart.
Acknowledgments
This work was funded by Comision Nacional de Ciencia y Tecnologia (CONICYT), Chile: FONDECYT 1120212 to SL, Anillo ACT1111 to SL and EJ; FONDAP 15130011 to SL; Red Internacional CONICYT 120003 to JAH, BAR, and SL; by the NIH (HL-120732, to JAH; HL100401, to JAH; HL-072016, to BAR; HL-097768, to BAR), AHA (0640084N, to JAH), AHA (SDG18440002, to JAH); CPRIT (RP110486P3, to JAH), and the Leducq Foundation (11CVD04, to JAH).
Abbreviations
- Ang II
angiotensin II
- E–C
excitation–contraction
- ER
endoplasmic reticulum
- ET-1
endothelin-1
- IGF-1
insulin-like growth factor-1
- INM
inner nuclear membrane
- InsP3
inositol 1,4,5-trisphosphate
- InsP3R
InsP3 receptor
- NE
nuclear envelope
- ONM
outer nuclear membrane
- PI4P
phosphatidylinositol-4-phosphate
- PIP2
phosphatidylinositol bisphosphate
- PLC
phospholipase C
- PS
perinuclear space
- RyR
ryanodine receptor
- SR
sarcoplasmic reticulum
Footnotes
Disclosures
None.
Contributor Information
Cristián Ibarra, Email: cristian.ibarra@astrazeneca.com.
Sergio Lavandero, Email: slavander@uchile.cl.
References
- 1.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–29. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 2.Bers DM. Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol. 2008;70:23–49. doi: 10.1146/annurev.physiol.70.113006.100455. [DOI] [PubMed] [Google Scholar]
- 3.Zhang S, Fritz N, Ibarra C, Uhlen P. Inositol 1,4,5-trisphosphate receptor subtype specific regulation of calcium oscillations. Neurochem Res. 2011;36:1175–11785. doi: 10.1007/s11064-011-0457-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berridge MJ. Inositol trisphosphate and calcium oscillations. Biochem Soc Symp. 2007:1–7. doi: 10.1042/BSS0740001. [DOI] [PubMed] [Google Scholar]
- 5.Uhlen P, Fritz N. Biochemistry of calcium oscillations. Biochem Biophys Res Commun. 2010;396:28–32. doi: 10.1016/j.bbrc.2010.02.117. [DOI] [PubMed] [Google Scholar]
- 6.Bers DM. Cardiac excitation–contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 7.Higazi DR, Fearnley CJ, Drawnel FM, Talasila A, Corps EM, Ritter O, et al. Endothelin-1-stimulated InsP3-induced Ca2+ release is a nexus for hypertrophic signaling in cardiac myocytes. Mol Cell. 2009;33:472–82. doi: 10.1016/j.molcel.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 8.Munoz JP, Collao A, Chiong M, Maldonado C, Adasme T, Carrasco L, et al. The transcription factor MEF2C mediates cardiomyocyte hypertrophy induced by IGF-1 signaling. Biochem Biophys Res Commun. 2009;388:155–60. doi: 10.1016/j.bbrc.2009.07.147. [DOI] [PubMed] [Google Scholar]
- 9.Olson EN. Gene regulatory networks in the evolution and development of the heart. Science. 2006;313:1922–7. doi: 10.1126/science.1132292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Janowski E, Berrios M, Cleemann L, Morad M. Developmental aspects of cardiac Ca(2+) signaling: interplay between RyR- and IP(3)R-gated Ca(2+) stores. Am J Physiol Heart Circ Physiol. 2010;298:H1939–50. doi: 10.1152/ajpheart.00607.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiong M, Parra V, Eisner V, Ibarra C, Maldonado C, Criollo A, et al. Parallel activation of Ca(2+)-induced survival and death pathways in cardiomyocytes by sorbitol-induced hyperosmotic stress. Apoptosis. 2010;15:887–903. doi: 10.1007/s10495-010-0505-9. [DOI] [PubMed] [Google Scholar]
- 12.Nakayama H, Bodi I, Maillet M, De Santiago J, Domeier TL, Mikoshiba K, et al. The IP3 receptor regulates cardiac hypertrophy in response to select stimuli. Circ Res. 2010;107:659–66. doi: 10.1161/CIRCRESAHA.110.220038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maillet M, van Berlo JH, Molkentin JD. Molecular basis of physiological heart growth: fundamental concepts and new players. Nat Rev Mol Cell Biol. 2013;14:38–48. doi: 10.1038/nrm3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Contreras-Ferrat AE, Toro B, Bravo R, Parra V, Vasquez C, Ibarra C, et al. An inositol 1,4,5-triphosphate (IP3)-IP3 receptor pathway is required for insulin-stimulated glucose transporter 4 translocation and glucose uptake in cardiomyocytes. Endocrinology. 151:4665–77. doi: 10.1210/en.2010-0116. [DOI] [PubMed] [Google Scholar]
- 15.Goonasekera SA, Molkentin JD. Unraveling the secrets of a double life: contractile versus signaling Ca2+ in a cardiac myocyte. J Mol Cell Cardiol. 2012;52:317–22. doi: 10.1016/j.yjmcc.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 16.Genka C, Ishida H, Ichimori K, Hirota Y, Tanaami T, Nakazawa H. Visualization of biphasic Ca2+ diffusion from cytosol to nucleus in contracting adult rat cardiac myocytes with an ultrafast confocal imaging system. Cell Calcium. 1999;25:199–208. doi: 10.1054/ceca.1999.0026. [DOI] [PubMed] [Google Scholar]
- 17.Kim CG, Park D, Rhee SG. The role of carboxyl-terminal basic amino acids in Gqalpha-dependent activation, particulate association, and nuclear localization of phospholipase C-beta1. J Biol Chem. 1996;271:21187–92. doi: 10.1074/jbc.271.35.21187. [DOI] [PubMed] [Google Scholar]
- 18.Ljubojevic S, Walther S, Asgarzoei M, Sedej S, Pieske B, Kockskamper J. In situ calibration of nucleoplasmic versus cytoplasmic Ca(2)+ concentration in adult cardiomyocytes. Biophys J. 2011;100:2356–66. doi: 10.1016/j.bpj.2011.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kockskamper J, Seidlmayer L, Walther S, Hellenkamp K, Maier LS, Pieske B. Endothelin-1 enhances nuclear Ca2+ transients in atrial myocytes through Ins(1,4,5)P3-dependent Ca2+ release from perinuclear Ca2+ stores. J Cell Sci. 2008;121:186–95. doi: 10.1242/jcs.021386. [DOI] [PubMed] [Google Scholar]
- 20.Wu X, Bers DM. Sarcoplasmic reticulum and nuclear envelope are one highly interconnected Ca2+ store throughout cardiac myocyte. Circ Res. 2006;99:283–91. doi: 10.1161/01.RES.0000233386.02708.72. [DOI] [PubMed] [Google Scholar]
- 21.Keminer O, Peters R. Permeability of single nuclear pores. Biophys J. 1999;77:217–28. doi: 10.1016/S0006-3495(99)76883-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paine PL, Moore LC, Horowitz SB. Nuclear envelope permeability. Nature. 1975;254:109–14. doi: 10.1038/254109a0. [DOI] [PubMed] [Google Scholar]
- 23.Paine PL. Nucleocytoplasmic movement of fluorescent tracers microinjected into living salivary gland cells. J Cell Biol. 1975;66:652–7. doi: 10.1083/jcb.66.3.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lipp P, Thomas D, Berridge MJ, Bootman MD. Nuclear calcium signalling by individual cytoplasmic calcium puffs. EMBO J. 1997;16:7166–73. doi: 10.1093/emboj/16.23.7166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.al-Mohanna FA, Caddy KW, Bolsover SR. The nucleus is insulated from large cytosolic calcium ion changes. Nature. 1994;367:745–50. doi: 10.1038/367745a0. [DOI] [PubMed] [Google Scholar]
- 26.Lee MA, Dunn RC, Clapham DE, Stehno-Bittel L. Calcium regulation of nuclear pore permeability. Cell Calcium. 1998;23:91–101. doi: 10.1016/s0143-4160(98)90107-5. [DOI] [PubMed] [Google Scholar]
- 27.Perez-Terzic C, Pyle J, Jaconi M, Stehno-Bittel L, Clapham DE. Conformational states of the nuclear pore complex induced by depletion of nuclear Ca2+ stores. Science. 1996;273:1875–7. doi: 10.1126/science.273.5283.1875. [DOI] [PubMed] [Google Scholar]
- 28.Kong SK, Tsang D, Leung KN, Lee CY. Nuclear envelope acts as a calcium barrier in C6 glioma cells. Biochem Biophys Res Commun. 1996;218:595–600. doi: 10.1006/bbrc.1996.0105. [DOI] [PubMed] [Google Scholar]
- 29.Danker T, Oberleithner H. Nuclear pore function viewed with atomic force microscopy. Pflugers Arch. 2000;439:671–81. doi: 10.1007/s004240000249. [DOI] [PubMed] [Google Scholar]
- 30.O’Brien EM, Gomes DA, Sehgal S, Nathanson MH. Hormonal regulation of nuclear permeability. J Biol Chem. 2007;282:4210–7. doi: 10.1074/jbc.M606300200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shirakawa H, Miyazaki S. Spatiotemporal analysis of calcium dynamics in the nucleus of hamster oocytes. J Physiol. 1996;494:29–40. doi: 10.1113/jphysiol.1996.sp021473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Allbritton NL, Oancea E, Kuhn MA, Meyer T. Source of nuclear calcium signals. Proc Natl Acad Sci U S A. 1994;91:12458–62. doi: 10.1073/pnas.91.26.12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chamero P, Villalobos C, Alonso MT, Garcia-Sancho J. Dampening of cytosolic Ca2+ oscillations on propagation to nucleus. J Biol Chem. 2002;277:50226–9. doi: 10.1074/jbc.C200522200. [DOI] [PubMed] [Google Scholar]
- 34.Colella M, Grisan F, Robert V, Turner JD, Thomas AP, Pozzan T. Ca2+ oscillation frequency decoding in cardiac cell hypertrophy: role of calcineurin/NFAT as Ca2+ signal integrators. Proc Natl Acad Sci U S A. 2008;105:2859–64. doi: 10.1073/pnas.0712316105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu Q, Deshpande S, Irani K, Ziegelstein RC. Ca2+ oscillation frequency regulates agonist-stimulated NF-kappaB transcriptional activity. J Biol Chem. 1999;274:33995–8. doi: 10.1074/jbc.274.48.33995. [DOI] [PubMed] [Google Scholar]
- 36.Dolmetsch RE, Xu K, Lewis RS. Calcium oscillations increase the efficiency and specificity of gene expression. Nature. 1998;392:933–6. doi: 10.1038/31960. [DOI] [PubMed] [Google Scholar]
- 37.Bading H, Hardingham GE, Johnson CM, Chawla S. Gene regulation by nuclear and cytoplasmic calcium signals. Biochem Biophys Res Commun. 1997;236:541–3. doi: 10.1006/bbrc.1997.7037. [DOI] [PubMed] [Google Scholar]
- 38.Li W, Llopis J, Whitney M, Zlokarnik G, Tsien RY. Cell-permeant caged InsP3 ester shows that Ca2+ spike frequency can optimize gene expression. Nature. 1998;392:936–41. doi: 10.1038/31965. [DOI] [PubMed] [Google Scholar]
- 39.Petersen OH, Gerasimenko OV, Gerasimenko JV, Mogami H, Tepikin AV. The calcium store in the nuclear envelope. Cell Calcium. 1998;23:87–90. doi: 10.1016/s0143-4160(98)90106-3. [DOI] [PubMed] [Google Scholar]
- 40.Guo A, Cala SE, Song LS. Calsequestrin accumulation in rough endoplasmic reticulum promotes perinuclear Ca2+ release. J Biol Chem. 2012;287:16670–80. doi: 10.1074/jbc.M112.340927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bootman MD, Thomas D, Tovey SC, Berridge MJ, Lipp P. Nuclear calcium signalling. Cell Mol Life Sci. 2000;57:371–8. doi: 10.1007/PL00000699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goldberg MW, Allen TD. Structural and functional organization of the nuclear envelope. Curr Opin Cell Biol. 1995;7:301–9. doi: 10.1016/0955-0674(95)80083-2. [DOI] [PubMed] [Google Scholar]
- 43.Lui PP, Kong SK, Fung KP, Lee CY. The rise of nuclear and cytosolic Ca2+ can be uncoupled in HeLa cells. Pflugers Arch. 1998;436:371–6. doi: 10.1007/s004240050645. [DOI] [PubMed] [Google Scholar]
- 44.Abrenica B, Gilchrist JS. Nucleoplasmic Ca(2+) loading is regulated by mobilization of perinuclear Ca(2+) Cell Calcium. 2000;28:127–36. doi: 10.1054/ceca.2000.0137. [DOI] [PubMed] [Google Scholar]
- 45.Xie X, Wu G, Lu ZH, Ledeen RW. Potentiation of a sodium–calcium exchanger in the nuclear envelope by nuclear GM1 ganglioside. J Neurochem. 2002;81:1185–95. doi: 10.1046/j.1471-4159.2002.00917.x. [DOI] [PubMed] [Google Scholar]
- 46.Xie X, Wu G, Lu ZH, Rohowsky-Kochan C, Ledeen RW. Presence of sodium–calcium exchanger/GM1 complex in the nuclear envelope of non-neural cells: nature of exchanger–GM1 interaction. Neurochem Res. 2004;29:2135–46. doi: 10.1007/s11064-004-6887-8. [DOI] [PubMed] [Google Scholar]
- 47.Echevarria W, Leite MF, Guerra MT, Zipfel WR, Nathanson MH. Regulation of calcium signals in the nucleus by a nucleoplasmic reticulum. Nat Cell Biol. 2003;5:440–6. doi: 10.1038/ncb980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guatimosim S, Amaya MJ, Guerra MT, Aguiar CJ, Goes AM, Gomez-Viquez NL, et al. Nuclear Ca2+ regulates cardiomyocyte function. Cell Calcium. 2008;44:230–42. doi: 10.1016/j.ceca.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 49.Malhas A, Goulbourne C, Vaux DJ. The nucleoplasmic reticulum: form and function. Trends Cell Biol. 2011;21:362–73. doi: 10.1016/j.tcb.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 50.Lui PP, Chan FL, Suen YK, Kwok TT, Kong SK. The nucleus of HeLa cells contains tubular structures for Ca2+ signaling with the involvement of mitochondria. Biochem Biophys Res Commun. 2003;308:826–33. doi: 10.1016/s0006-291x(03)01469-4. [DOI] [PubMed] [Google Scholar]
- 51.Alonso MT, Villalobos C, Chamero P, Alvarez J, Garcia-Sancho J. Calcium microdomains in mitochondria and nucleus. Cell Calcium. 2006;40:513–25. doi: 10.1016/j.ceca.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 52.Eisner V, Parra V, Lavandero S, Hidalgo C, Jaimovich E. Mitochondria fine-tune the slow Ca(2+) transients induced by electrical stimulation of skeletal myotubes. Cell Calcium. 2010;48:358–70. doi: 10.1016/j.ceca.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 53.Jaconi M, Bony C, Richards SM, Terzic A, Arnaudeau S, Vassort G, et al. Inositol 1,4,5-trisphosphate directs Ca(2+) flow between mitochondria and the endoplasmic/sarcoplasmic reticulum: a role in regulating cardiac autonomic Ca(2+) spiking. Mol Biol Cell. 2000;11:1845–58. doi: 10.1091/mbc.11.5.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Drago I, De Stefani D, Rizzuto R, Pozzan T. Mitochondrial Ca2+ uptake contributes to buffering cytoplasmic Ca2+ peaks in cardiomyocytes. Proc Natl Acad Sci U S A. 2012;109:12986–91. doi: 10.1073/pnas.1210718109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gomes DA, Leite MF, Bennett AM, Nathanson MH. Calcium signaling in the nucleus. Can J Physiol Pharmacol. 2006;84:325–32. doi: 10.1139/y05-117. [DOI] [PubMed] [Google Scholar]
- 56.Pusl T, Wu JJ, Zimmerman TL, Zhang L, Ehrlich BE, Berchtold MW, et al. Epidermal growth factor-mediated activation of the ETS domain transcription factor Elk-1 requires nuclear calcium. J Biol Chem. 2002;277:27517–27. doi: 10.1074/jbc.M203002200. [DOI] [PubMed] [Google Scholar]
- 57.Escobar M, Cardenas C, Colavita K, Petrenko NB, Franzini-Armstrong C. Structural evidence for perinuclear calcium microdomains in cardiac myocytes. J Mol Cell Cardiol. 2011;50:451–9. doi: 10.1016/j.yjmcc.2010.11.021. [DOI] [PubMed] [Google Scholar]
- 58.Ibarra C, Vicencio JM, Estrada M, Lin Y, Rocco P, Rebellato P, et al. Local control of nuclear calcium signaling in cardiac myocytes by perinuclear microdomains of sarcolemmal insulin-like growth factor 1 receptors. Circ Res. 2013;112:236–45. doi: 10.1161/CIRCRESAHA.112.273839. [DOI] [PubMed] [Google Scholar]
- 59.Ibarra C, Estrada M, Carrasco L, Chiong M, Liberona JL, Cardenas C, et al. Insulin-like growth factor-1 induces an inositol 1,4,5-trisphosphate-dependent increase in nuclear and cytosolic calcium in cultured rat cardiac myocytes. J Biol Chem. 2004;279:7554–65. doi: 10.1074/jbc.M311604200. [DOI] [PubMed] [Google Scholar]
- 60.Arantes LA, Aguiar CJ, Amaya MJ, Figueiro NC, Andrade LM, Rocha-Resende C, et al. Nuclear inositol 1,4,5-trisphosphate is a necessary and conserved signal for the induction of both pathological and physiological cardiomyocyte hypertrophy. J Mol Cell Cardiol. 2012;53:475–86. doi: 10.1016/j.yjmcc.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 61.Luo D, Yang D, Lan X, Li K, Li X, Chen J, et al. Nuclear Ca2+ sparks and waves mediated by inositol 1,4,5-trisphosphate receptors in neonatal rat cardiomyocytes. Cell Calcium. 2008;43:165–74. doi: 10.1016/j.ceca.2007.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nebel M, Schwoerer AP, Warszta D, Siebrands CC, Limbrock AC, Swarbrick JM, et al. Nicotinic acid adenine dinucleotide phosphate (NAADP)-mediated calcium signaling and arrhythmias in the heart evoked by beta-adrenergic stimulation. J Biol Chem. 2013;288:16017–30. doi: 10.1074/jbc.M112.441246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Morgan AJ, Galione A. Two-pore channels (TPCs): current controversies. Bioessays. 2014;36:173–83. doi: 10.1002/bies.201300118. [DOI] [PubMed] [Google Scholar]
- 64.Ehrlich BE. Functional properties of intracellular calcium-release channels. Curr Opin Neurobiol. 1995;5:304–9. doi: 10.1016/0959-4388(95)80042-5. [DOI] [PubMed] [Google Scholar]
- 65.Bezprozvanny I, Ehrlich BE. The inositol 1,4,5-trisphosphate (InsP3) receptor. J Membr Biol. 1995;145:205–16. doi: 10.1007/BF00232713. [DOI] [PubMed] [Google Scholar]
- 66.Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, et al. PKA phosphorylation dissociates FKBP12. 6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101:365–76. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 67.Coronado R, Morrissette J, Sukhareva M, Vaughan DM. Structure and function of ryanodine receptors. Am J Physiol. 1994;266:C1485–504. doi: 10.1152/ajpcell.1994.266.6.C1485. [DOI] [PubMed] [Google Scholar]
- 68.Kaftan EJ, Ehrlich BE, Watras J. Inositol 1,4,5-trisphosphate (InsP3) and calcium interact to increase the dynamic range of InsP3 receptor-dependent calcium signaling. J Gen Physiol. 1997;110:529–38. doi: 10.1085/jgp.110.5.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Perez PJ, Ramos-Franco J, Fill M, Mignery GA. Identification and functional reconstitution of the type 2 inositol 1,4,5-trisphosphate receptor from ventricular cardiac myocytes. J Biol Chem. 1997;272:23961–9. doi: 10.1074/jbc.272.38.23961. [DOI] [PubMed] [Google Scholar]
- 70.Garcia KD, Shah T, Garcia J. Immunolocalization of type 2 inositol 1,4,5-trisphosphate receptors in cardiac myocytes from newborn mice. Am J Physiol Cell Physiol. 2004;287:C1048–57. doi: 10.1152/ajpcell.00004.2004. [DOI] [PubMed] [Google Scholar]
- 71.Bootman MD, Fearnley C, Smyrnias I, MacDonald F, Roderick HL. An update on nuclear calcium signalling. J Cell Sci. 2009;122:2337–50. doi: 10.1242/jcs.028100. [DOI] [PubMed] [Google Scholar]
- 72.Bare DJ, Kettlun CS, Liang M, Bers DM, Mignery GA. Cardiac type 2 inositol 1,4,5-trisphosphate receptor: interaction and modulation by calcium/calmodulin-dependent protein kinase II. J Biol Chem. 2005;280:15912–20. doi: 10.1074/jbc.M414212200. [DOI] [PubMed] [Google Scholar]
- 73.Wu X, Zhang T, Bossuyt J, Li X, McKinsey TA, Dedman JR, et al. Local InsP3-dependent perinuclear Ca2+ signaling in cardiac myocyte excitation–transcription coupling. J Clin Invest. 2006;116:675–82. doi: 10.1172/JCI27374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zima AV, Bare DJ, Mignery GA, Blatter LA. IP3-dependent nuclear Ca2+ signalling in the mammalian heart. J Physiol. 2007;584:601–11. doi: 10.1113/jphysiol.2007.140731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gerasimenko OV, Gerasimenko JV, Tepikin AV, Petersen OH. ATP-dependent accumulation and inositol trisphosphate- or cyclic ADP-ribose-mediated release of Ca2+ from the nuclear envelope. Cell. 1995;80:439–44. doi: 10.1016/0092-8674(95)90494-8. [DOI] [PubMed] [Google Scholar]
- 76.Hennager DJ, Welsh MJ, DeLisle S. Changes in either cytosolic or nucleoplasmic inositol 1,4,5-trisphosphate levels can control nuclear Ca2+ concentration. J Biol Chem. 1995;270:4959–62. doi: 10.1074/jbc.270.10.4959. [DOI] [PubMed] [Google Scholar]
- 77.Santella L, Kyozuka K. Effects of 1-methyladenine on nuclear Ca2+ transients and meiosis resumption in starfish oocytes are mimicked by the nuclear injection of inositol 1,4,5-trisphosphate and cADP-ribose. Cell Calcium. 1997;22:11–20. doi: 10.1016/s0143-4160(97)90085-3. [DOI] [PubMed] [Google Scholar]
- 78.Santella L, De Riso L, Gragnaniello G, Kyozuka K. Separate activation of the cytoplasmic and nuclear calcium pools in maturing starfish oocytes. Biochem Biophys Res Commun. 1998;252:1–4. doi: 10.1006/bbrc.1998.9583. [DOI] [PubMed] [Google Scholar]
- 79.Kim JC, Son MJ, Subedi KP, Kim do H, Woo SH. IP3-induced cytosolic and nuclear Ca2+ signals in HL-1 atrial myocytes: possible role of IP3 receptor subtypes. Mol Cell. 2010;29:387–95. doi: 10.1007/s10059-010-0039-6. [DOI] [PubMed] [Google Scholar]
- 80.Marius P, Guerra MT, Nathanson MH, Ehrlich BE, Leite MF. Calcium release from ryanodine receptors in the nucleoplasmic reticulum. Cell Calcium. 2006;39:65–73. doi: 10.1016/j.ceca.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 81.Gerasimenko JV, Maruyama Y, Yano K, Dolman NJ, Tepikin AV, Petersen OH, et al. NAADP mobilizes Ca2+ from a thapsigargin-sensitive store in the nuclear envelope by activating ryanodine receptors. J Cell Biol. 2003;163:271–82. doi: 10.1083/jcb.200306134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fabiato A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am J Physiol. 1983;245:C1–C14. doi: 10.1152/ajpcell.1983.245.1.C1. [DOI] [PubMed] [Google Scholar]
- 83.Frank GB. The current view of the source of trigger calcium in excitation–contraction coupling in vertebrate skeletal muscle. Biochem Pharmacol. 1980;29:2399–406. doi: 10.1016/0006-2952(80)90341-x. [DOI] [PubMed] [Google Scholar]
- 84.Rizzuto R, Brini M, Murgia M, Pozzan T. Microdomains with high Ca2+ close to IP3-sensitive channels that are sensed by neighboring mitochondria. Science. 1993;262:744–7. doi: 10.1126/science.8235595. [DOI] [PubMed] [Google Scholar]
- 85.Bezprozvanny I, Watras J, Ehrlich BE. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991;351:751–4. doi: 10.1038/351751a0. [DOI] [PubMed] [Google Scholar]
- 86.Tovey SC, de Smet P, Lipp P, Thomas D, Young KW, Missiaen L, et al. Calcium puffs are generic InsP(3)-activated elementary calcium signals and are downregulated by prolonged hormonal stimulation to inhibit cellular calcium responses. J Cell Sci. 2001;114:3979–89. doi: 10.1242/jcs.114.22.3979. [DOI] [PubMed] [Google Scholar]
- 87.Remus TP, Zima AV, Bossuyt J, Bare DJ, Martin JL, Blatter LA, et al. Biosensors to measure inositol 1,4,5-trisphosphate concentration in living cells with spatiotemporal resolution. J Biol Chem. 2006;281:608–16. doi: 10.1074/jbc.M509645200. [DOI] [PubMed] [Google Scholar]
- 88.Clapham DE. Calcium signaling. Cell. 1995;80:259–68. doi: 10.1016/0092-8674(95)90408-5. [DOI] [PubMed] [Google Scholar]
- 89.Allbritton NL, Meyer T, Stryer L. Range of messenger action of calcium ion and inositol 1,4,5-trisphosphate. Science. 1992;258:1812–5. doi: 10.1126/science.1465619. [DOI] [PubMed] [Google Scholar]
- 90.Sanchez X, Carrasco MA, Vergara J, Hidalgo C. Inositol 1,4,5-triphosphate phosphatase activity in membranes isolated from amphibian skeletal muscle [corrected] FEBS Lett. 1991;279:58–60. doi: 10.1016/0014-5793(91)80250-7. [DOI] [PubMed] [Google Scholar]
- 91.Martelli AM, Bortul R, Tabellini G, Aluigi M, Peruzzi D, Bareggi R, et al. Reexamination of the mechanisms regulating nuclear inositol lipid metabolism. FEBS Lett. 2001;505:1–6. doi: 10.1016/s0014-5793(01)02752-1. [DOI] [PubMed] [Google Scholar]
- 92.Martelli AM, Gilmour RS, Bertagnolo V, Neri LM, Manzoli L, Cocco L. Nuclear localization and signalling activity of phosphoinositidase C beta in Swiss 3T3 cells. Nature. 1992;358:242–5. doi: 10.1038/358242a0. [DOI] [PubMed] [Google Scholar]
- 93.Mazzoni M, Bertagnolo V, Neri LM, Carini C, Marchisio M, Milani D, et al. Discrete subcellular localization of phosphoinositidase C beta, gamma and delta in PC12 rat pheochromocytoma cells. Biochem Biophys Res Commun. 1992;187:114–20. doi: 10.1016/s0006-291x(05)81466-4. [DOI] [PubMed] [Google Scholar]
- 94.Divecha N, Rhee SG, Letcher AJ, Irvine RF. Phosphoinositide signalling enzymes in rat liver nuclei: phosphoinositidase C isoform beta 1 is specifically, but not predominantly, located in the nucleus. Biochem J. 1993;289:617–20. doi: 10.1042/bj2890617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Neri LM, Borgatti P, Capitani S, Martelli AM. Nuclear diacylglycerol produced by phosphoinositide-specific phospholipase C is responsible for nuclear translocation of protein kinase C-alpha. J Biol Chem. 1998;273:29738–44. doi: 10.1074/jbc.273.45.29738. [DOI] [PubMed] [Google Scholar]
- 96.Zhang L, Malik S, Kelley GG, Kapiloff MS, Smrcka AV. Phospholipase C epsilon scaffolds to muscle-specific A kinase anchoring protein (mAKAPbeta) and integrates multiple hypertrophic stimuli in cardiac myocytes. J Biol Chem. 2011;286:23012–21. doi: 10.1074/jbc.M111.231993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wright CD, Chen Q, Baye NL, Huang Y, Healy CL, Kasinathan S, et al. Nuclear alpha1-adrenergic receptors signal activated ERK localization to caveolae in adult cardiac myocytes. Circ Res. 2008;103:992–1000. doi: 10.1161/CIRCRESAHA.108.176024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang L, Malik S, Pang J, Wang H, Park KM, Yule DI, et al. Phospholipase Cepsilon hydrolyzes perinuclear phosphatidylinositol 4-phosphate to regulate cardiac hypertrophy. Cell. 2013;153:216–27. doi: 10.1016/j.cell.2013.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cocco L, Martelli AM, Gilmour RS, Rhee SG, Manzoli FA. Nuclear phospholipase C and signaling. Biochim Biophys Acta. 2001;1530:1–14. doi: 10.1016/s1388-1981(00)00169-4. [DOI] [PubMed] [Google Scholar]
- 100.Bers DM. Membrane receptor neighborhoods: snuggling up to the nucleus. Circ Res. 2013;112:224–6. doi: 10.1161/CIRCRESAHA.112.300494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Proven A, Roderick HL, Conway SJ, Berridge MJ, Horton JK, Capper SJ, et al. Inositol 1,4,5-trisphosphate supports the arrhythmogenic action of endothelin-1 on ventricular cardiac myocytes. J Cell Sci. 2006;119:3363–75. doi: 10.1242/jcs.03073. [DOI] [PubMed] [Google Scholar]
- 102.Contreras-Ferrat AE, Toro B, Bravo R, Parra V, Vasquez C, Ibarra C, et al. An inositol 1,4,5-triphosphate (IP3)-IP3 receptor pathway is required for insulin-stimulated glucose transporter 4 translocation and glucose uptake in cardiomyocytes. Endocrinology. 2010;151:4665–77. doi: 10.1210/en.2010-0116. [DOI] [PubMed] [Google Scholar]
- 103.Lee DK, Lanca AJ, Cheng R, Nguyen T, Ji XD, Gobeil F, Jr, et al. Agonist-independent nuclear localization of the Apelin, angiotensin AT1, and bradykinin B2 receptors. J Biol Chem. 2004;279:7901–8. doi: 10.1074/jbc.M306377200. [DOI] [PubMed] [Google Scholar]
- 104.Zhong X, Liu J, Lu F, Wang Y, Zhao Y, Dong S, et al. Calcium sensing receptor regulates cardiomyocyte function through nuclear calcium. Cell Biol Int. 2012;36:937–43. doi: 10.1042/CBI20110594. [DOI] [PubMed] [Google Scholar]
- 105.Vicencio JM, Ibarra C, Estrada M, Chiong M, Soto D, Parra V, et al. Testosterone induces an intracellular calcium increase by a nongenomic mechanism in cultured rat cardiac myocytes. Endocrinology. 2006;147:1386–95. doi: 10.1210/en.2005-1139. [DOI] [PubMed] [Google Scholar]
- 106.Tadevosyan A, Vaniotis G, Allen BG, Hebert TE, Nattel S. G protein-coupled receptor signalling in the cardiac nuclear membrane: evidence and possible roles in physiological and pathophysiological function. J Physiol. 2012;590:1313–30. doi: 10.1113/jphysiol.2011.222794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tadevosyan A, Maguy A, Villeneuve LR, Babin J, Bonnefoy A, Allen BG, et al. Nuclear-delimited angiotensin receptor-mediated signaling regulates cardiomyocyte gene expression. J Biol Chem. 2010;285:22338–49. doi: 10.1074/jbc.M110.121749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Malhotra R, Sadoshima J, Brosius FC, 3rd, Izumo S. Mechanical stretch and angiotensin II differentially upregulate the renin–angiotensin system in cardiac myocytes in vitro. Circ Res. 1999;85:137–46. doi: 10.1161/01.res.85.2.137. [DOI] [PubMed] [Google Scholar]
- 109.Peters J, Farrenkopf R, Clausmeyer S, Zimmer J, Kantachuvesiri S, Sharp MG, et al. Functional significance of prorenin internalization in the rat heart. Circ Res. 2002;90:1135–41. doi: 10.1161/01.res.0000019242.51541.99. [DOI] [PubMed] [Google Scholar]
- 110.Dostal DE, Rothblum KN, Conrad KM, Cooper GR, Baker KM. Detection of angiotensin I and II in cultured rat cardiac myocytes and fibroblasts. Am J Physiol. 1992;263:C851–63. doi: 10.1152/ajpcell.1992.263.4.C851. [DOI] [PubMed] [Google Scholar]
- 111.Boivin B, Chevalier D, Villeneuve LR, Rousseau E, Allen BG. Functional endothelin receptors are present on nuclei in cardiac ventricular myocytes. J Biol Chem. 2003;278:29153–63. doi: 10.1074/jbc.M301738200. [DOI] [PubMed] [Google Scholar]
- 112.Merlen C, Farhat N, Luo X, Chatenet D, Tadevosyan A, Villeneuve LR, et al. Intracrine endothelin signaling evokes IP3-dependent increases in nucleoplasmic Ca(2+) in adult cardiac myocytes. J Mol Cell Cardiol. 2013;62:189–202. doi: 10.1016/j.yjmcc.2013.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Thomas PB, Liu EC, Webb ML, Mukherjee R, Hebbar L, Spinale FG. Exogenous effects and endogenous production of endothelin in cardiac myocytes: potential significance in heart failure. Am J Physiol. 1996;271:H2629–37. doi: 10.1152/ajpheart.1996.271.6.H2629. [DOI] [PubMed] [Google Scholar]
- 114.Wright CD, Wu SC, Dahl EF, Sazama AJ, O’Connell TD. Nuclear localization drives alpha1-adrenergic receptor oligomerization and signaling in cardiac myocytes. Cell Signal. 2008;24:794–802. doi: 10.1016/j.cellsig.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wu SC, Dahl EF, Wright CD, Cypher AL, Healy CL, O’Connell TD. Nuclear localization of alpha1A-adrenergic receptors is required for signaling in cardiac myocytes: an “inside-out” alpha1-AR signaling pathway. J Am Heart Assoc. 2014;3:e000145. doi: 10.1161/JAHA.113.000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.van Berlo JH, Maillet M, Molkentin JD. Signaling effectors underlying pathologic growth and remodeling of the heart. J Clin Invest. 2013;123:37–45. doi: 10.1172/JCI62839. [DOI] [PMC free article] [PubMed] [Google Scholar]