Abstract
A recent meta-analysis documented a significant statistical association between mild traumatic brain injury (mTBI) and attention deficit hyperactivity disorder (ADHD) (Adeyemo et al., 2014), but the direction of this effect was unclear. In this study, we hypothesized that ADHD would be an antecedent risk factor for mTBI. Participants were student athletes ages 12–25 who had sustained a mTBI and Controls of similar age and sex selected from studies of youth with and without ADHD. Subjects were assessed for symptoms of ADHD, concussion severity, and cognitive function. mTBI subjects had a significantly higher rate of ADHD than Controls, and in all cases the age of onset of ADHD was before mTBI onset. mTBI+ADHD subjects also had more severe concussion symptoms (fatigue and poor concentration) than mTBI-ADHD subjects. These results support ADHD as an antecedent risk factor for mTBI in student athletes and that its presence complicates the course of mTBI.
Keywords: traumatic brain injury, mild, attention deficit hyperactivity disorder, student athlete
Introduction
Traumatic brain injury (TBI) represents a diverse group of brain injuries that vary in cause, severity, and clinical outcomes affecting individuals from all socio-demographic strata including athletes and military personnel (Corps et al., 2015). Among TBIs, mild TBI (mTBI) is the most frequent type in the United States, representing 70–90% of traumatic brain injury complications (Langlois et al., 2006; Ruff, 2011). The Centers for Disease Control and Prevention estimates 1.5 million new cases of mTBI each year and described it in a 2003 report to the U.S. Congress as a silent epidemic (National Center for Injury Prevention and Control, 2003; Ruff, 2011).
In recent years, sport-related concussions in general and mTBI in particular have become an increasing public and legislative concern (Giza et al., 2013; Harmon et al., 2013). The increased focus on the morbidity of TBI in injured athletes has catalyzed public interest on the subject (Guskiewicz et al., 2005; Guskiewicz et al., 2007; Omalu et al., 2006; Omalu et al., 2005; Ruff, 2011). Given its increasing prevalence and potential for pervasive, deleterious sequelae (Corps et al., 2015), efforts to identify factors that put individuals at greater risk to develop an mTBI or complicate its course are of high clinical, scientific, and public health significance.
One such potential risk factor for mTBI is Attention-deficit/hyperactivity disorder (ADHD), a neurodevelopmental, childhood onset and persistent disorder associated with impulsivity and risk taking behavior. ADHD has a well-documented risk for accidents and injuries (Barkley et al., 1993; Barkley et al., 2010; Biederman et al., 2005; Lambert, 1995) that could include head injuries such as concussions and TBI.
To investigate the association between mTBI and ADHD, we recently conducted a systematic review and meta-analysis of studies that examined this relationship (Adeyemo et al., 2014). PubMed was searched for original studies that specifically evaluated the relationship between ADHD and mTBI. Our search identified five studies that fit our a priori inclusion and exclusion criteria comprising 3023 mTBI patients and 9,716 controls. Although the meta-analysis found a significant association between ADHD and mTBI, the majority of the available studies did specify the direction of effect between these disorders. This state of affairs calls for additional efforts aimed at further clarifications of the relationship and the directionality of effect between mTBI and ADHD.
An improved understanding of the nature of the association between ADHD and mTBI has important clinical, scientific and public health relevance. If ADHD is found to be an antecedent risk factor for mTBI, that would have important implications for identifying individuals at greater risk to develop mTBI or factors that could complicate its course. Such insights could lead to the development of early intervention strategies aimed at mitigating the development of mTBI in individuals at risk. If ADHD is found to complicate the course of mTBI, clarifying the relationship between mTBI and ADHD would promote a more optimal paradigm of care for patients with mTBI. Considering the high prevalence of both ADHD and mTBI, a further understanding of the nature of their association could have important public health relevance and inform future research and clinical decisions for patients with mTBI.
The main aim of the present study was to re-examine the association between ADHD and mTBI, attending to the shortcomings of the literature. To this end we assessed ADHD and mTBI in a sample of student athletes and compared them with an historical sample of subjects with and without ADHD matched for age and sex. Our research questions and associated hypotheses were as follows: 1) is ADHD an antecedent risk factor for mTBI? If this is the case, we would expect that pre-existing ADHD would be over-represented among athletes with mTBI relative to age and sex matched athletes without it. 2) Does ADHD complicate the course of mTBI? If this is the case, we would expect that individuals with ADHD would have a more compromised course of mTBI than other individuals sustaining a similar injury. 3) Does ADHD develop after mTBI (acquired or secondary ADHD)? If this is the case, we would expect that mTBIs would increase the risk for acquired ADHD relative to athletes without a history of concussions.
Methods
Participants
Mild TBI (mTBI) participants were male and female student athletes 12–25 years of age who had sustained a mTBI in the last 10 years (n = 29). A mTBI was defined as a traumatically induced physiological disruption of brain function as manifested by at least one of the following: any period of loss of consciousness; any loss of memory for events immediately before or after the accident; or any alteration in mental state at the time of the accident; and focal neurological deficits that may or may not be transient, but where the severity of the injury did not exceed loss of consciousness of approximately 30 minutes or less, or post-traumatic amnesia greater than 24 hours (Kay et al., 1993).
In order to limit confounding symptomatology, we excluded subjects with post-concussive neurological sequelae such as seizures or severe and frequent headaches within the past month. We also excluded subjects with a prior psychiatric disorder requiring hospitalization, a diagnosis of autism, psychosis, or bipolar disorder, or a lifetime diagnosis of epilepsy. Also excluded were subjects that underwent neurosurgery, suffered from any current serious chronic medical disease or major neurological disease, or had a history of significant alcohol or drug abuse. The study was approved by the Massachusetts General Hospital institutional review board and all subjects signed a written consent form. For minors, consent was signed by the parent or guardian and assent was obtained from the participating youth.
Eligible and consenting mTBI subjects completed a two-hour assessment battery to collect information regarding their medical and psychiatric history, including details about their head injury and ADHD symptoms. Participants <18 years of age were accompanied by a parent/guardian. When possible, parents of participants 18–24 years of age were contacted for an indirect report.
As comparisons, we used previously collected data from youth with and without ADHD of both sexes and their first-degree relatives ascertained from psychiatric and pediatric settings (Biederman et al., 1996; Biederman et al., 1999; Biederman et al., 2006a; Biederman et al., 2006b). Because ADHD was exclusionary for participating controls in our studies, we used as non-TBI comparisons the siblings of non-ADHD control probands that had no such exclusionary criteria. From this pool of available subjects, we selected an ADHD and Control group of similar age and sex to the mTBI cases on a 2:1 ratio. In this manner, two matches were randomly selected for each study subject. Complete methodological details for this sample have been previously reported (Biederman et al., 1996; Biederman et al., 1999; Biederman et al., 2006a; Biederman et al., 2006b). All protocols have been approved by the Massachusetts General Hospital Institutional Review Board and subjects signed a written consent for participation. For minors, consent was obtained from a parent or guardian and children signed a simplified, age-appropriate assent form.
Procedures for the assessment of ADHD were similar between the studies. They consisted of completing the ADHD module from the Kiddie Schedule for Affective Disorders and Schizophrenia Epidemiological version (K-SADS-E) (Orvaschel, 1994; Orvaschel et al., 1987) with the participants and independent interviews with the parent or guardian for minors. Whenever possible, indirect interviews were obtained from older subjects as well. When both direct and indirect interviews were obtained, data were combined by considering a diagnostic criterion positive if it was endorsed in either interview, consistent with our methods in previous studies (Biederman et al., 1999). We considered a full diagnosis of ADHD present if DSM diagnostic criteria were met (DSM-IV (American Psychiatric Association, 2000) for TBI subjects; DSM-III-R (American Psychiatric Association, 1987) for ADHD comparisons from previous studies); both diagnostic formulations are highly correlated (Lahey et al., 1994). Two mTBI subjects with ages of onset of 8 and 11 years, respectively, were given full ADHD diagnoses despite their late age of onset because their ages of onset were in grade school years and these ages are consistent with the DSM-V (American Psychiatric Association, 2013) age criteria of 12 years. The structured interview also provided information as to the age of onset of ADHD symptoms and associated severity Highly trained interviewers supervised by expert board certified child and adolescent psychiatrists administered the structured interviews. All interviews were audiotaped for quality control purposes.
Subjects also completed the Behavior Rating Inventory of Executive Function (BRIEF) self and parent report (participants ages <18 years) (Gioia et al., 2000) and the BRIEF-Adult self and informant report (for participants ≥18 years) (Roth et al., 2005). These are well-standardized instruments with excellent psychometric properties that assess behaviors associated with executive function deficits. The BRIEF and BRIEF-A include several subscales: Inhibition, Shifting, Emotional Control, Self-Monitoring, Initiation, Working Memory, Planning/Organizing, Task Monitoring, and Organization of Materials.
Full Scale IQ was estimated in the previous ADHD studies using a brief cognitive screen consisting of the Block Design and Vocabulary subtests of the WISC-R (Wechsler, 1974). In the mTBI study, estimates of IQ were based on the Vocabulary and Matrices subtests of the Wechsler Abbreviated Scale of Intelligence (Wechsler, 2011). Both measures are highly correlated.
TBI Specific Assessment Measures
British Columbia Post-Concussion Symptom Inventory
Questions from this scale were adapted for the purpose of this study to assess post-concussive symptoms and their duration. Specific questions included mechanism of impact, details of the injury, and duration and severity of post-concussive symptoms. Severity of each symptom was rated from 0 (Did not have this symptom at all) to 5 (Very severe problem). Subjects were asked to report the details of up to three concussions, and the highest average severity was used for each symptom (Iverson et al., 2006).
Immediate Post-concussion Assessment and Cognitive Testing (ImPACT) was used to assess post-concussive cognitive function. This is a 20-minute computerized neuropsychological battery (Lovell et al., 2000). The test is comprised of six modules that measure attention, memory, reaction time, and processing speed. Each module contributes to the calculation of four composite scores: Verbal Memory, Visual Memory, Reaction Time, and Processing Speed. Subjects participating remotely did not complete this assessment.
Statistical Analysis
For continuous variables, a Welch two-sample t-test was used to compare groups. For binary outcomes, a chi-squared test was carried out unless any cell counts were five or below, in which case Fisher’s Exact Test was used. A significance level of 0.05 was used throughout the analysis. Analysis was carried out using the R statistical programming language (R Core Team, 2014).
Results
Twenty-nine mTBI subjects were recruited for the study and compared with 80 comparators from our studies of youth with and without ADHD and their first-degree relatives. Subjects with mTBI were stratified by the presence or absence of ADHD and comparisons were made between mTBI subjects with ADHD (mTBI+ADHD; N=11), mTBI subjects without ADHD (mTBI-ADHD; N=18), ADHD comparators (ADHD; N=22) and control comparators (Controls; N=58). With the exception of ethnicity, there were no significant differences between mTBI subjects and Controls in age, family intactness, social class, or sex distribution characteristics (table 1). The mTBI subjects had a somewhat lower representation of Caucasian subjects (83% for mTBI vs 96% for Controls, p =0.09).
Table 1.
External Controls
| Control (N = 58) | TBI (N = 29) | Statistic | p | |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | |||
| Age | 17 (± 2.7) | 17.1 (± 2.9) | t = −0.2 | p = 0.8 |
|
| ||||
| SES | 1.7 (± 0.8) | 1.5 (± 0.5) | t = 0.9 | p = 0.4 |
|
| ||||
| Full IQ | 110.2 (± 13) | 112.4 (± 12.4) | t = −0.7 | p = 0.5 |
| N (%) | N (%) | |||
|
| ||||
| % Male | 30 (51.7%) | 15 (51.7%) | χ21 = 0.0 | p = 1 |
|
| ||||
| % Intact | 49 (84.5%) | 22 (75.9%) | χ21 = 0.5 | p = 0.5 |
|
| ||||
| % Caucasian | 51 (96.2%) | 24 (82.8%) | Fisher | p = 0.09 |
Due to missing data, the numbers of subjects available for analysis ranged from 21–29 (TBI) and 46–58 (Controls)
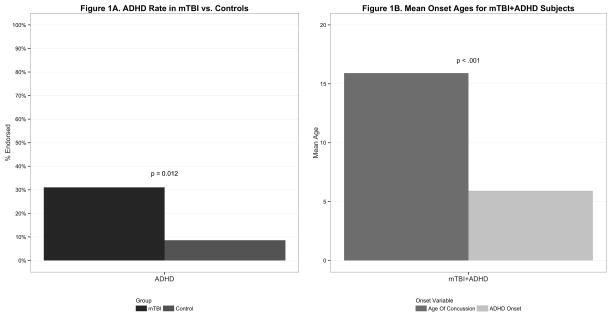
As shown in Figure 1, mTBI subjects had a significantly higher rate of ADHD than Controls without mTBI (31% vs 9%; p<0.012) (Figure 1 Panel A). The age of onset of ADHD was significantly earlier than the age of onset of mTBI (6 vs 16, p < 0.001) (Figure 1 Panel B) and in all cases, the age of onset of ADHD preceded the age of onset of mTBI. Only one mTBI subject had subsyndromal ADHD following mTBI.
Figure 1.
Figure 1A compares the percent of ADHD symptoms endorsed on the KSADS ADHD module by subjects with mTBI compared to Controls without mTBI.
Figure 1B compares the mean age of the first concussion to that of the mean onset of ADHD symptoms in subjects with mTBI+ADHD.
Clinical Correlates of ADHD in mTBI+ADHD and ADHD Subjects
As shown in Table 2, mTBI+ADHD subjects had similar ADHD characteristics to the ADHD comparison group.
Table 2.
Clinical Correlates of ADHD in mTBI+ADHD and ADHD Subjects
| mTBI+ADHD (N = 11) | ADHD (N = 22) | Statistic | p | |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | |||
| Total ADHD Symptoms | 12.6 (± 2.9) | 12.7 (± 4.3) | t = −0.0 | p = 1 |
|
| ||||
| Total Hyperactivity/Impulsive ADHD Symptoms | 6.9 (± 2.3) | 7.3 (± 2) | t = −0.5 | p = 0.7 |
|
| ||||
| Total Inattentive ADHD Symptoms | 5.7 (± 1.4) | 5.4 (± 3) | t = 0.4 | p = 0.7 |
|
| ||||
| ADHD Onset | 5.9 (± 2.9) | 11.6 (± 5.7) | t = −3.7 | p < .001 |
|
| ||||
| N (%) | N (%) | |||
| ADHD Impairment - % at least moderate | 7 (70%)† | 20 (95.2%) | Fisher | p = 0.09 |
|
| ||||
| ADHD Impairment - % Severe | 4 (40%)† | 6 (28.6%) | Fisher | p = 0.7 |
ADHD impairment data is missing for 1 mTBI subject
Clinical Correlates of mTBI in mTBI+ADHD and mTBI-ADHD
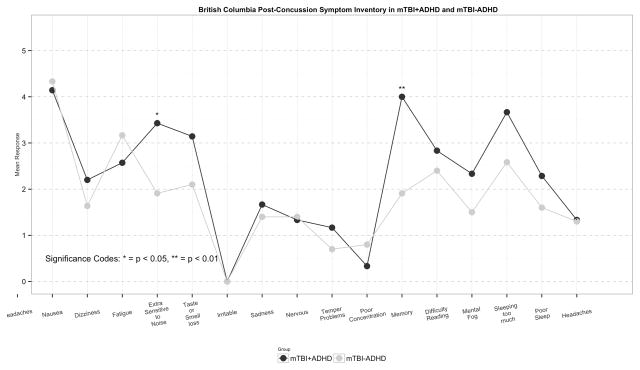
The mTBI+ADHD group had a greater percentage of subjects with >1 head injury than the mTBI-ADHD group (57.1 vs 30.8, p=0.4). Examination of individual items on the BC-PSI scale showed that out of the scale’s 16 items, Fatigue (3.4 vs 1.9, p=0.029) and Poor Concentration (4.0 vs 1.9, p=0.008) were significantly more severe in mTBI+ADHD compared with mTBI-ADHD subjects. Although not reaching statistical significance, Extra Sensitivity to Noise and Mental Fogginess were substantially more severe in mTBI+ADHD subjects (Figure 2).
Figure 2.
Figure 2 shows the mean severity of each symptom on the BC-PSI in subjects with mTBI with ADHD compared to mTBI subjects without ADHD. Severity of each symptom was rated from 0 (Did not have this symptom at all) to 5 (Very severe problem).
As shown in Table 3, mTBI+ADHD subjects had greater overall mean severity scores on the 16 items of the British Columbia Post-Concussion Symptom Inventory (BC-PSI) than mTBI-ADHD subjects (2.4 vs 1.8, p=0.1), had a larger percentage of subjects with >50% of the individual items scored (71.4 vs 30.8, p=0.2), and had a larger percentage of subjects with ≥ 5 items scored as severe (severity score ≥3) (100 vs 38.5, p=0.015). Only the latter comparison reached statistical significance. There were no meaningful differences in the ages of onset of mTBI between mTBI subjects with and without ADHD (16 vs 15, NS).
Table 3.
British Columbia Post-Concussive Symptom Inventory in mTBI+ADHD and mTBI-ADHD
| mTBI+ADHD (N = 11) | mTBI-ADHD (N = 18) | Statistic | p | |
|---|---|---|---|---|
| Average | 2.4 (± 0.8) | 1.8 (± 0.9) | t = 1.5 | p = 0.1 |
| Number of symptoms above 0 | 6.4 (± 5.6) | 4.8 (± 4.8) | t = 0.8 | p = 0.5 |
| More than 4 severe (sev > 2) | 7 (100%) | 5 (38.5%) | Fisher | p = 0.015** |
| More than half registered (sev > 0) | 5 (71.4%) | 4 (30.8%) | Fisher | p = 0.2 |
Due to missing data, the numbers of subjects available for analysis ranged from 7–11 (TBI+ADHD), and 12–18 (TBI-ADHD)
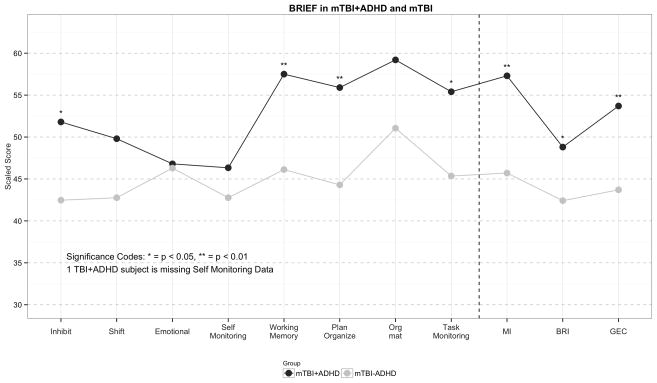
Although 3 of the 4 ImPACT items (Verbal Memory Composite, Visual Motor Speed Composite, and Reaction Time Composite) were substantially worse in mTBI+ADHD subjects than mTBI-ADHD subjects, these differences failed to attain statistical significance (Table 4). In contrast, compared with mTBI-ADHD participants, those with mTBI+ADHD were significantly more impaired on individual BRIEF subscale scores for Inhibit (51.8 vs 42.5, p=0.015), Working Memory (57.5 vs 46.1, p=0.001), and Planning/Organization (55.9 vs 44.3, p=0.009). We found the same pattern of results for the BRIEF composite scores: Metacognition Index (57.3 vs 45.7, p=0.006), Behavioral Regulation Index (48.8 vs 42.4, p=0.044) and Global Executive Composite (53.7 vs 43.7, p=0.006) (Figure 3).
Table 4.
ImPACT in mTBI+ADHD and mTBI-ADHD
| TBI+ADHD (N = 7) | TBI-ADHD (N = 13) | Statistic | p | |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | |||
| Visual Memory Composite (%ile) | 72.3 (± 30.4) | 72.1 (± 26.3) | t = 0.0 | p = 1 |
|
| ||||
| Visual Motor Speed Composite (%ile) | 62.9 (± 27) | 54.3 (± 30.2) | t = 0.6 | p = 0.5 |
|
| ||||
| Reaction Time Composite (%ile) | 60.4 (± 30.6) | 44.8 (± 33) | t = 1.1 | p = 0.3 |
|
| ||||
| Impulse Control Composite | 7.4 (± 4.5) | 6 (± 2.9) | t = 0.8 | p = 0.5 |
|
| ||||
| Total Symptom Score | 7 (± 5.5) | 9.6 (± 13.7) | t = −0.6 | p = 0.6 |
|
| ||||
| Cognitive Efficiency Index | 0.4 (± 0.1) | 0.4 (± 0.2) | t = 1.1 | p = 0.3 |
Figure 3.
Figure 3 compares the average scaled score for each BRIEF subscale score and each BRIEF composite score in subjects with mTBI and ADHD compared to mTBI subjects without ADHD. Higher scores suggest a higher level of executive dysfunction in the specific domain. The dashed line separates the subscale scores from the composite scores.
Discussion
Our results showed a significant overrepresentation of ADHD among student athletes who sustained a mTBI, confirming previous findings from a recent meta-analysis that provided strong evidence for such an association (Adeyemo et al., 2014). Our results showing a significantly earlier age of onset of ADHD compared to the age of the mTBI in patients with both disorders extend the findings from the meta-analysis by suggesting that ADHD may be an antecedent risk factor for mTBI. Notably, the presence of ADHD heralded a more protracted course of mTBI in student athletes in terms of severity of symptoms. These results support the study hypothesis that ADHD is an antecedent risk factor for mTBI in student athletes and that its presence complicates the course of mTBI.
While our meta-analysis provided strong evidence for an overall statistical association between ADHD and mTBI that could not be explained by publication biases or the effects of one single study, they were frustrated by the fact that most available studies did not specify which came first. Thus, by obtaining detailed chronological information regarding ages of onset of ADHD and mTBI, our current study could better address the directionality of effects.
Although our current results suggest that ADHD is an antecedent risk factor for mTBI, these findings are inconsistent with those of the sub-analyses of two previous studies that examined ADHD as a predictor of mTBI. One of the two studies had a very small sample (N=24 mTBI cases & N=24 controls) and used orthopedic controls as comparators (Max et al., 2004). Given that ADHD youth are at increased risk for injuries, orthopedic controls might be not be a good comparison group. In support of this idea, the prevalence of ADHD in these controls was 17%, much higher than the population rate of ADHD (Faraone et al., 2003). The second study suggesting that ADHD is not a risk factor for mTBI was large and used healthy controls (Fann et al., 2004), but its method of determining ADHD diagnoses prior to mTBI may have been insensitive because the rate of ADHD in their control group was only 0.7%, a much lower rate than that expected in the general population.
Also inconsistent with findings from the meta-analysis is the failure to support the hypothesis that ADHD is a complication of mTBI. In the current study, only one mTBI subject had an age of onset of ADHD symptoms after mTBI. This negative finding stands in contrast with those of the two previous studies included in the meta-analysis that yielded positive relative risks implicating mTBI as a risk factor for ADHD. One of these studies is the small Max et al. study using orthopedic controls (Max et al., 2004) and the second study (Keenan et al., 2008) used burn injury subjects as controls, a group that can also be expected to include accident-prone individuals.
The use of orthopedic and burns controls is of note, because whereas ADHD would be expected to cause accidents leading to orthopedic injuries, such accidents would not be expected to lead to ADHD. On the other hand, our results suggesting that ADHD is an antecedent risk factor for mTBI are consistent with an extensive literature linking ADHD with a wide range of accidents and injuries (Bonfield et al., 2013; Lam, 2002; Pastor et al., 2006; Swensen et al., 2004). More work is clearly needed to further examine the direction of effect between ADHD and mTBI. Future studies should also examine whether mTBI hastens the onset of ADHD in a genetically vulnerable individual and whether mTBI alters the course of ADHD beyond the acute recovery period.
While data from the meta-analysis was inconclusive as to whether individuals with ADHD have a different injury recovery trajectory, results from our study suggest that mTBI subjects with ADHD displayed a greater incidence and severity of mTBI symptoms when compared with mTBI subjects without ADHD. These findings correlate with current evidence that young adults and adolescents with ADHD who were admitted to a hospital following mTBI had worse functional outcomes than those who did not have ADHD (Bonfield et al., 2013). They are also consistent with findings reported by (Gerring et al.) that lesions in the thalamus and basal ganglia were greater in TBI subjects with ADHD symptoms.
Considering the large clinical and public health relevance of mTBI and given its high and increasing prevalence and significant consequences, further clarification of the relationship between mTBI and ADHD could have large implications The finding that ADHD increases the risk for TBI implies that its identification and treatment could mitigate the development of mTBI. Such knowledge may play an important role in providing a prognostic marker for severity and duration of mTBI course. Though cognitive dysfunction from traumatic brain injuries is frequently managed with stimulant medications, the American Medical Society for Sports Medicine’s position on Concussion finds no established role for stimulant medications in the treatment of cognitive deficits after a concussion (Harmon et al., 2013). ADHD, in contrast, is a treatable disorder that responds well to stimulant medications. Thus, if patients with traumatic brain injury meet diagnostic criteria for ADHD, interventions for ADHD could be considered.
The current study had a number of strengths. All mTBI’s in this study were a result of sports-related accidents, allowing us to more accurately attribute the injuries to risk-taking behaviors than to chance. The inclusion of an ADHD comparison group allowed for the evaluation of ADHD-associated risk factor in mTBI subjects. However, our findings should also be considered in light of methodological limitations. The relatively small sample size limited our statistical power to fully assess the relationship between mTBI and ADHD. Thus, our results should be considered preliminary until replicated in larger studies. Also, the comparators taken from our previous studies were not specifically assessed for mTBI. Future studies should compare mTBI participants to Controls that are specifically assessed for mTBI.
In addition, the comparators taken from previous studies did not complete the BRIEF or ImPACT, so we were unable to compare cognitive function of subjects with mTBI to that of subjects without mTBI. Although the mTBI sample had a larger representation of minorities than the comparator sample, the outcomes evaluated were not moderated by ethnicity. Finally, the sample was referred and largely Caucasian limiting its generalizability to community samples and other ethnic groups.
Conclusion
Despite these considerations, results from this study suggest that ADHD increases the risk of subsequent mTBI and compromises its course. They also suggest that this risk could be mediated by ADHD’s known risk for increasing accidents (Antshel et al., 2009; Biederman et al., 2006c). Further investigation of the relationship between mTBI and ADHD, and the direction of effect, is warranted.
Acknowledgments
This work was supported by NIH grants (J.B., grant numbers R01MH050657, R01HD036317); and by the Pediatric Psychopharmacology Research Council Fund. We would like to thank Elana Kagan for her contributions to the early stages of coordinating this study.
Sources of Funding
Dr. Joseph Biederman is currently receiving research support from the following sources: The Department of Defense, Food & Drug Administration, Ironshore, Lundbeck, Magceutics Inc., Merck, PamLab, Pfizer, Shire Pharmaceuticals Inc., SPRITES, Sunovion, Vaya Pharma/Enzymotec, and NIH. In 2015, Dr. Joseph Biederman has a US Patent Application pending (Provisional Number #61/233,686) through MGH corporate licensing, on a method to prevent stimulant abuse. In 2014, Dr. Joseph Biederman received honoraria from the MGH Psychiatry Academy for tuition-funded CME courses. He received research support from AACAP, Alcobra, Forest Research Institute, and Shire Pharmaceuticals Inc. Dr. Biederman received departmental royalties from a copyrighted rating scale used for ADHD diagnoses, paid by Ingenix, Prophase, Shire, Bracket Global, Sunovion, and Theravance; these royalties were paid to the Department of Psychiatry at MGH. In 2013, Dr. Joseph Biederman received an honorarium from the MGH Psychiatry Academy for a tuition-funded CME course. He received research support from APSARD, ElMindA, McNeil, and Shire. Dr. Biederman received departmental royalties from a copyrighted rating scale used for ADHD diagnoses, paid by Shire and Sunovion; these royalties were paid to the Department of Psychiatry at MGH. In 2012, Dr. Joseph Biederman received an honorarium from the MGH Psychiatry Academy and The Children’s Hospital of Southwest Florida/Lee Memorial Health System for tuition-funded CME courses. In previous years, Dr. Joseph Biederman received research support, consultation fees, or speaker’s fees for/from the following additional sources: Abbott, Alza, AstraZeneca, Boston University, Bristol Myers Squibb, Cambridge University Press, Celltech, Cephalon, Cipher Pharmaceuticals Inc., Eli Lilly and Co., Esai, Fundacion Areces (Spain), Forest, Fundación Dr. Manuel Camelo A.C., Glaxo, Gliatech, Hastings Center, Janssen, Juste Pharmaceutical Spain, McNeil, Medice Pharmaceuticals (Germany), Merck, MGH Psychiatry Academy, MMC Pediatric, NARSAD, NIDA, New River, NICHD, NIMH, Novartis, Noven, Neurosearch, Organon, Otsuka, Pfizer, Pharmacia, Phase V Communications, Physicians Academy, The Prechter Foundation, Quantia Communications, Reed Exhibitions, Shionogi Pharma Inc, Shire, the Spanish Child Psychiatry Association, The Stanley Foundation, UCB Pharma Inc., Veritas, and Wyeth.
Dr. Thomas Spencer has received research support from, has been a speaker for or on a speaker bureau or has been an Advisor of or on an Advisory Board of the following sources: Alcobra, Cephalon, Eli Lilly & Company, Glaxo-Smith Kline, Heptares, Impax, Ironshore, Janssen Pharmaceutical, Lundbeck, McNeil Pharmaceutical, Novartis Pharmaceuticals, Pfizer, Shire Laboratories, Inc, Sunovion, VayaPharma, the FDA, the National Institute of Mental Health and the Department of Defense. Dr. Spencer receives research support from Royalties and Licensing fees on copyrighted ADHD scales through MGH Corporate Sponsored Research and Licensing. Dr. Spencer has a US Patent Application pending (Provisional Number 61/233,686), through MGH corporate licensing, on a method to prevent stimulant abuse.
Dr. McGrath is a consultant for Immediate Post-Concussion Assessment and Cognitive Testing (ImPACT) applications.
Dr. Zafonte receives funding from the Department of Defense, the NIH, and the NIDRR. He receives publication royalties from Elsevier, Oakstone, and Demos.
In the past year, Dr. Faraone received income, travel expenses and/or research support from and/or has been on an Advisory Board for Pfizer, Ironshore, Shire, Akili Interactive Labs, CogCubed, Alcobra, VAYA Pharma, Neurovance, Impax, NeuroLifeSciences and research support from the National Institutes of Health (NIH). With his institution, he has US patent US20130217707 A1 for the use of sodium-hydrogen exchange inhibitors in the treatment of ADHD. In previous years, he received consulting fees or was on Advisory Boards or participated in continuing medical education programs sponsored by: Shire, Alcobra, Otsuka, McNeil, Janssen, Novartis, Pfizer and Eli Lilly. Dr. Faraone receives royalties from books published by Guilford Press: Straight Talk about Your Child’s Mental Health, Oxford University Press: Schizophrenia: The Facts and Elsevier, ADHD: Non-Pharmacologic Treatments.
Footnotes
Conflicts of Interest
Ms. Feinberg, Mr. Chan, Dr. Adeyemo, Ms. Woodworth, Dr. Panis, Dr. Bhatnagar, Dr. Uchida, Ms. Kenworthy, and Ms. Grossman have no conflicts to report.
References
- Adeyemo BO, Biederman J, Zafonte R, Kagan E, Spencer TJ, Uchida M, Kenworthy T, Spencer AE, Faraone SV. Mild traumatic brain injury and ADHD: A systematic review of the literature and meta-analysis. J Atten Disord. 2014;18:576–584. doi: 10.1177/1087054714543371. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association; A. P. Association, editor. Diagnostic and statistical manual of mental disorders. 5. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-III-R. 3. Washington, D.C: American Psychiatric Association; 1987. p. 567. Revised ed. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders: Fourth edition text revision (DSM-IV-TR) 4. Washington, DC: American Psychiatric Association; 2000. pp. 1–943. [Google Scholar]
- Antshel KM, Faraone SV, Maglione K, Doyle A, Fried R, Seidman L, Biederman J. Is adult attention deficit hyperactivity disorder a valid diagnosis in the presence of high IQ? Psychol Med. 2009;39:1325–35. doi: 10.1017/S0033291708004959. [DOI] [PubMed] [Google Scholar]
- Barkley RA, Guevremont DC, Anastopoulos AD, DuPaul GJ, Shelton TL. Driving-related risks and outcomes of attention deficit hyperactivity disorder in adolescents and young adults: A 3- to 5-year follow-up survey. Pediatrics. 1993;92:212–8. [PubMed] [Google Scholar]
- Barkley RA, Murphy KR, Fischer M. ADHD in adults: What the science says. New York, NY: Guilford Press; 2010. [Google Scholar]
- Biederman J, Faraone S, Milberger S, Guite J, Mick E, Chen L, Mennin D, Marrs A, Ouellette C, Moore P, Spencer T, Norman D, Wilens T, Kraus I, Perrin J. A prospective 4-year follow-up study of attention-deficit hyperactivity and related disorders. Arch Gen Psychiatry. 1996;53:437–46. doi: 10.1001/archpsyc.1996.01830050073012. [DOI] [PubMed] [Google Scholar]
- Biederman J, Faraone SV. Attention deficit hyperactivity disorder. Lancet. 2005;366:237–248. doi: 10.1016/S0140-6736(05)66915-2. [DOI] [PubMed] [Google Scholar]
- Biederman J, Faraone SV, Mick E, Williamson S, Wilens TE, Spencer TJ, Weber W, Jetton J, Kraus I, Pert J, Zallen B. Clinical correlates of ADHD in females: Findings from a large group of girls ascertained from pediatric and psychiatric referral sources. J Am Acad Child Adolesc Psychiatry. 1999;38:966–75. doi: 10.1097/00004583-199908000-00012. [DOI] [PubMed] [Google Scholar]
- Biederman J, Monuteaux M, Mick E, Spencer T, Wilens T, Klein K, Price JE, Faraone SV. Psychopathology in females with attention-deficit/hyperactivity disorder: A controlled, five-year prospective study. Biol Psychiatry. 2006a;60:1098–105. doi: 10.1016/j.biopsych.2006.02.031. [DOI] [PubMed] [Google Scholar]
- Biederman J, Monuteaux MC, Mick E, Spencer T, Wilens TE, Silva JM, Snyder LE, Faraone SV. Young adult outcome of attention deficit hyperactivity disorder: A controlled 10-year follow-up study. Psychol Med. 2006b;36:167–79. doi: 10.1017/S0033291705006410. [DOI] [PubMed] [Google Scholar]
- Biederman J, Petty C, Fried R, Fontanella J, Doyle AE, Seidman LJ, Faraone SV. Impact of psychometrically defined deficits of executive functioning in adults with attention deficit hyperactivity disorder. Am J Psychiatry. 2006c;163:1730–8. doi: 10.1176/ajp.2006.163.10.1730. [DOI] [PubMed] [Google Scholar]
- Bonfield CM, Lam S, Lin Y, Greene S. The impact of attention deficit hyperactivity disorder on recovery from mild traumatic brain injury. J Neurosurg Pediatr. 2013;12:97–102. doi: 10.3171/2013.5.PEDS12424. [DOI] [PubMed] [Google Scholar]
- Corps KN, Roth TL, McGavern DB. Inflammation and neuroprotection in traumatic brain injury. JAMA neurology. 2015 doi: 10.1001/jamaneurol.2014.3558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fann JR, Burington B, Leonetti A, Jaffe K, Katon WJ, Thompson RS. Psychiatric illness following traumatic brain injury in an adult health maintenance organization population. Arch Gen Psychiatry. 2004;61:53–61. doi: 10.1001/archpsyc.61.1.53. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Sergeant J, Gillberg C, Biederman J. The worldwide prevalence of ADHD: Is it an American condition? World Psychiatry. 2003;2:104–113. [PMC free article] [PubMed] [Google Scholar]
- Gerring J, Brady K, Chen A, Quinn C, Herskovits E, Bandeen-Roche K, Denckla MB, Bryan RN. Neuroimaging variables related to development of secondary attention deficit hyperactivity disorder after closed head injury in children and adolescents. Brain Inj. 2000;14:205–18. doi: 10.1080/026990500120682. [DOI] [PubMed] [Google Scholar]
- Gioia GA, Isquith PK, Guy SC, Kenworthy L. Brief behavior rating inventory of executive function: Manual. Lutz, Fl: Psychological Assessment Resources; 2000. [Google Scholar]
- Giza CC, Kutcher JS, Ashwal S, Barth J, Getchius TS, Gioia GA, Gronseth GS, Guskiewicz K, Mandel S, Manley G, McKeag DB, Thurman DJ, Zafonte R. Summary of evidence-based guideline update: Evaluation and management of concussion in sports: Report of the guideline development subcommittee of the american academy of neurology. Neurology. 2013 doi: 10.1212/WNL.0b013e31828d57dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guskiewicz KM, Marshall SW, Bailes J, McCrea M, Cantu RC, Randolph C, Jordan BD. Association between recurrent concussion and late-life cognitive impairment in retired professional football players. Neurosurgery. 2005;57:719–26. doi: 10.1093/neurosurgery/57.4.719. discussion 719–26. [DOI] [PubMed] [Google Scholar]
- Guskiewicz KM, Marshall SW, Bailes J, McCrea M, Harding HP, Jr, Matthews A, Mihalik JR, Cantu RC. Recurrent concussion and risk of depression in retired professional football players. Medicine Sci Sports Exerc. 2007;39:903–9. doi: 10.1249/mss.0b013e3180383da5. [DOI] [PubMed] [Google Scholar]
- Harmon KG, Drezner J, Gammons M, Guskiewicz K, Halstead M, Herring S, Kutcher J, Pana A, Putukian M, Roberts W. American medical society for sports medicine position statement: Concussion in sport. Clin J Sport Med. 2013;23:1–18. doi: 10.1097/JSM.0b013e31827f5f93. [DOI] [PubMed] [Google Scholar]
- Iverson GL, Zasler ND, Lange RT. Post-concussive disorders. In: Zasler ND, Katz HT, Zafonte RD, editors. Brain injury medicine: Principles and practice. New York: Demos Medical Publishing; 2006. pp. 373–405. [Google Scholar]
- Kay T, Harrington DE, Adams R, Andersen T, Berrol S, Cicerone K, Dahlberg C, Gerber D, Goka R, Harley P, Hilt J, Horn L, Lehmkuhl D, Malec J. Definition of mild traumatic brain injury. J Head Trauma Rehabil. 1993;8:86–87. [Google Scholar]
- Keenan HT, Hall GC, Marshall SW. Early head injury and attention deficit hyperactivity disorder: Retrospective cohort study. BMJ. 2008;337:a1984. doi: 10.1136/bmj.a1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahey B, Applegate B, McBurnett K, Biederman J, Greenhill L, Hynd G, Barkley R, Newcorn J, Jensen P, Richters J, Garfinkel B, Kerdyk L, Frick P, Ollendick T, Perez D, Hart E, Waldman I, Shaffer D. DSM-IV field trials for attention deficit hyperactivity disorder in children and adolescents. Am J Psychiatry. 1994;151:1673–1685. doi: 10.1176/ajp.151.11.1673. [DOI] [PubMed] [Google Scholar]
- Lam LT. Attention deficit disorder and hospitalization due to injury among older adolescents in new south wales, australia. J Atten Disord. 2002;6:77–82. doi: 10.1177/108705470200600204. [DOI] [PubMed] [Google Scholar]
- Lambert NM. Analysis of the driving histories of ADHD subjects. Washington, D.C: U.S. Department of Transportation, National Highway Traffic Safety Administration; 1995. pp. 1–21. [Google Scholar]
- Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: A brief overview. J Head Trauma Rehabil. 2006;21:375–8. doi: 10.1097/00001199-200609000-00001. [DOI] [PubMed] [Google Scholar]
- Lovell MR, Collins MW, Podell K, Powell J, Maroon J. Impact: Immediate post-concussion assessment and cognitive testing. 2000. [Google Scholar]
- Max J, Lansing AE, Koele SL, Castillo CS, Bokura H, Schachar R, Collings N, Williams KE. Attention deficit hyperactivity disorder in children and adolescents following traumatic brain injury. Dev Neuropsychol. 2004;25:159–77. doi: 10.1080/87565641.2004.9651926. [DOI] [PubMed] [Google Scholar]
- National Center for Injury Prevention and Control. Report to congress on mild traumatic brain injury in the United States: Steps to prevent a serious public health problem. Atlanta, GA: Centers for Disease Control and Prevention; 2003. [Google Scholar]
- Omalu BI, DeKosky ST, Hamilton RL, Minster RL, Kamboh MI, Shakir AM, Wecht CH. Chronic traumatic encephalopathy in a National Football League player: Part II. Neurosurgery. 2006;59:1086–92. doi: 10.1227/01.NEU.0000245601.69451.27. discussion 1092–3. [DOI] [PubMed] [Google Scholar]
- Omalu BI, DeKosky ST, Minster RL, Kamboh MI, Hamilton RL, Wecht CH. Chronic traumatic encephalopathy in a national football league player. Neurosurgery. 2005;57:128–34. doi: 10.1227/01.neu.0000163407.92769.ed. discussion 128–34. [DOI] [PubMed] [Google Scholar]
- Orvaschel H. Schedule for affective disorders and schizophrenia for school-age children epidemiologic version. 5. Ft. Lauderdale: Nova Southeastern University, Center for Psychological Studies; 1994. [Google Scholar]
- Orvaschel H, Puig-Antich J. Schedule for affective disorders and schizophrenia for school-age children: Epidemiologic version. Fort Lauderdale, FL: Nova University; 1987. [Google Scholar]
- Pastor PN, Reuben CA. Identified attention-deficit/hyperactivity disorder and medically attended, nonfatal injuries: US school-age children, 1997–2002. Ambul Pediatr. 2006;6:38–44. doi: 10.1016/j.ambp.2005.07.002. [DOI] [PubMed] [Google Scholar]
- R Core Team; R. F. f. S. Computing, editor. R: A language and environment for statistical computing. Vienna, Austria: 2014. [Google Scholar]
- Roth R, Isquith P, Gioia G. BRIEF-A Behavior Rating Inventory of Executive Function-Adult Version, publication manual. Lutz: Psychological Assessment Resources, Inc; 2005. [Google Scholar]
- Ruff RM. Mild traumatic brain injury and neural recovery: Rethinking the debate. NeuroRehabilitation. 2011;28:167–80. doi: 10.3233/NRE-2011-0646. [DOI] [PubMed] [Google Scholar]
- Swensen A, Birnbaum HG, Ben Hamadi R, Greenberg P, Cremieux PY, Secnik K. Incidence and costs of accidents among attention-deficit/hyperactivity disorder patients. J Adolesc Health. 2004;35:346.e1–9. [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechsler Intelligence Scale for Children-revised. New York: The Psychological Corporation; 1974. [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence - second edition (WASI-II) Bloomington, MN: NCS Pearson, Inc; 2011. [Google Scholar]