Abstract
Converging empirical data suggests a set of largely consistent personality traits exist in both human and nonhuman primates; despite these similarities, almost nothing is known concerning the neurobiological basis of these traits in nonhuman primates. The current study examined associations between chimpanzee personality traits and the grey matter volume and asymmetry of various frontal cortex regions in 107 captive chimpanzees. Chimpanzees rated as higher on Openness and Extraversion had greater bilateral grey matter volumes in the anterior cingulate cortex. Further, chimpanzee rated as higher on Dominance had larger grey volumes in the left anterior cingulate cortex and right Prefrontal Cortex (PFC). Finally, apes rated higher on Reactivity/Unpredictability had higher grey matter volumes in the right mesial PFC. All associations survived after applying False Discovery Rate (FDR) thresholds. Results are discussed in terms of current neuroscientific models of personality which suggest that the frontal cortex, and asymmetries in this region, play an important role in the neurobiological foundation of broad dispositional traits.
Keywords: Personality, Prefrontal cortex, Chimpanzees
1. Introduction
Within both the human and non-human primate literatures, the Five Factor Model (FFM; Freeman & Gosling, 2010; McCrae & Costa, 2008) is the most widely used model of personality. With regard to human samples, extensive factor-analytic research using both natural language adjectives (e.g., the lexical tradition) and theoretically-based personality questionnaires consistently reveal five robust broad personality dimensions across languages and cultures: Extraversion (e.g., energetic approach-oriented), Agreeableness (e.g., prosocial tendency towards others), Conscientiousness (e.g., impulse control abilities and attention to detail), Neuroticism (e.g., general tendency to experience negative emotions and distress), and Openness (e.g., open-mindedness, originality). In addition to extensive evidence of the replicability of this model among human samples, a large body of literature confirms the importance of these traits with respect to both psychological and physical health (e.g., Bogg & Roberts, 2004; Clark & Watson, 2008; Kotov et al., 2010; Miller & Lynam, 2001. Furthermore, among chimpanzees, a relatively smaller, but converging empirical literature, suggests the existence of largely similar personality traits as found among humans (i.e., FFM; Freeman & Gosling, 2010) organized in a similar hierarchical manner (Latzman et al., 2014; Latzman et al., in press). The comparative nature and importance of this nonhuman primate literature is particularly clear for research with chimpanzees, because of their close genetic similarity to humans.
Despite the recent interest in comparative personality among chimpanzees however, the vast majority of studies to date have focused on the description, construction, and validation of the personality construct as well as instruments designed to assess it. Although a relatively smaller literature addresses basic questions relating to the genetic basis of personality, surprisingly no study to date has examined the neurological correlates of non-human primate personality. Indeed, within the human literature, the study of personality neuroscience is becoming increasingly popular and influential (DeYoung, 2010). Reliable cross-species findings of associations between personality and specific brain regions would provide critical confirmatory evidence concerning a biologically-based explanatory model of personality. Given the growing excitement in trait personality within non-human primates (Freeman & Gosling, 2010), the elucidation of a largely parallel and robust trait structure (Freeman et al., 2013), and evidence of a genetic basis for individual variation across these traits (Adams, King, & Weiss 2012; Latzman et al., in press), investigation of the neuroanatomical correlates of chimpanzee personality is the next logical step, and thus is the primary goal of the current study.
1.1 Chimpanzees as a model species for comparative science
It is now widely accepted that humans and chimpanzees share many emotional processes resulting in an unparalleled animal model of human emotion (Phillips et al., 2014). In addition to an extremely high percentage of shared genetics, humans share a great deal of evolutionary history with chimpanzees. For example, chimpanzees live in complex social environments that require sophisticated social cognition and behavior to recruit social support, alliance formation, and recognition of emotion displays (de Waal, 1996).
Perhaps not surprisingly given our shared history, a small but converging empirical literature suggests chimpanzees demonstrate largely similar personality traits as found among humans (i.e., FFM; Freeman & Gosling, 2010), which can be organized in a similar hierarchical manner (Latzman et al., 2014; Latzman et al., in press). Indeed, recent factor analytic research, using a combination of top-down and bottom-up approaches, has led to the development of a comprehensive new rating scale for measuring personality in chimpanzees (Freeman et al., 2013). This work supports a robust five-factor solution largely paralleling the FFM reliably found with human samples: Reactivity/Undependability, Dominance, Extraversion, Openness, and Agreeableness. Although not typically labeled as such in the FFM, Dominance appears to parallel reverse-keyed Neuroticism; that is, Dominance and Neuroticism appear to lie on a bipolar dimension, each anchoring opposite poles of the dimension. Indeed, Dominance is reflected in low levels of fearfulness and timidity (Freeman et al., 2013). Reactivity/Unpredictability, however, is a dimension not previously found to emerge in the FFM, consisting of items that have previously been found to load on FFM Conscientiousness (e.g., impulsive, reverse-keyed), Agreeableness (e.g., deceptive, reverse-keyed), and Extraversion (e.g., calm, reverse-keyed) (Digman, 1990). All told, chimpanzee models are uniquely poised to provide access to highly complex processes underlying basic dispositional traits largely free from the typical socio-cultural confounds inherent in human studies (Nelson & Winslow, 2009). The strength of any animal model lies in its ability to have translational value between the animal model and humans. From this perspective, chimpanzees represent by far the best animal model species for investigations of personality, allowing for a better understanding of its evolutionary origins. In sum, the value of comparative chimpanzee research to the study of affective neuroscience is clear.
The majority of the comparative personality literature has focused confirming the existence and structure of chimpanzee personality, with few studies examining its neurological basis. There does exist a relatively small literature, however, beginning to address more basic questions regarding the genetic basis of personality. For example, similar to findings with human samples (Bouchard & McGue, 2003), some personality traits among chimpanzees have been found to be heritable (Adams, King, & Weiss, 2012; Latzman et al., in press) with this heritability attributable, at least in part, to specific genetic polymorphisms (Hopkins, Donaldson, & Young 2012; Hong et al., 2011). Given the fact that various genetic polymorphisms are expressed in different areas of the brain, the neurological basis for chimpanzee personality is assumed from these findings.
1.2 Personality neuroscience
Although our understanding of the neural mechanisms underlying the expression of personality dimensions is still in its infancy, and the specificity of associations are equivocal, recent human neuroimaging research confirms the importance of various brain regions in explaining the neurobiology of personality (DeYoung, 2010). Given that no research to date has investigated the neurological basis of personality in non-human primates, we relied upon this human literature as a basis for our tentative hypotheses. Among humans, one brain region that appears to be particularly important is the frontal cortex (FC), and particularly the anterior cingulate (ACC) and prefrontal (PFC) cortices.
1.2.1 Anterior Cingulate Cortex (ACC)
Activity of the ACC, an area associated with motivation and regulation of behavior through cognitive and affective mechanisms (Bush, Luu, & Posner, 2000), has been found to be associated with FFM Neuroticism (Eisenberger, Lieberman, & Satpute, 2005; Reuter et al., 2004), a trait that largely parallels low Dominance in chimpanzees (Freeman et al., 2013; Latzman et al., 2014; Latzman et al., in press), as well as Extraversion (Haas et al., 2006). For example, Neuroticism has been found to be associated with ACC activity at rest and in response to aversive or novel stimuli (Eisenberger, Lieberman, & Satpute, 2005; Reuter et al., 2004).
1.2.2 Prefrontal Cortex (PFC)
The PFC, located in the most anterior portion of the frontal lobe, is the most recently expanded portion of the brain and is most pronounced in primates (Rilling & Insel, 1999; Semendeferi et al., 1997). It is highly interconnected with other cortical, as well as subcortical, areas which allows for the control of a wide range of behaviors (Miller & Cohen, 2001). The functions of the PFC can be broadly summarized as carrying out goal-directed action with lesions to the PFC resulting in widespread deficits, including problems with motivation, attention, inhibition, planning, and memory (Koziol & Budding, 2009). This region may be particularly relevant in the context of comparative studies, as the functions subserved by the PFC are highly developed in both humans and great apes (Bianchi et al., 2013; Matsuzawa, 2001). Recent neuroimaging findings confirm the importance of this region in the explanation of various personality traits. Indeed, converging evidence from both structural and functional MRI studies suggest that this region in humans is broadly associated with FFM personality with more distinct correlates emerging between specific areas of the PFC and specific personality traits (DeYoung, 2010).
1.2.2.1 Orbital prefrontal cortex (oPFC)
Volume of the oPFC, a PFC region involved in decision making and coding of values of rewards (Bechara, Damasio, & Damasio, 2000) has been found to be associated with Extraversion (DeYoung et al., 2010; Rauch et al., 2005), a trait linked to the tendency to experience positive emotions, usually in the context of reward or expected reward (Clark & Watson, 2008). Similar findings emerge in the context of fMRI research, with Extraversion found to be associated with brain activity in the oPFC, among other areas, both at rest and in response to positive or rewarding stimuli (Canli et al., 2001; Cohen et al., 2005; Mobbs et al., 2005).
1.2.2.2 Mesial prefrontal cortex (mPFC)
Reduced volume of the mPFC, an area of the PFC involved in processing, representing, and integrating social and affective information (Blakemore, Winston, & Frith, 2004), has been found to be associated with Neuroticism (DeYoung, 2010), a trait that largely parallels reverse-keyed Dominance in chimpanzees (Freeman et al., 2013). In addition to volumetric findings, activity in the mPFC has also been found to be associated with Neuroticism, which is thought to reflect difficulties with emotion regulation (Williams et al., 2006). Further, mPFC activity has also been found to associate with Openness (DeYoung et al., 2009), although the consistency of this finding is not clear given various approaches to the measurement of this trait. Lastly, whereas Agreeableness has rarely been explicitly examined in the context of neuroimaging studies, empathy (an aspect of trait Agreeableness) has repeatedly been found to be associated with activity in the mPFC across a number of different paradigms (Chakrabarti, Bullmore, & Baron-Cohen, 2006).
1.2.2.3 Dorsal prefrontal cortex (dPFC)
Similar to other PFC areas, the dPFC, an area primarily responsible for motor planning, organization, and regulation, especially as related to impulse control (Miller & Cohen, 2001) has also been found to have distinct personality correlates. Indeed, dPFC activity has been found to be predicted by Agreeableness (Haas et al., 2007), a trait linked to prosocial, affiliative tendencies (McCrae & Costa, 2008). Additionally, dPFC activity has been found to be related to self-reported impulsivity (Brown et al., 2006), a key aspect of FFM Conscientiousness, and item-content associated with Reactivity/undependability in chimpanzees (Freeman et al., 2013).
1.2.3 Asymmetries of the frontal cortex
In addition to bilateral volumetric variation, a large extant theoretical and empirical literature reveals functional and anatomic hemispheric asymmetries are associated with many psychological and behavioral traits related to temperament and personality. Indeed, although a number of models have been proposed to explained the results (e.g., Davidson, 1998; Harmon-Jones, 2003), a converging set of findings suggests approach- (e.g., Extraversion) and avoidant (e.g., Neuroticism/low Dominance)-related temperament traits or emotions are differentially associated with the left and right FC, respectively. Not surprisingly, these differences in asymmetries also appear to be associated with psychiatric conditions. For example, rightward frontal asymmetry has been found to predict increased risk for depression (Nusslock, Shackman, Harmon-Jones, Allow, Coan, & Abramson, 2011). Taken together, the importance of quantifying this aspect of FC anatomy is clear from the potential role that asymmetries likely play in temperament and emotion, in addition to the importance of these asymmetries for understanding various behavioral and emotional outcomes.
1.3 Current Study
Despite a growing recognition of the critical importance of comparative personality research with non-human primates, research has yet to the neuroanatomical correlates of personality among chimpanzees. Such investigations are critically important in elucidating common biological systems underlying personality (Stockard, 1931) which, in turn, will provide important information concerning not only the basic neurobiologically-based architecture of personality, but also increase our understanding of the central role of basic dimensions of personality within the evolutionary process. The current study therefore aimed to examine associations between FFM chimpanzee personality and the volume of various FC regions. As no previous studies to date have examined associations between personality and the ACC and PFC among non-human primates, we based our hypotheses on the extant human personality neuroscience literature which were tentative given the relatively unequivocal and nascent state of the literature. Specifically, as reviewed above, we expected volume of the oPFC to be associated with Extraversion, mPFC volume to be associated with Openness and Agreeableness, as well as Dominance, the reverse-keyed chimpanzee equivalent of Neuroticism. With regard to the Agreeableness hypothesis, we based this on findings with regard to empathy, an aspect of FFM Agreeableness. Additionally, we hypothesized that dPFC volume would be associated with Agreeableness and Reactivity/undependability. We base the latter hypothesis on above-reviewed findings of associations with self-reported impulsivity, content that loads on the this dimension and also represents a central feature of FFM Conscientiousness. Lastly, we expected Dominance and Extraversion to be associated with ACC volume. In these studies, we focused on two aspects of cortical organization of the PFC regions, including the grey matter (GM) volume and the asymmetries in the regions. In addition to volumetric variation, as noted above, we also quantified FC asymmetries because of the potential role that asymmetries might play in emotion and behavior. Given previous findings of approach-related traits associated with leftward FC asymmetries and avoidant-related traits associated with rightward FC asymmetries, we expected Extraversion to evidence a significant leftward bias and Dominance to evidence a significant rightward bias.
2. Materials and Methods
2.1 Subjects
The sample consisted of 107 captive chimpanzees, including 50 males (Mage =22.43 ± 11.25) and 57 females (Mage = 19.87 ± 9.03) ranging in age from 8 to 50 years, housed at The University of Texas MD Anderson Cancer Center (UTMDACC). Chimpanzees were housed in social groups ranging from 5 to 14 individuals.
2.2 Assessment of personality
Through the consideration of both existing human personality literature as well as those traits that may be specific to chimpanzees, Freeman and colleagues (Freeman et al., 2013) used a combined top-down and bottom up approach to develop a 41-item personality questionnaire. Each item consists of a single trait accompanied by a behavioral definition and a Likert-type scale ranging from one ("least descriptive of the chimpanzee") to seven ("most descriptive of the chimpanzee"). Strong evidence was reported for five factors: Reactivity/unpredictability (e.g., Irritable, Temperamental/Moody), Dominance (e.g., Fearful [reversed], Timid [reversed]), Extraversion (e.g., Depressed [reversed], Solitary [reversed]), Openness (e.g., Human oriented, Inquisitive/Curious), and Agreeableness (e.g., Protective, Considerate). These scales have been found to evidence strong convergent and discriminant validity with various in vivo behavior has been demonstrated as has strong criterion validity with other scales previously validated across several different studies (Freeman et al., 2013). Further, reliability has been shown to be adequate both in terms of inter-rater reliability, as well as internal consistency, and factors have been found to demonstrate good external validity (Freeman et al., 2013).
Using this instrument, chimpanzees were rated by colony staff members that worked with the animals for an extended period of time and "feel that they have enough experience for an accurate rating." As with research using other chimpanzee personality questionnaires45, mean ratings across raters in the current study were used for all analyses. Consistent with previously published data on interrater reliabilities for personality ratings in chimpanzees (Weiss, King, & Hopkins, 2007), mean interrater reliability using ICC (3, k) across all items was .61. Further, consistent with previous findings (e.g., Freeman et al., 2013), the median internal consistency (Cronbach’s alpha) values across personality scales was .86, with values ranging from .48 (Agreeableness) to .94 (Reactivity/Undependendability).
2.3 Image Collection and Procedure
All chimpanzees were scanned during their annual physical examination which occurred within two-years of personality ratings. Magnetic resonance image (MRI) scans followed standard procedures at the UTMDACC and were designed to minimize stress. Thus, the animals were first sedated with ketamine (10 mg/kg) or telazol (3-5mg/kg) and were subsequently anaesthetized with propofol (40–60 mg/(kg/h)). They were then transported to the MRI scanning facility and placed in a supine position in the scanner with their head in a human-head coil. Upon completion of the MRI, chimpanzees were singly-housed for 2-24 hours to permit close monitoring and safe recovery from the anesthesia prior to returning to their home social group. All procedures were approved by the Institutional Animal Care and Use Committees at UTMDACC. The chimpanzees were scanned using a 1.5T G.E. echo-speed Horizon LX MR scanner (GE Medical Systems, Milwaukee, Wisconsin, USA). T1-weighted images were collected in the transverse plane using a gradient echo protocol (pulse repetition = 19.0 ms, echo time = 8.5 ms, number of signals averaged = 8, matrix size = 256 × 256, with 0.7 × 0.7 × 1.2 resolution).
Initially, each T1-weighted MRI scan was skull stripped and the volume segmented into grey matter, white matter and CSF using FSL (Analysis Group, FMRIB, Oxford, UK) (Smith et al., 2004; Zhang, Brady, & Smith, 2001). Next, each individual T-1 MRI scan was linearly registered to a previously constructed symmetrical template of the chimpanzee brain using procedures that have been described elsewhere (Li et al., 2010). The matrix derived from the linear registration was saved for later use. The region of interest masks (described below and in Hopkins & Avants, 2013) were drawn on the chimpanzee template brain using a mouse-controlled pointer and then transformed back to the individual native GM volume maps for each subject, using the inverse matrix of the original registration. The masks were then applied to the individual subjects’ segmented GM scan to derive volumes for each of the 4 regions within each hemisphere.
2.4 Regions of Interest
2.4.1 Prefrontal Cortex (PFC)
As described previously (i.e., Hopkins & Avants, 2013), landmarks used in previous research (Semendeferi & Damasio, 2000) were adopted in defining the orbital (oPFC), dorsal (dPFC) and mesial (mPFC) prefrontal cortex in serial coronal images (see Figure 1). Beginning posteriorly when the frontal orbital (FO) sulcus was first visible, for the oPFC, a line was drawn from the surface of the FO sulcus to its most medial point. A second line was then drawn from the medial point of FO to the tip of the rectus gyrus, which was then followed along the inferior outer surface of the cortex until intersecting with the lateral point of the FO sulcus. If the caudate and putamen were visible on the images, the line extending from the medial FO sulcus to the rectrus gyrus excluded these regions. The dPFC was outlined by laterally tracing on the surface of the brain from FO to the upper, dorsal most mesial point. From there, a line was drawn that connected the most medial point of all frontal sulci between the most mesial point and the FO sulcus. Finally, the mesial PFC was defined from the grey matter tip of the rectus gyrus to the upper dorsal end of the mesial surface, which included portions of the superior frontal gyrus but omitted any tissue belonging to the anterior cingulate cortex and subgenu region. The ACC regions were traced separately from the PFC.
Figure 1.
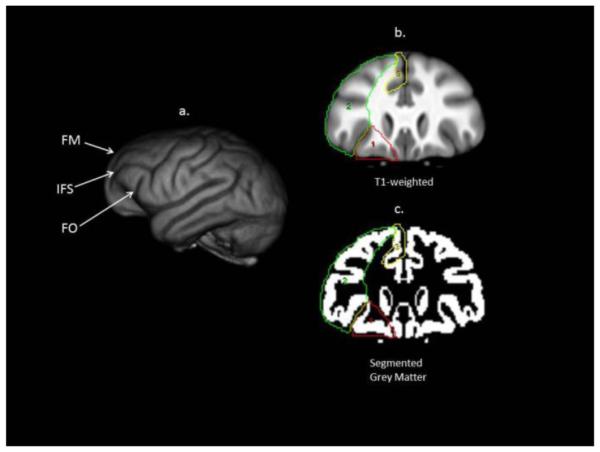
Prefrontal cortex regions of interest. This figure illustrates: a) 3D rendering of a chimpanzee brain; b & c) coronal view of template brain and segmented grey matter volume with the three object maps corresponding to the prefrontal cortex, outlined in red (orbital), green (dorsal), and yellow (mesial). See text for specific description of the landmarks used to define the regions. FM = fronto-marginal sulcus; IFC = inferior frontal sulcus, FO = front-orbital sulcus. Note, white matter is included on the ROIs depicted on the T1-weighted scan but when the object maps were applied to the segmented grey matter volume, only voxels corresponding to gray matter were included in the calculation of volume.
2.4.2 Anterior Cingulate Cortex (ACC)
The ACC was quantified in the sagittal and coronal planes following the procedure used in human subjects (Bush, Luu, & Posner, 2000) (see Figure 2) and in chimpanzees (Blatchley & Hopkins, 2010). For each subject, the mid-sagittal slice was identified. Moving 1 mm laterally in both the left and right hemisphere, a box was drawn around the corpus callosum (CC). The box surrounding the CC was then split at the anterior-posterior midpoint in order to divide the CC into anterior and posterior regions (Point A in Figure 2). Next, a vertical line was drawn perpendicular to the genu of the CC through the medial plane (Point B in Figure 2). The ACC was divided into three regions (see labels 3, 4 and 5 in Figure 2) and traced in the coronal plane. Region 24p was the gyrus that was superior to the CC and was bordered on the anterior by a vertical line (Point B in Figure 2) and on the posterior by a vertical line (Point A in Figure 2) (colored yellow, in Figure 2c). The superior border was the cingulate sulcus and the inferior border was the superior bank of CC (see Figure 2b). Region 24 was the portion of the cingulate that was anterior to the vertical line (Point B in Figure 2) and was bordered anteriorly by the cingulate sulcus and posteriorly by the vertical line (colored blue in Figure 2c). The superior border was the cingulate sulcus and the inferior border was the superior tip of the orbital frontal sulcus (see Figure 2b). The lateral border was the white matter belonging to the medial prefrontal cortex and the medial border was the mid-sagittal sulcus. Lastly, region 25 was that portion of the ACC that was inferior to the rostrum and genu of the CC. The anterior border was vertical line Point B in Figure 2 and the inferior border was the cingulate sulcus. The anterior border of region 25 was the rostrum and genu of the CC and the inferior border was the medial orbital sulcus (Figure 2b). The lateral border for area 25 was the internal capsule. To minimize potential Type I error due to the number of brain regions that were correlated with the different personality scales, we computed a single measure of grey matter volume for the entire anterior cingulate region. This was accomplished by summing the area 24, 24p and 25 GM volumes within each hemisphere.
Figure 2.
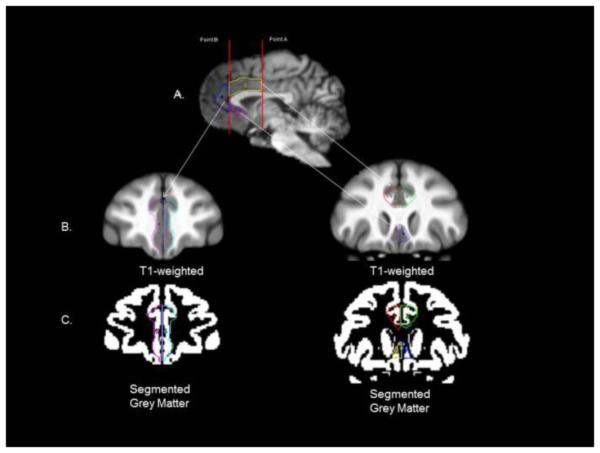
Anterior cingulate regions of interest. This figure illustrates: a) middle section view of the chimpanzee brain; b & c) coronal views of the chimpanzee T1-weighted and segmented grey matter brain with the anterior cingulate cortex (area 24) outlined in cyan and purple; c) coronal view of the chimpanzee brain with the anterior cingulate (red, green, area 24’) and subgenu (purple, blue) area 25 regions outlined on the scan. As above, white matter is included on the ROIs depicted on the T1-weighted scan but when the object maps were applied to the segmented grey matter volume, only voxels corresponding to gray matter were included in the calculation of volume
2.5 Data analysis
For each subject and hemisphere, we computed total GM volume for the dPFC, mPFC, oPFC and ACC. This was done by placing the object maps on the individual GM volumes and computing the number of grey matter voxels found within the region. Average GM volumes were derived by adding the left and right hemisphere volumes and dividing by two. For each region, we also computed asymmetry quotients (AQ) following the formula [AQ = (R − L) / ((R + L) *.5)] where R and L represent the left and right hemisphere volumes for each region. Positive AQ values reflect a right hemisphere bias and negative values reflect a left hemisphere bias. The absolute AQ values indicate the magnitude of the asymmetry, with larger values reflecting larger differences between the two hemispheres.
To assess the associations between the different personality constructs and brain measures, we first examined bivariate associations among personality, frontal cortex GM, and frontal asymmetries. We next conducted a series of multiple regression analyses with each of the FFM personality dimensions serving as the outcome measure, while sex and age (variables that have previously been found to associate with personality in chimpanzees; King Weiss, & Farmer, 2005; Weiss, King, & Hopkins, 2007) and either the adjusted volume of the PFC and ACC regions or AQ scores served as the predictor variables. To account for variation in head size and/or total GM volume, the GM volume of each region was divided by the total GM volume and multiplied by 100 for all analyses. Separate regression analyses were performed for the average adjusted GM volume and AQ measures so as to delineate the overall size compared to asymmetry predictors of individual variation in personality. To correct for multiple comparisons, effects were tested to determine if they survived a False Discovery Rate (FDR) threshold (Benjamini & Hochberg, 1995).
3. Results
3.1 Anatomical Analysis
Before assessing the association between the different brain measures and FFM personality, we performed a series of initial analyses on the PFC and ACC regions to test for sex differences and potential associations with age. For these analyses, we performed two multivariate analysis of covariance (MANCOVA) analyses with sex as the between-group factor and age as the covariate. The mean adjusted GM volumes for each region were the dependent variables in one analysis and the asymmetry quotient (AQ) scores were the dependent variables for the second analysis. Neither sex nor age was found to have significant effect on the mean adjusted volumes or AQ scores for the MANCOVA analyses.
Additionally, we tested whether the chimpanzees showed population-level asymmetries for each PFC region and the ACC using one sample t-tests on the AQ scores with the assumption that the population mean would be zero if the AQ scores were normally or bimodally distributed. Significant leftward asymmetries were found for the dPFC t(106)= −8.670, p < .001 and mPFC regions t(106)= −4.671, p < .001, while a significant rightward bias was found for the oPFC t(106)= 17.343, p < .001. No population-level bias was found for the ACC t(106)= −1.241, p = .217.
3.2 Bivariate analyses
Bivariate correlations among personality, raw frontal GM volumes, and frontal asymmetries are reported in Table 1. With the exception of Agreeableness, all personality traits evidenced moderate correlations with one another. Further, at the bivariate level, with the exception of Dominance, ACC GM volume was found to be significantly associated with all personality traits; this association was positive for Reactivity/Undependability and Extraversion and negative for Openness and Agreeableness. Further, both Openness and Agreeableness were negatively associated with GM volume of the dPFC and the mPFC. Mean FC GM was only significantly associated with Agreeableness.
Table 1.
Bivariate Correlations Among Personality, Frontal Cortex Grey Matter, and Frontal Asymmetries
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Personality | |||||||||||||
| 1. Reactivity/Unpredictability | |||||||||||||
| 2. Dominance | .39 | ||||||||||||
| 3. Extraversion | .45 | .27 | |||||||||||
| 4. Openness | .34 | .35 | .53 | ||||||||||
| 5. Agreeableness | −.11 | .06 | .16 | .24 | |||||||||
| Frontal GM | |||||||||||||
| 6. Dorsal PFC | .12 | −.11 | .16 | −.22 | −.22 | ||||||||
| 7. Orbital PFC | .06 | −.16 | .13 | −.14 | −.14 | .79 | |||||||
| 8. Medial PFC | .05 | −.14 | .05 | −.22 | −.22 | .81 | .74 | ||||||
| 9. ACC | .19 | −.10 | .26 | −.19 | −.19 | .82 | .62 | .66 | |||||
| 10. Mean FC GM | .12 | −.13 | .16 | .02 | −.21 | .98 | .88 | .85 | .84 | ||||
| Frontal Asymmetries | |||||||||||||
| 10. Dorsal PFC AQ | −.05 | −.05 | −.10 | −.02 | −.02 | .14 | .17 | .08 | .21 | .16 | |||
| 11. Orbital PFC AQ | .17 | .12 | .16 | .11 | .03 | .04 | .03 | −.01 | −.03 | .03 | .06 | ||
| 12. Medial PFC AQ | .16 | .16 | −.01 | .02 | −.06 | .08 | −.04 | .08 | .15 | .07 | .05 | −.03 | |
| 13. ACC AQ | −.04 | −.20 | −.11 | −.13 | −.13 | −.04 | −.02 | .04 | .01 | −.02 | −.10 | .16 | .08 |
| 14. Mean FC AQ | .13 | .06 | −.03 | −.01 | −.09 | .12 | .06 | .10 | .19 | .47 | .43 | .70 | .45 |
Note. N = 107. Significant correlations are shown in boldface. Correlations of ≥ I.19I are significant, p < .05; ≥ I.25I, p < .01. ; ≥ I.21I, p < .001. GM = grey matter. PFC = Prefrontal Cortex. ACC = Anterior Cingulate Cortex. FC = Frontal cortex. GM = Grey matter. AQ = Asymmetry Quotient.
3.2 Regression analyses
Table 2 summarizes the multiple R-values and associated F-values from the regression analyses for the mean adjusted GM volume and AQ scores for each personality trait. FFM personality dimensions served as dependent variables, while sex, age, and either the adjusted volume of the PFC and ACC regions or AQ scores served as the predictor variables. Further, a separate set of analyses was also run with the inclusion of GM of the visual cortex as a control region, and results did not differ. As such, we report on models that did not include this control region. For the adjusted GM volume analyses, significant positive R-values were found for Dominance, Extraversion, and Openness. With regard to associations between sex and age and personality, based on the partial r or beta values from these analyses (see Table 3), males were found to be higher on Dominance and Extraversion and older animals were found to be rated as lower on Reactivity/Unpredictability, Extraversion, and Openness. Further, chimpanzees with higher GM volumes in the ACC were rated as higher on Extraversion and Openness. Additionally, a borderline (p < .10) negative association emerged between average mPFC GM volume and Openness; chimpanzees with higher mPFC GM volumes were rated lower on Openness. After employing a FDR threshold for multiple comparisons, all associations survived.
Table 2.
Summary of Regression Analyses on Personality and Adjusted Frontal Grey Matter Volume and Asymmetry
| Personality trait | R | F | p-value |
|---|---|---|---|
| Average Adjusted GM | |||
| Reactivity/Unpredictability | .341 | 2.174 | .052 |
| Dominance | .411 | 3.345 | .005 |
| Extraversion | .637 | 11.249 | .001 |
| Openness | .357 | 2.4425 | .034 |
| Agreeableness | .262 | 1.221 | .302 |
| Asymmetry GM | |||
| Reactivity/Unpredictability | .389 | 2.938 | .011 |
| Dominance | .505 | 5.649 | .001 |
| Extraversion | .637 | 11.283 | .001 |
| Openness | .305 | 1.687 | .132 |
| Agreeableness | .245 | 1.053 | .396 |
Note. GM volumes adjusted for total GM as described in text.
All models control for age and sex.
GM = grey matter.
p < .05 shown in boldface.
Table 3.
Beta Values for each Personality Trait with the Frontal Grey Matter Volume and Asymmetry Scores, Sex and Age
| Predictor Variables | ||||||
|---|---|---|---|---|---|---|
| Sex | Age | dPFC | oPFC | mPFC | ACC | |
| Average GM | ||||||
| Reactivity/Unpredictability | −.055 | −.270* | .021 | −.061 | −.063 | .161 |
| Dominance | −.381* | .144 | .015 | −.100 | −.026 | .061 |
| Extraversion | −.254* | −.504* | −.034 | −.012 | −.131 | .191* |
| Openness | .112 | −.234* | −.086 | .158 | −.205 | .226* |
| Agreeableness | .190 | −.028 | −.177 | .160 | −.121 | .061 |
| Asymmetry GM | ||||||
| Reactivity/Unpredictability | −.034 | −.283* | −.060 | −.085 | .168 | −.097 |
| Dominance | −.372* | .149 | −.086 | −.023 | .233* | −.232* |
| Extraversion | −.247 | −.515* | .024 | −.016 | .075 | −.155 |
| Openness | .128 | −.223* | −.111 | −.036 | .061 | −.162 |
| Agreeableness | .203 | .019 | −.085 | .061 | −.061 | −.128 |
Note. dPFC = dorsal prefrontal cortex. oPFC = orbital prefrontal cortex. mPFC = mesial prefrontral cortex. ACC = anterior cingulate cortex. GM = grey matter. GM volumes adjusted for total GM as described in text. Values in boldface indicate Beta coefficients that survived critical FDR threshold for multiple comparisons. Beta coefficients that with a
are significant at p < .05.
With regard to asymmetries in the various brain regions, significant rightward multiple R-values were found for the Reactivity/Unpredictability, Dominance and Extraversion traits (see Tables 2 & 3). As with the previous average GM analyses, males were higher on Dominance and Extraversion and older animals were rater as lower on Reactivity/Unpredictability and Extraversion. Further, chimpanzees with greater rightward asymmetries in mPFC were found to have higher Reactivity/Undependability and Dominance scores, while chimpanzees with a greater leftward asymmetry in ACC had higher Dominance scores. Additionally, a borderline (p < .06) negative association between Extraversion and ACC AQ scores emerged; chimpanzees rated as higher on Extraversion had more leftward biased ACC asymmetries. After employing a FDR threshold for multiple comparisons, all associations survived.
4. Discussion
The current study represents the first study to date to examine associations between FFM personality and the FC, a brain region subserving functions known to be highly developed in both humans and great apes, and previously found to associate with human personality. Given the importance of elucidating common evolutionarily- and biologically-based models of personality, investigations of neurobiological correlates with chimpanzees, our closest non-human relatives, are particularly valuable. In this study, we focused on two aspects of cortical organization of the FC regions, including grey matter volume and asymmetry. Although not all hypotheses were confirmed, results of the current study provide support for the neuroanatomical basis of personality within the PFC and ACC. Results suggest a brain-based explanation for broad personality traits potentially indicating the evolutionary nature and conservation across species of general dispositions.
With regard to sex differences, males in the current study evidenced higher levels of both Extraversion and Dominance. Such results are consistent with findings by Weiss and colleagues (2007), albeit inconsistent with others who have failed to find sex differences across personality traits (e.g., King, Weiss, & Farmer, 2005). Additionally, whereas others have found significant age-related differences across personality traits (e.g., Weiss et al., 2007), or age-related differences for only Dominance (e.g., King et al., 2005), we found negative associations between age and both Reactivity/Undependability and Extraversion. The source of these disparate results is not known. As such, it will be important for research to continue to study not only age- and sex-related differences in personality, but also potential bases for these differences.
Consistent with expectations, mean adjusted FC GM volume was found to be associated with FFM personality. Specifically, after statistically controlling for age and sex, Dominance, Openness, and Extraversion were correlated with average GM volume; however, Agreeableness and Reactivity/Undependability were not. Given that Extraversion and Dominance (which parallels reversed FFM Neuroticism) are the two most affectively-based traits, the correlation with average GM volume potentially reflects the control of emotions in the service of goal-oriented behavior. When individual regions were examined, Extraversion and Openness were found to be associated with ACC GM volume and, Openness was found to be associated with lower mPFC volume (at a trend level). Although these associations were not significant following correction for multiple comparisons, these results nonetheless suggest that in addition to self-regulatory behaviors and emotional control, the ACC may also be important for the control of appetitive, approach-oriented, dispositional traits consistent with the trait Extraversion. It may be that the ACC is particularly important for regulating approach-oriented behavior and control of positive emotions. Indeed, the ACC is part of the classical Papez emotion circuit, and recent research indicates ACC activity is associated with emotion. The ACC is additionally part of a corticostriatal circuit involved in stimulus-reward learning with lesions to this area found to interfere with avoidance learning, resulting in increased expression of risky behaviors (Vogt, Finch, & Olson, 1992).
Given both theoretical and empirical literature suggesting the importance of examining FC asymmetries (Davidson, 1998; Harmon-Jones, 2003) and volumetric variation, associations between personality and asymmetries were also investigated. Consistent with expectations and previous findings that leftward versus rightward asymmetry correlates with approach versus avoidant temperaments, respectively, FC asymmetries were found to be associated with both Dominance and Extraversion. Unexpectedly, however, and contrary to the emotional valence hypothesis (Davidson, 1998), rightward asymmetry was associated with both traits. When individual regions were examined, however, results were more consistent with expectations. Specifically, Dominance, as well as Reactivity/Unpredictability, were associated with greater rightward asymmetries in the mPFC. Dominance was also associated with greater leftward asymmetry in the ACC. With regard to the former, these findings are consistent with previous findings among humans. Indeed, rightward ACC asymmetry has been found to be correlated with Harm Avoidance (Pujol et al., 2002), a trait dimension negatively correlated with Neuroticism (De Fruyt et al., 2000) (the human parallel of Dominance). Taken together, these results add to a nascent literature on asymmetries in nonhuman primates (Hopkins, 2013) and contribute to the larger literature on the importance of asymmetry in explaining temperamental variation among individuals.
4.1 Limitations
There are several limitations to this study. First, the cross-sectional correlational nature of the design does not allow for causal inferences to be made. That is, whether personality factors underlie variation in FC structure or vice versa cannot be inferred from these findings. Second, we used masks created with landmarks to define the FC that have been previously used in comparative studies with apes (Hopkins & Avants, 2013); but, it must be recognized that the morphological landmarks comprising the FC are not the same in humans and apes (Semendeferi & Damasio, 2000). Furthermore, the relationship between sulcal landmarks and cytoarchitectonic boundaries defining specific cortical regions are not particularly strong in either humans or apes (Schenker et al., 2010). Thus, we avoided using specific Broadmann area designations to describe our morphological regions of interest resulting in a need for further investigation in both human and ape. Third, for the purposes of limiting the number of statistical analyses, we combined the different regions within the ACC into a single measure. It has been well documented that the three main regions of the ACC (Areas 24, 24’ and 25) have distinct functions (Bush, Luu, & Posner, 2000) and it is likely that ACC-personality correlations may be mediated by which region is considered in the measurement.
Fourth, although commonly used in both the human and nonhuman primate neuroimaging literatures (Hopkins et al., 2014; Hopkins & Avants, 2013; Hopkins & Taglialatela, 2013), particularly with samples as large as the one in the current study, the template-based ROI approach has a number of limitations given the neuroanatomical variability among subjects. Nonetheless, it is likely a more conservative approach as it results in capturing only overlapping GM across subjects resulting in the potential for attenuated associations. Thus, although other approaches to ROI analyses exist (e.g., individually tracing ROIs for each subject in their native brain space), the more conservative approach employed in the current study underscores the robustness of our results. Fifth, this study, which represents a first step toward elucidating common evolutionarily- and biologically-based models of personality by examining associations with the PFC, necessarily has a corticocentric emphasis. Given that the cortex functions within a network of subcortical structures, and is part of several cortico-striatal circuits known to be important for emotion and decision making (Koziol & Budding, 2009), the corticocentric approach used in the current study is limited (Parvizi, 2009). Future research in this area should focus on the FC as well as its subcortical connectivity in order to elucidate the conservation or evolution of personality networks across species. Sixth, likely as a result of few items that are used to make up the scale, the Agreeableness scale evidenced a low internal consistency (Cronbach’s alpha = .48). Such a low internal consistency likely attenuated associations with frontal GM volume. Future research is therefore needed, potentially with more internally consistent assessments of Agreeableness, before we are able to be more confident in these findings. Finally, as the size of the social groups varied in the current study, it will also be important for future research to examine the effects of differential social housing on personality. It is important to note that the chimpanzees included in the current study have also lived in larger groups at various points in their lives as the facility in which the chimpanzees are housed varies the housing of the apes. The personality rating data presented here therefore represent a culmination of raters observations and interactions with the apes across these different social settings.
4.2 Conclusions
Limitations notwithstanding and although replication is needed, results of the current study provide support for the notion of FFM personality having both an evolutionary and biological basis. Such findings are critically important in advancing our understanding of human nature given the information these results provide for our comprehension of the evolution of the human brain and associated dispositions and behaviors (Rilling, 2013). Indeed, similar to findings in humans (DeYoung, 2010), our results confirm the importance of neuroscientific approaches to the study of basic dispositions (i.e., personality) and suggest that many of these associations are comparable in chimpanzees. Such findings further underscore the importance of chimpanzee models of trait personality for more fully elucidating the biological basis for individual variation in personality. Further, our results indicate that asymmetries in the FC and ACC regions are related to specific personality traits in chimpanzees, consistent with previous research implicating these regions in the control of emotion and behavior.
Highlights.
Investigated associations between personality and frontal cortex grey matter volume and asymmteries in chimpanzees
Openness and Extraversion associated with anterior cingulate cortex volume
Dominance associated with left anterior cingulate cortex and right prefrontal cortex volume
Reactivity/Unpredictability associated with right mesial prefrontal cortex volume
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams MJ, King JE, Weiss A. The majority of genetic variation in orangutan personality and subjective well-being is nonadditive. Behav. Genet. 2012;42:675–686. doi: 10.1007/s10519-012-9537-y. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cereb. Cortex. 2000;10:295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- Benjamin Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol. 1995;57:289–300. [Google Scholar]
- Bianchi S, et al. Synaptogenesis and development of pyramidal neuron dendritic morphology in the chimpanzee neocortex resembles humans. Proc. Natl. Acad. Sci. U.S.A. 2013;110:10395–10401. doi: 10.1073/pnas.1301224110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore S, Winston J, Frith U. Social cognitive neuroscience: Where are we heading? Trends In Cogn. Sci. 2004;8:216–222. doi: 10.1016/j.tics.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Blatchley BJ, Hopkins WD. Subgenual cingulate cortex and personality in chimpanzees (Pan troglodytes) Cogn., Affect. & Behav. Neurosci. 2010;10:414–421. doi: 10.3758/CABN.10.3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard TJ, McGue M. Genetic and environmental influences on human psychological differences. J. Neurobiol. 2003;54:4–45. doi: 10.1002/neu.10160. [DOI] [PubMed] [Google Scholar]
- Brown SM, Manuck SB, Flory JD, Hariri AR. Neural basis of individual differences in impulsivity: Contributions of corticolimbic circuits for behavioral arousal and control. Emotion. 2006;6:239–245. doi: 10.1037/1528-3542.6.2.239. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn. Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Canli T, Zhao Z, Desmond JE, Kang E, Gross J, Gabrieli JE. An fMRI study of personality influences on brain reactivity to emotional stimuli. Behav. Neurosci. 2001;115:33–42. doi: 10.1037/0735-7044.115.1.33. [DOI] [PubMed] [Google Scholar]
- Chakrabarti B, Bullmore E, Baron-Cohen S. Empathizing with basic emotions: Common and discrete neural substrates. Soc. Neurosci. 2006;1:364–384. doi: 10.1080/17470910601041317. [DOI] [PubMed] [Google Scholar]
- Clark L, Watson D. Handbook of personality: Theory and research. Guilford Press; New York, NY US: 2008. Temperament: An organizing paradigm for trait psychology. [Google Scholar]
- Cohen MX, Young J, Baek J, Kessler C, Ranganath C. Individual differences in extraversion and dopamine genetics predict neural reward responses. Cogn. Brain Res. 2005;25:851–861. doi: 10.1016/j.cogbrainres.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Anterior electrophysiological asymmetries, emotion, and depression: Conceptual and methodological conundrums. Psychophysiology. 1998;1998;35:607–614. doi: 10.1017/s0048577298000134. [DOI] [PubMed] [Google Scholar]
- De Fruyt FF, De Wiele L, Van Heeringen CC. Cloninger's psychobiological model of temperament and character and the Five-Factor Model of personality. Pers. Individ. Dif. 2000;29:441–452. [Google Scholar]
- de Waal FBM. Good Natured: the origins of right and wrong in humans and other animals. Harvard University Press; Cambridge, MA: 1996. [Google Scholar]
- DeYoung CG, et al. Testing predictions from personality neuroscience: Brain structure and the big five. Psychol. Sci. 2010;21:820–828. doi: 10.1177/0956797610370159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeYoung CG. Personality neuroscience and the biology of traits. Soc. Personal. Psychol. Compass. 2010;4:1165–1180. [Google Scholar]
- DeYoung CG, Shamosh NA, Green AE, Braver TS, Gray JR. Intellect as distinct from openness: Differences revealed by fMRI of working memory. J. Pers. Soc. Psychol. 2009;97:883–892. doi: 10.1037/a0016615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Digman JM. Personality structure: Emergence of the Five-Factor Model. Annu. Rev. Clin. Psychol. 1990;41:417–440. [Google Scholar]
- Eisenberger NI, Lieberman MD, Satpute AB. Personality from a controlled processing perspective: An fMRI study of neuroticism, extraversion, and self-consciousness. Cogn., Affect. & Behav. Neurosci. 2005;5:169–181. doi: 10.3758/cabn.5.2.169. [DOI] [PubMed] [Google Scholar]
- Freeman HD, et al. Developing a comprehensive and comparative questionnaire for measuring personality in chimpanzees using a simultaneous top-down/bottom-up design. Am. J. of Primatol. 2013;75:1042–1053. doi: 10.1002/ajp.22168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman HD, Gosling SD. Personality in nonhuman primates: A review and evaluation of past research. Am. J. of Primatol. 2010;72:653–671. doi: 10.1002/ajp.20833. [DOI] [PubMed] [Google Scholar]
- Haas BW, Omura K, Amin Z, Constable R, Canli T. Functional connectivity with the anterior cingulate is associated with extraversion during the emotional Stroop task. Soc. Neurosci. 2006;1:16–24. doi: 10.1080/17470910600650753. [DOI] [PubMed] [Google Scholar]
- Haas BW, Omura K, Constable R, Canli T. Emotional conflict and neuroticism: Personality-dependent activation in the amygdala and subgenual anterior cingulate. Behav. Neurosci. 2007;121:249–256. doi: 10.1037/0735-7044.121.2.249. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E. Clarifying the emotive functions of asymmetrical frontal cortical activity. Psychophys. 2003;40:838–848. doi: 10.1111/1469-8986.00121. [DOI] [PubMed] [Google Scholar]
- Hong K, et al. Polymorphism of the tryptophan hydroxylase 2 (TPH2) gene is associated with chimpanzee neuroticism. PLoS ONE. 2011;6 doi: 10.1371/journal.pone.0022144. doi:10.1371/journal.pone.0022144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Misiura M, Reamer LA, Schaeffer JA, Mareno MC, Schapiro SJ. Poor receptive joint attention skills are associated with atypical gray matter asymmetry in the posterior superior temporal gyrus of chimpanzees (Pan troglodytes) Front Psychol. 2014;5 doi: 10.3389/fpsyg.2014.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD. Neuroanatomical asymmetries and handedness in chimpanzees (Pan troglodytes): a case for continuity in the evolution of hemispheric specialization. Ann. N. Y. Acad. Sci. 2013;1288:17–35. doi: 10.1111/nyas.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Avants BB. Regional and hemispheric variation in cortical thickness in chimpanzees (Pan troglodytes) J. Neurosci. 2013;33:5241–5248. doi: 10.1523/JNEUROSCI.2996-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Taglialatela JP. Imitation of joint attention is associated with morphometric variation in the anterior cingulate cortex of chimpanzees (Pan troglodytes) Am J Primatol. 2013;75:441–449. doi: 10.1002/ajp.22120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Donaldson ZR, Young LJ. A polymorphic indel containing the RS3 microsatellite in the 5' flanking region of the vasopressin V1a receptor gene is associated with chimpanzee (Pan troglodytes) personality. Genes, Brain & Behav. 2012;11:552–558. doi: 10.1111/j.1601-183X.2012.00799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotov R, Gamez W, Schmidt F, Watson D. Linking “big” personality traits to anxiety, depressive, and substance use disorders: A meta-analysis. Psychol. Bull. 2010;136:768–821. doi: 10.1037/a0020327. [DOI] [PubMed] [Google Scholar]
- Koziol LF, Budding D. Subcortical structures and cognition: Implications for neuropsychological assessment. Springer Science + Business Media; New York, NY US: 2009. [Google Scholar]
- Latzman RD, Freeman HJ, Schapiro SJ, Hopkins WD. The contribution of genetics and early social rearing experiences to hierarchical personality dimensions in chimpanzees (Pan troglodytes) J Pers Soc Psy. Manuscript. doi: 10.1037/pspp0000040. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latzman RD, Hopkins WD, Keebaugh AC, Young LJ. Personality in Chimpanzees (Pan troglodytes): Exploring the Hierarchical Structure and Associations with the Vasopressin V1A Receptor Gene. PloS ONE. 2014;9(4):e95741. doi: 10.1371/journal.pone.0095741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman MD, Cunningham WA. Type I and Type II error concerns in fMRI research: re-balancing the scale. SCAN. 2009;4:423–428. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, et al. Chimpanzee (Pan troglodytes) precentral corticospinal system asymmetry and handedness: a diffusion magnetic resonance imaging study. PLoS ONE. 5 doi: 10.1371/journal.pone.0012886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzawa T. Primate origins of human cognition and behavior. Springer-Verlag Publishing; New York, NY US: 2001. [Google Scholar]
- McCrae RR, Costa PT., Jr . Sage handbook of personality theory and assessment. Sage Publications; Thousand Oaks, CA, US: 2008. Empirical and theoretical status of the five-factor model of personality traits. [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Mobbs D, Hagan CC, Azim E, Menon V, Reiss AL. Personality predicts activity in reward and emotional regions associated with humor. Proc. Natl. Acad. Sci. U.S.A. 2005;102:16502–16506. doi: 10.1073/pnas.0408457102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson EE, Winslow JT. Non-human primates: model animals for developmental psychopathology. Neuropsychopharm. 2009;34:90–105. doi: 10.1038/npp.2008.150. [DOI] [PubMed] [Google Scholar]
- Nusslock R, Shackman AJ, Harmon-Jones E, Alloy LB, Coan JA, Abramson LY. Cognitive vulnerability and frontal brain asymmetry: common predictors of first prospective depressive episode. J Abnorm Psychol. 2011;120:497–503. doi: 10.1037/a0022940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvizi J. Corticocentric myopia: old bias in new cognitive sciences. Trends in Cogn. Sci. 2009;13:354–359. doi: 10.1016/j.tics.2009.04.008. [DOI] [PubMed] [Google Scholar]
- Phillips KA, et al. Why primate models matter. Am J Primat Early View. 2014 doi: 10.1002/ajp.22281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol J, et al. Anatomical variability of the anterior cingulate gyrus and basic dimensions of human personality. Neuroimage. 2002;15:847–855. doi: 10.1006/nimg.2001.1004. [DOI] [PubMed] [Google Scholar]
- Rauch SL, et al. Orbitofrontal thickness, retention of fear extinction, and extraversion. Neuroreport. 2005;16:1909–1912. doi: 10.1097/01.wnr.0000186599.66243.50. [DOI] [PubMed] [Google Scholar]
- Reuter M, et al. Personality and emotion: test of Gray's personality theory by means of an fMRI study. Behav. Neurosci. 2004;118:462–469. doi: 10.1037/0735-7044.118.3.462. Rev. Neurosci. 24, 167-202 (2001) [DOI] [PubMed] [Google Scholar]
- Rilling JK. Comparative primate neuroimaging: insights into human brain evolution. Trends in Cogn. Sci. 2013 doi: 10.1016/j.tics.2013.09.013. doi: 10.1016/j.tics.2013.09.013. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Insel TR. The primate neocortex in comparative perspective using magnetic resonance imaging. J. Hum. Evol. 1999;37:191–223. doi: 10.1006/jhev.1999.0313. [DOI] [PubMed] [Google Scholar]
- Schenker NM, et al. Broca's area homologue in chimpanzees (Pan troglodytes): Probabilistic mapping, asymmetry, and comparison to humans. Cereb. Cortex. 2010;20:730–742. doi: 10.1093/cercor/bhp138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semendeferi K, Damasio H. The brain and its main anatomical subdivisions in living hominoids using magnetic resonance imaging. J. Hum. Evol. 2000;38:317–332. doi: 10.1006/jhev.1999.0381. [DOI] [PubMed] [Google Scholar]
- Semendeferi K, Damasio H, Frank R, Van Hoesen GW. The evolution of the frontal lobes: a volumetric analysis based on three dimensional reconstructions of the magnetic resonance scans of human and ape brains. J. Hum. Evol. 1997;32:375–388. doi: 10.1006/jhev.1996.0099. [DOI] [PubMed] [Google Scholar]
- Smith SM, et al. Advances in functional and structural MR image analysis and implementation of FSL. Neuroimage. 2004;23:208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Stockard CR. The physical basis of personality. Norton; New York: 1931. [Google Scholar]
- Vogt BA, Finch DM, Olson CR. Functional heterogeneity in cingulate cortex: the anterior executive and posterior evaluative regions. Cereb. Cortex. 1992;2:435–443. doi: 10.1093/cercor/2.6.435-a. [DOI] [PubMed] [Google Scholar]
- Weiss A, King JE, Hopkins WD. A cross-setting study of chimpanzee (Pan troglodytes) personality structure and development: zoological parks and Yerkes National Primate Research Center. Am. J. of Primatol. 2007;69:1264–1277. doi: 10.1002/ajp.20428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LM, et al. The mellow years?: Neural basis of improving emotional stability over age. J. Neurosci. 2006;26:6422–6430. doi: 10.1523/JNEUROSCI.0022-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans. Med. Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]


