Abstract
Retinoblastoma (RB) is the most common primary intraocular cancer in children, a nd the third most common cancer overall in infants. No molecular-targeted therapy for this lethal tumor exists. Since the tumor suppressor RB1, whose genetic inactivation underlies RB, is upstream of the epigenetic regulator EZH2, a pharmacologic target for many solid tumors, we reasoned that EZH2 might regulate human RB tumorigenesis. Histologic and immunohistochemical analyses were performed using an EZH2 antibody in sections from 43 samples of primary, formalin-fixed, paraffin embedded human RB tissue, cryopreserved mouse retina; and in whole cell lysates from human RB cell lines (Y79 and WERI-Rb1), primary human fetal RPE and fetal and adult retina, mouse retina and embryonic stem (ES) cells. While enriched during fetal human retinal development, EZH2 protein was not present in the normal postnatal retina. However, EZH2 was detected in all 43 analyzed human RB specimens, indicating that EZH2 is a fetal protein expressed in postnatal human RB. EZH2 expression marked single RB cell invasion into the optic nerve, a site of invasion whose involvement may influence the decision for systemic chemotherapy. To assess the role of EZH2 in RB cell survival, human RB and primary RPE cells were treated with two EZH2 inhibitors (EZH2i), GSK126 and SAH-EZH2 (SAH). EZH2i inhibitors impaired intracellular ATP production, an indicator of cell viability, in a time and dose-dependent manner, but did not affect primary human fetal RPE. Thus, aberrant expression of a histone methyltransferase protein is a feature of human RB. This is the first time this mechanism has been implicated for an eye, adnexal, or orbital tumor. The specificity of EZH2i toward human RB cells, but not RPE, warrants further in vivo testing in animal models of RB, especially those EZH2i currently in clinical trials for solid tumors and lymphoma.
INTRODUCTION
Retinoblastoma (RB) is the most common primary intraocular cancer in children, with an incidence of approximately 1 per 15,000 live births in the United States.1 RB is the third most common malignancy in infants younger than 1 year, and accounts for 12% of all infantile cancers per year.2 A blinding, disfiguring tumor associated with fatal metastasis, RB can be associated with pinealoblastoma, a pediatric brain tumor that carries a dismal prognosis. Mutations in the retinoblastoma (RB1) tumor suppressor gene underlie most causes of hereditary and sporadic forms of RB. Recently, a subset of early-onset, unilateral, aggressive RB tumors without mutations in RB1 has been identified. Diagnosed in 18% of infants with RB younger than 6 months of age, this form of RB is due to somatic amplification of the MYCN oncogene.3
Despite the centrality of RB1 and MYCN dysfunction in tumorigenesis, RB treatments such as laser photocoagulation, cryotherapy, enucleation, external beam radiation, systemic or local chemotherapy, such as intravitreal and intra-arterial chemotherapy via the ophthalmic artery, do not target these mutations specifically. Moreover, many of these therapies remain out of reach for the majority of affected children, who reside in non-developed countries. While the 5-year survival rate of children with RB in the United States is over 90%,4 the tumor is lethal in 50–70% of children in non-developed countries.5, 6 Even among survivors, RB treatments are associated with significant co-morbidities, including retinopathy, secondary cancers, neutropenia, and other local and systemic toxicities.7–11
Aside from changes in RB1 and MYCN, few other somatic genetic aberrations have been identified,12 limiting the development of novel, rationally-designed therapeutics. One relatively new avenue for rational drug development in cancer involves post-translational modification of chromatin, termed epigenetics. Epigenetic agents can alter how tightly histones bind DNA, which, in turn, determines the accessibility of the DNA to transcriptional machinery.13, 14 Thus, modulation of histones can change expression of oncogenes or tumor suppressors in cancers, even without the introduction of DNA mutations.14–17
In this work, we propose that the histone methyltransferase (HMT), EZH2/E zh2 (human/mouse), an enzyme that catalyzes trimethylation at lysine 27 on histone H3 (H3K27me3), is selectively expressed in RB tumors, required for tumor survival, and thus represents a novel therapeutic target.17 EZH2, which is expressed in several highly proliferative, aggressive tumors,18 triggers transcriptional silencing of tumor suppressors such as the cyclin-dependent kinase inhibitor p16/INK4a and p19/Arf.19–21 EZH2’s oncogenic actions are suppressed by RB1 and related protein P130,22, 23 and retinoblastoma formation in mice requires the joint inactivation of both RB1 and P130.24 SUZ12 mRNA, which encodes a protein that is a co-factor for EZH2, is upregulated in human RB, further implicating EZH2 in RB oncogenesis.25
Accordingly, herein we explore the clinical application of labeling retinoblastoma with EZH2 to aid in the detection of invasive RB cells. Currently histopathologic detection of single invasive RB cells to adjacent tissues remains difficult, even though invasion to the optic nerve is an indication for systemic chemotherapy following enucleation. Finally, our work raises the possibility that pharmacologic targeting of EZH2, the basis of two ongoing oncology clinical trials,26 may be a promising strategy toward the development of the first molecularly targeted therapies for RB.
MATERIALS AND METHODS
Immunofluorescence microscopy
Mouse retinal tissue sections and human retinoblastoma cell cultures were fixed in 4% paraformaldehyde and processed as previously described.27, 28 For immunofluorescence labeling, retinal tissue sections or retinoblastoma cell cultures blocked with 5% bovine serum albumin + 5% normal goat serum + 0.3% triton X-100 in PBS for 1 hour at room temperature. The blocking buffer was discarded, and the sections were washed three times with 1× PBS, before the addition of antibodies Ezh2 (1:200, Cell Signaling, Beverly, MA, #5246, rabbit monoclonal D2C9 clone) and CRX (1:500, Abnova, Taipei, Taiwan, H00001406-M02, mouse monoclonal M02 clone). Incubation was performed overnight at 4°C. Sections were washed three times, followed by incubation with secondary antibodies (1:200, Abcam, Cambridge, MA, ab150074 donkey anti-rabbit polyclonal Alexa Fluor 555; ab150105 donkey anti-mouse polyclonal Alexa Fluor 488; ab150073 donkey anti-rabbit polyclonal Alexa Fluor 488) conjugated with a fluorophore for 1 hour in the dark. The sections were washed again three times with 1× PBS for 30 minutes After staining with 4′,6-diamindino-2-phenyindole (DAPI, 2 mg/mL, Sigma, St. Louis, MO) to reveal cell nuclei, retinal sections were mounted and examined under fluorescence and confocal laser scanning microscopy (SP5; Leica, Wetzlar, Germany).
Fetal retina samples and cell culture of primary fetal RPE and human RB cell lines
Human retinoblastoma cell lines WERI-Rb1 (HTB-169) and Y79 (HTB-18) were obtained from American Type Culture Collection (ATCC, Manassas, VA) and cultured in RPMI-1640 media (ATCC, #30-2001) supplemented with fetal calf serum (10% for WERI-Rb1 and 20% for Y79). Human fetal retina was obtained from eyes at 19 weeks gestational age (Advanced Bioscience Resources, Inc., Alameda, CA), shipped on ice in RPMI media, and dissected within 26 hours post-mortem. The protocol of Maminishkis et al.29 was followed with the following modifications. After anterior segment removal, the eyecup was incubated in complete RPE media with 5% heat-inactivated fetal bovine serum (Life Technologies, Brown Deer, WI, #10082-147) and dispase (Worthington Biochem. Corp., Lakewood, NJ, LS02109) at 4.5 U/mL for 90 minutes at 37 degrees in a 5% CO2 incubator. After incubation, the retina naturally separated from the underlying RPE, and was freed from the optic nerve with scissors. Retina was used for Western blotting and immunohistochemistry. The eyecup was then inverted and placed on a plastic dome created by cutting the bottom off of a microcentrifuge tube. The inverted eyecup and plastic dome were pinned to a dissecting dish filled with silicone elastomer. RPE collected from 1 fetal eye was plated on one 25cm2 Primaria flask (Corning Inc., Corning, NY, 08-772-45). After 4–10 weeks, cells were split using trypsin again according to the protocol of Maminishkis et al., although some patches of tightly adherent RPE required a second trypsin incubation for up to an hour. Trypsinized cells were plated on 24-well transwells coated with human placental extracellular matrix (BD Biosciences, San Jose, CA) at a splitting density of 1:1.5, with each transwell estimated to have a surface area of 0.3cm2. Cells were grown on Transwells for 4–8 weeks prior to testing with EZH2 inhibitors. EZH2 peptide and small-molecule inhibitors SAH-EZH2 (#508320) and GSK126 (#15415) were purchased from Millipore (Billerica, MA) and Cayman Chemical (Ann Arbor, MI), respectively.
Collection and clinicopathologic analysis of primary human retinoblastoma tissues
After approval by the University of Michigan Institutional Review Board, we retrospectively searched the electronic pathology archives for all cases of enucleation for retinoblastoma without prior treatment, diagnosed between January 1988 and June 2014. 57 cases of enucleation for untreated retinoblastoma in 57 children during the study period were identified. Formalin-fixed paraffin-embedded (FFPE) blocks were available for 43 of 57 cases. Clinical data collected included age at the time of enucleation (in months), gender and laterality at the time of presentation. FFPE tissue blocks were cut in five-micrometer-thick sections and stained with hematoxylin and eosin. Slides were reviewed and histologic features recorded. The largest dimension of tumor in centimeters was determined by a combination of slide evaluation and review of the gross description. Histologic grade was determined by the estimated percentage of Flexner-Wintersteiner rosettes (1 = well differentiated, 2 = moderately differentiated, 3 = poorly differentiated, 4 = undifferentiated). Well differentiated tumors demonstrated rosettes in greater than 80% of fields examined, while poorly differentiated tumors contained rare rosettes or showed Homer-Wright rosettes. Moderately differentiated tumors were in between these two grades. Undifferentiated tumors showed no rosettes. The extent of optic nerve invasion and choroidal invasion was evaluated according to the guidelines presented in the American Joint Committee on Cancer (AJCC) Cancer Staging Manual, 7th edition. Briefly, the extent of optic nerve invasion was recorded as 1 = no invasion, 2 = anterior to lamina cribrosa, 3 = at lamina cribrosa and 4 = posterior to lamina cribrosa, while choroidal invasion was recorded as 0 = no invasion, 1 = focal invasion, 2 = massive invasion (at least 0.3 cm).
Immunohistochemistry
Immunohistochemical staining was performed on the DAKO Autostainer (DAKO, Carpinteria, CA) using DAKO Envision+ and diaminobenzadine (DAB) as the chromogen. Sections of deparaffinized human fetal week 19 retina or postnatal retinoblastoma sections were labeled with EZH2 (mouse monoclonal antibody, clone 11, 1:100, BD Biosciences, San Jose, CA, #612666) for 60 minutes at ambient temperature. Microwave epitope retrieval in 10 mM Tris/HCl, pH 9 containing 1 mM EDTA was used prior to staining. Appropriate negative (no primary antibody) and positive controls (reactive tonsil) were stained in parallel with each set of tumors studied. EZH2 immunostains were examined by light microscopy by two pathologists (L.L.W. and A.P.S.). Only EZH2 staining of the nucleus was marked as positive EZH2 expression. The staining was scored semiquantitatively and recorded based on percent nuclei staining (0 = negative, 1 = 1 – 25% immunoreactive cells, 2 = 26% – 50% immunoreactive cells, 3 = 51% – 75% immunoreactive cells, 4 = 76% – 100% immunoreactive cells) and intensity of staining (0 = negative, 1 = weak, 2 = moderate, 3 = strong). Corresponding sections were also labeled with hematoxylin and eosin, as previously described.12, 30, 31
Cell viability assay
Cell viability was assessed using CellTiter-Glo® Luminescent Cell Viability Kit (Promega, Madison, WI, #G7570) by measurement of intracellular ATP. Briefly, two retinoblastoma cell lines (5×104 cells per well in a 24-well plate) were treated with different doses of EZH2 inhibitors (SAH-EZH2 twice daily, GSK 126 daily). After 48 hours, 80 µl of cell suspensions were harvested and mixed with 20 µl of CellTiter-Glo® lysis buffer in a 96-well assay plate (Costar, Corning, Tewksbury, MA, #3912). After 10 min of incubation following manufacturer’s instructions, luminescence was recorded using a SpectraMax M5 plate reader. For primary RPE, cells were cultured in 24-transwell inserts with 125 µl of media per well and treated with EZH2 inhibitors for 48 hours. After removing 75 µl of cell culture media, 50 µl of CellTiter-Glo® lysis buffer was added to each transwell. 100 µl of cell lysate was transferred to a 96-well assay plate and incubated at room temperature for 10 min. Luminescence was then recorded using a Veritas TM microplate luminometer (Turner Biosystems, Sunnyvale, CA, #9100-102).
Western blot analysis
Cells treated with different doses of EZH2i at indicated time points were harvested and cell lysates were prepared using mPER mammalian protein extraction reagent (ThermoScientific, Waltham, MA, #78503). 20 µg of total protein was loaded per lane, run on a 12% SDS-PAGE gel and further transferred to PVDF membrane (Millipore, IPFL00010). The EZH2 antibody was obtained from Cell Signaling (clone D2C9), and β-actin (Cell Signaling, #4967S) was used to probe for equal loading.
Histone analysis
Histone extraction was performed using a kit according to manufacturer’s protocol (Abcam, catalog #: ab113476). Histone concentration was measured by BCA method, and 1 µg of histone was loaded on SDS-PAGE gel for western blot. The following antibodies were used: H3K27me3 (Millipore, #07-449), H3K4me3 (Millipore, #07-473) and total histone H3 (Abcam, #ab1791).
Statistical analysis
The chi-square test was used to determine statistical significance of categorical variables and analysis of variance (ANOVA) was used to analyze continuous variables. Associations among EZH2 staining percentage/intensity and clinicopathologic features were determined by Pearson’s correlation analysis. All statistical analyses were run using GraphPad Prism version 6.01 for Windows, GraphPad Software, La Jolla California USA, www.graphpad.com. A p-value <0.05 was considered statistically significant.
RESULTS
EZH2 Expression during Retinal Development
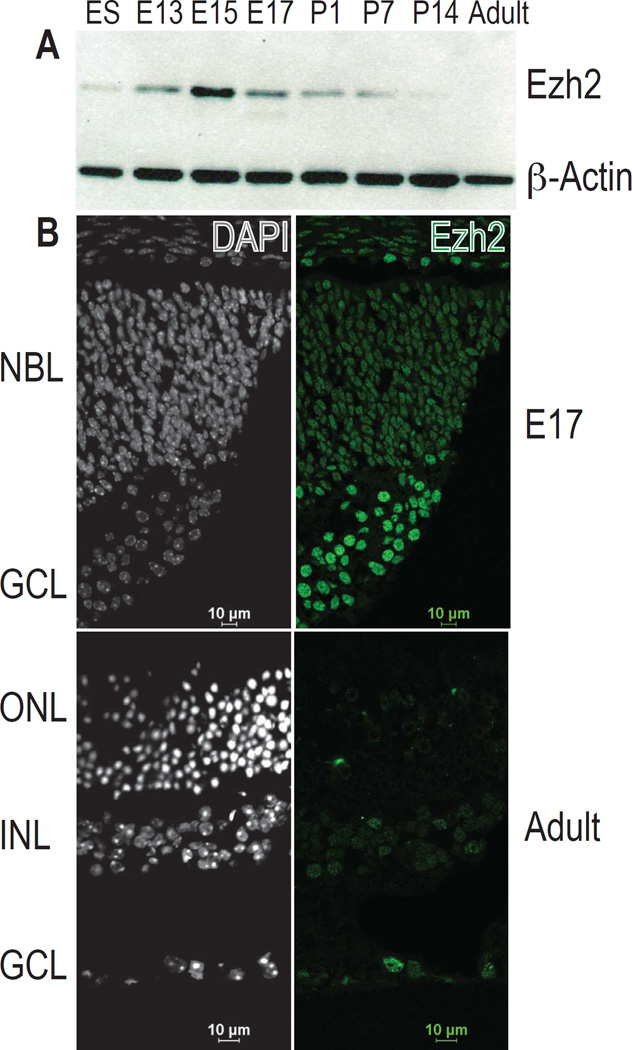
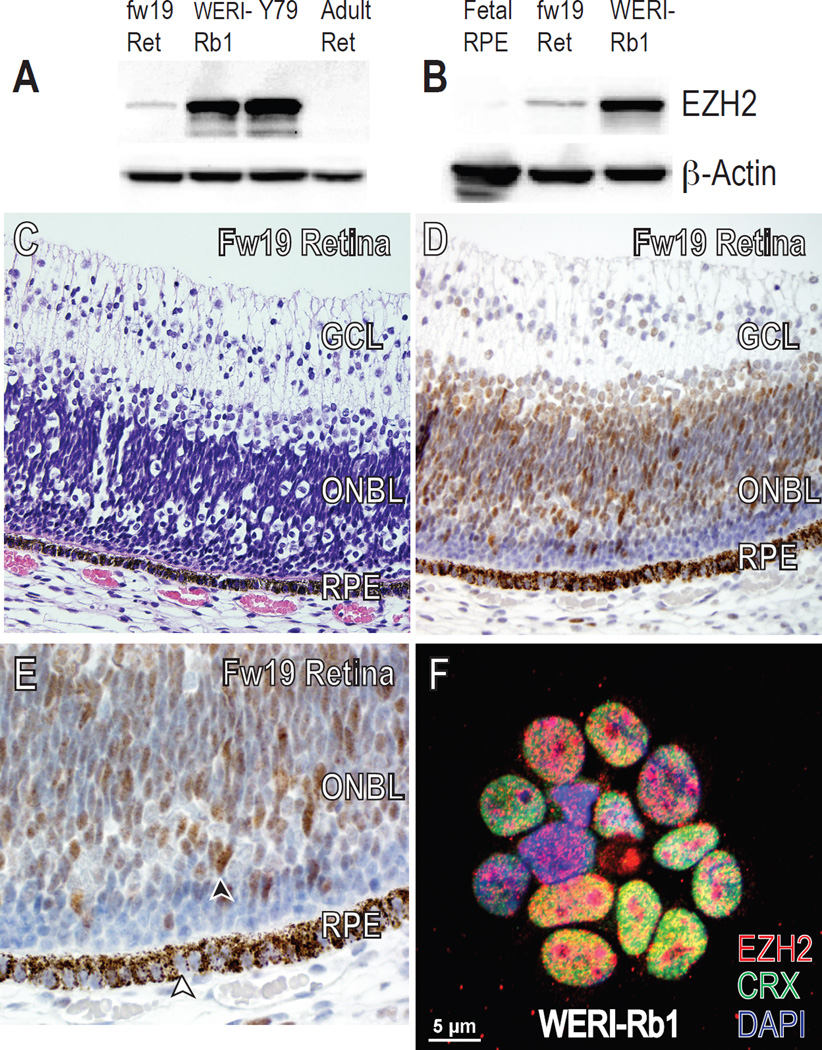
To determine the developmental expression of EZH2 in the mammalian retina, we extended our previous results28 by western blotting for Ezh2 in the mouse retina at various developmental timepoints and used mouse embryonic stem (ES) cells as a control. At its peak at embryonic day 15 to 17 (E15–17), Ezh2 protein levels were higher than at the ES cell stage. Levels in adult retina were nearly undetectable (Fig 1A). Immunofluorescence of Ezh2 levels at E17 versus adult mouse retina confirmed these findings (Fig 1B). Similar results were seen during human retinal development: retinal lysates from fetal week 19 (fw19) contained higher levels of EZH2 than the adult retina (Fig 2A). To confirm these results and to localize the protein in normal human retinal development, we performed immunohistochemistry in human fw19 retina, and found EZH2 to be expressed mainly in the outer neuroblastic layer, a known proliferative region of the fetal human retina (Fig 2C–E). We did not detect EZH2 in non-RB tumor infiltrated, normal postnatal human retina (Fig 3F). These results indicate the EZH2 is a fetal protein normally expressed during mouse and human retinal development.
Figure 1.
Ezh2 expression in the mouse retina. (A) Western blot of whole retina isolated from the indicated ages showed that Ezh2 protein levels from whole retina peaked during mid-gestation and decreased during postnatal and adult stages. β-actin was used as a loading control. (B) Immunofluorescence of frozen mouse retinal sections at indicated ages confirmed that Ezh2 protein (green) was more abundant in the retina during E17 than in the adult. NBL neuroblastic layer; GCL ganglion cell layer; ONL outer nuclear layer; INL inner nuclear layer; E embryonic day, P postnatal day.
Figure 2.
EZH2 expression in human cultured RB cells versus retina and RPE. (A, B) Western blot of human RB cell lines WERI-Rb1 and Y79, primary human fetal RPE cultures, and whole human retina isolated from midgestational and adult stages showed that EZH2 protein levels were highest in RB tumor cells, followed by fetal week (fw) 19 retina, and lowest in primary human fetal RPE cultures and adult retina. β-actin was used as a loading control. (C) Hematoxylin & eosin (H&E) and corresponding (D, E) EZH2 staining of fw19 human retina. (E) Black arrowhead indicates nuclear EZH2 staining of ONBL retinal progenitor cells while white arrowhead shows lack of nuclear EZH2 staining in RPE cells. RPE cells contain brown melanosome granules in their cytoplasm. Original magnification ×400 (C, D), ×600 (E). (F) Immunofluorescence of cultured WERI-Rb1 cells showed co-expression of EZH2 (red) and CRX (green) in all nuclei (DAPI, blue). Ret retina; GCL ganglion cell layer; ONBL outer neuroblastic layer; RPE retinal pigment epithelium.
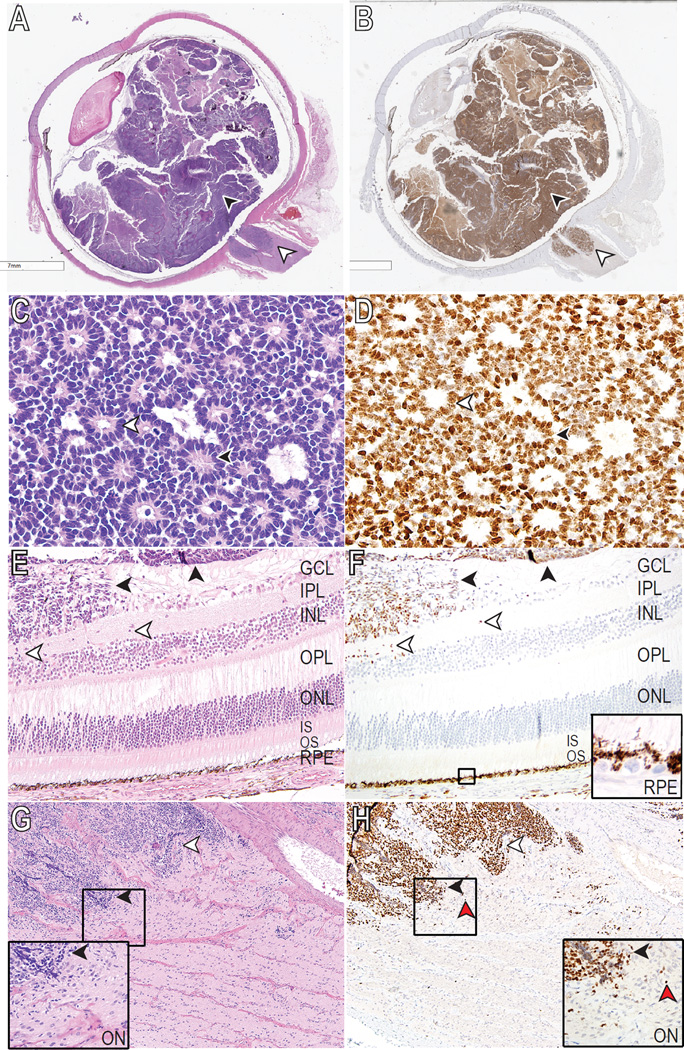
Figure 3.
EZH2 expression in human RB pathology samples. (A, C, E, G) Hematoxylin & eosin (H&E) and corresponding (B, D, F, H) EZH2 staining of human RB tumor samples. (A) Whole eye containing poorly-differentiated RB showed that both intraocular (black arrowheads) and extraocular (white arrowheads) portions of the tumor were positive for EZH2 (B). (C) Well-differentiated retinoblastoma with a combination of Homer Wright (black arrowhead) and Flexner-Wintersteiner (white arrowhead) rosettes. (D) EZH2 is expressed in the nuclei of retinoblastoma regardless of the type of rosette (white and black arrowheads). (E) Poorly differentiated RB (black arrowheads) infiltrating the superficial layers of the retina toward the inner nuclear layer (white arrowheads). (F) EZH2 highlights the infiltrating RB (arrowheads), but is absent in the remainder of the retina, including the RPE nuclei (inset). (G) Undifferentiated retinoblastoma demonstrating optic nerve invasion (black and white arrowheads) posterior to the lamina cribrosa. (H) EZH2 is positive in the invasive undifferentiated retinoblastoma and also highlights the single cell infiltration (red arrowhead) at the leading edges (black and white arrowheads) of the tumor (inset). Black boxes (F, G, H) show site magnified for insets. Original magnification ×400 (C, D), ×200 (E, F), ×100 (G, H). GCL ganglion cell layer; IPL inner plexiform layer; INL inner nuclear layer; OPL outer plexiform layer; ONL outer nuclear layer; IS/OS photoreceptor inner/outer segments; RPE retinal pigment epithelium; ON optic nerve.
EZH2 Expression in Human RB Cell Lines
Since many cancers aberrantly express fetal proteins in postnatal tissues, and because RB1 inhibits EZH2,22, 23 we suspected human RB cells obtained from the postnatal eye would express EZH2. We used western blotting to compare EZH2 protein levels from two human RB cell lines, WERI-Rb1 and Y79,32, 33 with primary human fetal RPE cultures and human retina from fw19 and adult. EZH2 levels were significantly higher in WERI-Rb1 and Y79 RB cells than in fw19 and adult human retina (Fig 2A) or primary human fetal RPE cultures (Fig 2B). The cell-of-origin for human RB has been suggested to be a CRX-positive, cone photoreceptor precursor.30, 31 Immunofluorescence confirmed that WERI-Rb1 cells express CRX and EZH2 (Fig 2F). These results demonstrate that EZH2 expression, normally transiently present during development, is expressed in human RB cells.
EZH2 Expression in Human RB Enucleation Samples
In vitro cancer cell lines, such as WERI-Rb1 and Y79, do not recapitulate some aspects of primary malignancies, and we considered the possibility that increased EZH2 protein in RB cell lines might not reflect EZH2 levels in primary human RB samples. To directly examine EZH2 protein levels in primary tumors, we performed a retrospective search of the electronic pathology archives, which yielded 43 cases of RB for which FFPE blocks were available. The mean age at enucleation was 21 months for all cases; 8 months for well-differentiated tumors, 17 months for moderately differentiated tumors, 30 months for poorly differentiated tumors and 33 months for undifferentiated tumors. All cases had negative optic nerve margins. The clinicopathologic features of these samples are summarized in Table 1.
Table 1.
Clinicopathologic Data from 43 Patients Enucleated for Retinoblastoma
| Clinicopathologic Features | n = 43 |
|---|---|
| Gender | |
| Male | 20 (47%) |
| Female | 23 (53%) |
| Age (months)a | 21 (2 – 53) |
| Laterality | |
| Unilateral | 34 (79%) |
| Bilateral | 9 (21%) |
| Tumor size (cm)a | 1.4 (0.7 – 1.8) |
| Histologic grade | |
| Well differentiated | 12 (28%) |
| Moderately differentiated | 11 (25%) |
| Poorly differentiated | 15 (35%) |
| Undifferentiated | 5 (12%) |
| Optic nerve invasion | |
| No invasion | 21 (49%) |
| Anterior to lamina cribrosa | 10 (23%) |
| At lamina cribrosa | 8 (19%) |
| Posterior to lamina cribrosa | 4 (9%) |
| Choroidal invasion | |
| No invasion | 34 (79%) |
| Focal | 4 (9%) |
| Massive | 5 (12%) |
Mean listed with the range shown in parentheses.
Following specimen collection, we probed for the presence of EZH2 protein by immunohistochemistry using human RB tumor sections from the 43 enucleations. All cases demonstrated nuclear EZH2 expression within tumor cells, with varying degrees of intensity (Supplementary Figure) and percent nuclei staining (Fig 3). Staining was restricted to RB cells, and spared tumor neovasculature and infiltrating polymorphonuclear leukocytes. Nearly all the cases (42 of 43, 98%) showed moderate to strong staining and 40 of 43 cases (93%) demonstrated staining in the majority of the tumor (Table 2). We identified a positive correlation between percentage and intensity of EZH2 staining (p < 0.001), but did not find any correlations between EZH2 and other clinicopathologic features (Supplementary Table). Other clinicopathologic correlations for non-EZH2 variables were found and are detailed in the supplementary material.
Table 2.
EZH2 Immunohistochemical Characteristics in 43 Eyes Enucleated for Retinoblastoma
| n | Percent nuclei staining | Staining intensity | |||||
|---|---|---|---|---|---|---|---|
| 1 – 25% | 26 – 50% | 51 – 75% | 76 –100% | Weak | Moderate | Strong | |
| 43 | 1 | 2 | 7 | 33 | 1 | 14 | 28 |
The presence of EZH2 protein delineated the entire extent of the intraocular tumor (Fig 3A, B, black arrowheads) as well as extraocular components, such as optic nerve invasion (Fig 3A, B, white arrowheads). EZH2 was present in tumor cells that formed characteristic RB histopathologic structures such as Homer Wright and Flexner-Wintersteiner rosettes (Fig 3C, D). The generally strong nuclear staining of RB tumor cells against the negative background provided an unprecedented histopathologic resolution that allowed detection of both bulk tumor (Fig 3E, F, black arrowheads) as well as single RB cell invasion into adjacent tissues, such as the inner plexiform layer (Fig 3E, F, white arrowheads) and optic nerve (Fig 3G, H, white arrowheads), and choroid (data not shown). EZH2 protein was not detected in non-tumor cells, including intact neural retina and RPE (Fig 3E, F). These data indicate that EZH2 is specifically expressed in primary human RB tumors, and that the presence of EZH2 resolves single-cell, metastatic RB cell invasion into adjacent structures.
While EZH2 expression appeared specific for RB cells, it did not mark all tumor cells. In three specimens, there were areas of EZH2-positive and negative RB cells (Fig 4A, B, black and white arrowheads, respectively). Interestingly, RB tumor cells that exhibited photoreceptor differentiation were negative for EZH2 (Fig 4A, inset, B). Two other specimens had unusual EZH2 staining patterns that were more likely related to specimen preparation. One tumor demonstrated loss of EZH2 staining in areas where tumor was not well preserved. The second tumor showed a loss of EZH2 staining only posteriorly, but this pattern did not correlate with specific histologic features. Taken together, these findings demonstrate that the presence of EZH2 protein specifically discriminates RB cells from normal tissue. Furthermore, differentiation state appeared to correlate with EZH2 expression, as foci of RB cells exhibiting photoreceptor differentiation were EZH2-negative.
Figure 4.
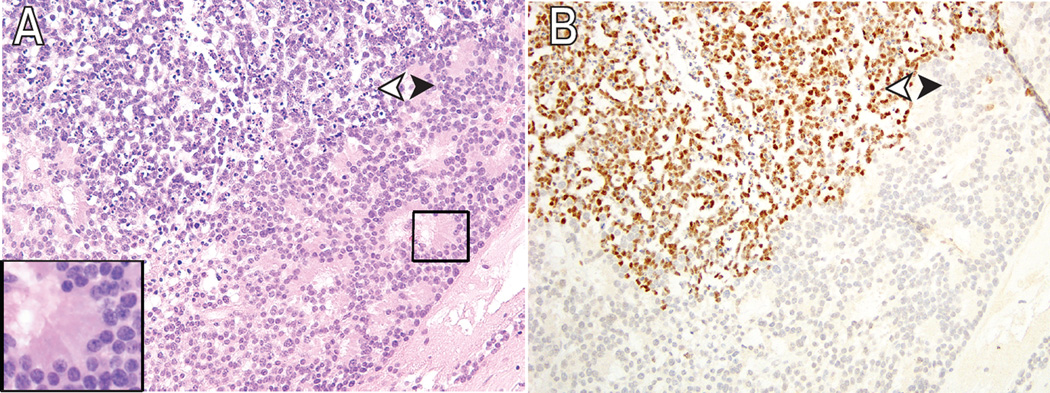
Differential expression of EZH2 in human RB pathology samples. (A) Moderately differentiated retinoblastoma (white arrowhead) with focus of photoreceptor differentiation (black arrowhead). The inset shows higher magnification of fleurettes. (B) EZH2 expression is absent in the foci of photoreceptor differentiation. Original magnification ×200.
Selectivity of EZH2 Inhibitors toward Human RB Cells
Since we found that EZH2 protein was selectively enriched in human RB cell lines and primary tumors, but not in non-tumor cells, we hypothesized that pharmacologic EZH2 inhibition might preferentially target primary RB cells but spare non-tumor cells of embryologic retinal origin, such as RPE.
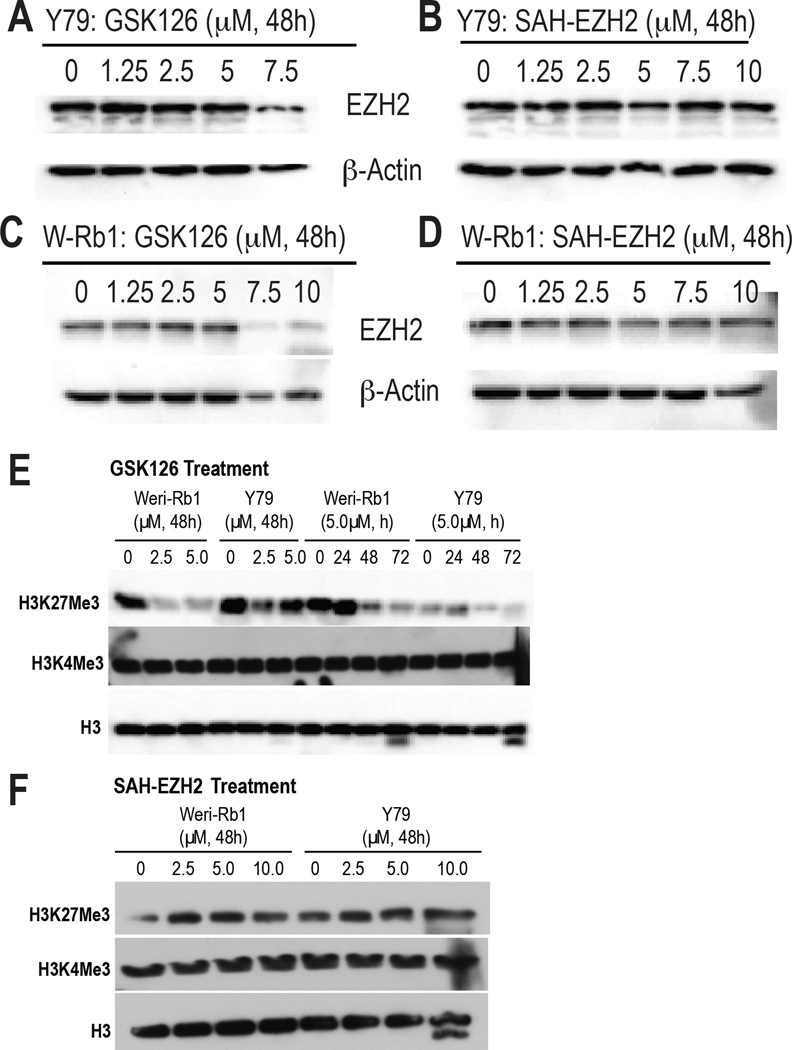
To test this possibility, we administered two recently developed EZH2 inhibitors (EZHi), the small molecule GSK126 and the peptide SAH-EZH2 (SAH), to WERI-Rb1, Y79, a nd primary human fetal RPE cells. GSK126 inhibits the enzymatic SET domain of EZH2, while SAH blocks interaction of EZH2 with its essential co-factor EED, an interaction which is required for Polycomb Repressive Complex 2 (PRC2)-mediated gene repression activity.34, 35 We used western blotting to assess the effect of the inhibitors, at previously validated IC50 concentrations,34, 35 on EZH2 in Y79 and WERI-Rb1 human RB cell lines (Fig 5A–D). At 48 h, we found that GSK126 concentrations of 7.5 µM or greater was sufficient to decrease EZH2 protein levels in Y79 and WERI-Rb1 cells (Fig 5A, C). Similar concentrations of SAH did not affect EZH2 protein levels in WERI-Rb1 and Y79 cells (Fig 5B, D).
Figure 5.
Effect of pharmacologic EZH2 inhibition on EZH2 and H3K27me3 levels in human RB cells. (A–D) Western blot of whole cell lysates of Y79 (A,B) and WERI-Rb1 (W-Rb1, C,D) following 48 h of treatment with indicated EZH2 inhibitors, GSK126 (A, C) and SAH-EZH2 (B, D). (E–F) Western blot of histone methylation status in whole cell lysates of WERI-Rb1 and Y79 following incubation with (E) indicated concentrations of GSK126 for 48 h or indicated periods of treatment at 5 µM of GSK126 or (F) indicated concentrations of SAH-EZH2 for 48 h. H3K27Me3 represents the histone methylation target of EZH2, whereas H3K4Me3 represents a site unaffected by the enzyme (negative control). H3 represents total histone 3 protein levels, which should also be unaffected by EZH2 modulation.
We considered the possibility that GSK126 and SAH may inhibit EZH2 activity without affecting its protein level.35, 36 To test this, we assessed levels of H3K27me3, a substrate for EZH2, with and without inhibitor treatment. We found that H3K27me3 levels, but not H3K4me3 levels (negative control), decreased in Weri-Rb1 and Y79 at GSK126 concentrations of 2.5 µM and higher (Fig 5E). In contrast, we did not find a change in H3K27me3 levels at SAH concentrations ranging from 2.5 to 10 µM (Fig 5F). These data indicate that GSK126 inhibits EZH2 enzymatic activity at H3K27me3 at doses that do not change EZH2 protein levels in human RB cell lines (< 7.5 µM), consistent with previous reports on other human tumor cell lines.35
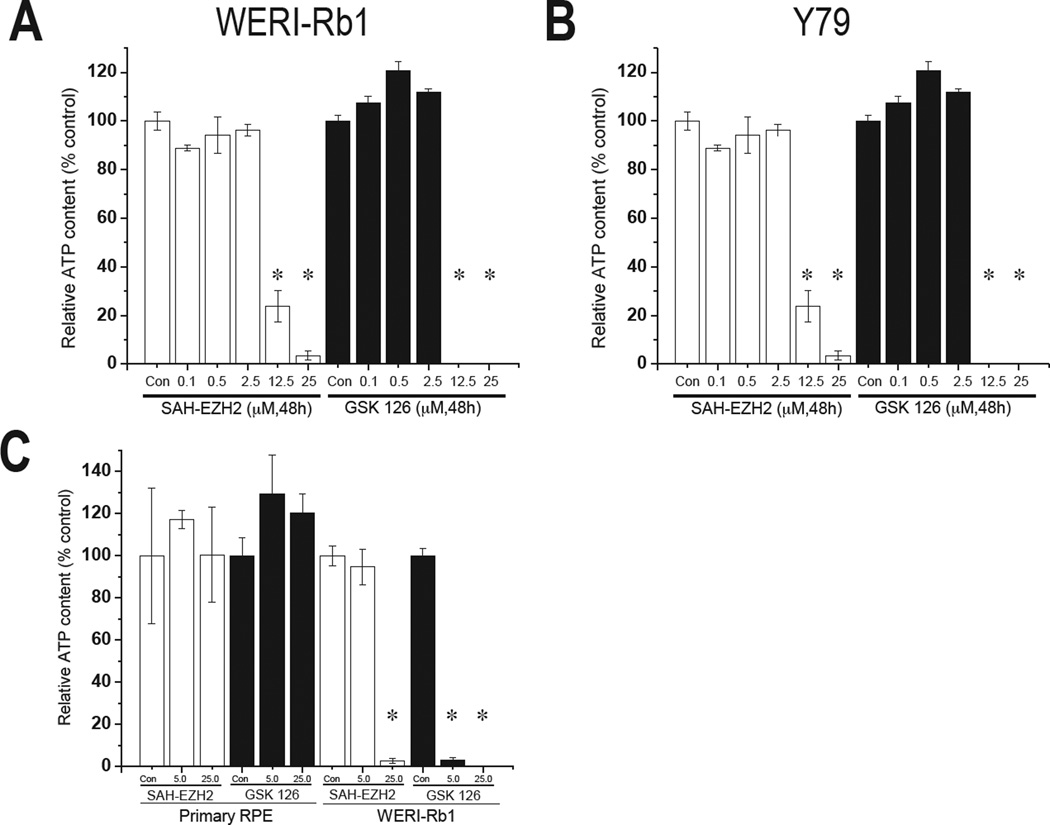
Based on the experiments above, we used a similar dose-response scheme to treat WERI-Rb1 and Y79 with EZH2i for 48h in an MTT assay. Both inhibitors decreased ATP content, an indicator of cell viability, in RB cells at concentrations above 5 µM, suggesting that EZH2 is required for viability in human RB cells (Fig 6). We reasoned that since primary human fetal RPE—which share a similar embryologic origin as RB—expressed much less EZH2 than RB cells (Fig 2B, D, E), they would be less sensitive to EZH2i. Indeed, we saw no change in viability of primary human fetal RPE even at 25 µM of SAH or GSK126, a level of EZH2i that greatly reduced viability in WERI-Rb1 cells (Fig 6C). From these data, we conclude that differential EZH2 protein levels predict response to EZH2i, and that EZH2i selectively impairs RB cell viability.
Figure 6.
Effect of pharmacologic EZH2 inhibition on viability in human RB cells and primary RPE. (A–C) Intracellular ATP, a direct measure of cell viability, was measured following co-culture of WERI-Rb1 (A) and Y79 (B) with the indicated concentrations of EZH2 inhibitors SAH-EZH2 (white bars) and GSK126 (black bars) for 48 h. (C) Culture of primary human fetal RPE with the indicated concentrations of SAH-EZH2 (white bars) and GSK126 (black bars) for 48 h. Human RB cells, but not primary RPE, were sensitive to high concentrations of EZH2 inhibitors. (* indicates significance at P < 0.05, compared to Con, control).
DISCUSSION
The discovery of the gene responsible for retinoblastoma, RB1, introduced the concept of a tumor suppressor to the field of cancer biology.37 Further, Knudson’s investigation of RB inheritance led to the “two-hit” hypothesis of cancer: a mechanism by which loss of heterozygosity (LOH) of a second allele subsequent to a germline mutation in the first allele leads to cancer.38 Interestingly, functional LOH can be caused not only by mutation within a second RB1 allele, but functional silencing of a normal, non-mutant RB1 copy by allele-specific DNA hypermethylation.39 This mechanism, epigenetic silencing by allele-specific DNA methylation, rather than mutation, also introduced a novel concept to cancer biology. Thus, the study of RB has contributed to key advances in the understanding of the genetic and epigenetic underpinnings of cancer overall.
Despite a 30-year understanding of RB, no rationally-targeted therapies based on the unique biology of RB exist. Currently, chemotherapy and enucleation form the backbone of RB therapy.8 While these therapies are effective in the US, the rate of treatment-related secondary cancers remains high, and in non-developed countries where therapeutic options are limited, RB mortality rates can reach 70%.5, 6, 8 A rationally-based therapeutic could alleviate treatment-related morbidity and provide an accessible treatment option in underserved areas.
In this study, we identify the HMT EZH2 protein as the first epigenetic enzyme to be dysregulated in human RB. Specifically, we found EZH2 was abundant in cultured human RB cell lines and RB tumor samples, but absent in non-proliferating cells of the normal fetal and postnatal retina. In fact, in eyes enucleated for advanced RB, EZH2 protein could be used as a marker to identify single RB cells invading into adjacent tissues such as the optic nerve. EZH2’s specific presence in RB tissue suggested the protein might be a therapeutic target. Indeed, we found that inhibitors of EZH2 killed human RB cells, but not primary human RPE cultures. Our findings raise the possibility that EZH2i may underpin a novel strategy against human RB, the first based on exploiting a targetable epigenetic enzyme specifically enriched in human RB.
In this work, we also extended our previous findings in the mouse retina28 to show, for the first time, that EZH2 is expressed transiently during human retinal development but completely repressed postnatally in non-tumorigenic retina. Indeed, EZH2 has been shown to be important for multiple developmental processes, as Ezh2 knockout mouse embryos die before completing gastrulation.40 EZH2’s dual role in development and cancer is well-demonstrated in the lung, where it is present during early lung development, downregulated by late gestation, and re-expressed in subtypes of lung carcinoma.41, 42 Thus, previous work is consistent with our finding that EZH2 is an oncoprotein that has a normally transient, developmentally-specific function.
Interestingly, we found that while EZH2 appears to be a specific marker for RB, it does not mark all RB cells. We noted that EZH2 protein is low or absent in focal regions of RB tumor that displayed enhanced photoreceptor differentiation. These areas do not have a high rate of cellular turnover and necrosis and therefore tend to persist in older patients.43 The three patients in this study showing foci of photoreceptor differentiation with absence of EZH2 staining had poorly or moderately differentiated tumors and were 20, 21 and 26 months of age. In a prior study, Eagle et al. evaluated photoreceptor differentiation in a cohort of enucleated eyes with retinoblastoma and found that photoreceptor differentiation was identified in tumors of all histologic grades and that the mean age of patients was 23 months44. The absence of EZH2 staining in the foci of photoreceptor differentiation is not surprising given photoreceptor differentiation is hypothesized to be a remnant of the precursor lesion (retinocytoma) rather than re-differentiation of the retinoblastoma45, 46. In our clinicopathologic analysis, EZH2 protein was not correlated with histologic grade. Nevertheless, a larger study of RB tumors may be consistent with the possibility that EZH2 may correlate to degree of cellular differentiation, as noted during retinal development and in other carcinomas.18 To this end, it would be interesting to assess EZH2 expression in a precursor RB variant, retinocytoma (retinoma), which we predict would have lower EZH2 expression than RB. Nevertheless, we remain uncertain of the significance EZH2+/high and EZH2−/low RB cell populations, and whether EZH2i may be more effective at eliminating EZH2+/high vs. EZH2−/low RB cells.
While we identified EZH2 protein in all RB enucleation samples, and that the proportion of EZH2 positivity correlated with staining intensity, we did not find a relationship between EZH2-related features and a variety of clinicopathologic features including age, laterality, tumor size, and optic nerve/choroidal invasion. A limitation of this study was that we were unable to analyze the relationship among EZH2 variables, tumor recurrence, and patient survival because the full clinical histories for many patients were not available. Nevertheless, we suspect that increased presence of EZH2 protein in RB tumors correlates with recurrence and poor prognosis, as it does in a variety of other cancers.18 A future study with more complete patient records, and possibly a larger number of patients, would be helpful to verify this hypothesis.
Lei et al. recently demonstrated that EZH2 mRNA levels, measured by quantitative real-time polymerase chain reaction (qRT-PCR), are significantly upregulated in RB.47 Furthermore, they found that EZH2 transcripts are specifically higher in tumors with high-risk features such as poor differentiation, choroidal invasion and orbital invasion. However, our study showed no association between EZH2 protein levels and histologic grade or high-risk invasive features. One explanation is that the upregulation of mRNA does not result in the sustained accumulation of EZH2 protein, due to post-translational modifications.48, 49 Another explanation may be the sensitivity of qRT-PCR versus immunohistochemistry in quantifying EZH2 levels.
A limitation of the Lei et al. study was the inability to localize EZH2 protein to specific cell types, as RNA was prepared from whole tissue extracts.47 In contrast, our immunohistochemical studies allowed localization of EZH2 protein specifically to the proliferating parts of fetal retina and postnatal RB cells. Our analysis allowed us to detect RB invasion into adjacent tissues, which has clinical implications. For instance, pathological evaluation of the tumor margin at the optic nerve and analysis of distal optic nerve for the presence of RB cells may influence therapeutic decision-making. Specifically, positive tumor margins or RB invasion toward distal segments of the optic nerve may lead to a decision to use systemic chemotherapy. We found that EZH2 staining may be helpful as a histopathologic tool because this protein can resolve both the bulk and single tumor cells invading adjacent tissues, which may be otherwise difficult to detect with standard H&E staining. However, only a minority of our cases displayed optic nerve invasion, and none had a positive margin. Also, since RB foci with fleurette photoreceptor differentiation were not stained with EZH2, EZH2 may not be sufficiently sensitive to detect all RB cells invading into the optic nerve. Therefore, a study involving invasive RB with positive margins containing fleurette components would be helpful in determining whether EZH2 would be helpful as a marker for metastatic invasion.
Intravitreal and intraophthalmic artery chemotherapy are emerging as globe-preserving therapies for advanced RB, for which affected eyes may otherwise undergo primary enucleation. However, one common agent, mephalan, has been reported to be toxic to the retina and choroid,9, 11 and may also cause systemic effects, such as neutropenia.10 Intravitreal and intraophthalmic artery approaches could be strengthened by delivery of agents that are more specific to the tumor while sparing non-involved tissues. Our preliminary data with EZH2i indicates that the high EZH2 expression specifically in RB tumor cells, but not primary human RPE, can be pharmacologically exploited. Indeed, a recent microarray profiling study of advanced human RB tumors has revealed EZH2 to be among the most downregulated genes in regressed tumors following systemic chemotherapy.50 Future studies should address local and systemic delivery and toxicities of EZH2i in animal models of RB.
Human RB has among the lowest mutational rates reported in cancer to date.12 The stable genome of RB limits the effectiveness of therapies that depend on targeting accumulated passenger mutations. Nearly all RB tumors are characterized by biallelic inactivation of RB1, but c arry few other mutations. Despite this genetic homogeneity, the behavior of individual RB tumors remains diverse, likely due to epigenetic variations. Thus, epigenetic regulators should be integrated into any approach toward novel RB therapeutics.12 Our goal was to identify a candidate epigenetic regulator whose dysregulation was not only common to RB and other solid tumors, but was also a rapidly actionable target.
EZH2 fits both these categories, and our group had the benefit of being the first to identify this protein in the mammalian retina.28 In this report, we reasoned that since EZH2 is expressed solely in RB tumor cells, but not in any other postnatal retinal cell type, RB tumor would be susceptible to EZH2 inhibition. Indeed, we identify two EZH2i, GSK126 and SAH-EZH2, as potential therapeutic candidates that selectively impair RB cell viability but spare non-tumor retinal cells. Yet, the precise mechanism by which these EZH2i acts on human RB cells may vary. At 5 µM, GSK126 did not affect EZH2 protein levels, but reduced H3K27me3 and viability in RB cells. This is consistent with the mechanism of action of GSK126 as a SET domain inhibitor that selectively reduces EZH2 enzymatic activity at its substrate, H3K27me3, before any effects on EZH2 protein level.35 In contrast, across a range of concentrations, SAH-EZH2 did not change EZH2 protein levels or H3K27me3 status, but did selectively inhibit RB cell viability at 12.5µM or higher without impairing fetal primary RPE viability. Inhibition of H3K27me3-independent EZH2 pathways has emerged as a potentially therapeutic target in castration-resistant prostate cancer and other advanced solid tumors.36 Given that SAH-EZH2 inhibits EZH2 activity by disrupting its interaction with EED, an essential co-factor, rather than directly blocking EZH2’s enzymatic pocket, a H3K27me3-independent mechanism may account for SAH-EZH2’s effects on RB cells in our assays. Thus, our report highlights two pharmacologic probes that can be used to interrogate H3K27me3-dependent and independent pathways in cancers that require EZH2 for survival.
EZH2i for cancer is a rapidly advancing field marked by deep commitments from a variety of biopharmaceutical companies.48, 51 Three clinical trials, NCT01897571, NCT02082977, a nd NCT02395601 (clinicaltrials.gov), are currently recruiting patients with subsets of B-cell lymphoma, pediatric malignant rhabdoid tumor (MRT), synovial sarcoma, and other advanced solid tumors for treatment with small-molecule EZH2i. These agents, EPZ-6438 (E7438) and GSK2816126, inhibit the enzymatic domain of EZH2 and are most closely related to GSK126 used in this study. In the case of B-cell lymphoma, patients must carry a hyperactivating EZH2 mutation, which has not been reported in human RB to date.3, 12 In contrast, MRT and synovial sarcoma are pediatric solid tumors that, like RB, do not carry an EZH2 mutation, but carry another mutation (SMARCB1, also known as INI1, hSNF5, and BAF47) that sensitizes the cancer to EZH2i.52, 53
Our work raises the possibility that if approved, these or newer generation EZH2i could be repurposed for evaluation in animal models of RB, and if promising, potentially toward human RB therapy. Finally, we suspect EZH2 dysregulation to be a common feature among various types of ophthalmic, adnexal, and orbital tumors. Thus EZH2i may offer a launching point toward the development of the first generation of rationally-designed, epigenetic therapies for ocular oncology.
Supplementary Material
Supplementary Table. We found associations for the following non-EZH2 variables: age at the time of enucleation and histologic grade (Pearson’s r = 0.718, p < 0.001); age at time of enucleation and extent of optic nerve invasion (r = 0.417, p = 0.005). These data indicated than an increase in age was associated with a decrease in tumor differentiation and greater extent of invasion into the optic nerve. Patient age was negatively and significantly associated with laterality of retinoblastoma at the time of enucleation (r = −0.331, p = 0.030), demonstrating that younger patients were more likely to present with bilateral tumors. Bilateral tumors were significantly associated with greater degrees of tumor differentiation (r = −0.384, p = 0.011). Tumor size and histologic grade were positively and significantly associated with the extent of optic nerve invasion (r = 0.457, p = 0.002 and r = 0.446, p = 0.003, respectively). Larger or more poorly differentiated tumors were associated with a greater extent of invasion into the optic nerve. Optic nerve invasion was also positively and significantly associated with choroidal invasion (r = 0.464, p = 0.002).
Supplementary Figure. Examples of weak, moderate, and strong EZH2 staining in human RB samples. (A,C,E) Hematoxylin & eosin (H&E) and corresponding (B, D, F) EZH2 staining in human RB samples. Representative weak (B), moderate (D), and strong (F) EZH2 staining is shown. Original magnification ×200. RB retinoblastoma.
Acknowledgments
This work was supported by the National Eye Institute (K12EY022299), a Career Development Award from the Research to Prevent Blindness, and Beat Blindness to R.C.R. J.M.L.M. and Q.Z. are supported by Department of Ophthalmology and Visual Sciences, University of Michigan Pre-Residency Research Fellowship, and J.M.L.M. is also supported by the Department of Ophthalmology and Visual Sciences T32 Visual Sciences Training Program grant from the National Eye Institute.
Footnotes
The authors have no conflicts of interest related to this subject matter to declare.
REFERENCES
- 1.Broaddus E, Topham A, Singh AD. Incidence of retinoblastoma in the USA: 1975–2004. Br J Ophthalmol. 2009;93(1):21–23. doi: 10.1136/bjo.2008.138750. [DOI] [PubMed] [Google Scholar]
- 2.Zhang J, Schweers B, Dyer MA. The first knockout mouse model of retinoblastoma. Cell Cycle. 2004;3(7):952–959. [PubMed] [Google Scholar]
- 3.Rushlow DE, Mol BM, Kennett JY, et al. Characterisation of retinoblastomas without RB1 mutations: genomic, gene expression, and clinical studies. Lancet Oncol. 2013;14(4):327–334. doi: 10.1016/S1470-2045(13)70045-7. [DOI] [PubMed] [Google Scholar]
- 4.Broaddus E, Topham A, Singh AD. Survival with retinoblastoma in the USA: 1975–2004. Br J Ophthalmol. 2009;93(1):24–27. doi: 10.1136/bjo.2008.143842. [DOI] [PubMed] [Google Scholar]
- 5.Boubacar T, Fatou S, Fousseyni T, et al. A 30-month prospective study on the treatment of retinoblastoma in the Gabriel Toure Teaching Hospital, Bamako, Mali. Br J Ophthalmol. 2010;94(4):467–469. doi: 10.1136/bjo.2009.159699. [DOI] [PubMed] [Google Scholar]
- 6.Chantada GL, Qaddoumi I, Canturk S, et al. Strategies to manage retinoblastoma in developing countries. Pediatr Blood Cancer. 2011;56(3):341–348. doi: 10.1002/pbc.22843. [DOI] [PubMed] [Google Scholar]
- 7.Friedman DN, Sklar CA, Oeffinger KC, et al. Long-term medical outcomes in survivors of extra-ocular retinoblastoma: the Memorial Sloan-Kettering Cancer Center (MSKCC) experience. Pediatr Blood Cancer. 2013;60(4):694–699. doi: 10.1002/pbc.24280. [DOI] [PubMed] [Google Scholar]
- 8.Wong JR, Morton LM, Tucker MA, et al. Risk of subsequent malignant neoplasms in long-term hereditary retinoblastoma survivors after chemotherapy and radiotherapy. J Clin Oncol. 2014;32(29):3284–3290. doi: 10.1200/JCO.2013.54.7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Francis JH, Schaiquevich P, Buitrago E, et al. Local and systemic toxicity of intravitreal melphalan for vitreous seeding in retinoblastoma: a preclinical and clinical study. Ophthalmology. 2014;121(9):1810–1817. doi: 10.1016/j.ophtha.2014.03.028. [DOI] [PubMed] [Google Scholar]
- 10.Dunkel IJ, Shi W, Salvaggio K, et al. Risk factors for severe neutropenia following intra-arterial chemotherapy for intra-ocular retinoblastoma. PLoS One. 2014;9(10):e108692. doi: 10.1371/journal.pone.0108692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tse BC, Kaste SC, Brennan R, et al. Enophthalmos and Choroidal Atrophy after Intraophthalmic Artery Chemotherapy for Retinoblastoma. Ophthalmology. 2014 doi: 10.1016/j.ophtha.2014.09.021. [DOI] [PubMed] [Google Scholar]
- 12.Zhang J, Benavente CA, McEvoy J, et al. A novel retinoblastoma therapy from genomic and epigenetic analyses. Nature. 2012;481(7381):329–334. doi: 10.1038/nature10733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rao RC, Chen DF, Miller JW. An epigenetic approach toward understanding ocular alphaherpesvirus pathogenesis and treatment. Int Ophthalmol Clin. 2011;51(4):117–133. doi: 10.1097/IIO.0b013e31822d6966. [DOI] [PubMed] [Google Scholar]
- 14.Rao RC, Dou Y. Hijacked in cancer: the KMT2 (MLL) family of methyltransferases. Nat Rev Cancer. 2015;15(6):334–346. doi: 10.1038/nrc3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rao RC, Hennig AK, Malik MT, et al. Epigenetic regulation of retinal development a nd disease. J Ocul Biol Dis Infor. 2011;4(3):121–136. doi: 10.1007/s12177-012-9083-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet. 2002;3(6):415–428. doi: 10.1038/nrg816. [DOI] [PubMed] [Google Scholar]
- 17.Schneider R, Bannister AJ, Kouzarides T. Unsafe SETs: histone lysine methyltransferases and cancer. Trends Biochem Sci. 2002;27(8):396–402. doi: 10.1016/s0968-0004(02)02141-2. [DOI] [PubMed] [Google Scholar]
- 18.Bachmann IM, Halvorsen OJ, Collett K, et al. EZH2 expression is associated with high proliferation rate and aggressive tumor subgroups in cutaneous melanoma and cancers of the endometrium, prostate, and breast. J Clin Oncol. 2006;24(2):268–273. doi: 10.1200/JCO.2005.01.5180. [DOI] [PubMed] [Google Scholar]
- 19.Bracken AP, Kleine-Kohlbrecher D, Dietrich N, et al. The Polycomb group proteins bind throughout the INK4A-ARF locus and are disassociated in senescent cells. Genes Dev. 2007;21(5):525–530. doi: 10.1101/gad.415507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ezhkova E, Pasolli HA, Parker JS, et al. Ezh2 orchestrates gene expression for the stepwise differentiation of tissue-specific stem cells. Cell. 2009;136(6):1122–1135. doi: 10.1016/j.cell.2008.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen H, Gu X, Su IH, et al. Polycomb protein Ezh2 regulates pancreatic beta-cell Ink4a/Arf expression and regeneration in diabetes mellitus. Genes Dev. 2009;23(8):975–985. doi: 10.1101/gad.1742509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bracken AP, Pasini D, Capra M, et al. EZH2 is downstream of the pRB-E2F pathway, essential for proliferation and amplified in cancer. EMBO J. 2003;22(20):5323–5335. doi: 10.1093/emboj/cdg542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bohrer LR, Chen S, Hallstrom TC, et al. Androgens suppress EZH2 expression via retinoblastoma (RB) and p130-dependent pathways: a potential mechanism of androgen-refractory progression of prostate cancer. Endocrinology. 2010;151(11):5136–5145. doi: 10.1210/en.2010-0436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ajioka I, Martins RA, Bayazitov IT, et al. Differentiated horizontal interneurons clonally expand to form metastatic retinoblastoma in mice. Cell. 2007;131(2):378–390. doi: 10.1016/j.cell.2007.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chakraborty S, Khare S, Dorairaj SK, et al. Identification of genes associated with tumorigenesis of retinoblastoma by microarray analysis. Genomics. 2007;90(3):344–353. doi: 10.1016/j.ygeno.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 26.Copeland RA. Molecular pathways: protein methyltransferases in cancer. Clin Cancer Res. 2013;19(23):6344–6350. doi: 10.1158/1078-0432.CCR-13-0223. [DOI] [PubMed] [Google Scholar]
- 27.Rao RC, Boyd J, Padmanabhan R, et al. Efficient serum-free derivation of oligodendrocyte precursors from neural stem cell-enriched cultures. Stem Cells. 2009;27(1):116–125. doi: 10.1634/stemcells.2007-0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rao RC, Tchedre KT, Malik MT, et al. Dynamic patterns of histone lysine methylation in the developing retina. Invest Ophthalmol Vis Sci. 2010;51(12):6784–6792. doi: 10.1167/iovs.09-4730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maminishkis A, Chen S, Jalickee S, et al. Confluent monolayers of cultured human fetal retinal pigment epithelium exhibit morphology and physiology of native tissue. Invest Ophthalmol Vis Sci. 2006;47(8):3612–3624. doi: 10.1167/iovs.05-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu XL, Fang Y, Lee TC, et al. Retinoblastoma has properties of a cone precursor tumor and depends upon cone-specific MDM2 signaling. Cell. 2009;137(6):1018–1031. doi: 10.1016/j.cell.2009.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu XL, Singh HP, Wang L, et al. Rb suppresses human cone-precursor-derived retinoblastoma tumours. Nature. 2014;514(7522):385–388. doi: 10.1038/nature13813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reid TW, Albert DM, Rabson AS, et al. Characteristics of an established cell line of retinoblastoma. J Natl Cancer Inst. 1974;53(2):347–360. doi: 10.1093/jnci/53.2.347. [DOI] [PubMed] [Google Scholar]
- 33.McFall RC, Sery TW, Makadon M. Characterization of a new continuous cell line derived from a human retinoblastoma. Cancer Res. 1977;37(4):1003–1010. [PubMed] [Google Scholar]
- 34.Kim W, Bird GH, Neff T, et al. Targeted disruption of the EZH2-EED complex inhibits EZH2- dependent cancer. Nat Chem Biol. 2013;9(10):643–650. doi: 10.1038/nchembio.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCabe MT, Ott HM, Ganji G, et al. EZH2 inhibition as a therapeutic strategy for lymphoma with EZH2-activating mutations. Nature. 2012;492(7427):108–112. doi: 10.1038/nature11606. [DOI] [PubMed] [Google Scholar]
- 36.Xu K, Wu ZJ, Groner AC, et al. EZH2 oncogenic activity in castration-resistant prostate cancer cells is Polycomb-independent. Science. 2012;338(6113):1465–1469. doi: 10.1126/science.1227604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cavenee WK, Dryja TP, Phillips RA, et al. Expression of recessive alleles by chromosomal mechanisms in retinoblastoma. Nature. 1983;305(5937):779–784. doi: 10.1038/305779a0. [DOI] [PubMed] [Google Scholar]
- 38.Knudson AG., Jr Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci U S A. 1971;68(4):820–823. doi: 10.1073/pnas.68.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sakai T, Toguchida J, Ohtani N, et al. Allele-specific hypermethylation of the retinoblastoma tumor-suppressor gene. Am J Hum Genet. 1991;48(5):880–888. [PMC free article] [PubMed] [Google Scholar]
- 40.O'Carroll D, Erhardt S, Pagani M, et al. The polycomb-group gene Ezh2 is required for early mouse development. Mol Cell Biol. 2001;21(13):4330–4336. doi: 10.1128/MCB.21.13.4330-4336.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Breuer RH, Snijders PJ, Smit EF, et al. Increased expression of the EZH2 polycomb group gene in BMI-1-positive neoplastic cells during bronchial carcinogenesis. Neoplasia. 2004;6(6):736–743. doi: 10.1593/neo.04160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Snitow ME, Li S, Morley MP, et al. Ezh2 represses the basal cell lineage during lung endoderm development. Development. 2015;142(1):108–117. doi: 10.1242/dev.116947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eagle RC., Jr The pathology of ocular cancer. Eye. 2013;27(2):128–136. doi: 10.1038/eye.2012.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eagle RC., Jr High-risk features and tumor differentiation in retinoblastoma: a retrospective histopathologic study. Archives of pathology & laboratory medicine. 2009;133(8):1203–1209. doi: 10.5858/133.8.1203. [DOI] [PubMed] [Google Scholar]
- 45.Dimaras H, Khetan V, Halliday W, et al. Loss of RB1 induces non-proliferative retinoma: increasing genomic instability correlates with progression to retinoblastoma. Human molecular genetics. 2008;17(10):1363–1372. doi: 10.1093/hmg/ddn024. [DOI] [PubMed] [Google Scholar]
- 46.Dimaras H, Khetan V, Halliday W, et al. Retinoma underlying retinoblastoma revealed after tumor response to 1 cycle of chemotherapy. Archives of ophthalmology. 2009;127(8):1066–1068. doi: 10.1001/archophthalmol.2009.178. [DOI] [PubMed] [Google Scholar]
- 47.Lei Q, Shen F, Wu J, et al. MiR-101, downregulated in retinoblastoma, functions as a tumor suppressor in human retinoblastoma cells by targeting EZH2. Oncol Rep. 2014;32(1):261–269. doi: 10.3892/or.2014.3167. [DOI] [PubMed] [Google Scholar]
- 48.Yamaguchi H, Hung MC. Regulation and Role of EZH2 in Cancer. Cancer Res Treat. 2014;46(3):209–222. doi: 10.4143/crt.2014.46.3.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chu CS, Lo PW, Yeh YH, et al. O-GlcNAcylation regulates EZH2 protein stability and function. Proc Natl Acad Sci U S A. 2014;111(4):1355–1360. doi: 10.1073/pnas.1323226111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nalini V, Segu R, Deepa PR, et al. Molecular Insights on Post-chemotherapy Retinoblastoma by Microarray Gene Expression Analysis. Bioinform Biol Insights. 2013;7:289–306. doi: 10.4137/BBI.S12494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bradley WD, Arora S, Busby J, et al. EZH2 Inhibitor Efficacy in Non-Hodgkin's Lymphoma Does Not Require Suppression of H3K27 Monomethylation. Chem Biol. 2014;21(11):1463–1475. doi: 10.1016/j.chembiol.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 52.Knutson SK, Warholic NM, Wigle TJ, et al. Durable tumor regression in genetically altered malignant rhabdoid tumors by inhibition of methyltransferase EZH2. Proc Natl Acad Sci U S A. 2013;110(19):7922–7927. doi: 10.1073/pnas.1303800110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Changchien YC, Tatrai P, Papp G, et al. Poorly differentiated synovial sarcoma is associated with high expression of enhancer of zeste homologue 2 (EZH2) J Transl Med. 2012;10:216. doi: 10.1186/1479-5876-10-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table. We found associations for the following non-EZH2 variables: age at the time of enucleation and histologic grade (Pearson’s r = 0.718, p < 0.001); age at time of enucleation and extent of optic nerve invasion (r = 0.417, p = 0.005). These data indicated than an increase in age was associated with a decrease in tumor differentiation and greater extent of invasion into the optic nerve. Patient age was negatively and significantly associated with laterality of retinoblastoma at the time of enucleation (r = −0.331, p = 0.030), demonstrating that younger patients were more likely to present with bilateral tumors. Bilateral tumors were significantly associated with greater degrees of tumor differentiation (r = −0.384, p = 0.011). Tumor size and histologic grade were positively and significantly associated with the extent of optic nerve invasion (r = 0.457, p = 0.002 and r = 0.446, p = 0.003, respectively). Larger or more poorly differentiated tumors were associated with a greater extent of invasion into the optic nerve. Optic nerve invasion was also positively and significantly associated with choroidal invasion (r = 0.464, p = 0.002).
Supplementary Figure. Examples of weak, moderate, and strong EZH2 staining in human RB samples. (A,C,E) Hematoxylin & eosin (H&E) and corresponding (B, D, F) EZH2 staining in human RB samples. Representative weak (B), moderate (D), and strong (F) EZH2 staining is shown. Original magnification ×200. RB retinoblastoma.