Abstract
Objective
Congenital deficiency of the principal boundary lubricant in cartilage (lubricin, encoded by the gene PRG4) increases joint friction and causes progressive joint failure. This study was undertaken to determine whether restoring lubricin expression would prevent, delay, or reverse the disease process caused by congenital deficiency.
Methods
Using genetically engineered lubricin deficient mice, we restored gene function before conception or at 3 weeks, 2 months, or 6 months after birth. We evaluated the effect of restoring gene function (i.e., expressing lubricin) on the tibial-femoral-patellar joints of mice, histologically and by ex vivo biomechanical testing.
Results
Restoring gene function prior to conception prevented disease. Restoring gene function at 3 weeks of age improved, but did not normalize, joint histology and whole joint friction; cyclic loading produced fewer activated caspase-3 containing chondrocytes when lubricin expression was restored at three weeks of age compared to non-restored littermates. Restoring lubricin expression in 2-month-old or 6-month-old mice had no beneficial effect on histology, whole joint friction, or activation of caspase-3 compared to non-restored littermates.
Conclusions
When boundary lubrication is congenitally deficient and cartilage becomes damaged, the window of opportunity for restoring lubrication and slowing disease progression is limited.
Introduction
In order to develop successful strategies for treating disease, it is important to know whether and when features of disease can be prevented, delayed, or reversed. These considerations are relevant to patients with congenital and acquired joint diseases. For example, nearly 50% of individuals with anterior cruciate ligament (ACL) injuries will develop signs and symptoms of arthritis within 15 years following their injury, independent of whether the injured joint has been surgically stabilized (1). This suggests it is either not possible to prevent progressive joint damage following many ACL injuries or that current treatment strategies are inadequate. Determining whether joint damage can be prevented or reversed, and identifying critical windows of opportunity for doing so, will be essential for providing optimal care and controlling health care costs.
Insights into processes involved in joint injury and repair may come from the study of individuals with the autosomal recessive disease Camptodactyly-Arthropathy-Coxa Vara-Pericarditis (CACP) syndrome and from animal models of this disease. CACP is a precocious joint failure disorder caused by genetic deficiency of lubricin (encoded by PRG4) (2), the major boundary lubricant in articulating joints (3). During development, lubricin is expressed by surface chondrocytes as soon as the joint cavitates (4). Subsequently, superficial zone chondrocytes and type B synoviocytes continue to express lubricin throughout life (4, 5). Patients with congenital (i.e., CACP) or acquired (e.g., inflammatory arthritis) deficiencies of lubricin have synovial fluid that fails to reduce friction in the boundary mode (6). Lubricin knockout (Prg4−/−) mice recapitulate features observed in patients with CACP, such as non-inflammatory synovial hyperplasia and precocious cartilage failure (4, 6). Importantly, damage to the cartilage surface in lubricin knockout mice is detectable by 2 weeks of age, and significantly increased whole joint friction is evident by 2 months of age (7). Thus congenital absence of lubricin damages the cartilage surface and alters whole joint mechanics early in life. However, complete cartilage failure can take a year to develop in Prg4−/− mice and decades to develop in patients with CACP (8). Thus, despite the absence of adequate boundary lubrication and the rapid appearance of joint damage, the progression to joint failure occurs at a much slower rate. This raises the possibility that any incipient traumatic event such as ACL or meniscal injury that causes cartilage surface damage will inevitably progress to cartilage failure over the long term (9). To determine whether the cartilage damage that results from congenital lubricin deficiency is treatable, we restored endogenous lubricin expression postnatally in lubricin knockout mice.
We created a strain of mice that have a reversible gene-trap in Prg4. This gene-trap blocks lubricin expression. Upon Cre-mediated excision of the gene-trap, lubricin expression is restored. We restored lubricin expression prior to conception, or at 3 weeks, 2 months, or 6 months of age and evaluated cartilage histology and whole joint friction in these mice and their non-restored littermates.
Materials and Methods
All animal studies were performed with the approval of the Institutional Animal Care and Use Committees at Boston Children’s Hospital and Rhode Island Hospital.
Animal models
Mice that are homozygous (Prg4−/−) or heterozygous (Prg4+/−) for lubricin loss-of-function alleles have been previously described (4). We generated mice with a new Prg4 allele (Prg4GT) (Figure 1)(Jax stock #025740). The Prg4GT allele was created by homologous recombination in 129 Sv/Ev ES cells (Figure 1A). The gene-trap, which consists of a loxP flanked artificial exon that contains a strong splice acceptor, LacZ coding sequence that is “in-frame” with Prg4, an internal ribosome entry site (IRES), a reverse tet transactivator (rTTA) cassette, poly-adenylation (pA) site, and a neomycin (NeoR) selection cassette driven by its own promoter, was derived from the gene-trap secretory vector pTT1TM (10). The Prg4GT allele is designed to produce membrane-anchored β-galactosidase and rTTA instead of lubricin in cells where Prg4 is normally expressed. The artificial exon can be excised with Cre-recombinase (i.e., Prg4GTR) to restore lubricin expression. We used two different Cre-recombinase alleles to excise the gene-trap. One allele, Tg(eII α-Cre) (Jax stock #003724), excises the gene-trap in the germline. The other allele, ROSA26CreERt2 (Jax stock #008463) causes widespread excision of the gene-trap when tamoxifen is given. Standard animal husbandry was performed to generate cohorts of male and female mice with the following genotypes, all on mixed B6/129 genetic backgrounds: Prg4−/−, Prg4+/−, Prg4GT/GT, Prg4GT/+, Prg4GT/−, Prg4GT/GT;ROSA26CreERt2/+, Prg4GT/+;ROSA26CreERt2/+, Prg4GTR/−, Prg4 GTR/GT.
Figure 1. Generation of mice with a genetically engineered, Cre-excisable, gene-trap (GT) in the second intron of Prg4.
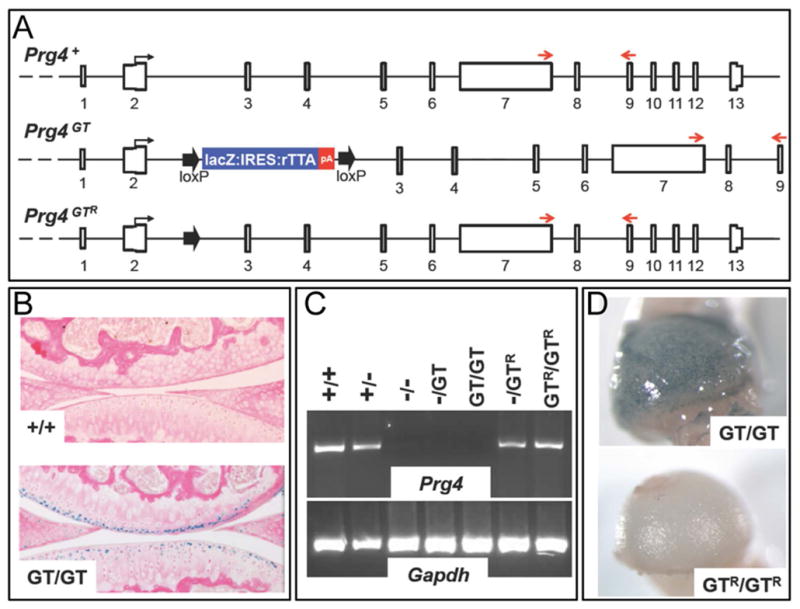
(A) Schematic depicting the genomic structure of the Prg4 wild-type (+), gene-trap (GT), and Cre-recombined gene-trap (GTR) alleles. The gene-trap is flanked by loxP sites (large arrows), which enable removal of the gene-trap with Cre-recombinase. (B) X-gal stained sagittal sections from the tibial-femoral joints of 2-month-old Prg4 wild-type (+/+) and homozygous gene-trap (GT/GT) mice. β-galactosidase activity is only seen in the superficial zone chondrocytes of the GT/GT mouse. (C) Semi-quantitative RT-PCR of total RNA extracted from joints of mice with genotypes containing different combinations of Prg4 wild type (+), knockout (−), gene-trap (GT), and Cre-recombined gene-trap (GTR) alleles. The PCR primers are located downstream of the gene-trap in Prg4 exons 7 and 9 (red arrows in panel A). Only mice with wild-type (+) or Cre-recombined (GTR) alleles have Prg4 mRNA transcript. (D) X-gal stained femoral heads from 1-month-old mice homozygous for Prg4 gene-trap (GT/GT) or Cre-recombined gene-trap (GTR/GTR) alleles. β-galactosidase activity at the femoral head surface of the GT/GT mouse is absent from the GTR/GTR mouse.
Induction of Cre-mediated recombination using tamoxifen
Mice with the genotypes Prg4GT/+, Prg4GT/GT, and Prg4GT/GT;ROSA26CreERt2/+ were given daily intraperitoneal injections of tamoxifen dissolved in corn oil (Sigma-Aldrich, US) at a dose of 0.1 mg/g body weight for 10 consecutive days beginning at 3 weeks, 2 months or 6 months of age and then permitted to age normally until they were euthanized.
Sample collection and processing
Mice were euthanized following CO2 inhalation and their joints were removed and processed immediately after.
Samples for knee joint histology were fixed in 4% (w/v) paraformaldehyde (PFA) in phosphate buffered saline (PBS) overnight, then rinsed several times in PBS and decalcified with either EDTA in PBS (14% v/v, pH 7.4) or a mild formic acid decalcifier (Immunocal, Decal, US) for ~ 2 weeks or 12 hours, respectively. Decalcified samples were again washed in PBS, and then dehydrated through a graded PBS-ethanol series, incubated in xylene, bisected in the sagittal plane with a razor, and infiltrated with paraffin. Six or 8 μm sagittal sections were collected from the center of the bisected joint outwards until the cruciate ligaments were no longer present, the tibial plateau became flattened, and the anterior and posterior portions of the meniscus were seen. Sections were stained with hematoxylin and eosin (H&E) following standard procedures (11).
Samples for X-gal staining were fixed briefly with 0.2% glutaraldehyde, 5 mM EDTA and 2 mM MgCl2 in PBS. The samples were then rinsed in PBS containing 2 mM MgCl2 and 0.02% NP-40. X-gal staining was performed overnight in the dark with constant shaking in X-gal staining solution (2 mg/ml X-Gal, 5 mM Potassium-ferro-cyanide, 5 mM Potassium-ferri-cyanide, 2 mM MgCl2, and 0.02% NP-40 in PBS). Following staining, samples were washed in PBS, post-fixed with PFA (4% w/v) and processed for histology as above.
Knee and femoral head cartilages were frozen in liquid nitrogen and homogenized in Trizol (Invitrogen, Life Technologies, US) to recover RNA. For reverse-transcriptase PCR (RT-PCR) assays, RNA was isolated using the PureLink RNA mini kit (Ambion, US) according to manufacturer’s instructions. Genomic DNA was removed by DNase I treatment. Taqman Reverse Transcriptase reagents (Applied Biosystems, Life Technologies, US) were used to generate cDNA with Oligo(dT). Semi-quantitative PCR was performed using forward and reverse primers located within exons 7 and 9, respectively, since this region of the Prg4 is downstream of the gene-trap and not subject to alternative splicing in wild-type mice. Gapdh served as an internal control. PCR products were separated on a 4% agarose gel and visualized using Sybr Safe (Invitrogen, USA). RNA sequencing and data interpretation were performed as previously described (12, 13).
Samples for biomechanical testing were harvested and soft tissue was removed around the tibial-femoral-patellar joint, leaving the joint capsule intact. Whole joint coefficient of friction (COF) was measured using a modified Stanton pendulum system, as previously described (7, 14, 15). During pendulum oscillation, motion was tracked with a Qualysis OPUS cameras and Track Manager software (Qualysis AB, Sweden) at a rate of 60 Hz. Oscillation data was processed with Visual3D software (C-Motion, Inc., US) and a custom MATLAB (MathWorks Inc., US) code was used to determine the peak amplitude of each cycle of oscillation and calculate COF using a Stanton linear decay model (14).
Ex vivo cyclic loading was performed for 60 minutes, with the joint submerged in Dulbecco’s Modified Eagle Medium (DMEM, Life Technologies, US) to prevent desiccation. Loading was achieved using an active pendulum that was continuously oscillated ±15° at a rate of 1.5 Hz in 15-minute increments (7). Whole joint COF was measured at the end of each increment at room temperature (RT) in air. At the end of the loading experiment, the specimen was fixed in 10% formalin. Prior to activated caspase-3 immunostaining, the specimen was decalcified at 4°C using a solution of 0.48M EDTA adjusted pH to 7.1. Decalcified specimens were paraffin embedded.
Quantitative histologic assessment of joint pathology
A tibial-femoral joint disease severity scoring system was devised based on several published scoring systems, including the OARSI histopathology initiative (16–18). An additional score for meniscus integrity was included. The scoring system is summarized in Supplemental Table 1. Photomicrographs of sagittal knee sections taken from mice with different Prg4 genotypes across several ages were used to develop a training and reference set of images encompassing normal and diseased joints. Briefly, scoring assessed surface morphology, synovial hyperplasia, chondrocyte proliferation, and meniscal architecture. The tibial plateau and the femoral condyle were scored separately. All samples were scored from H&E stained sections. Three scientists, who had been trained on the scoring system, were sent 2 photomicrographs for each experimental animal. The scientists were blinded with respect to the animals’ genotypes and treatment groups.
Activated caspase-3 immunostaining
Paraffin-embedded coronal sections (5 μm) were heated to 60°C for 30 min, deparaffinized, and hydrated in 3 changes of xylene alternating with alcohol. Sections were quenched in endogenous peroxidase in 3% hydrogen peroxide for 10 min and antigen retrieval was performed using a pepsin solution (ThermoScientific, US) following the manufacturer’s recommendation. A rabbit polyclonal antibody against active caspase-3 (ab13847, Abcam, US) at 1:50 dilution in staining buffer containing 8% (v/v) horse serum was added to the sections and incubated at 4°C overnight. After 3 washes with PBS, the sections were incubated with Cy3 goat anti-rabbit IgG (Molecular Probes, Life Technologies, US) at 1:50 dilution in staining buffer containing 8% (v/v) horse serum for 1 hr at RT, protected from light. The sections were washed 5 times with PBS and counterstained using Vectashield mounting media with DAPI (1.5ug/ml, Vector Laboratories Inc., US). Images were captured with a 10X objective, using a Roeper Scientific Photometrics CoolSNAP HQ2 monochrome camera (Roeper Scientific, Germany) connected to a Nikon Eclipse 90i microscope (Nikon, US). Fluorescent images were thresholded uniformly to reduce background auto-fluorescence and to adjust DAPI signal using Adobe Photoshop CS5 software (Adobe Systems Inc., US). Cells positive for activated caspase-3 were visualized at 100× magnification, using Image-Pro Plus 7.0 software. Activated caspase-3-positive chondrocytes and DAPI stained nuclei from the entire thickness of mouse cartilage on the medial and lateral tibial plateaus and femoral condyles were manually counted using corresponding H&E sections as a reference for cartilage depth by a blinded observer. The overall percentage of activated caspase-3-positive chondrocytes among the total number of chondrocytes was reported.
Digital droplet PCR to measure Cre-mediated recombination following tamoxifen administration in a 4-month-old mouse with the ROSA26CreERt2 allele
Tamoxifen was administered for 8 consecutive days to a 4-month-old mouse that was double homozygous for conditional Pik3caH1047R alleles (19) and ROSA26CreERt2 alleles. The mouse was euthanized 2 days later and DNA was extracted from multiple tissues including tail, cerebral cortex, and femoral head articular cartilage. A droplet digital PCR assay (20) was used to quantify the fraction of cells in the different tissues that had undergone Cre-mediated recombination.
The PCR primer pair (caagggagaggaatggtaagg and caactcaggcatgccggatcccaa) was used to generate a 719 bp amplimer from the conditional allele and a 265 bp amplimer from the recombined alleles. In the PCR mixture were also fluorescent probes that detect the recombined allele (FAM-cgaagttatgttaacttgttagacc-IABk) or the conditional allele (HEX-cgaagttatttgttagaccctt-IABk). Seven ng of each DNA sample was tested in duplicate in 20 μL reaction mixtures that were emulsified into approximately 14,000 droplets using a QX100 Droplet Generator (Bio-Rad, U.S.) according to the manufacturer’s instructions. PCR was performed with the following parameters: 10 min at 95°C, followed by 40 cycles of 30 sec at 94°C, 60 sec at 55°C, 120 sec at 72°C, and then kept at 12°C. Samples were analyzed using the QX100 Droplet Reader (Bio-Rad) and analyzed with QuantaSoft software (Bio-Rad).
Statistical Analyses
Statistical analyses for whole joint COF comparing genotypes and cyclic loading durations were performed using a two-way repeated measures ANOVA (α = 0.05) with Tukey’s multiple comparison test using Prism 6 Statistical Software (GraphPad Software Inc., La Jolla, CA). For total joint histology scores, the average of the three scientists’ scores for each animal was used for statistical analyses. Total joint histological scores between genotypes, treatment groups, and time points were compared using the nonparametric Mann-Whitney U test given the lack of normality and because they represent scores rather than continuous data. Inter-observer agreement in histological scores was measured with the intraclass correlation coefficient (ICC) with a 95% confidence interval (CI). Statistical analyses were performed using IBM SPSS Statistics (version 21.0, IBM, Armonk, NY). Two-tailed values of p < 0.05 were considered statistically significant. Comparisons between percentages of activated caspase-3-positive cells between different genotypes were analyzed using a one-way ANOVA (α=0.05) with Tukey’s multiple comparison test, using Prism 6 Statistical Software.
Due to their functional equivalence, Prg4GT/GT, Prg4GT/−, and Prg4−/− genotypes were combined into a single group during statistical analyses, as were Prg4+/−, Prg4GTR/−, Prg4 +/GT, and Prg4GTR/GT genotypes.
Results
Generation of mice with a reversible gene-trap in Prg4
Mice with the gene-trap allele (Figure 1A) were generated and bred to homozygosity and compound heterozygosity (i.e., Prg4GT/GT, Prg4GT/−, and Prg4GT/+). The gene-trap allele produced β-galactosidase instead of lubricin, as indicated by X-gal staining of Prg4+/+ and Prg4GT/GT cartilage (Figure 1B). Furthermore, Prg4GT/GT mice did not express wild-type Prg4 messenger RNA, as indicated by RT-PCR using Prg4 primers in exons that are downstream of the gene-trap (Figure 1C) and by RNA sequencing (Supplemental Figure 1). To determine whether Cre-mediated excision of the gene-trap restored endogenous Prg4 mRNA expression, we excised the gene-trap in germ cells using the Tg(eII α-Cre) allele to generate mice with a Prg4GTR allele (Figure 1A) and bred the Prg4GTR allele to homozygosity and compound heterozygosity (Prg4GTR/GTR and Prg4GTR/−). Compared to Prg4GT/GT articular chondrocytes, Prg4GTR/GTR articular chondrocytes expressed lubricin mRNA (Figure 1C) and no longer expressed β-galactosidase (Figure 1D). We performed RNA sequencing on knee joint cartilage from 2- and 3-week-old Prg4+/+, Prg4GT/GT, Prg4GTR/−, Prg4+/−, Prg4GTR/GT, and Prg4+/GT mice, and confirmed that the GT allele is a null allele (Supplemental Figure 1A and B) and the abundance (Supplemental Figure 1B) and splice-forms (data not shown) from the GTR allele are the same as those from the wild-type allele.
The Prg4GT allele is a loss-of-function allele, whereas the Prg4GTR allele appears “wild-type”
The histological appearances of tibial-femoral cartilages from 2-month-old Prg4−/− and Prg4GT/− mice had similar cartilage changes (Figure 2A), consistent with the Prg4GT allele preventing lubricin expression. Conversely, the histological appearances of cartilage in Prg4+/− and Prg4GTR/− mice were the same (Figure 2B), and both were indistinguishable from wild-type mice, indicating that the Prg4GTR functioned like a wild-type allele. By 2 months of age, protein deposition on the cartilage surface was apparent in Prg4−/− and Prg4GT/−, but not in Prg4+/− and Prg4GTR/− mice (Figure 2). Consistent with the abnormal appearance of the articular cartilage, lubricin deficient (Prg4GT/GT and Prg4GT/−) mice had higher whole joint COF compared to lubricin sufficient (Prg4+/− and Prg4GTR/−) mice (Figure 3A and Table 1). In addition the lubricin deficient joints exhibited a progressive increase in COF during ex vivo cyclic loading compared to lubricin sufficient joints (Figure 3B). The total joint scores from 150 mice with different Prg4 genotypes and ages (data not shown) were used to determine the intraclass correlation coefficient (ICC) among the 3 scorers; the ICC was 0.922 (95% confidence interval 0.897 to 0.941), indicating high correlation. As expected, the total joint scores of 2-month-old lubricin sufficient (Prg4+/− and Prg4GT/+) mice were significantly better than the scores in lubricin deficient (Prg4GT/GT, Prg4GT/−, and Prg4−/−) mice (Figure 3C and Table 1). Additionally, the total joint scores of lubricin deficient mice significantly worsened from 2 months to 6 months of age and from 6 months to 9 months of age (Figure 3C and Table 1) In contrast, no worsening in the total joint score was observed when lubricin sufficient mice were examined at 2 months and 6 months of age (p = 0.628), although there was greater variability in the total joint scores of the 6-month-old mice (Figure 3C and Table 1).
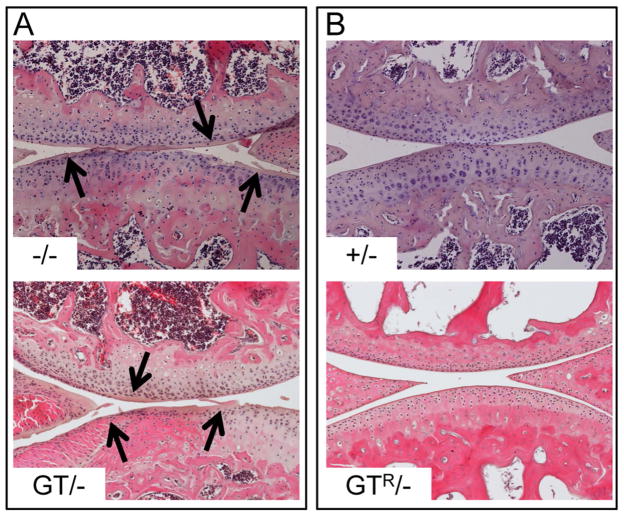
Figure 2. The Prg4 gene-trap allele is a loss-of-function allele whose function is fully restored following Cre-excision.
(A) H&E stained tibial-femoral joints from 2-month-old Prg4 knockout (−/−) and compound heterozygous gene-trap/knockout (GT/−) mice. Note protein deposition along the cartilage surface (arrows), consistent with lubricin deficiency. (B) H&E stained tibial-femoral joints from 2-month-old Prg4 heterozygous knockout (+/−) and compound heterozygous Cre-recombined/knockout (GTR/−) mice. The articular cartilage in these mice appears normal in that the superficial zone chondrocyte nuclei are located adjacent to the cartilage surface, the internal edges of the menisci are sharp, and there is no protein deposition along the surface. All images were taken at 100X magnification.
Figure 3. Tibial-femoral-patellar whole joint coefficient of friction (COF) measurements and total joint scores are affected by animal age and Prg4 genotype.
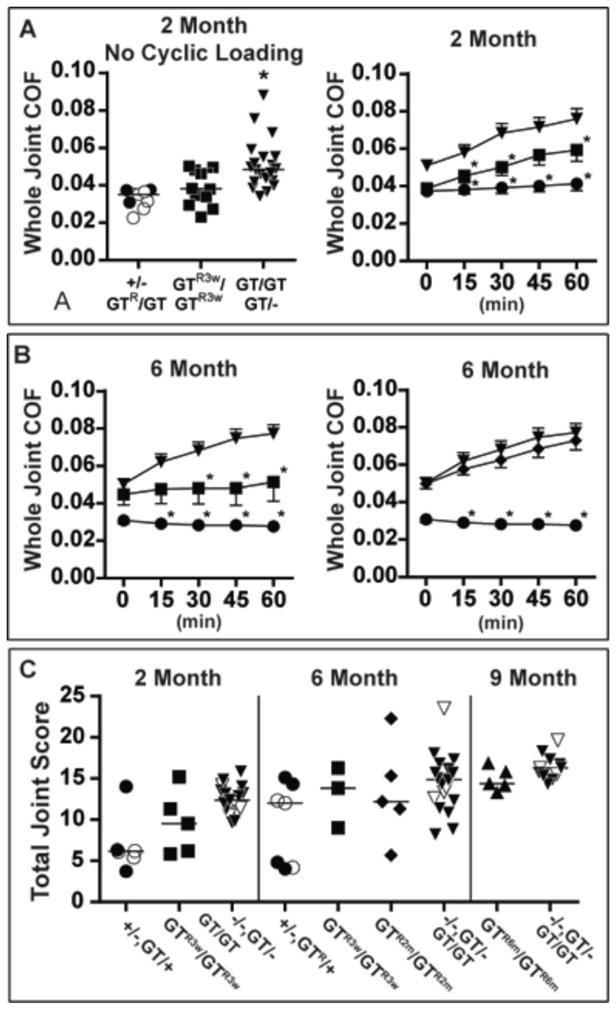
(A) left panel - scatter plot depicting individual COFs in 2-month-old lubricin sufficient (+/−, ○ and GTR/GT, ●), deficient (GT/− and GT/GT, ▼), and re-expressed at 3 weeks of age (GTR3w/GTR3w, ■) mice. Horizontal bars indicate the median COF. Cohort sizes and p values for between group comparisons are reported in Table 1. right panel –Mean COF (±SEM) during ex vivo cyclic loading of 2-month-old lubricin sufficient (GTR/GT, ●, n = 3), deficient (GT/−, and GT/GT, ▼, n = 20), and re-expressed at 3 weeks of age (GTR3w/GTR3w, ■, n = 12) mice. Asterisks indicate COF is significantly lower (p < 0.05) in lubricin sufficient and GTR3w/GTR3w mice compared to deficient mice. (B) left panel – Mean COF (±SEM) during cyclic loading in 6-month-old lubricin sufficient (GTR/−, ●, n = 3), deficient (GT/−, and GT/GT, ▼, n = 14), and re-expressed at 3 weeks of age (GTR3w/GTR3w, ■, n = 7,) mice. Asterisks indicate COF is significantly lower in lubricin sufficient and GTR3w/GTR3w mice compared to deficient mice. right panel - same COF measurements as in the left panel, except for mice whose lubricin was re-expressed at 2 months of age (GTR2m/GTR2m, ◆, n = 14). Asterisks indicate COF is significantly lower in lubricin sufficient compared to GTR2m/GTR2m and deficient mice. (C) Scatter plot depicting individual total joint scores at 2 months, 6 months, or 9 months of age in lubricin sufficient (+/−, ○ or GT/+), deficient (−/ −, ▽, GT/− and GT/GT, ▼), re-expressed at 3 weeks of age (GTR3w/GTR3w, ■), re-expressed at 2 months of age (GTR2m/GTR2m, ◆), and re-expressed at 6 months of age (GTR6m/GTR6m, ▲) mice. Horizontal bars indicate the median joint score. Cohort sizes and p values for between group comparisons are reported in Table 1.
Table 1.
Table of Statistical Comparisons
| Coefficient of Friction1 | p value2 |
|---|---|
| 2 mo LS (9) vs 6 mo LS (3) | 0.792 |
| 2 mo LD (20) vs 6 mo LD (14) | 0.863 |
| 2 mo LS (9) vs 2 mo LD (20) | < 0.001 |
| 2 mo LS (9) vs 2 mo GTr3w (12) | 0.217 |
| 2 mo GTR3w (12) vs 2 mo LD (20) | 0.003 |
| 6 mo LS (3) vs 6 mo LD (14) | 0.006 |
| 6 mo LS (3) vs 6 mo GTR3w (7) | 0.069 |
| 6 mo GTR3w (7) vs 6 mo LD (14) | 0.270 |
| 6 mo GTR2m (5) vs 6 mo LD (14) | 0.937 |
| Total Joint Histology | p value3 |
| 2 mo LS (6) vs 6 mo LS (7) | 0.628 |
| 2 mo LD (7) vs 6 mo LD (20) | 0.020 |
| 6 mo LD (20) vs 9 mo LD (11) | 0.032 |
| 2 mo LS (6) vs 2 mo LD (17) | 0.006 |
| 2 mo LS (6) vs 2 mo GTR3w (6) | 0.180 |
| 2 mo GTR3w (6) versus 2 mo LD (17) | 0.062 |
| 6 mo LS (7) vs 6 mo LD (20) | 0.026 |
| 6 mo GTR3w (3) vs 6 mo LD (20) | 0.698 |
| 6 mo GTR2m (5) vs 6 mo LD (20) | 0.488 |
| 9 mo GTR6m (5) vs 9 mo LD (11) | 0.145 |
| Activated caspase-3 | p value4 |
| 2 mo LS (3) vs 2 mo LD (19) | <0.001 |
| 2 mo GTR3w (12) vs 2 mo LD (19) | <0.001 |
| 2 mo GTR3w (12) vs LS (3) | 0.96 |
| 6 mo LS (3) vs 6 mo LD (13) | <0.001 |
| 6 mo GTR3w (3) vs 6 mo LD (13) | <0.001 |
| 6 mo GTR3w (6) vs 6 mo LS (3) | 0.92 |
| 6 mo GTR2m (12) vs 6 mo LD (13) | 0.77 |
| 6 mo GTR2m (12) vs 6 mo LS (3) | 0.001 |
| 6 mo GTR3w (6) vs 6 mo GTR2m (12) | <0.001 |
Significant p values are highlighted in bold
LS – lubricin sufficient mice have genotypes +/−, GTR/−, GTR/GT, or +/GT
LD – lubricin deficient mice have genotypes −/−, GT/−, or GT/GT
GTR3w – gene-trap reversed at 3 weeks of age
GTR2m – gene-trap reversed at 2 months of age
GTR6m – gene-trap reversed at 6 months of age
Number of animals is within parentheses
measured without ex vivo cyclic loading (time = 0)
p values determined using a two-way ANOVA
p values determined using a non-parametric Mann-Whitney U test
p values determined using a one-way ANOVA
Partial histological and biomechanical benefit of restoring lubricin expression in 3-week-old animals
Having demonstrated that reversing the Prg4GT allele (i.e., Prg4GTR) prior to conception prevented cartilage damage, we next determined whether restoring lubricin expression after birth would be beneficial. To induce Cre-mediated excision of the gene-trap after birth, we employed the ROSA26CreERt2 allele. We generated Prg4GT/+, Prg4GT/GT, and Prg4GT/GT;ROSA26CreERt2/+ mice, and at 3 weeks of age administered 10 daily IP injections of tamoxifen or vehicle-alone. We then examined knee joints biomechanically (Figure 3A, B and Table 1) and histologically (Figures 3C, 4 and Table 1) when the mice reached 2 months and 6 months of age. We confirmed that 10 days of tamoxifen led to efficient excision of the gene-trap in the superficial zone chondrocytes of Prg4GT/GT;ROSA26CreERt2/+ mice by performing X-gal staining at 2 months of age (Figure 4B).
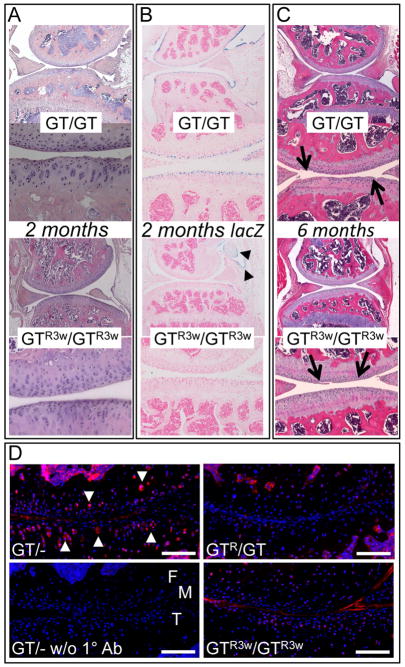
Figure 4. Restoring Prg4 expression in 3-week-old mice may delay, but does not prevent, the histologic appearance of cartilage damage.
(A – C) Sections from mice with the median total histology score for each genotype and treatment group. (A) H&E and (B) X-gal stained tibial-femoral joints from 2-month-old mice that are homozygous for the gene-trap allele (GT/GT) or homozygous for a gene trap allele that had been Cre-excised at 3-weeks-of-age (GTR3w/GTR3w). Note the normal appearance of the cartilage surface and the absence of X-gal staining chondrocytes in the 2-month-old GTR3w/GTR3w mice. Images taken at 40X (above each genotype) and 200X (below each genotype) magnification. Some regions of synovium (arrowheads) still express LacZ indicating that Cre-mediated recombination of the gene-trap allele in synovium is incomplete. (C) H&E stained sections from 6-month-old mice that are homozygous for the gene-trap allele (GT/GT) or homozygous for a gene trap allele that had been Cre-excised at 3-weeks-of-age (GTR3w/GTR3w). Protein deposition (arrows) is present on the surface of the GTR3w/GTR3w cartilage (arrows) although the morphology of the superficial zone chondrocytes is less severely affected compared to GT/GT cartilage. (D) Immunostaining for activated caspase-3 in 2-month-old lubricin deficient (GT/−), sufficient (GTR/GT), and re-expressed at 3 weeks of age (GTR3w/GTR3w) mice following 60 minutes of ex vivo cyclic loading. Cell nuclei are labeled with DAPI. Omission of primary antibody (w/o 1° Ab) in GT/− cartilage shows no staining; the locations of the femoral condyle (F), meniscus (M), and tibial (T) are also indicated in this section. Arrowheads locate examples of chondrocytes that are positive for activated caspase-3. Scale bars indicate 100 μm. Cohort sizes and p values for between group comparisons for the percentage of activated caspase-3 containing chondrocytes are reported in Table 1.
At 2 months of age the knee joints of Prg4GT/GT;ROSA26CreERt2/+ mice that were treated with tamoxifen at 3 weeks of age (Prg4GTR3w/GTR3w) had lower COF (p < 0.003) and trended toward having better appearing cartilage histology and joint scores (p = 0.062) than lubricin deficient mice (Figure 3 and Table 1). The histologic appearance appeared worse than that of mice with an inherited functional (e.g., Prg4+ and Prg4GTR) allele (Figures 2B, 3C, and 4), although this difference did not achieve statistical significance (Table 1). Biomechanical testing showed that restoring lubricin expression at 3 weeks of age resulted in a significant reduction in COF compared to littermates with non-functional alleles (Figure 3A, B), and an insignificant increase compared to mice with inherited functional alleles (Figure 3A, B). Interestingly, however, compared to mice with inherited functional alleles, ex vivo cyclic loading in the Prg4GTR3w/GTR3w mice was associated with a progressive increase in COF (Figure 3B). We determined the percentage of chondrocytes that were positive for activated caspase-3 following cyclic loading and found significantly fewer cells exhibited activated caspase-3 in Prg4GTR3w/GTR3w mice compared to lubricin deficient littermates (Figure 4D and Table 1), and observed no difference between Prg4GTR3w/GTR3w and PrgGTR/− mice (p = 0.96).
At 6 months of age the joint COF in Prg4GTR3w/GTR3w mice became higher than that of mice with inherited functional alleles and non-significantly different from lubricin deficient mice (Figure 3B and Table 1). However, 6-month-old Prg4GTR3w/GTR3w mouse joints did not have the progressive increase in COF with cyclic loading that occurred in their lubricin deficient littermates (Figure 3B), and the percentage of activated caspase-3-positive chondrocytes in cyclically loaded 6-month-old Prg4GTR3w/GTR3w joints was less than in lubricin deficient joints (Table 1). We could no longer detect a quantifiable improvement in the cartilage histology of the 6-month-old Prg4GTR3w/GTR3w mice compared to lubricin deficient mice (Figures 3C, 4C, and Table 1).
No histological or biomechanical benefit from restoring Prg4 expression in 2-month-old mice
Having found partial benefit from restoring expression in 3-week-old mice, we next restored expression in 2-month-old mice (Prg4GTR2m/GTR2m), aged the animals until they were 6 months old, and examined their joints histologically and biomechanically. Cartilage histology, COF, and the percentage of cells positive for activated caspase-3 were no better in Prg4GTR2m/GTR2m mice than in lubricin deficient mice (Figures 3, 5, and Table 1), with most of the activated caspase-3 positive cells located in the middle, rather than superficial, articular cartilage zone. Similarly, we observed no histological benefit of restoring lubricin expression at 6 months of age when joints were examined at 9 months of age (Figure 3C and Table 1). Similar to what others have reported previously (21), we confirmed the ROSA26CreERt2 allele remains active in older mice by performing RNA sequencing on joints from Prg4GTR6m/GTR6m immediately after they received 10 consecutive days of tamoxifen (Supplemental Figure 1B) and by using a ddPCR assay to demonstrate that > 98% of articular cartilage chondrocytes underwent Cre-mediated recombination in a 4-month-old mouse immediately after receiving 8 consecutive days of tamoxifen (Supplemental Figure 1C).
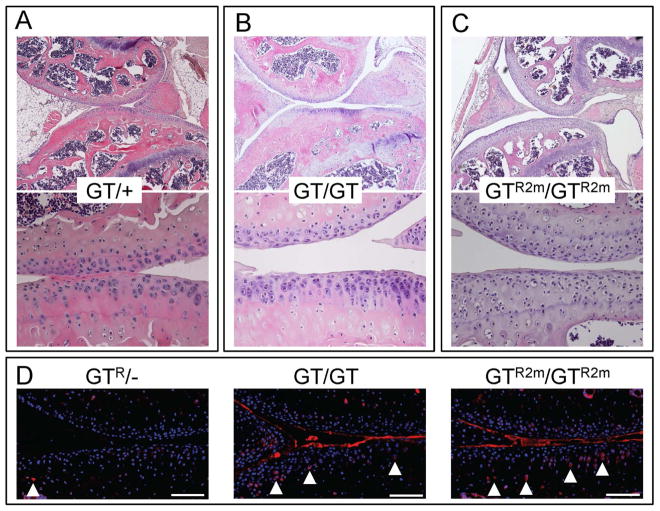
Figure 5. Restoring Prg4 expression in 2-month-old mice does not improve cartilage histology.
(A–C) H&E stained sections from 6-month-old mice with the median total histology score for each genotype and treatment group. Images taken at 40X and 200X magnification. (A) heterozygous gene-trap mouse (GT/+). (B) homozygous gene-trap mouse (GT/GT). (C) homozygous mouse whose gene-trap was Cre-excised at 2-months-of-age (GTR2m/GTR2m). (D) Immunostaining for activated caspase-3 in 6-month-old lubricin sufficient (GTR/−), deficient (GT/GT), and re-expressed at 2 months of age (GTR2m/GTR2m) mice following 60 minutes of ex vivo cyclic loading. Cell nuclei are labeled with DAPI. Arrowheads locate examples of chondrocytes that are positive for activated caspase-3. Scale bars indicate 100 μm. Cohort sizes and p values for between group comparisons for the percentage of activated caspase-3 containing chondrocytes are reported in Table 1.
Discussion
Degenerative joint disease is common (22, 23). In the context of a severe joint injury, the initiating event is known (24, 25). However, in most patients with osteoarthritis an incipient event, if one exists, is not known. We employed a genetic model of progressive joint failure (i.e., lubricin deficient mice), caused by inadequate boundary lubrication at the cartilage surface, to determine whether cartilage damage can be ameliorated by restoring the boundary lubricant and, if so, whether there exists a window of opportunity for successfully intervening. These questions are of primary importance for individuals with CACP, who have congenital lubricin deficiency, and may be important for individuals with acquired deficiency of lubricin, as can occur during injury, infection, and inflammatory disease (26).
We restored lubricin expression in lubricin deficient mice using a Cre-excisable Prg4 gene-trap (Figure 1). We first showed that removal of the gene-trap recreates a wild-type allele (Figure 2). We then evaluated mice in which we removed the gene-trap at 3 weeks of age and observed an improved, but not normal, histological appearance of their tibial-femoral cartilage at 2 months of age (Figures 3 and 4). The whole joint COF in these mice was also improved, but not normal, at 2 months of age (Figure 3); however, we could no longer detect an overall improvement in the histologic appearance or COF of the articular cartilage in the 6-month-old mice (Figures 3 and 4). Histological appearance, or at least the total joint scoring system we employed, may not accurately reflect joint function in these animals since even lubricin sufficient mice developed higher and more variable joint scores as they aged. Biomechanical function during cyclic loading with measurement of activated caspase-3 may provide a more sensitive method for assessing the effect, or lack thereof, of lubricin restoration (Figures 3 and 4). Based on biomechanical testing and activated caspase-3 staining, restoring lubricin expression in 3-week-old mice produced some beneficial effect.
Restoring lubricin expression in 2-month-old mice produced no apparent benefit (Figures 3 and 5). These data imply that the therapeutic window of opportunity for preventing irreversible cartilage damage in congenital lubricin deficiency is small, at least in this animal model. The relationship between therapeutic windows in mice and humans is unknown. However, it seems likely that early intervention will be required in order to prevent or delay cartilage failure in humans with CACP.
Recent findings indicate that lubricin may promote cell preservation by providing anabolic signaling (27) and preventing apoptosis. A significant reduction in the percentage of activated caspase-3 positive cells in mice recombined at 3 weeks of age compared to lubricin deficient mice indicates the reestablishment of cellular protection following recombination. As the sole cell type in cartilage, chondrocytes play specialized roles in the maintenance of cartilage extracellular matrix. The majority of activated caspase-3 positive chondrocytes were rounded, upper middle zone cells, located just below the flattened superficial chondrocytes. Based on previous findings in bovine cartilage (28–30) and studies regarding the structure of collagen in mouse cartilage (31), we hypothesize that the junction of tangential to columnar collagen II bundles in this region results in elevated cartilage strain that damages rounded chondrocytes, which are not designed to withstand shear stress. Furthermore, elevated stress may contribute to a number of transduction pathways, including the expression of catabolic and apoptotic factors (32–39), which accelerate cartilage deterioration in the disease state.
Our results may be relevant for common forms of degenerative joint disease. Excessive friction has been shown to cause apoptosis in bovine cartilage bearings (15, 40). We observed excessive caspase-3 activation following cyclic loading in lubricin deficient mice (Figures 4 and 5, and Table 1) and in gene-trap mice whose lubricin expression was restored at 2 months of age (Figure 5). Thus, patients with a transient deficiency of boundary lubrication may sustain cartilage damage that will progress, even after lubricin levels return to normal. If transiently increased friction within a joint is an incipient event for degenerative joint disease, then strategies for preventing or rapidly reducing friction are needed. Five published studies have reported beneficial effects of administering lubricin following joint injury in animal models (41–45). However, each of these studies had limited follow-up - between 28 and 70 days following joint injury - so it remains uncertain whether adding lubricin has really prevented or only delayed joint failure. Interestingly, constitutive overexpression of lubricin has been demonstrated to prevent cartilage damage in a mouse model of traumatic joint injury, although the length of follow-up was less than 6 weeks (27).
The strength of our study is in its genetic approach. By placing the gene-trap into the Prg4 locus, we were able to restore endogenous lubricin mRNA expression when the gene-trap was excised. Also, since lubricin mRNA has several different splice-forms, our genetic approach restored expression of all. However, we have not demonstrated that lubricin protein is produced when the gene-trap was excised since we have not been able to generate antibodies (46) or find a commercially available antibody that reliably immunodetects mouse lubricin when Prg4−/− mice are used as an appropriate negative control. Instead, we inferred that protein was being expressed based on the normal phenotypes of Prg4GTR/GTR and Prg4GTR/− mice, the re-appearance of Prg4 mRNA by RT-PCR (Figure 1) and RNA sequencing in mice with the Prg4GTR allele (Supplemental Figure 1), and the loss of β-galactosidase expression in chondrocytes and most synoviocytes where the Prg4GT allele had been Cre-excised (Figure 4).
There are several limitations of our study. First, some cohorts were underpowered to achieve statistical significance. Faster and more reliable imaging techniques are being developed to assess cartilage morphology, which should make studies involving larger sized cohorts more feasible. Second, scattered areas of β-galactosidase expression were still present in the synovium of treated mice, indicating we had not restored lubricin expression in every type B synoviocyte and, therefore, may not have attained normal lubricin levels in the synovial fluid. Third, the genetic background of the animals used in this study was mostly C57BL/6J, which heals full thickness cartilage defects less well than other strains (47). Therefore, mice with other genetic backgrounds might have had better responses to lubricin re-expression. Finally, despite having an ICC of > 0.9, the histological scoring system we employed may be insensitive at detecting chondroprotective effects. In a pre-clinical rodent OA lubricin supplementation study, a modified Mankin histologic scoring system failed to show improvement but urine type II collagen degradation products were significantly lower in the rodents treated with exogenous lubricin (42). Longer-term follow-up and more sensitive measures of joint structure and function may be needed to better assess therapeutic outcome.
Future work is also needed to determine whether restoration of lubricin expression earlier than 3 weeks of age can reverse disease or prevent disease progression. It is possible that lubricin assumes a critical function during joint cavitation that is irreplaceable after birth, although the cartilage of newborn lubricin deficient mice appeared normal by electron microscopy (6). Also, the present studies were performed in mice that were congenitally deficient for lubricin, as are patients with CACP. Performing studies in mice whose joints had formed when lubricin was sufficient and then developed a transient deficiency would better inform us regarding the importance of preventing transient deficiency in traumatic injuries and inflammatory disorders.
To conclude, our data suggest that early restoration of lubricin expression may slow disease progression in patients with CACP, who congenitally lack this protein. Further studies are required to determine the relevance of our findings to patients experiencing transient acquired deficiency of lubricin due to traumatic injury or inflammation.
Supplementary Material
(A) Screen shot from the Integrated Genomics Viewer of RNA sequencing data obtained from 2 Prg4GT/GT and 2 Prg4+/+ mice that map to exons 7 to 11. Histograms depicting read depths (up to 4000 reads) for the RNA sequencing data are shown (horizontal arrows). Read depth averages ~ 2000X for the +/+ mice and < 10 for the GT/GT mice. Black and red arrowheads, indicate examples of 50-basepair RNA sequencing reads mapping to exons and introns, respectively. Vertical arrows point to examples of reads spanning exons, consistent with mRNA splicing. Very few examples of exon spanning reads are found in the GT/GT data. (B) Histogram depicting abundance of Prg4 wild-type (+), gene-trap (GT), and gene-trap excised (GTR) transcripts in 2 – 3 week old mouse joints determined by RNA sequencing, as well as Prg4 abundance in a mouse whose gene-trap had been excised at 6 months of age (GTR6m). Prg4 transcript abundance is normalized to aggrecan (Acan), type II collagen (Col2a1), and type IX collagen alpha 1 chain (Col9a1) abundance and set at 100% for the wild-type Prg4 allele. The GT allele produces less than 0.1% of wild-type transcript whereas the GTR allele produces between 80 and 110% of wild-type transcript. Extracted mRNA from the joints of 6-month-old GTR6m mice contains only 4 – 8% of the Prg4 transcript found in 3-week-old wild-type and GTR mice. A likely explanation for this observation is that most superficial zone chondrocytes have already died in mice that lacked lubricing till 6 months of age. N= the number of joints from which independent RNA sequencing libraries were generated; libraries were made from 2 animals with each Prg4 allele. (C) Scatterplots depicting ddPCR results for Cre-mediated recombination of a conditional allele (Pik3ca) in cartilage, tail, and cerebral cortex from a 4-month-old mouse that had just been given 8 consecutive days of tamoxifen. DNA from a mouse with the conditional allele and no ROSA26CreERt2 allele serves as the control. The X-axis indicates fluorescence intensity in droplets for the probe targeting the non-recombined amplimer and the Y-axis indicates fluorescence intensity in droplets for the probe targeting the Cre-recombined amplimer. Individual droplets that contain no amplimer are pseudocolored black, non-recombined amplimer orange, Cre-recombined amplimer blue. The percentages amplimer-containing droplets that have Cre-recombined alleles are indicated beneath each tissue studied. Note Cre-mediated recombination has occurred in > 98% of the articular cartilage chondrocytes. The bar graph indicates the numbers of cells from each tissue for which DNA was analyzed to determine the Cre-mediated recombination rate.
Acknowledgments
The National Institutes of Health grant AR050180 supported this work. GDJ discloses that he has a patent for the use of lubricin in joints and a Small Business Technology Transfer award to explore the commercialization of intra-articular lubricin injections.
The National Institutes of Health grant AR050180 supported this work. The authors thank Dr. Minjie Zhang for helping process cartilage samples for ddPCR and RNA sequencing.
Footnotes
Author contributions and disclosures
AH generated and characterized the mouse cohorts, conducted lubricin restoration experiments, performed histologic sectioning for joint scoring and, with KAW, wrote the initial draft of this manuscript. KAW conducted lubricin restoration experiments, and performed biomechanical testing, activated caspase-3 immunostaining, and joint histology scoring. YC created and characterized mice with the Prg4GT allele and, with UMA, performed RNA sequencing. JMA performed joint histology scoring. PS characterized the mouse cohorts. LXZ performed biomechanical testing. SH and SGL performed the ddPCR experiments. DZ performed statistical analyses. GDJ performed joint histology scoring and, with MLW, conceived and designed the experiments. All authors contributed to, read, and approved the submitted version of the manuscript. GDJ discloses that he has a patent for the use of lubricin in joints and a Small Business Technology Transfer award to explore the commercialization of intra-articular lubricin injections. All other authors have no disclosures.
References
- 1.von Porat A, Roos EM, Roos H. High prevalence of osteoarthritis 14 years after an anterior cruciate ligament tear in male soccer players: a study of radiographic and patient relevant outcomes. Annals of the rheumatic diseases. 2004;63(3):269–73. doi: 10.1136/ard.2003.008136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marcelino J, Carpten JD, Suwairi WM, Gutierrez OM, Schwartz S, Robbins C, et al. CACP, encoding a secreted proteoglycan, is mutated in camptodactyly-arthropathy-coxa vara-pericarditis syndrome. Nature genetics. 1999;23(3):319–22. doi: 10.1038/15496. [DOI] [PubMed] [Google Scholar]
- 3.Jay GD, Britt DE, Cha CJ. Lubricin is a product of megakaryocyte stimulating factor gene expression by human synovial fibroblasts. The Journal of rheumatology. 2000;27(3):594–600. [PubMed] [Google Scholar]
- 4.Rhee DK, Marcelino J, Baker M, Gong Y, Smits P, Lefebvre V, et al. The secreted glycoprotein lubricin protects cartilage surfaces and inhibits synovial cell overgrowth. The Journal of clinical investigation. 2005;115(3):622–31. doi: 10.1172/JCI200522263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schumacher BL, Hughes CE, Kuettner KE, Caterson B, Aydelotte MB. Immunodetection and partial cDNA sequence of the proteoglycan, superficial zone protein, synthesized by cells lining synovial joints. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 1999;17(1):110–20. doi: 10.1002/jor.1100170117. [DOI] [PubMed] [Google Scholar]
- 6.Jay GD, Torres JR, Rhee DK, Helminen HJ, Hytinnen MM, Cha CJ, et al. Association between friction and wear in diarthrodial joints lacking lubricin. Arthritis and rheumatism. 2007;56(11):3662–9. doi: 10.1002/art.22974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drewniak EI, Jay GD, Fleming BC, Zhang L, Warman ML, Crisco JJ. Cyclic loading increases friction and changes cartilage surface integrity in lubricin-mutant mouse knees. Arthritis and rheumatism. 2012;64(2):465–73. doi: 10.1002/art.33337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Albuhairan I, Al-Mayouf SM. Camptodactyly-arthropathy-coxavara-pericarditis syndrome in Saudi Arabia: clinical and molecular genetic findings in 22 patients. Seminars in arthritis and rheumatism. 2013;43(2):292–6. doi: 10.1016/j.semarthrit.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Anderson DD, Chubinskaya S, Guilak F, Martin JA, Oegema TR, Olson SA, et al. Post-traumatic osteoarthritis: improved understanding and opportunities for early intervention. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2011;29(6):802–9. doi: 10.1002/jor.21359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friedel RH, Plump A, Lu X, Spilker K, Jolicoeur C, Wong K, et al. Gene targeting using a promoterless gene trap vector (“targeted trapping”) is an efficient method to mutate a large fraction of genes. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(37):13188–93. doi: 10.1073/pnas.0505474102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmitz N, Laverty S, Kraus VB, Aigner T. Basic methods in histopathology of joint tissues. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2010;18 (Suppl 3):S113–6. doi: 10.1016/j.joca.2010.05.026. [DOI] [PubMed] [Google Scholar]
- 12.Ayturk UM, Jacobsen CM, Christodoulou DC, Gorham J, Seidman JG, Seidman CE, et al. An RNA-seq protocol to identify mRNA expression changes in mouse diaphyseal bone: applications in mice with bone property altering Lrp5 mutations. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2013;28(10):2081–93. doi: 10.1002/jbmr.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kozhemyakina E, Zhang M, Ionescu A, Ayturk UM, Ono N, Kobayashi A, et al. Identification of a Prg4-positive articular cartilage progenitor cell population. Arthritis & rheumatology. 2015 doi: 10.1002/art.39030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drewniak EI, Jay GD, Fleming BC, Crisco JJ. Comparison of two methods for calculating the frictional properties of articular cartilage using a simple pendulum and intact mouse knee joints. Journal of biomechanics. 2009;42(12):1996–9. doi: 10.1016/j.jbiomech.2009.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Waller KA, Zhang LX, Elsaid KA, Fleming BC, Warman ML, Jay GD. Role of lubricin and boundary lubrication in the prevention of chondrocyte apoptosis. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(15):5852–7. doi: 10.1073/pnas.1219289110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glasson SS, Chambers MG, Van Den Berg WB, Little CB. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2010;18 (Suppl 3):S17–23. doi: 10.1016/j.joca.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 17.Coles JM, Zhang L, Blum JJ, Warman ML, Jay GD, Guilak F, et al. Loss of cartilage structure, stiffness, and frictional properties in mice lacking PRG4. Arthritis and rheumatism. 2010;62(6):1666–74. doi: 10.1002/art.27436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cook JL, Kuroki K, Visco D, Pelletier JP, Schulz L, Lafeber FP. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the dog. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2010;18 (Suppl 3):S66–79. doi: 10.1016/j.joca.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 19.Kinross KM, Montgomery KG, Kleinschmidt M, Waring P, Ivetac I, Tikoo A, et al. An activating Pik3ca mutation coupled with Pten loss is sufficient to initiate ovarian tumorigenesis in mice. The Journal of clinical investigation. 2012;122(2):553–7. doi: 10.1172/JCI59309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hindson BJ, Ness KD, Masquelier DA, Belgrader P, Heredia NJ, Makarewicz AJ, et al. High-throughput droplet digital PCR system for absolute quantitation of DNA copy number. Analytical chemistry. 2011;83(22):8604–10. doi: 10.1021/ac202028g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ripoche D, Gout J, Pommier RM, Jaafar R, Zhang CX, Bartholin L, et al. Generation of a conditional mouse model to target Acvr1b disruption in adult tissues. Genesis. 2013;51(2):120–7. doi: 10.1002/dvg.22352. [DOI] [PubMed] [Google Scholar]
- 22.Neogi T, Zhang Y. Epidemiology of osteoarthritis. Rheumatic diseases clinics of North America. 2013;39(1):1–19. doi: 10.1016/j.rdc.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hochberg MC, Yerges-Armstrong L, Yau M, Mitchell BD. Genetic epidemiology of osteoarthritis: recent developments and future directions. Current opinion in rheumatology. 2013;25(2):192–7. doi: 10.1097/BOR.0b013e32835cfb8e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Louboutin H, Debarge R, Richou J, Selmi TA, Donell ST, Neyret P, et al. Osteoarthritis in patients with anterior cruciate ligament rupture: a review of risk factors. The Knee. 2009;16(4):239–44. doi: 10.1016/j.knee.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 25.Brown TD, Johnston RC, Saltzman CL, Marsh JL, Buckwalter JA. Posttraumatic osteoarthritis: a first estimate of incidence, prevalence, and burden of disease. Journal of orthopaedic trauma. 2006;20(10):739–44. doi: 10.1097/01.bot.0000246468.80635.ef. [DOI] [PubMed] [Google Scholar]
- 26.Elsaid KA, Fleming BC, Oksendahl HL, Machan JT, Fadale PD, Hulstyn MJ, et al. Decreased lubricin concentrations and markers of joint inflammation in the synovial fluid of patients with anterior cruciate ligament injury. Arthritis and rheumatism. 2008;58(6):1707–15. doi: 10.1002/art.23495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruan MZ, Erez A, Guse K, Dawson B, Bertin T, Chen Y, et al. Proteoglycan 4 expression protects against the development of osteoarthritis. Science translational medicine. 2013;5(176):176ra34. doi: 10.1126/scitranslmed.3005409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buckley MR, Bergou AJ, Fouchard J, Bonassar LJ, Cohen I. High-resolution spatial mapping of shear properties in cartilage. Journal of biomechanics. 2010;43(4):796–800. doi: 10.1016/j.jbiomech.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buckley MR, Gleghorn JP, Bonassar LJ, Cohen I. Mapping the depth dependence of shear properties in articular cartilage. Journal of biomechanics. 2008;41(11):2430–7. doi: 10.1016/j.jbiomech.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 30.Wong BL, Bae WC, Gratz KR, Sah RL. Shear deformation kinematics during cartilage articulation: effect of lubrication, degeneration, and stress relaxation. Molecular & cellular biomechanics: MCB. 2008;5(3):197–206. [PMC free article] [PubMed] [Google Scholar]
- 31.Hughes LC, Archer CW, ap Gwynn I. The ultrastructure of mouse articular cartilage: collagen orientation and implications for tissue functionality. A polarised light and scanning electron microscope study and review. European cells & materials. 2005;9:68–84. doi: 10.22203/ecm.v009a09. [DOI] [PubMed] [Google Scholar]
- 32.Hashimoto S, Nishiyama T, Hayashi S, Fujishiro T, Takebe K, Kanzaki N, et al. Role of p53 in human chondrocyte apoptosis in response to shear strain. Arthritis and rheumatism. 2009;60(8):2340–9. doi: 10.1002/art.24706. [DOI] [PubMed] [Google Scholar]
- 33.Healy ZR, Lee NH, Gao X, Goldring MB, Talalay P, Kensler TW, et al. Divergent responses of chondrocytes and endothelial cells to shear stress: cross-talk among COX-2, the phase 2 response, and apoptosis. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(39):14010–5. doi: 10.1073/pnas.0506620102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee MS, Sun MT, Pang ST, Ueng SW, Chen SC, Hwang TL, et al. Evaluation of differentially expressed genes by shear stress in human osteoarthritic chondrocytes in vitro. Chang Gung medical journal. 2009;32(1):42–50. [PubMed] [Google Scholar]
- 35.Lee MS, Trindade MC, Ikenoue T, Goodman SB, Schurman DJ, Smith RL. Regulation of nitric oxide and bcl-2 expression by shear stress in human osteoarthritic chondrocytes in vitro. Journal of cellular biochemistry. 2003;90(1):80–6. doi: 10.1002/jcb.10611. [DOI] [PubMed] [Google Scholar]
- 36.Lee MS, Trindade MC, Ikenoue T, Schurman DJ, Goodman SB, Smith RL. Effects of shear stress on nitric oxide and matrix protein gene expression in human osteoarthritic chondrocytes in vitro. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2002;20(3):556–61. doi: 10.1016/S0736-0266(01)00149-8. [DOI] [PubMed] [Google Scholar]
- 37.Ding L, Heying E, Nicholson N, Stroud NJ, Homandberg GA, Buckwalter JA, et al. Mechanical impact induces cartilage degradation via mitogen activated protein kinases. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2010;18(11):1509–17. doi: 10.1016/j.joca.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu F, Wang P, Kontrogianni-Konstantopoulos A, Konstantopoulos K. Prostaglandin (PG)D(2) and 15-deoxy-Delta(12,14)-PGJ(2), but not PGE(2), mediate shear-induced chondrocyte apoptosis via protein kinase A-dependent regulation of polo-like kinases. Cell death and differentiation. 2010;17(8):1325–34. doi: 10.1038/cdd.2010.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu F, Wang P, Lee NH, Goldring MB, Konstantopoulos K. Prolonged application of high fluid shear to chondrocytes recapitulates gene expression profiles associated with osteoarthritis. PloS one. 2010;5(12):e15174. doi: 10.1371/journal.pone.0015174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Waller KA, Zhang LX, Fleming BC, Jay GD. Preventing friction-induced chondrocyte apoptosis: comparison of human synovial fluid and hylan G-F 20. The Journal of rheumatology. 2012;39(7):1473–80. doi: 10.3899/jrheum.111427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jay GD, Elsaid KA, Kelly KA, Anderson SC, Zhang L, Teeple E, et al. Prevention of cartilage degeneration and gait asymmetry by lubricin tribosupplementation in the rat following anterior cruciate ligament transection. Arthritis and rheumatism. 2012;64(4):1162–71. doi: 10.1002/art.33461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jay GD, Fleming BC, Watkins BA, McHugh KA, Anderson SC, Zhang LX, et al. Prevention of cartilage degeneration and restoration of chondroprotection by lubricin tribosupplementation in the rat following anterior cruciate ligament transection. Arthritis and rheumatism. 2010;62(8):2382–91. doi: 10.1002/art.27550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Teeple E, Elsaid KA, Jay GD, Zhang L, Badger GJ, Akelman M, et al. Effects of supplemental intra-articular lubricin and hyaluronic acid on the progression of posttraumatic arthritis in the anterior cruciate ligament-deficient rat knee. The American journal of sports medicine. 2011;39(1):164–72. doi: 10.1177/0363546510378088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Flannery CR, Zollner R, Corcoran C, Jones AR, Root A, Rivera-Bermudez MA, et al. Prevention of cartilage degeneration in a rat model of osteoarthritis by intraarticular treatment with recombinant lubricin. Arthritis and rheumatism. 2009;60(3):840–7. doi: 10.1002/art.24304. [DOI] [PubMed] [Google Scholar]
- 45.Elsaid KA, Zhang L, Waller K, Tofte J, Teeple E, Fleming BC, et al. The impact of forced joint exercise on lubricin biosynthesis from articular cartilage following ACL transection and intra-articular lubricin’s effect in exercised joints following ACL transection. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2012;20(8):940–8. doi: 10.1016/j.joca.2012.04.021. [DOI] [PubMed] [Google Scholar]
- 46.Ai M, Cui Y, Sy MS, Lee DM, Zhang LX, Larson KM, et al. Anti-lubricin monoclonal antibodies created using lubricin-knockout mice immunodetect lubricin in several species and in patients with healthy and diseased joints. PloS one. 2015;10(2):e0116237. doi: 10.1371/journal.pone.0116237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rai MF, Hashimoto S, Johnson EE, Janiszak KL, Fitzgerald J, Heber-Katz E, et al. Heritability of articular cartilage regeneration and its association with ear wound healing in mice. Arthritis and rheumatism. 2012;64(7):2300–10. doi: 10.1002/art.34396. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Screen shot from the Integrated Genomics Viewer of RNA sequencing data obtained from 2 Prg4GT/GT and 2 Prg4+/+ mice that map to exons 7 to 11. Histograms depicting read depths (up to 4000 reads) for the RNA sequencing data are shown (horizontal arrows). Read depth averages ~ 2000X for the +/+ mice and < 10 for the GT/GT mice. Black and red arrowheads, indicate examples of 50-basepair RNA sequencing reads mapping to exons and introns, respectively. Vertical arrows point to examples of reads spanning exons, consistent with mRNA splicing. Very few examples of exon spanning reads are found in the GT/GT data. (B) Histogram depicting abundance of Prg4 wild-type (+), gene-trap (GT), and gene-trap excised (GTR) transcripts in 2 – 3 week old mouse joints determined by RNA sequencing, as well as Prg4 abundance in a mouse whose gene-trap had been excised at 6 months of age (GTR6m). Prg4 transcript abundance is normalized to aggrecan (Acan), type II collagen (Col2a1), and type IX collagen alpha 1 chain (Col9a1) abundance and set at 100% for the wild-type Prg4 allele. The GT allele produces less than 0.1% of wild-type transcript whereas the GTR allele produces between 80 and 110% of wild-type transcript. Extracted mRNA from the joints of 6-month-old GTR6m mice contains only 4 – 8% of the Prg4 transcript found in 3-week-old wild-type and GTR mice. A likely explanation for this observation is that most superficial zone chondrocytes have already died in mice that lacked lubricing till 6 months of age. N= the number of joints from which independent RNA sequencing libraries were generated; libraries were made from 2 animals with each Prg4 allele. (C) Scatterplots depicting ddPCR results for Cre-mediated recombination of a conditional allele (Pik3ca) in cartilage, tail, and cerebral cortex from a 4-month-old mouse that had just been given 8 consecutive days of tamoxifen. DNA from a mouse with the conditional allele and no ROSA26CreERt2 allele serves as the control. The X-axis indicates fluorescence intensity in droplets for the probe targeting the non-recombined amplimer and the Y-axis indicates fluorescence intensity in droplets for the probe targeting the Cre-recombined amplimer. Individual droplets that contain no amplimer are pseudocolored black, non-recombined amplimer orange, Cre-recombined amplimer blue. The percentages amplimer-containing droplets that have Cre-recombined alleles are indicated beneath each tissue studied. Note Cre-mediated recombination has occurred in > 98% of the articular cartilage chondrocytes. The bar graph indicates the numbers of cells from each tissue for which DNA was analyzed to determine the Cre-mediated recombination rate.