Abstract
Wound healing is usually facilitated by the use of a wound dressing that can be easily applied to cover the wound bed, maintain moisture, and avoid bacterial infection. In order to meet all of these requirements, we developed an in situ forming biodegradable hydrogel (iFBH) system composed of a newly developed combination of biodegradable poly (ethylene glycol) maleate citrate (PEGMC) and poly (ethylene glycol) diacrylate (PEGDA). The in situ forming hydrogel systems are able to conform to the wound shape in order to cover the wound completely and prevent bacterial invasion. A 2k factorial analysis was performed to examine the effects of polymer composition on specific properties, including the curing time, Young’s modulus, swelling ratio and degradation rate. An optimized iFBH formulation was achieved from the systematic factorial analysis. Further, in vitro biocompatibility studies using adult human dermal fibroblasts (HDFs) confirmed that the hydrogels and degradation products are not cytotoxic. The iFBH wound dressing was conjugated and functionalized with antimicrobial peptides as well. Evaluation against bacteria both in vitro and in vivo in rats demonstrated that the peptide-incorporated iFBH wound dressing offered excellent bacteria inhibition and promoted wound healing. These studies indicated that our in situ forming antimicrobial biodegradable hydrogel system is a promising candidate for wound treatment.
Keywords: Biodegradable hydrogel, antimicrobial peptide, factorial analysis, wound healing, formulation
INTRODUCTION
Wound healing is a dynamic, complex process consisting of many cellular and molecular events that can be categorized as the inflammatory, proliferative, and remodeling (maturation) phases. Usually, normal wounds can heal in a definite time period; however, certain conditions, such as bacterial infection, renal disease, ischemia, diabetics and local hypoxia, can hinder the wound healing process, which result in the development of a complex wound. These complex wounds lengthen the healing time, and, in some instances, can result in life-threatening situations1–3. In order to manage these complex wounds, many tissue engineered grafts, such as Apligraf® and Dermagraft®, have been previously developed4. However, the lack of antimicrobial properties and expensive nature of these grafts illustrates the need for alternatives in the field of wound dressing.
Among all wound dressing materials, hydrogels possess a wide range of capabilities, such as swelling, in situ gelling capacity, drug/growth factor delivery, and hydrophilicity, that makes them an attractive alternative to traditional treatment approaches. Swelling avoids the formation of fluid filled pockets, which minimizes the risk of bacterial infection. A crosslinked network provides a good platform for the controlled delivery of drugs and/or growth factors, while in situ gelling provides ease of applicability, ensuring the complete closure of wound. Moreover, hydrophilicity maintains moisture at the wound site to possibly enhance epithelial cell migration and support necrotic tissue debridement1,3,5,6. Therefore, hydrogels have been extensively studied, particularly poly(ethylene glycol) (PEG)-based hydrogels. PEG-based hydrogels are biologically inert with resistance to protein adsorption. Poly(ethylene glycol) diacrylate (PEGDA) hydrogels, a class of PEG-based hydrogels, can present tunable physical/mechanical properties, such as stiffness and swelling ratio for various drug delivery systems7,8. Although promising, PEGDA hydrogel alone fails to meet the ideal regenerative medicine requirements, as hydrogels should present a favorable temporary substrate chemical and physical environment for tissue growth and regeneration9. Our lab recently developed a novel citric acid derived biodegradable hydrogel, poly(ethylene glycol) maleate citrate (PEGMC)10. PEGMC is cytocompatible, biodegradable, and in situ crosslinkable with tunable degradability and mechanical properties11. Additionally, pendent carboxyl groups provided by citric acid can be utilized for conjugation with peptides, antibodies, and other biomolecules to provide additional functionality.
Herein, we develop an in situ forming biodegradable hydrogel (iFBH) system using a copolymer network of PEGMC and PEGDA as a biodegradable dressing for the treatment of skin wounds. A factorial analysis on the effects of PEGMC concentration, concentration of a short chain PEGDA, molecular weights of a long chain PEGDA, and the amount of initiators was conducted. These factors were systematically studied to optimize the hydrogels’ properties, such as swelling, degradation, curing time, and mechanical stiffness. Antimicrobial properties are also desirable for the ideal wound dressing12. Thus, antimicrobial agents, including antibiotics, silver nanoparticles, and antimicrobial peptides, have been widely incorporated into wound dressings. Compared to traditional antibiotics, antimicrobial peptides have broader inhibition activity against most bacteria. Antimicrobial peptides kill bacteria more rapidly and can target multiple bacteria cellular processes13–15. They can also be easily conjugated onto hydrogels. Therefore, antimicrobial peptides, including CHRG01, ABU-CHRG01 (ABU), Temporin-A (TEMP-A), and Ala5-Tritrp7 (ALA5), were conjugated onto the PEGMC/PEGDA hydrogels to provide anti-infection functions. Preliminary in vivo studies were also performed in this study using the in situ forming antimicrobial biodegradable hydrogel (iFABH) on a rat skin wound model in order to demonstrate its potential as a biodegradable wound dressing.
EXPERIMENTAL SECTION
Materials
Poly(ethylene glycol) (PEG200, PEG4600 and PEG8000 with molecular weights MW=200, 4600, and 8000Da, respectively), citric acid, maleic acid, poly(ethylene glycol) diacrylate (PEGDA700, MW=700 Da), and all other chemicals were purchased from Sigma Aldrich or Alfa Aesar. All of the antimicrobial peptides, CHRG01, ABU-CHRG01 (ABU), Temporin-A (TEMP-A), and Ala5-Tritrp7 (ALA5), were custom-made by Anaspec Inc. with N-terminals available for conjugation to PEGMC. Peptide sequences are: CHRG01, KSSTRGRKSSRRKK-NH2; ABU, Aminobutyric acid-KSSTRGRKSSRRKK-NH2; TEMP-A, FLPLIGRVLSGIL-NH2; and ALA5, VRRFAWWWPFLRR-NH2.
Synthesis of PEGMC
PEGMC was synthesized by a polycondensation reaction as described previously10. Briefly, a mixture of PEG200:maleic acid:citric acid with a molar ratio of 1:0.6:0.4 was melted at 160°C in a 100 ml flask under a nitrogen atmosphere. The temperature was then reduced to 140°C and the reaction proceeded under 50 mTorr pressure for 6 hours. The resulting pre-polymer was dissolved in deionized water. The polymer solution was filtered and dialyzed against 500Da molecular-weight-cut-off dialysis membranes for purification. The purified polymer solution was then lyophilized and stored in a refrigerator at 4°C before use.
Synthesis of PEGDA4600 and PEGDA8000
The long chain PEGDA was synthesized according to the protocol described by Durst et al7. Briefly, 2 mmol of PEG (4600 or 8000Da) was dissolved in dichloromethane and 1.3 ml triethyl amine was added to the solution. Later, 7.5 mmol acryloyl chloride was dissolved in dichloromethane and added to the reaction drop-wise. This reaction was then kept for continuous stirring in a dark and inert environment for 2 days. After 2 days, the solution was washed with K2CO3 (2M) to remove the hydrochloride acid and then dehydrated using 2 g of anhydrous MgSO4. The synthesized polymers are named PEGDA4600 and PEGDA8000.
Preparation of in situ forming biodegradable hydrogel (iFBH)
iFBHs with different formulations, as listed in Table 1, were prepared by free radical polymerization. First, the PEGMC solution was neutralized to pH 7.0, followed by the addition of two different PEGDA polymers. The short chain PEGDA acts as a crosslinker since it has a smaller MW (700Da) and provides most of the double bonds for crosslinking. The long chain PEGDA acts as a structural mediator with a higher MW (~4600 or 8000Da), since it has a significant impact on the structures of hydrogels and their physical properties, including swelling and mechanical properties. The mixture was then purged with N2 for 20 minutes. Afterwards, ammonium persulfate (APS) and tetramethylethylenediamine (TEMED) were added to form a gel. These hydrogels were then cut and lyophilized for 24 hours for further studies.
Table 1.
Formulations of iFBH systems that were used for the factorial analysis.
| Formulation Number | Formulation Factors | |||
|---|---|---|---|---|
|
| ||||
| A: PEGMC Concentration (mg/ml) | B: PEGDA MW (Da)* | C: APS Concentration (mg/ml) | D: PEGDA700 Concentration (mg/ml) | |
| 1 | 200 | 4600 | 16 | 64 |
| 2 | 200 | 4600 | 32 | 32 |
| 3 | 200 | 8000 | 16 | 32 |
| 4 | 200 | 8000 | 32 | 64 |
| 5 | 400 | 4600 | 16 | 64 |
| 6 | 400 | 4600 | 32 | 32 |
| 7 | 400 | 8000 | 16 | 32 |
| 8 | 400 | 8000 | 32 | 64 |
The concentrations of PEGDA4600 and 8000 were 50 mg/ml
Characterization of iFBHs
All pre-polymers were characterized by Fourier transform infrared spectroscopy (FTIR) to confirm the chemical structures. The curing time of the iFBHs was examined by keen visual observation. Briefly, a magnetic stir bar was placed in the pre-hydrogel solution prepared and the solution was kept on a magnetic stir plate at ~ 120 rpm. Upon addition of TEMED and APS, the time to solidification was recorded as the curing time (n=4).
Next, the swelling behavior of the iFBHs was studied. The dry weights (WD) of all the hydrogels (n=4) were obtained and the samples were then immersed in 5 ml of 10mM PBS (pH 7.4) for 24 hours. Samples were then taken out of PBS and any extra liquid on the surface of the samples was removed using filter papers. The swollen weight (WS) of the samples was measured. The swelling ratio was then calculated using Equation (1)
| (1) |
In vitro degradation of the hybrid iFBH system was studied over a period of 28 days with incubation at 37°C in 10mM PBS. The PBS solutions were changed every day. The dry weights of the hydrogel samples before (W0) and after (Wt) incubation were measured. Degradation was calculated in terms of percentage weight loss using Equation (2)
| (2) |
Cross-sectional morphology of iFBH samples was observed using scanning electron microscopy (SEM) to visualize the surface morphology changes before and after degradation in PBS. Briefly, lyophilized hydrogel samples were sputter-coated with silver and then examined under a Hitachi S-30000N VP SEM.
Mechanical properties of different hydrogels were tested using a MTS® Insight™ II mechanical tester equipped with a 10 N load cell (Eden Praire, MN). Briefly, hydrogels were cut into strips (n=4) with sizes of 3 mm (thickness) × 5 mm (width) × 12 mm (length). The testing gauge was 15 mm. The tensile tests were performed at 100 mm/min and Young’s Modulus was calculated at the initial elastic region (<10% elongation).
Factorial analysis of formulation factors
A systematic two level (2k) factorial analysis was performed using Design-Ease 8® (Stat-Ease, Inc.), which is a design of experiments (DOE) software, as described previously16. The software provided outputs in the forms of half-normal probability plots, surface diagrams, and mathematical equations. These outputs were analyzed to optimize the properties of the iFBH system. For the factorial analysis, the formulation factors (independent variables) were the concentration of PEGMC, the concentration of the short chain PEGDA700, the molecular weight of the long chain PEGDA (PEGDA4600 and PEGDA8000), and the concentration of the initiator APS. For each formulation factor, realistic high-level and low-level values were input into Design Ease, as displayed in Table 1. Design Ease then output in random order the formulations that were to be tested. The response factors (dependent variables) chosen were curing time, swelling ration, degradation rate, and Young’s Modulus.
In vitro cytocompatibility of the iFBH compounds and degradation products
PEGMC, APS, and degradation products of the hybrid hydrogel system were tested for cytocompatibility on human dermal fibroblasts cells (HDFs). HDFs were cultured in Dulbeco’s Modified Eagle Medium (DMEM) media with 10% Fetal Bovine Serum (FBS) and 1% Penicillin and streptomycin (Pen Strip) at 37°C and 5% CO2. PEGMC was UV sterilized before being added to complete media. Serial solutions of PEGMC in DMEM were prepared, ranging from 0–10 mg/ml. HDFs were then treated with these solutions in 96 well tissue culture plates for 24 hours. After incubation, CellTiter 96® Aqueous One Solution Cell Proliferation (MTS) assays were performed as per the manufacturer’s instruction to determine cell viability. Similarly, HFDs were treated with APS at concentrations of 0, 5, 10, 50, 100, and 200 μg/ml for 24 hours and their viabilities were measured by MTS assays. To evaluate the cytotoxicity of degradation products, hydrogels that were made of 200 mg PEGMC, 64 mg of PEGDA700, 50 mg of PEGDA8000, and 16 mg of APS were degraded in PBS for 24 hours. Effluents were then collected and freeze-dried in order to obtain degradation products. After UV sterilization, they were added to HDFs with concentrations of 0.25, 0.74, 2.22, 6.67, and 20 mg/ml. Finally, the samples were analyzed by MTS assays after 24 hours of incubation.
Formation and characterization of in situ forming antimicrobial biodegradable hydrogel system(iFABH)
Antimicrobial biodegradable hydrogel systems were prepared using antimicrobial peptide conjugated PEGMC as the imperative component. The synthesis route and final composition are demonstrated in Scheme 1. First, PEGMC was conjugated with different cytocompatible antimicrobial peptides, including CHRG01, ABU, TEMP-A and ALA5, via the carbodiimide coupling technique as previously described16. Briefly, 1 wt% PEGMC dissolved in 1M MES buffer was treated with 10mM of 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide (EDC) and 10mM N-hydroxysuccinimide (NHS). After 30 minutes of activation, 1 mg of peptide was added to the PEGMC solution. This solution was then kept overnight with gentle agitation. Later, the resulting solution was dialyzed and lyophilized to obtain peptide conjugated PEGMC. The conjugation was confirmed by FTIR. The antimicrobial hydrogel was then formulated with PEGMC-peptide using a combination determined by the factorial analysis, in which 200 mg/ml peptide conjugated PEGMC, 64 mg PEGDA700, 50 mg PEGDA8000, and 16 mg APS were added to 1 ml DI water to form a hydrogel.
Scheme 1.
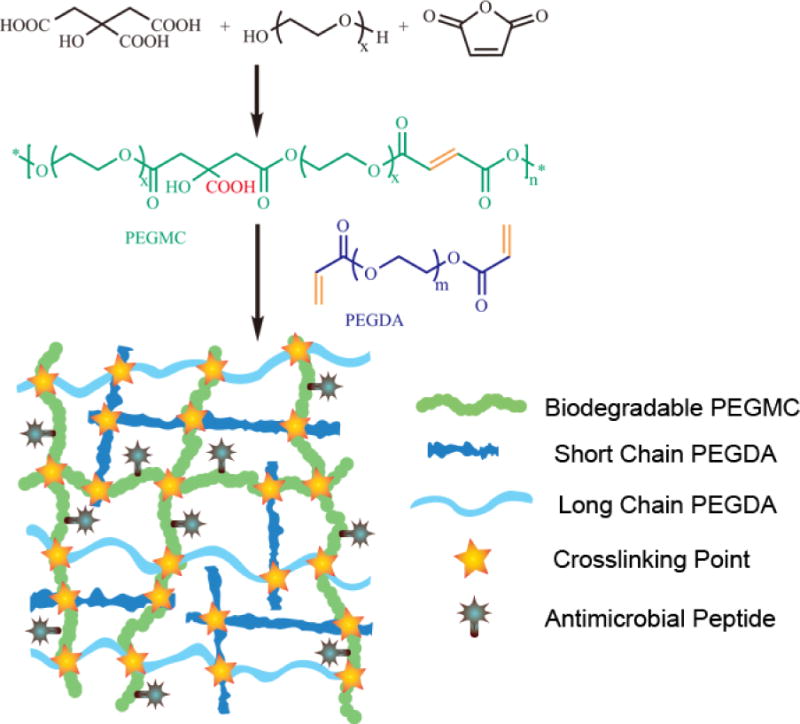
Synthesis route of PEGMC and a schematic illustration of the in situ forming biodegradable antibacterial hydrogel (iFABH). Biodegradable PEGMC provides a degradable backbone and carboxyl groups that can be used to conjugate antimicrobial peptides. Short chain PEGDA provides the most double bonds for crosslinking.
For antimicrobial studies, Gram-positive Staphylococcus aureus (S. aureus, ATCC strain 25923), which has been shown to cause wound infection12,17, were used. Prior to each experiment, bacteria were cultured in Trypticase Soy Broth for 18 hours at 37°C. Eluted media of PEGMC with peptides and without peptides (negative control) were incubated with bacteria (1 × 106 CFU/ml) for 24 hours. At a predetermined time, the effectiveness of the antimicrobial peptides in killing bacteria was determined by measuring the optical density (OD, λ=650 nm, n=4) via a pour plating technique as described earlier18. 25 μg/ml of ampicillin solution, which is typically used in cell culture medium, served as the positive control. Inhibition zone study was also performed to assess the antimicrobial behavior. The different hydrogels, including an ampicillin encapsulated hydrogel as the positive control, were placed on agar plates containing freshly cultured S. aureus and incubated at 37°C for 16–18 hours. After incubation, the area of the zone of inhibition around the samples was imaged and measured.
In vivo wound healing assessment
Preliminary in vivo wound healing studies of iFABHs were conducted using a normal rat skin wound model as previously described19,20. All Sprague-Dawley rats were treated and used in accordance with the protocol approved by the University of Texas at Arlington Animal Care and Use Committee (IACUC). Briefly, all rats (female, 2 months old, weighing around 240 g) were anesthetized by injection of ketamine (40 mg/kg) and xylazine (5 mg/kg). The skin of each rat was shaved and disinfected using 70% ethanol. Five wounds were created along the dorsal side of the skin on each rat using a 5 mm diameter biopsy puncher. Three of the wounds were control groups (PEGMC, PEGMC-AMP, Hydrofera Blue) and two were experimental groups, which were ABU and TEMP-A conjugated iFABH systems. Once the wounds were created, the wound area was cleaned and filled with hydrogel solution. Specifically, 200 mg peptide conjugated PEGMC, 64 mg PEGDA700, and 50 mg PEGDA8000 were dissolved in 1 ml nitrogen purged PBS as part A, and 16 mg APS were dissolved in 1 ml nitrogen purged PBS as part B. Parts A and B were mixed and injected together onto the wound bed through catheters. The in situ gelling property of the hydrogel enabled the formation of a gel shortly after it was applied on the wound. Further, after 24 hours, S. aureus bacterial suspension with seeding density of 2.8×106 CFUs/250 μl/cm2 was applied to each of the gels21. The behavior of the animals and the wound areas were closely observed for 2 weeks. After 14 days of observation, they were sacrificed and the tissue surrounding the wound area was removed and fixed by 10% neutral buffed formalin. Tissue samples were embedded in paraffin before sectioning. Histological studies by hematoxylin and eosin (H&E) staining and Masson’s Trichrome staining were performed.
2.10 Statistical Analysis
All experiments were conducted with n=4 and statistical analysis was performed for determination of significance with a 95% confidence interval (p<0.05) using one-way ANOVA.
RESULTS AND DISCUSSION
Characterization of hydrogel structure
Our previously developed PEGMCs are biodegradable; in situ crosslinkable polymers have been shown to be suitable for drug/growth factor/cell delivery, and tissue engineering10,11. First, PEGMC was synthesized via a simple polycondensation reaction of citric acid, maleic acid, and PEG. FTIR spectra (Figure S1 Supporting Information) confirmed the presence of characteristic peaks for functional groups from the degradable ester bond of citric acid and maleic acid (−C=O at 1690–1750 cm−1), the hydroxyl group (−OH at 3570 cm−1), and the double bond from maleic acid (−C=C at 1645 cm−1)10.
Hydrogels with various compound combinations as listed in Table 1 were prepared. The formation and degradation of the hydrogel system were observed using SEM. As seen in Figure S2A (Supporting Information), wrinkled sheet structure of iFBH is reflected on day 1, which is followed by a flat surface over a period of 28 days (Figure S2B, C, and D) due to erosion.
Factorial analysis of iFBH formations
Existing studies have shown that the composition and preparation method might have an effect on the morphology, swelling behavior, and protein release of hydrogels22,23. For use in wound healing, the applicability, mechanical integrity, biocompatibility, and swelling capacity of the hydrogels are important factors that need to be optimized23,24. Thus, in this study, curing time, Young’s modulus, degradation (percent weight loss), and swelling ratio were taken as response factors (dependent variables) as they relate to applicability, stiffness and durability, long-term biocompatibility and comfort, and swelling capacity, respectively. The formulation factors (independent variables) used were PEGMC concentration, long chain PEGDA MW, APS concentration, and PEGDA700 concentration.
First, the curing time of the system was studied in order to quantify the in situ gelling capacity of the hydrogel. For each formulation listed in Table 1, the curing time was recorded in Design-Ease 8®. A half-normal probability was obtained, as shown in Figure 1A. The magnitude of the effect a formulation factor had on the curing time increases the further distance it is to the right of the line. Negative (inversely related) effects are shown in blue and positive (directly related) effects are shown in orange. From Figure 1A, it can be determined that PEGDA MW, PEGDA700 concentration, and APS concentration have increasingly negative effects on the curing time. The surface diagram, Figure 1B, also displays the same trends, as the shortest curing time corresponds to the lowest PEGDA MW and highest APS concentration. It is also apparent from the surface diagram that APS concentration has a more prominent effect on curing time than PEGDA MW does, as the slope of the edge of the plot corresponding to APS concentration is steeper than that of PEGDA MW. In addition, the half-normal probability plot, Figure 1A, also shows that PEGMC does not have much of an effect on curing time. These results are consistent with the fact that more double bonds in the hydrogel system facilitate faster crosslinking. Design Ease 8® outputs Equation 3 here, which gives a prediction of the curing time based on the values of the formulation factors:
| (3) |
where A is the PEGMC concentration (mg/ml), B is the long chain PEGDA molecular weight (Da), C is the APS concentration (mg/ml), and D is the PEGDA700 concentration (mg/ml).
Figure 1.
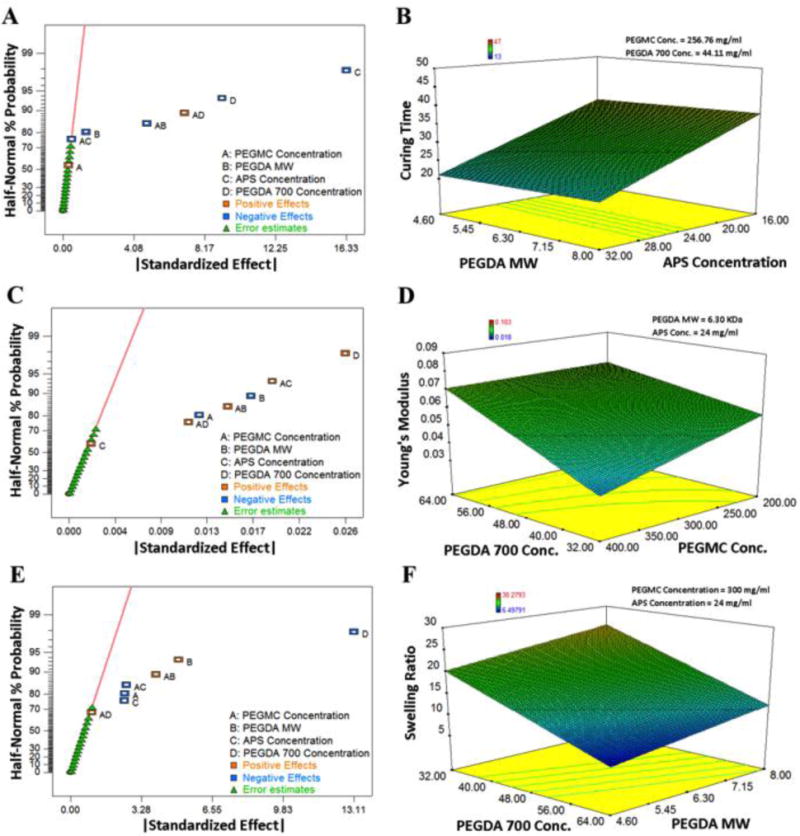
Half-normal probability plots and three-dimensional (3D) surface diagrams of the iFBH system for (A–B) curing time (s), (C–D) Young’s modulus (MPa), and (E–F) swelling ratio (%).
Second, Young’s modulus was studied in order to evaluate the stiffness and durability of the hydrogel. From Figures 1C–D, it can be determined that PEGDA700 concentration has the largest effect on Young’s modulus and it is a positive effect. Also, the PEGMC concentration and PEGDA MW have increasingly negative effects on Young’s modulus. However, APS concentration does not have a significant effect. Similar results, in regards to the negative effect of the molecular weight of long chain PEGDA on the stiffness of hydrogels, were obtained by Temenoff et al25 for oligo(poly(ethylene glycol) fumarate hydrogels. Equation 4 gives a prediction of Young’s Modulus based on the values of the formulation factors.
| (4) |
where A, B, C, and D are same as in Equation 3.
Third, the effects the formulation factors had on the swelling ratios were evaluated. Based on Figures 1E–F, APS concentration, PEGMC concentration, and PEGDA700 concentration have increasingly negative effects on the swelling ratio. This is because they are all prone to increasing the crosslinking rate. However, the MW of the long chain PEGDA has a positive effect on the swelling ratio, which is similar to what was observed by Sabnis et al in a PEGDA only system23. Further, we also found that the crosslinker PEGDA700 concentration has the greatest effect on swelling ratio. Equation 5 gives a prediction for the swelling ratio based on the values of the formulation factors.
| (5) |
where A, B, C, and D are same as in Equation 3.
Fourth, the weight loss of the hydrogel was examined at three different time points to evaluate the degradation process. At Day 1 (Figure 2A–B), APS concentration and PEGMC concentration had negative effects on percent weight loss, while PEGDA MW had a positive effect. At Day 14 (Figure 2C-D), PEGDA700 concentration and APS concentration had negative effects on percent weight loss, while PEGDA MW again had a positive effect. At Day 28 (Figure 2E–F), the weight losses were in the range of 44.4% to 83.8% for different formulations. In addition, APS concentration and PEGDA700 concentration had negative effects on percent weight loss, while PEGMC concentration and PEGDA MW both had positive effects. However, the magnitudes of all of the effects were much smaller at Day 28 than at Days 1 and 14. The mixed effects of different formulation factors are probably due to that the degradation is driven by PEGMC; however, the influences of swelling and hydrophilicity are also significant. Equation 6 was obtained to predict the percent weight loss at 28 days based on the values of the formulation factors.
| (6) |
where A, B, C, and D are same as in Equation 3.
Figure 2.
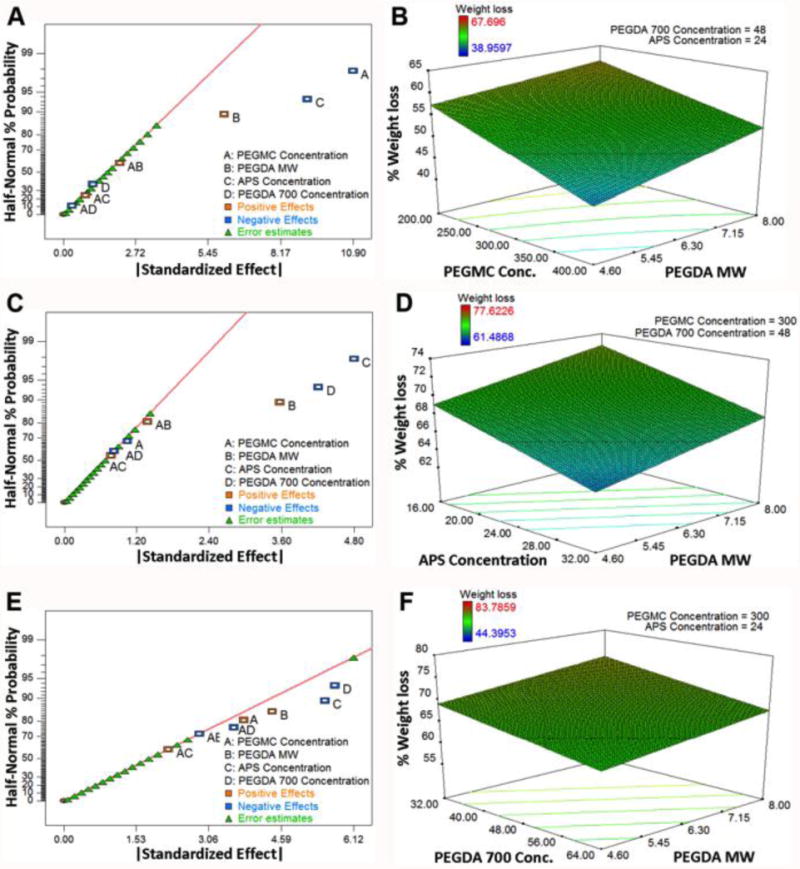
Half-normal probability plots and 3D surface diagrams for degradation (%weight loss) of the iFBH system after (A–B) Day 1, (C–D) Day 14, and (E–F) Day 28.
Table 2 summarizes these results. For each response factor, the formulation factors that affect it are listed in order of the magnitude of their effect, with the formulation factor that has the greatest effect listed first. Based on the numeric response of our 2k factorial analysis, an iFBH formulation that consists of 200 mg PEGMC, 64 mg PEGDA700, 50 mg PEGDA8000, 16 mg APS was found to be the optimum formulation for ideal wound dressing applications. This formulation was also selected for antimicrobial hydrogel fabrication and further in vitro and in vivo studies.
Table 2.
Response factors and the effects of formulation factors determined by factorial analysis. ↑ indicates a positive effect and ↓ indicate a negative effect.
| Response factors | Formulation factors (Most dominant factor on the left) | |||
|---|---|---|---|---|
| Curling Time | APS Concentration↓ | PEGDA700 Concentration↓ | PEGDA MW↓ | |
| Young’s Modulus | PEGDA700 Concentration↑ | PEGDA MW↓ | PEGMC Concentration↓ | |
| Swelling Ratio | PEGDA700 Concentration↓ | PEGDA MW↑ | PEGMC Concentration↓ | APS Concentration↓ |
| Percent Weight Loss, Day 1 | PEGMC Concentration↓ | APS Concentration↓ | PEGDA MW↑ | |
| Percent Weight Loss, Day 14 | APS Concentration↓ | PEGDA700 Concentration↓ | PEGDA MW↑ | |
| Percent Weight Loss, Day 28 | PEGDA700 Concentration↓ | APS Concentration↓ | PEGDA MW↑ | PEGMC Concentration↑ |
Cytocompatibility of the iFBH components and degradation products
Biocompatibility studies were conducted on human dermal fibroblasts (HDFs), as fibroblasts play a vital role in wound healing and skin regeneration26,27. PEGDA hydrogels have been previously found to be cytocompatible28. PEGMC, APS, and degradation products were tested to validate their compatibility. PEGMC showed cytocompatibility with more than 90% cell viability up to a 5 mg/ml concentration and reduced cell viability to 75% for the 10 mg/ml concentration when compared with the tissue culture plate control (Figure 3A). According to the results, APS proved to be cytocompatible up to 50 μg/ml and significant cell death was observed at concentrations higher than 100 μg/ml (Figure 3B). This is consistent with a previous study of APS cytotoxicity29. Additionally, hydrogel effluents that were released after the hydrolysis of iFBH were analyzed. From 0 to 2.22 mg/ml of effluent concentrations, there was no significant decrease observed in cell viability, but at 6.67 mg/ml and 20 mg/ml there was significant reduction in cell viability (Figure 3C). Thus, the cytocompatibility studies indicate that the materials used in the hydrogel formation are cytocompatible with a wide range of concentrations.
Figure 3.
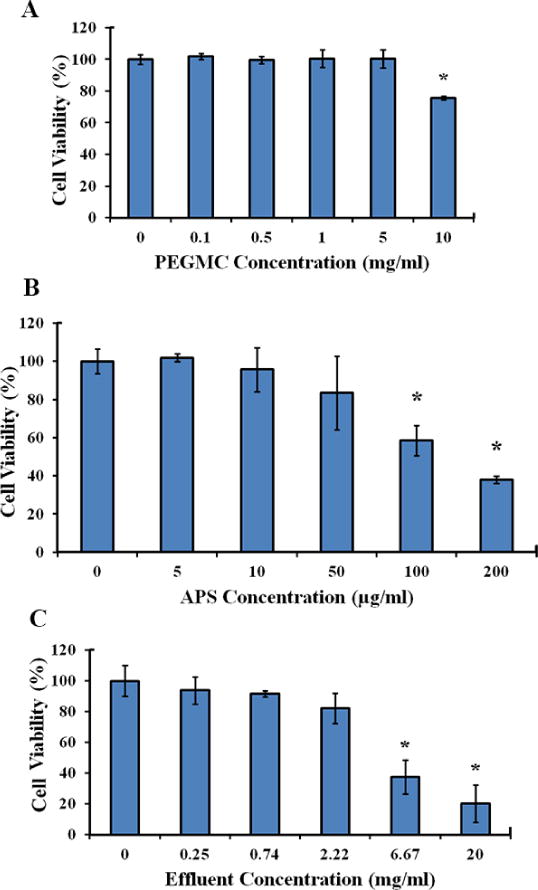
HDF cell viability with incubation with the iFBH system and its components. Evaluation of in vitro human dermal fibroblast viability for (A) PEGMC, (B) APS, and (C) effluents of the iFBH system at different concentrations with 24 hours of incubation. (*, p<0.05, compared to controls of concentration=0)
Formation of antimicrobial biodegradable hydrogel system
In order to prevent the infection of wound sites, we designed iFABH systems consisting of different antimicrobial peptides30. By taking advantage of free carboxyl groups on PEGMC, we conjugated four antimicrobial peptides (CHRG01, ABU, TEMP-A, and ALA5) onto our polymers. The formation of the antimicrobial PEGMC was confirmed by FTIR (Figure S1). FTIR for peptide conjugated PEGMC shows an additional characteristic peak of -CONH around 1560 cm−1. Furthermore, the peptide conjugated PEGMC was used to fabricate antimicrobial hybrid hydrogels with the optimized formulation from the previous factorial analysis to form iFABH, which consists of 200 mg PEGMC, 64 mg PEGDA700, 50 mg PEGDA8000, 16 mg APS.
In vitro antimicrobial property assessment
The wound healing dressings that were developed consist of antimicrobial peptides that aid in controlling the bacterial growth without using any antibiotics, thus avoiding the toxicity and antibiotic resistance that could develop with constant antibiotic use31–33. As an alternative to antibiotics, nano-silver is also being used, but its excessive use can be highly toxic, causing damage to DNA by direct interactions34. Antimicrobial peptides, however, have been investigated as an ideal entity for combating infection because they are a part of the innate epithelial chemical shield35,36.
Four different antimicrobial peptides (CHRG01, ABU, TEMP-A and ALA5) conjugated iFABHs were tested with S. aureus. As seen in Figure 4, ABU, TEMP-A conjugated hydrogels, and the positive control PEGMC-AMP showed significantly less bacterial growth in comparison to negative controls for a period of 24 hours, with 61.2±17.0%, 70.5±15.8%, and 57.7±13.3% bacteria survival rates, respectively. ChRG01 and ALA5 conjugated iFABHs did not exhibit antibacterial activities. Also, PEGMC itself had moderate bacterial inhibition with 79.8±14.7% survival; however, the commercial antimicrobial wound dressing, Hydrofera Blue, did not show significant inhibition of bacteria growth. Results from inhibition zone study showed that PEGMC with either of the antimicrobial peptides or ampicillin showed significantly higher antimicrobial activity compared to the negative control (PLGA scaffolds) (Figure S3). ABU and TEMP-A containing hydrogels (2.47±0.08 and 2.95±0.08 cm2 zones of inhibition, respectively) were tested here and in later in vivo studies since they showed better bacteria killing behavior in a previous OD study. Interestingly, PEGMC by itself again demonstrated antimicrobial activities with a 1.71±0.17 cm2 zone of inhibition. As shown in Figure S3, the hydrogels containing the ABU and TEMP-A peptides showed significant improvement of bacteria inhibition and comparable results with gels containing ampicillin (2.82±0.06 cm2 zone of inhibition). Thus, ABU and TEMP-A incorporated iFABHs were used for further in vivo studies.
Figure 4.
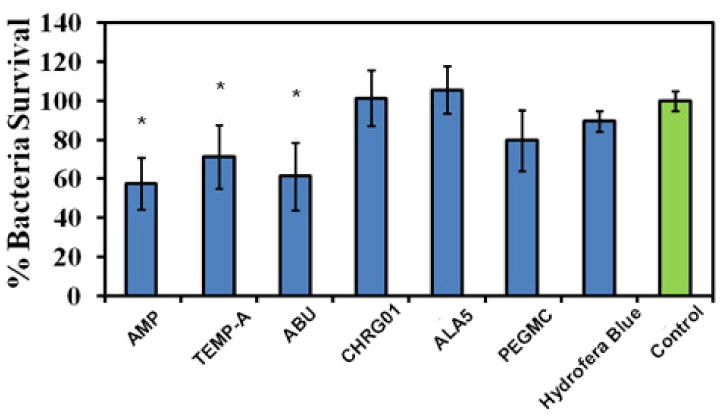
Antimicrobial properties of the iFBH system. S.aureus survival rate as the percentage to the negative control (growth medium alone), CHRG01, ABU, TEMP-A, and ALA5-conjugated PEGMC, pristine PEGMC, commercial dressing (Hydrofera Blue), and ampicillin (AMP)-loaded PEGMC (positive control). ABU and TEMP-A-conjugated PEGMC and PEGMC-AMP showed a significant decrease compared to the negative control (*, p<0.05, compared to control).
In vivo wound healing evaluation
To evaluate the in vivo wound healing performance of our antimicrobial hydrogels, a rat model with full thickness wounds created on the back was used, as shown in Figure S4. PEGMC/PEGDA solution as the first component and APS as the second component were mixed and filled into the wound bed; then iFABHs were formed quickly and stabilized in a few minutes (Figure S4). Pristine PEGMC, ampicillin incorporated PEGMC, and commercially available Hydrofera Blue served as controls. However, Hydrofera Blue was difficult to fix on the wound sites unless fibrin glue was applied (Figure S4). Our in situ forming hybrid hydrogels was much easier to apply onto the wound area compared to Hydrogera Blue. After 24 hours of implantation, S. aureus bacterial suspension was applied on top of each sample. All hydrogel samples kept the wound bed hydrated until the wound completely closed. No infection or scab formation was observed for the antimicrobial composite hydrogels. After 14 days of implantation, full epidermis regeneration was noticeable for all samples (Figure S4). Hydrogel residues were rarely observed at the wound site, suggesting that they degraded during the healing process. The surrounding tissue was excised, sectioned and histologically stained by H&E and Masson’s Trichrome staining.
Representative histological staining images are displayed in Figure 5. All wound sites were closed, as indicated by the full regeneration of epidermal tongue after 14 days. The thicknesses of granulation tissue for ABU, TEMP-A hydrogels, and pristine PEGMC were similar with no bacteria applied (Figure 5A–C); however, with the bacteria added, ABU and TEMP-A containing hydrogels exhibited much less inflammation and granulation tissue formation due to the bacteria-killing behavior (Figure 5K–M). Compared to the Hydrofera Blue dressing (Figure 5E, 6O), our antimicrobial peptide conjugated hydrogels also showed less granulation formed with and without bacteria added. In addition, the positive control PEGMC-AMP promoted wound healing and inhibited inflammation in the most effective manner (Figure 5D, 5I, 5N, and 5S), since more inflammatory cells were replaced by fibroblasts and skin tissue remodeling began to appear. Masson’s Trichrome staining after 14 days of healing also suggested more collagen deposition and a higher amount of myofibroblast formation for ABU and TEMP-A hydrogels compared to pristine PEGMC and Hydrofera Blue, especially with bacteria applied (Figure 5F–J, 5P–T), indicating a faster wound healing process at 14 days19. In particular, ABU-conjugated iFABH demonstrated that healing was established at the remodeling phase with normal skin functions starting to resume after 14 days.
Figure 5.
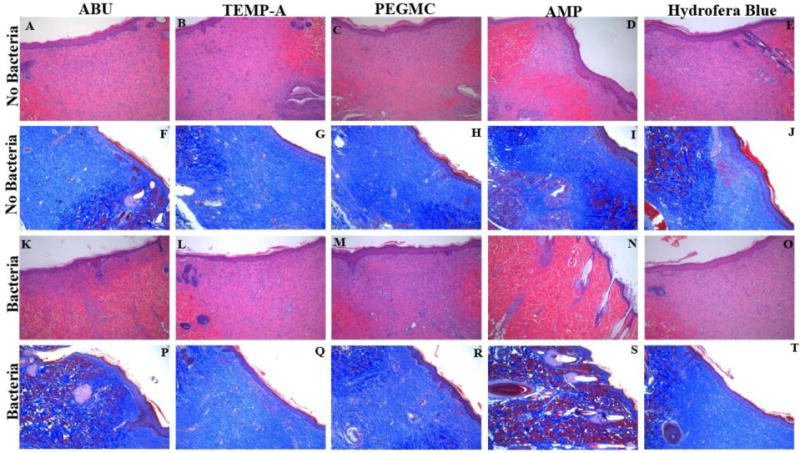
Representative histological images of skin wound samples treated by different dressings, including ABU-conjugated iFABH, TEMP-A-conjugated iFABH, pristine PEGMC, ampicillin (AMP)-loaded PEGMC, and a commercial dressing (Hydrofera Blue) for 7 days (H&E staining, row 1 and row 3) and 14 days (Masson’s Trichrome staining, row 2 and row 4) with and without bacterial challenges.
DISCUSSION
In this study, we intended to develop an optimized PEGMC/PEGDA combined hydrogel system with ideal mechanical/physical performance and biodegradability and also to subsequently endow the hydrogel antimicrobial properties with biocidal peptides for wound healing applications, as illustrated in Scheme 1. The successes of polymer synthesis and peptide conjugation were confirmed by FTIR. Traditional PEGDA hydrogels are non-degradable37, while pure PEGMC hydrogel degrades rapidly in a few days and lacks the integrity to support tissue growth10,11. As a combination, iFBHs had controlled degradation rates with different compounds. In addition, breakdown of PEGMC chains possibly generated some free PEGDA chains that can be dissolved in water and physiological solutions. Thus, the PEGMC/PEGDA hydrogel system is a partially biodegradable blend that is more suitable for wound healing applications with tunable mechanical properties and degradation rates.
To optimize the iFBH formulations, we conducted a 2k factorial analysis. The formulation factors, including PEGMC concentration, long chain PEGDA MW, APS concentration, and PEGDA700 concentration, were varied to study their effects on the curing time, Young’s modulus, swelling ratio, and degradation rates at different time points. For different response factors, the influences of each of the formulation factors were summarized in Table 2. Based on our factorial analysis results, an iFBH formulation that consists of 200 mg PEGMC, 64 mg PEGDA700, 50 mg PEGDA8000, and 16 mg APS was determined to be optimal for wound healing applications.
Key components including APS and PEGMC of the iFBH formulation were also found to be cytocompatible at used concentrations by testing on human dermal fibroblasts. The iFBH degradation product was cytocompatible as well at concentrations lower than 2 mg/ml. With conjugation of different antimicrobial peptides, iFABHs were formed with different bacteria inhibition activities. Interestingly, PEGMC itself showed moderate bacteria inhibition capability. This result is in agreement with our previous studies on the evaluation of antimicrobial properties of citrate-based polymers, since citric acid itself is a biocidal agent38. Additionally, the incorporation of antimicrobial peptides could further improve the antimicrobial performance of the hydrogels38. In particular, iFABHs with ABU and TEMP-A incorporated were most effective at suppressing the S. aureus proliferation. Previous studies have shown good stability and maintenance of the bio-functionalities of peptides after conjugated onto hydrogels by carbodiimide chemistry39,40. Our study also confirmed that chemically conjugated hydrogels with antimicrobial peptides could be effective materials for preventing infection, which is critical for wound healing purposes.
The iFABH can be formed in situ on the wound bed. By using a two-component injection of PEGMC/PEGDA solution and APS solution simultaneously, a layer of hydrogel can be formed to cover the wound area. The iFABH is much easier to apply compared to commercialized Hydrofera Blue. In vivo studies demonstrated that iFABHs with ABU and TEMP-A peptides showed less inflammation than Hydrofera Blue with and without the challenges of bacteria on a rat skin wound model. Histological analysis proved that our antimicrobial hybrid hydrogels are an effective dressing to promote wound healing, especially under the circumstances of possible infections. Since PEGMC degrades completely in 2–4 weeks10, the degradation window is long enough to maintain antimicrobial peptides on the wound bed to prevent infections. Thus, in situ formed iFABH are ideal degradable materials for wound treatment by covering the wound bed completely and preventing bacterial infections.
CONCLUSIONS
A novel citric acid based PEGMC/PEGDA hybrid hydrogel system was successfully synthesized. The biodegradable hydrogel system has in situ gelling capabilities. A mathematical factorial analysis was performed to determine how formulation factors, such as the molecular weight and concentration of PEGDA, concentration of PEGMC, and concentration of APS, affect the physical and mechanical properties of the resulting hydrogels. To achieve the ideal curing time, Young’s Modulus, swelling ratio, and degradation, an optimized formulation was determined based on the mathematical analysis. The optimum iFABH was further found to be cytocompatible and biocidal with the conjugation of antimicrobial peptides. A preliminary in vivo study illustrated the easy usage of PEGMC/PEGDA hydrogels on the rat skin wound model. These antimicrobial hydrogels also promoted wound healing and prevented infections. Thus, our biodegradable in situ gelling PEGMC/PEGDA hydrogels could be a promising material for wound dressing applications.
Supplementary Material
Acknowledgments
The work was supported in part by the Norman Hackerman Advanced Research Program (ARP), National Institutes of Health grants (HL 118498, EB 012575 and CA 182670), and National Science Foundation Awards (DMR 1313553 and CMMI 1266116). The authors also acknowledge Dr. Hong Weng and Dr. Liping Tang from the University of Texas at Arlington for their guidance in animal studies.
ABBREVIATIONS
- iFBH
in situ forming biodegradable hydrogel
- iFABH
in situ forming antimicrobial biodegradable hydrogel
- PEGDA
poly (ethylene glycol) diacrylate
- PEGMC
poly (ethylene glycol) maleate citrate
- APS
ammonium persulfate
- TEMED
tetramethylethylenediamine
- HDF
human dermal fibroblast
- ABU
ABU-CHRG01
- TEMP-A
Temporin-A
- ALA5
Ala5-Tritrp7
- AMP
ampicillin
- EDC
1-Ethyl-3-(3-dimethylaminopropyl)-carbodiimide
- NHS
N-Hydroxysuccinimide
- DOE
design of experiments
- SEM
scanning electron microscopy
- FTIR
Fourier transform infrared spectroscopy
- OD
optical density
Footnotes
Supporting Information. Additional figures and results are shown in Supporting Information.
References
- 1.Habeeba Park CC, Sharon Henry, Adrian Barbul. Complex Wounds and Their Management. Surg Clin N Am. 2010;90(6):1181–94. doi: 10.1016/j.suc.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Fonder MA, Lazarus GS, Cowan DA, Aronson-Cook B, Kohli AR, Mamelak AJ. Treating the chronic wound: A practical approach to the care of nonhealing wounds and wound care dressings. J Am Acad Dermato. 2008;58(2):185–206. doi: 10.1016/j.jaad.2007.08.048. [DOI] [PubMed] [Google Scholar]
- 3.Ferreira MC, Tuma P, Jr, Carvalho VF, Kamamoto F. Complex wounds. Clinics. 2006;61(6):571–8. doi: 10.1590/s1807-59322006000600014. [DOI] [PubMed] [Google Scholar]
- 4.Marston WA. Dermagraft, a bioengineered human dermal equivalent for the treatment of chronic nonhealing diabetic foot ulcer. Expert Rev Med Devices. 2004;1(1):21–31. doi: 10.1586/17434440.1.1.21. [DOI] [PubMed] [Google Scholar]
- 5.Balakrishnan B, Mohanty M, Umashankar PR, Jayakrishnan A. Evaluation of an in situ forming hydrogel wound dressing based on oxidized alginate and gelatin. Biomaterials. 2005;26(32):6335–6342. doi: 10.1016/j.biomaterials.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 6.Keast DH, Orsted H. The basic principles of wound care. Ostomy Wound Manage. 1998;44(8):24–8. 30–1. [PubMed] [Google Scholar]
- 7.Durst CA, Cuchiara MP, Mansfield EG, West JL, Grande-Allen KJ. Flexural characterization of cell encapsulated PEGDA hydrogels with applications for tissue engineered heart valves. Acta Biomaterialia. 2011;7(6):2467–2476. doi: 10.1016/j.actbio.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burmania JA, Martinez-Diaz GJ, John Kao W. Synthesis and physicochemical analysis of interpenetrating networks containing modified gelatin and poly(ethylene glycol) diacrylate. J Biomed Mater Res A. 2003;67A(1):224–234. doi: 10.1002/jbm.a.10106. [DOI] [PubMed] [Google Scholar]
- 9.Chen S-H, Tsao C-T, Chang C-H, Lai Y-T, Wu M-F, Chuang C-N, Chou H-C, Wang C-K, Hsieh K-H. Assessment of reinforced poly(ethylene glycol) chitosan hydrogels as dressings in a mouse skin wound defect model. Materials Science and Engineering: C. 2013;33(5):2584–2594. doi: 10.1016/j.msec.2013.02.031. [DOI] [PubMed] [Google Scholar]
- 10.Gyawali D, Nair P, Zhang Y, Tran RT, Zhang C, Samchukov M, Makarov M, Kim HKW, Yang J. Citric acid-derived in situ crosslinkable biodegradable polymers for cell delivery. Biomaterials. 2010;31(34):9092–9105. doi: 10.1016/j.biomaterials.2010.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gyawali D, Nair P, Kim HKW, Yang J. Citrate-based biodegradable injectable hydrogel composites for orthopedic applications. Biomaterials Science. 2013;1(1):52–64. doi: 10.1039/C2BM00026A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ong S-Y, Wu J, Moochhala SM, Tan M-H, Lu J. Development of a chitosan-based wound dressing with improved hemostatic and antimicrobial properties. Biomaterials. 2008;29(32):4323–4332. doi: 10.1016/j.biomaterials.2008.07.034. [DOI] [PubMed] [Google Scholar]
- 13.Hoover DM, Wu Z, Tucker K, Lu W, Lubkowski J. Antimicrobial Characterization of Human {beta}-Defensin 3 Derivatives. Antimicrob. Agents Chemother. 2003;47(9):2804–2809. doi: 10.1128/AAC.47.9.2804-2809.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marr AK, Gooderham WJ, Hancock RE. Antibacterial peptides for therapeutic use: obstacles and realistic outlook. Curr Opin Pharmacol. 2006;6(5):468–72. doi: 10.1016/j.coph.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 15.Hancock REW, Sahl H-G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat Biotech. 2006;24(12):1551–1557. doi: 10.1038/nbt1267. [DOI] [PubMed] [Google Scholar]
- 16.Tengvall P, Jansson E, Askendal A, Thomsen P, Gretzer C. Preparation of multilayer plasma protein films on silicon by EDC/NHS coupling chemistry. Colloids Surf B Biointerfaces. 2003;28(4):261–272. [Google Scholar]
- 17.Atiyeh BS, Costagliola M, Hayek SN, Dibo SA. Effect of silver on burn wound infection control and healing: review of the literature. Burns. 2007;33(2):139–48. doi: 10.1016/j.burns.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 18.Su SH, Eaton JW, Venezia RA, Tang L. Interactions of vancomycin resistant enterococci with biomaterial surfaces. ASAIO J. 1998;44(6):770–5. doi: 10.1097/00002480-199811000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Xie Z, Paras CB, Weng H, Punnakitikashem P, Su L-C, Vu K, Tang L, Yang J, Nguyen KT. Dual growth factor releasing multi-functional nanofibers for wound healing. Acta Biomaterialia. 2013;9(12):9351–9359. doi: 10.1016/j.actbio.2013.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dorsett-Martin WA. Rat models of skin wound healing: A review. Wound Repair and Regeneration. 2004;12(6):591–599. doi: 10.1111/j.1067-1927.2004.12601.x. [DOI] [PubMed] [Google Scholar]
- 21.Bracho DO, Barsan L, Arekapudi SR, Thompson JA, Hen J, Stern SA, Younger JG. Antibacterial properties of an iron-based hemostatic agent in vitro and in a rat wound model. Acad Emerg Med. 2009;16(7):656–60. doi: 10.1111/j.1553-2712.2009.00439.x. [DOI] [PubMed] [Google Scholar]
- 22.Abreu FOMS, Bianchini C, Forte MMC, Kist TBL. Influence of the composition and preparation method on the morphology and swelling behavior of alginate-chitosan hydrogels. Carbohydr Polym. 2008;74(2):283–289. [Google Scholar]
- 23.Sabnis A, Wadajkar AS, Aswath P, Nguyen KT. Factorial analyses of photopolymerizable thermoresponsive composite hydrogels for protein delivery. Nanomedicine. 2009;5(3):305–315. doi: 10.1016/j.nano.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jayakumar R, Prabaharan M, Sudheesh Kumar PT, Nair SV, Tamura H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol Adv. 2011;29(3):322–37. doi: 10.1016/j.biotechadv.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 25.Temenoff JS, Athanasiou KA, Lebaron RG, Mikos AG. Effect of poly(ethylene glycol) molecular weight on tensile and swelling properties of oligo(poly(ethylene glycol) fumarate) hydrogels for cartilage tissue engineering. J Biomed Mater Res A. 2002;59(3):429–437. doi: 10.1002/jbm.1259. [DOI] [PubMed] [Google Scholar]
- 26.Ghosh K, Ren XD, Shu XZ, Prestwich GD, Clark RA. Fibronectin functional domains coupled to hyaluronan stimulate adult human dermal fibroblast responses critical for wound healing. Tissue Eng. 2006;12(3):601–13. doi: 10.1089/ten.2006.12.601. [DOI] [PubMed] [Google Scholar]
- 27.Froget S, Barthelemy E, Guillot F, Soler C, Coudert MC, Benbunan M, Dosquet C. Wound healing mediator production by human dermal fibroblasts grown within a collagen-GAG matrix for skin repair in humans. Eur Cytokine Netw. 2003;14(1):60–4. [PubMed] [Google Scholar]
- 28.Zhong C, Wu J, Reinhart-King CA, Chu CC. Synthesis, characterization and cytotoxicity of photo-crosslinked maleic chitosan–polyethylene glycol diacrylate hybrid hydrogels. Acta Biomaterialia. 2010;6(10):3908–3918. doi: 10.1016/j.actbio.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 29.Shin H, Temenoff JS, Mikos AG. In Vitro Cytotoxicity of Unsaturated Oligo[poly(ethylene glycol) fumarate] Macromers and Their Cross-Linked Hydrogels. Biomacromolecules. 2003;4(3):552–560. doi: 10.1021/bm020121m. [DOI] [PubMed] [Google Scholar]
- 30.Kazemzadeh-Narbat M, Kindrachuk J, Duan K, Jenssen H, Hancock REW, Wang R. Antimicrobial peptides on calcium phosphate-coated titanium for the prevention of implant-associated infections. Biomaterials. 2010;31(36):9519–9526. doi: 10.1016/j.biomaterials.2010.08.035. [DOI] [PubMed] [Google Scholar]
- 31.Campoccia D, Montanaro L, Speziale P, Arciola CR. Antibiotic-loaded biomaterials and the risks for the spread of antibiotic resistance following their prophylactic and therapeutic clinical use. Biomaterials. 2010;31(25):6363–77. doi: 10.1016/j.biomaterials.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 32.Sibbald RG, Orsted H, Schultz GS, Coutts P, Keast D. Preparing the wound bed 2003: focus on infection and inflammation. Ostomy Wound Manage. 2003;49(11):23–51. [PubMed] [Google Scholar]
- 33.Hurdle JG, O’Neill AJ, Chopra I, Lee RE. Targeting bacterial membrane function: an underexploited mechanism for treating persistent infections. Nat Rev Microbiol. 2011;9(1):62–75. doi: 10.1038/nrmicro2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chaloupka K, Malam Y, Seifalian AM. Nanosilver as a new generation of nanoproduct in biomedical applications. Trends Biotechnol. 2010;28(11):580–8. doi: 10.1016/j.tibtech.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 35.Niyonsaba F, Ushio H, Nakano N, Ng W, Sayama K, Hashimoto K, Nagaoka I, Okumura K, Ogawa H. Antimicrobial peptides human beta-defensins stimulate epidermal keratinocyte migration, proliferation and production of proinflammatory cytokines and chemokines. J Invest Dermatol. 2007;127(3):594–604. doi: 10.1038/sj.jid.5700599. [DOI] [PubMed] [Google Scholar]
- 36.Namjoshi S, Caccetta R, Benson HA. Skin peptides: biological activity and therapeutic opportunities. J Pharm Sci. 2008;97(7):2524–42. doi: 10.1002/jps.21198. [DOI] [PubMed] [Google Scholar]
- 37.Browning MB, Cosgriff-Hernandez E. Development of a Biostable Replacement for PEGDA Hydrogels. Biomacromolecules. 2012;13(3):779–786. doi: 10.1021/bm201707z. [DOI] [PubMed] [Google Scholar]
- 38.Su L-C, Xie Z, Zhang Y, Nguyen KT, Yang J. Study on the Antimicrobial Properties of Citrate-Based Biodegradable Polymers. Front Bioeng Biotechnol. 2014;2:23. doi: 10.3389/fbioe.2014.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.He X, Ma J, Jabbari E. Effect of Grafting RGD and BMP-2 Protein-Derived Peptides to a Hydrogel Substrate on Osteogenic Differentiation of Marrow Stromal Cells. Langmuir. 2008;24(21):12508–12516. doi: 10.1021/la802447v. [DOI] [PubMed] [Google Scholar]
- 40.Lin C-C, Anseth KS. Controlling Affinity Binding with Peptide-Functionalized Poly(ethylene glycol) Hydrogels. Advanced Functional Materials. 2009;19(14):2325–2331. doi: 10.1002/adfm.200900107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


