To the editor:
Endothelial cells respond to vascular damage by secreting concatemers of the adhesive glycoprotein von Willebrand factor (VWF) to capture blood platelets and promote hemostasis. VWF is contained in large rod-shaped secretory granules called Weibel-Palade bodies (WPBs), and how VWF is stored in and released from these organelles is of considerable interest.1 Recently, a novel mechanism was described for VWF release in which an actomyosin ring forms around the WPB several seconds after its fusion with the plasma membrane to squeeze VWF from the WPB.2 This new mechanism was described in experiments using the potent secretagogue phorbol 12-myristate 13-acetate (PMA), and has received considerable attention.3-5 However, the mechanism of action of PMA differs in several key respects from that of physiological secretagogues, such as histamine, that elevate intracellular free calcium ion concentrations ([Ca2+]i). PMA action is characterized by a slow onset (tens of seconds to minutes) but a protracted (hours) period of WPB fusion that occurs without an increase in [Ca2+]i. VWF is released slowly from the WPB after fusion with the plasma membrane (tens of seconds), and secretion is prevented by inhibition of protein kinase C,6 myosin IIB (MyoIIB),2 or actin disruption or stabilization.7 In contrast, histamine (or ionomycin) triggers a rapid (<1 second) but transient (10-30 seconds) burst in WPB exocytosis.8 Ca2+-mediated VWF secretion is not blocked by protein kinase C inhibition6 or actin disruption,9 and early optical studies indicated that the initial expulsion of VWF occurs on a subsecond time scale (see Erent et al8 and references therein). On the basis of these observations we asked whether the actomyosin process represents a general mechanism for VWF release from WPBs. Our new data suggest not.
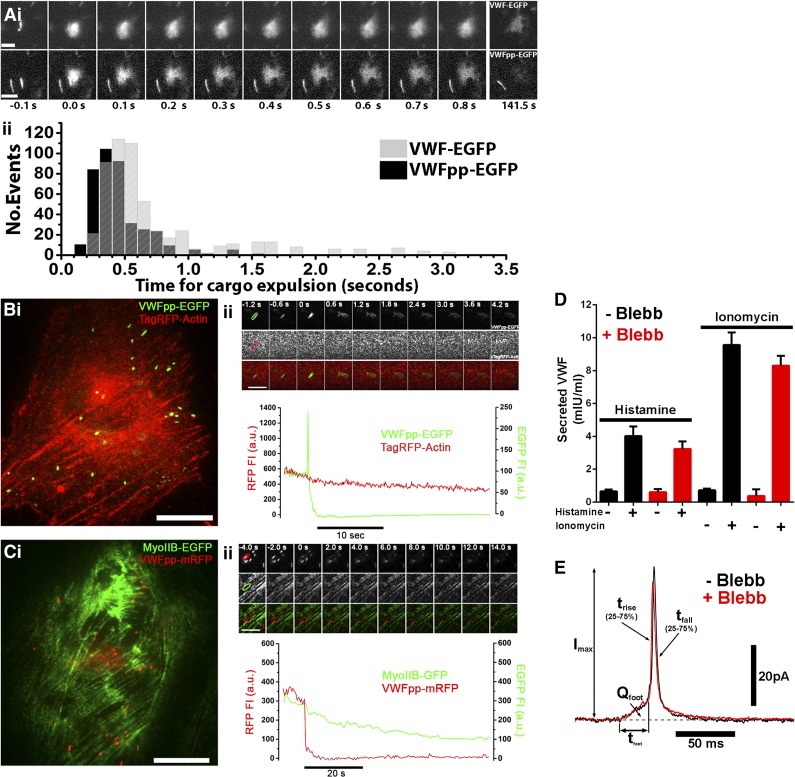
First, live-cell imaging of fluorescent VWF–enhanced green fluorescent protein (VWF-EGFP) or VWF-propeptide-EGFP (VWFpp-EGFP) expulsion from individual WPBs shows this process to be fast (Figure 1Ai-ii). Second, dual-color imaging of endothelial cells coexpressing VWFpp-EGFP or VWFpp-mCherry and either TagRFP-actin (Figure 1Bi-ii) or MyoIIB-GFP (Figure 1Ci-ii) revealed no evidence of redistribution or accumulation of actin or MyoIIB to WPBs undergoing exocytosis. Third, MyoIIB inhibition by blebbistatin did not prevent histamine-evoked VWF secretion (Figure 1D), and we have reconfirmed that actin disruption or stabilization fails to prevent Ca2+-mediated VWF secretion. Because myosin II may subtly regulate the opening of secretory granule fusion pores (reviewed in Porat-Shliom et al3), we also performed amperometry studies but found no major effects of MyoIIB inhibition on WPB fusion pore formation or expansion (Figure 1E). Together, the data suggest that expulsion of VWF from WPBs during Ca2+-driven WPB exocytosis does not involve actomyosin.
Figure 1.
Fast actomyosin-independent VWF expulsion from WPBs during Ca2+-mediated exocytosis. In the absence of flow, secreted VWF concatemers form irregular-shaped patches on the cell surface and disperse slowly into solution. Importantly, the initial expulsion of VWF from WPBs and the subsequent dispersal from the cell surface constitute separate processes. (Ai) Montages of individual WPB exocytotic events taken from live-cell videos of histamine-stimulated human umbilical vein endothelial cells (HUVECs) expressing VWF-EGFP (top) or VWFpp-EGFP (bottom). Images were acquired at 10 frames per second. The first frame in which an increase in EGFP fluorescence due to fusion was detected is set to t = 0 seconds. Bars represent 2 μm. (Aii) Histograms of the pooled times, from t = 0 seconds to expulsion, indicated by formation of irregular patches of cell surface VWF-EGFP (gray bars) or VWFpp-EGFP (black bars) from individual WPBs after stimulation with histamine or ionomycin. For VWF-EGFP, n = 543 fusion events (100 μM histamine, n = 183, 10 cells; 1 µM ionomycin, n = 310, 11 cells); for VWFpp-EGFP, n = 402 fusion events (100 μM histamine, n = 92, 7 cells; 1 μM ionomycin, n = 310, 13 cells). (Bi) Dual-color imaging of a single HUVEC coexpressing VWFpp-EGFP and TagRFP-actin. Bar represents 10 μm. (Bii) Upper: Image montage of a single WPB undergoing exocytosis during ionomycin stimulation; EGFP fluorescence (top), RFP fluorescence (middle), merge image (bottom). Images were acquired at 30 frames per second, and selected frames (times indicated) are shown. Bar represents 2 µm. Lower: Mean fluorescence intensity (FI) within the color-coded regions of interest indicated on the first frame of the upper panel top row, plotted against time. WPB fusion is associated with a sharp increase in EGFP fluorescence due to EGFP-dequenching.8 Note that there was no evidence of RFP-actin accumulation prior to or during WPB exocytosis (n = 136 fusion events, 12 cells). (Ci-ii) Same as for panel Bi-ii, but in HUVECs coexpressing MyoIIB-GFP and VWFpp-mRFP. Note that there was no evidence of MyoIIB-EGFP accumulation prior to or during WPB exocytosis (n = 24 fusion events, 5 cells). Also note that RFP fluorescence is not pH sensitive, and exocytosis, therefore, is marked only by a fall in WPB associated RFP fluorescence. (D) Blebbistatin (Blebb) treatment (25 µM for 20 minutes; red bars) does not alter basal (−), histamine-evoked (100 μM; +), or ionomycin-evoked (1 µM; +) VWF secretion. Plots show data pooled from 3 independent experiments each carried out in triplicate (mean ± standard error of the mean). (E) Examples of individual WPB current spikes recorded by amperometry in control (black) and Blebb-pretreated (red) HUVECs. The kinetics of current spike foot signals and main spike rise times provide information about fusion pore formation and expansion. Mean (± standard error of the mean) pre–foot spike parameters, including foot signal duration (tfoot; control: 13.72 ± 3.31 ms, n = 216, 44 cells; Blebb: 9.85 ± 1.09 ms, n = 256, 50 cells) and total foot signal charge (Qfoot; control: 0.057 ± 0.013 pC; Blebb: 0.0636 ± 0.0147 pC), were no different (Student t test). Mean main spike parameters, including spike rise time (trise; control: 3.22 ± 0.17 ms, n = 317 spikes, 44 cells; Blebb: 3.26 ± 0.15 ms, n = 384 spikes, 50 cells), peak amplitude (Imax; control: 29.14 ± 1.31 pA; Blebb: 27.96 ± 1.11 pA), and spike decay time (tfall; control: 15.08 ± 1.09 ms; Blebb: 13.64 ± 0.84 ms), were not different (Student t test). a.u., arbitrary unit; GFP, green fluorescent protein; RFP, red fluorescent protein.
What other mechanism might account for fast actomyosin-independent VWF expulsion during Ca2+-mediated exocytosis? Studies of mucins, large multimeric glycoproteins closely related to VWF, suggest that the subsecond expulsion of these charged polymers from mucin granules is driven by ionic fluxes and water entry (discussed in Erent et al8). VWF, like mucins, is stored at high concentration, and the condensation and aggregation of these proteins are facilitated by charge shielding by cationic species including hydrogen ion (H+) and Ca2+. The acidic lumen of the WPB is particularly important for VWF expulsion. Loss of H+ following fusion pore formation precedes postfusion changes in WPB morphology and rapid VWF expulsion, and both processes can be blocked simply by lowering the external pH close to that of the prefusion mature WPB.10 Thus, VWF expulsion from WPBs during histamine- or Ca2+-mediated exocytosis utilizes a beautifully simple mechanism that depends, in essence, on the chemistry of VWF and the intracellular processes that ensure its condensation and aggregation for storage at high concentration.
Is this mechanism likely to be of physiological relevance? Following injury, endothelial cells must function rapidly to minimize blood loss. The earliest event within these endothelial cells will be an increase in [Ca2+]i produced either by cell damage, physicomechanical stimulation, or acute activation by physiological mediators generated locally at the injury site (eg, thrombin, histamine, fibrin, adenine nucleotides, and peptidoleukotrienes). Together, these mediators ensure rapid VWF expulsion to the endothelial cell surface to capture platelets and facilitate hemostasis.
Clearly, there is more than one way to unpack a WPB. Further studies will be needed to clarify the specific physiological conditions subserved by actomyosin-dependent and -independent VWF delivery to the endothelial cell surface.
Authorship
Acknowledgments: The authors thank Dr Justin Molloy (Francis Crick Institute Mill Hill Laboratory, London, United Kingdom) for assistance with total internal reflection fluorescence microscopy and for critical reading of the manuscript, and Dr Matthew J. Hannah for critical comments on the manuscript. MyoIIB-GFP was kindly provided by Dr R. S. Adelstein (National Institutes of Health National Heart, Lung, and Blood Institute, Bethesda, MD). T.C. was funded by the United Kingdom Medical Research Council grant U117573808. R.B. was supported by a European Hematology Association Research Fellowship.
Contribution: I.L.C., E.C., N.H., R.B., G.M., and T.C. designed and performed the research and analyzed the data; and I.L.C., N.H., R.B., and T.C. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for E.C. is Department of Neuroscience, University of Wisconsin, Madison, WI.
The current affiliation for N.H. is National Heart and Lung Institute, Imperial College, London, United Kingdom.
The current affiliation for R.B. is Department of Plasma Proteins, Sanquin-AMC Landsteiner Laboratory, Amsterdam, The Netherlands.
Correspondence: T. Carter, Cardiovascular and Cell Science Research Institute, St George’s University, Cranmer Terrace, London SW17 0RE, United Kingdom; e-mail: tcarter@sgul.ac.uk.
References
- 1.Springer TA. von Willebrand factor, Jedi knight of the bloodstream. Blood. 2014;124(9):1412–1425. doi: 10.1182/blood-2014-05-378638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nightingale TD, White IJ, Doyle EL, et al. Actomyosin II contractility expels von Willebrand factor from Weibel-Palade bodies during exocytosis. J Cell Biol. 2011;194(4):613–629. doi: 10.1083/jcb.201011119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Porat-Shliom N, Milberg O, Masedunskas A, Weigert R. Multiple roles for the actin cytoskeleton during regulated exocytosis. Cell Mol Life Sci. 2013;70(12):2099–2121. doi: 10.1007/s00018-012-1156-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nightingale TD, Cutler DF, Cramer LP. Actin coats and rings promote regulated exocytosis. Trends Cell Biol. 2012;22(6):329–337. doi: 10.1016/j.tcb.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Marks MS, Heijnen HF, Raposo G. Lysosome-related organelles: unusual compartments become mainstream. Curr Opin Cell Biol. 2013;25(4):495–505. doi: 10.1016/j.ceb.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carew MA, Paleolog EM, Pearson JD. The roles of protein kinase C and intracellular Ca2+ in the secretion of von Willebrand factor from human vascular endothelial cells. Biochem J. 1992;286(2):631–635. doi: 10.1042/bj2860631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nightingale TD, Pattni K, Hume AN, Seabra MC, Cutler DF. Rab27a and MyRIP regulate the amount and multimeric state of VWF released from endothelial cells. Blood. 2009;113(20):5010–5018. doi: 10.1182/blood-2008-09-181206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erent M, Meli A, Moisoi N, et al. Rate, extent and concentration dependence of histamine-evoked Weibel-Palade body exocytosis determined from individual fusion events in human endothelial cells. J Physiol. 2007;583(1):195–212. doi: 10.1113/jphysiol.2007.132993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vischer UM, Barth H, Wollheim CB. Regulated von Willebrand factor secretion is associated with agonist-specific patterns of cytoskeletal remodeling in cultured endothelial cells. Arterioscler Thromb Vasc Biol. 2000;20(3):883–891. doi: 10.1161/01.atv.20.3.883. [DOI] [PubMed] [Google Scholar]
- 10.Babich V, Knipe L, Hewlett L, et al. Differential effect of extracellular acidosis on the release and dispersal of soluble and membrane proteins secreted from the Weibel-Palade body. J Biol Chem. 2009;284(18):12459–12468. doi: 10.1074/jbc.M809235200. [DOI] [PMC free article] [PubMed] [Google Scholar]