Structured Summary
Background
Olmesartan-associated enteropathy (OAE) is characterized by diarrhea, nausea, vomiting, abdominal pain, weight loss, and severe sprue-like enteropathy, all of which is resolved after olmesartan medoxomil discontinuation.
Aim
To determine the mechanistic similarities with celiac sprue.
Methods
Duodenal biopsies were extracted from OAE patients before (n=11) or after (n=17) discontinuation of olmesartan medoxomil (on or off olmesartan medoxomil). There were 7 “on/off” paired samples. Formalin fixed biopsies were stained for CD8, CD4, FoxP3, IL-15R, and psmad 2/3. Caco2 cells (human colonic epithelial line) were treated with olmesartan medoxomil and stained for IL-15, IL-15R, and ZO-1.
Results
In the “on olmesartan medoxomil” duodenal biopsies, a significant increase in the numbers of CD8+ cells and the number of cells that are FoxP3+ (a regulatory T cell marker) are present in the duodenum as compared to the duodenal biopsies from patients who discontinued olmesartan medoxomil. IL15R expression is also increased with olmesartan medoxomil use. Evaluation of the effect of olmesartan medoxomil upon Caco-2 cells demonstrated that IL15 expression is increased in response to olmesartan medoxomil treatment. Further, ZO-1, a tight junction protein, is disrupted in olmesartan medoxomil-treated Caco-2 cells.
Conclusions
OAE shares many features with celiac disease, including symptoms and immunopathogenic pathways, such as increased numbers of CD8+ cells and corresponding overexpression of IL15 by epithelial cells. Taken together, the treatment of epithelial cells with olmesartan medoxomil induces a response by intestinal epithelial cells that is similar to the innate effect of gluten upon the epithelium of celiac patients.
Keywords: Olmesartan, enteropathy, IL-15, angiotensin, diarrhea, celiac disease
Introduction
Olmesartan-associated enteropathy (OAE) is an enteropathy associated with the use of olmesartan medoxomil that resolves with the discontinuation of the drug1. It is characterized by diarrhea, nausea, vomiting, abdominal pain, and weight loss. The occurrence of OAE in individuals who routinely use olmesartan medoxomil is currently unknown but is thought to be rare. At Mayo Clinic, 35 individuals have been diagnosed with OAE in the last 5 years, and a national study done in France identified 31 OAE patients2, 3. There have also been multiple case reports from around the world4–11. Although the frequency for OAE appears to be low, it is imperative to rapidly identify these individuals, because complications of OAE are severe, including renal failure, and suspension of the drug leads to resolution of symptoms and mucosal healing12, 13. Olmesartan is an angiotensin II receptor blocker (ARB) and is administered as a prodrug (olmesartan medoxomil)14. Of these, only olmesartan medoxomil has been consistently reported to be associated with enteropathy1. Olmesartan differs from most other ARBs in the attachment of the medoxomil moiety. The one other ARB with medoxomil, azilsartan, was approved by the US FDA on February 25, 2011 and is not widely used. As yet, no associations between azilsartan and enteropathy have been reported. Histopathologically and clinically, OAE appears to have many similarities to celiac disease and some similarities to autoimmune enteropathy (AIE)1, 13, 15, 16.
To determine the mechanisms that occur in OAE, we did a set of analyses that were inspired by the marked similarities in both the clinical symptoms and histopathology shared between celiac disease and OAE. As a starting point, we first took duodenal biopsies from the OAE patients before and/or after they discontinued their use of olmesartan medoxomil (on or off olmesartan medoxomil) and then characterized the cell types of the infiltrate.
Materials and Methods
Diagnostic Criteria for OAE
The following three criteria were used for diagnosis of OAE1.
Chronic diarrhea (>4 weeks) while taking olmesartan medoxomil
An alternate cause for the enteropathy could not be established after a systematic diagnostic evaluation that included investigation for disorders associated with nonresponsive celiac disease as previously reported by our group.
Clinical improvement after discontinuation of olmesartan medoxomil.
OAE Patients
Duodenal biopsies from 26 OAE patients were used for the immunohistochemistry analyses. Of the 26 OAE patients, 11 were men, 15 were women. For the alternate diagnosis evaluation, the medical histories of 35 OAE patients were reviewed. For this, the ratio of males to females was again 0.7 to 1.03.
Extraction of Duodenal Biopsies
For the diagnostic evaluation, a small bowel biopsy was done before withdrawal of the drug (on olmesartan) and one after discontinuing the use of olmesartan (“off olmesartan”). “Off olmesartan” biopsies were defined as biopsies performed at least 30 days after the date of suspension of olmesartan with a mean of 3 months after date of suspension. Duodenal tissue was immediately placed into formalin and later embedded into paraffin. There were 7 paired small bowel biopsy samples, with which a biopsy was taken while the patient was on olmesartan medoxomil (on) and another paired biopsy was taken after the same patient discontinued olmesartan medoxomil (off). There were an additional four “on” samples and an additional 10 “off” samples that did not have a corresponding paired sample.
Immunohistochemistry
Immunohistochemical staining was done at the Mayo Pathology Resource Core Facility. Antibodies used were purified anti CD8 (DAKO), purified anti CD4 (DAKO), purified anti FoxP3 (Abcam), purified anti granzymeB (DAKO), purified anti IL-15R (Biorbyt), and purified anti psmad2/3 (Santa Cruz Biotechnology).
Treatment of Caco-2 Cells with Olmesartan medoxomil, Olmesartan Acid, diacetyl (medoxomil) Telmisartan, and Losartan
Caco2 cells purchased from ATCC (American Type Culture Collection) were treated with trypsin (0.05% Gibco) and then cultured in media for 7 days on tissue culture microscope slides (Nunc Lab-Tek II Chamber Slide System-Thermo Scientific). Media was EMEM (Eagles Minimum Essential Medium-American Type Culture Collection) supplemented with 10% Fetal Bovine Serum and penicillin/streptomycin. Olmesartan medoxomil (Benicar) was ground into fine particles and then resuspended into HCl (pH 6.8). Losartan (Thermo Fisher Scientific) was resuspended into phosphate buffered saline (PBS). Olmesartan Acid was resuspended in DMSO(Dimethyl Sulfoxide-Sigma Aldrich), as well as telmisartan (Thermo Fisher Scientific). Diacetyl (Sigma-Aldrich), the three different ARBs and DMSO were then added to the Caco 2 cells at 30 micromolar concentration in 2 ml of fresh media. Exposure times were between 30 minutes to 4 hours and are described in each figure legend.
Immunofluorescent Analysis
Caco-2 cells were fixed in 3.7% formaldehyde and stained with purified anti ZO-1 (Invitrogen), purified anti IL-15 (BioRad), or purified anti IL-15R (BiOrbyt). The secondary antibody used was FITC conjugated anti rabbit IgG (Jackson Immunoresearch). Nuclei were stained using DAPI (4',6-Diamidino-2-phenylindole-Sigma Aldrich). Final evaluation and image capture of the staining was done with a confocal laser microscope (Zeiss LSM 780) using Zen 2010 software. Immunofluorescence quantitation was done using ImageJ (1.48v) software, available from NIH.
Statistical Analysis of Immunohistochemistry
For the CD8, CD4, FoxP3, psmad 2/3, and IL15R staining, the average number of positive cells in a villous/crypt unit was calculated after counting the total number of positive cells for three villous/crypt units, wherein a villous/crypt unit of area is defined as the area of one villous and the area below that villous extending through the crypt layer. For the epithelial staining of IL15R, and the intracellular staining of psmad 2/3 in the lamina propria, an intensity score was calculated. For the psmad 2/3 staining, 0 was no staining, 1 and 2 were given for weak and intermediate intensity, and 3 was the highest intensity of staining. For the IL15R staining, 0 was given for no staining, 1 was intermediary intensity, and 2 was the highest intensity of staining. The statistical significance of the differences in total number of positive cells of each staining in a villous/crypt unit between the on and off olmesartan groups was assessed by the Mann-Whitney rank-sum test. The on group consisted of 11 samples and the off group 17 samples for CD4, CD8, FoxP3, and psmad 2/3 staining The differences in intensity scores between the two groups were assessed by the Mann-Whitney rank-sum test. Graphpad Prism6 was used to conduct the analyses.
Results
Distribution of CD8+ and CD4+ Cells
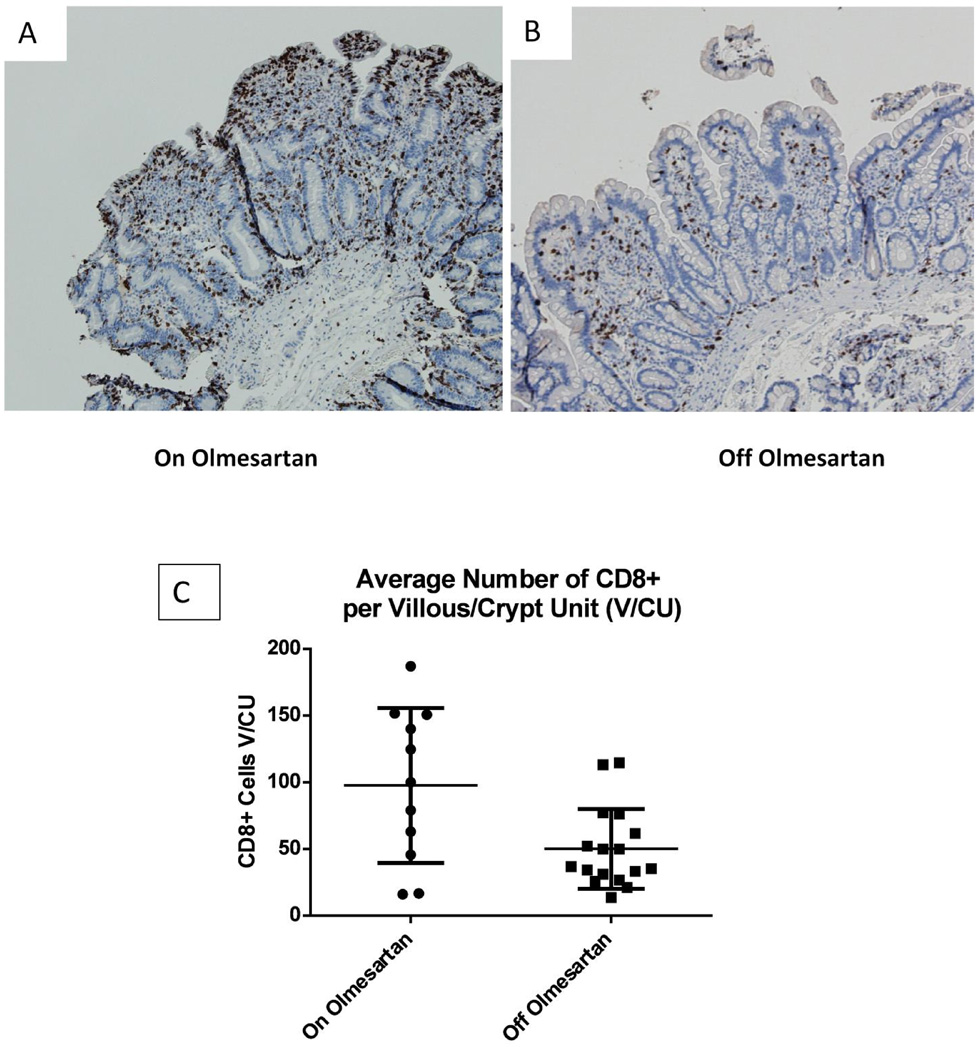
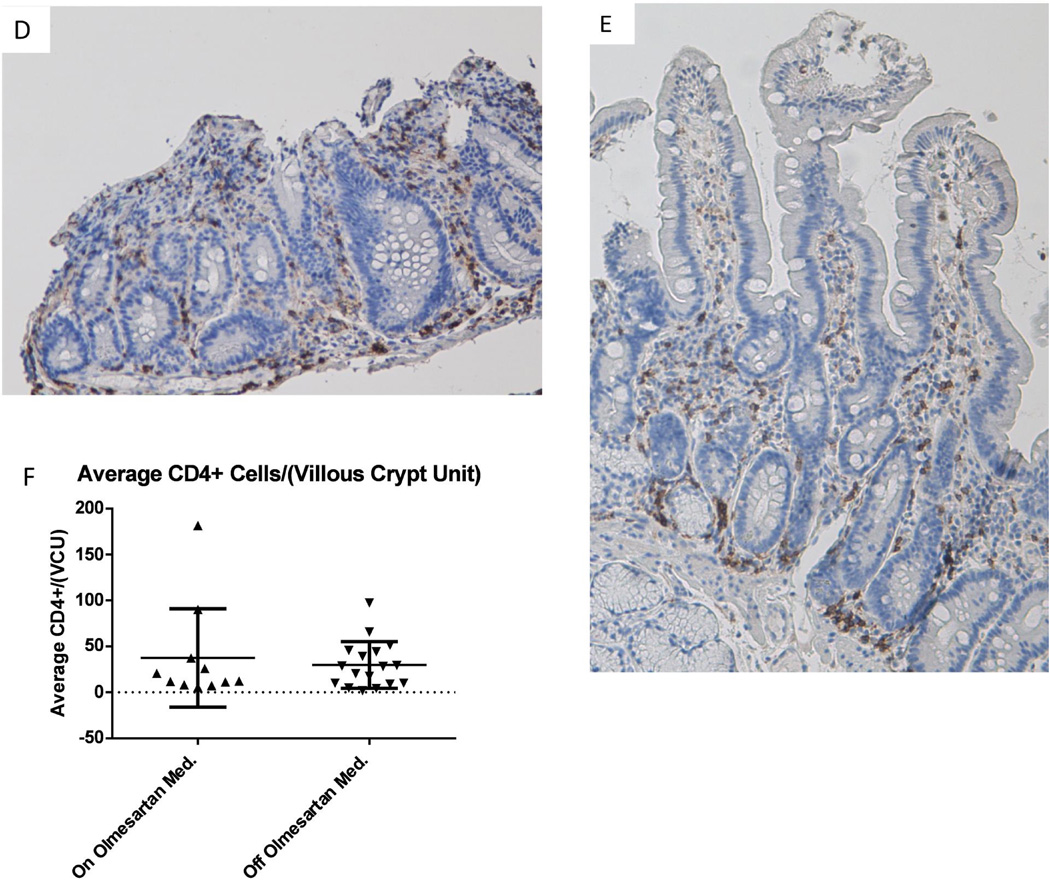
Previously we had published that lymphocyte infiltration of the small bowel (duodenum) occurred in patients with OAE1. In order to determine the cellular composition of the lymphocytic infiltration, anti CD8 (Figures 1A–1C) and anti CD4 (Figures 1D–1F) staining was done on duodenal biopsies from OAE patients while they were on olmesartan medoxomil (Figures 1A and 1D) or off olmesartan medoxomil (Figures 1B and 1E). Results from the CD8 staining demonstrate a significant increase (p<0.05) in the number of CD8+ cells in the duodenum of OAE patients on the drug as compared to off the drug (Figure 1C). This analysis was done on unpaired samples (11 on olmesartan medoxomil, 17 off olmesartan medoxomil). In contrast, the analysis of CD4+ cells did not reveal a significant difference (p=0.67) in the number of CD4+ cells in the small intestine of OAE patients, comparing on and off olmesartan medoxomil (Figure 1F).
Figure 1.
Distribution of CD8+ and CD4+ cells in duodenal biopsies: Biopsies were extracted from the duodenum of a representative OAE patient while on olmesartan medoxomil (A) and off olmesartan medoxomil (B). Anti CD8 staining (brown color) is depicted in panels A and B (10× magnification). The average total number of CD8+ cells in each villous/crypt unit was counted for unpaired samples and graphed in panel C, which displays the mean and standard deviation of the CD8+ cells per villous/(crypt unit) for unpaired samples from patients on or off olmesartan medoxomil. A significant increase in the number of CD8+ cells occurred while on olmesartan medoxomil (p<0.05 unpaired t test with Welch’s correction). In addition, biopsies were also stained with anti CD4 (brown color, panels D and E) (20× magnification). Panel F displays the mean and standard deviation of the number of CD4+ cells in each villous/crypt unit for unpaired samples from patients on or off olmesartan medoxomil. There was no significant change between on or off olmesartan (p=0.67) using the unpaired t test with Welch’s correction.
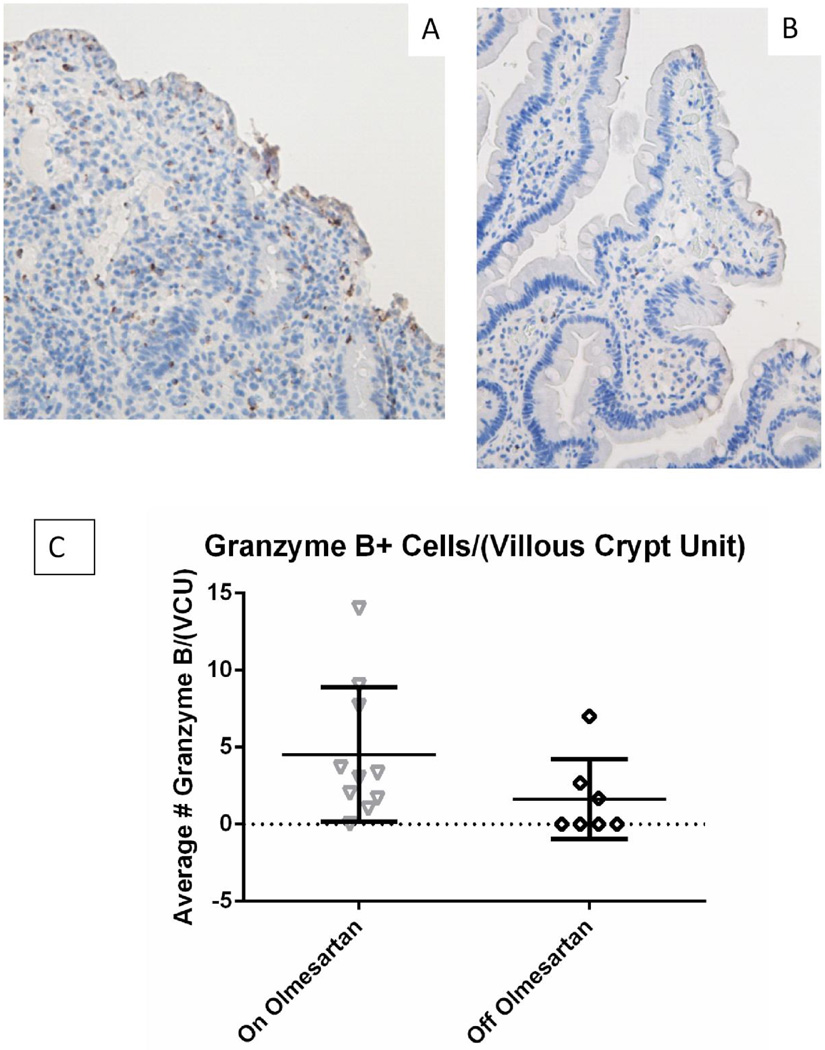
Distribution of Granzyme B+ Cells
We next evaluated the expression of granzyme B to determine the potential presence of cytotoxic T lymphocytes (CTLs). Figure 2A shows greater numbers of granzyme B+ cells while on olmesartan medoxomil, as compared to off (Figure 2B), indicating that increased numbers of CTLs are present in the infiltrates of the duodenum of OAE patients. However this was not statistically significant (Figure 2C) (p=0.1 unpaired t test using Welch’s correction).
Figure 2.
Granzyme B expression in duodenal biopsies: Duodenal biopsies from a representative OAE patient while on (A) and off (B) olmesartan medoxomil were stained with anti granzyme B (brown). Panels A and B were taken at 20× magnification. The average total number of granzyme B+ cells in each villous/crypt unit was counted for unpaired samples and graphed in panel C. Panel C displays the mean and standard deviation. There was a numerical increase of granzyme B+ cells, but this was not statistically significant (p=0.1) using unpaired t test using Welch’s correction.
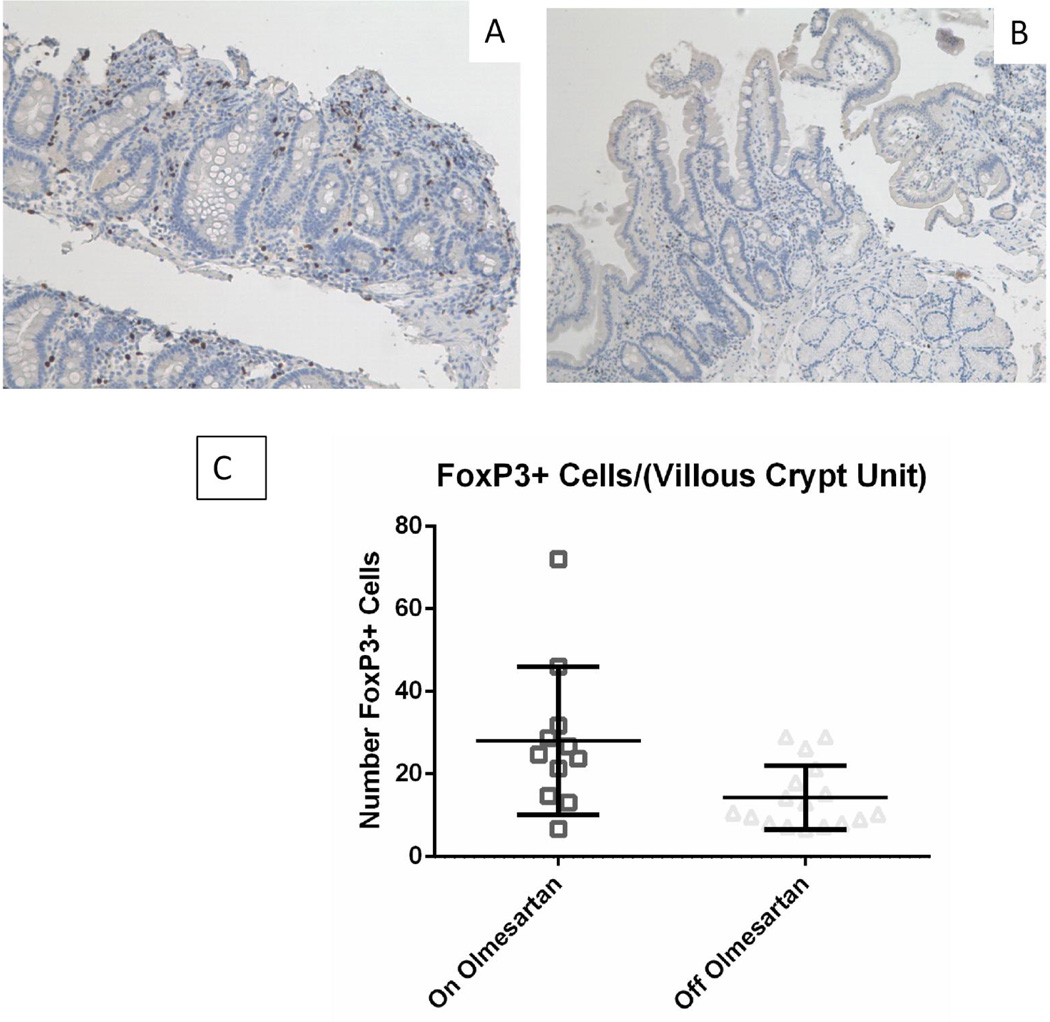
Distribution of FoxP3+ Cells
To determine if the OAE patients have a deficiency in their intestinal regulatory T cells, we next addressed whether olmesartan medoxomil treatment decreased the number of FoxP3+ cells. Figures 3A (on olmesartan medoxomil) and 3B (off olmesartan medoxomil) demonstrate that the patients did have a significant increase in the number of FoxP3+ cells (Figure 3C) (p<0.05 unpaired t test with Welch’s correction), despite the concurrent inflammation whilst on olmesartan medoxomil. This increase in FoxP3+ cells with olmesartan medoxomiluse would indicate that the FoxP3+ cells were not able to effectively suppress the inflammation induced by olmesartan medoxomil.
Figure 3.
FoxP3 expression in duodenum: Duodenal biopsies from a representative OAE patient while on (A) or off (B) olmesartan were stained with anti FoxP3 (brown). Panel C shows the mean with standard deviation of unpaired on and off samples. There was a statistically significant increase in the number of FoxP3+ cells with the use of olmesartan medoxomil (p<0.05), using the unpaired t test with Welch’s correction.
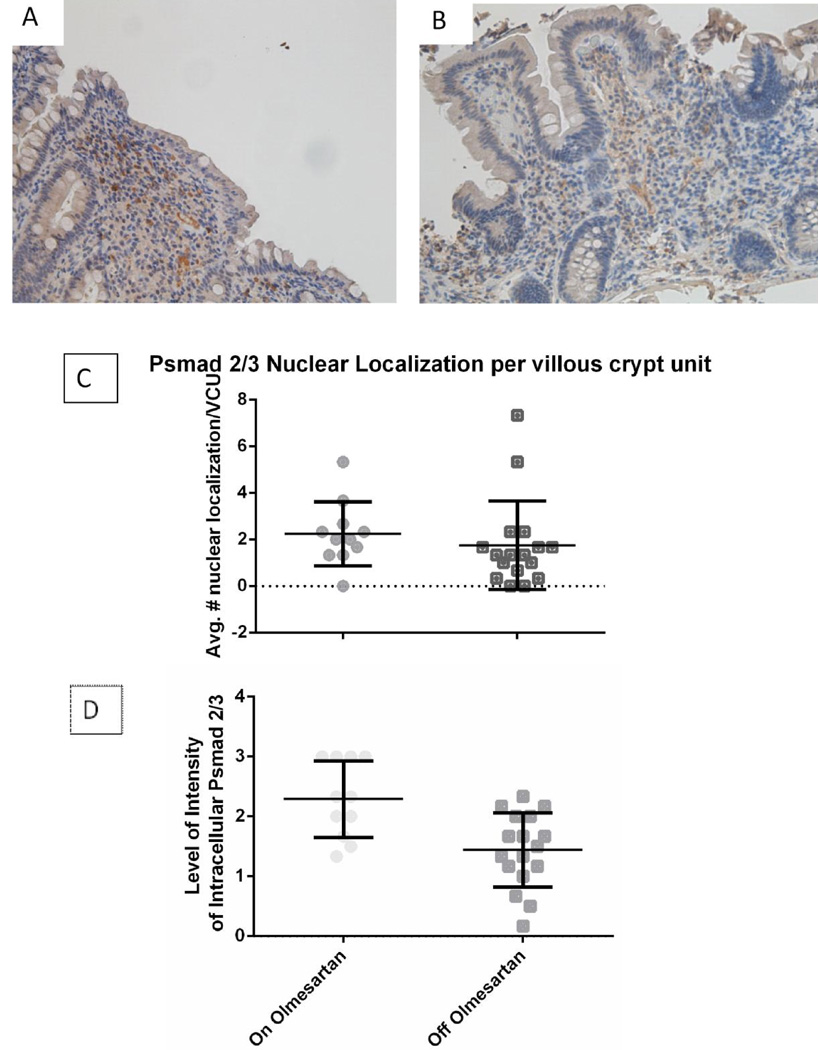
TGFβ Signaling Through Phosphorylation of smad 2/3
One method by which regulatory T cells are rendered non-functional, is the disruption of the TGFβR signaling pathway. Phosphorylated smad 2/3 (psmad 2/3) localized within the cell indicates that the TGFβ signaling pathway is activated, and nuclear translocation of psmad 2/3 indicates successful signaling. Staining of the duodenal biopsies for psmad 2/3 found that nuclear localization was at the same level in both of the groups (Figure 4A, 4B). Intracellular localization on the other hand was significantly increased while on olmesartan medoxomil as compared to off olmesartan medoxomil (Figure 4C, 4D) (p<0.01). This would indicate that the TGFβ signaling pathway is active in the patients while they are taking olmesartan medoxomil.
Figure 4.
Phosphorylation of smad 2/3: Duodenal biopsies from a representative OAE patient while on (A) or off (B) olmesartan medoxomil were stained with anti psmad 2/3 (brown). Panels C and D show the mean with standard deviation for nuclear (C) and intracellular (D) localization of unpaired duodenal biopsies from patients on or off olmesartan. There was no statistically significant difference with the nuclear localization between on and off olmesartan medoxomil (p=0.43); however, there was a statistically significant increase in the intracellular localization with the use of the drug, using unpaired t test with Welch’s correction (p<0.01).
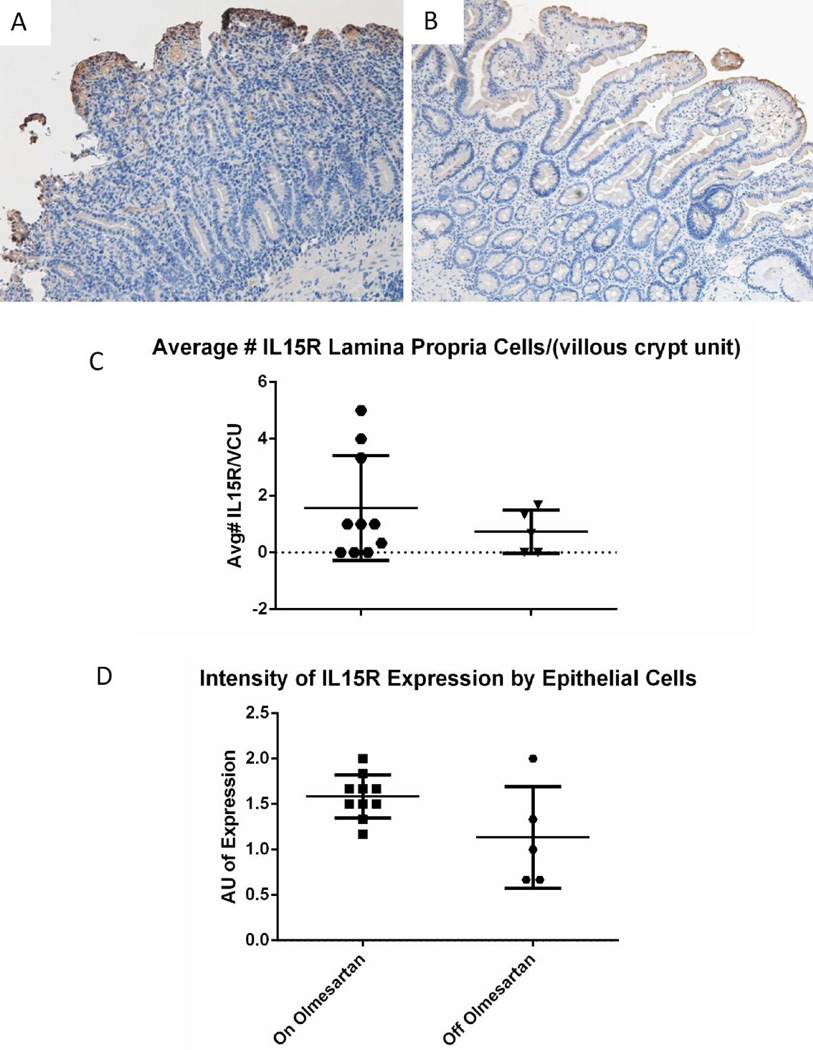
Expression of IL-15R
In refractory celiac disease, CD8+ T cells are rendered less sensitive to regulatory T cell suppression, due to an overexpression of IL-1517. In addition, celiac patients have an overexpression of IL-15 and IL-15R, which contribute to the activation of CTLs and subsequent killing of epithelial cells18. To determine if IL-15R expression was altered with the use of olmesartan medoxomil, immunohistochemistry staining for IL-15R was performed on duodenal biopsies of OAE patients. As Figures 5A and 5B demonstrate, IL15R is upregulated while on olmesartan medoxomil, as compared to off olmesartan medoxomil, which is similar to celiac disease. Figure 5C shows that IL15R is not significantly increased by lamina propria cells (p=0.2), but is significantly increased by epithelial cells (Figure 5D) (p<0.05), while on olmesartan medoxomil.
Figure 5.
IL15R expression/distribution: Duodenal biopsies from a representative OAE patient while on (A) or off (B) olmesartan were stained with anti IL15R (brown). Panel C shows the average number of IL15R+ cells in the lamina propria of unpaired duodenal biopsies from patients on or off olmesartan, whilst panel D shows the average intensity of anti IL15R staining of the epithelial cells of unpaired duodenal biopsies. The observed increase in the lamina propria with the use of the drug was not statistically significant (P=0.2) using unpaired t test; however, the increased intensity of staining of the intestinal epithelium was statistically significant (p<0.05) using unpaired t test.
Response of Caco2 Cells to Olmesartan Medoxomil
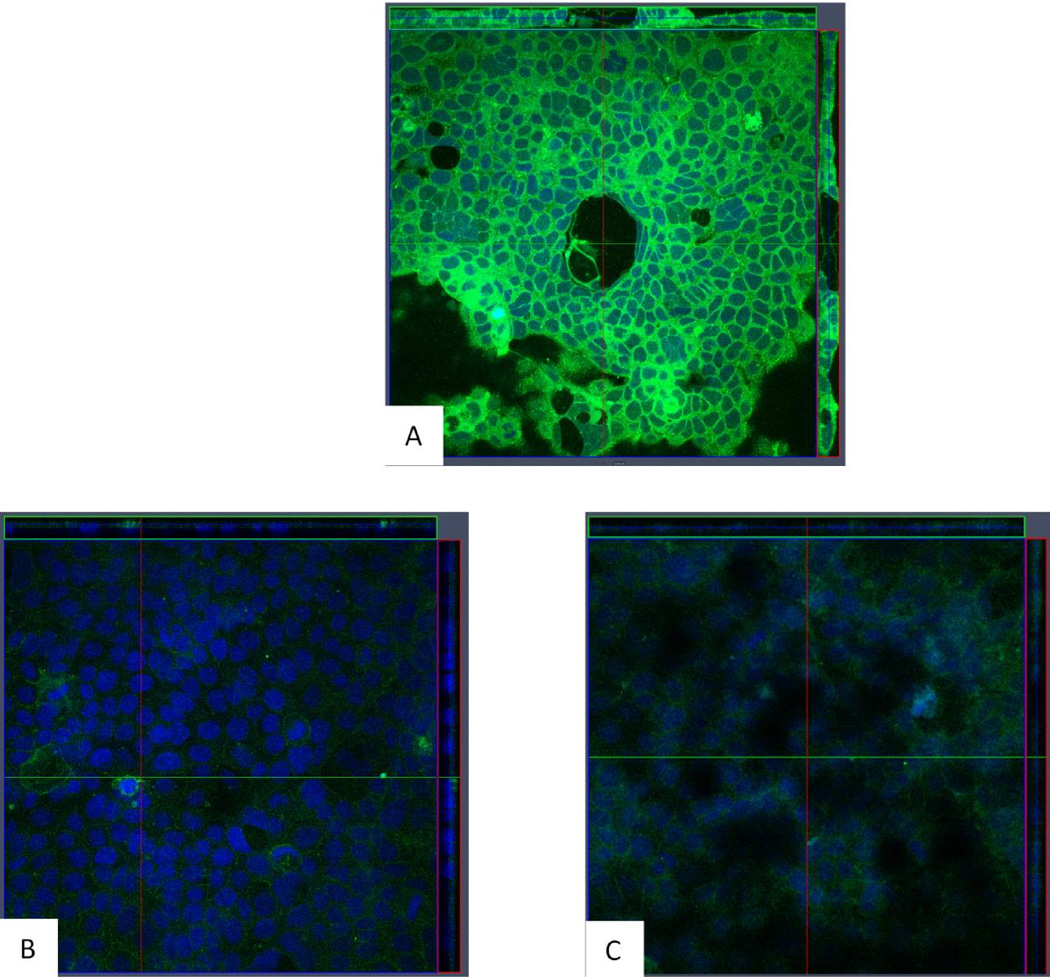
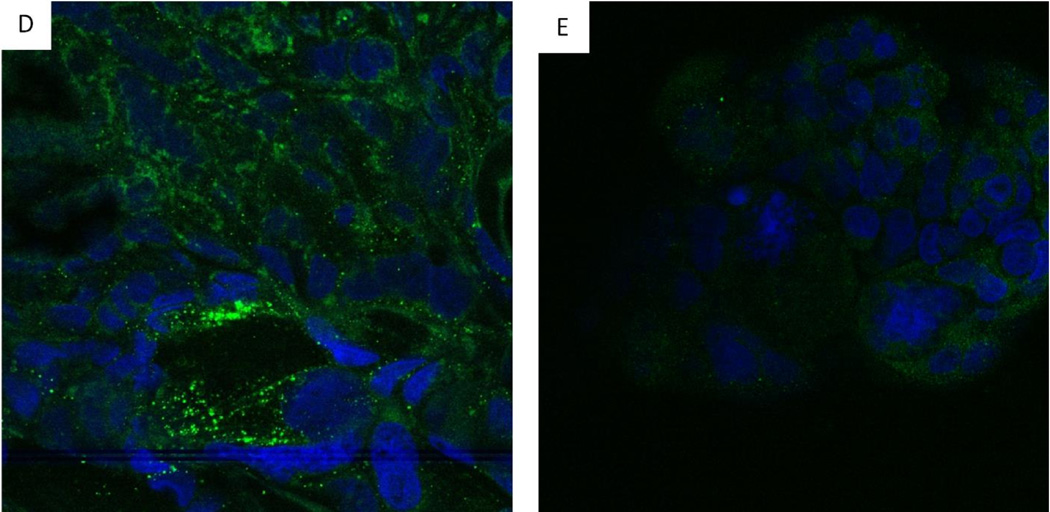
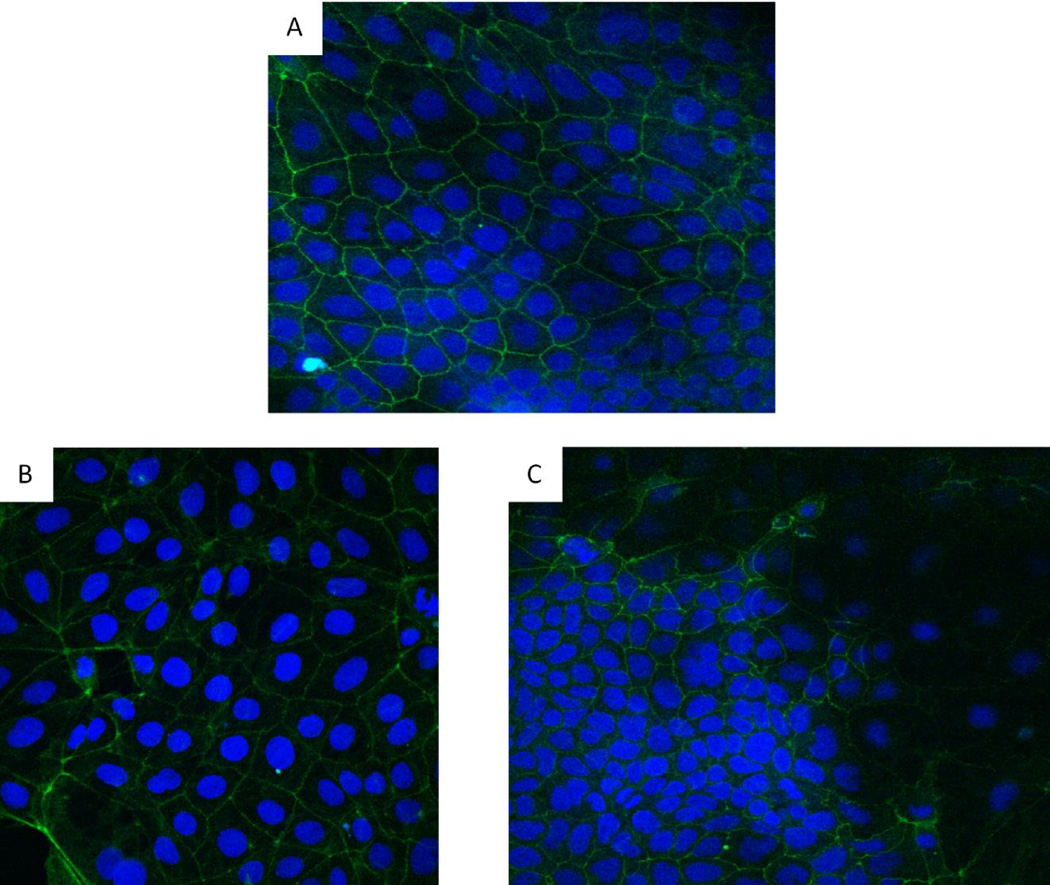
Because many OAE patients were diagnosed with colitis, we used a colonic epithelial cell line (Caco 2) to determine if olmesartan medoxomil could directly impact the function of colonic epithelial cells1, 3. To do this, Caco-2 cells were treated with olmesartan medoxomil, losartan, or telmisartan. Since one feature of celiac disease is aberrantly increased production of IL-15, we first determined if exposure of Caco 2 cells to these ARBs results in the expression and/or release of IL-15. Olmesartan medoxomil at 30 mM clearly induces the expression and/or release of IL-15 after a 4 hour exposure (Figure 6A), whereas losartan (Figure 6B) and telmisartan (Figure 6C) at 30 mM do not. We also did not see any expression of IL15 with treatment of the diluent for olmesartan alone (data not shown). Caco-2 cells also increased their expression of IL15R after exposure to olmesartan medoxomil (Figure 6D), but not after exposure to losartan (Figure 6E). Untreated Caco-2 cells had the expected distribution pattern of ZO-1 (Figure 7A), which was somewhat altered at 30 minutes after treatment with olmesartan medoxomil (Figure 7B), and clearly disrupted after four hours (Figure 7C).
Figure 6.
IL-15 and IL15R expression by olmesartan medoxomil treated Caco-2 cells. Caco-2 cells were cultured for 5–7 days and then treated with 30 micromolar olmesartan (A), 30 micromolar Losartan, (B), or 30 micromolar Telmisartan for four hours (C). Confocal laser microscope orthogonal images of anti IL-15 staining are depicted, with anti-IL15 (green) and DAPI (blue). Magnification 40×. In addition, Caco-2 cells were cultured for 5–7 days, treated for an additional 60 hours with 30 micromolar olmesartan medoxomil (D) or losartan (E) and then stained for IL-15R (green).
Figure 7.
Redistribution of tight junction protein ZO-1. Caco-2 cells were cultured for 5–7 days and stained for ZO-1 (Panel A). Panels B and C are Caco-2 cells treated with 30 micromolar olmesartan medoxomil for 30 minutes (B) or 4 hours (C). FITC conjugated anti ZO-1 and DAPI (blue) were used for staining. Magnification (40×). ZO-1 is depicted by green, and nucleic acid by blue.
Response of Caco 2 Cells to Olmesartan Acid and Diacetyl (Medoxomil)
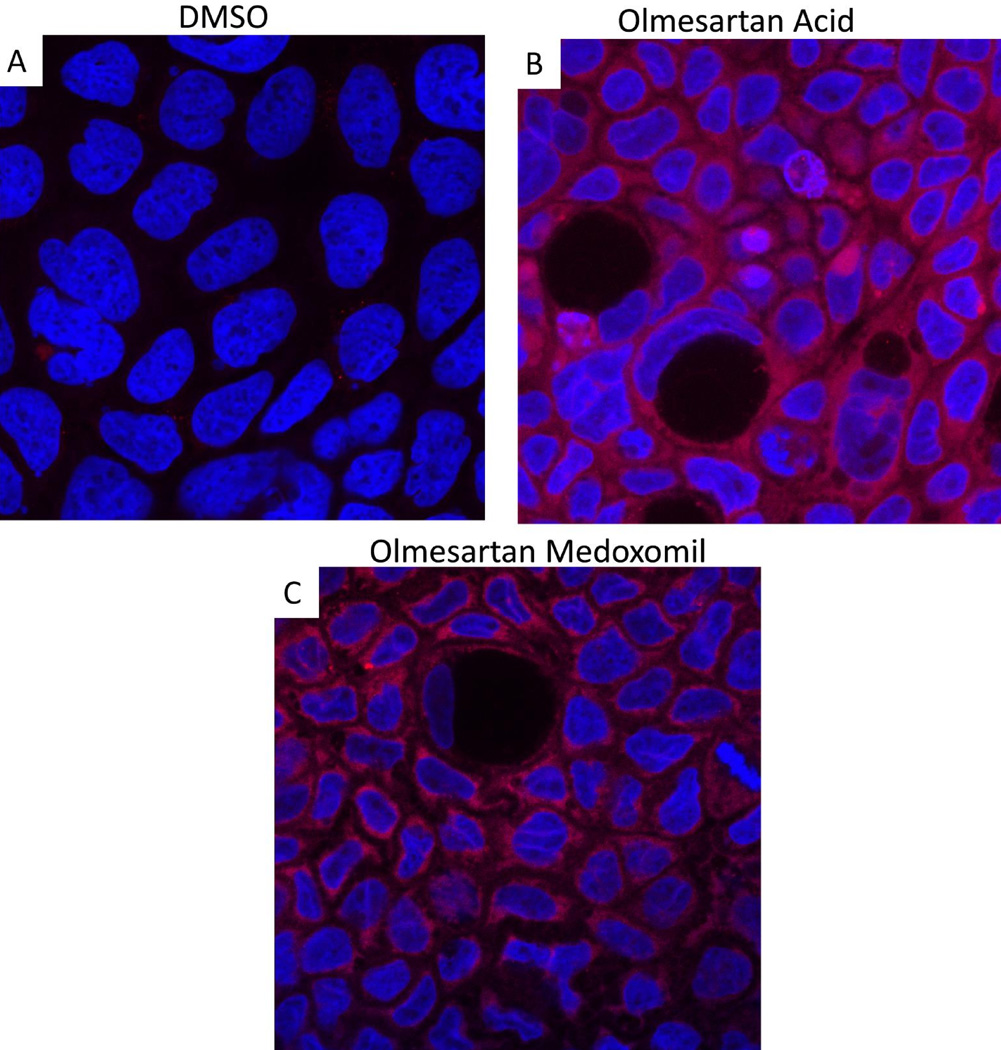
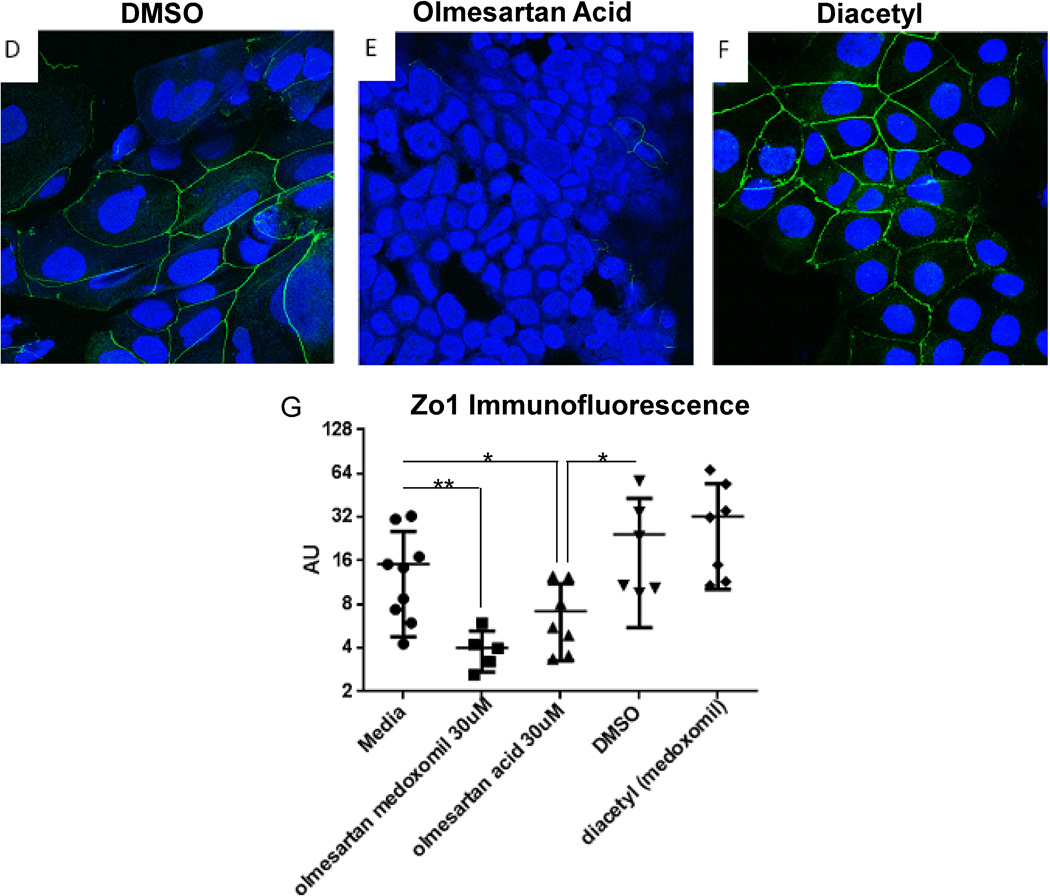
As displayed in Figure 8, olmesartan acid alone was also able to induce increased expression of IL-15 by Caco-2 cells (panels A and B). Treatment with the diluent alone, DMSO, did not induce expression of IL-15 (panel A), but olmesartan acid did (panel B). Corresponding staining of IL-15 after treatment with olmesartan medoxomil is provided as a direct comparison (panel C). As compared to DMSO alone (panel D), olmesartan acid was also able to disrupt the ZO-1 tight junction protein pattern (panel E). Staining with diacetyl (medoxomil) did not result in a disruption of ZO-1 (panel F). Using ImageJ software, the immunofluorescence was quantitated, and this is displayed in panel G.
Figure 8.
Olmesartan Acid alone can increase IL-15 and disrupt ZO-1. Caco-2 cells were cultured for 5–7 days, treated with 30 micromolar olmesartan acid, 30 micromolar DMSO, and/or 30 micromolar diacetyl (medoxomil) for four hours and then stained for IL-15 (red) (A–C) or ZO-1 (green) (D–F). Panels A and D are DMSO (diluent for olmesartan acid) alone. Panels B and E are olmesartan acid and panel C is olmesartan medoxomil. Panel F is diacetyl alone. Panel G is a graph displaying the immunofluorescence of ZO-1 staining of Caco2 cells treated with the different reagents, where each dot represents a different well of Caco-2 cells treated with the listed reagent.
Discussion
Patients with olmesartan-associated enteropathy (OAE) have often been misdiagnosed with celiac disease or more specifically refractory celiac disease, due to the failure to respond to a gluten free diet. Our data shows that OAE shares many of the pathogenic pathways present in celiac disease. In OAE, there is a clear increase in CD8+ cells while the patient is taking olmesartan medoxomil. The additional increase in the number of granzyme B positive cells would indicate that CTLs are increased in the villous crypt units of the patients while on olmesartan medoxomil and may play a role in the destruction of the epithelium, especially since granzyme B + cells are increased in both the lamina propria and the epithelial layer while on olmesartan medoxomil. This increase in granzyme B+ cells is similar to untreated celiac patients and refractory celiac patients19.
In addition, no significant change in the number of CD4+ cells was observed between those on olmesartan medoxomil and those off. In celiac disease, gluten specific CD4+ T cells develop and expand, leading to increased levels of interferon gamma (IFNγ) in the intestine and anti gluten antibodies in the serum20. The activation and expansion of inflammatory CD4+ T cells is mediated by HLA DQ2 and/or HLA DQ8, and as such, over 95% of all celiac patients are either DQ2 and/or DQ8 positive. At some point afterwards, B cells begin to produce anti tissue transglutaminase IgA (IgG in IgA deficient individuals). So far, no OAE patients have been identified that are positive for antibodies against tTG1, 13. Also of interest, was the high incidence of DQ2+ individuals in OAE (71%). Thus, although the number of CD4+ cells in the villous crypt unit did not change on or off olmesartan medoxomil, it is still possible that DQ2 could play a role in the pathogenesis of OAE. However, we continue to identify OAE patients that are neither DQ2 nor DQ8 positive (>4). Therefore, DQ2 positivity is not required for developing OAE1.
The ongoing inflammation suggests a loss of regulation of inflammation; the observation that FoxP3+ cells were significantly increased in the lamina propria of individuals on olmesartan medoxomil suggests that regulatory T cells were present, and indeed expanded, but lacked the ability to suppress the inflammation. Immune regulation in the intestine is crucially dependent upon TGFβ, and it has been speculated the ARBs may inhibit TGFβ signaling. Our results with the psmad 2/3 staining, in which there was no difference in psmad 2/3 nuclear localization between the on and off groups would suggest that TGFβR signaling is occurring at the same level on or off olmesartan medoxomil, implying that TGFβR signaling is occurring correctly and that olmesartan medoxomil is not inhibiting TGFβ signaling.
An additional pathway in which lamina propria lymphocytes are rendered unresponsive to regulatory T cells is the IL-15 pathway. Previous studies have demonstrated that overexpression of IL-15 and IL15R occurs in refractory sprue patients and overexpression of IL-15 in mice results in enteropathy that is diet independent18, 21, 22. The enteropathy that occurs in the transgenic mice that overexpresses IL-15 in the intestine is also associated with the influx of a large number of CD8+ cells22(22). Another publication demonstrated that in celiac patients, IL-15 interferes with the suppressive ability of regulatory T cells23. Gluten can stimulate epithelial cells to over express IL15, and may be why celiac patients as a group overexpress IL-15 and/or have sensitivity to IL-1523, 24. Disruption of the tight junction complexes of epithelial cells through disruption of ZO-1 localization, also occurs as an innate immune response to gluten in celiac disease25. Our observation that olmesartan medoxomil can increase the expression of both IL-15 and IL15R by Caco2 cells, and that OAE patients on olmesartan medoxomil have increased levels of IL15R would suggest that the enteropathy associated with olmesartan medoxomil use is a consequence of increased IL15 expression induced by olmesartan medoxomil. All together then, many of the mechanistic pathways present in OAE pathogenesis are similar to those of innate immune responses to gliadin in celiac disease26. These pathways would include the increased numbers of CD8+ cells, the increased expression of IL-15R, and a state of non-responsiveness to increased numbers of regulatory T cells. A central thread to all of these mechanisms is the intestinal epithelial cell, which is targeted by CTLs in celiac disease.
In addition, our observations that directly treating Caco 2 cells with telmisartan and losartan neither increased IL-15 production nor disrupted tight junction protein complexes indicate that these deleterious OAE effects are not associated with all of the ARBs. This is supported by the findings of one study that found that telmisartan and losartan do not appear to be associated with enteropathy27. Further, the fact that olmesartan acid by itself can disrupt Zo1 in intestinal epithelial cells as well as induce increased expression of IL15, and that medoxomil by itself (diacetyl) does not, would suggest that olmesartan acid by itself is causing the pathology in OAE. However, many more experiments need to be done to prove that only olmesartan acid is causing all of pathology in OAE and to determine if any other ARBs exert any of the pathology associated mechanistic pathways induced by olmesartan acid.
In summary, a small number of patients will develop enteropathy in response to olmesartan medoxomil ; this enteropathy is not gluten dependent, and both the stomach and colon of many OAE patients are also affected in addition to the small intestine. Our work suggests that epithelial cells respond to olmesartan medoxomil, and more specifically, the olmesartan acid portion of the olmesartan medoxomil increases the expression of IL-15 and disrupts the tight junction protein ZO-1. Since some studies have demonstrated that refractory celiac sprue patients also have aberrantly high expression of IL-15, one potential unifying theory for OAE is that these patients in certain circumstances were unable to downregulate the IL-15 expression induced by olmesartan medoxomil, and therefore later developed enteropathy.
One limitation was our inability to safely rechallenge the patients with olmesartan medoxomil due to the severity of the illness; therefore, we cannot conclusively state that olmesartan medoxomil use directly causes the increase in the numbers of CD8+ cells that we had observed in OAE patients while on olmesartan medoxomil. We were also unable to do paired analyses because of the small number of cases in which paraffin embedded tissue was still available for research use from both on and off olmesartan medoxomil. We continue to identify patients with OAE and are conducting further analyses on other potential genetic causes as well as the mechanistic pathways that contribute to CD8+ cell recruitment by olmesartan medoxomil treated epithelial cells.
Acknowledgements
We would like to acknowledge the Mayo Pathology Resource Core, especially Ms. LouAnn Gross for her work with the immunohistochemistry staining and Mr. Eugene Krueger in the Optical Microscopy Core within the Mayo Clinic Center for Cell Signaling in the Department of Gastroenterology for help with the confocal laser microscope imaging. We would also like to thank Ms. April Huseby with the use of Image J software and the quantitation of immunofluorescence.
Declaration of funding interests:
This study was funded in part by a grant from the National Institutes of Health (R01 DK057892 to J.A.M.)
Footnotes
Authorship statement:
Guarantor of the article: Joseph A. Murray, MD
Specific author contributions: Study concept and design (EV Marietta, A Rubio-Tapia); data acquisition (EV Marietta, A Nadeau, AK Cartee, I Singh, A Rubio-Tapia); data analysis (EV Marietta, A Nadeau, I Singh, RS Choung, I Singh, T-T Wu, A Rubio-Tapia, JA Murray); manuscript drafting (EV Marietta); critical revision of manuscript (A Rubio-Tapia, JA Murray); statistical analysis (EV Marietta, RS Choung).
All authors reviewed and approved the final manuscript.
Statement of Interests:
Authors' declaration of personal interests:
There are no personal interests to disclose.
References
- 1.Rubio-Tapia A, Herman ML, Ludvigsson JF, et al. Severe spruelike enteropathy associated with olmesartan. Mayo Clin Proc. 2012;87(8):732–738. doi: 10.1016/j.mayocp.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marthey L, Cadiot G, Seksik P, et al. Olmesartan-associated enteropathy: results of a national survey. Aliment Pharmacol Ther. 2014;40(9):1103–1109. doi: 10.1111/apt.12937. [DOI] [PubMed] [Google Scholar]
- 3.Cartee AK, Nadeau A, Rubio-Tapia A, Herman ML, Murray JA. Characterizing olmesartan-induced enteropathy risk factors, severity, and symptom resolution, a follow-up to the 2012 case series. Gastroenterology. 2014;146(Supplement 5):S-603–S-604. [Google Scholar]
- 4.van Beurden YH, Nijeboer P, Janssen J, Verbeek WH, Mulder CJ. Diarrhoea and malabsorption due to olmesartan use. Ned Tijdschr Geneeskd. 2014;158:A7370. [PubMed] [Google Scholar]
- 5.Nielsen JA, Steephen A, Lewin M. Angiotensin-II inhibitor (olmesartan)-induced collagenous sprue with resolution following discontinuation of drug. World J Gastroenterol. 2013;19(40):6928–6930. doi: 10.3748/wjg.v19.i40.6928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agudo Fernandez S. Sprue-like enteropathy due to olmesartan: A case report. Gastroenterol Hepatol. 2015;38(2):108–109. doi: 10.1016/j.gastrohep.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 7.Fiorucci G, Puxeddu E, Colella R, Paolo Reboldi G, Villanacci V, Bassotti G. Severe spruelike enteropathy due to olmesartan. Rev Esp Enferm Dig. 2014;106(2):142–144. doi: 10.4321/s1130-01082014000200011. [DOI] [PubMed] [Google Scholar]
- 8.Abdelghany M, Gonzalez L, 3rd, Slater J, Begley C. Olmesartan associated sprue-like enteropathy and colon perforation. Case Rep Gastrointest Med. 2014;2014:494098. doi: 10.1155/2014/494098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nunge D, Eoche M, Fumery M, Nguyen-Khac E, Gras V, Andrejak M. Severe enteropathy with villous atrophy olmesartan medoxomil-associated. Therapie. 2013;68(6):419–421. doi: 10.2515/therapie/2013057. [DOI] [PubMed] [Google Scholar]
- 10.Stanich PP, Yearsley M, Meyer MM. Olmesartan-associated sprue-like enteropathy. J Clin Gastroenterol. 2013;47(10):894–895. doi: 10.1097/MCG.0b013e31829a27e6. [DOI] [PubMed] [Google Scholar]
- 11.Dreifuss SE, Tomizawa Y, Farber NJ, Davison JM, Sohnen AE. Spruelike enteropathy associated with olmesartan: an unusual case of severe diarrhea. Case Rep Gastrointest Med. 2013;2013:618071. doi: 10.1155/2013/618071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeGaetani M, Tennyson CA, Lebwohl B, et al. Villous atrophy and negative celiac serology: a diagnostic and therapeutic dilemma. Am J Gastroenterol. 2013;108(5):647–653. doi: 10.1038/ajg.2013.45. [DOI] [PubMed] [Google Scholar]
- 13.Theophile H, David XR, Miremont-Salame G, Haramburu F. Five cases of sprue-like enteropathy in patients treated by olmesartan. Dig Liver Dis. 2014;46(5):465–469. doi: 10.1016/j.dld.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 14.Michel MC, Foster C, Brunner HR, Liu L. A systematic comparison of the properties of clinically used angiotensin II type 1 receptor antagonists. Pharmacol Rev. 2013;65(2):809–848. doi: 10.1124/pr.112.007278. [DOI] [PubMed] [Google Scholar]
- 15.Gentile NM, Murray JA, Pardi DS. Autoimmune enteropathy: a review and update of clinical management. Curr Gastroenterol Rep. 2012;14(5):380–385. doi: 10.1007/s11894-012-0276-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Otsubo K, Kanegane H, Kamachi Y, et al. Identification of FOXP3-negative regulatory T-like (CD4(+)CD25(+)CD127(low)) cells in patients with immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome. Clin Immunol. 2011;141(1):111–120. doi: 10.1016/j.clim.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 17.Korneychuk N, Ramiro-Puig E, Ettersperger J, et al. Interleukin 15 and CD4(+) T cells cooperate to promote small intestinal enteropathy in response to dietary antigen. Gastroenterology. 2014;146(4):1017–1027. doi: 10.1053/j.gastro.2013.12.023. [DOI] [PubMed] [Google Scholar]
- 18.Malamut G, El Machhour R, Montcuquet N, et al. IL-15 triggers an antiapoptotic pathway in human intraepithelial lymphocytes that is a potential new target in celiac disease-associated inflammation and lymphomagenesis. J Clin Invest. 2010;120(6):2131–2143. doi: 10.1172/JCI41344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Di Sabatino A, Ciccocioppo R, Cupelli F, et al. Epithelium derived interleukin 15 regulates intraepithelial lymphocyte Th1 cytokine production, cytotoxicity, and survival in coeliac disease. Gut. 2006;55(4):469–477. doi: 10.1136/gut.2005.068684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jabri B, Sollid LM. Mechanisms of disease: immunopathogenesis of celiac disease. Nat Clin Pract Gastroenterol Hepatol. 2006;3(9):516–525. doi: 10.1038/ncpgasthep0582. [DOI] [PubMed] [Google Scholar]
- 21.Yokoyama S, Watanabe N, Sato N, et al. Antibody-mediated blockade of IL-15 reverses the autoimmune intestinal damage in transgenic mice that overexpress IL-15 in enterocytes. Proc Natl Acad Sci U S A. 2009;106(37):15849–15854. doi: 10.1073/pnas.0908834106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohta N, Hiroi T, Kweon MN, et al. IL-15-dependent activation-induced cell death-resistant Th1 type CD8 alpha beta+NK1.1+ T cells for the development of small intestinal inflammation. J Immunol. 2002;169(1):460–468. doi: 10.4049/jimmunol.169.1.460. [DOI] [PubMed] [Google Scholar]
- 23.Zanzi D, Stefanile R, Santagata S, et al. IL-15 interferes with suppressive activity of intestinal regulatory T cells expanded in Celiac disease. Am J Gastroenterol. 2011;106(7):1308–1317. doi: 10.1038/ajg.2011.80. [DOI] [PubMed] [Google Scholar]
- 24.Harris KM, Fasano A, Mann DL. Monocytes differentiated with IL-15 support Th17 and Th1 responses to wheat gliadin: implications for celiac disease. Clin Immunol. 2010;135(3):430–439. doi: 10.1016/j.clim.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drago S, El Asmar R, Di Pierro M, et al. Gliadin, zonulin and gut permeability: Effects on celiac and non-celiac intestinal mucosa and intestinal cell lines. Scand J Gastroenterol. 2006;41(4):408–419. doi: 10.1080/00365520500235334. [DOI] [PubMed] [Google Scholar]
- 26.Abadie V, Jabri B. IL-15: a central regulator of celiac disease immunopathology. Immunol Rev. 2014;260(1):221–234. doi: 10.1111/imr.12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Samo S, Stobaugh DJ, Ehrenpreis ED. Investigation of the link between intestinal adverse events and olmesartan using the Food and Drug Administration Adverse Event Reporting System. Gastroenterology. 2014;146(Supplement 5):S-604. [Google Scholar]