Abstract
Objective
The aim of this study was to determine whether selected damage-associated molecular patterns (DAMPs) present in osteoarthritic (OA) joints excite nociceptors through toll-like receptor (TLR)-4.
Methods
The ability of S100A8 and α2-macroglobulin to excite nociceptors was determined by measuring: (1) Release of monocyte chemoattractant protein (MCP)-1 by cultured dorsal root ganglion (DRG) cells; (2) Intracellular calcium (Ca)i levels in cultured DRG neurons from naïve mice or mice 8 weeks after destabilization of the medial meniscus (DMM). The role of TLR4 was assessed using Tlr4−/− cells or a TLR4 inhibitor. (Ca)i levels in neurons within ex vivo intact DRG were measured using Pirt-GCaMP3 mice. Neuronal Tlr4 expression was determined by in situ hybridization. DMM surgery was performed in wild-type and Tlr4−/− mice; mechanical allodynia was monitored, and joint damage was assessed histologically after 16 weeks.
Results
Both naïve and DMM DRG neurons expressed Tlr4. Both S100A8 and α2-macroglobulin stimulated release of the pro-algesic chemokine, MCP-1, by DRG cultures and neurons rapidly responded to S100A8 and α2-macroglobulin with increased (Ca)i. Blocking TLR4 inhibited these effects. Neurons within intact DRG responded to the TLR4 agonist, lipopolysaccharide. In both calcium-imaging assays, it was primarily the nociceptor population of neurons that responded to TLR4 ligands. Tlr4−/− mice were not protected from mechanical allodynia or from joint damage associated with DMM.
Conclusion
Our experiments suggest a role for TLR4 signaling in the excitation of nociceptors by selected DAMPs. Further research is needed to delineate the importance of this pathway in relation to OA pain.
Keywords: pain, osteoarthritis, innate immunity, mouse models, other
INTRODUCTION
Pain represents a protective response to potentially damaging stimuli and is therefore essential to health. Under normal circumstances, both the stimuli that cause pain and the ensuing response are relatively transient in nature. However, pain may also be experienced in the context of chronic pathology. In these chronic pain syndromes, nerves that normally transmit painful information to the brain undergo numerous changes in their properties, resulting in hypersensitivity to painful stimulation or in allodynia - defined as pain in response to a normally innocuous stimulus - or even in pain in the absence of a stimulus (1).
Chronic joint disease is frequently accompanied by pain. Notably, osteoarthritis (OA), the most common form of arthritis, is a major source of chronic pain (2). The lifetime risk of developing symptomatic knee OA is approximately 45% based on Johnston County Osteoarthritis Project data (3) and worldwide, OA is a leading cause of chronic pain (4). Treatment options for OA pain include acetaminophen, nonsteroidal anti-inflammatory drugs, opioids, and intra-articular therapy with glucocorticoid or hyaluronan preparations, but their efficacy is limited and chronic use can be associated with adverse effects (5).
In order to develop improved analgesics, a better understanding of mechanisms that generate and maintain OA pain is necessary. Current evidence suggests that ongoing nociceptive input from the joint contributes to the maintenance of OA knee pain (6). The pathology of knee OA affects all joint tissues, including cartilage, subchondral bone, menisci, ligaments, and synovium (7). With the exception of articular cartilage, joint tissues are innervated by sensory neurons. The cell bodies of these afferents are contained in the dorsal root ganglia (DRG). These neurons are pseudounipolar, extending one axon toward the periphery and the other to the dorsal horn of the spinal cord, where synapses are formed with second-order neurons. Nociceptive neurons, either small unmyelinated C-fibers or medium-sized lightly myelinated Aδ-fibers, are a subtype of sensory afferents that detect mechanical, chemical or thermal noxious stimuli in the innervated tissues (1). Therefore, it is of great interest to define mediators in the OA joint that can sensitize nociceptors and lead to changes that contribute to pain. Numerous inflammatory mediators, including a number of cytokines and chemokines present in the arthritic joint, are capable of activating nociceptors, and may thus contribute to joint pain (8–10).
In addition to traditional inflammatory cytokines like IL-1β, IL-6, and TNF-α, damage-associated molecular patterns (DAMPs) (11, 12) have been implicated in driving the chronic, low-level inflammation associated with OA (11, 13). DAMPs can activate pattern recognition receptors (PRR) on chondrocytes and synovial macrophages, thus promoting cartilage degradation and synovitis in OA (14). These PRR include toll-like receptors (TLR) and the receptor for advanced glycation end products (RAGE). In particular, TLR4 has been highlighted as a potential target for disease-modifying OA drugs (15).
Since it has recently been reported that DRG neurons also express PRR (16–19), including TLR4 (16, 20, 21), we hypothesized that DAMPs generated in the OA joint can directly excite nociceptors and thus provide a putative link between tissue damage and pain development. To test this hypothesis, we chose to investigate two endogenous activators of TLR4 present in OA joints, namely, S100A8 (22) and α2-macroglobulin (23). Synovial biopsies taken from patients with early, symptomatic knee OA had significantly elevated expression of the alarmin, S100A8, which correlated with the presence of synovitis (24). Patients undergoing total joint replacement also had abundant S100A8 synovial expression (24). The plasma protein, α2-macroglobulin, is detectable in synovial fluid in both early- and late-stage knee OA (23, 25–27). S100A8 and α2-macroglobulin were shown to promote catabolic pathways in macrophages through TLR4 (22, 23). Therefore, we investigated the ability of these DAMPs to excite sensory neurons through TLR4 and promote a pro-algesic state. We have previously shown that wild-type mice develop sustained mechanical allodynia for 16 weeks after destabilization of the medial meniscus (DMM), but not after sham surgery, indicating that maintenance of mechanical allodynia is associated with joint damage in this model (28, 29). Therefore, we tested the role of TLR4 in the development of mechanical allodynia, a behavioral measure of sensitization, in the DMM model.
MATERIALS AND METHODS
Animals and surgery
A total of 125 mice were used. All animal experiments were approved by the Institutional Animal Care and Use Committees at Rush University Medical Center and Northwestern University. Animals were housed with food and water ad libitum and kept on 12-hour light cycles. Wild-type C57BL/6, Tlr4−/− mice (Jackson and courtesy of Dr. David Hackam, University of Pittsburgh, C57BL/6 background (30)), and Pirt-GCaMP3 mice (courtesy of Dr. Xinzhong Dong, Johns Hopkins University, C57BL/6 background (31)) were used. DMM surgery was performed as previously described (29, 32) in the right knee of 10-week old male mice. Briefly, after medial parapatellar arthrotomy, the anterior fat pad was dissected to expose the anterior medial meniscotibial ligament, which was severed. The knee was flushed with saline and the incision closed.
DRG cell culture and stimulations
Cells were isolated from knee-innervating dorsal root ganglia (L3-L5) of 3–4 mice (male or female naïve C57BL/6 or Tlr4−/− mice at least 10 weeks of age or male C57BL/6 mice 8 weeks after DMM surgery), plated onto glass coverslips, and cultured in adult neurogenic medium, as previously described (29). Overnight stimulations from days 3–4 were carried out with S100A8 (0–1 μg/mL, Prospec, East Brunswick, NJ) or α2-macroglobulin (0–100 μg/mL, EMD Millipore, Billerica, MA) ± the selective small molecule TLR4 inhibitor, Tak242 (1 μM, EMD Millipore). Stimulation with 10 ng/mL IL-1α (Peprotech, Rocky Hill, NJ) was used as a positive control. Similar results were obtained ± polymyxin B (inhibitor of lipopolysaccharide (LPS) and used to control for endotoxin contamination) (10 μg/mL, Sigma-Aldrich, St. Louis, MO). In addition, endotoxin levels in S100A8 and α2-macroglobulin were confirmed to be <0.01 EU/μg by LAL assay (Fisher Scientific, Pittsburgh, PA). As a positive control for TLR4 activation, stimulations were performed using the TLR4 ligand LPS (1 μg/mL) (Ultra-pure LPS from E. coli O111:B4 purified so that only TLR4 is activated (33); InvivoGen, San Diego, CA, cat#tlrl-3pelps). Supernatants were collected for protein analysis. Three independent experiments were performed per stimulus.
Protein analysis of supernatant
Total protein levels were determined by BCA assay (Thermo Fisher Scientific, Inc., Rockford, IL), and MCP-1 protein levels were determined via ELISA (R&D Systems Inc, Minneapolis, MN).
In situ hybridization
Ipsilateral L3-L5 DRG were harvested, embedded, and sectioned as previously described (29). For the generation of Tlr4 probes, a 405-bp Tlr4 cDNA fragment (GenBank no. NM_021297) was cloned by PCR by using mouse brain cDNA. The resulting PCR product was subcloned into a pGEM-T Easy Vector (Promega Madison, WI) and verified by restriction analysis and automated DNA sequencing (Perkin Elmer, Boston, MA). The Tlr4 template was linearized with Xba I to generate an antisense probe by using SP6 polymerase. The sense probe was linearized with Hind III by using T7 polymerase. In situ hybridization histochemistry for Tlr4 was performed by using digoxigenin-labeled riboprobes (Roche Applied Science, Indianapolis, IN) as previously described (34).
In vitro calcium imaging
The response of cultured DRG neurons to selected DAMPs was recorded though intracellular Ca2+-imaging, following standard protocols using Fura-2AM (2 μM; Life Technologies, Grand Island, NY) (29, 35). S100A8 (1 μg/mL) or α2-macroglobulin (100 μg/mL) was applied for 3 min by adding 0.5–1 mL of solution to the bath chamber. Cells were washed with balanced salt solution before applying controls (potassium (50 mM) and capsaicin (10 μM)). LPS (1 μg/mL) was used as a positive control for TLR4 activation. Three independent experiments, each using DRG pooled from 4 naïve wild-type C57BL/6, three independent experiments each using DRG pooled from 4 wild-type C57BL/6 mice 8 weeks after DMM surgery, and two independent experiments using DRG pooled from 4 Tlr4−/− naïve mice, were performed.
Ex vivo calcium imaging of intact DRG
Intact DRG (L4 or L5) were isolated from naïve male or female Pirt-GCaMP3 mice and equilibrated in artificial cerebrospinal fluid (ACSF) (31) bubbled with 95% O2/5% CO2 on ice. After 30 minutes, explants were placed in a perfusion chamber within ACSF and imaged using a CSU-X1 spinning disk confocal microscope (Intelligent Imaging Innovations, Inc., Denver, CO) at 20x magnification at the 488 nm wavelength. Explants were stimulated by injecting 10 μL of LPS solution into a continuously running perfusion chamber with a volume of 1 mL (LPS = 50 μg/mL). Positive controls (potassium and capsaicin) were applied as for in vitro calcium imaging. Image analysis was performed using an ImageJ (36) macro to determine change in fluorescence intensity with time. Neurons with spontaneous responses to perfusion buffer were excluded.
von Frey testing
Wild-type or Tlr4−/− mice were tested for mechanical allodynia using von Frey fibers as previously described (29). Baseline thresholds were assessed prior to surgery, and weeks 2, 4, 8, 12, and 16 after DMM. Results are representative of two independent experiments.
Histopathology of the knee
Sixteen weeks after DMM, histopathology of the knee was evaluated based on OARSI recommendations (39) (Alison Bendele, Bolder BioPATH, Inc., Boulder CO). Joints were fixed in 10% formalin, decalcified, embedded in the frontal plane, sectioned, and stained with Toluidine blue, as described (29). Medial and lateral femoral condyles and tibial plateaux were scored for severity of cartilage degeneration on a scale of 0–5, with 5 representing the most damage (maximum summed score=60). Scoring of the osteophytes on the medial and lateral sides (largest on tibial or femoral surface under evaluation) and categorization into small, medium and large was done with an ocular micrometer and scored from 0–3 (3 = large).
Statistical analysis
For MCP-1 stimulation experiments, one-way ANOVA with Bonferroni post-tests or unpaired t-tests assuming equal variances were used to compare the groups of interest. For calcium imaging experiments, Fisher’s exact tests were used to compare the number of responses in capsaicin-sensitive neurons versus non-capsaicin-sensitive neurons. For von Frey testing, one-way ANOVA with Bonferroni post-tests was used to compare each time point to time 0. For joint histopathology, an unpaired t-test assuming equal variance was used to compare Tlr4−/− mice to wild-type mice. A p-value < 0.05 was considered significant for all tests. All analyses were carried out using GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego, CA). Results are presented as mean ± standard error of the mean.
RESULTS
MCP-1 production by cultured DRG cells
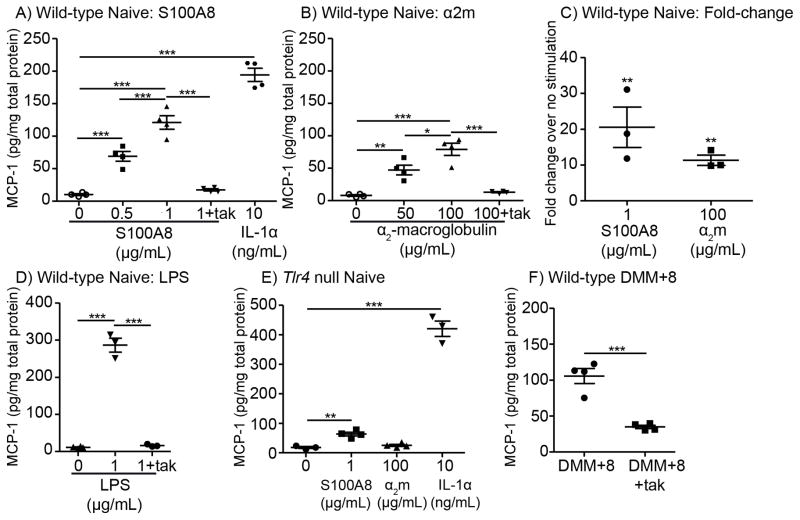
In order to determine whether selected DAMPs can promote a pro-algesic state in DRG cells, we stimulated primary cultures of DRG cells and measured release of the chemokine MCP-1 (CCL2) into culture medium, since we previously found that MCP-1 is upregulated by DRG neurons in the DMM model and acts as a key mediator of pain (29). Overnight stimulation of DRG cells with S100A8 or α2-macroglobulin induced release of MCP-1, in a concentration-dependent manner (Figs 1A,B). IL-1α was included as a positive control for MCP-1 stimulation (Fig 1A). A subset of experiments was performed in the presence of polymyxin B to test for endotoxin contamination. Similar results were found with or without polymyxin B (Supp. Fig 1). In three independent experiments, both S100A8 and α2-macroglobulin induced significantly greater amounts of MCP-1 compared to unstimulated cells (Fig 1C).
Figure 1.
Candidate osteoarthritis DAMPs induce MCP-1 production by DRG cells primarily through TLR4. A) Wild-type DRG cells were stimulated with 0, 0.5, or 1 μg/mL S100A8 ± the small molecule TLR4 inhibitor Tak242 (Tak, 1 μM) or with 10 ng/mL IL-1α. Representative of three independent experiments. B) Wild-type DRG cells were stimulated with 0, 50, or 100 μg/mL α2-macroglobulin, ± Tak242 (Tak, 1 μM). Representative of three independent experiments. C) Fold-change plot combines data from the three independent experiments for S100A8 and α2-macroglobulin (α2m); **p<0.01 vs no stimulation. D) Wild-type DRG cells were stimulated with 1 μg/mL LPS ± 1 μM Tak242. Representative of two independent experiments. E) Tlr4−/− DRG cells were stimulated with 1 μg/mL S100A8, 100 μg/mL α2-macroglobulin, or 10 ng/mL IL-1α. Representative of three independent experiments for S100A8 and two experiments for α2-macroglobulin. F) DRG cells taken from mice 8 weeks after surgery were incubated ± 1 μM Tak242. Representative of three independent experiments. *p<0.05, **p<0.01, ***p<0.001; mean±SEM.
Role of Toll-like receptor 4 in MCP-1 production
Since the TLR4 pathway has been reported to mediate cytokine production in response to these DAMPs in other cell types, including macrophages and phagocytes (22, 23, 40), we investigated whether the observed MCP-1 upregulation was mediated through TLR4. A small molecule TLR4 inhibitor, Tak242 (1 μM), significantly inhibited MCP-1 production induced by S100A8 (83±2% inhibition, in 3 independent experiments) and by α2-macroglobulin (88±2% inhibition, in 3 independent experiments) (Fig 1A,B). The pattern-associated molecular pattern (PAMP) lipopolysaccharide (TLR4-selective LPS from E. coli O111:B4 (33)) was tested as a positive control for the ability of the TLR4 pathway to induce MCP-1 release. LPS stimulated the production of MCP-1 19.0±6.6-fold over unstimulated cells, and Tak242 completely blocked LPS-stimulated MCP-1 release (Fig 1D).
In Tlr4−/− cells, stimulation of MCP-1 production by S100A8 and by α2-macroglobulin was inhibited to a similar extent as seen with Tak242, while IL-1α still provoked robust stimulation of MCP-1 (Fig 1E). Specifically, S100A8 stimulated only 3.0±0.3-fold production of MCP-1 compared to unstimulated Tlr4−/− cells, whereas α2-macroglobulin stimulation was completely inhibited (1.4±0.03-fold compared to unstimulated Tlr4−/− cells).
Together, these results suggest that S100A8 and α2-macroglobulin primarily signal through TLR4 on DRG neurons.
Blocking TLR4 reduces MCP-1 produced by DRG cells harvested 8 weeks after DMM surgery
We have previously shown that, 8 weeks after DMM surgery, cultured DRG cells produce increased amounts of MCP-1 compared to sham and to age-matched naïve neurons (29). Here, we confirmed our previous results, finding that DRG cells taken from DMM mice 8 weeks after surgery produce more MCP-1 than naïve cells (Fig 1A,F). Incubating DMM-DRG cultures with Tak242 significantly reduced MCP-1 production, suggesting that part of the upregulated expression of MCP-1 by these cells in OA is driven by activation of TLR4 (Fig 1F).
DRG neurons express Tlr4
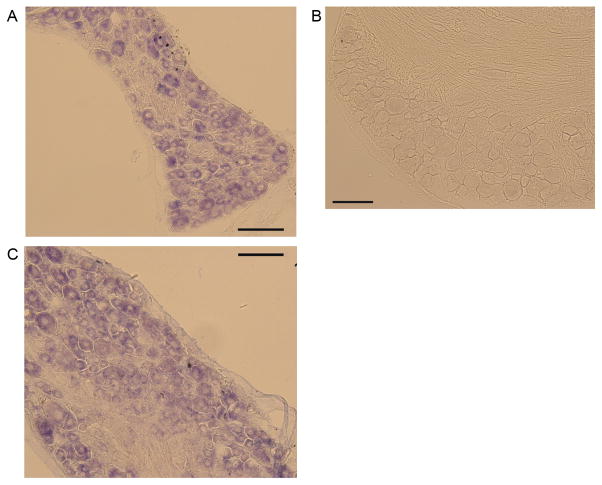
In order to determine which cells in the DRG express Tlr4, in situ hybridization was performed. Staining DRG sections from naïve mice with the anti-sense probe revealed that Tlr4 was widely expressed by small-to-medium-diameter neurons (Fig 2A), which is consistent with previous reports utilizing an immunohistochemical approach (20, 21). A sense probe was used as a negative control (Fig 2B). Similar numbers and sizes of neurons expressed Tlr4 8 weeks after DMM compared to naïve expression (Fig 2C).
Figure 2.
A) Representative image of in situ hybridization using an anti-sense probe for Tlr4 in DRG sections taken from wild-type naïve mice, n=2. B) Sense probe control. C) Representative image of in situ hybridization using an anti-sense probe for Tlr4 in DRG sections taken from wild-type DMM mice 8 weeks after surgery, n=2. Magnification 20x. Scale bars, 100 μm.
Calcium imaging in cultured naïve DRG neurons
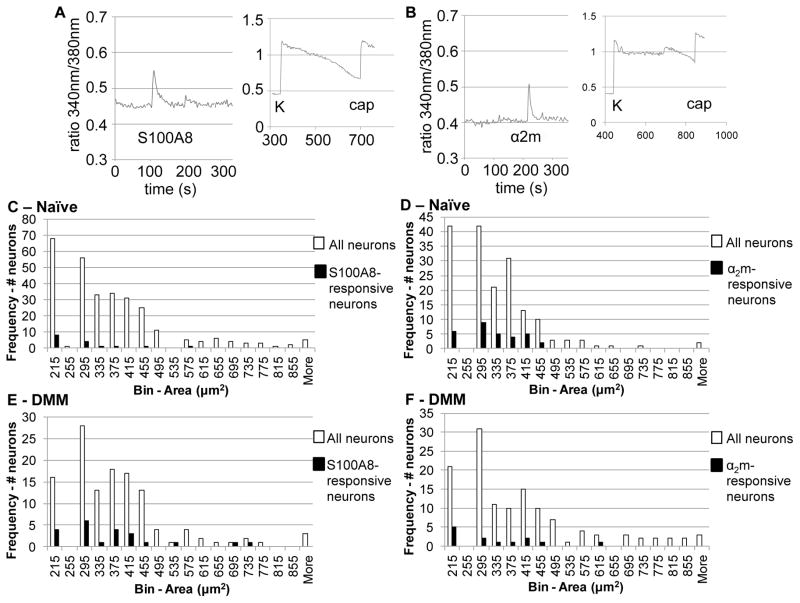
We next sought to determine whether S100A8 and α2-macroglobulin can directly excite DRG neurons by examining their ability to elicit calcium signals. Cultured DRG neurons rapidly responded to S100A8 (1 μg/mL) and to α2-macroglobulin (100 μg/mL), as indicated by increased intracellular calcium ((Ca)i) levels, suggesting that DRG neurons express receptors for these proteins (Fig 3A,B). Response to potassium (K) depolarization to activate voltage-dependent Ca-channels was used to confirm that each traced cell was a viable sensory neuron. Both S100A8 and α2-macroglobulin induced responses in small-to-medium-diameter neurons, which is consistent with the size of nociceptors (Fig 3C,D).
Figure 3.
Candidate osteoarthritis DAMPs induce intracellular calcium (Ca)i increases in cultured primary DRG neurons. Representative traces showing (Ca)i increases in a wild-type DRG neuron in response to A) S100A8 and to B) α2-macroglobulin (α2m). Insets show response to 50 mM potassium (K), which was used to verify neuronal identity and viability, and response to 10 μM capsaicin (cap), which was used to identify TRPV1-expressing nociceptors. Histograms showing areas of naïve neurons (in μm2) responding to C) S100A8 and to D) α2-macroglobulin relative to histograms showing the areas of all imaged neurons, both responsive and non-responsive. Histograms showing areas of neurons from DMM mice 8 weeks after surgery (in μm2) responding to E) S100A8 and to F) α2-macroglobulin relative to histograms showing the areas of all imaged neurons, both responsive and non-responsive.
Further, we divided responses into two categories, responses by capsaicin-sensitive neurons (cap; marker for transient receptor potential vanilloid-1 (TRPV1)-expressing nociceptive neurons) or by non-capsaicin-sensitive neurons. Both S100A8 and α2-macroglobulin induced more responses in capsaicin-sensitive neurons compared to non-capsaicin-sensitive neurons (Table 1), although only α2-macroglobulin induced significantly greater responses in capsaicin-sensitive neurons. LPS-induced (Ca)i increases were also primarily observed in capsaicin-sensitive neurons (Table 1).
Table 1.
Calcium imaging of DRG neurons
| Type of Imaging | Protein tested | (# neuronal responses)/(total # capsaicin-sensitive neurons), (%) | (# neuronal responses)/(total # non-capsaicin-sensitive neurons), (%) | (# neuronal responses)/(total # neurons), (%) | p-value (capsaicin-sensitive vs non-capsaicin-sensitive) | number of independent experiments |
|---|---|---|---|---|---|---|
| Naïve in vitro | S100A8 (1 μg/mL) | 10/127 (8%) | 4/134 (3%) | 14/261 (5%) | 0.1011 | 3 |
| DMM+8 in vitro | S100A8 (1 μg/mL) | 18/82 (22%) | 5/52 (10%) | 23/134 (17%) | 0.0984 | 3 |
| Naïve in vitro | α2-macroglobulin (100 μg/mL) | 23/98 (23%) | 10/120 (8%) | 33/218 (15%) | 0.0023 | 3 |
| DMM+8 in vitro | α2-macroglobulin (100 μg/mL) | 15/76 (20%) | 7/81 (9%) | 22/157 (14%) | 0.0646 | 3 |
| Naïve in vitro | LPS (1 μg/mL) | 5/52 (10%) | 1/54 (2%) | 6/106 (6%) | 0.11 | 1 |
| Naïve ex vivo | LPS (50 μg/mL) | 30/104 (29%) | 0/82 (0%) | 30/186 (16%) | <0.0001 | 2 |
In DRG neurons taken from Tlr4−/− mice, responses to S100A8 and α2-macroglobulin were seen in a reduced number of neurons compared to wild-type neurons (Data combining two independent experiments: S100A8: 3/113 neurons (2.7%); α2-macroglobulin 1/99 neurons (1.0%); compare to wild-type responses in column 5 of Table 1), again confirming that S100A8 and α2-macroglobulin primarily signal through TLR4 on DRG neurons.
Calcium imaging in cultured DMM DRG neurons
We also tested whether DRG neurons taken from DMM mice 8 weeks after surgery could be directly excited by S100A8 or by α2-macroglobulin. Increased numbers of neurons taken from DMM mice responded to S100A8 compared to naïve neurons (p=0.0004, Table 1). In contrast, similar numbers of neurons from DMM mice responded to α2-macroglobulin compared to naïve neurons, perhaps reflecting differences in the way these two stimuli engage and signal through the TLR4 receptor. In both cases, responses were primarily in small-to-medium-diameter, capsaicin-sensitive neurons, similar to the naïve findings (Fig 3E,F; Table 1).
Ex vivo calcium imaging in intact DRG explants
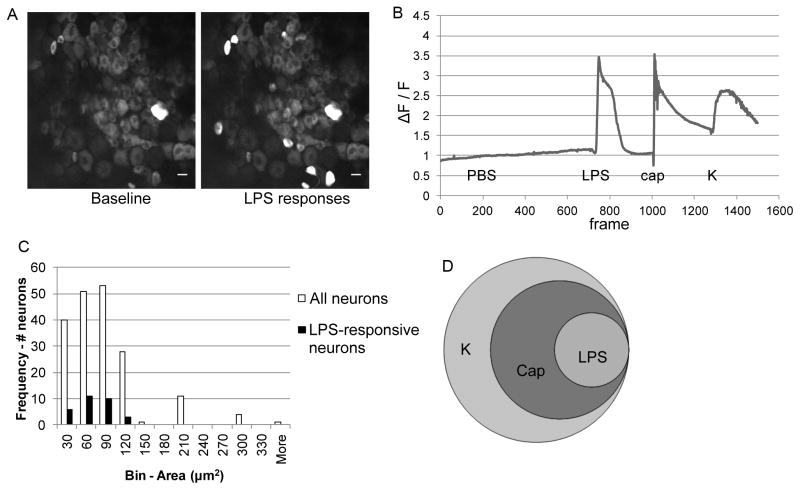
In order to produce a more complete picture of the effects of TLR4 activation on sensory ganglia, ex vivo calcium imaging was performed using intact DRG explants from naïve Pirt-GCaMP3 mice. Pirt-GCaMP3 mice express a genetically-encoded fluorescent calcium indicator (GCaMP3) in almost all primary sensory neurons via the Pirt promoter, which is selective for DRG neurons and absent from other peripheral tissues, glia, and the central nervous system (31). In contrast to in vitro calcium imaging of cultured cells, this technique visualizes (Ca)i changes by DRG neurons in situ, providing additional evidence that TLR4 expression and activation by DRG neurons is not a cell-culture artifact. Using this technique, LPS induced rapid (Ca)i increases in DRG neurons (representative screen shot and trace shown in Fig 4A,B, Videos 1–4). Responses to LPS were seen in small-to-medium-diameter neurons (Fig 4C). All neurons responding to LPS also responded to capsaicin (Table 1, Fig 4D). Together, this is consistent with LPS inducing responses specifically in TRPV1-expressing nociceptors.
Figure 4.
The TLR4 agonist LPS induces intracellular calcium (Ca)i increases in neurons contained within intact DRG. Representative A) images (scale bar = 10 μm) and B) traces of Pirt-GCaMP3 DRG neuron responses to LPS (50 μg/mL). The trace plots the relative change in fluorescence (ΔF/F), indicative of (Ca)i increases. Response to 10 μM capsaicin (cap) was used to identify TRPV1-expressing nociceptors, and response to 50 mM potassium (K) was used to verify neuronal identity and viability. Representative of two independent experiments. C) Histogram showing areas of neurons (μm2) responding to LPS compared to histogram showing size of all imaged neurons. D) Venn diagram showing the relationship among LPS-, capsaicin- (cap), and potassium- (K) responding neurons.
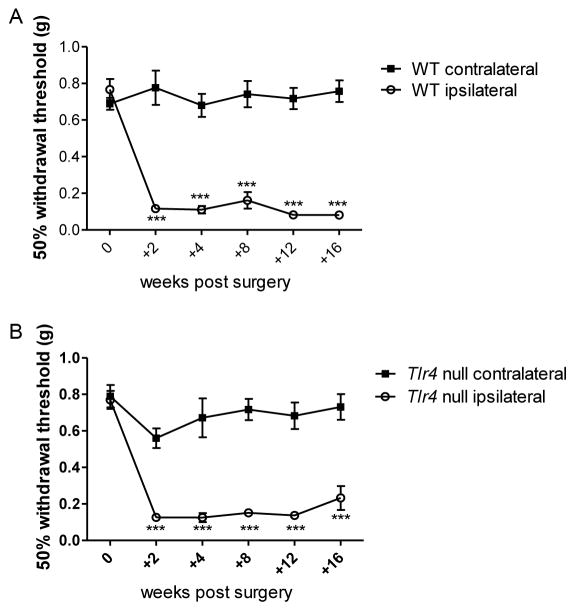
Role of TLR4 in mechanical allodynia associated with experimental osteoarthritis
In order to assess whether TLR4 is necessary for the development of mechanical allodynia, DMM surgery was performed in Tlr4−/− mice and mechanical allodynia was monitored for 16 weeks. Mechanical allodynia developed by 2 weeks after surgery and was maintained through 16 weeks, similar to what was seen in wild-type mice (29) (Fig 5A,B, representative of two independent experiments). In addition, 16 weeks after DMM, Tlr4−/− mice (cartilage degeneration score = 19.1±3.6; osteophyte score = 1.9±0.3; n=9) showed similar joint damage as wild-type mice (cartilage degeneration score = 19.1±2.6; osteophyte score = 1.9±0.3; n=11).
Figure 5.
Wild-type (WT) (A) and Tlr4−/− mice (B) develop ipsilateral mechanical allodynia (n=6–12 mice/time point) through 16 weeks post DMM surgery. ***p<0.001 vs time 0. mean±SEM. Representative of two independent experiments.
DISCUSSION
In these experiments, we have demonstrated for the first time that DRG neurons can respond to representative molecules from two classes of DAMPs present in osteoarthritic joints: alarmins, represented by S100A8, and plasma proteins, represented by α2-macroglobulin. TLR4 is the primary receptor through which S100A8 and α2-macroglobulin excite DRG neurons, as shown by blockade using a small-molecule TLR4 inhibitor and Tlr4−/− mice. Our previous results demonstrated that MCP-1 and its receptor, CCR2, are upregulated by DRG neurons 8 weeks after DMM surgery (29). In addition, Ccr2−/− mice are protected from persistent pain in the DMM model. The ability of DAMPs such as S100A8 and α2-macroglobulin to upregulate MCP-1 production in DRG neurons, and the ability of a TLR4 inhibitor to reduce MCP-1 released by DRG cells 8 weeks after DMM surgery, suggests that the TLR4 pathway may be an important upstream pathway for the promotion of pain. However, Tlr4−/− mice were not protected from developing mechanical allodynia associated with joint damage after DMM surgery, indicating that pathways other than TLR4 contribute to the development of OA-associated mechanical allodynia in this model.
Chondrocytes demonstrate increased S100a8 mRNA expression during the first two weeks after DMM, while chondrocyte immunostaining in load-bearing areas of cartilage was lost during this time (41). This led the authors of that study to speculate that S100A8 is secreted into the extracellular space where it may act as a cytokine-like molecule during early OA (41). Extracellular homodimeric S100A8 and S100A9, but not the heterodimer S100A8/A9, have been shown to induce catabolic responses in human (42) and ovine (41) chondrocytes. S100A8 and S100A9 stimulation of chondrocyte monolayers from OA patients upregulated MCP-1 expression, and S100A9 stimulation was mediated through TLR4 signaling (S100A8 stimulation of MCP-1 was not tested for TLR4 dependency in that study) (42). S100A8 has also been associated with RAGE signaling under certain circumstances (43). Here, we demonstrate that S100A8-stimulated MCP-1 release by DRG neurons is primarily mediated by TLR4. Our findings suggest that, in addition to acting on chondrocytes, extracellular S100A8 may be able to act directly on joint nociceptors in early OA.
Synovial fluid levels of the endoproteinase inhibitor, α2-macroglobulin, are elevated in both early- and late-stage OA (23, 25–27). α2-Macroglobulin inhibits a wide range of proteases present in synovial fluid, including MMPs and ADAMTSs (27, 44, 45) and intra-articular administration of α2-macroglobulin has been found to inhibit cartilage degradation in the rat anterior cruciate ligament transection model, consistent with its protease-inhibiting ability (46). Conversely, this protein may also promote inflammation by stimulating macrophages to produce cytokines (47) through TLR4 signaling (23). Here, α2-macroglobulin stimulated DRG cells to produce MCP-1 through TLR4.
Using calcium imaging assays, we demonstrated that in both naïve and DMM cells (Ca)i increases in response to S100A8 and to α2-macroglobulin occur in small-to-medium-diameter neurons, and that the majority of responses occur in neurons that express TRPV1 (capsaicin-sensitive neurons). Concordantly, in situ hybridization showed that mainly small-to-medium-diameter neurons express Tlr4, both in naive mice and in mice 8 weeks after DMM. Together, these findings suggest that it is the nociceptor population that is able to directly respond to these DAMPs. This is in line with recent studies showing that the majority of calcium responses by cultured neurons to another TLR4 agonist, HMGB1, were in capsaicin-sensitive neurons (16, 48). In addition, 6% of neurons responded to HMGB1, which again, is consistent with the percentage of naïve neurons shown here to respond to S100A8 (5%) and to α2-macroglobulin (15%). Although the numbers of neurons responding to S100A8 and to α2-macroglobulin were decreased in Tlr4−/− mice, a few neurons still responded, indicating that other pathways, including RAGE, may also facilitate responses to these DAMPs.
Through the use of genetically modified Pirt-GCaMP3 mice, it was possible to visualize (Ca)i increases in DRG neurons within seconds of application of LPS, a TLR4 ligand, while neurons were still contained in intact DRGs. Intact DRG are more relevant to the in vivo situation, providing evidence that these functional responses to TLR4 ligands are not an artifact of cell isolation and culture and that neurons are able to directly respond to these stimuli. These results support previous work in cell culture showing TLR4-mediated LPS-induced (Ca)i increases in dissociated DRG neurons (16, 20, 49, 50). Again, the fact that responses were seen primarily in capsaicin-sensitive neurons suggests that LPS-responsive neurons are a subpopulation of TRPV1-expressing nociceptors, which is consistent with recent reports studying calcium fluxes induced by LPS in cell culture (16, 20). Although we have not identified the source of the (Ca)i signal in cell culture or whole ganglion experiments, published observations that activation of TLR4 can depolarize populations of DRG neurons indicate that a rise in (Ca)i represents direct excitation of neurons by this mechanism (20).
Collectively, our findings suggest a role for TLR4 in mediating nociceptor sensitization by tissue damage products such as S100A8 and α2-macroglobulin. Although our experiments and other reports clearly establish that the cell bodies of DRG neurons express TLR4 (16, 20, 21), their presence and location on joint termini needs to be investigated.
Based on our in vitro findings, we tested whether Tlr4−/− mice developed mechanical allodynia, an evoked measure of sensitization, after DMM. We found that Tlr4−/− mice developed secondary mechanical allodynia in the presence of experimental OA after DMM and this was maintained for 16 weeks, just like in wild-type mice (29). This may reflect the great complexity of the OA joint milieu, where many tissue damage products are generated in the course of progressive disease. In addition to the TLR4 pathway, OA DAMPs can signal through TLR2 and RAGE (11), and other pro-algesic cytokines and chemokines signal through additional pathways, which often involve the transcription factor NFκB (9, 51, 52). Our results are consistent with the idea that it is unlikely that OA-associated pain would arise from damage products signaling through a single pathway. Further, secondary mechanical allodynia in the hindpaw also reflects central sensitization (53). Although we focused on the role of TLR4 in the peripheral nervous system, there is evidence that this receptor can play a role in regulation of neuro-immune processes in the central nervous system. In a nerve transection model (54) and an inflammatory arthritis model (55), mechanical allodynia and spinal microglial activation were reduced in Tlr4−/− mice. Likewise, intrathecal injection of a TLR4 antagonist reduced mechanical allodynia associated with several acute inflammatory models (56). Here, we did not observe a reduction in mechanical allodynia in Tlr4−/− mice in the DMM model, which may be due to differences in both peripheral and central levels of inflammation in the DMM model compared to the models above.
It is interesting to note that Tlr4−/− mice developed similar joint damage as wild-type mice by 16 weeks after surgery. This is consistent with previous reports that state that S100a9−/− mice (which are also functional S100a8−/− mice (57)) were not protected from joint damage after DMM (24, 58). In another murine surgical OA model, the partial meniscectomy model, Tlr4−/− mice also developed similar joint destruction compared to wild-type mice (59). In contrast, in collagenase-induced arthritis, which has more synovitis than the DMM model, synovial expression of S100A8 and S100A9 has been shown to be upregulated for prolonged periods (24, 58), and S100a9−/− mice were protected from synovitis, cartilage degradation, and osteophyte formation in this model (24, 58). Together, this suggests that in a joint environment with low-level inflammation, other pathways besides the TLR4 pathway are likely driving joint damage. Tlr4−/− mice have yet to be tested in the collagenase-induced model.
In summary, our experiments suggest a role for TLR4 signaling in mediating the excitation of DRG neurons by the DAMPs, S100A8 and α2-macroglobulin, which may contribute to nociceptor sensitization in OA. Further research is needed to understand the relative importance of this pathway in comparison to other PRR pathways in the development of mechanical allodynia and other OA-associated pain behaviors. In addition, the complex and diverse actions of DAMPs on a variety of cell types indicate that more research is needed to understand how intervening in these PRR pathways at different stages of disease and in varying inflammatory environments augments both pain and joint damage.
Supplementary Material
Video 1: Representative video demonstrating neuronal responses to the application of PBS (control) (10 μL) during ex vivo calcium imaging. Responses are indicated by an increase in fluorescence intensity in neuronal cell bodies.
Video 2: Representative video demonstrating neuronal responses to the application of LPS (10 μL = 50 μg) during ex vivo calcium imaging. Responses are indicated by an increase in fluorescence intensity in neuronal cell bodies.
Video 3: Representative video demonstrating neuronal responses to the application of capsaicin (10 μL of 10 mM stock) during ex vivo calcium imaging. Responses are indicated by an increase in fluorescence intensity in neuronal cell bodies.
Video 4: Representative video demonstrating neuronal responses to the application of potassium solution (50 mM) during ex vivo calcium imaging. Responses are indicated by an increase in fluorescence intensity in neuronal cell bodies.
Supplemental Figure 1: Wild-type DRG cells were stimulated with 0 or 1 μg/mL S100A8 ± the small molecule TLR4 inhibitor Tak242 (Tak, 1 μM) with or without 10 μg/mL polymyxin B (PMB). Representative of two independent experiments. By two-way ANOVA, S100A8 signficantly upregulated MCP-1 production, p<0.0001 with or without PMB. There was no difference among the conditions comparing with PMB to without PMB, p=0.8695.
Acknowledgments
Rachel Miller was supported by an Arthritis Foundation Postdoctoral Fellowship and by the US National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) (F32AR062927). Anne-Marie Malfait (R01AR064251 and R01AR060364) and Richard Miller (R01AR064251) were supported by NIAMS.
References
- 1.Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–84. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neogi T. The epidemiology and impact of pain in osteoarthritis. Osteoarthritis Cartilage. 2013;21:1145–53. doi: 10.1016/j.joca.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murphy L, Schwartz TA, Helmick CG, Renner JB, Tudor G, Koch G, et al. Lifetime risk of symptomatic knee osteoarthritis. Arthritis Rheum. 2008;59:1207–13. doi: 10.1002/art.24021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cross M, Smith E, Hoy D, Nolte S, Ackerman I, Fransen M, et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73:1323–30. doi: 10.1136/annrheumdis-2013-204763. [DOI] [PubMed] [Google Scholar]
- 5.Hochberg MC, Altman RD, April KT, Benkhalti M, Guyatt G, McGowan J, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken) 2012;64:465–74. doi: 10.1002/acr.21596. [DOI] [PubMed] [Google Scholar]
- 6.Malfait AM, Schnitzer TJ. Towards a mechanism-based approach to pain management in osteoarthritis. Nat Rev Rheumatol. 2013;9:654–64. doi: 10.1038/nrrheum.2013.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 2012;64:1697–707. doi: 10.1002/art.34453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McDougall JJ, Linton P. Neurophysiology of arthritis pain. Curr Pain Headache Rep. 2012;16:485–91. doi: 10.1007/s11916-012-0300-0. [DOI] [PubMed] [Google Scholar]
- 9.Miller RE, Miller RJ, Malfait AM. Osteoarthritis joint pain: The cytokine connection. Cytokine. 2014 doi: 10.1016/j.cyto.2014.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schaible HG. Mechanisms of chronic pain in osteoarthritis. Curr Rheumatol Rep. 2012;14:549–56. doi: 10.1007/s11926-012-0279-x. [DOI] [PubMed] [Google Scholar]
- 11.Liu-Bryan R, Terkeltaub R. Emerging regulators of the inflammatory process in osteoarthritis. Nature Reviews Rheumatology. 2015;11:35–44. doi: 10.1038/nrrheum.2014.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sokolove J, Lepus CM. Role of inflammation in the pathogenesis of osteoarthritis: latest findings and interpretations. Ther Adv Musculoskelet Dis. 2013;5:77–94. doi: 10.1177/1759720X12467868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldring MB, Otero M. Inflammation in osteoarthritis. Current opinion in rheumatology. 2011;23:471–8. doi: 10.1097/BOR.0b013e328349c2b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu-Bryan R. Synovium and the innate inflammatory network in osteoarthritis progression. Curr Rheumatol Rep. 2013;15:323. doi: 10.1007/s11926-013-0323-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gomez R, Villalvilla A, Largo R, Gualillo O, Herrero-Beaumont G. TLR4 signalling in osteoarthritis-finding targets for candidate DMOADs. Nat Rev Rheumatol. 2014 doi: 10.1038/nrrheum.2014.209. [DOI] [PubMed] [Google Scholar]
- 16.Allette YM, Due MR, Wilson SM, Feldman P, Ripsch MS, Khanna R, et al. Identification of a functional interaction of HMGB1 with Receptor for Advanced Glycation End-products in a model of neuropathic pain. Brain Behav Immun. 2014 doi: 10.1016/j.bbi.2014.06.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu T, Xu ZZ, Park CK, Berta T, Ji RR. Toll-like receptor 7 mediates pruritus. Nat Neurosci. 2010;13:1460–2. doi: 10.1038/nn.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qi J, Buzas K, Fan H, Cohen JI, Wang K, Mont E, et al. Painful pathways induced by TLR stimulation of dorsal root ganglion neurons. J Immunol. 2011;186:6417–26. doi: 10.4049/jimmunol.1001241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shibasaki M, Sasaki M, Miura M, Mizukoshi K, Ueno H, Hashimoto S, et al. Induction of high mobility group box-1 in dorsal root ganglion contributes to pain hypersensitivity after peripheral nerve injury. Pain. 2010;149:514–21. doi: 10.1016/j.pain.2010.03.023. [DOI] [PubMed] [Google Scholar]
- 20.Due MR, Piekarz AD, Wilson N, Feldman P, Ripsch MS, Chavez S, et al. Neuroexcitatory effects of morphine-3-glucuronide are dependent on Toll-like receptor 4 signaling. J Neuroinflammation. 2012;9:200. doi: 10.1186/1742-2094-9-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Y, Zhang H, Kosturakis AK, Jawad AB, Dougherty PM. Toll-like receptor 4 signaling contributes to Paclitaxel-induced peripheral neuropathy. J Pain. 2014;15:712–25. doi: 10.1016/j.jpain.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vogl T, Tenbrock K, Ludwig S, Leukert N, Ehrhardt C, van Zoelen MA, et al. Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat Med. 2007;13:1042–9. doi: 10.1038/nm1638. [DOI] [PubMed] [Google Scholar]
- 23.Sohn DH, Sokolove J, Sharpe O, Erhart JC, Chandra PE, Lahey LJ, et al. Plasma proteins present in osteoarthritic synovial fluid can stimulate cytokine production via Toll-like receptor 4. Arthritis Res Ther. 2012;14:R7. doi: 10.1186/ar3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Lent PL, Blom AB, Schelbergen RF, Sloetjes A, Lafeber FP, Lems WF, et al. Active involvement of alarmins S100A8 and S100A9 in the regulation of synovial activation and joint destruction during mouse and human osteoarthritis. Arthritis Rheum. 2012;64:1466–76. doi: 10.1002/art.34315. [DOI] [PubMed] [Google Scholar]
- 25.Gobezie R, Kho A, Krastins B, Sarracino DA, Thornhill TS, Chase M, et al. High abundance synovial fluid proteome: distinct profiles in health and osteoarthritis. Arthritis Res Ther. 2007;9:R36. doi: 10.1186/ar2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hadler NM, Johnson AM, Spitznagel JK, Quinet RJ. Protease inhibitors in inflammatory synovial effusions. Ann Rheum Dis. 1981;40:55–9. doi: 10.1136/ard.40.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tortorella MD, Arner EC, Hills R, Easton A, Korte-Sarfaty J, Fok K, et al. Alpha2-macroglobulin is a novel substrate for ADAMTS-4 and ADAMTS-5 and represents an endogenous inhibitor of these enzymes. J Biol Chem. 2004;279:17554–61. doi: 10.1074/jbc.M313041200. [DOI] [PubMed] [Google Scholar]
- 28.Malfait AM, Ritchie J, Gil AS, Austin JS, Hartke J, Qin W, et al. ADAMTS-5 deficient mice do not develop mechanical allodynia associated with osteoarthritis following medial meniscal destabilization. Osteoarthritis Cartilage. 2010;18:572–80. doi: 10.1016/j.joca.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 29.Miller RE, Tran PB, Das R, Ghoreishi-Haack N, Ren D, Miller RJ, et al. CCR2 chemokine receptor signaling mediates pain in experimental osteoarthritis. Proc Natl Acad Sci U S A. 2012;109:20602–7. doi: 10.1073/pnas.1209294110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sodhi CP, Shi XH, Richardson WM, Grant ZS, Shapiro RA, Prindle T, Jr, et al. Toll-like receptor-4 inhibits enterocyte proliferation via impaired beta-catenin signaling in necrotizing enterocolitis. Gastroenterology. 2010;138:185–96. doi: 10.1053/j.gastro.2009.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim YS, Chu Y, Han L, Li M, Li Z, Lavinka PC, et al. Central terminal sensitization of TRPV1 by descending serotonergic facilitation modulates chronic pain. Neuron. 2014;81:873–87. doi: 10.1016/j.neuron.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glasson SS, Blanchet TJ, Morris EA. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthritis Cartilage. 2007;15:1061–9. doi: 10.1016/j.joca.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 33.Hirschfeld M, Ma Y, Weis JH, Vogel SN, Weis JJ. Cutting edge: repurification of lipopolysaccharide eliminates signaling through both human and murine toll-like receptor 2. J Immunol. 2000;165:618–22. doi: 10.4049/jimmunol.165.2.618. [DOI] [PubMed] [Google Scholar]
- 34.Tran PB, Banisadr G, Ren D, Chenn A, Miller RJ. Chemokine receptor expression by neural progenitor cells in neurogenic regions of mouse brain. The Journal of comparative neurology. 2007;500:1007–33. doi: 10.1002/cne.21229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oh SB, Tran PB, Gillard SE, Hurley RW, Hammond DL, Miller RJ. Chemokines and glycoprotein120 produce pain hypersensitivity by directly exciting primary nociceptive neurons. J Neurosci. 2001;21:5027–35. doi: 10.1523/JNEUROSCI.21-14-05027.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–5. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 38.Dixon WJ. Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol. 1980;20:441–62. doi: 10.1146/annurev.pa.20.040180.002301. [DOI] [PubMed] [Google Scholar]
- 39.Glasson SS, Chambers MG, Van Den Berg WB, Little CB. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthritis Cartilage. 2010;18 (Suppl 3):S17–23. doi: 10.1016/j.joca.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 40.van Lent PL, Grevers LC, Schelbergen R, Blom A, Geurts J, Sloetjes A, et al. S100A8 causes a shift toward expression of activatory Fcgamma receptors on macrophages via toll-like receptor 4 and regulates Fcgamma receptor expression in synovium during chronic experimental arthritis. Arthritis Rheum. 2010;62:3353–64. doi: 10.1002/art.27654. [DOI] [PubMed] [Google Scholar]
- 41.Zreiqat H, Belluoccio D, Smith MM, Wilson R, Rowley LA, Jones K, et al. S100A8 and S100A9 in experimental osteoarthritis. Arthritis Res Ther. 2010;12:R16. doi: 10.1186/ar2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schelbergen RF, Blom AB, van den Bosch MH, Sloetjes A, Abdollahi-Roodsaz S, Schreurs BW, et al. Alarmins S100A8 and S100A9 elicit a catabolic effect in human osteoarthritic chondrocytes that is dependent on Toll-like receptor 4. Arthritis Rheum. 2012;64:1477–87. doi: 10.1002/art.33495. [DOI] [PubMed] [Google Scholar]
- 43.Leclerc E, Fritz G, Vetter SW, Heizmann CW. Binding of S100 proteins to RAGE: an update. Biochim Biophys Acta. 2009;1793:993–1007. doi: 10.1016/j.bbamcr.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 44.Cawston TE, Mercer E. Preferential binding of collagenase to alpha 2-macroglobulin in the presence of the tissue inhibitor of metalloproteinases. FEBS Lett. 1986;209:9–12. doi: 10.1016/0014-5793(86)81074-2. [DOI] [PubMed] [Google Scholar]
- 45.Tchetverikov I, Lohmander LS, Verzijl N, Huizinga TW, TeKoppele JM, Hanemaaijer R, et al. MMP protein and activity levels in synovial fluid from patients with joint injury, inflammatory arthritis, and osteoarthritis. Ann Rheum Dis. 2005;64:694–8. doi: 10.1136/ard.2004.022434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang S, Wei X, Zhou J, Zhang J, Li K, Chen Q, et al. Identification of alpha2-macroglobulin as a master inhibitor of cartilage-degrading factors that attenuates the progression of posttraumatic osteoarthritis. Arthritis Rheumatol. 2014;66:1843–53. doi: 10.1002/art.38576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chu CT, Howard GC, Misra UK, Pizzo SV. Alpha 2-macroglobulin: a sensor for proteolysis. Ann N Y Acad Sci. 1994;737:291–307. doi: 10.1111/j.1749-6632.1994.tb44319.x. [DOI] [PubMed] [Google Scholar]
- 48.Feldman P, Due MR, Ripsch MS, Khanna R, White FA. The persistent release of HMGB1 contributes to tactile hyperalgesia in a rodent model of neuropathic pain. J Neuroinflammation. 2012;9:180. doi: 10.1186/1742-2094-9-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Diogenes A, Ferraz CC, Akopian AN, Henry MA, Hargreaves KM. LPS sensitizes TRPV1 via activation of TLR4 in trigeminal sensory neurons. J Dent Res. 2011;90:759–64. doi: 10.1177/0022034511400225. [DOI] [PubMed] [Google Scholar]
- 50.Hou L, Wang X. PKC and PKA, but not PKG mediate LPS-induced CGRP release and [Ca(2+)](i) elevation in DRG neurons of neonatal rats. J Neurosci Res. 2001;66:592–600. doi: 10.1002/jnr.1249. [DOI] [PubMed] [Google Scholar]
- 51.Bowles RD, Mata BA, Bell RD, Mwangi TK, Huebner JL, Kraus VB, et al. In vivo luminescence imaging of NF-kappaB activity and serum cytokine levels predict pain sensitivities in a rodent model of osteoarthritis. Arthritis Rheumatol. 2014;66:637–46. doi: 10.1002/art.38279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roman-Blas JA, Jimenez SA. NF-kappaB as a potential therapeutic target in osteoarthritis and rheumatoid arthritis. Osteoarthritis Cartilage. 2006;14:839–48. doi: 10.1016/j.joca.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 53.Sagar DR, Staniaszek LE, Okine BN, Woodhams S, Norris LM, Pearson RG, et al. Tonic modulation of spinal hyperexcitability by the endocannabinoid receptor system in a rat model of osteoarthritis pain. Arthritis Rheum. 2010;62:3666–76. doi: 10.1002/art.27698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tanga FY, Nutile-McMenemy N, DeLeo JA. The CNS role of Toll-like receptor 4 in innate neuroimmunity and painful neuropathy. Proc Natl Acad Sci U S A. 2005;102:5856–61. doi: 10.1073/pnas.0501634102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Christianson CA, Dumlao DS, Stokes JA, Dennis EA, Svensson CI, Corr M, et al. Spinal TLR4 mediates the transition to a persistent mechanical hypersensitivity after the resolution of inflammation in serum-transferred arthritis. Pain. 2011;152:2881–91. doi: 10.1016/j.pain.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sorge RE, LaCroix-Fralish ML, Tuttle AH, Sotocinal SG, Austin JS, Ritchie J, et al. Spinal cord Toll-like receptor 4 mediates inflammatory and neuropathic hypersensitivity in male but not female mice. J Neurosci. 2011;31:15450–4. doi: 10.1523/JNEUROSCI.3859-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Manitz MP, Horst B, Seeliger S, Strey A, Skryabin BV, Gunzer M, et al. Loss of S100A9 (MRP14) results in reduced interleukin-8-induced CD11b surface expression, a polarized microfilament system, and diminished responsiveness to chemoattractants in vitro. Mol Cell Biol. 2003;23:1034–43. doi: 10.1128/MCB.23.3.1034-1043.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schelbergen RF, de Munter W, van den Bosch MH, Lafeber FP, Sloetjes A, Vogl T, et al. Alarmins S100A8/S100A9 aggravate osteophyte formation in experimental osteoarthritis and predict osteophyte progression in early human symptomatic osteoarthritis. Ann Rheum Dis. 2014 doi: 10.1136/annrheumdis-2014-205480. [DOI] [PubMed] [Google Scholar]
- 59.Nasi S, Ea HK, Chobaz V, van Lent P, Liote F, So A, et al. Dispensable role of myeloid differentiation primary response gene 88 (MyD88) and MyD88-dependent toll-like receptors (TLRs) in a murine model of osteoarthritis. Joint Bone Spine. 2014;81:320–4. doi: 10.1016/j.jbspin.2014.01.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video 1: Representative video demonstrating neuronal responses to the application of PBS (control) (10 μL) during ex vivo calcium imaging. Responses are indicated by an increase in fluorescence intensity in neuronal cell bodies.
Video 2: Representative video demonstrating neuronal responses to the application of LPS (10 μL = 50 μg) during ex vivo calcium imaging. Responses are indicated by an increase in fluorescence intensity in neuronal cell bodies.
Video 3: Representative video demonstrating neuronal responses to the application of capsaicin (10 μL of 10 mM stock) during ex vivo calcium imaging. Responses are indicated by an increase in fluorescence intensity in neuronal cell bodies.
Video 4: Representative video demonstrating neuronal responses to the application of potassium solution (50 mM) during ex vivo calcium imaging. Responses are indicated by an increase in fluorescence intensity in neuronal cell bodies.
Supplemental Figure 1: Wild-type DRG cells were stimulated with 0 or 1 μg/mL S100A8 ± the small molecule TLR4 inhibitor Tak242 (Tak, 1 μM) with or without 10 μg/mL polymyxin B (PMB). Representative of two independent experiments. By two-way ANOVA, S100A8 signficantly upregulated MCP-1 production, p<0.0001 with or without PMB. There was no difference among the conditions comparing with PMB to without PMB, p=0.8695.