Abstract
Background
Studies suggest that a single prophylactic dose of amoxicillin reduces early implant complications, but it is unclear whether other antibiotics are also effective. This study compared the local antimicrobial and anti-inflammatory effects resulting from a single dose of azithromycin or amoxicillin prior to surgical placement of one-stage dental implants.
Methods
Healthy adult patients requiring one-stage dental implant placement were randomly allocated to receive either 2g amoxicillin (n=7) or 500mg azithromycin (n=6) prior to surgery. Peri-implant crevicular fluid (PICF) samples from the new implant and gingival crevicular fluid (GCF) from adjacent teeth were sampled on postoperative days 6, 13 and 20. Inflammatory mediators in the samples were analyzed by immunoassay and antibiotic levels were measured by bioassay.
Results
On day 6, azithromycin concentrations in GCF and PICF were 3.39±0.73μg/ml and 2.77±0.90μg/ml, respectively, while amoxicillin was below the limit of detection. During early healing, patents in the azithromycin group exhibited a significantly greater decrease in GCF volume (p=0.03, ANOVA). At specific times during healing, the azithromycin group exhibited significantly lower levels of IL-6 and IL-8 in GCF than the amoxicillin group and exhibited significantly lower levels of G-CSF, IL-8, MIP-1β and IP-10 in PICF.
Conclusions
Azithromycin was available at the surgical site for a longer period of time than amoxicillin, and patients taking azithromycin exhibited lower levels of specific pro-inflammatory cytokines and chemokines in GCF and PICF. Thus, preoperative azithromycin may enhance resolution of postoperative inflammation to a greater extent than amoxicillin.
Keywords: Inflammation, wound healing, antibiotic prophylaxis, cytokines, chemokines
Introduction
Dental implants can undergo early failure when there are complications in wound healing after placement. In some instances, excessive inflammation leads to formation of scar tissue or inhibition of bone apposition around the implant.1 Early failures have not been consistently included in analyses of implant loss, but they appear to occur more frequently than late failures2,3 and are most likely to affect patients with diabetes, a history of smoking, or poor bone conditions.4, 5 There is evidence that prophylactic antibiotics can help control localized infections and facilitate a more predictable outcome. A large-scale prospective study found a significantly higher implant survival rate in patients who had taken preoperative antibiotics.6 Although some studies found no benefit from antibiotic prophylaxis,7 evidence-based reviews have concluded that a single preoperative dose of antibiotic reduces implant failure.4, 8, 9 The regimen used most commonly is 2 grams of amoxicillin (AMX) administered one hour before implant placement.8 For patients who are allergic to penicillins, azithromycin (AZM, 500mg) is recommended as a prophylactic regimen for dental procedures.10 In contrast to AMX, AZM concentrations in gingival crevicular fluid (GCF) are typically 40 times higher than in serum and are sustained for up to two weeks.11 AZM and other macrolides produce potentially useful immunomodulatory effects. Macrolides inhibit pro-inflammatory cytokine production when used to treat chronic inflammatory airway disease.12 The mechanisms by which this occurs are not fully understood, but macrolides inhibit the activation of nuclear factor- kappa B (NF-KB) and activating protein-1 (AP-1), which regulate the expression of IL-6, TNF-α, IL-1β and other pro-inflammatory cytokines.13-15 Macrolides may also inhibit mitogen-activated protein kinase and extracellular-regulated kinase, resulting in decreased IL-8 production.16 In the gingiva, related anti-inflammatory effects have been observed in patients with minimal bacterial plaque and clinically healthy gingiva. AZM triggers a significant reduction in the rate of GCF flow in parallel with reductions the amounts of IL-1β, IL-8, TNF-α, and VEGF in GCF.17
The purpose of this study was to assess the resolution of postoperative peri-implant inflammation during early healing in the presence of two different systemic antibiotic regimens, and compare it to the resolution of inflammation at adjacent periodontal sites. Based on previous observations,11, 17 we hypothesized that a single dose of AZM prior to implant placement would produce more profound inhibition of inflammatory mediator production and yield more sustained antimicrobial activity in PICF during the postoperative course.
Materials and Methods
Power analysis and study population
Previous work with AZM suggested that the current study should be powered to detect a 0.25μl difference in GCF volume, a 55pg difference in GCF IL-8 content and an 80pg difference in GCF TNF-α content on postoperative day 6.17 While this previous study did not provide any guidance relative to PICF, an assumption was made that PICF would yield findings that were similar to GCF. Based on these assumptions, an estimated total sample size of twelve patients was required to achieve a power of 0.8. The sample size was increased to accommodate an expected attrition rate of 25%. Forty patients were assessed for eligibility. Ultimately, sixteen healthy patients who elected a treatment plan that included one-stage placement of a dental implant in the Ohio State University Graduate Periodontology Clinic were enrolled between July, 2012 and April, 2013. The patients were non-smokers aged 21 or older and had two natural teeth adjacent to the site where the implant was to be placed. They had no history of a drug allergy or systemic disease, were not pregnant, and had not taken any other drugs in the month prior to the study. Written informed consent was obtained under a protocol approved by the Ohio State University Biomedical Sciences IRB.
Study design
Patients were randomly divided into control (AMX) and experimental (AZM) groups according to the order in which they were enrolled. A research pharmacist compounded sequentially numbered drug regimens of similar appearance containing either a single 2g dose of AMX or a 500mg dose of AZM. Within each block of four patients, an equal number of patients was allocated to the two groups. The coding of each package remained sealed until completion of data collection.
One hour prior to surgery, baseline clinical assessments of Gingival Index (GI18), Plaque Index (PI19) and GCF volume were carried out for the teeth adjacent to the site where the implant was to be placed. Baseline GCF samples were collected from tooth surfaces adjacent to the implant site with filter paper strips‡ as previously described.20 Briefly, the teeth were isolated with cotton rolls, supragingival plaque (if present) was removed, and the sites were gently dried with air. GCF was collected for 30 s with filter paper strips positioned at the orifice of the crevice and measured with a calibrated measurement device§. To assure collection of an adequate sample volume, two strips were collected from each of the four adjacent surfaces. There was a delay of at least 10 min between consecutive samples to permit the crevice to refill with fluid. The eight strips were pooled and frozen for later analysis. Patients then took their assigned dose of antibiotic.
A one-stage implant surgical protocol, which involves placing the implant and the healing abutment in a single procedure, was utilized. The main determinant of whether an implant could be placed in one stage was availability of sufficient alveolar bone to provide implant stability. Stability was assessed by resonance frequency analysis deviceκ. Consistent with a previous study,21 one-stage surgery was deemed to be inappropriate if the Implant Stability Quotient of the newly-placed implant was <50. Implants were placed at bone level, and a healing abutment of standardized height was utilized. The surgical site was closed using expanded PTFE sutures. Patients were asked to use an antimicrobial rinse containing 0.12% chlorhexidine twice daily and to avoid using non-steroidal anti-inflammatory analgesics. They were provided with acetaminophen tablets (500mg) and instructed to take one tablet every 6 h as needed for pain. Patients returned for follow-up on days 6, 13 and 20. Modified PI (mPI22) was assessed at each implant site and PI was assessed at adjacent natural tooth sites. Four surfaces of each implant site (distobuccal, distolingual, mesiobuccal, and mesiolingual) and two sites of each adjacent natural tooth were evaluated. GCF or PICF samples were collected from the same four surfaces at the respective sites. Again, two strips were collected from each site. Thus, a total of eight strips from the implant and eight strips from adjacent natural tooth sites were collected at each follow-up visit and pooled separately. In addition, a 3 ml sample of peripheral blood was obtained on day 6. Samples of GCF, PICF and serum were stored at -20° C. At the time of analysis, GCF and PICF were eluted from each pool of paper strips with a 200μL volume of phosphate buffered saline as previously described.20
Measurement of biological mediators in PICF and GCF
Each pooled sample was subjected to analysis by a multiplex bead-based immunoassay for twenty human cytokines, chemokines and growth factors¶ according to the manufacturer's directions. The content of these mediators was expressed as the total amount (in picograms) recovered from each pool of 30s GCF or PICF samples, which each consisted of eight filter paper strips. Although this is a common approach for reporting biomarkers in crevice fluid,23 the concentration (in ng/ml) of selected biomarkers was also calculated.
Measurement of AMX and AZM content in PICF and GCF
Approximately 75% of the eluted PICF and GCF sample volume was processed for measurement of AZM or AMX content. In addition, AZM and AMX were assayed in peripheral blood samples from day 6. GCF and PICF eluates and blood serum samples were treated with 40μl of 0.5g/ml Na2CO3 and extracted three times with 1 ml of diethyl ether. The extracts were dried under streaming nitrogen, reconstituted in acetonitrile, and applied to sterile paper disks#. After evaporation of the acetonitrile, the AZM or AMX content of the disks was determined with an agar diffusion bioassay, using Kocuria rhizophila** as the indicator organism.11 The assay was calibrated over the range of 2 to 12ng with authentic AZM and AMX standards††.
Statistical analysis
In the tables and figure legends, the methods of statistical analysis are described in the footnotes.
Results
Study population
In the AMX group, one patient dropped out due to failure to comply with the appointment schedule. In the AZM group, two patients were excluded from analysis. One patient sustained physical trauma to the surgical site from an accident two days after implant placement. Another patient exhibited unusually poor postoperative healing and, upon testing, was found to have severe undiagnosed type 2 diabetes. The characteristics of the thirteen patients who completed the study are presented in Table 1. There were no significant intergroup differences with respect to mean age, gender composition or median GI scores at natural tooth surfaces adjacent to the implant placement sites. Patients in both groups started the study with a thin film of plaque at the gingival margin.
Table 1. Description of Patient Groups.
| Characteristic | AMX Group | AZM Group |
|---|---|---|
| Number of Patients | 7 | 6 |
| Age* | 51.0 ± 5.7 | 62.0 ± 4.1 |
| Gender (M/F)† | 3/4 | 4/2 |
| Implant Location (mand/max)† | 5/2 | 4/2 |
| Implant placement | Bone level (7) | Bone level (6) |
| Acetaminophen tablets taken* | 1.5 ± 0.85 | 1.8 ± 1.1 |
| GI, day 0‡ | 0.00 (0.00, 0.00) | 1.25 (0.00, 1.50) |
| PI, day 0‡ | 1.00 (1.00, 1.375) | 1.00 (0.00, 1.00) |
| PI, day 20‡ | 0.00 (0.00, 0.875) | 0.75 (0.00. 1.00) |
| mPI, day 6‡ | 0.00 (0.00, 0.375) | 0.00 (0.00, 0.00) |
| mPI, day 20‡ | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) |
| Implant failures (24 months) | 0/7 | 0/6 |
Presented as mean ± SEM. Difference between groups is not statistically significant (P>0.05, t-test)
Proportions not significantly different from that expected from random occurrence (P>0.05, Fisher Exact test)
Presented as median (25%, 75%). Difference between groups is not statistically significant (P>0.05, Mann-Whitney rank sum test)
Changes in plaque at the surgical sites
At periodontal sites adjacent to the implant, both groups exhibited an initial decrease in PI on day 6 after implant placement, and PI remained below baseline levels on days 13 and 20 (not shown). At implant sites, neither group exhibited a significant change in their modified PI scores over the course of the study. Both groups started and ended the study with relatively low levels of plaque.
Antibiotic concentrations in serum, GCF and PICF
The mean AZM concentrations in samples of serum, GCF and PICF obtained on day 6 were 0.015±0.002μg/ml, 3.39±0.73μg/ml and 2.77±0.90μg/ml, respectively. Concentrations in GCF and PICF were significantly higher than in serum, but not significantly different from each other (P<0.05, Holm-Sidak test). On day 13, the mean AZM concentrations in GCF and PICF were 2.24±0.70μg/ml and 2.03±0.38μg/ml, respectively. On day 20, AZM, was not consistently detected in GCF or PICF. In the AMX group, serum antibiotic concentrations were below the limit of detection on day 6. Since AMX levels are often lower in GCF than in serum,24 antibiotic levels were assumed to be below the limit of detection in GCF and PICF.
GCF and PICF volumes
In both groups, GCF volume at sites adjacent to the implant increased on day 6 and decreased thereafter (Fig 1A). Baseline GCF volume was higher in the AZM group. On days 6, 13 and 20, however, the GCF volumes of the AZM group were consistently lower than those of the AMX group. To examine these differences more closely and facilitate comparisons, the changes from baseline were analyzed (Fig 1A inset). ANOVA revealed a statistically significant difference between the two groups (P=0.03).
Figure 1.
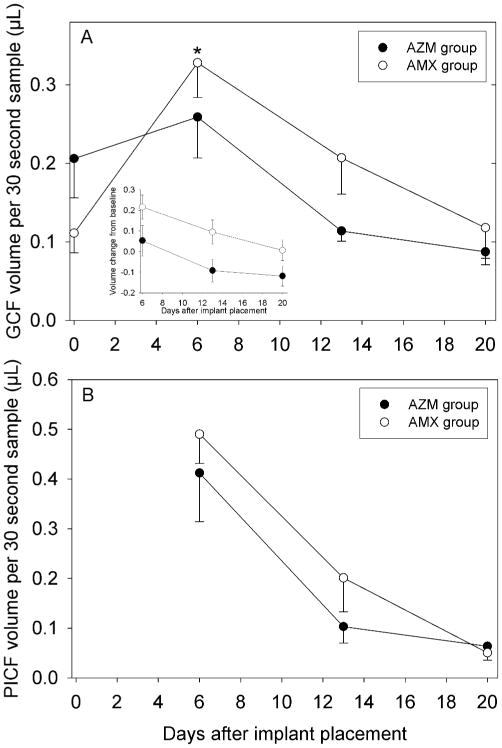
Effect of a preoperative dose of AZM or AMX on crevicular fluid volumes during early healing after implant placement. Data are presented as mean and SEM. Panel A: Changes in GCF volume at tooth surfaces adjacent to implant placement sites. Both groups exhibited significant changes in volume over time (P<0.05, repeated measures ANOVA). In the inset, the data were analyzed as change from baseline to adjust for differences in baseline values. A significant group effect was observed (P=0.03, ANOVA). Panel B: Changes in PICF volume at implant placement sites. Both groups exhibited significant changes in volume over time (P<0.05, repeated measures ANOVA).
Both groups exhibited a statistically significant decrease in PICF volume after day 6 (Fig 1B). The PICF sample volumes from the AZM group were somewhat lower than those of the AMX group on days 6 and 13, but their volumes converged on day 20. The differences observed on days 6 and 13 were not statistically significant (P>0.05, t-test).
Analysis of mediators in GCF
Table 2 presents a summary of changes from baseline inflammatory mediator levels over the course of the study, and detailed longitudinal data are presented for IL-6 and IL-8. Baseline IL-6 content in GCF was similar in both groups (Fig 2A). IL-6 increased sharply in both groups on day 6, and then decreased to similar levels by day 20. GCF IL-6 content was significantly higher in the AMX group on day 6 (P=0.002, t-test). A similar pattern was observed when the data were expressed as concentration (Table 3). There was little difference in IL-6 concentration between groups at baseline or on day 20, and only the AMX group exhibited a significant increase in IL-6 on day 6.
Table 2. Summary of Changes in Inflammatory Mediator Content in GCF (baseline to day 20) and PICF (day 6 to day 20)*.
| Mediator (category†) | GCF (with AZM pre-med) | GCF (with AMX pre-med) | PICF (with AZM pre-med) | PICF (with AMX pre-med) |
|---|---|---|---|---|
| IL-1β (PI) | NS | NS | NS | Decreased, days 13 & 20‡ |
| IL-6 (PI) | Increased, day 6‡ | Increased, day 6‡ | Decreased, day 20‡ | Decreased, days 13 & 20‡ |
| IL-12 (p70) (PI) | NS | Increased, days 6 & 20‡ | NS | Increased on day 20‡ |
| IL-17 (PI) | ND | ND | ND | ND |
| TNF-α (PI) | Increased on days 6 & 13‡ | NS | Decreased on day 20‡ | Decreased on day 20‡ |
| G-CSF/CSF3 (PI) | Decreased, days 6 to 20‡ | NS | Decreased, days 13 & 20‡ | NS |
| GM-CSF/CSF2 (PI) | ND | ND | ND | ND |
| IL-8/CXCL8 (C) | NS | Increased, day 6‡ | NS§ | Decreased, days 13 & 20‡ |
| MIP-1α/CCL3 (C) | NS | Increased, day 6‡ | Decreased, day 20‡ | Decreased, days 13 & 20‡ |
| MIP-1β/CCL4 (C) | Increased, day 6‡ | Increased, day 6‡ | Decreased, days 13 & 20‡ | Decreased, days 13 & 20‡ |
| RANTES/CCL5 (C) | NS | Increased, days 6 to 20‡ | NS | Decreased, day 20‡ |
| Eotaxin/CCL11 (C) | NS | NS | NS | Decreased, days 13 & 20‡ |
| IP-10/CXCL10 (C) | NS | NS | NS§ | NS |
| PDGF-BB (GF) | Decreased, days 13 & 20‡ | NS | NS | Decreased, days 13 & 20‡ |
| VEGF (GF) | NS | NS# | NS | Increased, day 20‡ |
| FGF (GF) | ND | ND | ND | ND |
| IL-10 (AI) | NS | Increased, day 6‡ | NS | NS |
| IL-1ra (AI) | NS | NS | NS | Increased, day 20‡ |
| IFN-γ (T1) | ND | ND | ND | ND |
| IL-4 (T2) | NS | NS | NS | Decreased, days 13 & 20‡ |
Abbreviations: NS, no significant changes; ND, not consistently detected
Content units: pg/30 second sample
Indicates primary effect: PI, pro-inflammatory; C, chemokine; GF, growth factor; AI, anti-inflammatory; T1, TH1; T2, TH2 cytokine
P<0.05, repeated measures ANOVA; P<0.05 in Holm-Sidak post-hoc test
P<0.05, repeated measures ANOVA, but no significant changes by Holm-Sidak post-hoc test
Figure 2.
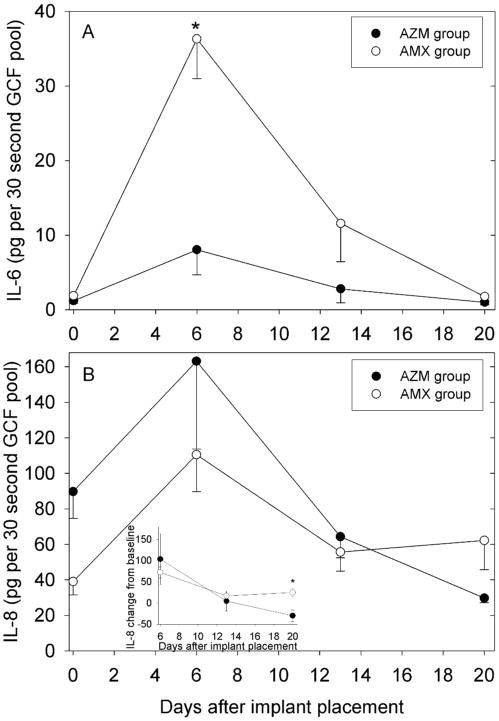
Effect of a preoperative dose of AZM or AMX on changes in inflammatory mediator content of GCF from adjacent teeth during early healing after implant placement. Data are presented as mean and SEM values recovered from a pool of eight samples per patient. Panel A: IL-6 content of pooled GCF samples. The asterisk denotes a statistically significant differences between groups (P= 0.002, ANOVA, adjusted by Tukey-Kramer). Panel B: Changes in the IL-8 content of pooled GCF. In the inset, the data were analyzed as change from baseline to adjust for differences in baseline values. The asterisk denotes a significant difference between groups (P=0.03, ANOVA, adjusted by Tukey-Kramer).
Table 3. Effect of a single preoperative dose of AZM or AMX on inflammatory mediator concentrations in GCF and PICF during healing after implant placement*.
| Fluid | Mediator | AMX grp day 0 | AMX grp day 6 | AMX grp day 13 | AMX grp day 20 | AZM grp day 0 | AZM grp day 6 | AZM grp day 13 | AZM grp day 20 |
|---|---|---|---|---|---|---|---|---|---|
| GCF | IL-6 | 98.2 ± 37.2 | 589 ± 115† | 264 ± 89.5 | 88.4 ± 21.5 | 26.7 ± 7.9 | 112 ± 69.4 | 142 ± 97.2 | 71.2 ± 31.3 |
| GCF | IL-8 | 1645 ± 378 | 1905 ± 342 | 1882 ± 458 | 3294 ± 736 | 1616 ± 317 | 2309 ± 340 | 2813 ± 391 | 2068 ± 475 |
| PICF | G-CSF | N/A | 92.0 ± 23.8 | 267 ± 121 | 348 ± 47.6 | N/A | 170 ± 53.9 | 212 ± 55.5 | 268 ± 60.1 |
| PICF | IL-8 | N/A | 6561 ± 1856 | 5263 ± 2457 | 12162 ± 5496‡ | N/A | 5509 ± 1527 | 2959 ± 1194 | 2104 ± 603‡ |
| PICF | MIP-1β | N/A | 542 ± 111 | 970 ± 324 | 1438 ± 443‡ | N/A | 437 ± 132 | 397 ± 88.9 | 334 ± 149‡ |
| PICF | IP-10 | N/A | 1312 ± 846 | 1362 ± 648 | 2187 ± 1326‡ | N/A | 263 ± 99.0 | 262 ± 83.1 | 221 ± 55.9‡ |
Concentration units: ng/ml
denotes a statistically significant difference from day 0 (P<0.05, repeated measures ANOVA; P<0.05, Holm-Sidak post-hoc test)
denotes statistically significant differences between groups (P ≤ 0.05, Mann-Whitney rank sum test)
The baseline IL-8 content of GCF was somewhat higher in the AZM group (Fig 2B). In both groups, there was an increase in IL-8 content on day 6, followed a decrease to similar levels on day 13. On day 20, IL-8 content in the AZM group was below that of the AMX group and below its own baseline levels. To facilitate comparisons, the changes from baseline were analyzed (Fig 2B, inset). ANOVA revealed a statistically significant difference in between the two groups on day 20 (P=0.03). The results were somewhat different when the data were expressed as concentration (Table 3). Baseline IL-8 concentrations were similar in the two groups, but IL-8 was lower in the AZM group on day 20.
No statistically significant differences in the content of G-CSF, MIP-1β, IP-10, TNF-α or IL-1β were observed between the two groups at any time point (data not shown). However, a borderline significant decrease in TNF-α content in the AZM group relative the AMX group was observed on day 20 (P=0.104, t-test). No significant differences in the anti-inflammatory mediators IL-10 and IL-1 receptor antagonist were observed between the two groups (data not shown).
Analysis of mediators in PICF
Table 2 presents a summary of changes from day 6 to day 20, and detailed longitudinal data are presented for G-CSF, IL-8, MIP-1β and IP-10. The G-CSF content of PICF samples from the AMX group was essentially unchanged from day 6 to day 20 (Fig 3A). In contrast, the AZM group exhibited somewhat higher levels of G-CSF on day 6 and decreased to significantly lower levels than in the AMX group on days 13 and 20 (P<0.05, t-test). When expressed as concentration, G-CSF levels were also lower in the AZM group on days 13 and 20, but the differences were not statistically significant (Table 3).
Figure 3.
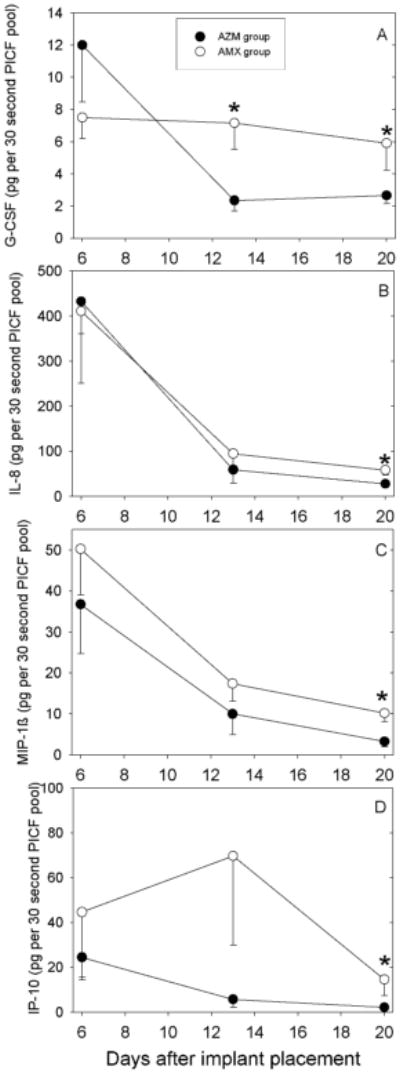
Effect of a single preoperative dose of AZM or AMX on changes in the content of G-CSF (Panel A), IL-8 (Panel B), MIP-1β (Panel C) and IP-10 (Panel D) in PICF during early healing after one-stage implant surgery. Data are presented as mean - SEM. Statistically significant differences between groups are denoted by asterisks (P<0.05, t-test).
The IL-8 content was similar in both groups on day 6 and decreased dramatically between day 6 and day 13 (Fig 3B). On day 20, the IL-8 content in PICF from the AZM group was significantly lower than in the AMX group. Similarly, IL-8 concentration on day 20 was significantly lower in the AZM group (Table 3). Regardless of whether the results were expressed as content or concentration, mean IL-8 levels in PICF were at least twice those found in GCF.
In both groups, the amount of MIP-1β in PICF decreased progressively from day 6 to day 20 (Fig 3C). The MIP-1β content in PICF samples from the AZM group was consistently lower than that of the AMX group, and this difference was statistically significant on day 20. Consistent with this finding, MIP-1β concentration was significantly lower in the AZM group on day 20 (Table 3). MIP-1β content was highly variable on day 6, but the mean amounts measured in PICF were roughly twice those found in GCF (not shown).
Patients in the AZM group exhibited a gradual decrease in IP-10 content from day 6 to day 20 (Fig 3D). In the AMX group, IP-10 levels increased from day 6 to day 13 and decreased thereafter. IP-10 content was significantly lower in the AZM group on day 20. When expressed as concentration, IP-10 was consistently lower in the AZM group. The difference between the two groups was statistically significant on day 20 (table 3). No statistically significant differences in the content of the pro-inflammatory cytokines IL-6, TNF-α and IL-1β were observed between the two groups at any time point (data not shown). The amount of IL-6 found in PICF was somewhat higher than in GCF on days 6 and 13, and the amount of TNF-α in PICF was more than twice that found in GCF on day 6 (data not shown). Lastly, no significant differences in the anti-inflammatory cytokines IL-10 and IL-1 receptor antagonist were observed between the two groups (data not shown).
Discussion
Comparison of AMX and AZM groups
With respect to mean age, gender distribution, and baseline plaque levels, the two groups were reasonably comparable. Despite the randomization of patients, the baseline median GI score and mean GCF flow rate at natural tooth sites adjacent to implant placement sites were somewhat higher in patients in the AZM group.
Bioavailability of AZM and AMX
AMX was not detected in the blood samples from day 6. This was not surprising, since pharmacokinetic studies have shown that oral AMX produces peak blood levels 1 to 2h after administration and exhibits a short half-life. Approximately 60% of an oral dose of AMX is excreted in the urine within 6 to 8h.25 Since AMX yields similar concentrations in blood serum and GCF,24 it is reasonable to assume that AMX levels in the much smaller volumes of GCF and PICF were well below the limit of detection. This is consistent with the report of Khoury et al.,26 in which GCF AMX levels were below detectable levels several hours after a single 2g pre-surgical dose of AMX.
In contrast, samples from the AZM group contained measurable amounts of antibiotic on days 6 and 13. The mean concentrations observed on day 6 in serum and GCF (0.015 and 3.39 μg/ml, respectively) were slightly lower than those reported in a study in which the patients received a total dose of 750 mg AZM.11 The observed AZM concentrations in PICF were slightly lower than in GCF, but were not significantly different. On day 13, the AZM concentrations in GCF and PICF (2.24 and 2.03 μg/ml, respectively) were similar and slightly lower than the concentrations observed on day 6. Variations in post-surgical healing may have contributed to the relatively high variability of AZM measurements in GCF and PICF. These findings confirm that AZM has a greater potential to influence postoperative healing around a new implant because it is eliminated less rapidly than AMX.
Antibiotic effects on plaque and clinical signs of inflammation
There is a strong positive correlation between histological signs of inflammation and GCF volume.27 Changes from the baseline rate of GCF flow after implant placement were of significantly lower magnitude in the AZM group. In contrast, the AMX group exhibited a significant increase in GCF flow rate on day 6. This suggests that the post-surgical inflammatory response was more constrained in the AZM group. Neither group exhibited significant changes in plaque accumulation on natural teeth or implants. This was not surprising, since patents in both groups were asked to use an antimicrobial mouthrinse after implant placement.
Peri-implant sites, unlike the adjacent natural tooth sites, had no pre-existing junctional epithelium to enhance soft tissue adhesion to the implant. However, the AZM group consistently expressed lower volumes of PICF than the AMX group on days 6 and 14. Although this difference was not significant, it suggests that post-surgical healing progressed more rapidly in the AZM group. The observed PICF volumes per 30s sample are comparable to those previously reported.26, 28 The data suggest that resolution of postoperative inflammation and healing of the junctional epithelium occurred more rapidly in the AZM group.
Cytokines and chemokines in GCF and PICF
A previous report suggested that there is a tendency toward higher cytokine production around dental implants than around natural teeth.29 A recent study that compared implants and adjacent teeth with respect to clinical and biological parameters of wound healing also supports this finding. The authors observed higher levels of pro-inflammatory cytokines around implants than around periodontal sites.30 Consistent with these reports, PICF levels of TNF-α, IL-8 and MIP-1β were at least 2-fold higher than their respective levels in GCF on day 6 of the present study. Collectively, these results indicate that peri-implant tissues exhibit a more robust response to surgical trauma than periodontal tissues surrounding adjacent teeth.
A major objective of this study was to determine if AZM and AMX had different effects on the production of inflammatory mediators during early healing after implant placement. In comparison with the AMX group, the AZM group exhibited significantly lower IL-6 in GCF on day 6 and a more significant decrease from baseline GCF IL-8 content on day 20. In PICF obtained from newly-placed implants, the AZM group samples contained significantly lower amounts of G-CSF, IL-8, MIP-1β and IP-10 on day 20 and a significantly lower amount of G-CSF on day 13. The pattern was similar when the levels of IL-8, MIP-1β and IP-10 were expressed as concentrations. All of these mediators are classified as target genes of NF-KB, a family of DNA binding proteins that controls the transcription of many pro-inflammatory cytokines, chemokines, and adhesion molecules. Studies have shown that erythromycin and AZM suppress the activation of NF-KB and AP-1.13, 31 The effect of macrolide antibiotics on the expression of inflammatory mediators has been characterized in the medical literature, especially in the treatment of respiratory inflammatory diseases. Erythromycin suppresses the activation of IL-6 in human bronchial epithelial cells.13 Several other cytokines, chemokines or growth factors are reportedly inhibited by macrolides, including TNF-α, GM-CSF, MIP-1, IL-8, RANTES, eotaxin, and vascular endothelial growth factor (VEGF).32, 33 Aside from its effects on NF-KB, there is some evidence that down-regulation of cytokine expression by macrolides may be related to inhibition of bacteria or inhibition of neutrophil activation.34
To fully appreciate the net effect of AZM and AMX on the inflammatory response after implant surgery, their effects on the balance of pro- and anti-inflammatory mediators at the healing sites must be considered. In GCF, patients in the AMX group exhibited significant increases from baseline with respect to the pro-inflammatory mediators IL-12, IL-8, MIP-1β and RANTES (Table 2), a dramatic increase in IL-6 (Fig 2) and an increase in the anti-inflammatory mediator IL-10 (Table 2). Similar increases were not observed in the AZM group, perhaps because all of these mediators are target genes of NF-KB. It is more difficult to judge the net effect on mediators in PICF samples, because the first samples were collected on day 6 rather than at baseline (day 0). At various times during early healing, however, the AMX group exhibited significantly higher levels of the pro-inflammatory mediators G-CSF, IL-8, MIP-1β and IP-10 than the AZM group. During the same period (day 6 to day 20), the AMX group exhibited a significant increase in IL-1 receptor antagonist (an anti-inflammatory mediator and NF-KB target gene), but the AZM group did not. In summary, the AMX group exhibited significant increases in a broader range of pro- and anti-inflammatory mediators during early healing when compared to the AZM group.
Shortcomings of the study
To gain a better appreciation of the influence of prophylactic antibiotics on healing after implant surgery, it would have been useful to incorporate a control (placebo) arm into the design of this study. Since the standard protocol in our clinic is to utilize prophylactic AMX, it is possible that the IRB would have considered the omission of a prophylactic regimen to be below the standard of care.
Although the study successfully demonstrated that the AZM group exhibited decreased GCF volume and lower IL-8 levels in GCF and PICF, the power was too low to detect significant differences in some comparisons. Based on the actual findings, approximately 29 patients per group would be needed to detect a significant difference in PICF volume between the AMX and AZM groups with a statistical power of 0.80. Similarly, the AZM group exhibited only a borderline significant decrease in GCF TNF-α content relative to the AMX group, and there was no significant difference in PICF TNF-α content between the two groups. However, the study yielded several positive findings that had not been anticipated. In comparison to the AMX group, the AZM group exhibited significantly lower levels of IL-6 in GCF and significantly lower levels of G-CSF, MIP-1β and IP-10 in PICF. It is reasonable to be cautious about extending the results of this small study to wider populations.
Conclusions
The primary purpose of a prophylactic antibiotic is to produce transient suppression of bacteria in blood and at wound sites in individuals who are susceptible to infection. The results indicate that AZM is available at the surgical site for a longer period of time than AMX. Perhaps the most remarkable finding of the current study is that a single prophylactic dose of AZM appeared to alter several potentially important aspects of inflammation and early healing after implant surgery. During the healing process, soft tissue attaches to the implant and to adjacent natural teeth. This helps exclude bacteria and facilitates resolution of inflammation. Clinically, these healing processes are accompanied by a reduction in the volume of PICF and GCF. Patients in the AZM group exhibited lower volumes of GCF and PICF at many study time points, suggesting that healing and resolution of inflammation progressed more rapidly in the AZM group. The lower levels of G-CSF, IL-6, IL-8, MIP-1β and IP-10 observed in the AZM group could potentially have contributed to decreased mobilization of granulocytic precursors and decreased recruitment of immune and inflammatory cells to the healing surgical site. Although recruitment of neutrophils cells plays an essential role in healing, reduction of excessive infiltration is associated with accelerated wound closure in mice35 and could potentially contribute to a lower incidence of complications in healing.
To the best of our knowledge, no previous study has evaluated the presence of AZM using an early wound healing protocol around one-stage dental implants. A pilot study has been published using a similar protocol to evaluate the effectiveness of AMX as a prophylactic antibiotic and its role in early wound healing around newly-placed one-stage dental implants.26 Comparing patients taking AMX with controls, the study found no significant difference in PICF volume 7 days after implant placement. Similarly, no previous study has evaluated the effect of AZM on clinical and biochemical parameters of healing around newly placed dental implants. Our results suggest that AZM has a favorable influence on healing, but further studies are indicated to fully characterize the extent to which AZM alters the complex processes associated with the inflammatory response.
Acknowledgments
The authors thank the Investigational Drug Service at the Ohio State University Wexner Medical Center for preparation of antibiotic regimens and Drs. Michael Beck and Pin-Chuang Lai of the Ohio State University College of Dentistry for statistical guidance and help with clinical sample collection, respectively. This study was supported by grant R21 DE018804 from the National Institute for Dental and Craniofacial Research (Bethesda, MD), a seed grant from the Ohio State University College of Dentistry, and the Ohio State University Center for Clinical and Translational Science (supported by grant UL1TR001070 from the National Center for Advancing Translational Sciences, Bethesda, MD).
Footnotes
Periopaper, OraFlow Inc, Smithtown, NY, USA
Periotron 6000, IDE Interstate, Amityville, NY, USA
Osstell AB, Goteborg, Sweden
Bio-Plex #L5001F72WN, Bio-Rad Laboratories Inc., Hercules, CA, USA
BD Biosciences, Sparks, MD, USA
ATCC 9341, American Type Culture Collection, Manassas, VA, USA
US Pharmacopeia, Rockville, MD, USA
Conflict of interest statement: The authors declare no conflicts of interest in this study
References
- 1.Esposito M, Thamsen P, Ericson LE, Lekholm U. Histopathologic observations on early implant failures. Int J Oral Maxillofac Implants. 1999;14:798–810. [PubMed] [Google Scholar]
- 2.Palma-Carrio C, Maestre-Ferrin L, Penarrocha-Oltra D, Penarrocha-Diago MA, Penarrocha-Diago M. Risk factors associated with early failure of dental implants. A literature review. Medica Oral Patol Oral y Cirugia Bucal. 2011;16:e514–517. doi: 10.4317/medoral.16.e514. [DOI] [PubMed] [Google Scholar]
- 3.Koldsland OC, Scheie AA, Aass AM. Prevalence of implant loss and the influence of associated factors. J Periodontol. 2009;80:1069–1075. doi: 10.1902/jop.2009.080594. [DOI] [PubMed] [Google Scholar]
- 4.Esposito M, Worthington HV, Loli V, Coulthard P, Grusovin MG. Interventions for replacing missing teeth: antibiotics at dental implant placement to prevent complications. Cochrane Database Syst Rev. 2010;7:CD004152. doi: 10.1002/14651858.CD004152.pub3. [DOI] [PubMed] [Google Scholar]
- 5.Alsaadi G, Quirynen M, Michiles K, Teughels W, Komarek A, van Steenberghe D. Impact of local and systemic factors on the incidence of failures up to abutment connection with modified surface oral implants. J Clin Periodontol. 2008;35:51–57. doi: 10.1111/j.1600-051X.2007.01165.x. [DOI] [PubMed] [Google Scholar]
- 6.Laskin DM, Dent CD, Morris HF, Ochi S, Olson JW. The influence of preoperative antibiotics on success of endosseous implants at 36 months. Ann Periodontol. 2000;5:166–74. doi: 10.1902/annals.2000.5.1.166. [DOI] [PubMed] [Google Scholar]
- 7.Anitua E, Aguirre JJ, Gorosabel A, et al. A multicentre placebo-controlled ramdomised clinical trial of antibiotic prophylaxis for placement of dental implants. Eur J Oral Implantol. 2009;2:283–292. [PubMed] [Google Scholar]
- 8.Esposito M, Grusovin MG, Worthington HV. Interventions for replacing missing teeth: antibiotics at dental implant placement to prevent complications. Cochrane Database Syst Rev. 2013;7:CD004152. doi: 10.1002/14651858.CD004152.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharaf B, Jandali-Rifai M, Susarla SM, Dodson TB. Do Perioperative Antibiotics Decrease Implant Failure? J Oral Maxillofac Surg. 2011;69:2345–2350. doi: 10.1016/j.joms.2011.02.095. [DOI] [PubMed] [Google Scholar]
- 10.Wilson W, Taubert KA, Gewitz M, et al. Prevention of infective endocarditis: Guidelines from the American Heart Association. Circulation. 2007;116:1736–1754. doi: 10.1161/CIRCULATIONAHA.106.183095. [DOI] [PubMed] [Google Scholar]
- 11.Jain N, Lai PC, Walters JD. Effect of gingivitis on azithromycin concentrations in gingival crevicular fluid. J Periodontol. 2012;83:1122–1128. doi: 10.1902/jop.2012.110558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giamarellos-Bourboulis EJ. Macrolides beyond the conventional antimicrobials: a class of potent immunomodulators. Int J Antimicrob Agents. 2008;31:12–20. doi: 10.1016/j.ijantimicag.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Desaki M, Okazaki H, Sunazuka T, Omura S, Yamamoto K, Takizawa H. Molecular mechanisms of anti-inflammatory action of erythromycin in human bronchial epithelial cells: Possible role in the signaling pathway that regulates nuclear factor kappa B activation. Antimicrob Agents Chemother. 2004;48:1581–1585. doi: 10.1128/AAC.48.5.1581-1585.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kikuchi T, Hagiwara K, Honda Y, et al. Clarithromycin suppresses lipopolysaccharide-induced interleukin-8 production by human monocytes through AP-1 and NF-kappa B transcription factors. J Antimicrob Chemother. 2002;49:745–755. doi: 10.1093/jac/dkf008. [DOI] [PubMed] [Google Scholar]
- 15.Matsumura Y, Mitani A, Suga T, et al. Azithromycin may inhibit interleukin-8 through suppression of Rac1 and a nuclear factor-kappa B pathway in KB cells stimulated with lipopolysaccharide. J Periodontol. 2011;82:1623–1631. doi: 10.1902/jop.2011.100721. [DOI] [PubMed] [Google Scholar]
- 16.Shinkai M, Foster GH, Rubin BK. Macrolide antibiotics modulate ERK phosphorylation and IL-8 and GM-CSF production by human bronchial epithelial cells. Am J Physiol-Lung Cell and Molec Physiol. 2006;290:L75–85. doi: 10.1152/ajplung.00093.2005. [DOI] [PubMed] [Google Scholar]
- 17.Ho W, Eubank T, Leblebicioglu B, Marsh C, Walters J. Azithromycin decreases crevicular fluid volume and mediator content. J Dent Res. 2010;89:831–835. doi: 10.1177/0022034510368650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loe H, Silness J. Periodontal Disease in Pregnancy. I. Prevalence and Severity. Acta Odontol Scand. 1963;21:533–551. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- 19.Silness J, Loe H. Periodontal Disease in Pregnancy. II. Correlation between Oral Hygiene and Periodontal Condition. Acta Odontol Scand. 1964;22:121–135. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 20.Conway TB, Beck FM, Walters JD. Gingival fluid ciprofloxacin levels at healthy and inflamed human periodontal sites. J Periodontol. 2000;71:1448–1452. doi: 10.1902/jop.2000.71.9.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nedir R, Bischof M, Szmukler-Moncler S, et al. Predicting osseointegration by means of implant primary stability. Clin Oral Impl Res. 2004;15:520–528. doi: 10.1111/j.1600-0501.2004.01059.x. [DOI] [PubMed] [Google Scholar]
- 22.Mombelli A, van Oosten MA, Schurch E, Jr, Lang NP. The microbiota associated with successful or failing osseointegrated titanium implants. Oral Microbiol Immunol. 1987;2:145–151. doi: 10.1111/j.1399-302x.1987.tb00298.x. [DOI] [PubMed] [Google Scholar]
- 23.Guentsch A, Kramesberger M, Sroka A, et al. Comparison of gingival crevice fluid sampling methods in patients with severe chronic periodontitis. J Periodontol. 2011;82:1051–1060. doi: 10.1902/jop.2011.100565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Winkelhoff AJ, Rams TE, Slots J. Systemic antibiotic therapy in periodontics. Periodontol 2000. 1996;10:45–78. doi: 10.1111/j.1600-0757.1996.tb00068.x. [DOI] [PubMed] [Google Scholar]
- 25.Spyker DA, Rugloski RJ, Vann RL, O'Brien WM. Pharmacokinetics of amoxicillin: dose dependence after intravenous, oral, and intramuscular administration. Antimicrob Agents Chemother. 1977;11:132–141. doi: 10.1128/aac.11.1.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khoury SB, Thomas L, Walters JD, Sheridan JF, Leblebicioglu B. Early wound healing following one-stage dental implant placement with and without antibiotic prophylaxis: a pilot study. J Periodontol. 2008;79:1904–1912. doi: 10.1902/jop.2008.070670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Griffiths GS. Formation, collection and significance of gingival crevice fluid. Periodontol 2000. 2003;31:32–42. doi: 10.1034/j.1600-0757.2003.03103.x. [DOI] [PubMed] [Google Scholar]
- 28.Gunday S, Topcu A, Guncu G, Akman A, Karabulut E, Yamilik N. Analysis of potential factors affecting peri-implant sulcus fluid (PISF) volume. Clin Dent Res. 2011;35:12–24. [Google Scholar]
- 29.Nowzari H, Botero JE, DeGiacomo M, Villacres MC, Rich SK. Microbiology and cytokine levels around healthy dental implants and teeth. Clin Implant Dent Relat Res. 2008;10:166–73. doi: 10.1111/j.1708-8208.2007.00076.x. [DOI] [PubMed] [Google Scholar]
- 30.Emecen-Huja P, Eubank TD, Shapiro V, Yildiz V, Tatakis DN, Leblebicioglu B. Peri-implant versus periodontal wound healing. J Clin Periodontol. 2013;40:816–824. doi: 10.1111/jcpe.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aghai ZH, Kode A, Saslow JG, et al. Azithromycin suppresses activation of nuclear factor-kappa B and synthesis of pro-inflammatory cytokines in tracheal aspirate cells from premature infants. Pediat Res. 2007;62:483–488. doi: 10.1203/PDR.0b013e318142582d. [DOI] [PubMed] [Google Scholar]
- 32.Kohyama T, Takizawa H, Kawasaki S, Akiyama N, Sato M, Ito K. Fourteen-member macrolides inhibit interleukin-8 release by human eosinophils from atopic donors. Antimicrob Agents Chemother. 1999;43:907–911. doi: 10.1128/aac.43.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sato E, Nelson DK, Koyama S, Hoyt JC, Robbins RA. Erythromycin modulates eosinophil chemotactic cytokine production by human lung fibroblasts in vitro. Antimicrob Agents Chemother. 2001;45:401–406. doi: 10.1128/AAC.8.2.401-406.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reato G, Cuffin AM, Tullio V, et al. Immunomodulating effect of antimicrobial agents on cytokine production by human polymorphonuclear neutrophils. Int J Antimicrob Agents. 2004;23:150–154. doi: 10.1016/j.ijantimicag.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 35.Dovi JV, He LK, DiPietro LA. Accelerated wound closure in neutrophil-depleted mice. J Leukocyte Biol. 2003;73:448–455. doi: 10.1189/jlb.0802406. [DOI] [PubMed] [Google Scholar]


