SUMMARY
Previous data on the associations between nocturnal oxygen saturation parameters and carotid atherosclerosis are conflicting. We examined the prospective associations of nocturnal oxygen saturation (SaO2) and cardiovascular disease (CVD) risk factors with carotid intima-media thickness (IMT) and plaques. We used data on 689 Wisconsin Sleep Cohort participants who had baseline overnight polysomnography followed by carotid ultrasonography a mean (standard deviation) of 7.8 (2.5) years later. Far wall common carotid IMT was measured using B-mode ultrasound. Bilateral common, bifurcation, and internal carotid artery segments were evaluated for plaque score. Participants were 56 (8) years old (55% male); 32% had hypertension and mean body-mass index was 31 (7) kg/m2. Mean and minimum nocturnal SaO2 were 95% (2) and 86% (7), respectively. Mean percent sleep time with SaO2 <90% was 2% (8). Both mean (odds ratio [OR]=0.60 lower plaque count per 5% higher mean SaO2, 95% CI=0.38–0.96, p=0.033) and minimum SaO2 (OR=0.88 lower plaque count per 5% higher minimum SaO2, 95% CI=0.80–0.97, p=0.013) predicted carotid plaque score after adjusting for age, sex and body-mass index. Minimum SaO2 predicted future plaque score after adding adjustment for traditional CVD risk factors (OR=0.90 lower plaque count per 5% higher minimum SaO2, 95% CI=0.81–0.99, p=0.038). Mean SaO2 was not associated with carotid IMT after CVD risk factor adjustment. We conclude that minimum nocturnal SaO2 is an independent predictor of future carotid plaque burden. Other nocturnal SaO2 parameters are not associated with future carotid IMT or plaques after adjusting for traditional CVD risk factors.
Keywords: Atherosclerosis, Carotid arteries, Epidemiology, Sleep apnea, Ultrasound
Introduction
Obstructive sleep apnea (OSA) is associated with increased risk of cardiovascular disease (CVD) and is characterized by repeated episodes of airway obstruction with a concomitant decrease in oxygen saturation (Campos-Rodriguez et al., 2005; Marin et al., 2005; Somers et al., 2008; Young et al., 2008). Repetitive hypoxic insults may lead to endothelial dysfunction, arterial injury, and eventually, atherosclerosis (Jelic et al., 2008; Kraiczi et al., 2001; Yokoe et al., 2003). OSA is an important public health concern due to its rising prevalence, likely related to the obesity epidemic (Peppard et al., 2013; Peppard et al., 2000). Carotid artery intima-media thickness (IMT) and carotid artery plaques are established markers of subclinical arterial disease that predict future CVD events (Inaba et al., 2012; Den Ruijter et al., 2012; Stein et al., 2008a).
We have previously shown that the apnea-hypopnea index (AHI) is associated with greater future carotid atherosclerosis (Gunnarsson et al., 2014). However, existing data on associations between nocturnal oxygen saturation parameters and carotid atherosclerosis are limited to small cross-sectional and case-control studies that have shown conflicting results (Baguet et al., 2009; Baguet et al., 2005; Drager et al., 2010; Drager et al., 2005; Minoguchi et al., 2005; Ozdemir et al., 2013; Protogerou et al., 2007; Saletu et al., 2006; Schulz et al., 2005; Suzuki et al., 2004; Szabóová et al., 2007; Tan et al., 2012). Also, very few studies have looked at the relationships between oxygen saturation parameters and plaques (Baguet et al., 2005; Saletu et al., 2006; Schulz et al., 2005). Thus, we undertook this study to investigate the prospective associations of baseline nocturnal oxygen saturation parameters with carotid IMT and plaque score using data from the Wisconsin Sleep Cohort Study, a large cohort study of the natural history of OSA in adults. We hypothesized that the severity of oxygen desaturation would predict higher future carotid IMT and carotid plaques and that the associations would persist after adjusting for body-mass index and other CVD risk factors.
Methods
Participants and Study Procedures
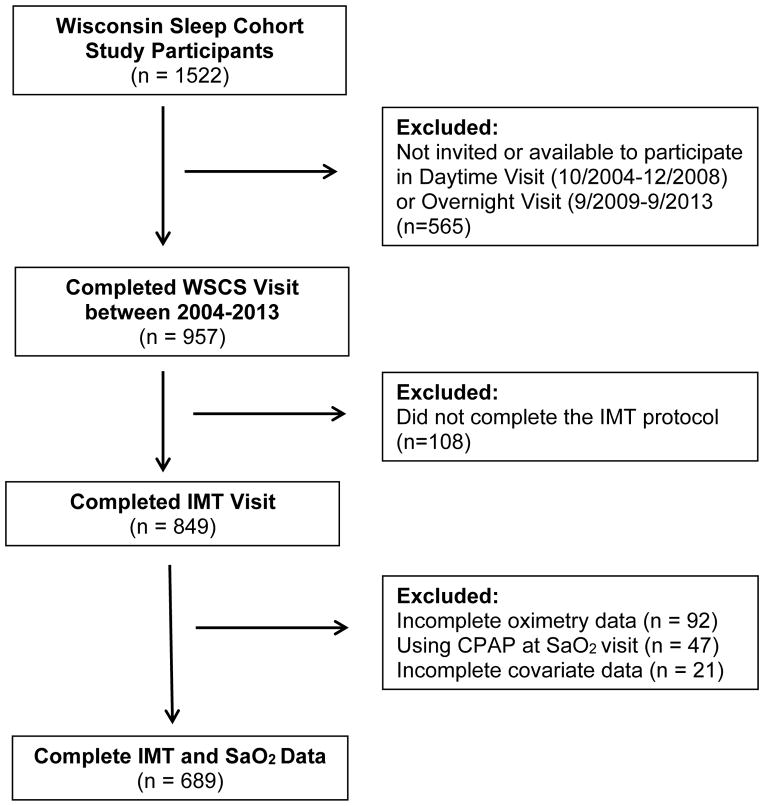
The study was approved by the UW Health Sciences Institutional Review Board. All subjects provided free informed consent for participation in all study activities. The design of the parent study, the Wisconsin Sleep Cohort Study (WSCS), begun in 1989, has been reported previously (Peppard et al., 2013). The protocol for the current investigation of nocturnal saturation and subclinical carotid atherosclerosis was added in 2004. Subjects underwent in-lab overnight polysomnography from August 2000 to September 2013 (See Figure 1). Methods for performing and interpreting these polysomnograms have been described elsewhere (Young et al., 1993). Note that between 1988 and 2000, sleep studies were scored using a paper-based system; since 2000, studies have been scored on a computer. All statistical modeling adjusts for the scoring changes; this removes instrumentation-related influences on sleep-disordered breathing assessments after the year 2000. The polysomnography variables of interest were mean nocturnal oxygen saturation (mean SaO2), minimum nocturnal oxygen saturation (minimum SaO2) defined as the lowest saturation reached during sleep, and percentage of time spent asleep with oxygen saturation below 90% (TST<90%). Arterial oxygen saturation (SaO2) was measured using pulse oximetry (Datex-Ohmeda 3900 Pulse Oximeter). All values of SaO2 <50% were considered to be measurement error and were excluded. Only five subjects had minimum saturation <55%. These were considered valid measurements and were included.
Figure 1.
Participant Flow Diagram
The overnight protocol included measurement of height and weight, and blood pressure assessment by auscultatory sphygmomanometry (average of 2 sitting measurements at 5-minute intervals). A questionnaire was completed at each visit that included items about past medical history, current and past medication use, sleep habits, lifestyle habits (including cigarette smoking history, alcohol use, and physical activity), socio-demographic characteristics, and use of positive airway pressure (CPAP). The morning after the sleep study, a venipuncture was performed for fasting plasma glucose and lipids.
Carotid Artery Ultrasound and Measurements
Carotid ultrasound studies were performed from October 2004, through September 2013, on 849 participants. Approximately half of the participants completed two carotid ultrasound visits in this time period. To maximize the time between polysomnography and carotid ultrasound visits, the measurements from the second visit were used in the analysis. Ninety-two participants were excluded for not having SaO2 data at least two years prior to the IMT measures. Participants that were using CPAP for the treatment of OSA at the time of the polysomnography (n=47) or were missing covariate data (n=21) were also excluded from this analysis. The final sample size was 689 participants with complete IMT and SaO2 data (Figure 1). The mean (standard deviation) follow-up time between first polysomnogram study and carotid ultrasound was 7.8 (2.5) years.
B-mode ultrasound images of each carotid artery were obtained using an 8.0 MHz linear array transducer (8L5, Acuson Sequoia C512, Siemens Medical Solutions, Malvern, PA), using a standardized imaging protocol (Stein et al., 2004). Mean carotid IMT was measured from the distal 1 cm of the far walls of each common carotid artery from three different angles on each side. IMT measurements at each angle were performed in triplicate and averaged. Carotid artery plaque was defined as a focal wall thickening with carotid IMT ≥1.5 mm or a distinct protrusion into the lumen with a thickness 50% greater than that of the adjacent wall (Stein et al., 2008a). Carotid plaques were evaluated from transverse and longitudinal views of all carotid artery segments. The carotid plaque score (CPS) was defined as the total number of segments with carotid plaque (range 0–12). Ultrasound examinations were interpreted and measured by a single experienced investigator with excellent reproducibility, blinded to all subject information (Stein et al., 2008b; Stein et al., 2004).
Statistical Analysis
Statistical analyses were performed with SAS, version 9.2 (SAS Institute Incorporated, Cary, NC). Spearman correlations were computed as initial associations between nocturnal oxygen saturation parameters and carotid IMT variables. Primary outcome variables were: 1) mean carotid IMT (modeled with linear regression) and 2) carotid plaque score (modeled with ordinal logistic regression). For both outcomes, we evaluated associations with baseline nocturnal oxygen saturation parameters and covariates assessed at the first polysomnography study. We chose to describe these associations with 5% increments in the oxygen saturation parameters because it was clinically meaningful and beyond normal measurement variations. Baseline variables included age, sex, body-mass index, systolic blood pressure, current smoking status, serum high-density lipoprotein (HDL) and self-reported use of lipid-lowering medications. These baseline variables were used to predict carotid ultrasound measures assessed an average of 7.8 years after baseline measurements.
Meaningful deviations from modeling assumptions were checked for each presented model. For ordinal logistic regressions of the carotid plaque score outcome, the proportional odds assumption was met when statistically significant covariates were included in the model and when the upper end of the plaque score distribution was grouped together into one “high” level (i.e., 9–12 plaques were grouped into a single category, ≥9 plaques). Thus, plaque score models included only statistically significant covariates and the plaque score outcome variable was grouped as described above.
Results
At baseline, the mean (standard deviation) age of the 689 participants was 56 (8) years (55% male, 97% white) (Table I). Mean body-mass index was 31 (7) kg/m2, 32% were taking anti-hypertensive medications, and 11% were smokers. At the carotid ultrasound assessment visit (7.8 [2.5] years later), the participants’ mean age was 64 (8) years; mean body-mass index was 31 (7) kg/m2 and 51% were taking anti-hypertensive medications. The mean carotid IMT was 0.76 (0.16) mm and the average carotid plaque score was 2.5 (2.5).
Table 1.
Descriptive Statistics for Participant Demographics and Key Study Variables
| Variable | At Baseline Sleep Study Visit | At Carotid Ultrasound Visit |
|---|---|---|
| Age – years | 56 (8) | 64 (8) |
| Male – N (%) | 375 (54%) | - |
| Race – % White | 97% | - |
| Body-mass index – kg/m2 | 31(7) | 31 (7) |
| Apnea-Hypopnea Index – events/hour | 6.4 (8.7) | |
| N (%) | ||
| <5 | 421 (61%) | - |
| 5–15 | 190 (28%) | - |
| 15–30 | 56 (8%) | - |
| ≥30 | 22 (3%) | - |
| Systolic blood pressure – mmHg | 125 (15) | 126 (14) |
| High-density lipoprotein cholesterol – mg/dL | 52 (14) | - |
| Glucose – mg/dL | 103 (24) | - |
| Current smoker – N (%) | 79 (11%) | 52 (8%) |
| Antihypertensive medication use – N (%) | 220 (32%) | 348 (51%) |
| Lipid-lowering medication use – N (%) | 156 (22%) | 306 (44%) |
| Diabetes medication use – N (%) | 40 (6%) | 86 (12%) |
| Mean % O2 saturation | 95 (2) | - |
| Minimum % O2 saturation | 86 (7) | - |
| Percent sleep time with SaO2<90% | 2 (8) | - |
| Mean CCA intima-media thickness – mm | - | 0.76 (0.16) |
| Presence of carotid plaque | - | 477 (69%) |
| Carotid plaque score – count | - | 2.5 (2.5) |
CCA = Common carotid artery
All values are mean (standard deviation) unless noted otherwise.
Associations between Nocturnal Oxygen Saturation Parameters and Future Carotid IMT
Both minimum (r=−0.11; p=0.004) and mean (r=−0.10; p=0.007) nocturnal SaO2 were inversely correlated with future IMT. In linear regression models adjusted for age and sex, only minimum SaO2 predicted IMT (β=−0.009 mm per 5% increment in minimum SaO2, 95% CI=−0.017 – −0.001, p=0.023). This association no longer was statistically significant after adding body-mass index and traditional CVD risk factors (p=0.991) to the model (Table 2). Surprisingly, mean SaO2 was associated with higher IMT after covariate adjustment, though this association probably is spurious (see discussion). Percent time with SaO2 <90% was not associated with future carotid IMT.
Table 2.
Associations of Baseline Nocturnal Oximetry Parameters with Future Carotid Intima-Media Thickness and Carotid Plaque Score*
| Predictor | IMT (mm) β†, (95% CI) |
p | Plaque Score (count) OR‡, (95% CI) |
p |
|---|---|---|---|---|
| Minimum SaO2 | 0.000 (−.008, 0.008) | 0.991 | 0.90 (0.81, 0.99) | 0.038 |
| Mean SaO2 | 0.045 (0.005, 0.084) | 0.026 | 0.77 (0.47, 1.26) | 0.301 |
| TST<90% | 0.000 (−0.001, 0.002) | 0.804 | 1.00 (0.99, 1.02) | 0.655 |
CI = confidence interval; IMT = intima-media thickness; OR = odds ratio; SaO2 = arterial oxygen saturation; TST<90% =percentage of time spent asleep with oxygen saturation below 90%
Models adjusted for: Age, sex, body-mass index, systolic blood pressure, current smoking, serum high-density lipoprotein cholesterol, and use of lipid-lowering medications
Millimeter (mm) per 5% increment in nocturnal oxygen saturation parameter or TST<90%
Relative odds of greater plaque scores per 5% increment in nocturnal oxygen saturation parameter or TST<90%
Associations between Baseline Mean and Future Carotid Plaque Score
Both minimum (r=−0.13; p=0.001) and mean (r=−0.15; p<0.001) SaO2 were inversely correlated with future carotid plaque score. In ordinal logistic regression models adjusted for age, sex and body-mass index, both mean SaO2 (OR=0.60 per 5% higher mean SaO2, 95% CI 0.38–0.96, p=0.033) and minimum SaO2 (OR=0.88 per 5% higher minimum SaO2, 95% CI 0.80–0.97, p=0.013) predicted risk of higher carotid plaque score. TST<90% was not associated with carotid plaque score (p=0.66). Minimum SaO2 was the only saturation parameter that remained a significant predictor of carotid plaque score after adjusting for traditional CVD risk factors (OR=0.90 per 5% higher minimum SaO2, 95% CI 0.81–0.99, p=0.038) (Table 2). Other significant predictors of carotid plaque score in the final model included age, body-mass index (BMI), systolic blood pressure, high density lipoprotein cholesterol, lipid medication use and smoking (Table 3).
Table 3.
Multivariable Associations of Minimum Sa02 and other Cardiovascular Disease Risk Factors With Future Carotid Plaque Score (fully adjusted model)
| Predictor | Plaque Score Odds Ratio (95% CI) |
p |
|---|---|---|
| Age | 1.09 (1.07 – 1.11) | <0.001 |
| Female sex | 0.87 (0.63 – 1.18) | 0.373 |
| Body-mass index | 0.97 (0.95 – 1.00) | 0.031 |
| Systolic blood pressure | 1.02 (1.01 – 1.03) | <0.001 |
| High-density lipoprotein cholesterol | 0.99 (0.97 – 1.00) | 0.010 |
| Lipid medication use | 2.02 (1.46 – 2.81) | <0.001 |
| Current smoker | 2.27 (1.49 – 3.47) | <0.001 |
| Minimum SaO2 | 0.90 (0.81 – 0.99) | 0.038 |
CI = confidence interval; SaO2 = arterial oxygen saturation
Including blood pressure and diabetes medication use in the model did not change the association between minimum SaO2 and carotid plaques (OR 0.98, 95% CI 0.96–1.00, p=0.043), but including these two variables meant that proportional odds assumption for ordinal logistic regression model was not met. Thus, we decided to leave these variables out of the final model.
The association between minimum SaO2 and carotid plaque score remained significant after adjusting for mean SaO2 level at rest before the sleep study and other possible causes of hypoxia, such as self-reported history of emphysema, obstructive lung disease, asthma or congestive heart failure. Also, inclusion of CPAP users did not change the results significantly (OR=0.89 per 5% higher minimum SaO2, 95% CI 0.81–0.98, p=0.023). Similarly, in models including interaction terms, neither age nor sex interacted with minimum SaO2 to predict plaque score.
Discussion
This is the first large cohort study to show that minimum nocturnal oxygen saturation is independently associated with future carotid artery plaques. This finding has important clinical implications because plaques are powerful predictors of CVD risk (Inaba et al., 2012). The associations of other parameters of nocturnal oxygen saturation with carotid atherosclerosis (i.e. mean SaO2 and TST<90%) were confounded by BMI and other CVD risk factors. These findings are in keeping with our previous study which showed that AHI, another marker of OSA severity, was a stronger, independent predictor of future plaques than of IMT (Gunnarsson et al., 2014). Similarly, we found that minimum oxygen saturation predicted carotid IMT after adjusting for age and sex, but this association disappeared after the addition of BMI. This may be because BMI is more closely correlated with carotid wall thickening than with plaque development.
We believe that the observed association between higher mean SaO2 and higher later carotid IMT was spurious. The effect size was very small and likely was due to residual confounding effects of drugs that affect carotid wall thickness more than plaque, such as use of antihypertensive and lipid-lowering medications.
Of note, our results remained robust after adding interaction terms for age and sex and also after adjustments for self-reported history of COPD and congestive heart failure. This supports our theory that the observed increase in plaques is related to OSA rather than other causes of nocturnal hypoxia. Adding CPAP users to our models did not change the association with minimum Sa02 significantly. However, it is important to note that the CPAP group was too small (n=47) to draw any meaningful conclusions about the effects of CPAP treatment on carotid atherosclerosis. Also, we were unable to determine any definitive threshold points for minimum SaO2 predicting increased carotid plaques, most likely because there were not enough subjects with nocturnal oxygen saturation below 80%. Of note, we excluded all subjects with minimum SaO2 <50% due to high likelihood that these were artifactually low values. Excluding true hypoxemic episodes with Sa02 <50% would be expected to weaken our observed association between minimum SaO2 and carotid plaque score. As a sensitivity test, we looked at the effect of excluding the five subjects with minimum SaO2 <55% and found that the strong trend between minimum SaO2 and carotid plaques remained, but the level of significance decreased, as expected (p=0.072).
Previous data on the relationship between nocturnal oxygen saturation and carotid atherosclerosis come from relatively small case-control and cross-sectional studies on patients with known or suspected OSA (Baguet et al., 2009; Baguet et al., 2005; Drager et al., 2010; Drager et al., 2005; Minoguchi et al., 2005; Ozdemir et al., 2013; Protogerou et al., 2007; Saletu et al., 2006; Schulz et al., 2005; Suzuki et al., 2004; Szabóová et al., 2007; Tan et al., 2012). It therefore is challenging to compare our findings with these older studies because our data come from randomly selected community dwelling adults with much higher mean and minimum oxygen saturations and a lower percentage of TST<90%, so some true associations may have been missed. However, the majority of these studies showed that either minimum (Drager et al., 2005; Schulz et al., 2005; Suzuki et al., 2004; Tan et al., 2012), mean (Baguet et al., 2009; Baguet et al., 2005; Schulz et al., 2005; Tan et al., 2012) or TST<90% (Baguet et al., 2009; Minoguchi et al., 2005; Saletu et al., 2006; Schulz et al., 2005; Suzuki et al., 2004; Szabóová et al., 2007) or two or more of these oxygen saturation parameters were associated with increased carotid IMT. Only one study showed no relationship (Drager et al., 2010). It is important to appreciate that only one-half of the studies explored these relationships with adjustments for covariates. Thus, it is not clear whether the oxygen saturation parameters contributed independently to carotid IMT in the studies that were based on simple correlations.
There have been very few studies that examined OSA-associated hypoxia and carotid plaques and their results are conflicting (Baguet et al., 2005; Protogerou et al., 2007; Saletu et al., 2006). One study found that both minimum and mean SaO2 predicted carotid plaques (Baguet et al., 2005). Two other studies found no relationship between TST<90% and plaques but did not comment on mean or minimal SaO2 and plaques (Protogerou et al., 2007; Saletu et al., 2006). However, studies examining coronary arteries have shown that OSA is associated with increased coronary artery plaque scores (Kent et al., 2013; Weinreich et al., 2013).
Intermittent hypoxemia causes increased systemic inflammation and sympathetic activity leading to endothelial dysfunction (Jelic et al., 2008; Kraiczi et al., 2001; Yokoe et al., 2003). Hypoxemia also is related to atherosclerotic plaque progression in animal models (Jun et al., 2010; Savransky et al., 2007). Inflammation, sympathetic activation, and endothelial dysfunction may explain, in part, the observed relationship between minimum SaO2 and carotid plaques. However, we did not have measurements of inflammatory markers to address this directly.
It is intriguing that minimum SaO2 but not mean SaO2 or TST <90% were independently related to carotid plaque score. One study showed that compared to minimum SaO2, TST <90% might be a better predictor of increased inflammation in patients with OSA (Yokoe et al., 2003), but to our knowledge, no such study has been performed in community dwelling subjects. It is possible that in a cohort derived from the general community with a very low prevalence of severe OSA; lowest nocturnal oxygen level (i.e. minimum SaO2), rather than mean SaO2 or TST <90%, better reflects the endothelial damage caused by nocturnal hypoxia and thus is a stronger stimulator of atherosclerosis.
The strengths of this study include the large size cohort, prospective design, and inclusion of multiple covariates. Limitations include that participants were 97% Caucasian which reduces the generalizability of our findings. Because we did not have carotid artery measurements at the time of the baseline polysomnography, we were unable to assess carotid IMT progression. We also did not have hemoglobin A1C levels to adjust for diabetes mellitus control. Diagnoses of COPD, asthma and congestive heart failure were self-reported without confirmatory tests or clinical assessment at the time of baseline assessments.
CONCLUSION
In a large cohort of community-dwelling subjects, minimal nocturnal oxygen saturation is an independent risk factor for increased future carotid plaque burden. This has important clinical implications because carotid plaque burden strongly predicts CVD events. Thus, early recognition and treatment of OSA and associated risk factors could affect future CVD risk.
Acknowledgments
The authors thank the following for their valuable assistance: Jean Einerson, MS (lab coordination), Laurel Finn, MS (data quality control and analysis); Kristin Hansen, BS (data acquisition); Carol M Mitchell, PhD (data quality); Kathryn Pluff, BS (subject recruitment, data collection, laboratory management); Amanda Rasmuson, MS (laboratory management, data quality control, data collection); Nicole Salzieder, BS (data collection); Kathy Stanback, BA (data collection); Robin Stubbs, BS (data quality control, information technology support); Mary Sundstrom, BS (data collection); Joanne Weber, BS (data acquisition); and Steven Weber, PhD (subject communications, technical consulting).
Funding
NIH grants R01HL62252, R01AG036838, R01AG14124, and UL1RR025011, K23HL094760, T32HL07936, S10RR021086. The funding institutes played no role in the design and conduct of the study; no role in the collection, management, analysis, or interpretation of the data; and no role in the preparation, review, or approval of the manuscript.
Footnotes
Access to data
James H. Stein and Paul E. Peppard had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Conflicts of interest
Dr. Stein received royalties from the University of Wisconsin Alumni Research Foundation for intellectual property related to carotid wall thickness and “vascular age.” The authors have no potential conflicts of interest related to financial interests, activities, relationships, or affiliations.
Contributions
Conception and design: PEP, TY, KMH, JHS
Acquisition of data: PEP, CEK, TY, KMH, JHS
Analysis and interpretation of data: SIG, PEP, JHB, JHS, MP
Drafting of manuscript: SIG, JHS
Critical revision of manuscript for important intellectual content: All authors
Statistical analysis: PEP, JHB, MP
Administrative and technical support: PEP, CEK, EWH, JHS
Obtaining funding: PEP, TY, JHS
References
- Baguet JP, Hammer L, Lévy P, et al. The severity of oxygen desaturation is predictive of carotid wall thickening and plaque occurrence. Chest. 2005;128:3407– 3412. doi: 10.1378/chest.128.5.3407. [DOI] [PubMed] [Google Scholar]
- Baguet JP, Nadra M, Barone-Rochette G, Ormezzano O, Pierre H, Pépin JL. Early cardiovascular abnormalities in newly diagnosed obstructive sleep apnea. Vasc Health Risk Manag. 2009;5:1063–1073. doi: 10.2147/vhrm.s8300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos-Rodriguez F, Peña-Griñan N, Reyes-Nuñez N, et al. Mortality in obstructive sleep apnea-hypopnea patients treated with positive airway pressure. Chest. 2005;128:624–633. doi: 10.1378/chest.128.2.624. [DOI] [PubMed] [Google Scholar]
- Drager LF, Bortolotto LA, Lorenzi MC, Figueiredo AC, Krieger EM, Lorenzi-Filho G. Early signs of atherosclerosis in obstructive sleep apnea. Am J Respir Crit Care Med. 2005;172:613–618. doi: 10.1164/rccm.200503-340OC. [DOI] [PubMed] [Google Scholar]
- Drager LF, Bortolotto LA, Maki-Nunes C, et al. The incremental role of obstructive sleep apnoea on markers of atherosclerosis in patients with metabolic syndrome. Atherosclerosis. 2010;208:490–495. doi: 10.1016/j.atherosclerosis.2009.08.016. [DOI] [PubMed] [Google Scholar]
- Gunnarsson SI, Peppard PE, Korcarz CE, et al. Obstructive sleep apnea is associated with future subclinical carotid artery disease: thirteen-year follow-up from the Wisconsin Sleep Cohort. Arterioscler Thromb Vasc Biol. 2014;34:2338–2342. doi: 10.1161/ATVBAHA.114.303965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba Y, Chen JA, Bergmann SR. Carotid plaque, compared with carotid intima- media thickness, more accurately predicts coronary artery disease events: a meta- analysis. Atherosclerosis. 2012;220:128–133. doi: 10.1016/j.atherosclerosis.2011.06.044. [DOI] [PubMed] [Google Scholar]
- Jelic S, Padeletti M, Kawut SM, et al. Inflammation, oxidative stress, and repair capacity of the vascular endothelium in obstructive sleep apnea. Circulation. 2008;117:2270–2278. doi: 10.1161/CIRCULATIONAHA.107.741512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun J, Reinke C, Bedja D, et al. Effect of intermittent hypoxia on atherosclerosis in apolipoprotein E-deficient mice. Atherosclerosis. 2010;209:381–386. doi: 10.1016/j.atherosclerosis.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent BD, Garvey JF, Ryan S, Nolan G, Dodd JD, McNicholas WT. Severity of obstructive sleep apnoea predicts coronary artery plaque burden: a coronary computed tomographic angiography study. Eur Respir J. 2013;42:1263–1270. doi: 10.1183/09031936.00094812. [DOI] [PubMed] [Google Scholar]
- Kraiczi H, Caidahl K, Samuelsson A, Peker Y, Hedner J. Impairment of vascular endothelial function and left ventricular filling: association with the severity of apnea induced hypoxemia during sleep. Chest. 2001;119:1085–1091. doi: 10.1378/chest.119.4.1085. [DOI] [PubMed] [Google Scholar]
- Marin JM, Carrizo SJ, Vicente E, Agusti AGN. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- Minoguchi K, Yokoe T, Tazaki T, et al. Increased carotid intima-media thickness and serum inflammatory markers in obstructive sleep apnea. Am J Respir Crit Care Med. 2005;172:625–630. doi: 10.1164/rccm.200412-1652OC. [DOI] [PubMed] [Google Scholar]
- Ozdemir C, Conkbayir I, Kuru A, et al. Correlation between the intima-media thickness and Framingham risk score in patients with sleep apnea syndrome. J Thorac Dis. 2013;5:751–757. doi: 10.3978/j.issn.2072-1439.2013.11.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased Prevalence of Sleep-Disordered Breathing in Adults. Am J Epidemiol. 2013;177:1006–1014. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000;284:3015– 3021. doi: 10.1001/jama.284.23.3015. [DOI] [PubMed] [Google Scholar]
- Protogerou AD, Laaban JP, Czernichow S, et al. Structural and functional arterial properties in patients with obstructive sleep apnoea syndrome and cardiovascular comorbidities. J Hum Hypertens. 2007;22:415–422. doi: 10.1038/sj.jhh.1002318. [DOI] [PubMed] [Google Scholar]
- Den Ruijter HM, Peters SAE, Anderson TJ, et al. Common carotid intima-media thickness measurements in cardiovascular risk prediction: a meta-analysis. JAMA. 2012;308:796–803. doi: 10.1001/jama.2012.9630. [DOI] [PubMed] [Google Scholar]
- Saletu M, Nosiska D, Kapfhammer G, et al. Structural and serum surrogate markers of cerebrovascular disease in obstructive sleep apnea (OSA): association of mild OSA with early atherosclerosis. J Neurol. 2006;253:746–752. doi: 10.1007/s00415-006-0110-6. [DOI] [PubMed] [Google Scholar]
- Savransky V, Nanayakkara A, Li J, et al. Chronic Intermittent Hypoxia Induces Atherosclerosis. Am J Respir Crit Care Med. 2007;175:1290–1297. doi: 10.1164/rccm.200612-1771OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz R, Seeger W, Fegbeutel C, et al. Changes in extracranial arteries in obstructive sleep apnoea. Eur Respir J. 2005;25:69–74. doi: 10.1183/09031936.04.00030004. [DOI] [PubMed] [Google Scholar]
- Somers VK, White DP, Amin R, et al. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. J Am Coll Cardiol. 2008;52:686– 717. doi: 10.1016/j.jacc.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Stein JH, Fraizer MC, Aeschlimann SE, Nelson-Worel J, McBride PE, Douglas PS. Vascular age: integrating carotid intima-media thickness measurements with global coronary risk assessment. Clin Cardiol. 2004;27:388– 392. doi: 10.1002/clc.4960270704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein JH, Korcarz CE, Hurst RT, et al. American Society of Echocardiography Carotid Intima-Media Thickness Task Force Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr. 2008;21:93–111. doi: 10.1016/j.echo.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Stein JH, Tzou WS, DeCara JM, et al. Usefulness of increased skin cholesterol to identify individuals at increased cardiovascular risk (from the Predictor of Advanced Subclinical Atherosclerosis study) Am J Cardiol. 2008;101:986–991. doi: 10.1016/j.amjcard.2007.11.044. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Nakano H, Maekawa J, et al. Obstructive sleep apnea and carotid-artery intima-media thickness. Sleep. 2004;27:129–133. doi: 10.1093/sleep/27.1.129. [DOI] [PubMed] [Google Scholar]
- Szabóová E, Tomori Z, Donic V, Petrovicová J, Szabó P. Sleep apnoea inducing hypoxemia is associated with early signs of carotid atherosclerosis in males. Respir Physiol Neurobiol. 2007;155:121–127. doi: 10.1016/j.resp.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Tan TY, Liou CW, Friedman M, Lin HC, Chang HW, Lin MC. Factors associated with increased carotid intima-media thickness in obstructive sleep apnea/hypopnea syndrome. The Neurologist. 2012;18:277–281. doi: 10.1097/NRL.0b013e3182675344. [DOI] [PubMed] [Google Scholar]
- Weinreich G, Wessendorf TE, Erdmann T, et al. Heinz Nixdorf Recall (HNR) study group Association of obstructive sleep apnoea with subclinical coronary atherosclerosis. Atherosclerosis. 2013;231:191–197. doi: 10.1016/j.atherosclerosis.2013.09.011. [DOI] [PubMed] [Google Scholar]
- Yokoe T, Minoguchi K, Matsuo H, et al. Elevated levels of C-reactive protein and interleukin-6 in patients with obstructive sleep apnea syndrome are decreased by nasal continuous positive airway pressure. Circulation. 2003;107:1129–1134. doi: 10.1161/01.cir.0000052627.99976.18. [DOI] [PubMed] [Google Scholar]
- Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31:1071–1078. [PMC free article] [PubMed] [Google Scholar]
- Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]