Abstract
Background
Uric acid (UA) is associated with high blood pressure in adolescents and left ventricular hypertrophy (LVH) and cardiovascular disease (CVD) in adults. We sought to determine if UA is independently associated with CVD risk factors and left ventricular mass (LVM) over time in hypertensive youth.
Methods
One-year prospective observational study of hypertensive children aged 3-19 years. Cross-sectional and longitudinal associations of serum UA with CVD risk factors and LVM were explored.
Results
At baseline: mean age 13.8 years, mean UA 5.5 mg/dL, 24 % with elevated UA, 51 % overweight/obese, and 39 % with LVH. Measures of adiposity, low high-density lipoprotein cholesterol, high-sensitivity CRP, LVM and LVH were all significantly associated with elevated UA at baseline, but not with change over time. Each 1 mg/dL increase in baseline UA was associated with 2.5 g/m2.7 greater LVM index at follow-up (95% CI 0.64, 4.39; p=0.01); after adjustment for age, sex, race, body mass index z-score, change in UA, time, BP and medication use, this association was no longer significant.
Conclusions
Hypertensive children with elevated UA have a higher prevalence of obesity-related CVD risk factors. Among hypertensive children, UA may be a marker of adiposity and not an independent CVD risk factor.
Keywords: blood pressure, cardiovascular disease, left ventricular hypertrophy, obesity, longitudinal, children, uric acid
Introduction
Cardiovascular disease (CVD) remains the leading cause of mortality worldwide [1]. Primary prevention and treatment of traditional cardiovascular disease (CVD) risk factors is the mainstay of efforts aimed at diminishing the morbidity and mortality related to CVD in adults. As CVD risk factors are increasingly prevalent among children and adolescents, identifying at risk children would aid in minimizing the burden of adult CVD. In fact, the American Heart Association's recent policy statement emphasizes that CVD risk factor prevention should begin in childhood [2].
In recent years, the prevalence of hyperuricemia has increased in children [3]. Adult studies have described an association between serum uric acid (UA) and increased CVD mortality [4-6] independent of traditional CVD risk factors [7]. A common precursor to adult CVD events is left ventricular hypertrophy (LVH) [8]. This pathological remodeling of the heart is also associated with elevated UA in adults [9, 10]. Further, when LVH and hyperuricemia are present together, the risk for CVD events increases substantially [6].
While there is some evidence that lowering UA may reduce blood pressure (BP) in adolescents [11], it is not currently a therapeutic target for hypertension treatment in these patients nor in adults. Further, the longitudinal association of serum UA with CVD risk factors and target organ damage in hypertensive children has not been well described. We therefore sought to investigate the association of UA with CVD risk factors and LVH, defined as increased left ventricular mass index (LVMI). We also explored the hypothesis that BP mediates the relationship between serum UA and LVMI over time.
Methods
Study subjects and study design
This was a secondary analysis of data obtained during a 1-year prospective observational study designed to evaluate the impact of CVD risk factors on regression of LVH over time among hypertensive children. Participants were recruited from an urban pediatric subspecialty clinic between August 2010 and June 2013. To be eligible, children had to be 3-21 years old and diagnosed with hypertension according to established guidelines prior to the study visit. Children with chronic kidney disease ≥stage 2, congenital heart disease or cancer were ineligible. Informed consent and assent were obtained at the baseline visit. The institutional review board at Johns Hopkins University School of Medicine approved this study.
Data collection
Anthropometric measurements
At baseline and 12-months, children had their height measured with a stadiometer to the nearest 0.1 cm and their weight with light clothing measured by a calibrated standing balance scale to the nearest 0.1 kg. Body mass index (BMI) was calculated as (kg/m2). Height, weight, BMI percentiles and z-scores were calculated using Centers for Disease Control and Prevention normative growth data [12-14]. Children were categorized as normal weight (BMI percentile <85 %) or overweight/obese (BMI percentile ≥85 %). Waist circumference was measured 1 cm above the patients’ navel on exhale using a Gulick tape measure which applies known tension. Hip circumference was measured at the widest girth around the hip and buttocks in triplicate; the averages were categorized as above/below age-sex-race specific 90th percentiles [15].
Blood pressure measurements
After five minutes of rest, a research nurse obtained three clinic BP measurements with an aneroid sphygmomanometer, 30 seconds apart. BP cuffs were chosen based on mid-arm circumference. Individuals responsible for BP measurement underwent training and passed yearly certification evaluations. The three BP measurements were averaged to provide one overall measurement for each visit.
Children also underwent 24-hour ambulatory BP monitoring (ABPM; Spacelabs 90210) according to published guidelines [16]. Every twenty minutes an oscillometric BP was obtained. Awake and sleep times were determined by diary.
To allow for comparison of degree of BP elevation among all participants, BPs were indexed by dividing the average systolic (and diastolic) BP by the corresponding 95th percentile systolic (and diastolic) BP [16, 17]. For participants 18 years of age and older, 140 mmHg was used as the denominator for systolic BP index (SBPi) and 90 mmHg was used for the denominator for diastolic BP index (DBPi) for both clinic and awake BP measurements. A BP index ≥1 indicates an elevated BP; each 0.1 increment above (or below) 1 represents a 10 % increase (or decrease) in BP from the BP threshold for hypertension.
Laboratory assessment
A random blood sample and a 24-hour urine collection were obtained at baseline and 12-months for analysis of UA (mg/dL), high-sensitivity C-reactive protein (hsCRP; mg/dL), lipids [total cholesterol, low-density lipoprotein, high-density lipoprotein (HDL), triglycerides; all mg/dL], hemoglobin A1c (%), plasma renin activity (ng/mL/h), serum aldosterone (ng/dL) and sodium concentration (mg/day). HsCRP concentration was determined using highly sensitive immunonoturbidimetry. Hemoglobin A1c was measured by high performance liquid chromatography. Aldosterone and plasma renin activity were measured at Quest Diagnostics using liquid chromatography tandem mass spectrometry (LC/MS/MS). Urine sodium concentration was measured using a Hitachi automated clinical chemistry analyzer.
Serum UA was determined by enzymatic spectrophotometry. Children were categorized as having elevated or normal serum UA based on age-sex-race 95th percentile norms [18, 19]. All routine clinical tests, except for plasma renin activity and serum aldosterone, were performed in the Johns Hopkins Hospital clinical laboratory.
Echocardiography
Participants underwent standardized two-dimensional guided M-mode echocardiography at baseline and 12-month follow-up. Measurements were digitally recorded and read by a study cardiologist for standardization (KWH). Three diastolic measurements of the left ventricular (LV) dimension, intraventricular septum and LV posterior wall were made and averaged.
LV Mass (LVM) was calculated using the Devearaux equation [20]: LVM (g) = 0.81 [1.04 (intraventricular septal thickness + posterior wall thickness + LV end diastolic internal dimension)3– (LV end diastolic internal dimension)3]+ 0.06. LVMI was calculated as: (LVM/ height in meters2.7). LVH was defined as LVMI ≥95th percentile [21].
Statistical analysis
Cross-sectional analyses
Baseline clinical variables were compared between those with and without elevated UA at baseline. Categorical variables were compared using Fisher's exact tests and continuous variables were compared using Student's t-tests for normally distributed data and Wilcoxon Rank Sum for non-normally distributed data. Linear and logistic regression was utilized to evaluate the association between continuous and categorical measurements of UA and LVMI and between UA and LVH, respectively. To evaluate whether BP mediated this potential relationship, two multivariable regression models were conducted: one adjusting for age, sex, race and BMI z-score, and another further adjusting for awake systolic BP index (SBPi), diastolic BP index (DBPi) and if they were taking an angiotensin converting enzyme inhibitor (ACEi), angiotensin II receptor blocker (ARB) or diuretic.
Longitudinal analyses
To explore longitudinal associations, delta variables were created for UA, LVM, LVMI and selected risk factors by subtracting the baseline value from the 12-month value. Associations of (1) baseline exposures, and (2) change in CVD risk factors over time with the change in UA over time were explored with univariate linear regression analyses. The relationship of UA at baseline with LVMI at follow-up was explored with multivariable linear regression adjusting for age at baseline, sex, race, baseline BMI z-score, change in UA and time between visits. As above, we ran an additional model further adjusting for 12-month awake SBPi, DBPi and ACEi/ARB/diuretic use at 12-months. Finally, the same covariates were included in logistic regression analyses with LVH at follow-up as the outcome.
We also conducted several secondary exploratory analyses. We investigated for a longitudinal relationship of baseline UA with both LVMI and LVH by categorizing UA in the following ways: (1) divided into tertiles; (2) dichotomized as ≥ or <sex-specific observed mean; and (3) dichotomized as ≥ or <5.5 mg/dL, based on evidence that serum UA ≥5.5 mg/dL may be predictive of primary hypertension in otherwise healthy children [22]. As the normal values for UA and LVMI vary substantially by age, we also conducted stratified analyses to determine if age modified the effect of UA on LV size.
Several participants had missing UA data (8 at baseline; 3 at 12 months). This missing data was due to random lab processing error; therefore, we conducted multiple imputation utilizing all available information on related covariates [23]. These imputed values were then analyzed as described above, with sensitivity analyses conducted to demonstrate the impact of multiple imputation compared to complete case analyses.
Statistical and database software used includes STATA 11.2 (Stata Corporation, College Station, TX) and REDCAP (Research Electronic Data Capture) hosted at Johns Hopkins University. Statistical significance for all analyses was set at two-sided p <0.05.
Results
Cross-sectional results at baseline
Fifty-three children were enrolled and completed the baseline visit, with 49 children returning for the 12-month follow-up visit. Of the 4 children who did not complete all study visits, 2 dis-enrolled and 2 were lost to follow-up. At baseline, the mean age of the 49 children who completed both baseline and 12-month assessments was 13.8 years [standard deviation (SD) 3.9]. The average length of follow-up was 12.8 months (SD 2.1). 51 % were overweight/obese, and 39 % had LVH. Forty-one children had baseline UA data. The mean UA overall was 5.5 mg/dL (SD 1.7), with mean UA for females being 4.7 mg/dL (SD 1.2) and males 6.1 mg/dL (SD 1.8) (p= 0.008). 24 % of participants had elevated UA. Table 1 compares demographic characteristics and CVD risk factors between children with normal and elevated UA at baseline. Children with elevated UA had greater markers of adiposity than those with a normal UA. They also had lower HDL-cholesterol, higher hsCRP, greater LVM and a greater prevalence of LVH than those with normal UA. In addition, they were older and had a higher plasma renin activity than children with normal UA. While non-significant, children with elevated UA excreted 1.4 grams more sodium per day than those with normal UA (4.6 vs. 3.2 grams, p=0.32). There were no statistically significant differences in sex, race, mean clinic or awake BP (mmHg or index), or LVMI between the groups. In addition, other CVD risk factors such as birth weight, metabolic syndrome diagnosis (defined in Table 1) and non-HDL cholesterol were not different between the groups.
TABLE 1.
Demographic and Cardiovascular Disease Risk Factors Among Hypertensive Children with Normal and Elevated Serum Uric Acid Levels
| Mean (±SD), Median (IQR) or n (%) | Overall N=41 | Normal UAa N=31 | Elevated UAa N=10 | P |
|---|---|---|---|---|
| Age, y | 13.8 ± 4.1 | 12.9 ± 4.3 | 16.6 ± 1.5 | <0.001 |
| Male, % | 26 (63%) | 20 (65%) | 6 (60%) | 1.00 |
| African American, % | 16 (39%) | 12 (39%) | 4 (40%) | 1.00 |
| Birth weight (kg) | 3.2 ± 0.8 N=37 |
3.1 ±0.8 N=29 |
3.2 ± 1.2 N=8 |
0.83 |
| Weight percentile | 78.3 ± 24.8 | 73.2 ± 26.5 | 93.9 ± 7.1 | <0.001 |
| BMI (kg/m2) | 25.3 ± 7.8 | 22.9 ± 5.9 | 32.7 ± 8.3 | 0.004 |
| BMI percentile | 78.4 ± 23.9 | 73.1 ± 25.2 | 94.8 ± 5.7 | <0.001 |
| BMI z-score | 1.2 ± 1.0 | 0.93 ± 1.0 | 1.9 ± 0.6 | 0.001 |
| Overweight/Obeseb, % | 21 (51%) | 12 (39%) | 9 (90%) | 0.009 |
| Clinic SBP (mmHg) | 121.1 ± 12.5 N=28 |
119.3 ± 12.2 N=21 |
126.5 ± 12.7 N=7 |
0.22 |
| 95th percentile Clinic SBPi | 1.0 ± 0.1 N=28 |
1.0 ± 0.1 N=21 |
0.9 ± 0.1 N=7 |
0.86 |
| Awake SBP (mmHg) | 129.1 ± 10.8 N=37 |
128.8 ± 11.0 N=29 |
130.4 ± 10.9 N=8 |
0.72 |
| 95th percentile Awake SBPi | 1.0 ± 0.1 N=37 |
1.0 ± 0.1 N=29 |
1.0 ± 0.1 N=8 |
0.26 |
| Clinic DBP (mmHg) | 72.8 ± 10.2 N=28 |
70.7 ± 8.9 N=21 |
79.2 ± 11.9 N=7 |
0.12 |
| 95th percentile Clinic DBPi | 0.9 ± 0.1 N=28 |
0.9 ± 0.1 N=21 |
0.9 ± 0.1 N=7 |
0.32 |
| Awake DBP (mmHg) | 72.4 ± 7.3 N=37 |
73.0 ± 7.8 N=29 |
69.9 ± 4.1 N=8 |
0.14 |
| 95th percentile Awake DBPi | 0.9 ± 0.1 N=37 |
0.9 ± 0.1 | 0.8 ± 0.1 | 0.16 |
| Metabolic syndromec | 11 (27%) | 6 (19%) | 5 (50%) | 0.10 |
| Waist circumference ≥ 90th percentile | 11 (27%) | 6 (19%) | 5 (50%) | 0.10 |
| Hypertriglyceridemia, % | 15 (37%) | 10 (32%) | 5 (50%) | 0.45 |
| Low HDL, % | 12 (29%) | 6 (19%) | 6 (60%) | 0.04 |
| Non-HDL (mg/dL) | 106.3 ± 35.0 | 105.0 ± 39.8 | 110.5 ± 15.4 | 0.52 |
| HDL (mg/dL) | 52.0 ± 15.6 | 56.1 ± 15.0 | 39.5 ± 10.3 | <0.001 |
| Lipoprotein (a) | 84.2 ± 106.9 N=40 |
98.6 ± 119.0 N=30 |
41.3 ± 32.9 | 0.02 |
| 24-hr Urinary Sodium Excretion (mg) | 3534.5 ± 1912 N=32 |
3235.3 ± 1262.0 N=25 |
4603.3 ± 3288.7 N=7 |
0.32 |
| Serum aldosterone (ng/dL) | 2 (1,6) | 3 (1,8) | 2 (0.5, 6) | 0.46 |
| Plasma Renin activity (ng/mL/h) | 4.1 (2.5, 9.0) | 3.6 (2.2, 7.9) | 9.7 (3.9, 27.9).4 | 0.02 |
| Aldosterone-Renin Ratio | 0.7 (0.14, 1.6) | 0.93 (0.22, 2.7) | 0.2 (0.1, 0.7) | 0.03 |
| Taking ACEi/ARB, % | 18 (44%) | 10 (32%) | 8 (80%) | 0.01 |
| hsCRP (mg/L) n=40 | 0.5 (0.2, 1.5) | 0.4 (0.2, 1.1) N=30 |
1.5 (0.5, 3.2) | 0.009 |
| LVM (g) | 133.2 ± 53.2 | 120.0 ± 46.0 | 174.3 ± 55.3 | 0.02 |
| LVMI (g/m2.7) | 38.1 ± 9.8 | 37.2 ±9.1 | 40.6 ±12.0 | 0.43 |
| LVH, % | 16 (39%) | 9 (29%) | 7 (70%) | 0.03 |
SD indicates standard deviation; IQR, interquartile range; UA, uric acid; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; BPi, blood pressure index = (BP/95th percentile BP or 140/90 if ≥ 18 yrs); HDL, high density lipoprotein cholesterol; ACEi, angiotensin converting enzyme inhibitor; ARB, angiotensin II receptor blocker; hsCRP, high sensitivity C-reactive protein; LVM, left ventricular mass; LVMI, left ventricular mass index = LVM/ht(m2.7); LVH, left ventricular hypertrophy = LVMI≥95th percentile
Normal UA defined as a serum UA <95th percentile for age, race and sex using Harriet Lane Handbook cutoffs for <12 years [18]; NHANES for 12-19 years [19]. Elevated UA is defined as ≥95th percentile.
Overweight/obese defined as BMI ≥85th percentile
Metabolic syndrome defined if any three of the following were present: BMI> 97th percentile; hypertriglyceridemia (triglycerides >110 mg/dL); low HDL (HDL cholesterol <40); systolic/diastolic blood pressure >90th percentile; waist circumference >90th percentile.
Table 2 displays the association of baseline UA with measures of LV size at baseline. In univariate regression, each 1 mg/dL greater baseline UA was associated with 20.2 grams greater LVM (95% CI 12.9, 27.5; p <0.001) and 1.5 times greater odds of LVH (95% CI 1.0, 2.3; p=0.03). However, these associations were no longer significant after adjustment for age, sex, race/ethnicity, BMI z-score, and further adjusted for wake SBPi and DBPi and ACEi/ARB/diuretic use.
TABLE 2.
Association of Serum Uric Acid with Left Ventricular Size at Baseline
| Univariate Linear Models | Adjusted Multivariable Linear Modelsa | Adjusted Multivariable Linear Modelsb | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Exposure | Outcome | β Coefficient | 95% CI | P | β Coefficient | 95% CI | P | β Coefficient | 95% CI | P |
| UA (mg/dL) | LVM (g) | 20.2 | 12.9, 27.5 | <0.001 | 5.0 | −2.6, 12.7 | 0.19 | 3.2 | −5.9, 12.3 | 0.48 |
| UA (mg/dL) | LVMI (g/m2.7) | 1.2 | −0.4, 2.8 | 0.14 | −0.1 | −2.3, 2.1 | 0.93 | −0.9 | −3.5, 1.7 | 0.49 |
| Elevated UAc | LVMI (g/m2.7) | 3.6 | −3.0, 10.1 | 0.28 | 1.2 | −6.2, 8.5 | 0.75 | −0.3 | −8.8, 8.2 | 0.94 |
| Univariate Logistic Models | Adjusted Multivariable Logistic Modelsa | Adjusted Multivariable Logistic Modelsb | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | ||
| UA (mg/dL) | LVH | 1.6 | 1.1, 2.4 | 0.02 | 1.3 | 0.7, 2.3 | 0.40 | 1.0 | 0.5, 2.1 | 0.89 |
| Elevated UAc | LVH | 5.0 | 1.1, 24.1 | 0.04 | 3.3 | 0.4, 25.3 | 0.25 | 2.2 | 0.2, 23.4 | 0.51 |
CI indicates confidence interval; UA, uric acid; LVM, left ventricular mass; LVMI, left ventricular mass index = LVM/ht(m2.7); OR, odds ratio; LVH, left ventricular hypertrophy = LVMI≥95th percentile
Data adjusted for age, sex, race/ethnicity, and body mass index z-score
Data adjusted as above plus wake systolic blood pressure index and wake diastolic blood pressure index (blood pressure index=wake BP/95th percentile wake BP or 140/90 for those ≥18 yrs; values ≥1 are elevated), and if taking ACEi/ARB/Diuretic at baseline
Elevated UA if ≥ age-sex-race specific 95th percentile values
Longitudinal results
At 12-month follow-up, mean UA was 5.7mg/dL (SD 1.6) and mean change from baseline was 0.2 mg/dL (SD 0.9; range −1.9, 2.3). Children <12 years old at baseline experienced a greater increase in UA (0.7 mg/dL, SD 0.8) compared to those ≥12 years old (−0.02 mg/dL, SD 0.8; p=0.02). The change in UA over time also differed by sex, although this difference was not statistically significant: mean increase in UA among males was 0.3 mg/dL (SD 0.1) and among females was 0.1 mg/dL (SD 0.6; p=0.5).
At 12-month follow-up, the prevalence of elevated UA, overweight/obesity and LVH was similar to baseline values; 20 % had elevated UA, 57 % were overweight/obese and 53 % had LVH (all p>0.05 when compared to baseline). Overall, BMI z-score increased by 0.1 (SD 0.3; p=0.02) from baseline. Individuals who were overweight/obese at baseline had a lesser change in BMI z-score compared to those who were of healthy weight at baseline (0.03 (SD 0.2) vs. 0.2 (SD 0.4); p=0.09). On average, the cohort had good BP control throughout, with uncontrolled hypertension only present among 38 % of participants at baseline and 35 % at follow-up. The majority (90 %) of children were prescribed an antihypertensive medication. Seven children were on an ARB both at baseline and at 12-month follow-up. In addition, 5 children were taking a diuretic at baseline, 6 at follow-up and only 2 of the children prescribed a diuretic were not also on an ACEi/ARB. Age and serum aldosterone at baseline were the only patient characteristics associated with UA over time (Table 3).
TABLE 3.
Association of Cardiovascular Disease Risk Factors with Change in Uric Acid Over Time
| Baseline Variables | Change in Variables over Time | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusteda | Unadjusted | Adjusteda | |||||||||
| Characteristic | Δ UA | 95% CI | P | Δ UA | 95% CI | P | Δ UA | 95% CI | P | Δ UA | 95% CI | P |
| Age, y | −0.1 | −0.2, 0.01 | 0.03 | −0.1 | −0.2, 0.01 | 0.03 | ||||||
| Male Sex | 0.2 | −0.6, 1.0 | 0.65 | 0.2 | −0.1, 0.9 | 0.55 | ||||||
| AA Race | 0.5 | −0.2, 1.2 | 0.12 | 0.6 | −0.1, 1.2 | 0.08 | ||||||
| BMI z-score | 0.01 | −0.4, 0.4 | 0.95 | −0.1 | −0.4, 0.3 | 0.66 | 0.3 | −0.7, 1.3 | 0.55 | 0.34 | −0.5, 1.3 | 0.41 |
| Wake SBP index | −0.3 | −4.3, 3.7 | 0.89 | −0.4 | −4.2, 3.3 | 0.81 | −0.1 | −4.2, 4.1 | 0.97 | −0.6 | −4.4, 3.1 | 0.74 |
| Wake DBP index | 0.6 | −2.8, 4.0 | 0.71 | 0.4 | −3.1, 3.8 | 0.83 | 0.5 | −4.0, 5.0 | 0.82 | −0.02 | −3.7, 3.6 | 0.99 |
| HbA1c, % | 0.3 | −0.4, 1.0 | 0.35 | 0.1 | −0.7, 0.9 | 0.76 | −0.03 | −0.7, 0.8 | 0.93 | −0.1 | −0.8, 0.5 | 0.70 |
| Non-HDL cholesterol | 0.01 | −0.003, 0.02 | 0.18 | 0.01 | −0.003, 0.01 | 0.20 | −0.002 | −0.01, 0.01 | 0.70 | −0.001 | −0.01, 0.01 | 0.81 |
| 24-hour urinary sodium excretion (g) | −0.001 | −0.0002, 0.0006 | 0.24 | −0.0004 | −0.0002, 0.0001 | 0.61 | 0.001 | −0.0001, 0.0001 | 0.27 | 0.00002 | −0.0001, 0.0001 | 0.70 |
| hsCRP | −0.04 | −0.3, 0.2 | 0.66 | −0.04 | −0.2, 0.2 | 0.71 | −0.03 | −0.2, 0.12 | 0.71 | −0.004 | −0.1, 0.1 | 0.96 |
| Plasma Renin Activity | −0.02 | −0.04, 0.01 | 0.18 | −0.01 | −0.03, 0.02 | 0.50 | −0.003 | −0.02, 0.01 | 0.75 | −0.004 | −0.02, 0.01 | 0.63 |
| Serum aldosterone | −0.02 | −0.1, 0.001 | 0.05 | −0.02 | −0.04, 0.003 | 0.09 | 0.02 | −0.002, 0.04 | 0.09 | 0.02 | −0.01, 0.04 | 0.13 |
| Aldosterone-Renin Ratio | −0.04 | −0.2, 0.1 | 0.38 | −0.03 | −0.1, 0.1 | 0.43 | 0.04 | −0.04, 0.1 | 0.38 | 0.03 | −0.04, 0.10 | 0.37 |
| LVMI (g/m2.7) | 0.003 | −0.03, 0.03 | 0.87 | −0.0001 | −0.03, 0.3 | 0.99 | −0.02 | −0.1, 0.02 | 0.35 | −0.003 | −0.04, 0.04 | 0.89 |
UA indicates uric acid; AA indicates African American; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HbA1c, hemoglobin A1c; HDL; high density lipoprotein; hsCRP; high sensitivity C-reactive protein; LVMI, left ventricular mass andex = LVM/ht(m2.7)
Adjusted for age, sex, and race/ethnicity
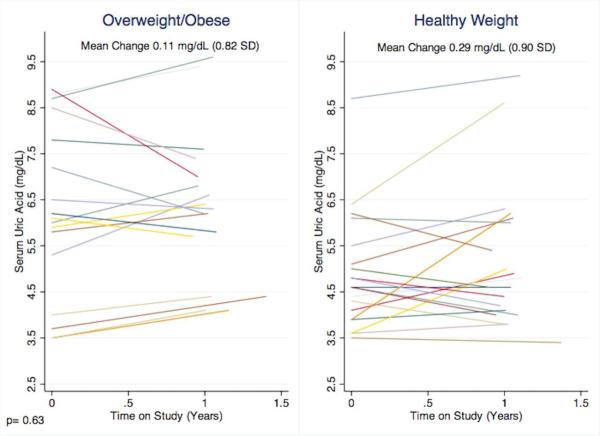
Given the association of markers of adiposity with elevated UA at baseline, we conducted stratified analyses based on characterization as overweight/obese vs. healthy weight at baseline. Compared to participants of healthy weight, overweight/obese participants at baseline had a higher serum UA (6.2 mg/dL vs. 4.9 mg/dL, p=0.01) but no difference in change over time (Figure 1). Further, increasing BMI z-score was not associated with change in UA: participants with an increase in BMI z-score over time demonstrated a serum UA increase of 0.2 mg/dL and those with a stable/decreasing BMI z-score over time demonstrated a serum UA increase of 0.3 mg/dL (p=0.8).
Figure 1.
Individual participant's change in uric acid stratified by weight category at baseline.
In unadjusted analyses, baseline UA was positively associated with LVMI over time; each 1 mg/dL greater baseline UA was associated with a 2.5 g/m2.7 greater LVMI at follow-up (95% CI 0.6, 4.4; p=0.01). However, after adjustment this association was no longer significant. Analyses with LVH as the outcome revealed similar results (Table 3).
Exploratory secondary analyses revealed no longitudinal relationship between UA and LVMI or LVH when UA was divided into tertiles; dichotomized into ≥ or <sex-specific observed mean; or when dichotomized as ≥ or <5.5 mg/dL. In addition, stratified analyses separating children into different age groups (≥12 vs. < 12 years; ≥8 vs. <8 years) revealed similar non-significant results.
Discussion
In this 1-year observational study of hypertensive children, we found that a quarter of children had an elevated UA and that over 25 % had other comorbid CVD risk factors, most of which were related to overweight/obesity. These more traditional CVD risk factors - adiposity, low HDL, hsCRP, LVM and LVH - were significantly associated with elevated UA, as were older age and variables related to the renin-angiotensin-aldosterone system. Of these, adiposity had the strongest relationship with UA. In fact, overweight/obesity was the most prevalent CVD risk factor in our cohort, with a striking 51 % of children being overweight/obese at baseline and 57 % overweight/obese at 1 year follow-up, despite standard of care guidance regarding weight loss and lifestyle modifications. Not only did the overweight/obese children have a higher UA overall, practically all (90 %) of those with an elevated UA were overweight/obese, a remarkable difference from the group with normal UA.
Interestingly, BP was not associated with UA in either the cross-sectional or prospective analyses. Given prior research in the field [11, 22, 24], we expected to find an independent relationship between BP and UA among the children in our study. The postulated biological mechanisms underlying this relationship have been related to UA inducing oxidative stress, activating inflammatory pathways, increasing vascular smooth muscle proliferation and endothelial dysfunction, and stimulating the renin-angiotensin-aldosterone system [25].
The lack of an association between UA and BP in our cohort may be related to the fact that all enrolled children were being treated for hypertension; there were no untreated normotensive children included. It may be that once BP is already elevated above normal, UA may have a lesser role in further elevating BP. In fact, our previous study that demonstrated increasing levels of UA are associated with elevated BP [26] included healthy US adolescents with a wide range of BPs (normal and elevated), not just elevated BP as in this cohort of children. Another possibility for the lack of an observed association is our comparatively small sample size. More studies are required to further investigate this relationship.
Despite the significant cross-sectional baseline associations, multivariable analyses revealed that serum UA was not independently associated with CVD risk factors over time, nor was it associated with LVM or LVH. There are several potential explanations for these findings. It may be that, as suggested above, among treated hypertensive children UA has a lesser role in the development of target organ damage. It may also be that the cardiovascular system only exhibits notable changes after a longer exposure to elevated UA. Multiple prior studies suggesting UA's effect in adults coursed over several years [27], whereas we only looked at the relationship between UA, LVMI and CVD risk factors over one year. In fact, we noted very little change in the measured CVD risk factors over the 1-year observation period. Specifically, UA values remained similar over time as did BMI z-scores and BP. If time exposed is important in pathogenesis, then a longer period of observation in childhood, or following youth into adulthood may be necessary to discern the pathological effects of UA on CVD risk factors and end-organs. Another possibility is related to small sample size; studying more children would increase statistical power to detect an association if present. Finally, it may be that UA is merely a marker of overweight/obesity, and not an independent CVD risk factor. As such, controlling for overweight/obesity in a multivariable analysis would necessarily diminish any observed univariate relationship.
One finding that should be highlighted is the high dietary sodium intake among all children in the cohort, and the greater intake found among those with elevated UA. The overall mean sodium intake, as ascertained by 24-hour urinary excretion of sodium that estimates intake the day prior, was 3.5 grams/day. This intake is similar to the mean daily intake reported from National Health and Nutrition Examination Survey data in 2012, where the average sodium intake reported by 8-18 year olds was 3.4 g/day [28]. The children in our study with elevated UA had 1.4 gram/day greater sodium excretion than those with normal UA levels. While not statistically significant, we feel this deserves mention as it adds to the literature regarding the impact of dietary sodium on CVD risk among children. Further, it is consistent with the adult literature which has shown higher sodium intake to be independently associated with larger increases in serum uric acid over time [29].
Our study has several important limitations. First, our cohort size is small and from a tertiary care sub-specialty clinic, thus may not be a general representation of all hypertensive children. Second, our 1-year observational period may be too short to detect significant associations. Furthermore, we were unable to get fasting insulin samples, and therefore could not fully explore the relationship between UA and LVMI. Finally, as an observational study, it is possible that other unmeasured exposures and risk factors may have influenced our findings and could explain some of our results.
Notwithstanding these limitations, this study benefits from the prospective analysis of change in UA over time. To our knowledge, this study is the longest follow-up in children and adolescents looking at the relationship of UA and CVD risk factors over time. In addition, the study population provided data from a racially diverse group of hypertensive children with standardized anthropometric, BP and echocardiography measurements. We were able to follow a population of hypertensive individuals who were largely free of the multiple CVD co-morbidities found in adults. This allows for a less confounded investigation into the association of UA and CVD risk factors.
In summary, CVD risk factors such as overweight/obesity, hypertension and left ventricular hypertrophy are highly prevalent among children and adolescents. UA, a putative CVD risk factor in adults, is associated with a higher prevalence of obesity-related CVD risk factors among hypertensive children. Uric acid may have a role in CVD risk-assessment. Future studies with longer length of follow-up are needed to determine if uric acid is merely a marker of adiposity or an independent CVD risk factor.
Acknowledgements
This study is supported by grants from by the American Society of Nephrology, National Kidney Foundation of MD, American Heart Association, Thomas Wilson Sanitarium for Children of Baltimore City, the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number K23HL119622. It was also made possible by Grant Number UL1 RR 025005 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the NCRR or NIH.
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
References
- 1. [July 22, 2014];Cardiovascular diseases (CVDs) Fact Sheet No. 317. 2013 (2014, at http://www.who.int/mediacentre/factsheets/fs317/en/)
- 2.Weintraub WS, Daniels SR, Burke LE, Franklin BA, Goff DC, Jr, Hayman LL, Lloyd-Jones D, Pandey DK, Sanchez EJ, Schram AP, Whitsel LP, American Heart Association Advocacy Coordinating Committee. Council on Cardiovascular Disease in the Young. Council on the Kidney in Cardiovascular Disease. Council on Epidemiology and Prevention. Council on Cardiovascular Nursing. Council on Arteriosclerosis. Thrombosis and Vascular Biology. Council on Clinical Cardiology, and Stroke Council Value of primordial and primary prevention for cardiovascular disease: a policy statement from the American Heart Association. Circulation. 2011;124:967–990. doi: 10.1161/CIR.0b013e3182285a81. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen S, Choi HK, Lustig RH, Hsu CY. Sugar-sweetened beverages, serum uric acid, and blood pressure in adolescents. J Pediatr. 2009;154:807–813. doi: 10.1016/j.jpeds.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fang J, Alderman MH. Serum uric acid and cardiovascular mortality the NHANES I epidemiologic follow-up study, 1971-1992. National Health and Nutrition Examination Survey. JAMA. 2000;283:2404–2410. doi: 10.1001/jama.283.18.2404. [DOI] [PubMed] [Google Scholar]
- 5.Niskanen LK, Laaksonen DE, Nyyssönen K, Alfthan G, Lakka HM, Lakka TA, Salonen JT. Uric acid level as a risk factor for cardiovascular and all-cause mortality in middle-aged men: a prospective cohort study. Arch Intern Med. 2004;164:1546–1551. doi: 10.1001/archinte.164.14.1546. [DOI] [PubMed] [Google Scholar]
- 6.Iwashima Y, Horio T, Kamide K, Rakugi H, Ogihara T, Kawano Y. Uric acid, left ventricular mass index, and risk of cardiovascular disease in essential hypertension. Hypertension. 2006;47:195–202. doi: 10.1161/01.HYP.0000200033.14574.14. [DOI] [PubMed] [Google Scholar]
- 7.Kanbay M, Segal M, Afsar B, Kang DH, Rodriguez-Iturbe B, Johnson RJ. The role of uric acid in the pathogenesis of human cardiovascular disease. Heart. 2013;99:759–766. doi: 10.1136/heartjnl-2012-302535. [DOI] [PubMed] [Google Scholar]
- 8.Hill JA, Olson EN. Cardiac plasticity. N Engl J Med. 2008;358:1370–1380. doi: 10.1056/NEJMra072139. [DOI] [PubMed] [Google Scholar]
- 9.Mitsuhashi H, Yatsuya H, Matsushita K, Zhang H, Otsuka R, Muramatsu T, Takefuji S, Hotta Y, Kondo T, Murohara T, Toyoshima H, Tamakoshi K. Uric acid and left ventricular hypertrophy in japanese men. Circ J. 2009;73:667–672. doi: 10.1253/circj.cj-08-0626. [DOI] [PubMed] [Google Scholar]
- 10.Krishnan E, Hariri A, Dabbous O, Pandya BJ. Hyperuricemia and the echocardiographic measures of myocardial dysfunction. Congest Heart Fail. 2012;18:138–143. doi: 10.1111/j.1751-7133.2011.00259.x. [DOI] [PubMed] [Google Scholar]
- 11.Feig DI, Soletsky B, Johnson RJ. Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension: a randomized trial. JAMA. 2008;300:924–932. doi: 10.1001/jama.300.8.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Disease Control Clinical Growth Charts [March 15, 2010];2000 (at http://www.cdc.gov/growthcharts/clinical_charts.htm)
- 13.Centers for Disease Control and Prevention Growth Charts and Z-score Data Files [September 10, 2012]; (at http://www.cdc.gov/growthcharts/zscore.htm)
- 14.Centers for Disease Control and Prevention Body Mass Index-for-Age Percentiles [September 29, 2012];2 to 20 years: Girls. (at http://www.cdc.gov/growthcharts/data/set2clinical/cj41c074.pdf)
- 15.Fernandez JR, Redden DT, Pietrobelli A, Allison DB. Waist circumference percentiles in nationally representative samples of African-American, European-American, and Mexican-American children and adolescents. J Pediatr. 2004;145:439–444. doi: 10.1016/j.jpeds.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 16.Urbina E, Alpert B, Flynn J, Hayman L, Harshfield GA, Jacobson M, Mahoney L, McCrindle B, Mietus-Snyder M, Steinberger J, Daniels S, American Heart Association Atherosclerosis, Hypertension, and Obesity in Youth Committee Ambulatory blood pressure monitoring in children and adolescents: recommendations for standard assessment: a scientific statement from the American Heart Association Atherosclerosis, Hypertension, and Obesity in Youth Committee of the council on cardiovascular disease in the young and the council for high blood pressure research. Hypertension. 2008;52:433–451. doi: 10.1161/HYPERTENSIONAHA.108.190329. [DOI] [PubMed] [Google Scholar]
- 17.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(2 Suppl 4th Report):555–576. [PubMed] [Google Scholar]
- 18.Tschudy MM, Arcara KM, Johns Hopkins Hospital. Children's Medical and Surgical Center., ScienceDirect (Online service) The Harriet Lane handbook a manual for pediatric house officers. 19th ed. Mosby Elsevier; Philadelphia, PA: 2012. p. xvi.p. 1132. [10] p. of plates. [Google Scholar]
- 19.Shatat IF, Abdallah RT, Sas DJ, Hailpern SM. Serum uric acid in U.S. adolescents: distribution and relationship to demographic characteristics and cardiovascular risk factors. Pediatr Res. 2012;72:95–100. doi: 10.1038/pr.2012.47. [DOI] [PubMed] [Google Scholar]
- 20.Devereux RB, Alonso DR, Lutas EM, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–458. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 21.Khoury PR, Mitsnefes M, Daniels SR, Kimball TR. Age-specific reference intervals for indexed left ventricular mass in children. J Am Soc Echocardiogr. 2009;22:709–714. doi: 10.1016/j.echo.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 22.Feig DI, Johnson RJ. Hyperuricemia in childhood primary hypertension. Hypertension. 2003;42:247–252. doi: 10.1161/01.HYP.0000085858.66548.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greenland S, Finkle WD. A critical look at methods for handling missing covariates in epidemiologic regression analyses. Am J Epidemiol. 1995;142:1255–1264. doi: 10.1093/oxfordjournals.aje.a117592. [DOI] [PubMed] [Google Scholar]
- 24.Grayson PC, Kim SY, LaValley M, Choi HK. Hyperuricemia and incident hypertension: a systematic review and meta-analysis. Arthritis Care Res (Hoboken) 2011;63:102–110. doi: 10.1002/acr.20344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feig DI. The role of uric acid in the pathogenesis of hypertension in the young. J Clin Hypertens (Greenwich) 2012;14:346–352. doi: 10.1111/j.1751-7176.2012.00662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loeffler LF, Navas-Acien A, Brady TM, Miller ER, 3rd, Fadrowski JJ. Uric acid level and elevated blood pressure in US adolescents: National Health and Nutrition Examination Survey, 1999-2006. Hypertension. 2012;59:811–817. doi: 10.1161/HYPERTENSIONAHA.111.183244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. N Engl J Med. 2008;359:1811–1821. doi: 10.1056/NEJMra0800885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang Q, Zhang Z, Kuklina EV, Fang J, Ayala C, Hong Y, Loustalot F, Dai S, Gunn JP, Tian N, Cogswell ME, Merritt R. Sodium intake and blood pressure among US children and adolescents. Pediatrics. 2012;130:611–619. doi: 10.1542/peds.2011-3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Forman JP, Scheven L, de Jong PE, Bakker SJ, Curhan GC, Gansevoort RT. Association between sodium intake and change in uric acid, urine albumin excretion, and the risk of developing hypertension. Circulation. 2012;125:3108–3116. doi: 10.1161/CIRCULATIONAHA.112.096115. [DOI] [PMC free article] [PubMed] [Google Scholar]