Abstract
Given a decision that requires less than half a second for evaluating the characteristics of the incoming pitch and generating a motor response, hitting a baseball potentially requires unique perception-action coupling to achieve high performance. We designed a rapid perceptual decision making experiment modeled as a Go/No-Go task, yet tailored to reflect a real scenario confronted by a baseball hitter. For groups of experts (Division I baseball players) and novices (non-players) we recorded electroencephalography (EEG) while they performed the task. We analyzed evoked EEG single-trial variability, contingent negative variation (CNV), and pre-stimulus alpha power with respect to the expert vs. novice groups. We found strong evidence for differences in inhibitory processes between the two groups, specifically differential activity in supplementary motor areas (SMA), indicative of enhanced inhibitory control in the expert (baseball player) group. We also found selective activity in the fusiform gyrus (FG) and orbital gyrus in the expert group, suggesting an enhanced perception-action coupling in baseball players that differentiates them from matched controls. In sum, our results show that EEG correlates of decision formation can be used to identify neural markers of high-performance athletes.
Keywords: contingent negative variation, electroencephalography, perception-action coupling, fusiform gyrus, supplemental motor cortex
Introduction
Deciding whether or not to swing at a baseball is a complex task where a 1/3-success rate is worth millions of dollars a year and a likely spot in the Baseball Hall of Fame. This interceptive action under severe time constraints requires that a batter predicts the location of a 3 inch diameter ball by extracting anticipatory cues from the opponent’s actions (Abernethy, 1996; Burroughs, 1984), integrating these with perceptual cues from the spin and trajectory of the ball, and finally estimating the time at which the ball will reach the plate. While accumulating and integrating this evidence, the batter is deciding on the execution or inhibition of the interceptive action (i.e. whether or not to swing). The extreme difficulty of this task together with the skill needed to perform it at even a modest success rate has led to the hypothesis that professional baseball players have, like other high-performing athletes, developed performance improving perceptual and cognitive abilities relative to non-athletes (Miura et al., 2010; Yarrow et al., 2009).
Several studies have investigated potential neural correlates indicative of perceptual and cognitive performance enhancement specific to baseball players. For example, Radlo et al., using electroencephalography (EEG), showed that more advanced players had faster reaction times (RTs) and greater P300 latencies when classifying pitch types compared to intermediate players (Radlo et al., 2001). Other groups have used the Go/No-Go reaction time task (Donders, 1969) to examine the neural basis of inhibition in baseball players. Kida et al. (2005) investigated the RTs of baseball players in a Go/No-Go task and found that skilled baseball players could execute the response to the Go stimulus more quickly than less skilled baseball players, tennis players, and non-athletes (Kida et al., 2005). Nakamoto and Mori repeated the Go/No-Go task to examine whether baseball players’ shorter RTs were influenced by stimulus–response compatibility (SRC) effects (Nakamoto and Mori, 2008). Specifically, they found that baseball players’ simple RT, i.e. reaction time when there is no perceptual decision needed, was not faster than that of matched controls, but for baseball-specific stimuli, the stimulus-response compatibility mediated a faster response time among experts. Additionally, basing their findings on previous work linking No-Go frontal P300 strength to response inhibition, they found greater P300 amplitudes in baseball players when the SRC was similar to baseball batting. More recently, the same group showed that baseball players, performing a Go/No-Go task in which the subjects needed to coincide their response to the arrival of a moving object, had larger amplitude N2s and P300s compared to controls (Nakamoto and Mori, 2012).
There also has been substantial work pointing to athletes employing “embodied cognition”. Cognition is said to be “embodied” when it acutely depends upon features of the physical body of an agent, that is, when aspects of the agent’s body plays a significant causal or physically constitutive role in cognitive processing. For instance, Holt and Beilock (2006) performed an experiment with two groups of athletes (ice hockey and football players) and a novice control group where subjects had to evaluate the plausibility of action-related sentences representative of everyday or sport-specific situations. They found that subjects responded most quickly to items that matched the sentence-implied actions for everyday and non-sport-specific actions, however, only the athletes showed faster response times for their respective sport-specific scenarios. Similar sport-specific experiments that have offered evidence of embodied cognition include golfers (Witt and Linkenauger, 2008), American football players (Witt and Dorsch, 2009), and baseball players (Witt and Proffitt, 2005). Recently, there has been empirical evidence that supports the idea that the perception of objects in the sporting environment is embodied (Gray, 2014).
Another likely difference between expert baseball players and novices is in their respective abilities for task-specific perception-action coupling. Perception-action coupling involves tightly integrating perceptual processing with action generation. It is likely linked to the development of neural substrates, which improve with training, that enable rapid and reliable predictions from incoming perceptual information. Moshe Bar’s “visual prediction theory” is consistent with perception-action coupling in the visual domain, and points to specific cortical areas likely central to differentiating experts from novices. Specifically, Bar (Bar, 2009a, b; Cheung and Bar, 2012; Kveraga et al., 2011) notes the role of the orbitofrontal cortex (OFC) in multimodal associations and links this capability with heightened prediction capability in visual experts. He furthermore provides evidence that the OFC is part of a larger visual expertise network that includes the fusiform face area (FFA). Bar hypothesizes that the associations driven in part by the orbitofrontal cortex combine with the visual expertise driven in part by the FFA to produce superior prediction capabilities in visual experts. The fusiform gyrus (FG), an area that includes the FFA, is best know for its face selectivity (Grill-Spector et al., 2004; Kanwisher et al., 1997; Liu et al., 2010), though more recently, studies have shown that the FFA responds to dynamic biological motion (Peelen et al., 2006; Sokolov et al., 2012) and non-face objects, if those objects are associated with expertise (Bilalić et al., 2011; Bilalic et al., 2012; Gauthier et al., 1999; McGugin et al., 2012; Rossion et al., 2004; Tong et al., 2008; Xu, 2005) suggesting a role for FFA, and potentially OFC, in perception-action coupling for expertise-driven rapid visual decisions.
Previous studies comparing baseball players and non-players have not looked for any preparatory neural differences during the pre-stimulus interval. However, there have been many studies investigating the preparatory neural activity during the pre-shot period of shooting, archery, putting, and dart throwing. Many of these studies have focused on the spectral power (Hatfield et al., 2004), specifically the alpha band (8–12 Hz); for a review see (Miura et al., 2010). In addition to power fluctuations in EEG oscillatory bands, the contingent negative variation (CNV), another pre-stimulus preparatory signal, has been linked to athletic skill (Hung et al., 2004). The CNV has been connected with both motor preparation and cognitive processes including attention, expectancy, motivation, and arousal (Brunia and Damen, 1988; Ikeda et al., 1996; van Boxtel and Brunia, 1994). In this study, we plan to analyze neural preparatory signals of alpha power and CNV to compare the pre-stimulus responses of the experts to the novices.
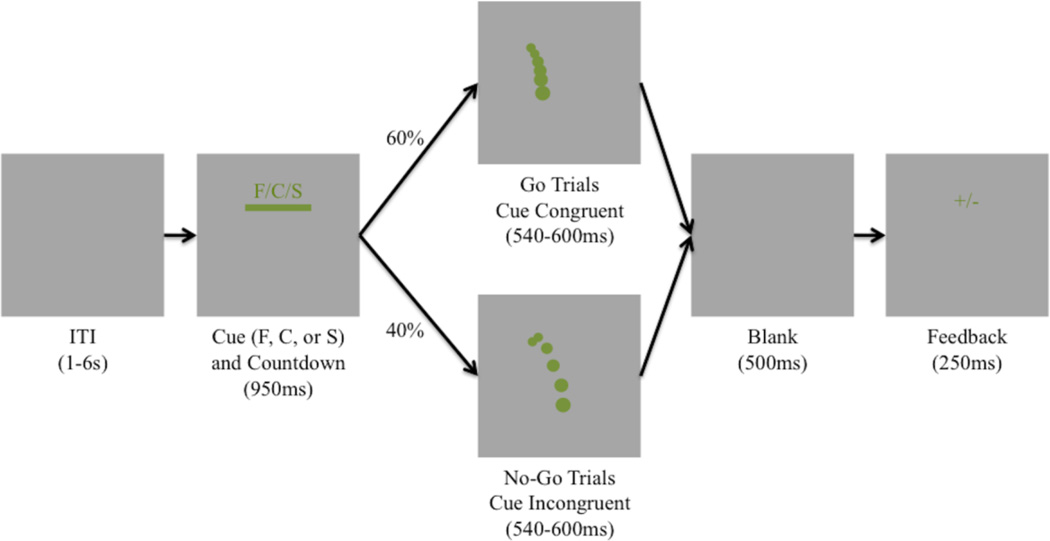
In this paper, we use high-spatial density EEG and single-trial analysis in order to capture variability within and across individuals in a way that allows us to more fully test whether cortical areas, consistent with perception-action coupling and embodied cognition, show activity that differentiates expert baseball hitters from novice controls. We compare EEG activity measured from Division I Collegiate baseball players (experts) to a set of matched novice controls for a novel Go/No-Go task that resembles an in-game baseball-batting situation. Using simulated baseball trajectories (Sherwin et al., 2012), we designed an experiment to match the fraction of a second a batter has to recognize the pitch “type” and decide whether or not to “swing”, given his “target” pitch. In the experiment, target pitches are cues presented to the subject prior to the pitch, indicating the type of pitch that should elicit a “Go” response. This mirrors the in-game situation of a batter “sitting on a pitch”. A mismatch between the player’s target pitch and the resulting pitch can lead to no swing, a late swing on faster than expected pitches, or an early swing on slower than expected pitches. Figure 1 illustrates the paradigm.
Figure 1.
Schematic Illustration of the modified stimuli Go/No-Go Paradigm. Each trial starts with a 950ms countdown bar along with a letter (F/C/H) pitch cue. At the end of the countdown, an animated green ball will appear following a simulated baseball trajectory. For 60% of the trials this trajectory will be congruent with the cue letter while 40% of the time it will be incongruent. After the animation of the pitch disappears (540–600ms), there is 500ms of a blank screen before the subject is given feedback (“+” for correct responses or “−” for incorrect responses).
Methods
Subjects
19 subjects, 9 collegiate Division I baseball players (mean age − 19.9 ± 1.1 yrs) and 10 non-player novices (mean age −21.2 ± 1.6 yrs), participated in the experiment. None of the novice subjects had any collegiate baseball experience. All of the expert baseball players were active players on a collegiate baseball team. All subjects reported normal or corrected vision and no history of neurological problems. All novices were right handed, while one of the baseball players was left-handed. Informed consent was obtained from all participants in accordance with the guidelines and approval of the Columbia University Institutional Review Board.
Stimuli Overview
Similar to our previous work (Sherwin et al., 2012), we simulated each pitch via a differential equation solver in Matlab 2010a (Mathworks, Natick, MA, USA) (see Pitch Simulations below) and presented these using PsychToolbox (Brainard, 1997). Pitches were simulated using 6-coupled differential equations (Adair, 1990; Armenti, 1992):
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
| (6) |
| (7) |
The first three equations specify the change in spatial location in each direction, which equals the velocity of the baseball. The last four equations specify the accelerations due to the drag (F(v)), the Magnus force (B), and gravity (g) acting on the baseball. After specifying the initial conditions (x0, y0, z0, vx0, vy0, vz0, ω(rotational frequency)), the 6 ordinary differential equations were solved in MATLAB. Each of the three pitches -fastball, curveball, and slider -- has well-defined initial conditions. To create each pitch, we varied the initial velocity and the rotation angle. All initial velocities were sampled from the same uniform distribution (78 ± 3 mph), though each pitch had its own rotation angle distribution (Fastball (270°± 5°), Curveball (50°± 5°), Slider (0°± 5°)). For each simulated pitch, an isoluminant green circle was plotted on a gray background for every frame of the trajectory. The size of the circle increased as it approached the viewer, so as to give the illusion of depth. When the ball crossed “home plate”, the circle disappeared.
Behavioral Paradigm
Subjects viewed 5 blocks of 90 simulated baseball pitch trials on a computer monitor with a jittered inter-stimulus interval (ISI) of mean = 3s, SE = 225ms. Subjects sat at a distance of 51” from the screen. The simulated view was that of the catcher sitting on a standard baseball diamond, i.e. at the end point of the pitch trajectory (horizontal view 3.93°, vertical view 1.12°). The subject was presented with a pitch chosen at pseudorandom (“fastballs”, “curveballs”, and “sliders”), where the initial conditions of the trajectory were also jittered so that no two pitches from the same category followed the exact same trajectory. We used these stimuli to create a Go/No-Go paradigm. Preceding the pitch, a horizontal bar (S1) (horizontal view 3.93°, vertical view 0.28°) appeared onscreen for 950ms, during which time the horizontal length of the bar shrunk at a constant rate until it disappeared. The bar shrank from either left to right, or vice versa, with equal pseudorandom uniform probability. While the bar was onscreen, a single-letter cue indicated above it the possible pitch trajectory to follow (‘F’ for fastball, ‘C’ for curveball, or ‘S’ for slider; horizontal view 0.28°, vertical view 0.28°). Once the bar shrank completely in length, the pitch trajectory (S2) began from that point on the screen.
Subjects were instructed to press a button on a keyboard only if the trajectory cue matched the actual pitch trajectory. For instance, if the cue above the horizontal bar was an ‘F’ and a fastball trajectory followed, then the subject would be expected to execute a button response (i.e., a ‘Go’). If a curveball or slider trajectory were to follow the same cue, then the subject would be expected to refrain from executing a button response (i.e., a ‘No-Go’). For this experiment, 60% of trials were ‘Go’ trials and 40% were ‘No-Go’ trials in order to control for the oddball effect in a more standard Go/No-Go paradigm. Subjects were told they must respond while the ball was still on the screen. Visual feedback was presented after the trajectory’s completion in the form of a cross (‘+’) for correct responses and dash (‘−’) for incorrect responses. A ‘Go’ response was not considered correct unless the cue matched the trajectory and the button response occurred before the end of the trajectory (i.e. before the simulated ball disappeared from the screen). All button responses were right handed using the index finger, regardless of subject’s handedness. Subjects were instructed to respond, “as fast and as accurately as possible.” Subjects performed an initial training and practice phase where they had to score an accuracy of at least 60%. After subjects reached this performance, we began recording EEG for 5 blocks of the experiment.
Data Acquisition and Preprocessing
EEG data was acquired in an electrostatically shielded room (ETS-Lindgren, Glendale Heights, IL, USA) using a BioSemi Active Two AD Box ADC-12 (BioSemi, The Netherlands) amplifier from 64 Ag/AgCl scalp electrodes arranged in the 10–20 System. Data were sampled at 2048 Hz. A software-based 0.5 Hz high pass filter was used to remove DC drifts, a 60 Hz (harmonic) notch filter to minimize line noise artifacts, and a 100 Hz low pass filter were applied before resampling the data to 256 Hz. These filters were designed to be linear-phase to minimize delay distortions. Stimulus events – i.e., countdown, pitch type, responses – were recorded on separate channels.
Independent components analysis (ICA) was run using EEGLAB (Delorme and Makeig, 2004) and FastICA (Hyvarinen, 1999) algorithm to remove eye-blink artifacts. Data were then re-referenced to the average across all electrodes. In S2 stimulus-locked epoching (−1500ms to 2000ms), the average baseline was removed using data from − 200ms to 0ms. An automatic artifact epoch rejection algorithm from EEGLAB was run to remove all epochs that exceeded a probability threshold of 5 standard deviations from the average. Trials where the subject’s RT was earlier than 100ms from pitch onset were excluded from further analysis.
Behavioral Analysis
Percent error rates and RTs were analyzed. Errors were broken down into both omissions and commissions, i.e., no-responses and late responses in Go trials, and button presses in No-Go trials. Repeated- measures ANOVAs on each behavioral measure were carried out using Trial type (two levels: Go, No-Go) as the within-subject factor and group (expert/novice) as the between subject factor. Post hoc comparisons were also made in order to determine the significance of contrasts by applying the Bonferroni procedure (alpha=0.05).
Data Analysis
Our primary analysis focused on a single-trial approach to discriminate between a set of stimulus or response conditions given the EEG data. This type of analysis allows one to identify neural correlates of task performance expressed by differences in both the mean and single-trial variability. First, we considered only behaviorally correct trials. Regularized logistic regression was used as a classifier to find an optimal projection for discriminating between behaviorally correct Go and behaviorally correct No-Go trials over a specific temporal window (Parra et al., 2005). This approach has been previously applied to identify discriminant neural components underlying rapid perceptual decisionmaking (Goldman et al., 2009; Sherwin et al., 2012; Walz et al., 2013a). Specifically, we defined a training window starting at either a pre-stimulus or post-stimulus onset time τ, with a duration of δ, and used logistic regression to estimate a spatial weighting vector wτ that maximally discriminates between EEG sensor array signals X for each class (e.g., Go vs. No-Go trials):
| (8) |
In eqn. 8, X is an N×T matrix (N sensors and T time samples). The result is a ‘discriminating component’ that is specific to activity correlated with each condition, while minimizing activity correlated with both task conditions. For our experiments, the duration of the training window (δ) was 50ms and the center of the window (τ) was varied across time in 25ms steps. We used the re-weighted least squares algorithm to learn the optimal discriminating spatial weighting vector (Jordan and Jacobs, 1994). We also estimated the electrical coupling coefficients aτ as,
| (9) |
This equation describes the electrical coupling of the discriminating component (i.e. forward models) that explains most of the sensor activity. We quantified the performance of the linear discriminator by the area under the receiver operator characteristic (ROC) curve, referred to here as AUC, using a leave-one-out procedure. We used the ROC AUC metric to characterize the discrimination performance as a function of sliding our training window from 0ms pre-stimulus to 1000ms post-stimulus (i.e, varying τ).
We quantified the statistical significance of AUC in each window (τ) using a label permutation procedure. Specifically, we randomized the truth labels (i.e. trial was a Correct Go or a Correct No-Go) for each trial and retrained the classifier. This was done 1000 times for each subject at the 500ms window, giving a total of 19000 permutations. The AUC values from these permutations were used to establish a p-value for the mean AUC at each time window. We then controlled for multiple comparisons using a Bonferroni correction at p<0.05. All significant results are thus reported at p<0.05 corrected for multiple comparisons.
Component-informed Source Localization
We used source localization (sLoreta) (Pascual-Marqui et al., 1999) to estimate the most likely cortical source distributions that differentiated experts from novices for Correct Go, Correct No-Go, and Incorrect No-Go trials. Specially, on a subject-by-subject basis, we selected the window, τ, at which the LOO AUC value was the maximum for the Correct Go versus Correct No-Go comparison and Correct No-Go versus Incorrect No-Go — e.g. times at which the neural components were most discriminative.
Using these markers in time, we trial-averaged the EEG sensor data in the 50ms window (τ) across all epochs that were Correct Go trials, Correct No-Go trials, or Incorrect No-Go creating three grand average ERPs for each subject. The Correct No-Go timings were taken from the Correct Go versus Correct No-Go discrimination. Using these grand average ERP values, we then used sLoreta to solve for the most likely current source distribution in the cortex based on the EEG sensor data and array topology registered to a standard brain. We used these distributions in separate analyses to compare the activation differences between experts and novices using a two-group independent T-test with variance smoothing=0.1.
Contingent Negative Variation (CNV) and Pre-Stimulus Alpha Power
The Contingent Negative Variation (CNV) is a slow negatively deflecting ERP in which the amplitude increases during the time interval between a first warning stimulus (S1) and a second imperative stimulus (S2) on which a decision needs to be executed. For estimating the CNV, data were baseline corrected (−200ms to 0ms) from S1 and then re-epoched around S2. CNV was measured by taking the average amplitude at the Cz electrode during the final 200ms of the S1-S2 interval (Hung et al., 2004). To estimate the pre-pitch occipital alpha power, we followed a similar analysis method as Lou et al (Lou et al., 2014), in which a visual rapid perceptual decision-making paradigm was also used. For each subject, we selected the ICA component with a posterior scalp distribution and the highest ratio of alpha-power (8–12 Hz) to that of surrounding frequencies (6–14 Hz). This ICA component was bandpass filtered from 8–12Hz after which we applied the Hilbert transform to obtain the temporal magnitude and phase. Finally for each trial, we took the average alpha magnitude during the final 200ms of the S1-S2 interval.
Similar to analyzing the behavioral results, we analyzed mean pre-pitch CNV amplitude and alpha power using repeated-measures ANOVAs on each measure using Trial type (four levels: Correct Go, Correct No-Go, Incorrect No-Go, Incorrect Go) as the within-subject factor and group (expert/novice) as the between-subject factor. The Greenhouse-Geisser (GG) epsilon correction was applied to adjust the degrees of freedom of the F ratios where necessary.
Results
Behavioral Performance
Table 1 presents group data for response times and error rates for Go and No-Go trials while SI Table 1, 2 show the average number of trials for each trial type. A two-way ANOVA on the response times showed a significant effect for the Group (F(1,17)=26.98,p<0.0001, η2=0.607) and the Group x Trial interaction (F(1,17)=5.64,p=0.03, ,η2=0.0087). Trial type (F(1,17)=2.6,p=0.13,η2=0.004) did not pass our significance threshold of p<0.05. The significant main effect for the Group indicates that experts have faster response times compared to novices. The significant Group x Trial interaction indicates experts decrease their reaction times for incorrect trials while novices have no significant difference in response time between correct and incorrect trials.
Table 1.
Mean behavioral response times (RT) and Error Rates for experts and novices. Standard deviations are in parenthesis.
| Go Trials |
No-Go Trials |
|||
|---|---|---|---|---|
| RT (ms) | Error Rate (%) | RT (ms) | Error Rate (%) | |
| Experts | 445 (20) | 8.4 (2.9) | 439 (21) | 44 (8.5) |
| Novices | 489 (18) | 22.7 (7) | 490 (21) | 46 (11) |
The two-way ANOVA for error rates showed a significant main effect for Group (F(1,17)=9.55,p=0.007, η2=0.226),Trial Type (F(1,17)=132.7,p<0.001, η2=0.79), and the Group x Trial interaction (F(1,17)=5.89,p=0.027, η2=0.14). These results indicated that experts commit fewer errors and that both groups commit fewer errors for Go compared to NoGo trials.
Single-trial Analysis of Discriminating EEG Components
For this analysis, we only considered behaviorally correct trials. However, the same analysis was run for the comparisons of Correct Go versus Incorrect No-Go and Correct No-Go versus Incorrect No-Go trials and reported in the Supplemental Information (SI Figures 1, 2). Classification analysis using Incorrect Go trials were not run because of the small number of trials for experts (see SI Table 1, 2) as well as the mixing of incorrect omissions and late commissions that make up the Incorrect Go trials.
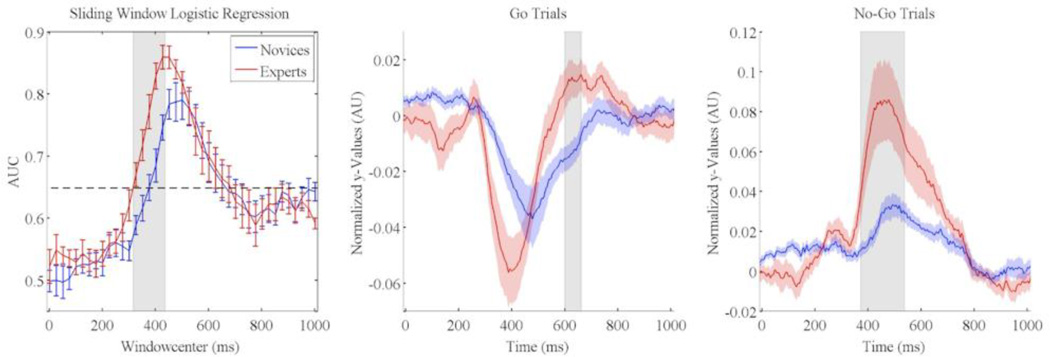
Figure 2 (left panel) shows the mean (across subjects, separated by group) performance (area under the ROC curve: AUC) for stimulus-locked EEG components discriminative of Correct Go vs. Correct No-Go discrimination trials. We found that experts and novices had similarly shaped discrimination curves, however experts exhibited an earlier rise and larger peak than novices. Both groups showed no significant early discrimination (discrimination before 300ms), however discrimination rose sharply to a maximum AUC of 0.86 at 450ms for experts and 0.79 at 500ms for novices. The expert’s discrimination curve also was shifted 75ms earlier relative to that for the novices. To test for significant discrimination differences between experts and novices, we computed an independent groups t-test at each window. Shaded regions indicated significant differences (p<0.05 FDR corrected) in discrimination activity between experts and novices. Experts show significantly higher discrimination than novices from 325 to 425ms.
Figure 2.
Stimulus-locked EEG discrimination results for Correct Go versus Correct No-Go trials for Novices (blue) and experts (red) (Left panel). Each AUC curve shows the mean and standard error bars computed using leave-one-out discrimination. The significance line (dotted) is corrected for multiple comparisons (line at p=0.05 Bonferonni corrected for 41 time window comparisons). Grey shading indicates which time points showed a significant difference between experts and novices (independent groups t-test at each window and an FDR correction for multiple windows). Stimulus-locked averaged normalized y-values for Go and No-Go trials are plotted in the middle and right panels. Grey shading indicates which time points showed a significant difference between experts and novices (p<0.05 FWE corrected).
While we see significant differences between experts and novices in the discrimination space, we also wanted to see if this stronger discrimination is due to expert/novice differences in the Go or No-Go trials. To this end, each subject’s max discriminating classifier was used to create normalized y-values averaged across trials from 0 to 1000ms from onset of stimulus using eq. 8. Each subject’s time series was then averaged within group for both Go (Figure 2 middle panel) and No-Go (Figure 2 right panel) trials. For Go trials, novices had significantly lower (stronger Go response) y’s from 598 to 660ms (p<0.05 FWE corrected), while for No-Go trials; experts had significantly higher y’s from 371 to 535ms (p<0.05 FWE corrected).
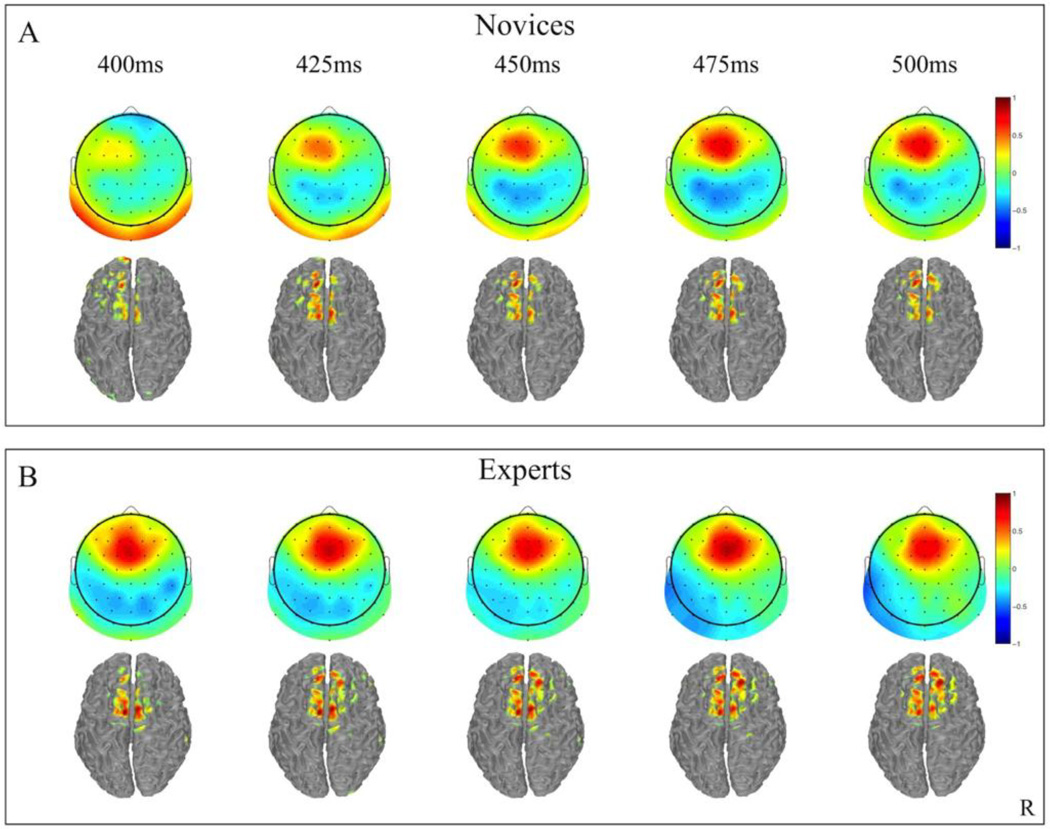
Group mean stimulus-locked forward models, shown as scalp and cortical source plots, are given in Figure 3. Plots are of selected time points (400–500ms) for both novices and experts, where the center of the discrimination window is indicated at the top of each column. Dark red and blue colors indicate strong correlation of the discriminatory component with the measured scalp activity. The two main sources of discrimination are consistently located in the central frontal and parietal regions of the scalp plots.
Figure 3.
Group averaged stimulus-locked forward models (aτ), shown as scalp maps and cortical distributions, for novices (A) and experts (B). Only behaviorally correct Go and No-Go trials are used to estimate these forward models. Red indicates areas with stronger positive weighting for No-Go trials and blue shading indicates areas for more positive weighting for Go trials. The center time of each window is given above each plot.
Source Localization
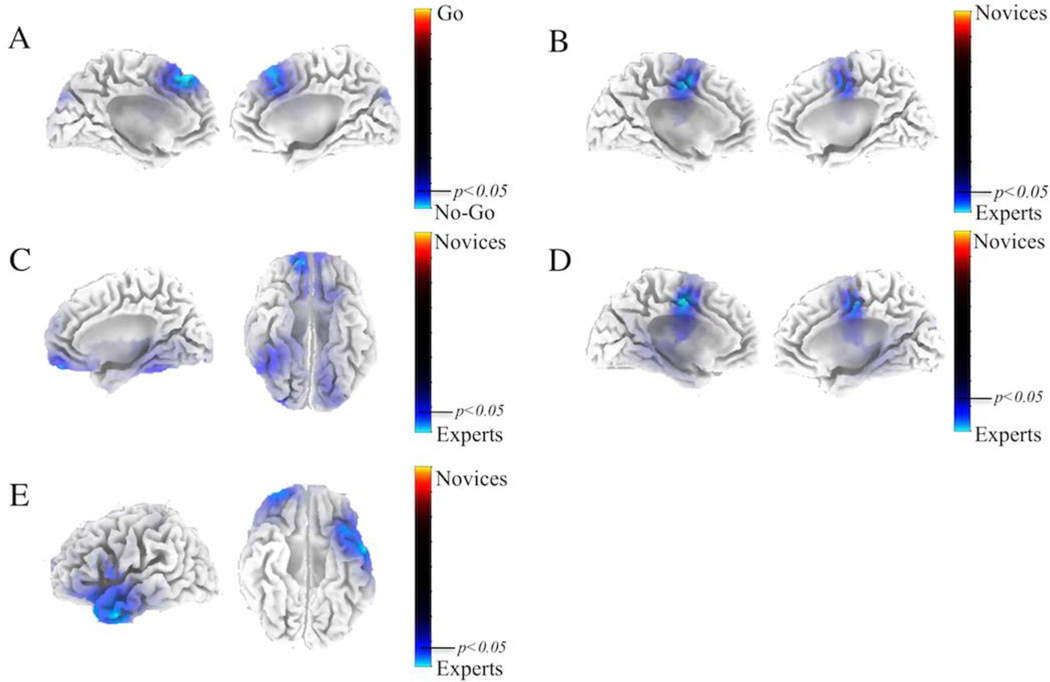
We identified the temporal windows having the maximum AUC for each subject (see Methods), and found that experts had a mean maximum AUC at 447 +/− 45ms while the novices had a mean maximum AUC at 495 +/− 55ms (t=2.043,p=0.057, Cohen’s d=0.9385, Independent Groups T-test) for the Correct Go versus Correct No-Go discrimination. For the Correct No-Go versus Incorrect No-Go discrimination, experts had a mean maximum AUC at 475 +/− 54ms while the novices had a mean maximum AUC at 494 +/− 46ms (t=0.7612,p=0.45, Cohen’s d=0.3497, Independent Groups T-test) (Figure S1). Using the EEG data from these subject-specific time windows, we solved for the source distributions using sLoreta (see Methods). For the first source localization, we performed a paired t-test for Correct Go vs. Correct No-Go across both experts and novices. The resulting t-distribution is shown in Figure 4A. We did a permutation test (10000 permutations) to establish significance levels (p < 0.05) for the null hypothesis of no difference in activity between Go and No-Go trials. We found significant cortical source distributions in Brodmann areas 6 and 8, which include the frontal eye fields and the pre-supplemental motor area (preSMA) (peak MNI coordinates- X=−5mm, Y=40mm, Z=50mm).
Figure 4.
sLoreta source distributions. (A) shows the source localization for the paired t-test for Correct Go vs. Correct No-Go across both experts and novices. Blue regions indicate significant source localizations for No-Go trials. (B) shows the group t-test between experts and novices of the within subject paired difference between Correct Go and Correct No-Go. Blue regions indicate source localization areas where experts have increased activation compared to the novices. (C) shows the differences between experts and novices for Correct Go trials, (D) shows the differences between experts and novices for the Correct No-Go trials, and (E) shows the differences between experts and novices for Incorrect No-Go trials. Again, for C, D, and E blue regions indicate experts are stronger than novices. For all tests, 10000 permutations were run to generate significance at the p<0.05 multiple comparison corrected level.
For the second source localization, we performed a group t-test between experts and novices of the within subject paired difference between Correct Go and Correct No-Go source distributions. As for the first source localization, we did permutation testing to establish significance. Figure 4B shows significant regions where experts have stronger cortical neural generators than novices (peak MNI coordinates – X=−5mm, Y=−5mm, Z=50mm). Significant regions include the Anterior Cingulate Cortex (ACC) and Supplementary Motor Area (SMA). Next, we conducted group t-tests for differences between experts and novices in Correct Go, Correct No-Go, and Incorrect No-Go trials separately. For Correct Go trials (Figure 4C), experts had significantly stronger cortical source distributions (peak MNI coordinates – X=15mm, Y=55mm, Z=−15mm) in frontal orbital gyrus (BA 11) and fusiform gyrus (BA 37), while in Correct No-Go trials (Figure 4D) a similar cluster to Figure 4B is shown, with activation in the SMA and ACC (peak MNI coordinates – X=−5mm, Y=−10mm, Z=50mm). Finally, experts had significantly stronger cortical source distributions for Incorrect No-Go trials (peak MNI coordinates – X=−55mm, Y=0mm, Z=−30mm) in the middle temporal gyrus (BA 21), superior temporal gyrus (BA 38), and superior frontal gyrus (BA 10/11).
Pre-Stimulus Alpha Power
We performed a two-way ANOVA for pre-stimulus Alpha power, however results were insignificant for all factors analyzed. Experts had a lower alpha power magnitude but insignificant difference from novices (F(1,17)=0.750,p=0.40, η2=0.0415). Trial type (F(3,51)=0.28, p=0.83, η2=0.00028) and the interaction of Group x Trial Type (F(3,51)=2.72, p=0.054, η2=0.0028) was insignificant.
Contingent Negative Variation (CNV)
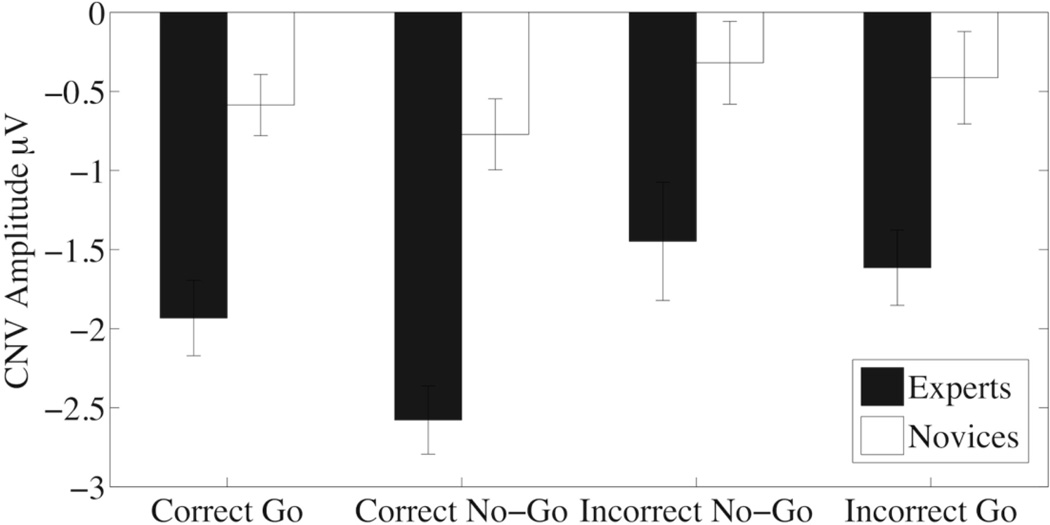
Figure 4 shows the average CNV amplitude for experts and novices across the four trial types. A two way ANOVA for CNV amplitude showed significant main effect for the Group (F(1,17)=30.66,p=0.00004, η2=0.4512) and Trial type (F(3,51)=4.756,p=0.0053,η2=0.132, GGe=0.648), while the Group x Trial interaction (F(3,51)=0.948,p=0.42,η2=0.029) did not pass our significance threshold. The significant main effect for Group indicates that experts have larger CNV amplitudes across trial types compared to novices. An analysis of the main effect of trial type showed Correct No-Go trials were significantly different from Incorrect No-Go trials (z=3.526, p=0.003) and significantly different from Incorrect Go trials (z=3.005, p=0.019). We included an additional contrast for correct versus incorrect trials that was also significant (z=3.195, p=0.0098).
Discussion
In this study, we identified spatio-temporal neural correlates that differentiate baseball players and novice controls in a Go/No-Go task. This task simulates an important aspect of hitting, though neither group (player or controls) was trained on the specific task. Group differences in neural activity manifested themselves in a combination of temporally precise discriminating neural components, scalp forward model topology, spatial distribution of neuronal sources, and pre-stimulus preparatory neural activity. Below we discuss these results within the context of the perception-action coupling and embodied cognition hypotheses.
Our behavioral observations—that experts have faster response times than novices for Go trials (Table 1)—confirms Kida et al. (2005) and Nakamoto and Mori (2008) reaction time findings. Nakamoto and Mori reasoned that faster response times for baseball players were a result of the SRC effects of the stimulus paradigm and stronger inhibition responses of the players. They showed that the players’ response times were the fastest when the stimulus-response mapping was the most similar to a baseball task and that the frontal P300 amplitude was larger in the players when compared to controls.
Our single-trial results (Figure 2) also support Nakamoto’s reasoning. EEG discrimination for experts’ Correct Go versus Correct No-Go trials is significantly higher than for novices, beginning at 325ms post-stimulus. An analysis of the scalp topologies of the forward model (Figure 3) shows stronger frontal inhibitory components of the experts than the novices. This frontal inhibitory component is most likely a frontal P300. The averaged event-related potentials for the No-Go trials for expert and novices at Fz showing the frontal P300 are plotted in Figure S3. By splitting the discrimination plot into the Go and No-Go components, we again confirm that the significant differences between experts and novices is in the No-Go trials and therefore in the inhibition response. In addition, response locked (Figure S6) and post-hoc analyses (Figure S7–Figure S10) confirm that the single-trial results are not confounded by behavioral differences in responses times. With these results, we confirm previous findings that the stronger frontal inhibitory components may contribute to the experts’ faster reaction times (Nakamoto and Mori, 2008, 2012; Nakata et al., 2012).
Source localization revealed greater activation for correct No-Go trials compared to correct Go trials in the preSMA across both expert and novice groups (Figure 4A). Since our experimental design reduces the “oddball confound,” these results suggest the preSMA is an important structure in the inhibition response. Our findings add to the growing literature supporting the theory that the preSMA is critical for inhibition Albert et al. (2013).
While the source localization comparing correct No-Go to correct Go trials confirms previous experimental results, our finding that expert baseball players have higher activation in the SMA during successfully inhibited trials compared to novices is completely novel. Understanding the functional and anatomical differences between the preSMA and SMA can help elucidate why experts would recruit the SMA more than the novices for inhibitory actions (Figure 4B/C).
The rostral preSMA and the caudal SMA make up the main components of the supplemental motor cortex (SMC) (Nachev et al., 2008). Commonly, the vertical commissure anterior line (y = 0) serves as an anatomical landmark to distinguish between these two regions of the SMC (Picard and Strick, 1996). The general functional dissociation between the SMA and preSMA is that the SMA is implicated in motor response execution, while the preSMA is involved in response inhibition. Whereas the SMA has strong connections with the primary motor cortex and spinal cord, the pre-SMA has substantial connections with the prefrontal cortex and caudate. These patterns of connectivity support the key roles of SMA and preSMA in movement planning/execution and inhibitory control, respectively. While the evidence for preSMA activation during Go/No-Go tasks is substantial, evidence for SMA activation during inhibition tasks is sparse (Wardak, 2011).
Previous studies have shown response inhibition consistently involves the pre-SMA, and the only evidence for the involvement of SMA came from one micro-lesion study in humans (Sumner et al., 2007). However, studies using monkeys have shown that the SMA has a role in the reactive control of movement, by showing that some neurons in the SMA influence movement cancellation (Chen et al., 2010; Scangos and Stuphorn, 2010). Following the initial human micro-lesion study of Sumner et al. (2007), Boy et al. (2010) found that automatic inhibitory mechanisms that suppress automatic motor activations evoked by cues are supported by GABA concentration in the SMA, as measured with magnetic resonance spectroscopy (Boy et al., 2010a). The authors further support this conclusion in a companion fMRI study that showed that SMA signals, but not preSMA signals, were modulated in the inhibitory task (Boy et al., 2010b). Finally, a group of recent fMRI experiments identified areas involved in proactive control of movements by analyzing suppression of movements that are represented in the motor system but not performed. These representations were created through motor imagery (Kasess et al., 2008) or by observing the actions of others (Dinomais et al., 2009) and they found SMA activation when motor execution had to be suppressed.
These final two studies raise the issue of whether the baseball players’ discrimination and higher activation of the SMA are linked to an embodied cognition of the visual stimulus. Our results – that expert baseball players recruit the SMA during inhibition more than novices – suggests that the baseball players are actively inhibiting their response as if they need to stop their physical “swing.”
While source localization data for No-Go trials revealed stronger activation in the SMA for experts, comparisons using the Go trials revealed stronger activations in the frontal orbital gyrus (BA 11) and Fusiform Gyrus (BA 37) for experts (Figure 4C). The combined activations of the FG and orbitofrontal cortex in correct Go trials fits with Bar’s theory of visual prediction and expertise being mediated by similar neural structures (Bar, 2009a, b; Cheung and Bar, 2012; Kveraga et al., 2011). Our results for differential activation in experts during correct Go trials, i.e., trials in which a successful trajectory prediction was made and acted upon, reinforce Bar’s hypothesis of visual expertise and prediction being tightly coupled cortical phenomena –i.e. evidence of enhanced perception-action coupling in hitters.
The baseball players have become experts in pitch tracking through years of practice. Over time, as with car recognition (McGugin et al., 2012) or chess expertise (Bilalić et al., 2011), the expert baseball players’ fusiform gyri may have become selective to differentiate baseball pitch-like objects. Thus, when performing a task that was meant to be a simulation of baseball pitches, the experts’ fusiform areas were more activated than the novices.
These results confirm and add to previous research showing that stronger inhibitory EEG potentials correlate with faster reaction times (Nakamoto and Mori, 2008; Nakata et al., 2012). Nakata et al. (2012) showed that only stronger inhibitory amplitude components correlated with faster reaction times in a Go/NoGo task. We believe that the SMA network is recruited as part of the inhibitory circuitry for experts — thereby increasing the frontal inhibitory component—and that this allows for better control of one’s response and therefore faster reaction times. For Go trials, the OFG and FG have been shown to be crucial in visual object recognition for both accuracy (Bar et al., 2001) and reaction times (Chaumon et al., 2014). We believe this combination of task specific enhanced motor inhibition and visual object recognition helps in modulating faster reaction times for experts.
To check if the same cortical differences between experts and novices occurred in error trials, we ran a separate analysis with Incorrect No-Go trials. As with previous results with Correct Go and No-Go trials, we show that the experts are activating a separate cortical network for error trials (Figure 4E). Specifically, experts showed stronger activations in the middle temporal gyrus (BA 21) and superior temporal gyrus (BA 38).
Some caution is warranted while interpreting the source localization results as the inversion solution is an ill-posed problem and we used a low-resolution sLoreta technique to solve for the potential neural sources. Further analysis of the spatio-temporal dynamics differentiating experts and novices can possibly be assessed using simultaneously acquired EEG-fMRI (Goldman et al., 2009; Walz et al., 2013b).
While previous studies have shown differences in alpha band power between high performance athletes and novices, our results did not show a significant difference in alpha power between the baseball players and novices. The lack of significant findings can be a result of our analysis method, our experimental paradigm, and possibly the subject population. Studies that have found differences in preparatory alpha oscillations have used a variety of scalp locations to find differences in their subject populations, while in our experiment we only measured occipital alpha power. Our experimental paradigm allowed for a relatively short ∼1sec preparatory period, while experiments with riflemen and golfers that showed differences between experts and novices had much longer preparatory periods of ∼3s. Our experiment’s short preparation time may not have been long enough for the oscillation power differences to separate enough to be significant.
Whereas most studies have looked at spectral power for comparing high performance athletes, Hung et al. found differences in broadband power of table tennis players by measuring the ERP during their preparatory period (i.e. the CNV) before a cued stimulus (Hung et al., 2004). When we looked at CNV, we saw large significant differences between groups and trial types (Figure 5). We found that baseball players had significantly greater amplitude CNV compared to that of the novices. We also found that Correct No-Go trials had significantly greater amplitude CNVs compared to Incorrect Go and Incorrect No-Go trials and that correct trials had higher CNVs than incorrect trials. In a post-hoc analysis, we correlated Correct No-Go CNV amplitude with No-Go behavioral accuracy while controlling for expertise (r=−0.472, p=0.048) indicating that even after controlling for expertise, higher amplitude CNV correlates with better performance. These results suggest that the amount of motor preparation and other cognitive processes, such as attention, expectancy and motivation, devoted to the task before the visual stimulus can affect performance and be a marker for expertise.
Figure 5.
Average Contingent Negative Variation (CNV) amplitude for experts and novices for the four trial type conditions with standard error bars. Experts have larger CNV amplitudes across trial types compared to novices (p=0.00004). Across expertise groups, Correct No-Go trials were significantly different from Incorrect No-Go trials (p=0.003) and significantly different from Incorrect Go trials (p=0.019). In addition, correct versus incorrect trials were also significant (p=0.0098).
Conclusion
Considered together, these results indicate a different cognitive process unfolding for expert and novice baseball batters as they perform the baseball-like task studied here. These spatio-temporal neural differences, beginning as early as 200ms before the pitch trajectory starts and lasting up to 700ms afterwards, provide evidence for a enhanced perception-action coupling in the expert group. We find evidence that these neural differences could translate into higher behavioral accuracies and faster response times in experts. Furthermore, for cases when an overt response is not observable (e.g., a No-Go), we find a neural marker for expertise originating in the SMA, i.e., a marker for knowing when not to swing.
Supplementary Material
Highlights.
Experts in baseball have superior behavioral results compared to novices in a novel Go-NoGo task.
Baseball experts have stronger inhibition responses measured by single-trial EEG analysis.
Source localization reveals stronger cortical activation in the SMA for experts during inhibition.
Results show possible evidence for enhanced perception-action coupling in baseball players.
Contingent Negative Variations during pre-stimulus are stronger in baseball experts.
Acknowledgments
This work was supported by grants from the Army Research Office (W911NF-11-1-0219), the National Institutes of Health (R01-MH085092), the Army Research Laboratory under Cooperative Agreement Number W911NF-10-2-0022 and in part by an appointment to the U.S. Army Research Laboratory Postdoctoral Fellowship Program administered by the Oak Ridge Associated Universities through a contract with the U.S. Army Research Laboratory. The views and conclusions contained in this document are those of the authors and should not be interpreted as representing the official policies, either expressed or implied, of the Army Research Laboratory of the US Government. The US Government is authorized to reproduce and distribute reprints for Government purposes notwithstanding any copyright notation herein. Jordan Muraskin and Jason Sherwin are co-founders of deCervo, a company that provides neural profiles for athletes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abernethy B. Training the visual-perceptual skills of athletes. Insights from the Study of Motor Expertise. Am J Sports Med. 1996;24:S89–S92. [PubMed] [Google Scholar]
- Adair RK. The physics of baseball. 1990:110. [Google Scholar]
- Armenti A. The Physics of sports. New York: American Institute of Physics; 1992. [Google Scholar]
- Bar M. Predictions: a universal principle in the operation of the human brain. Introduction. Philos Trans R Soc Lond B Biol Sci. 2009a;364:1181–1182. doi: 10.1098/rstb.2008.0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar M. The proactive brain: memory for predictions. Philos Trans R Soc Lond B Biol Sci. 2009b;364:1235–1243. doi: 10.1098/rstb.2008.0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar M, Tootell RB, Schacter DL, Greve DN, Fischl B, Mendola JD, Rosen BR, Dale AM. Cortical mechanisms specific to explicit visual object recognition. Neuron. 2001;29:529–535. doi: 10.1016/s0896-6273(01)00224-0. [DOI] [PubMed] [Google Scholar]
- Bilalić M, Langner R, Ulrich R, Grodd W. Many faces of expertise: fusiform face area in chess experts and novices. J Neurosci. 2011;31:10206–10214. doi: 10.1523/JNEUROSCI.5727-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilalic M, Turella L, Campitelli G, Erb M, Grodd W. Expertise modulates the neural basis of context dependent recognition of objects and their relations. Hum Brain Mapp. 2012;33:2728–2740. doi: 10.1002/hbm.21396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boy F, Evans CJ, Edden RA, Singh KD, Husain M, Sumner P. Individual differences in subconscious motor control predicted by GABA concentration in SMA. Curr Biol. 2010a;20:1779–1785. doi: 10.1016/j.cub.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boy F, Husain M, Singh KD, Sumner P. Supplementary motor area activations in unconscious inhibition of voluntary action. Exp Brain Res. 2010b;206:441–448. doi: 10.1007/s00221-010-2417-x. [DOI] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spatial vision. 1997;10:433–436. [PubMed] [Google Scholar]
- Brunia CH, Damen EJ. Distribution of slow brain potentials related to motor preparation and stimulus anticipation in a time estimation task. Electroencephalogr Clin Neurophysiol. 1988;69:234–243. doi: 10.1016/0013-4694(88)90132-0. [DOI] [PubMed] [Google Scholar]
- Burroughs WA. Visual Simulation Training of Baseball Batters. International Journal of Sport Psychology. 1984;15:117–126. [Google Scholar]
- Chaumon M, Kveraga K, Barrett LF, Bar M. Visual predictions in the orbitofrontal cortex rely on associative content. Cereb Cortex. 2014;24:2899–2907. doi: 10.1093/cercor/bht146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Scangos KW, Stuphorn V. Supplementary motor area exerts proactive and reactive control of arm movements. J Neurosci. 2010;30:14657–14675. doi: 10.1523/JNEUROSCI.2669-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung OS, Bar M. Visual prediction and perceptual expertise. Int J Psychophysiol. 2012;83:156–163. doi: 10.1016/j.ijpsycho.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Dinomais M, Minassian AT, Tuilier T, Delion M, Wilke M, N’Guyen S, Richard I, Aube C, Menei P. Functional MRI comparison of passive and active movement: possible inhibitory role of supplementary motor area. Neuroreport. 2009;20:1351–1355. doi: 10.1097/WNR.0b013e328330cd43. [DOI] [PubMed] [Google Scholar]
- Donders FC. On the speed of mental processes. Acta psychologica. 1969:412–431. doi: 10.1016/0001-6918(69)90065-1. [DOI] [PubMed] [Google Scholar]
- Gauthier I, Tarr MJ, Anderson aW, Skudlarski P, Gore JC. Activation of the middle fusiform ‘face area’ increases with expertise in recognizing novel objects. Nat Neurosci. 1999;2:568–573. doi: 10.1038/9224. [DOI] [PubMed] [Google Scholar]
- Goldman RI, Wei CY, Philiastides MG, Gerson AD, Friedman D, Brown TR, Sajda P. Single-trial discrimination for integrating simultaneous EEG and fMRI: identifying cortical areas contributing to trial-to-trial variability in the auditory oddball task. Neuroimage. 2009;47:136–147. doi: 10.1016/j.neuroimage.2009.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray R. Embodied perception in sport. International Review of Sport and Exercise Psychology. 2014:37–41. [Google Scholar]
- Grill-Spector K, Knouf N, Kanwisher N. The fusiform face area subserves face perception, not generic within-category identification. Nat Neurosci. 2004;7:555–562. doi: 10.1038/nn1224. [DOI] [PubMed] [Google Scholar]
- Hatfield BD, Haufler AJ, Hung TM, Spalding TW. Electroencephalographic studies of skilled psychomotor performance. Journal of Clinical Neurophysiology. 2004;21:144–156. doi: 10.1097/00004691-200405000-00003. [DOI] [PubMed] [Google Scholar]
- Hung TM, Spalding TW, Maria DLS, Hatfield BD. Assessment of reactive motor performance with event-related brain potentials: attention processes in elite table tennis players. Journal of sport & exercise physiology. 2004:317–337. [Google Scholar]
- Hyvarinen A. Fast and robust fixed-point algorithms for independent component analysis. IEEE Trans Neural Netw. 1999;10:626–634. doi: 10.1109/72.761722. [DOI] [PubMed] [Google Scholar]
- Ikeda A, Luders HO, Collura TF, Burgess RC, Morris HH, Hamano T, Shibasaki H. Subdural potentials at orbitofrontal and mesial prefrontal areas accompanying anticipation and decision making in humans: a comparison with Bereitschaftspotential. Electroencephalogr Clin Neurophysiol. 1996;98:206–212. doi: 10.1016/0013-4694(95)00239-1. [DOI] [PubMed] [Google Scholar]
- Jordan MI, Jacobs RA. Hierarchical Mixtures of Experts and the Em Algorithm. Neural Computation. 1994;6:181–214. [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasess CH, Windischberger C, Cunnington R, Lanzenberger R, Pezawas L, Moser E. The suppressive influence of SMA on M1 in motor imagery revealed by fMRI and dynamic causal modeling. Neuroimage. 2008;40:828–837. doi: 10.1016/j.neuroimage.2007.11.040. [DOI] [PubMed] [Google Scholar]
- Kida N, Oda S, Matsumura M. Intensive baseball practice improves the Go/Nogo reaction time, but not the simple reaction time. Brain Res Cogn Brain Res. 2005;22:257–264. doi: 10.1016/j.cogbrainres.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Kveraga K, Ghuman AS, Kassam KS, Aminoff EA, Hamalainen MS, Chaumon M, Bar M. Early onset of neural synchronization in the contextual associations network. Proc Natl Acad Sci U S A. 2011;108:3389–3394. doi: 10.1073/pnas.1013760108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Harris A, Kanwisher N. Perception of face parts and face configurations: an FMRI study. Journal of cognitive neuroscience. 2010;22:203–211. doi: 10.1162/jocn.2009.21203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou B, Li Y, Philiastides MG, Sajda P. Prestimulus alpha power predicts fidelity of sensory encoding in perceptual decision making. Neuroimage. 2014;87:242–251. doi: 10.1016/j.neuroimage.2013.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGugin RW, Gatenby JC, Gore JC, Gauthier I. High-resolution imaging of expertise reveals reliable object selectivity in the fusiform face area related to perceptual performance. Proc Natl Acad Sci U S A. 2012;109:17063–17068. doi: 10.1073/pnas.1116333109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura A, Nakata H, Kudo K, Yoshie M. Characteristics of the athletes’ brain: evidence from neurophysiology and neuroimaging. Brain research reviews. 2010;62:197–211. doi: 10.1016/j.brainresrev.2009.11.006. [DOI] [PubMed] [Google Scholar]
- Nachev P, Kennard C, Husain M. Functional role of the supplementary and pre-supplementary motor areas. Nat Rev Neurosci. 2008;9:856–869. doi: 10.1038/nrn2478. [DOI] [PubMed] [Google Scholar]
- Nakamoto H, Mori S. Effects of stimulus-response compatibility in mediating expert performance in baseball players. Brain Res. 2008;1189:179–188. doi: 10.1016/j.brainres.2007.10.096. [DOI] [PubMed] [Google Scholar]
- Nakamoto H, Mori S. Experts in fast-ball sports reduce anticipation timing cost by developing inhibitory control. Brain and Cognition. 2012;80:23–32. doi: 10.1016/j.bandc.2012.04.004. [DOI] [PubMed] [Google Scholar]
- Nakata H, Sakamoto K, Kakigi R. The relationship between reaction time and response variability and somatosensory No-go potentials. Eur J Appl Physiol. 2012;112:207–214. doi: 10.1007/s00421-011-1973-5. [DOI] [PubMed] [Google Scholar]
- Parra LC, Spence CD, Gerson AD, Sajda P. Recipes for the linear analysis of EEG. Neuroimage. 2005;28:326–341. doi: 10.1016/j.neuroimage.2005.05.032. [DOI] [PubMed] [Google Scholar]
- Pascual-Marqui RD, Lehmann D, Koenig T, Kochi K, Merlo MC, Hell D, Koukkou M. Low resolution brain electromagnetic tomography (LORETA) functional imaging in acute, neuroleptic-naive, first-episode, productive schizophrenia. Psychiatry Res. 1999;90:169–179. doi: 10.1016/s0925-4927(99)00013-x. [DOI] [PubMed] [Google Scholar]
- Peelen MV, Wiggett AJ, Downing PE. Patterns of fMRI activity dissociate overlapping functional brain areas that respond to biological motion. Neuron. 2006;49:815–822. doi: 10.1016/j.neuron.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Picard N, Strick PL. Motor areas of the medial wall: a review of their location and functional activation. Cereb Cortex. 1996;6:342–353. doi: 10.1093/cercor/6.3.342. [DOI] [PubMed] [Google Scholar]
- Radlo SJ, Janelle CM, Barba DA, Frehlich SG. Perceptual decision making for baseball pitch recognition: Using P300 latency and amplitude to index attentional processing. Research quarterly for exercise and sport. 2001;72:22–31. doi: 10.1080/02701367.2001.10608928. [DOI] [PubMed] [Google Scholar]
- Rossion B, Kung C-C, Tarr MJ. Visual expertise with nonface objects leads to competition with the early perceptual processing of faces in the human occipitotemporal cortex. 2004 doi: 10.1073/pnas.0405613101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scangos KW, Stuphorn V. Medial frontal cortex motivates but does not control movement initiation in the countermanding task. J Neurosci. 2010;30:1968–1982. doi: 10.1523/JNEUROSCI.4509-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin J, Muraskin J, Sajda P. You Can’t Think and Hit at the Same Time: Neural Correlates of Baseball Pitch Classification. Front Neurosci. 2012;6:177. doi: 10.3389/fnins.2012.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolov AA, Erb M, Gharabaghi A, Grodd W, Tatagiba MS, Pavlova MA. Biological motion processing: the left cerebellum communicates with the right superior temporal sulcus. Neuroimage. 2012;59:2824–2830. doi: 10.1016/j.neuroimage.2011.08.039. [DOI] [PubMed] [Google Scholar]
- Sumner P, Nachev P, Morris P, Peters AM, Jackson SR, Kennard C, Husain M. Human medial frontal cortex mediates unconscious inhibition of voluntary action. Neuron. 2007;54:697–711. doi: 10.1016/j.neuron.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong MH, Joyce CA, Cottrell GW. Why is the fusiform face area recruited for novel categories of expertise? A neurocomputational investigation. Brain Res. 2008;1202:14–24. doi: 10.1016/j.brainres.2007.06.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Boxtel GJ, Brunia CH. Motor and non-motor components of the Contingent Negative Variation. Int J Psychophysiol. 1994;17:269–279. doi: 10.1016/0167-8760(94)90069-8. [DOI] [PubMed] [Google Scholar]
- Walz JM, Goldman RI, Carapezza M, Muraskin J, Brown TR, Sajda P. Simultaneous EEG-fMRI reveals a temporal cascade of task-related and default-mode activations during a simple target detection task. Neuroimage. 2013a doi: 10.1016/j.neuroimage.2013.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz JM, Goldman RI, Carapezza M, Muraskin J, Brown TR, Sajda P. Simultaneous EEG-fMRI Reveals Temporal Evolution of Coupling between Supramodal Cortical Attention Networks and the Brainstem. J Neurosci. 2013b;33:19212–19222. doi: 10.1523/JNEUROSCI.2649-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardak C. The Role of the Supplementary Motor Area in Inhibitory Control in Monkeys and Humans. Journal of Neuroscience. 2011;31:5181–5183. [Google Scholar]
- Witt JK, Dorsch TE. Kicking to bigger uprights: Field goal kicking performance influences perceived size. Perception. 2009;38:1328–1340. doi: 10.1068/p6325. [DOI] [PubMed] [Google Scholar]
- Witt JK, Linkenauger SA. Putting to a bigger hole: Golf performance relates to perceived size. Psychonomic bulletin & review. 2008;15:581–585. doi: 10.3758/pbr.15.3.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt JK, Proffitt DR. See the ball, hit the ball apparent ball size is correlated with batting average. Psychological Science. 2005;16:937–938. doi: 10.1111/j.1467-9280.2005.01640.x. [DOI] [PubMed] [Google Scholar]
- Xu Y. Revisiting the role of the fusiform face area in visual expertise. Cereb Cortex. 2005;15:1234–1242. doi: 10.1093/cercor/bhi006. [DOI] [PubMed] [Google Scholar]
- Yarrow K, Brown P, Krakauer JW. Inside the brain of an elite athlete: the neural processes that support high achievement in sports. Nat Rev Neurosci. 2009;10:585–596. doi: 10.1038/nrn2672. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.