Abstract
The tumor microenvironment plays an important role in the progression of melanoma, the prototypical immunologic cutaneous malignancy. The triggering receptor expressed on myeloid cells (TREM) family of innate immune receptors modulates inflammatory and innate immune signaling. It has been investigated in various neoplastic diseases, but not in melanoma. This study examines the expression of TREM‐1 (a proinflammatory amplifier) and TREM‐2 (an anti‐inflammatory modulator and phagocytic promoter) in human cutaneous melanoma and surrounding tissue. Indirect immunofluorescence staining was performed on skin biopsies from 10 melanoma patients and staining intensity was semiquantitatively scored. Expression of TREM‐1 and TREM‐2 was higher in keratinocytes than melanoma tissue (TREM‐1: p < 0.01; TREM‐2: p < 0.01). Whereas TREM‐2 was the dominant isoform expressed in normal keratinocytes, TREM‐1 expression predominated in melanoma tissue (TREM‐1 to TREM‐2 ratio: keratinocytes = 0.78; melanoma = 2.08; p < 0.01). The increased TREM ratio in melanoma tissue could give rise to a proinflammatory and protumor state of the microenvironment. This evidence may be suggestive of a TREM‐1/TREM‐2 paradigm in which relative levels dictate inflammatory and immune states, rather than absolute expression of one or the other. Further investigation regarding this paradigm is warranted and could carry prognostic or therapeutic value in treatment for melanoma.
Keywords: melanoma, triggering receptor expressed on myeloid cells, TREM‐1, TREM‐2, Inflammation, tumor microenvironment
Introduction
Malignant melanoma is the deadliest cutaneous malignancy and carries serious public health implications with its rapidly increasing incidence in the United States and worldwide.1, 2 While malignant transformation of melanocytes is largely influenced by environmental ultraviolet (UV) exposure, tumorigenesis is not fully explained by UV radiation alone.3, 4 Immunologic and inflammatory processes have a significant influence as well. In fact, malignant melanoma is considered one of the prototypical immunogenic tumors and its progression depends on both indirect cytokine signaling, and direct interactions between tumor cells, peritumoral stromal cells, and inflammatory cells.5, 6 Notably, cells of the immune system have both antitumor and tumor‐promoting effects on melanoma.7
Immunotherapy constitutes the current forefront of novel cancer therapy,8 creating a continued need for identification of new molecular targets and biomarkers, particularly in melanoma research. The triggering receptor expressed on myeloid cells (TREM) family of innate immune receptors has been identified as playing pivotal roles in modulating inflammatory and innate immune signaling, creating implications in numerous diseases.9, 10 There is growing interest for these receptors in neoplastic diseases, including prognosis, evaluating disease progression, and even potential therapy.11 TREM‐1 has been indicated as a proinflammatory amplifier, with activation inducing secretion of tumor necrosis factor α, interleukin‐8, and monocyte chemoattractant protein 1 in addition to neutrophil degranulation.12 Conversely, TREM‐2 generally functions as an anti‐inflammatory modulator and phagocytic promoter.13 The high mobility group box‐1 (HMGB1) protein14 is a putative TREM‐1 ligand and is increased in expression following UV irradiation of epidermal keratinocytes.15 This potentially tumor‐promoting relationship warrants exploration of TREM in melanoma. To the knowledge of the authors, the role of TREM‐1 and TREM‐2 has not yet been explored in melanoma. In the present study, we examined the expression of TREM receptors in melanoma and surrounding tissue.
Methods
Sample selection
The study was performed on retrospectively procured punch and shave biopsy specimens removed in 2007 from consecutive patients with cutaneous melanoma at Creighton University Medical Center. Skin biopsies were fixed in formalin and embedded in paraffin blocks from which sections were cut for immunohistochemical analysis. A pathologist confirmed the diagnosis of melanoma and areas of unaffected skin and melanoma were identified in each biopsy specimen. The Creighton University Institutional Review Board approved the research protocol of this study.
Indirect immunofluorescence
Deparaffinization, rehydration, and antigen retrieval were performed prior to immunostaining. Sections were blocked/permeabilized by incubating for 2 hours in solution containing PBS, 0.25% Triton X‐100, and 5% (v/v) donkey serum. The following primary antibodies were used in a 1:50 dilution with PBS for overnight incubation at 4°C: anti‐TREM‐1 (sc‐19309, SantaCruz Biotech, Dallas, TX, USA) and anti‐TREM‐2 (sc‐48764, SantaCruz Biotech, Dallas, TX, USA). After washing with PBS, secondary antibodies (Alexa Fluor 594 donkey anti‐goat IgG and Alexa Fluor 488 goat anti‐rabbit IgG, Jackson ImmunoResearch Laboratories, West Grove, PA, USA) diluted 1:200 in PBS with 0.1% Triton X‐100, 1% donkey serum were applied and incubated for 2 hours without light. DAPI‐containing mounting media (Vectashield H‐1500, Vector Laboratories, Burlingame, CA, USA) was used for nuclear staining. Negative controls were performed using no primary antibody. Staining intensity was semiquantitatively scored on a 0 to 4 scale. Statistical analysis was performed using the Mann–Whitney U‐test to compare two independent groups of data in Microsoft Excel 14.4 statistical software package.
Results
Clinical features of the analyzed specimens are summarized in Table 1. A total of 10 patients were included in this study. The basal cells, melanocytes, sebaceous glands, and sweat glands of normal tissue had minimal to no TREM expression. Both TREM‐1 and TREM‐2 were apparent in the extranuclear spaces of melanoma tissue and the keratinocytes of the normal interfollicular and follicular epithelium (Figure 1).
Table 1.
Clinical features of included specimens
| Clinical characteristic (N = 10) | |
|---|---|
| Mean age | 61.3 |
| M/F ratio | 6/4 (1.5) |
| Histological pattern | |
| SS | 5 (50%) |
| Lentigo maligna (2 in situ) | 3 (30%) |
| In situ, unspecified | 2 (20%) |
| Clark level | |
| 1 | 4 (40%) |
| 2 | 4 (40%) |
| 3 | 0 (0%) |
| 4 | 2 (20%) |
| Primary tumor staging | |
| pTis | 4 (40%) |
| pT1a | 4 (40%) |
| pT1b | 2 (20%) |
| Excluding in situ (N = 6) | |
| Mean tumor thickness | 0.38 mm |
| Ulceration absent | 6 (100%) |
| Mitotic rate low | |
| (<6 mitoses/mm2) | 6 (100%) |
| Angiolymphatic invasion absent | 6 (100%) |
| Host inflammation | |
| Nonbrisk | 5 (83%) |
| Absent | 1 (17%) |
| Regression absent | 6 (100%) |
| Microscopic satellites absent | 6 (100%) |
| Precursor lesion | |
| Absent | 3 (50%) |
| Dermal melanocytic nevus | 3 (50%) |
Figure 1.
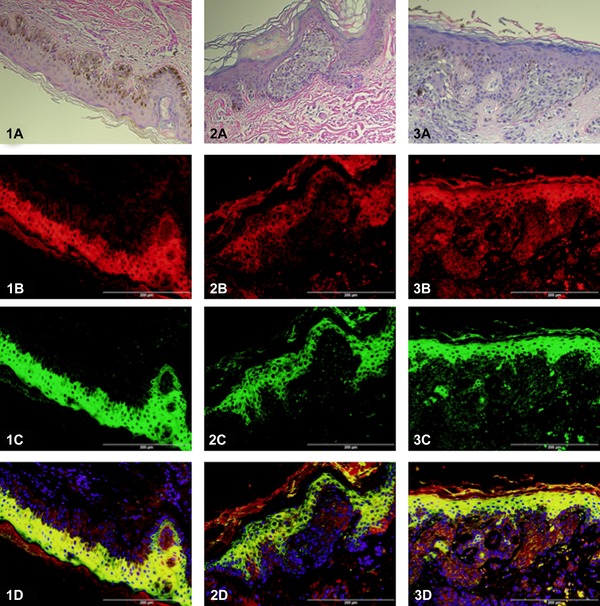
Expression of TREM‐1 and TREM‐2 in skin biopsies. Images 1(A–D) are from an in situ melanoma (Clark's level 1) of unspecified histological subclassification. The remaining images are from superficial spreading melanoma evaluated as Clark's level 2 (images 2A–D) and level 4 (images 3A–D). Images are show with hematoxylin and eosin staining (A). In the immunofluorescent images, TREM‐1 is reported by red (B) and TREM‐2 is reported by green (C). In the combined images (D), DAPI nuclear counterstaining is shown and yellow indicates colocalization of TREM‐1 and TREM‐2.
The keratinocytes expressed much higher absolute levels of both TREM‐1 and TREM‐2 than the melanoma tissue (TREM‐1: p < 0.01; TREM‐2: p < 0.01). Interestingly, there was a marked difference in TREM‐1 to TREM‐2 ratios between the two cell types (Figure 2). However, when the immunostaining of TREM‐1 and TREM‐2 were compared between the different histological subclassification, based on superficial spreading versus lentigo maligna versus in situ not otherwise specified, no statistical difference was observed. While TREM‐2 was the predominant isoform expressed in normal keratinocytes, the areas of melanoma expressed higher levels of the TREM‐1 isoform (TREM‐1 to TREM‐2 ratio: keratinocytes = 0.78; melanoma = 2.08; p < 0.01). Notably, the TREM‐1 in melanoma with a lower Clark's level seemed to be a mixture of primarily extracellular with some cytoplasmic expression, whereas the higher Clarks’ level melanoma was observed to have generalized extranuclear expression (not significantly different).
Figure 2.
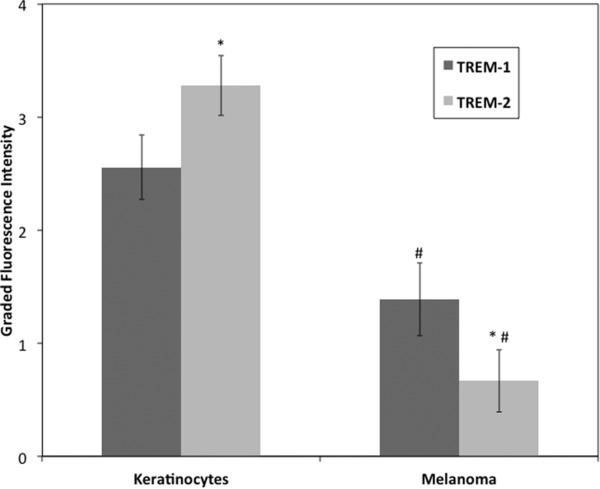
Comparison of TREM‐1 and TREM‐2 fluorescence intensity in keratinocytes and melanoma. Both TREM isoforms were expressed in normal keratinocytes and areas of melanoma at differing levels with higher expression of TREM‐2 than TREM‐1 in keratinocytes (*p < 0.01) and higher expression of TREM‐1 than TREM‐2 in melanoma (# p ≤ 0.01). Interestingly, the ratio of TREM‐1 to TREM‐2 was different between the two, with melanoma tissue having predominantly TREM‐1 expression. This may be indicative of a proinflammatory state in the tumor microenvironment. Values are shown as mean graded fluorescence intensity with 95% confidence intervals.
Discussion
Two of the four major histological types of melanoma were represented in this study: superficial spreading melanoma (constituting approximately 70% of melanomas) and lentigo maligna melanoma (representing 4–10% of melanomas). All included melanomas were of low stage (less than pT2) with no evidence of metastasis and/or advanced progression, reflective of the current trend of earlier diagnosis of melanoma.16 The included specimens did exhibit some heterogeneity in the Clark's level, an older classification system that still has clinical value in the staging of thin (1 mm or less) melanomas.17
Expression of TREM‐1 is notoriously upregulated in a number of inflammatory diseases.11 In skin, TREM‐1 has been documented to be present in the normal epidermis and increased in psoriasis.18 The present experiment found concurrent expression of TREM‐2 and TREM‐1 in normal keratinocytes of the epidermis, with a predominance of the former (TREM‐1 to TREM‐2 ratio = 0.78). Interestingly, while expression of both TREM isoforms was lower in melanoma tissue, TREM‐1 was exhibited at higher levels relative to TREM‐2 (TREM‐1 to TREM‐2 ratio = 2.08). Considering its known functions in inflammatory signaling amplification, imbalance favoring TREM‐1 expression could mediate the proinflammatory, and thus protumor state of the microenvironment in melanoma. While increased expression of TREM‐1 has been implicated in many types of malignancy, there has been little investigation of TREM‐2, let alone the ratio of the two, in neoplastic disease.
While the comparison described above is not of melanoma to nonmalignant melanocytes, the comparison of melanoma to keratinocytes suggests that nonneoplastic cells could have higher levels of TREM expression, whereas the TREM ratio determines the microenvironment. Some exploration of this ratio exists in nonneoplastic disease. In a cell culture model of acute lung injury, an alteration of the TREM‐1 to TREM‐2 ratio has been observed due to both an increase in TREM‐1 and a decrease in TREM‐2 upon LPS‐induction of the disease.19 Administration of vasoactive intestinal peptide normalized the TREM ratio, demonstrating anti‐inflammatory functionality. Similarly, a shift in this ratio toward TREM‐1 expression is noted to correlate with severity of chronic obstructive pulmonary disease.20
Immune cells involved with tumors can modulate the local microenvironment and ultimately influence cancer progression, response to therapy, and prognosis.8 For example, high numbers of peritumoral T lymphocytes in metastatic malignant melanoma is associated with lower progression of disease,21 whereas M2 polarized tumor associated macrophages are associated with a poor prognosis due to their promotion of regulatory T cells and inhibition of CD8+ T lymphocyte antitumor properties.22, 23, 24 Such associated immune cells could influence the levels of TREM within the melanoma tissue and deserves further exploration.
The two TREM isoforms have been generalized to exhibit opposing effects, with TREM‐1 amplifying inflammatory signaling and TREM‐2 inhibiting inflammation. The currently presented data alludes to the existence of a more intricate TREM‐1/TREM‐2 paradigm in which the relative ratio of expression, rather than the absolute amount of expression of each, influences the local inflammatory state. However, there is paucity in the available literature regarding such a balance between these two receptors. While this study is limited by the semiquantitative nature and small scale, the results represent a rationale for further exploration of this potential paradigm. In conclusion, our results are the first to investigate levels of both TREM‐1 and TREM‐2 in melanoma tissue. The observed alteration in the TREM‐1 to TREM‐2 ratio in melanoma compared to normal local tissue warrants further investigation into the possibility of a TREM‐1/TREM‐2 paradigm in malignancy. Such a paradigm carries strong clinical potential including novel therapeutic modalities.
Financial and competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with financial interest or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was utilized in the production of this manuscript.
Acknowledgments
This work was supported by research grants R01 HL116042, R01 HL112597, and R01 HL120659 to DK Agrawal from the Office of the Director, National Institutes of Health, and National Heart, Lung and Blood Institute, NIH USA. The content of this original research article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1. Siegel R, Ma J, Zou Z, Jemal A. Cancer Statistics, 2014. CA Cancer J Clin 2014; 64(1): 9–29. [DOI] [PubMed] [Google Scholar]
- 2. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010; 127: 2893–2917. [DOI] [PubMed] [Google Scholar]
- 3. Ando H, Niki Y, Ito M, Akiyama K, Matsui MS, Yarosh DB, Ichihashi M. Melanosomes are transferred from melanocytes to keratinocytes through the processes of packaging, release, uptake, and dispersion. J Invest Dermatol 2012; 132(4): 1222–1229. [DOI] [PubMed] [Google Scholar]
- 4. Hodis E, Watson IR, Kryukov GV, Arold ST, Imielinski M, Theurillat JP, Nickerson E, Auclair D, Li L, Place C, et al. A landscape of driver mutations in melanoma. Cell 2012; 150: 251–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer 2005; 5(4): 263–274. [DOI] [PubMed] [Google Scholar]
- 6. Vasaturo A, Verweij D, Heinzerling L, Vries J De, Blokx W, Figdor CG. Immune infiltrates impact on the prediction of prognosis and response to immunotherapy of melanoma patients. J Transl Med 2015; 13(Suppl 1): P12. [Google Scholar]
- 7. Ladányi A. Prognostic and predictive significance of immune cells infiltrating cutaneous melanoma. Pigment Cell Melanoma Res 2015. Mar 26 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 8. Nguyen AH, Berim IG, Agrawal DK. Cellular and molecular immunology of lung cancer: therapeutic implications. Expert Rev Clin Immunol 2014; 10(12): 1711–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pelham CJ, Pandya AN, Agrawal DK. Triggering receptor expressed on myeloid cells receptor family modulators : a patent review. Expert Opin Ther Pat 2014; 24: 1383–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pelham C, Agrawal D. Emerging roles for triggering receptor expressed on myeloid cells receptor family signaling in inflammatory diseases. Expert Rev Clin Immunol 2014; 10(2): 243–256. [DOI] [PubMed] [Google Scholar]
- 11. Nguyen AH, Berim IG, Agrawal DK. Chronic inflammation and cancer: emerging roles of triggering receptors expressed on myeloid cells. Expert Rev Clin Immunol 2015; 11(7): 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bouchon A, Dietrich J, Colonna M. Cutting edge: inflammatory responses can be triggered by TREM‐1, a novel receptor expressed on neutrophils and monocytes. J Immunol 2000; 164(10): 4991–4995. [DOI] [PubMed] [Google Scholar]
- 13. Sharif O, Knapp S. From expression to signaling: roles of TREM‐1 and TREM‐2 in innate immunity and bacterial infection. Immunobiology 2008; 213(9–10): 701–713. [DOI] [PubMed] [Google Scholar]
- 14. Ellerman JE, Brown CK, De Vera M, Zeh HJ, Billiar T, Rubartelli A, Lotze MT. Masquerader: high mobility group box‐1 and cancer. Clin Cancer Res 2007; 13(10): 2836–2848. [DOI] [PubMed] [Google Scholar]
- 15. Bald T, Quast T, Landsberg J, Rogava M, Glodde N, Lopez‐Ramos D, Kohlmeyer J, Riesenberg S, van den Boorn‐Konijnenberg D, Hömig‐Hölzel C, et al. Ultraviolet‐radiation‐induced inflammation promotes angiotropism and metastasis in melanoma. Nature 2014; 507: 109–113. [DOI] [PubMed] [Google Scholar]
- 16. Nikolaou V, Stratigos AJ. Emerging trends in the epidemiology of melanoma. Br J Dermatol 2014; 170: 11–19. [DOI] [PubMed] [Google Scholar]
- 17. Balch CM, Soong S, Atkins MB. An evidence‐based staging system for. CA Cancer J Clin 2004; 54(3): 131–149. [DOI] [PubMed] [Google Scholar]
- 18. Hyder LA, Gonzalez J, Harden JL, Johnson‐Huang LM, Zaba LC, Pierson KC, Eungdamrong NJ, Lentini T, Gulati N, Fuentes‐Duculan J, et al. TREM‐1 as a potential therapeutic target in psoriasis. J Invest Dermatol 2013; 133: 1742–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sun G‐Y, Guan C‐X, Zhou Y, Liu Y‐P, Li S‐F, Zhou H‐F, Tang C‐Y, Fang X. Vasoactive intestinal peptide re‐balances TREM‐1/TREM‐2 ratio in acute lung injury. Regul Pept 2011; 167(1): 56–64. [DOI] [PubMed] [Google Scholar]
- 20. Byers DE, Wu K, Jin X, Benoit L, Schechtman K, Yusen RD, Pierce RA, Holtzman MJ. Trem‐2/trem‐1 imbalance reflects alternative (m2) macrophage activation and disease severity in COPD. Am J Respir Crit Care Med. 2013; 187: A4141. [Google Scholar]
- 21. Tjin EPM, Krebbers G, Meijlink KJ, van de Kasteele W, Rosenberg EH, Sanders J, Nederlof PM, van de Wiel BA, Haanen JB , Melief CJ, et al. Immune‐escape markers in relation to clinical outcome of advanced melanoma patients following immunotherapy. Cancer Immunol Res 2014; 2(6): 538–546. [DOI] [PubMed] [Google Scholar]
- 22. Allavena P, Sica A, Solinas G, Porta C, Mantovani A. The inflammatory micro‐environment in tumor progression: the role of tumor‐associated macrophages. Crit Rev Oncol Hematol 2008; 66(1): 1–9. [DOI] [PubMed] [Google Scholar]
- 23. Sica A, Schioppa T, Mantovani A, Allavena P. Tumour‐associated macrophages are a distinct M2 polarised population promoting tumour progression: potential targets of anti‐cancer therapy. Eur J Cancer 2006; 42(6): 717–727. [DOI] [PubMed] [Google Scholar]
- 24. Jensen TO, Schmidt H, Møller HJ, Høyer M, Maniecki MB, Sjoegren P, Christensen IJ, Steiniche T. Macrophage markers in serum and tumor have prognostic impact in American joint committee on cancer stage I/II melanoma. J Clin Oncol 2009; 27(20): 3330–3337. [DOI] [PubMed] [Google Scholar]


