Abstract
Objective
To examine the cost-effectiveness of the HITIDES intervention.
Design
Randomized controlled effectiveness and implementation trial comparing depression collaborative care with enhanced usual care.
Setting
Three Veterans Health Administration (VHA) HIV clinics in the Southern US.
Subjects
249 HIV-infected patients completed the baseline interview; 123 were randomized to the intervention and 126 to usual care.
Intervention
HITIDES consisted of an off-site HIV depression care team that delivered up to 12 months of collaborative care. The intervention used a stepped-care model for depression treatment and specific recommendations were based on the Texas Medication Algorithm Project and the VA/Department of Defense Depression Treatment Guidelines.
Main outcome measure(s)
Quality-adjusted life years (QALYs) were calculated using the 12-Item Short Form Health Survey, the Quality of Well Being Scale, and by converting depression-free days to QALYs. The base case analysis used outpatient, pharmacy, patient, and intervention costs. Cost-effectiveness was calculated using incremental cost effectiveness ratios (ICERs) and net health benefit (NHB). ICER distributions were generated using nonparametric bootstrap with replacement sampling.
Results
The HITIDES intervention was more effective and cost-saving compared to usual care in 78% of bootstrapped samples. The intervention NHB was positive and therefore deemed cost-effective using an ICER threshold of $50,000/QALY.
Conclusions
In HIV clinic settings this intervention was more effective and cost-saving compared to usual care. Implementation of off-site depression collaborative care programs in specialty care settings may be a strategy that not only improves outcomes for patients, but also maximizes the efficient use of limited healthcare resources.
INTRODUCTION
Depression is the single most common mental health condition seen in non-mental health settings. [1] Collaborative care for depression is effective [2–13] and cost-effective in adult primary care, [14–21] but many patients are seen outside primary care. It is less clear whether collaborative care for depression is effective in specialty care, few studies have been completed on this topic to date.[22, 23] Even more unclear is whether collaborative care for depression is cost-effective outside of primary care, because the cost profiles of specialty care providers and the services they provide are significantly different from those seen in primary care.
We chose Human Immunodeficiency Virus (HIV) as our test case because it is similar to a primary care setting in that many HIV providers often provide whole person care, not just HIV care. Also, depression is associated with non-adherence to HIV medication regimens and decreased immune functioning which can lead to accelerated HIV progression and increased risk of mortality.[24–33] Because depression can be effectively managed, it is a modifiable risk factor for the progression of HIV.[34–36] We chose the Veterans Health Administration (VHA) because it is the largest provider of HIV care in the nation[37] and it has a long history of mental health delivery innovation. As previously reported, the HIV Translating Initiatives for Depression Into Effective Solutions (HITIDES) intervention described in more detail below resulted in a significant increase in depression-free days and decrease in HIV symptom severity compared to usual care.[22] To our knowledge this is the first cost-effectiveness analysis of a collaborative care intervention for depression set in a specialty physical healthcare setting.
METHODS
STUDY SETTING AND ENROLLMENT PROCEDURES
The intervention, methods, and clinical outcomes of the HITIDES study have been described in detail elsewhere.[22] To summarize, the HITIDES study was a randomized controlled implementation and effectiveness trial comparing depression collaborative care with enhanced usual care in three VA HIV specialty clinics.[38] Depression screening was implemented as part of usual care at all sites.[22]
USUAL CARE DESCRIPTION
All clinic site healthcare providers participating in the study received one hour of training in the detection and management of depression in patients with HIV and were also instructed in referral procedures for specialty mental healthcare at their site. These procedures included the suggestion of at least one failed depression treatment trial before referral. Usual care consisted of depression treatment by HIV or mental health clinicians without involvement from the HITIDES depression care team.
HITIDES INTERVENTION DESCRIPTION
A more detailed description of the intervention has been published elsewhere.[22] The HITIDES intervention involved collaboration between on-site HIV providers and an off-site HITIDES depression team comprised of a registered nurse depression care manager (DCM), clinical pharmacist, and psychiatrist (J.M.P). The HITIDES depression care support team was located off-site at the Central Arkansas Veterans Healthcare System in Little Rock, AR and met weekly or as needed either in-person or via telephone to discuss patients who were not responding to current depression treatment. All clinical communications with care providers took place in the electronic medical record progress notes. The DCM was solely responsible for communication with patients which was done exclusively via telephone. The HITIDES care team provided treatment suggestions to the clinicians responsible for direct patient care; all treatment decisions were ultimately left to on-site treatment providers.
Patients received the following intervention components from the DCM via a telephone encounter: participant education and activation,[39] assessment of treatment barriers and possible resolutions, depression symptom and treatment monitoring, substance abuse monitoring, and instruction in self-management (e.g., encouraging patients to exercise and participate in social activities).[4, 40] The DCM used standardized instruction scripts, which were supported by the Web-based decision support system NetDSS (available at https://www.netdss.net) during these telephone encounters.[41] The intervention used a stepped-care model for depression treatment[2] and specific treatment recommendations were based on the Texas Medication Algorithm Project[42] and the VA/Department of Defense Depression Treatment Guidelines.[43]
DATA COLLECTION
Baseline, 6- and 12-month data were collected by telephone interviewers who were blinded to treatment assignment and used computer-assisted assessments. At baseline, demographics, depression history, and chronic physical health conditions were measured using the Depression Outcomes Module.[44, 45] Mental health comorbidity was measured using the Mini International Neuropsychiatric Interview.[46, 47] Acceptability of antidepressant treatment was measured using an item developed for the Quality Improvement for Depression studies.[6, 48] Follow-up data-collection interviews were completed for 226/249 participants (90.8%) at 6 months and 215/249 (86.3%) at 12 months.
Depression-free days (DFDs) were calculated from the 20-item Symptom Checklist (SCL-20).[49] SCL-20≤0.5 was considered depression-free (1.0) and ≥2.0 was considered fully symptomatic (0.0) and scores in between were assigned a linear proportional value between 1.0 and 0.0. Disease-specific DFD-derived quality-adjusted life years (QALYs) were calculated by assigning 0.6 (traditional) or 0.8 (conservative) for patients who were fully symptomatic (SCL-20 ≥ 2.0), 1.0 for patients that were asymptomatic (SCL-20 ≤ 0.5), and assigning a linear proportional value for values in between. Therefore, 0.4 (traditional) and 0.2 (conservative) corresponded to the potential improvement in QALYs from fully depressed to fully asymptomatic. DFDs and DFD QALYs were calculated using area under the curve calculations of baseline, 6-month, and 12-month data.[50, 51] Generic QALYs were calculated using the SF-12 standard gamble to QALY conversion formula[52] and the QWB scale.[53, 54]
Intervention costs, healthcare expenditures, and patient costs were collected to assess the cost of the intervention from a societal perspective. Intervention costs included both fixed and variable costs. We included only DCM training as a net fixed intervention cost because the other fixed intervention costs were attributed to participants in both the intervention and usual care groups. Variable intervention costs included the time spent by intervention personnel delivering the intervention (e.g. time spent preparing and delivering the intervention, entering progress notes into the medical record, and attending intervention team meetings). These costs were calculated separately for the DCM, clinical pharmacist, and psychiatrist based on an hourly rate calculated from their respective VA salaries and fringe costs. Total intervention costs were estimated at $557 per intervention participant ($68,503/123).
Healthcare expenditures were assessed using VA Decision Support System data. This system uses an activity-based costing allocation method and includes fixed direct, variable direct, and fixed indirect costs. While the cost estimates have not been validated via micro-costing, DSS provides a useful proxy for encounter cost that is helpful to researchers. Outpatient expenditures for the base case analysis were organized in the following groups by clinic type (i.e., primary stop code): primary care, infectious disease, mental health, substance abuse, other medical specialty, and ancillary (including laboratory orders and radiography). Outpatient medication data were divided into HIV-related, depression-related, and other. Inpatient encounter data were used for secondary cost per QALY analyses. Patient travel and time expenditures were calculated based on self-reported time spent at 6- and 12-month follow-up interviews and income information collected at baseline. Expenditures were not discounted because of the relatively short 12-month time horizon of the study.
STATISTICAL ANALYSIS
We utilized an intent-to-treat analysis at the patient level. We performed a power calculation assuming an 11% difference in the percentage of responders between intervention and usual care using a 1-tailed t test (α = .05). A sample size of 280 (140 subjects per arm) would provide 74% power. Independent variables with missing values were imputed using multiple imputation methods.[55] Owing to the large number of available covariates and the use of multiple imputation methods, only those covariates found to significantly predict dependent variables at p<0.10 in bivariate analyses were included in multivariate analyses. After model specification was finalized, healthcare costs for the year prior to patient baseline assessment were added as a covariate to expenditure models.
Due to skewness from several high cost outliers the expenditure outcomes were non-normally distributed, so generalized linear models (GLMs) were utilized.[56] We ran 7 GLMs with normal, gamma, or inverse normal distributions and identity, logarithm, or square root link functions using a consistent specification of independent variables. The GLM regression with a gamma distribution and identity link function fit the expenditure data most appropriately. Using a similar procedure, the GLM regression with an inverse normal distribution and log link fit the DFD QALY data best, while gamma with identity link was used for both SF-12 and QWB derived QALYs.
Based on the coefficients from the GLM regressions for the specified independent variables and the covariate values for each participant, we calculated two predicted expenditures for each participant to determine the incremental treatment effect on costs.[57] The first expenditure prediction was if the participant had been randomized to the intervention (factual for intervention patients and counterfactual for usual care), and the second expenditure prediction was as if the participant had been randomized to usual care (counterfactual for intervention patients and factual for usual care). The difference between these two expenditure predictions represented the incremental effect of the intervention on expenditures for a particular participant because all covariate effects were identical for the two estimates for a given patient. We then averaged the difference between the two predicted values for each participant and across all participants to generate an incremental effect in the entire sample.
The point estimate of the original sample will be used for means [58]; however, typical standard error estimation methods do not apply to incremental cost-effectiveness ratios (ICERs) for two reasons. First, the possibility of having zero or near zero denominators is non-negligible. Second, expenditure and effectiveness estimates are rarely independent.[58] Therefore, we ran 1000 replications of nonparametric bootstrap with replacement model to generate an empirical joint distribution of incremental expenditures and QALYs.[58, 59] We then constructed acceptability curves representing the probability of falling below ICER thresholds ranging from 0 to $100,000 per QALY for each clinical outcome: DFD-derived QALYs (0.4 [traditional] and 0.2 [conservative]), SF-12 standard gamble QALYs, and QWB-SA QALYs.[60]
In addition, we calculated the net health benefit (NHB) as suggested by Stinnett and Mullahy[61] to assist in the interpretation of [61] a negative (ICER). [58] NHB is calculated by dividing the marginal cost of the program by a cost-effectiveness threshold (e.g. $50,000/QALY) and subtracting the result from the marginal effectiveness of the program (e.g. QALY difference). If the NHB is positive then the intervention is deemed cost-effective compared to the threshold used and should be selected for implementation. Otherwise, more health improvements could be attained by forgoing the intervention and investing in programs that are at least marginally cost-effective.
Results
Baseline sociodemographic, clinical, and depression-related variables are presented in Table 1. In general, patients were middle-aged, predominantly African-American, single, males with high levels of physical and mental health comorbidity in addition to moderate HIV symptoms. The only group differences at baseline were intervention patients had lower QWB-SA scores (0.44 vs. 0.49, p<0.01) and higher physical health comorbidity scores (3.8 vs. 3.2, p<0.05).
Table 1.
Baseline Participant Sociodemographic and Clinical Characteristics
| Group | ||||
|---|---|---|---|---|
| Variable | Intervention (n=123) | Usual Care (n=126) | ||
| Sociodemographic | ||||
| Age, mean (SD), y | 49.8 | (8.7) | 49.8 | (10.5) |
| Male sex | 120 | (97.6) | 122 | (96.8) |
| African American race | 78 | (63.4) | 77 | (61.6) |
| Single/never married | 103 | (83.7) | 98 | (77.8) |
| High school graduate or higher | 118 | (95.9) | 113 | (89.7) |
| Annual income ≥ $20,000 | 60 | (50.8) | 52 | (42.6) |
| Clinical | ||||
| SF-12V PCS score, mean (SD) | 41.5 | (12.5) | 39.5 | (11.6) |
| SF-12V MCS score, mean (SD) | 34.3 | (10.5) | 35.1 | (11.0) |
| SCL-20 score, mean (SD) | 1.8 | (0.6) | 1.9 | (0.7) |
| QWB-SA score, mean (SD)** | 0.49 | (0.1) | 0.44 | (0.1) |
| Physical health comorbidity score, mean (SD)* | 3.2 | (2.3) | 3.8 | (2.3) |
| PHQ-9, mean (SD) | 15.7 | (4.2) | 16 | (4.7) |
| Major depression | 92 | (74.8) | 98 | (77.8) |
| Panic disorder | 10 | (8.1) | 18 | (14.3) |
| Generalized anxiety disorder | 74 | (60.2) | 76 | (60.3) |
| Posttraumatic stress disorder | 34 | (27.6) | 40 | (31.7) |
| At-risk drinking | 19 | (15.4) | 26 | (20.6) |
| Any inpatient mental health admission | 33 | (26.8) | 32 | (25.4) |
| Any past depression treatment | 98 | (79.7) | 98 | (77.8) |
| Any depression treatment in past 6 mos. | 68 | (55.7) | 67 | (53.2) |
| Depression treatment type | ||||
| Watchful waiting acceptable | 88 | (71.5) | 85 | (67.5) |
| Antidepressant medication acceptable | 88 | (72.1) | 87 | (69.6) |
| Individual counseling acceptable | 108 | (87.8) | 113 | (89.7) |
| Group counseling acceptable | 66 | (53.5) | 76 | (60.3) |
| Bothersome HIV symptoms, mean (SD) | 7.8 | (4.1) | 8 | (4.3) |
| Current anti-HIV prescription | 99 | (80.5) | 99 | (78.6) |
| Skipped anti-HIV medication in past 4 d | 23 | (23.2) | 28 | (28.3) |
| Anti-HIV medication adherence, mean % (SD) | 93.5 | (16.2) | 91.2 | (20.1) |
| Current AD prescription | 75 | (61.0) | 78 | (61.9) |
| Skipped AD in past 4 d | 22 | (29.3) | 20 | (25.6) |
| AD regimen adherence, mean % (SD) | 85.4 | (30.5) | 86.4 | (31.1) |
Abbreviations: AD, antidepressant; HIV, human immunodeficiency virus; MCS, mental component summary; PCS, physical component summary; PHQ-9, 9-item Patient Health Questionnaire; QWB-SA, Quality of Well-Being Self-administered Scale; SCL-20, 20-item Hopkins Symptom Checklist; SF-12V, Medical Outcomes Study Veterans 12-Item Short-Form Health Survey.
Unless otherwise indicated, data are expressed as number (percentage) of participants. Percentages reflect the following missing data: race, 1 usual care participant; annual income, 5 intervention and 4 usual care participants; any depression treatment in the past 6 months, 1 intervention participant; and antidepressant acceptable, 1 intervention and 1 usual care participant.
P .01 for intervention vs usual care.
P .05 for intervention vs usual care.
The PHQ-9 was used as depression screening measure. The SCL-20 was used as the depression outcome measure.
Mental health comorbidity was identified using the Mini International Neuropsychiatric Interview.
Table 2 summarizes intervention and healthcare costs incurred by patients in the intervention and usual care groups. Healthcare costs were broken into outpatient (e.g. primary care, infectious disease, mental health, etc.) and pharmacy costs (HIV-related, depression-related, and other). The only statistically significant unadjusted difference in healthcare costs either before or after the intervention was higher post-intervention infectious disease outpatient costs for the intervention group ($3427 vs. $2585), indicating that intervention patients had more infectious disease visits than usual care patients. Total unadjusted healthcare expenditures increased an average $1150 for usual care patients and decreased $840 for intervention patients. After adjustment for case mix variables the overall intervention was cost saving, specifically including outpatient and pharmacy costs resulted in cost savings of $1368 (p<0.01) (Table 3). When inpatient costs were added for a secondary analysis the cost savings for the intervention was $534, but no longer statistically significant. Inpatient costs were included in a secondary analysis because of the generally highly skewed distribution for these costs; this approach is consistent with the literature.[17–19, 62, 63]
Table 2.
Unadjusted Mean Intervention and Healthcare Costs
| INTERVENTION COSTS, $ | ||||||||
|---|---|---|---|---|---|---|---|---|
| Hours | Rate | Cost | Fringe | Total | Per Intervention Patient (N=123) | |||
| FIXED INTERVENTION COST | ||||||||
| DCM Training | 40 | 48 | 1,910 | 477 | 2,387 | 19 | ||
| Total Fixed | 40 | 48 | 1,910 | 477 | 2,387 | 19 | ||
| VARIABLE INTERVENTION COST | ||||||||
| Psychiatrist (Weekly Meetings) | 28 | 107 | 2,998 | 749 | 3,747 | 30 | ||
| Pharmacist (Weekly Meetings) | 25 | 63 | 1,586 | 396 | 1,982 | 16 | ||
| Pharmacist (Consultations) | 30 | 63 | 1,878 | 470 | 2,348 | 19 | ||
| DCM (Weekly Meetings) | 28 | 48 | 1,341 | 335 | 1,676 | 14 | ||
| DCM (Baseline Encounter) | 179 | 48 | 8,522 | 2,130 | 10,652 | 87 | ||
| DCM (Follow-up Encounters | 766 | 48 | 36,569 | 9,142 | 45,711 | 372 | ||
| Total Variable | 1056 | 52,893 | 13,223 | 66,116 | 538 | |||
| TOTAL INTERVENTION COST | ||||||||
| Total | 1096 | 54,802 | 13,701 | 68,503 | 557 | |||
| HEALTHCARE UTILIZATION COSTS, $ | ||||||||
| Pre-intervention (Mean) | Post-intervention (Mean) | |||||||
| Usual Care | Intervention | X2 p>|t| | KW* p>|t| | Usual Care | Intervention | X2 p>|t| | KW* p>|t| | |
| PHARMACY | ||||||||
| HIV | 6,109 | 5,797 | 0.936 | 0.223 | 6,372 | 4,550 | 0.467 | 0.134 |
| Depression | 215 | 116 | 0.232 | 0.637 | 265 | 157 | 0.278 | 0.737 |
| Other | 2,393 | 2,209 | 0.616 | 0.580 | 1,698 | 1,481 | 0.490 | 0.226 |
| TOTAL | 8,718 | 8,122 | 0.881 | 0.326 | 8,335 | 6,188 | 0.396 | 0.342 |
| OUTPATIENT | ||||||||
| Substance abuse | 283 | 337 | 0.760 | 0.869 | 389 | 202 | 0.168 | 0.721 |
| Other specialty | 685 | 458 | 0.174 | 0.573 | 707 | 754 | 0.804 | 0.845 |
| Primary care | 328 | 384 | 0.565 | 0.887 | 282 | 293 | 0.902 | 0.568 |
| Mental health | 672 | 657 | 0.942 | 0.610 | 1,188 | 994 | 0.451 | 0.849 |
| Infectious disease | 2,648 | 2,624 | 0.907 | 0.950 | 2,585 | 3,427 | 0.003 | 0.006 |
| Ancillary (laboratory, radiography, etc.) | 11,044 | 10,153 | 0.822 | 0.650 | 10,744 | 7,931 | 0.293 | 0.907 |
| TOTAL | 18,561 | 15,362 | 0.481 | 0.964 | 20,093 | 16,456 | 0.303 | 0.788 |
| INPATIENT | ||||||||
| TOTAL | 5386 | 4622 | 0.693 | 0.312 | 6738 | 4795 | 0.424 | 0.630 |
| PATIENT COSTSc | ||||||||
| TOTAL | 3 | 5 | 0.439 | 0.642 | 3 | 3 | 0.664 | 0.333 |
| TOTAL, $ | ||||||||
| TOTAL maina | 27,286 | 23,504 | 0.646 | 0.745 | 28,447 | 22,657 | 0.310 | 0.725 |
| TOTAL secondaryb | 32,667 | 28,111 | 0.593 | 0.637 | 35,168 | 27,443 | 0.225 | 0.827 |
Main analysis consisted of pharmacy, outpatient, and patient costs only
Secondary analysis consisted of pharmacy, outpatient, inpatient, and patient costs
Patient costs include wait and travel time incurred by patients to receive care
Kruskal-Wallis
Table 3.
Adjusted Mean Incremental Cost per QALY Ratios and Net Health Benefit (Original Sample)
| Quality adjusted life year (QALY) method | QALY Difference (Int-UC) | Mean ICER Outpatient and Pharmacy | Mean ICER Outpatient, Pharmacy, and Inpatient | Net Health Benefit (QALYs) |
|---|---|---|---|---|
| Depression free days (DFD 0.4 [traditional]) (fully depressed=0.6)a | 0.020 | −67,663 | −26,416 | 0.048 |
| Depression free days (DFD 0.2 [conservative]) (fully depressed=0.8)a | 0.011 | −125,004 | −48,803 | 0.038 |
| SF-12V standard gamble conversionb | 0.010 | −131,418 | −51,307 | 0.038 |
| Quality of Well-Being self-administeredc | 0.009 | −147,014 | −57,395 | 0.037 |
| Disease specific measure | DFD Difference (Int-UC) | Net Health Benefit (DFDs) | ||
| Depression-free days (DFD)a | 19 | −71 | −28 | 156 |
| Costing method | Cost Difference (Int-UC) | |||
| Outpatient and pharmacy ($)d | −1,368 | |||
| Outpatient, inpatient, and pharmacy ($)e | −534 |
Case mix variables were baseline 20-item Hopkins Symptom Checklist (SCL-20) score, physical health comorbidity, HIV symptom index, marital status, annual household income, comorbid mental health, current HIV medication, any inpatient mental health visit and any depression treatment in the past 6 months
Case mix variables were baseline 20-item Hopkins Symptom Checklist (SCL-20) score, physical health comorbidity, HIV symptom index, education, annual household income, comorbid mental health, current HIV medication, any inpatient mental health visit, and any depression treatment in the past 6 months
Case mix variables were baseline 20-item Hopkins Symptom Checklist (SCL-20) score, physical health comorbidity, HIV symptom index, marital status, education, annual household income, comorbid mental health, current HIV medication, any inpatient mental health visit, and any depression treatment in the past 6 months
Case mix variables were baseline 20-item Hopkins Symptom Checklist (SCL-20) score, physical health comorbidity, HIV symptom index, gender, race, depression, PTSD, current HIV medication
Case mix variables were baseline 20-item Hopkins Symptom Checklist (SCL-20) score, physical health comorbidity, HIV symptom index, marital status, annual household income, comorbid mental health, current HIV medication, any inpatient mental health visit, and any depression treatment in the past 6 months
As reported previously, the intervention resulted in 19.3 (p<0.01) additional DFDs over usual care.[22] DFD QALYs were calculated by varying the QALY estimate associated with depression improving from fully depressed to fully asymptomatic (0.2 [traditional] to 0.4 [conservative]). Using the most commonly reported DFD to QALY conversion (DFD 0.4 [traditional]) resulted in 0.020 incremental QALYs and the more conservative approach (DFD 0.2 [conservative]) resulted in 0.011 incremental QALYs for the intervention in the original sample (Table 3 [case mix variables are listed in the table notes]). We also calculated incremental generic QALYs using SF-12 standard gamble (0.010 greater for the intervention) and the QWB-SA (0.009 greater for the intervention). Although the intervention resulted in significantly more DFDs none of the QALY measures (DFD-derived or generic) were statistically different between the intervention and usual care group. This was not unexpected as the findings of the clinical effectiveness trial note there were significant differences at 6-month follow-up but not at 12-months.[22]
All mean ICERs taken from the original sample were negative (Table 3). Each of the NHB calculations using the $50,000/QALY threshold were positive for the intervention ranging from 0.037 QALYs for the QWB-SA QALYs to 0.048 QALYs for the DFD 0.4 to QALY conversion (Table 3). NHB analysis of the disease-specific DFD measure was also positive further supporting the cost-effectiveness of the intervention (156 additional DFDs).
Figure 1 gives the ICER distribution for the bootstrapped sample. Using the $50,000/QALY threshold, the base case analysis is cost-effective for 97% of the samples. Treatments that show ICERs less than $20,000/QALY are typically recommended for rapid dissemination into healthcare systems.[62] In our base case analysis, there is a 96.4% probability that the HITIDES intervention will cost less than $20,000/QALY and 77.8% probability that it will be cost saving. The acceptability curves for all four QALY measures are presented in Figure 2 and the probability of being less than $50,000/QALY varies between 82–97%, depending on the QALY measure.
Figure 1.
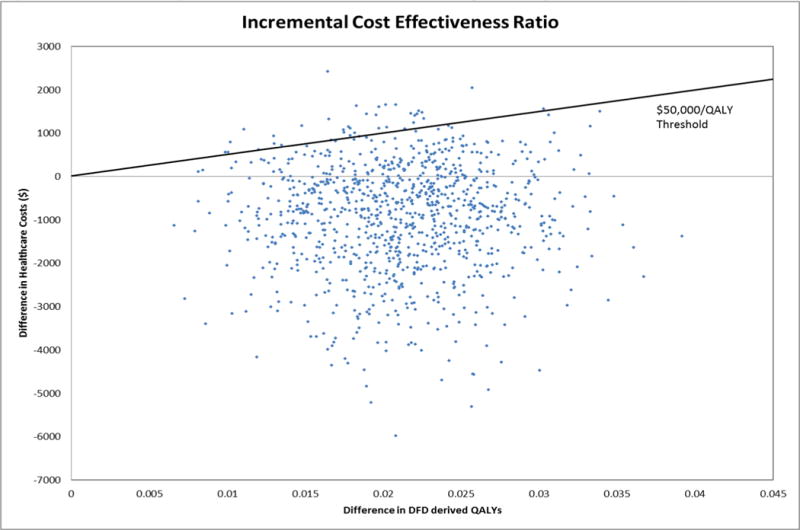
ICER of Bootstrap Distribution for Base Case (DFD-derived QALYs; Outpatient and Pharmacy costs)
Figure 2.
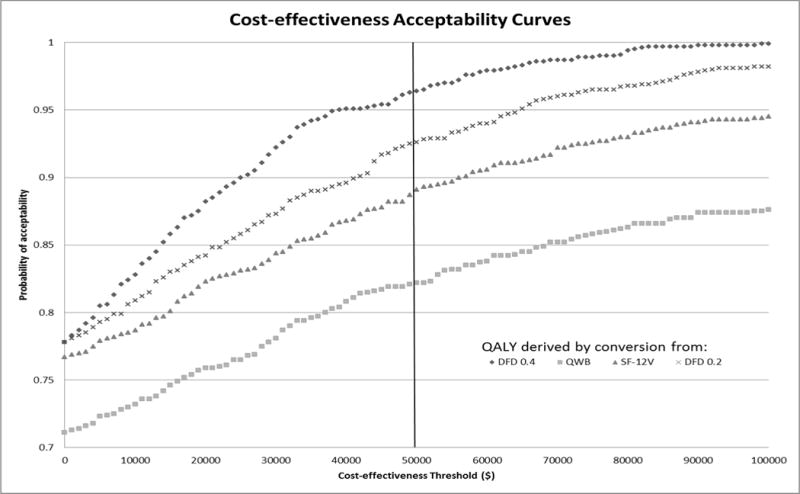
Acceptability Curves for all QALY Measures (Bootstrapped Sample)
Discussion
The HITIDES intervention demonstrated improved outcomes and decreased costs compared to usual care over one year. Whereas depression and HIV symptom severity differences were statistically significant at 6-months, the QALY differences over 12-months were not. As Glick notes however, the lack of significant QALY differences represents “‘absence of evidence of a difference’ and not ‘evidence of absence of a difference’”.[64] For this reason, healthcare economists recommend evaluating the joint distribution of cost and effectiveness (e.g., incremental cost-effectiveness plane or acceptability curve) in order to identify situations where the examination of clinical effect and cost simultaneously indicates clear advantages for one intervention over another.[64–66] As evidenced by our bootstrapped sample, despite the small QALY difference, the intervention was cost-effective in 97% of replications.
Cost per QALY estimates for collaborative depression care in non-veteran populations range from $3,303/QALY to $67,225/QALY adjusted to 2013 dollars and using only outpatient costs.[19, 20, 49, 63, 67] In the VA, cost per quality estimates range from $67,965/QALY to $103,319/QALY, adjusted to 2013 dollars.[17, 68] These cost per QALY estimates indicate that the collaborative care interventions cost more and resulted in better outcomes than usual care.
Other studies have examined subsamples of patients with depression.[19] Katon et al found that a multi-condition collaborative treatment program for depression, diabetes, and coronary heart disease was both effective (114 additional DFDs, 0.335 QALYS over a two year period) and cost-saving.[69] In another subgroup of patients with depression and diabetes, collaborative care was associated with substantially lower non-mental health medication and outpatient costs and cost per QALY ratios ranged from $261 to $524 per QALY (2013 dollars).[62] These findings of cost-effective or cost-savings interventions in complex primary care patients suggests that collaborative care interventions may be particularly cost-effective for comorbid high cost patients. This situation is especially true in the HIV clinic where the HITIDES intervention was implemented. Total outpatient and pharmacy costs averaged $25,381 in the year prior to the intervention; this is substantially higher than that seen in the multi-condition cohort ($10,026).
The cost savings associated with the HITIDES intervention appears to be attributable to lower HIV medication costs and ancillary (e.g. laboratory, radiography, etc.) costs. Couple this finding with the fact that the intervention group had more HIV clinic visits and lower HIV symptom severity[22] and the interpretation of these findings could be that HIV symptoms were better controlled in intervention patients requiring less expensive HIV medication and laboratory monitoring. Of note, mental health costs are not significantly different between the two groups before or after the intervention signifying no substitution for mental health care by the intervention. The implication of this finding is that a wider roll-out of this intervention in VA HIV clinics could result in improved outcomes and cost savings. Further, given the demographic similarity between VA and non-VA HIV clinics,[70] similar results may be possible in non-VA HIV clinic settings.
Since the NHB of the HITIDES intervention is positive then it is cost-effective compared to a “marginally cost-effective” program and should be selected for implementation. NHB findings for all outcome measures were positive, providing additional benefits to Veterans, supporting the case for implementation. Further, interventions that result in cost-effectiveness ratios less than $20,000 per QALY are recommended for rapid implementation into healthcare systems and the HITIDES intervention certainly meets this criterion.[71]
Collaborative care approaches to depression management in primary care settings have been shown to be cost effective and associated with greater patient satisfaction outcomes.[3, 69] However, HIV clinics may be considered the patient’s medical home and may not be located in primary care clinics. Therefore, considering available resources, HIV clinics could obtain depression collaborative care from on-site resources (within the HIV clinic or a nearby primary care clinic) or an off-site collaborative care team used in the HITIDES study. Another alternative is a hybrid team with both on-site and off-site collaborative care resources but the hybrid team was not tested in this study. While cost savings is not a prerequisite for implementation of a program to improve the mental health of patients,[72] the impressive results from the HITIDES intervention shifts the question from whether to implement to how best to implement this program. The depression collaborative care literature supports both on-site and off-site depression care teams.[73] The HITIDES intervention used an off-site team to cover three specialty clinics that differed across many characteristics (e.g. size, location, HIV provider mix, etc.).[38] The use of a single, centrally located care manager whose time could be devoted solely to this intervention may enhance intervention fidelity and introduce efficiencies in both training and supervision costs.
This study has several limitations worth noting. First, although the VA is the largest single provider of HIV care in the world and largest managed care organization in the US, the results of this study may not be generalizable to systems of care that are less integrated or that do not use electronic medical records. However, as the healthcare system changes these differences may be diminish. While the demographic and clinical characteristics of VA patients are typically different from patients in other healthcare settings, this limitation is less important for patients with HIV where the population differences are less prominent. Additionally, the DSS cost data only includes care received in the VA system. While comprehensive HIV care was provided to both the usual care and intervention groups in the study, any care received outside the VA system would not be represented in our findings. This would be especially concerning for an older group of study subjects with eligibility for both Veterans benefits and Medicare, but with an average age around 50 in this study this concern is diminished. The HITIDES intervention utilized an off-site intervention team; the relationship or generalizability of this approach to that of an on-site team is unknown. Our base case analysis used the DFD to QALY conversion formula that has been used in other depression collaborative care studies; however, there is no gold standard effectiveness measure for depression studies. Therefore, several QALY measures were used including the DFD to QALY conversion and generic QALY measures. Our results suggest that the DFD 0.2 [conservative] to QALY conversion is more consistent with the results from generic QALY measures.
In conclusion, in a specialty physical health clinic this depression collaborative care intervention (HITIDES) was effective and cost-saving. This finding is consistent with other primary care depression collaborative care results in subgroups of patients with expensive physical health comorbidities. Implementation of off-site depression collaborative care programs in specialty care clinics or to targeted patients based on clinical characteristics may be a strategy that not only improves outcomes for patients, but also maximizes the efficient use of limited healthcare resources.
Acknowledgments
Sources of Funding: Funded by VA QUERI SDP (MNT 05-152)
Footnotes
Conflicts of Interest: no potential conflicts of interest
Contributor Information
Jacob T Painter, Email: JTPainter@uams.edu, Center for Mental Healthcare and Outcomes Research, Central Arkansas Veterans Healthcare System; Division of Pharmaceutical Evaluation and Policy, University of Arkansas for Medical Sciences, 2200 Fort Roots Drive (152/NLR), North Little Rock, Arkansas 72114, Phone: 501-257-1740, Fax: 501-257-1707.
John C Fortney, Email: FortneyJohnC@uams.edu, Center for Mental Healthcare and Outcomes Research &, South Central Mental Illness Research, Education and Clinical Centers, Central Arkansas Veterans Healthcare System &, Psychiatric Research Institute, University of Arkansas for Medical Sciences.
Allen L Gifford, Email: Allen.Gifford@va.gov, VA New England Healthcare System, Center for Healthcare Quality, Outcomes, and Economic Research, Bedford, Massachusetts.
David Rimland, Email: David.Rimland@va.gov, Atlanta VA Medical Center &, Department of Infectious Disease, Emory University, School of Medicine, Atlanta, Georgia.
Thomas Monson, Email: Thomas.Monson@va.gov, Department of Infectious Disease, Central Arkansas Veterans Healthcare System.
Maria C. Rodriguez-Barradas, Email: Maria.Rodriguez-Barradas@va.gov, Michael E. DeBakey VA Medical Center &, Department of Medicine – Infectious Disease, Baylor College of Medicine.
Jeffrey M Pyne, Email: JMPyne@uams.edu, Center for Mental Healthcare and Outcomes Research &, South Central Mental Illness Research, Education and Clinical Centers, Central Arkansas Veterans Healthcare System;, Psychiatric Research Institute, University of Arkansas for Medical Sciences.
References
- 1.Robinson WD, Geske JA, Prest LA, Barnacle R. Depression treatment in primary care. The Journal of the American Board of Family Practice. 2005;18:79–86. doi: 10.3122/jabfm.18.2.79. [DOI] [PubMed] [Google Scholar]
- 2.Katon W, Von Korff M, Lin E, Simon G, Walker E, Unutzer J, et al. Stepped collaborative care for primary care patients with persistent symptoms of depression: a randomized trial. Arch Gen Psychiatry. 1999;56:1109–1115. doi: 10.1001/archpsyc.56.12.1109. [DOI] [PubMed] [Google Scholar]
- 3.Katon W, Robinson P, Von Korff M, Lin E, Bush T, Ludman E, et al. A multifaceted intervention to improve treatment of depression in primary care. Arch Gen Psychiatry. 1996;53:924–932. doi: 10.1001/archpsyc.1996.01830100072009. [DOI] [PubMed] [Google Scholar]
- 4.Simon GE, VonKorff M, Rutter C, Wagner E. Randomised trial of monitoring, feedback, and management of care by telephone to improve treatment of depression in primary care. BMJ. 2000;320:550–554. doi: 10.1136/bmj.320.7234.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rost K, Nutting P, Smith J, Werner J, Duan N. Improving depression outcomes in community primary care practice: A randomized trial of the QuEST intervention. Quality Enhancement by Strategic Teaming. Journal of General Internal Medicine. 2001;16:143–149. doi: 10.1111/j.1525-1497.2001.00537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wells KB, Sherbourne C, Schoenbaum M, Duan N, Meredith L, Unutzer J, et al. Impact of disseminating quality improvement programs for depression in managed primary care: a randomized controlled trial. JAMA. 2000;283:212–220. doi: 10.1001/jama.283.2.212. [DOI] [PubMed] [Google Scholar]
- 7.Finley PR, Rens HR, Pont JM, Gess SL, Louie C, Bull SA, et al. Impact of collaborative care model upon depression in primary care: A randomized controlled trial. Pharmacotherapy. 2003;23:1175–1185. doi: 10.1592/phco.23.10.1175.32760. [DOI] [PubMed] [Google Scholar]
- 8.Adler DA, Bungay KM, Wilson IB, Pei Y, Supran S, Peckham E, et al. The impact of a pharmacist intervention on 6-month outcomes in depressed primary care patients. General Hospital Psychiatry. 2004;26:199–209. doi: 10.1016/j.genhosppsych.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 9.Dobscha SK, Corson K, Hickam DH, Perrin NA, Kraemer DF, Gerrity MS. Depression decision support in primary care: a cluster randomized trial. Ann Intern Med. 2006;145:477–487. doi: 10.7326/0003-4819-145-7-200610030-00005. [DOI] [PubMed] [Google Scholar]
- 10.Bruce ML, Ten Have TR, Reynolds CF, III, Katz II, Schulberg HC, Mulsant BH, et al. Reducing suicidal ideation and depressive symptoms in depressed older primary care patients: A randomized controlled trial. Journal of the American Medical Association. 2004;291(9):1081–1091. doi: 10.1001/jama.291.9.1081. [DOI] [PubMed] [Google Scholar]
- 11.Alexopoulos GS, Katz IR, Bruce ML, Heo M, Have TT, Raue P, et al. Remission in depressed geriatric primary care patients: A report from the PROSPECT Study. American Journal of Psychiatry. 2005;162(4):718–724. doi: 10.1176/appi.ajp.162.4.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hedrick SC, Chaney EF, Felker B, Liu CF, Hasenberg N, Heagerty P, et al. Effectiveness of collaborative care depression treatment in Veterans’ Affairs primary care. J Gen Intern Med. 2003 Jan;18:9–16. doi: 10.1046/j.1525-1497.2003.11109.x. [see comment] 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Unutzer J, Katon W, Callahan CM, Williams JW, Jr, Hunkeler E, Harpole L, et al. Collaborative care management of late-life depression in the primary care setting: a randomized controlled trial. JAMA. 2002;288:2836–2845. doi: 10.1001/jama.288.22.2836. [DOI] [PubMed] [Google Scholar]
- 14.Pyne JM, Rost KM, Farahati F, Tripathi SP, Smith J, Williams DK, et al. One size fits some: the impact of patient treatment attitudes on the cost-effectiveness of a depression primary-care intervention. Psychol Med. 2005;35:839–854. doi: 10.1017/s0033291704003332. [DOI] [PubMed] [Google Scholar]
- 15.Pyne JM, Smith J, Fortney J, Zhang M, Williams DK, Rost K. Cost-effectiveness of a primary care intervention for depressed females. J Affect Disord. 2003;74:23–32. doi: 10.1016/s0165-0327(02)00115-5. [DOI] [PubMed] [Google Scholar]
- 16.Simon GE, Ludman EJ, Rutter C. Incremental benefit and cost of telephone care management and telephone psychotherapy for depression in primary care. Arch Gen Psychiatry. 2009;66:1081–1089. doi: 10.1001/archgenpsychiatry.2009.123. [DOI] [PubMed] [Google Scholar]
- 17.Liu CF, Hedrick SC, Chaney EF, Heagerty P, Felker B, Hasenberg N, et al. Cost-effectiveness of collaborative care for depression in a primary care veteran population. Psychiatric Services. 2003;54:698–704. doi: 10.1176/appi.ps.54.5.698. [DOI] [PubMed] [Google Scholar]
- 18.Simon GE, Von Korff M, Ludman EJ, Katon WJ, Rutter C, Unutzer J, et al. Cost-effectiveness of a program to prevent depression relapse in primary care. Med Care. 2002;40:941–950. doi: 10.1097/00005650-200210000-00011. [DOI] [PubMed] [Google Scholar]
- 19.Simon GE, Manning WG, Katzelnick DJ, Pearson SD, Henk HJ, Helstad CS. Cost-effectiveness of systematic depression treatment for high utilizers of general medical care. Arch Gen Psychiatry. 2001;58:181–187. doi: 10.1001/archpsyc.58.2.181. [DOI] [PubMed] [Google Scholar]
- 20.Schoenbaum M, Unutzer J, Sherbourne C, Duan N, Rubenstein LV, Miranda J, et al. Cost-effectiveness of practice-initiated quality improvement for depression: results of a randomized controlled trial. JAMA. 2001;286:1325–1330. doi: 10.1001/jama.286.11.1325. [DOI] [PubMed] [Google Scholar]
- 21.Von Korff M, Katon W, Bush T, Lin EH, Simon GE, Saunders K, et al. Treatment costs, cost offset, and cost-effectiveness of collaborative management of depression. Psychosom Med. 1998;60:143–149. doi: 10.1097/00006842-199803000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Pyne JM, Fortney JC, Curran GM, Tripathi S, Atkinson JH, Kilbourne AM, et al. Effectiveness of collaborative care for depression in human immunodeficiency virus clinics. Arch Intern Med. 2011;171:23–31. doi: 10.1001/archinternmed.2010.395. [DOI] [PubMed] [Google Scholar]
- 23.Walker J, Sharpe M. Depression Care for People with Cancer: a collaborative care intervention. Gen Hosp Psychiatry. 2009;31:436–441. doi: 10.1016/j.genhosppsych.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 24.Evans DL, Ten Have TR, Douglas SD, Gettes DR, Morrison M, Chiappini MS, et al. Association of depression with viral load, CD8 T lymphocytes, and natural killer cells in women with HIV infection. Am J Psychiatry. 2002;159:1752–1759. doi: 10.1176/appi.ajp.159.10.1752. [DOI] [PubMed] [Google Scholar]
- 25.Farinpour R, Miller EN, Satz P, Selnes OA, Cohen BA, Becker JT, et al. Psychosocial risk factors of HIV morbidity and mortality: findings from the Multicenter AIDS Cohort Study (MACS) J Clin Exp Neuropsychol. 2003;25:654–670. doi: 10.1076/jcen.25.5.654.14577. [DOI] [PubMed] [Google Scholar]
- 26.Gordillo V, del Amo J, Soriano V, Gonzalez-Lahoz J. Sociodemographic and psychological variables influencing adherence to antiretroviral therapy. AIDS. 1999;13:1763–1769. doi: 10.1097/00002030-199909100-00021. [DOI] [PubMed] [Google Scholar]
- 27.Ickovics JR, Hamburger ME, Vlahov D, Schoenbaum EE, Schuman P, Boland RJ, et al. Mortality, CD4 cell count decline, and depressive symptoms among HIV-seropositive women: longitudinal analysis from the HIV Epidemiology Research Study. JAMA. 2001;285:1466–1474. doi: 10.1001/jama.285.11.1466. [DOI] [PubMed] [Google Scholar]
- 28.Leserman J, Petitto JM, Gu H, Gaynes BN, Barroso J, Golden RN, et al. Progression to AIDS, a clinical AIDS condition and mortality: psychosocial and physiological predictors. Psychol Med. 2002;32:1059–1073. doi: 10.1017/s0033291702005949. [DOI] [PubMed] [Google Scholar]
- 29.Leserman J. HIV disease progression: depression, stress, and possible mechanisms. Biol Psychiatry. 2003;54:295–306. doi: 10.1016/s0006-3223(03)00323-8. [DOI] [PubMed] [Google Scholar]
- 30.Lyketsos CG, Hoover DR, Guccione M, Senterfitt W, Dew MA, Wesch J, et al. Depressive symptoms as predictors of medical outcomes in HIV infection. Multicenter AIDS Cohort Study. JAMA. 1993;270:2563–2567. [PubMed] [Google Scholar]
- 31.Mayne TJ, Vittinghoff E, Chesney MA, Barrett DC, Coates TJ. Depressive affect and survival among gay and bisexual men infected with HIV. Arch Intern Med. 1996;156:2233–2238. [PubMed] [Google Scholar]
- 32.Moskowitz JT. Positive affect predicts lower risk of AIDS mortality. Psychosom Med. 2003;65:620–626. doi: 10.1097/01.psy.0000073873.74829.23. [DOI] [PubMed] [Google Scholar]
- 33.Singh N, Squier C, Sivek C, Wagener M, Nguyen MH, Yu VL. Determinants of compliance with antiretroviral therapy in patients with human immunodeficiency virus: prospective assessment with implications for enhancing compliance. AIDS Care. 1996;8:261–269. doi: 10.1080/09540129650125696. [DOI] [PubMed] [Google Scholar]
- 34.Brown JL, Vanable PA. Cognitive-behavioral stress management interventions for persons living with HIV: a review and critique of the literature. Ann Behav Med. 2008;35:26–40. doi: 10.1007/s12160-007-9010-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Horberg MA, Silverberg MJ, Hurley LB, Towner WJ, Klein DB, Bersoff-Matcha S, et al. Effects of depression and selective serotonin reuptake inhibitor use on adherence to highly active antiretroviral therapy and on clinical outcomes in HIV-infected patients. J Acquir Immune Defic Syndr. 2008;47:384–390. doi: 10.1097/QAI.0b013e318160d53e. [DOI] [PubMed] [Google Scholar]
- 36.Safren SA, O’Cleirigh C, Tan JY, Raminani SR, Reilly LC, Otto MW, et al. A randomized controlled trial of cognitive behavioral therapy for adherence and depression (CBT-AD) in HIV-infected individuals. Health Psychol. 2009;28:1–10. doi: 10.1037/a0012715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Czarnogorski M, Halloran C, James, Pedati C, Dursa EK, Durfee J, Martinello R, et al. Expanded HIV Testing in the US Department of Veterans Affairs, 2009–2011. American journal of public health. 2013;103:e40–e45. doi: 10.2105/AJPH.2013.301376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Curran GM, Pyne J, Fortney JC, Gifford A, Asch SM, Rimland D, et al. Development and implementation of collaborative care for depression in HIV clinics. AIDS Care. 2011;23:1626–1636. doi: 10.1080/09540121.2011.579943. [DOI] [PubMed] [Google Scholar]
- 39.Rost K, Nutting PA, Smith J, Werner JJ. Designing and implementing a primary care intervention trial to improve the quality and outcome of care for major depression. Gen Hosp Psychiatry. 2000;22:66–77. doi: 10.1016/s0163-8343(00)00059-1. [DOI] [PubMed] [Google Scholar]
- 40.Fortney JC, Pyne JM, Edlund MJ, Robinson DE, Mittal D, Henderson KL. Design and implementation of the telemedicine-enhanced antidepressant management study. Gen Hosp Psychiatry. 2006;28:18–26. doi: 10.1016/j.genhosppsych.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 41.Fortney JC, Pyne JM, Steven CA, Williams JS, Hedrick RG, Lunsford AK, et al. A web-based clinical decision support system for depression care management. The American Journal of Managed Care. 2010;16:849. [PMC free article] [PubMed] [Google Scholar]
- 42.Suehs B, Argo TR, Bendele SD, Crismon ML, Trivedi MH, Kurian B. Texas Medication Algorithm Project Procedural Manual: Major Depressive Disorder Algorithms. Texas Department of State Health Services. 2008 [Google Scholar]
- 43.MDD Working Group. VA/DoD Clinical Practice Guideline for Management of Major Depressive Disorder (MDD) 2.0. Washington, DC; 2008. [Google Scholar]
- 44.Smith GR, Burnam A, Burns BJ, Cleary PD, Rost K. Depression Outcomes Module (DOM) In: First MB, Ross R, editors. Handbook of Psychiatric Measures. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 45.Rost K, Smith GR, Burnam MA, Burns BJ. Measuring the outcomes of care for mental health problems: The case of depressive disorders. Medical Care. 1992;30(5,suppl):MS266–MS273. doi: 10.1097/00005650-199205001-00026. [DOI] [PubMed] [Google Scholar]
- 46.Lecrubier Y, Sheehan DV, Weiller E, Amorim P, Bonora I, Sheehan KH, et al. The Mini International Neuropsychiatric Interview (MINI): A short diagnostic structured interview: Reliability and validity according to the CIDI. European Psychiatry. 1997;12:224–231. [Google Scholar]
- 47.Sheehan DV, Lecrubier Y, Sheehan KH, Janavs J, Weiller E, Keskiner A, et al. The validity of the Mini International Neuropsychiatric Interview (MINI) according to the SCID-P and its reliability. European Psychiatry. 1997;12:232–241. [Google Scholar]
- 48.Rost KM, Nutting P, Smith J, Werner J, Duan N. Improving depression outcomes in community primary care practice: A randomized trial of the QuEST intervention. J Gen Intern Med. 2001;16:143–149. doi: 10.1111/j.1525-1497.2001.00537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Simon GE, Katon WJ, VonKorff M, Unutzer J, Lin EH, Walker EA, et al. Cost-effectiveness of a collaborative care program for primary care patients with persistent depression. Am J Psychiatry. 2001;158:1638–1644. doi: 10.1176/appi.ajp.158.10.1638. [DOI] [PubMed] [Google Scholar]
- 50.Ganiats T, Browner D, Kaplan R. Comparison of two methods of calculating Quality-adjusted Life Years. Qual Life Res. 1996;5:162–164. doi: 10.1007/BF00435981. [DOI] [PubMed] [Google Scholar]
- 51.Lave JR, Frank RG, Schulberg HC, Kamlet MS. Cost-effectiveness of treatments for major depression in primary care practice. Archives of General Psychiatry. 1998;55:645–651. doi: 10.1001/archpsyc.55.7.645. [DOI] [PubMed] [Google Scholar]
- 52.Brazier JE, Roberts J. The estimation of a preference-based measure of health from the SF-12. Med Care. 2004;42:851–859. doi: 10.1097/01.mlr.0000135827.18610.0d. [DOI] [PubMed] [Google Scholar]
- 53.Kaplan RM, Bush JW, Berry CC. Health status: Types of validity and the index of well-being. Health Services Research. 1976;11:478–507. [PMC free article] [PubMed] [Google Scholar]
- 54.Pyne JM, Patterson TL, Kaplan RM, Gillin JC, Koch WL, Grant I. Assessment of the quality of life of patients with major depression. Psychiatr Serv. 1997;48:224–230. doi: 10.1176/ps.48.2.224. [DOI] [PubMed] [Google Scholar]
- 55.Royston P. STATA Journal 5. Multiple imputation of missing values: Update of ICE. 2005:527–536. [Google Scholar]
- 56.Lumley T, Diehr P, Emerson S, Chen L. The importance of the normality assumption in large public health data sets. Annu Rev Public Health. 2002;23:151–169. doi: 10.1146/annurev.publhealth.23.100901.140546. [DOI] [PubMed] [Google Scholar]
- 57.Kleinman LC, Norton EC. What’s the Risk? A simple approach for estimating adjusted risk measures from nonlinear models including logistic regression. Health Serv Res. 2009;44:288–302. doi: 10.1111/j.1475-6773.2008.00900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Briggs AH, Wonderling DE, Mooney CZ. Pulling cost-effectiveness analysis up by its bootstraps: a non-parametric approach to confidence interval estimation. Health Economics. 1997;6:327–340. doi: 10.1002/(sici)1099-1050(199707)6:4<327::aid-hec282>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 59.Anderson JP, Bush JW, Chen M, Dolenc D. Policy space areas and properties of benefit-cost/utility analysis. JAMA. 1986;255:794–795. [PubMed] [Google Scholar]
- 60.Hunink MGM, Bult JR, de Vries J, Weinstein MC. Uncertainty in decision models analyzing cost-effectiveness: the joint distribution of incremental costs and effectiveness evaluated with a nonparametric bootstrap method. Med Decis Making. 1998;18:337–346. doi: 10.1177/0272989X9801800312. [DOI] [PubMed] [Google Scholar]
- 61.Stinnett AA, Mullahy J. Net health benefits: a new framework for the analysis of uncertainty in cost-effectiveness analysis. Med Decis Making. 1998;18:S68–S80. doi: 10.1177/0272989X98018002S09. [DOI] [PubMed] [Google Scholar]
- 62.Katon W, Unutzer J, Fan MY, Williams JW, Jr, Schoenbaum M, Lin EH, et al. Cost-effectiveness and net benefit of enhanced treatment of depression for older adults with diabetes and depression. Diabetes Care. 2006;29:265–270. doi: 10.2337/diacare.29.02.06.dc05-1572. [DOI] [PubMed] [Google Scholar]
- 63.Pyne JM, Rost KM, Zhang M, Williams DK, Smith J, Fortney J. Cost-effectiveness of a primary care depression intervention. J Gen Intern Med. 2003;18:432–441. doi: 10.1046/j.1525-1497.2003.20611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Glick HA, Doshi JA, Sonnad SS, Polsky D. Economic evaluation in clinical trials. Oxford University Press; 2014. [Google Scholar]
- 65.Claxton K. The irrelevance of inference: a decision-making approach to the stochastic evaluation of health care technologies. Journal of health economics. 1999;18:341–364. doi: 10.1016/s0167-6296(98)00039-3. [DOI] [PubMed] [Google Scholar]
- 66.Briggs AH, O’Brien BJ. The death of cost-minimization analysis? Health Econ. 2001;10:179–184. doi: 10.1002/hec.584. [DOI] [PubMed] [Google Scholar]
- 67.Katon WJ, Schoenbaum M, Fan MY, Callahan CM, Williams J, Jr, Hunkeler E, et al. Cost-effectiveness of improving primary care treatment of late-life depression. Arch Gen Psychiatry. 2005;62:1313–1320. doi: 10.1001/archpsyc.62.12.1313. [DOI] [PubMed] [Google Scholar]
- 68.Pyne JM, Fortney JC, Tripathi SP, Maciejewski ML, Edlund MJ, Williams DK. Cost-effectiveness analysis of a rural telemedicine collaborative care intervention for depression. Arch Gen Psychiatry. 2010;67:812–821. doi: 10.1001/archgenpsychiatry.2010.82. [DOI] [PubMed] [Google Scholar]
- 69.Katon W, Russo J, Lin EH, Schmittdiel J, Ciechanowski P, Ludman E, et al. Cost-effectiveness of a multicondition collaborative care intervention: a randomized controlled trial. Arch Gen Psychiatry. 2012;69:506–514. doi: 10.1001/archgenpsychiatry.2011.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Valdiserri R. The State of Care for Veterans with HIV/AIDS. Palo Alto, California: U.S. Department of Veteran Affairs, Public Health Strategic Health Care Group, Center for Quality Management in Public Health; 2009. [Google Scholar]
- 71.Gold MR, Siegel JE, Russell LB, Weinstein MC. Cost-Effectiveness in Health and Medicine. New York: Oxford University Press, Inc.; 1996. p. 425. [Google Scholar]
- 72.Sturm R. Economic grand rounds: the myth of medical cost offset. Psychiatric Services. 2001;52:738–740. doi: 10.1176/appi.ps.52.6.738. [DOI] [PubMed] [Google Scholar]
- 73.Simon GE, Ludman EJ. Should mental health interventions be locally grown or factory-farmed? American Journal of Psychiatry. 2013;170:362–365. doi: 10.1176/appi.ajp.2013.13010043. [DOI] [PMC free article] [PubMed] [Google Scholar]


