Abstract
Fungal infections due to Candida and Aspergillus species cause extensive morbidity and mortality, especially among immunosuppressed patients, and antifungal therapy is critical to patient management. Yet only a few drug classes are available to treat invasive fungal diseases, and this problem is compounded by the emergence of antifungal resistance. Echinocandin drugs are the preferred choice to treat candidiasis. They are the first cell wall–active agents and target the fungal-specific enzyme glucan synthase, which catalyzes the biosynthesis of β-1,3-glucan, a key cell wall polymer. Therapeutic failures occur rarely among common Candida species, with the exception of Candida glabrata, which are frequently multidrug resistant. Echinocandin resistance in susceptible species is always acquired during therapy. The mechanism of resistance involves amino acid changes in hot-spot regions of Fks subunits of glucan synthase, which decrease the sensitivity of the enzyme to drug. Cellular stress response pathways lead to drug adaptation, which promote the formation of resistant fks strains. Clinical factors promoting echinocandin resistance include empiric therapy, prophylaxis, gastrointestinal reservoirs, and intra-abdominal infections. A better understanding of the echinocandin resistance mechanism, along with cellular and clinical factors promoting resistance, will promote more effective strategies to overcome and prevent echinocandin resistance.
Keywords: echinocandin, caspofungin, micafungin, FKS, glucan synthase, chitin synthase
Introduction
Fungal infections are a major global health problem, with more than 300 million people afflicted, resulting in nearly 1.35 million deaths annually.1 Invasive fungal infections are a consequence of underlying diseases and conditions such as AIDS, cancer, organ transplantation, and corticosteroid therapies, with most deaths resulting from infection with Cryptococcus, Candida, and Aspergillus species.1 In all cases, the successful management of patients with invasive fungal disease requires antifungal therapy. Yet treatment options are restricted, as current antifungal drugs comprise only limited chemical classes represented by polyenes, azoles, flucytosine, and echinocandins.2 Azole drugs (e.g., fluconazole, itraconazole, voriconazole, posaconazole, and isavuconazole) inhibit the biosynthesis of the plasma membrane sterol ergosterol; polyene drugs (e.g., amphotericin B) are pore-forming molecules that bind to ergosterol in the plasma membrane; flucytosine (5-FC) blocks pyrimidine metabolism and DNA synthesis; and the echinocandin drugs (e.g., caspofungin, anidulafungin, and micafungin) are cell wall–active antifungal agents that inhibit the biosynthesis of critical glucan polymers. The echinocandins drugs are now preferred first-line therapy for patients with invasive candidiasis,3 and it has been reported that more than 60% of candidemia patients receive an echinocandin during therapy.4 Given the clinical importance of echinocandins, this review focuses on emerging resistance to echinocandin class drugs and underlying mechanisms.
Echinocandin class drugs
Echinocandin drugs are lipopeptide molecules that non-competitively inhibit β-1,3-D-glucan synthase, which is responsible for the biosynthesis of β-1,3-D-glucan, a principal structural component of fungal cell walls.5 The echinocandin drugs were approved by the U.S. Food and Drug Administration (FDA) for the treatment of esophageal and invasive candidiasis, candidemia, and as empirical therapy in febrile neutropenic patients and prophylaxis in patients undergoing hematopoietic stem cell transplantation (HSCT).6,7 The Infectious Diseases Society of America (IDSA) recommends an echinocandin for most neutropenic patients with candidemia. In the non-neutropenic patient population, echinocandins are recommended in place of an azole, and they are preferred in patients with moderately severe to severe illness, as well as in patients with recent azole exposure.3 Echinocandin drugs are broadly active against most prominent Candida species, where they display in vitro fungicidal activity.8,9 In contrast, they are fungistatic against susceptible molds like the Aspergillus species, where they lyse the apical tips of expanding hyphae, alter hyphal morphology, and modify cell wall composition and organization.10,11 The echinocandins have a limited spectrum and they are inactive against Mucormycetes, Cryptococcus spp., or Fusarium spp. Echinocandins are highly active against azole-resistant yeasts such as C. glabrata and C. krusei,12,13,14 as well as to some Candida biofilms,15–18 since their mechanism of action is unrelated to azoles and they are not substrates for multidrug efflux systems found in highly azole-resistant strains.14
The echinocandins have an outstanding therapeutic index with a low potential for renal or hepatic toxicity or serious drug–drug interactions.19,20 Their low toxicity may reflect the fact that β-1,3-D-glucan synthase, the echinocandin target, is a fungal-specific enzyme not found in humans. All echinocandins have low oral bioavailability and are administered intravenously. Echinocandin drugs are highly serum protein bound, which affects in vitro potency.21–23 They have a prominent Cmax but are largely AUC/MIC (area under the curve/minimum inhibitory concentration) drugs. They distribute well into tissues, but poorly into the CNS and eye.24 The β-1,3-D-glucan synthase target comprises a GTP-binding protein, Rho, which helps regulate the biosynthetic capacity of glucan synthase25 and a catalytic subunit, Fks, encoded by three related genes, FKS1, FKS2, and FKS3. FKS1 is essential in C. albicans26,27 and most other Candida spp., while FKS1 and FKS2 are functionally redundant in C. glabrata.28 FKS3 is expressed at a very low level relative to the other genes,29 and it does not appear to be a major contributor to overall biosynthetic capacity.
Breakpoints and the epidemiology of resistance
The predominant Candida species causing invasive infections are highly susceptible to echinocandin drugs.30,31 The Clinical and Laboratory Standards Institute (CLSI) and the European Committee on Antimicrobial Susceptibility Testing (EUCAST) have established standardized microbroth dilution susceptibility tests for Candida and echinocandins (36–38), which show uniformly potent activity against most Candida species.30,31 From 2003 to 2007, 8271 isolates of Candida spp. were obtained from over 100 centers worldwide and tested with the CLSI M27-A3 broth microdilution method to define wild-type populations and epidemiological cutoff values (ECV). MIC values of ≤ 0.06 mg/ml and ECV values ≤ 0.25 mg/ml were obtained for all major Candida species against the three echinocandin drugs.24 Higher MIC values are observed for C. parapsilosis, 0.25–2 μg/ml; and C. guilliermondii, 0.5–2 μg/ml. Both CLSI and EUCAST have established species and drug-specific clinical breakpoints (CBP) for echinocandin drugs,32,33 and epidemiological cutoff values have been defined for anidulafungin and micafungin against common Candida species.24 EUCAST has not established caspofungin breakpoints and does not recommend caspofungin MIC testing for clinical assessment, owing to interlaboratory testing variability,32 and the CLSI has raised caution when using caspofungin testing, especially with C. glabrata.
Candida spp. isolates resistant to echinocandin drugs are increasingly reported.29,34–45 However, the overall prevalence remains low at approximately 2–3% with C. albicans and most other Candida spp.46–49 Candida glabrata is the major exception for which some centers report high levels of (8–13%) resistance.50–52 The emergence of high resistance in C. glabrata follows from epidemiologic shifts at some centers in which this Candida species is the predominant bloodstream organism recovered from patients, due largely to the rising use of echinocandins and azoles for prophylaxis.53,54 Echinocandin resistance typically emerges after prolonged therapy,43 although it has been reported shortly after initiation of therapy.44,55 Echinocandin resistance of 8.0–9.3% was reported in a recent SENTRY program among 1669 bloodstream isolates (BSI) of C. glabrata.56 Similarly, over a 10-year period, echinocandin resistance in C. glabrata rose from 2–3% to > 13%.50 Alarmingly, the rise in echinocandin resistance among C. glabrata was accompanied by a parallel increase in azole resistance, resulting in multidrug-resistant strains that in some cases are untreatable (Fig. 1). Overall, resistance rates in C. glabrata vary from 3% to 10%, depending on geography and host population.50,51,57–59 The rapid acquisition of mechanism-specific echinocandin resistance by C. glabrata during therapy in an azole-resistant background leading to multidrug resistance with an unfavorable outcome is concerning.
Figure 1.
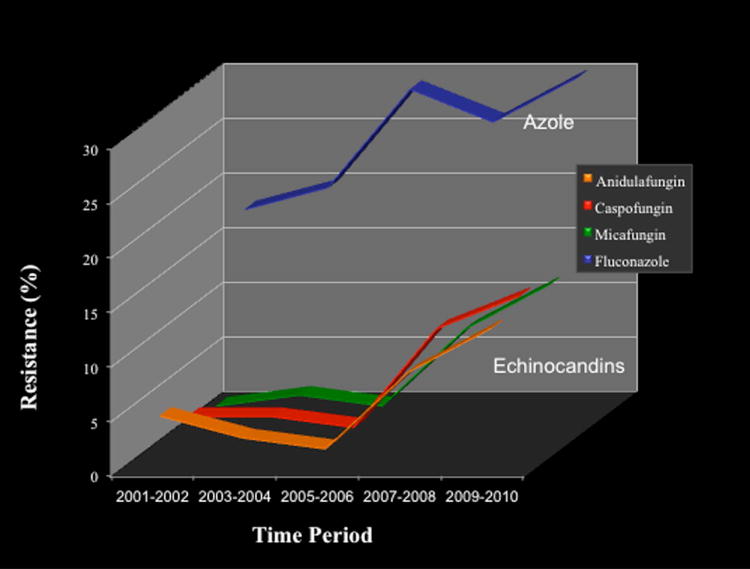
A 10-year profile for antifungal resistance of Candida glabrata isolates to azole and echinocandin drugs. Adapted from Ref. 50.
Acquired resistance mechanism
It is well established that mutations in the FKS genes encoding the catalytic subunits of glucan synthase confer echinocandin resistance in otherwise susceptible Candida species resulting in therapeutic breakthrough infections.60 Amino acid substitutions in Fks subunits induce elevated MIC values (10–100 fold) and reduce the sensitivity of glucan synthase (IC50) to drug by as much as 3000-fold.29,36,61 Prominent mutations in FKS genes are associated with poor pharmacodynamic responses and diminished clinical outcome.62,63 The presence of an FKS mutation is an independent risk factor for echinocandin failure in patients with C. glabrata infections, and the presence of an FKS mutation was superior to MIC in predicting clinical response, especially when caspofungin is used for testing.63
Amino acid substitutions associated with resistance occur in two limited but highly conserved hot-spot regions of Fks36,64,65 encompassing residues Phe641–Pro649 and Arg1361 (or equivalent) in C. albicans and most other Candida spp. Amino acid substitutions at Ser645 and Phe641 cause the most pronounced phenotypes7,29,36,66 and they are the most abundant, accounting for more than 75% of resistance in C. albicans.7 These fks mutant strains are largely insensitive to drug and respond poorly or not at all in pharmacodynamic studies of murine models of infection.67–70 In C. glabrata, resistance occurs in homologous regions of FKS1 and FKS2.29,61 Amino acid substitutions occur at twice the frequency in Fks2 relative to Fks1,7,29,66 and modifications at amino acid positions Ser629 and Ser663 and Fks2 position F659S confer the highest IC50 and MIC values. In some cases, nonsense mutations occur in either FKS1 or FKS2 (C. glabrata), which confer prominent resistance.29,61,71 Resistance conferring amino acid substitutions can alter the catalytic capacity of glucan synthase,36 which is sensed by the cell, resulting in altered gene expression for FKS1 and FKS2, which may impact susceptibility.28,29 FKS2 expression is also downregulated by the immunosuppressant tacrolimus (FK506),72 and it can be used to overcome resistance conferred by FKS2 via suppression of gene expression.28 The FKS echinocandin-resistance mechanism resulting in elevated MIC values and clinical failures is widely observed in Candida species including C. tropicalis, C. krusei and C. kefyr55,73,74 (Fig. 1A). Mapping of the mutational hot spots on a topology map for Fks1 indicates that amino acid substitution occur near the extracellular membrane surface within transmembrane segments 6 and 7 (Fig. 1B). The external location may suggest a potential interaction site for echinocandin drugs that does not require entry into the cell.75
Hot spot polymorphisms and inherent reduced susceptibility
Mutations in the FKS hotspot regions cause a range of phenotypes, which correlate with modification of drug–target interactions, resulting in altered kinetic inhibition (IC50).29,36 Some mutations, like those at positions Phe641 and Ser645 in C. albicans and related species, confer the most pronounced phenotypes. However, other mutations in the hot spot region, especially those near the C-terminal end of hot spot 1, cause less pronounced phenotypes. Notable examples are the naturally occurring polymorphisms at Pro649 in the C. parapsilosis complex (C. parapsilosis sensu stricto, C. orthopsilosis, and C. metapsilosis) and at Met633 and Ala634 in C. guilliermondii, which display inherently high MIC values relative to other Candida species.24,54,76 Intrinsic reduced susceptibility carries an uncertain clinical significance, as these infecting strains are often successfully treated with echinocandins at established dosages,77–79 but it varies with patient population.80–82 It has been shown for C. parapsilosis that glucan synthase is 10- to 50-fold less sensitive to the echinocandins relative to enzymes from C. albicans,83 which accounts for the higher MIC values. But the enzyme, while less sensitive, is still inhibited at typical therapeutic drug concentrations, which accounts for clinical response. A third region defined by W695 (outside clinical hot spots 1 and 2) of Saccharomyces cerevisiae Fks1 was found,84 but it is not associated with clinical failures.
Fks defects and fitness
In C. albicans, amino acid substitutions in Fks1 conferring echinocandin resistance carry a fitness cost, as they compete much less efficiently with isogenic FKS wild-type strains in murine models of infection.28,29,85 This is largely due to the fact that these substitutions decrease the catalytic Vmax for the biosynthesis of β-1,3-D-gliucan,29,36 which alters the cell wall composition and morphology, making mutant cells somewhat less fit relative to wild type.85 Collectively, this data is consistent with the observation that horizontal transmission is rarely, if ever, encountered, and resistance involves de novo acquired resistance.
Biofilms
Like bacteria, fungal biofilms are organized in a complex communal structure consisting of cells embedded within an extensive polysaccharide matrix.86 In Candida and Aspergillus species, the extracellular biofilm matrix is composed predominantly of β-glucan, which sequesters drugs and effectively decreases their concentration at the level of the cell membrane.87 Genetic or chemical modulation of extracellular glucan production enhances cellular susceptibility to antifungal agents.88 A number of global transcriptional regulators such as Rlm and Smi1 and genes such as FSK regulate glucan formation, leading to resistant biofilms.88
Stress adaptation and resistance emergence
The fungal cell wall is a dynamic structure that changes during growth and development, and requires remodeling of the crosslinking of β-1,3- and β-1,6-glucans. As the cell wall is critical to fungal cell survival, agents like echinocandin drugs that alter cell wall integrity induce significant cellular stress. In response, fungi possess a repertoire of adaptive response mechanisms that protect against such destabilizing environmental stresses.60,89 Echinocandins induce a set of genes from the protein kinase C (PKC) cell integrity–signaling pathway,90 as well as those required for cell wall maintenance and architecture. Stress signals at the cell surface are transmitted to Rho1 GTPase, which mobilizes a variety of effectors. Activation of cell wall integrity signaling alters the production of various carbohydrate polymers of the cell wall, along with cell wall architecture and remodeling.91 Inhibition of glucan biosynthesis by the echinocandins induces PKC, Ca2+/calcineurin/Crz1, and HOG (high osmolarity glycerol),92,93 which mediate the response. Hsp90 is also induced, leading to tolerance to echinocandin drugs through its client proteins calcineurin and Mkc194,95 and co-chaperone Sgt1.95–97 Hsp90 orchestrates cellular stress response circuitry that has a profound impact on both azole and echinocandin resistance, and genetic or chemical modulation of Hsp90 reduces echinocandin tolerance.95,97–101 Echinocandin action also induces compensatory increases in chitin synthesis, which maintains the structural integrity of the cell wall, as chitin replaces β-1,3-glucan and decreases sensitivity to the drug.92,93,102–104 Compensatory increases in chitin are coordinated by the PKC, HOG, and calcineurin signaling pathways.92 For most Candida species, activation of Chs2 and Chs8 enables survival in the presence of fungicidal levels of echinocandins.93,105 In C. glabrata, the terminal MAPK of the PKC signaling pathway, Slt2, controls chitin increase in response to echinocandins.106 Increases in chitin levels have been linked to paradoxical growth observed at very high echinocandin levels exceeding normal therapeutic doses.107–109 Finally, membrane sphingolipids can interact with echinocandins and modulate enzyme sensitivity to the drug.110,111 Overall, adaptive cellular responses stabilize cells in the presence of the drug, which affords cells time to escape drug action by forming FKS hot-spot mutations (Fig. 3).
Figure 3.
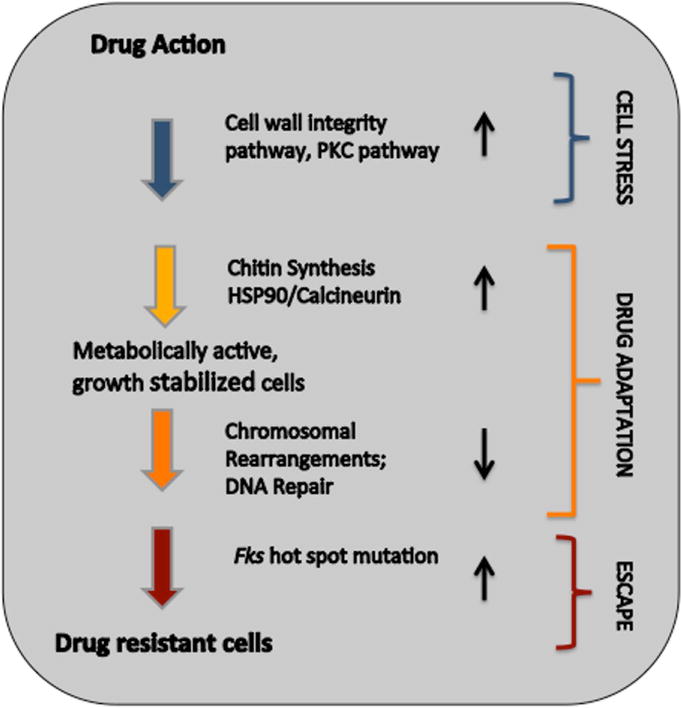
Schema depicting critical stages in evolution of drug resistance following drug exposure.
Clinical reservoirs and microbial factors driving resistance
The gastrointestinal tract is a normal commensal site for Candida species, and genotyping confirms that colonizing isolates are often the infecting strain for most patients with invasive disease.112 Candida colonization of the gastrointestinal tract is associated with a mixed bacterial and fungal biofilm.113 Drug penetration into the glucan matrix of the biofilm is irregular,87 and there are varying levels of drug exposure resulting in the emergence of drug-tolerant and fks resistant mutants. In the presence of drug, these resistant cells proliferate and are available to cause systemic infections. The biofilm acts as a reservoir that seeds resistant infections. Another important reservoir involves intra-abdominal candidiasis, which occurs in 40% of patients with repeated gastrointestinal surgery, perforation. or necrotizing pancreatitis.114 These high-burden infections, along with poor drug penetration, are a critical reservoir that promotes resistance.
Like most anti-infectives, a close association exists between drug exposure and the emergence of resistance. It is well-established that FKS-mediated resistance is directly linked to prior, prolonged, and/or repeated drug exposure.43,115–117 As total drug exposure is an important driver of resistance, there is some concern that antifungal prophylaxis, which is intended to prevent infection, may promote the development of breakthrough resistance. The echinocandin drugs have favorable pharmacokinetics and safety profiles that are well suited for prophylaxis. Micafungin is FDA approved for prophylaxis of Candida infections in patients undergoing hematopoietic stem cell transplantation (HSCT) or expected to be neutropenic for at least 10 days,118 and the European Society of Clinical Microbiology and Infectious Diseases guidelines also recommend micafungin for prophylaxis against Candida infections in allogeneic HSCT adult and pediatric patients, as well as in pediatric patients with acute myeloid and recurrent leukemia.119 Both micafungin and caspofungin have been used for prophylaxis in adults and children;120–124 meta-analyses affirm that prophylaxis reduces the incidence of invasive fungal infections.125,126 However, the increasing use of echinocandin drugs for prophylaxis is a concern for increasing resistance. Low-dose prophylaxis has been linked to emergence of echinocandin resistance in C. glabrata,127 as well as in C. albicans.128 The expanding use of echinocandin prophylaxis among patients at high risk for invasive fungal infections is likely to fuel an increase in the frequency of isolates that are resistant to multiple classes of antifungal drugs.
Finally, the association between increasing echinocandin and azole resistance in C. glabrata, resulting in multidrug-resistant strains, is a major concern. Selection pressure from prophylaxis and therapy affecting high-burden reservoirs contributes greatly. Microbial factors play a critical role in this process. In contrast to the echinocandins, azole drug resistance resulting in clinical failure may be caused by a variety of genetic changes, most of which affect the expression of fungal drug transporters or the structure and/or expression of fungal drug targets.129 Chromosomal instability is rapidly observed following exposure to azoles or echinocandins, whereby Candida cells can undergo unequal division to produce aneuploid progeny.130 This intrinsic property of yeasts strongly suggests that cellular stress increases genetic diversity by altering genome integrity.131 Stress-induced aneuploidy depends on the function of a stress-inducible protein chaperone HSP90, which through its client proteins influences chromosome segregation and cell cycle progression.98,132 Aneuploidy can also promote a variety of other genomic rearrangements and mutagenic lesions leading to altered drug phenotypes.133
Conclusions
Overall, echinocandin resistance is uncommon, as most infecting Candida species retain drug susceptibility. Yet, acquired drug resistance resulting in therapeutic failures, especially among immunosuppressed patients on long-duration or repeated therapy, is a significant factor in certain clinical high-risk settings. The recent emergence of multidrug resistance to azole and echinocandin class drugs among C. glabrata strains is worrisome. The FKS mechanism conferring stable drug resistance is well defined, but it is important to identify critical genetic factors that promote prominent fks mutant genotypes. Fungi robustly respond to stress through a variety of compensatory mechanisms, as well as through chromosomal modifications, that stabilize cells in response to drug and facilitate escape through the formation of characteristic FKS hot-spot mutations. Finally, total drug exposure and the expanding use of prophylaxis, along with host microbial reservoirs that limit drug access, are important contributors to resistance emergence in critically ill patients. Given these considerations, there is an opportunity to enhance initiation of appropriate therapy by incorporating molecular resistance testing to shorten diagnostic delays and limit resistance emergence by reassessing therapeutic dosing strategies and improved stewardship.
Figure 2.
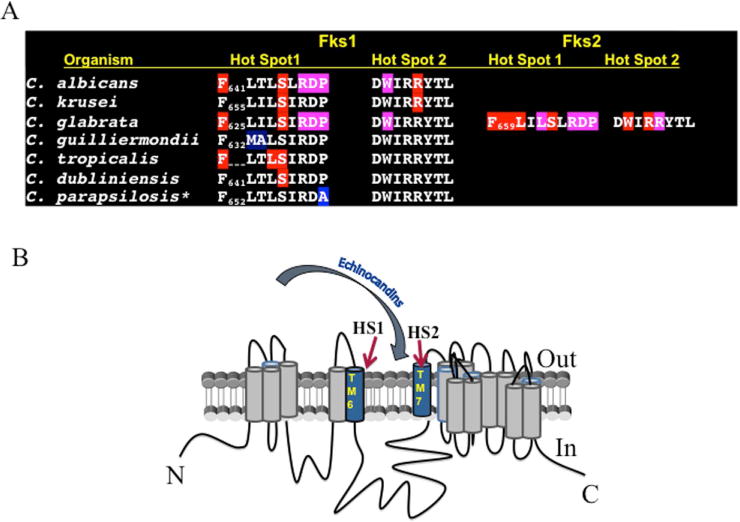
(A) Spectrum of Fks amino acid changes conferring clinical resistance. Amino acid sequences of Fks hot-spot sequences for major Candida species and positions associated with prominent resistance (red), weaker resistance (purple) and naturally occurring reduced susceptibility (blue). (B) Topology model for glucan synthase and predicted positions of amino acid substitutions conferring echinocandin resistance. Adapted from Ref. 75.
Acknowledgments
D.S.P. is supported by NIH Grant AI109025 and by Astellas.
References
- 1.Brown GD, et al. Hidden killers: human fungal infections. Sci Transl Med. 2012;4:165rv113. doi: 10.1126/scitranslmed.3004404. [DOI] [PubMed] [Google Scholar]
- 2.Odds FC, Brown AJ, Gow NA. Antifungal agents: mechanisms of action. Trends Microbiol. 2003;11:272–279. doi: 10.1016/s0966-842x(03)00117-3. [DOI] [PubMed] [Google Scholar]
- 3.Pappas PG, et al. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:503–535. doi: 10.1086/596757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cleveland AA, et al. Changes in incidence and antifungal drug resistance in candidemia: results from population-based laboratory surveillance in Atlanta and Baltimore, 2008–2011. Clin Infect Dis. 2012;55:1352–1361. doi: 10.1093/cid/cis697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Onishi J, et al. Discovery of novel antifungal (1,3)-beta-D-glucan synthase inhibitors. Antimicrobial Agents and Chemotherapy. 2000;44:368–377. doi: 10.1128/aac.44.2.368-377.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turner MS, Drew RH, Perfect JR. Emerging echinocandins for treatment of invasive fungal infections. Expert Opin Emerg Drugs. 2006;11:231–250. doi: 10.1517/14728214.11.2.231. [DOI] [PubMed] [Google Scholar]
- 7.Perlin DS. Current perspectives on echinocandin class drugs. Future Microbiol. 2011;6:441–457. doi: 10.2217/fmb.11.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barchiesi F, et al. Comparison of the fungicidal activities of caspofungin and amphotericin B against Candida glabrata. Antimicrobial Agents and Chemotherapy. 2005;49:4989–4992. doi: 10.1128/AAC.49.12.4989-4992.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ernst EJ, et al. In vitro pharmacodynamic properties of MK-0991 determined by time-kill methods. Diagn Microbiol Infect Dis. 1999;33:75–80. doi: 10.1016/s0732-8893(98)00130-8. [DOI] [PubMed] [Google Scholar]
- 10.Bowman JC, et al. Efficacy of caspofungin against Aspergillus flavus, Aspergillus terreus, and Aspergillus nidulans. Antimicrobial Agents and Chemotherapy. 2006;50:4202–4205. doi: 10.1128/AAC.00485-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bowman JC, et al. The antifungal echinocandin caspofungin acetate kills growing cells of Aspergillus fumigatus in vitro. Antimicrobial Agents and Chemotherapy. 2002;46:3001–3012. doi: 10.1128/AAC.46.9.3001-3012.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pfaller MA, et al. Caspofungin activity against clinical isolates of fluconazole-resistant Candida. J Clin Microbiol. 2003;41:5729–5731. doi: 10.1128/JCM.41.12.5729-5731.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bachmann SP, Patterson TF, Lopez-Ribot JL. In vitro activity of caspofungin (MK-0991) against Candida albicans clinical isolates displaying different mechanisms of azole resistance. J Clin Microbiol. 2002;40:2228–2230. doi: 10.1128/JCM.40.6.2228-2230.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Niimi K, et al. Overexpression of Candida albicans CDR1, CDR2, or MDR1 does not produce significant changes in echinocandin susceptibility. Antimicrobial Agents and Chemotherapy. 2006;50:1148–1155. doi: 10.1128/AAC.50.4.1148-1155.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bachmann SP, et al. Antifungal combinations against Candida albicans biofilms in vitro. Antimicrobial Agents and Chemotherapy. 2003;47:3657–3659. doi: 10.1128/AAC.47.11.3657-3659.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferreira JA, et al. Biofilm formation and effect of caspofungin on biofilm structure of Candida species bloodstream isolates. Antimicrobial Agents and Chemotherapy. 2009;53:4377–4384. doi: 10.1128/AAC.00316-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuhn DM, et al. Antifungal susceptibility of Candida biofilms: unique efficacy of amphotericin B lipid formulations and echinocandins. Antimicrobial Agents and Chemotherapy. 2002;46:1773–1780. doi: 10.1128/AAC.46.6.1773-1780.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simitsopoulou M, et al. Species-specific and drug-specific differences in susceptibility of Candida biofilms to echinocandins: characterization of less common bloodstream isolates. Antimicrobial Agents and Chemotherapy. 2013;57:2562–2570. doi: 10.1128/AAC.02541-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen SC, Slavin MA, Sorrell TC. Echinocandin antifungal drugs in fungal infections: a comparison. Drugs. 2011;71:11–41. doi: 10.2165/11585270-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 20.Kofla G, Ruhnke M. Pharmacology and metabolism of anidulafungin, caspofungin and micafungin in the treatment of invasive candidosis: review of the literature. Eur J Med Res. 2011;16:159–166. doi: 10.1186/2047-783X-16-4-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Odabasi Z, et al. Effects of serum on in vitro susceptibility testing of echinocandins. Antimicrobial Agents and Chemotherapy. 2007;51:4214–4216. doi: 10.1128/AAC.01589-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paderu P, et al. Serum differentially alters the antifungal properties of echinocandin drugs. Antimicrobial Agents and Chemotherapy. 2007;51:2253–2256. doi: 10.1128/AAC.01536-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wiederhold NP, et al. In vivo efficacy of anidulafungin and caspofungin against Candida glabrata and association with in vitro potency in the presence of sera. Antimicrobial Agents and Chemotherapy. 2007;51:1616–1620. doi: 10.1128/AAC.00105-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pfaller MA, et al. Wild-type MIC distributions and epidemiological cutoff values for the echinocandins and Candida spp. Journal of Clinical Microbiology. 2010;48:52–56. doi: 10.1128/JCM.01590-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mazur P, Baginsky W. In vitro activity of 1,3-beta-D-glucan synthase requires the GTP-binding protein Rho1. J Biol Chem. 1996;271:14604–14609. doi: 10.1074/jbc.271.24.14604. [DOI] [PubMed] [Google Scholar]
- 26.Mio T, et al. Cloning of the Candida albicans homolog of Saccharomyces cerevisiae GSC1/FKS1 and its involvement in beta-1,3-glucan synthesis. J Bacteriol. 1997;179:4096–4105. doi: 10.1128/jb.179.13.4096-4105.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thompson JR, et al. A glucan synthase FKS1 homolog in cryptococcus neoformans is single copy and encodes an essential function. J Bacteriol. 1999;181:444–453. doi: 10.1128/jb.181.2.444-453.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katiyar SK, et al. Fks1 and Fks2 are functionally redundant but differentially regulated in Candida glabrata: implications for echinocandin resistance. Antimicrobial Agents and Chemotherapy. 2012;56:6304–6309. doi: 10.1128/AAC.00813-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garcia-Effron G, et al. Effect of Candida glabrata FKS1 and FKS2 mutations on echinocandin sensitivity and kinetics of 1,3-beta-D-glucan synthase: implication for the existing susceptibility breakpoint. Antimicrobial Agents and Chemotherapy. 2009;53:3690–3699. doi: 10.1128/AAC.00443-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pfaller MA, et al. Multicenter study of anidulafungin and micafungin MIC distributions and epidemiological cutoff values for eight Candida species and the CLSI M27-A3 broth microdilution method. Antimicrobial Agents and Chemotherapy. 2014;58:916–922. doi: 10.1128/AAC.02020-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pfaller MA, et al. Echinocandin and triazole antifungal susceptibility profiles for clinical opportunistic yeast and mold isolates collected from 2010 to 2011: application of new CLSI clinical breakpoints and epidemiological cutoff values for characterization of geographic and temporal trends of antifungal resistance. Journal of Clinical Microbiology. 2013;51:2571–2581. doi: 10.1128/JCM.00308-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arendrup MC, et al. Breakpoints for antifungal agents: An update from EUCAST focussing on echinocandins against Candida spp. and triazoles against Aspergillus spp. Drug Resistance Updates. 2013;16:81–95. doi: 10.1016/j.drup.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 33.Pfaller MA, et al. Clinical breakpoints for the echinocandins and Candida revisited: integration of molecular, clinical, and microbiological data to arrive at species-specific interpretive criteria. Drug Resistance Updates. 2011;14:164–176. doi: 10.1016/j.drup.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 34.Cleary JD, et al. Reduced Candida glabrata susceptibility secondary to an FKS1 mutation developed during candidemia treatment. Antimicrobial Agents and Chemotherapy. 2008;52:2263–2265. doi: 10.1128/AAC.01568-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garcia-Effron G, et al. Novel FKS mutations associated with echinocandin resistance in Candida species. Antimicrobial Agents and Chemotherapy. 2010;54:2225–2227. doi: 10.1128/AAC.00998-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garcia-Effron G, Park S, Perlin DS. Correlating echinocandin MIC and kinetic inhibition of fks1 mutant glucan synthases for Candida albicans implications for interpretive breakpoints. Antimicrobial Agents and Chemotherapy. 2009;53:112–122. doi: 10.1128/AAC.01162-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kahn JN, et al. Acquired echinocandin resistance in a Candida krusei isolate due to modification of glucan synthase. Antimicrobial Agents and Chemotherapy. 2007;51:1876–1878. doi: 10.1128/AAC.00067-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Laverdiere M, et al. Progressive loss of echinocandin activity following prolonged use for treatment of Candida albicans oesophagitis. J Antimicrob Chemother. 2006;57:705–708. doi: 10.1093/jac/dkl022. [DOI] [PubMed] [Google Scholar]
- 39.Miller CD, et al. Progressive esophagitis caused by Candida albicans with reduced susceptibility to caspofungin. Pharmacotherapy. 2006;26:877–880. doi: 10.1592/phco.26.6.877. [DOI] [PubMed] [Google Scholar]
- 40.Garcia-Effron G, et al. Caspofungin-resistant Candida tropicalis strains causing breakthrough fungemia in patients at high risk for hematologic malignancies. Antimicrobial Agents and Chemotherapy. 2008;52:4181–4183. doi: 10.1128/AAC.00802-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wiederhold NP, et al. Pyrosequencing to detect mutations in FKS1 that confer reduced echinocandin susceptibility in Candida albicans. Antimicrobial Agents and Chemotherapy. 2008;52:4145–4148. doi: 10.1128/AAC.00959-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pfeiffer CD, et al. Breakthrough invasive candidiasis in patients on micafungin. Journal of Clinical Microbiology. 2010;48:2373–2380. doi: 10.1128/JCM.02390-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thompson GR, 3rd, et al. Development of caspofungin resistance following prolonged therapy for invasive candidiasis secondary to Candida glabrata infection. Antimicrobial Agents and Chemotherapy. 2008;52:3783–3785. doi: 10.1128/AAC.00473-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lewis JS, 2nd, et al. Rapid Emergence of Echinocandin Resistance in Candida glabrata Resulting in Clinical and Microbiologic Failure. Antimicrobial Agents and Chemotherapy. 2013;57:4559–4561. doi: 10.1128/AAC.01144-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dannaoui E, et al. Candida spp. with Acquired Echinocandin Resistance, France, 2004–2010(1) Emerg Infect Dis. 2012;18:86–90. doi: 10.3201/eid1801.110556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pfaller MA, et al. Echinocandin and triazole antifungal susceptibility profiles for Candida spp., Cryptococcus neoformans, and Aspergillus fumigatus: application of new CLSI clinical breakpoints and epidemiologic cutoff values to characterize resistance in the SENTRY Antimicrobial Surveillance Program (2009) Diagn Microbiol Infect Dis. 2011;69:45–50. doi: 10.1016/j.diagmicrobio.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 47.Pfaller MA, et al. Candida bloodstream infections: comparison of species distribution and resistance to echinocandin and azole antifungal agents in Intensive Care Unit (ICU) and non-ICU settings in the SENTRY Antimicrobial Surveillance Program (2008–2009) Int J Antimicrob Agents. 2011;38:65–69. doi: 10.1016/j.ijantimicag.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 48.Pfaller MA, et al. Geographic variations in species distribution and echinocandin and azole antifungal resistance rates among Candida bloodstream infection isolates: report from the SENTRY Antimicrobial Surveillance Program 2008 to 2009. Journal of Clinical Microbiology. 2011;49:396–399. doi: 10.1128/JCM.01398-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Castanheira M, et al. Low prevalence of fks1 hot spot 1 mutations in a worldwide collection of Candida strains. Antimicrobial Agents and Chemotherapy. 2010;54:2655–2659. doi: 10.1128/AAC.01711-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alexander BD, et al. Increasing echinocandin resistance in Candida glabrata: clinical failure correlates with presence of FKS mutations and elevated minimum inhibitory concentrations. Clin Infect Dis. 2013;56:1724–1732. doi: 10.1093/cid/cit136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pfaller MA, et al. Frequency of decreased susceptibility and resistance to echinocandins among fluconazole-resistant bloodstream isolates of Candida glabrata. Journal of Clinical Microbiology. 2012;50:1199–1203. doi: 10.1128/JCM.06112-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Farmakiotis D, Tarrand JJ, Kontoyiannis DP. Drug-Resistant Candida glabrata Infection in Cancer Patients. Emerging Infectious Diseases. 2014;20:1833–1840. doi: 10.3201/eid2011.140685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lortholary O, et al. Recent exposure to caspofungin or fluconazole influences the epidemiology of candidemia: a prospective multicenter study involving 2,441 patients. Antimicrobial Agents and Chemotherapy. 2011;55:532–538. doi: 10.1128/AAC.01128-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tortorano AM, et al. A 1-year prospective survey of candidemia in Italy and changing epidemiology over one decade. Infection. 2013;41:655–662. doi: 10.1007/s15010-013-0455-6. [DOI] [PubMed] [Google Scholar]
- 55.Fekkar A, et al. Rapid emergence of echinocandin resistance during Candida kefyr fungemia treatment with caspofungin. Antimicrobial Agents and Chemotherapy. 2013;57:2380–2382. doi: 10.1128/AAC.02037-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pfaller MA, et al. Frequency of Decreased Susceptibility and Resistance to Echinocandins Among Fluconazole-Resistant Bloodstream Isolates of Candida glabrata: Results from the SENTRY Antimicrobial Surveillance Program 2006–2010 and the Centers for Disease Control and Prevention Population-Based Surveillance 2008–2010. J Clin Microbiol. 2012;50:1199–203. doi: 10.1128/JCM.06112-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Eschenauer GA, et al. Real-world experience with echinocandin MICs against Candida species in a multicenter study of hospitals that routinely perform susceptibility testing of bloodstream isolates. Antimicrobial Agents and Chemotherapy. 2014;58:1897–1906. doi: 10.1128/AAC.02163-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Arendrup MC, Perlin DS. Echinocandin resistance: an emerging clinical problem? Curr Opin Infect Dis. 2014;27:484–492. doi: 10.1097/QCO.0000000000000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pham CD, et al. Role of FKS Mutations in Candida glabrata: MIC values, echinocandin resistance, and multidrug resistance. Antimicrob Agents Chemother. 2014;58:4690–4696. doi: 10.1128/AAC.03255-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Perlin DS. Resistance to echinocandin-class antifungal drugs. Drug Resistance Updates. 2007;10:121–130. doi: 10.1016/j.drup.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Katiyar S, Pfaller M, Edlind T. Candida albicans and Candida glabrata clinical isolates exhibiting reduced echinocandin susceptibility. Antimicrobial Agents and Chemotherapy. 2006;50:2892–2894. doi: 10.1128/AAC.00349-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lackner M, et al. Position and numbers of FKS mutations in C. albicans selectively influence in vitro and in vivo susceptibility to echinocandin treatment. Antimicrobial Agents and Chemotherapy. 2014;58:3626–3635. doi: 10.1128/AAC.00123-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shields RK, et al. The presence of an FKS mutation rather than MIC is an independent risk factor for failure of echinocandin therapy among patients with invasive candidiasis due to Candida glabrata. Antimicrobial Agents and Chemotherapy. 2012;56:4862–4869. doi: 10.1128/AAC.00027-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Johnson ME, Katiyar SK, Edlind TD. A new Fks hotspot for acquired echinocandin resistance in yeast, and its contribution to intrinsic resistance of Scedosporium species. Antimicrobial Agents and Chemotherapy. 2011;55:3774–3781. doi: 10.1128/AAC.01811-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Katiyar SK, Edlind TD. Role for Fks1 in the intrinsic echinocandin resistance of Fusarium solani as evidenced by hybrid expression in Saccharomyces cerevisiae. Antimicrobial Agents and Chemotherapy. 2009;53:1772–1778. doi: 10.1128/AAC.00020-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Perlin DS. Echinocandin-resistant Candida: molecular methods and phenotypes. Curr Fungal Infect Rep. 2011;5:113–119. [Google Scholar]
- 67.Arendrup MC, et al. Differential In vivo activity of Anidulafungin, Caspofungin and Micafungin against C. glabrata with and without FKS resistance mutations. Antimicrobial Agents and Chemotherapy. 2012;56:2435–2442. doi: 10.1128/AAC.06369-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Howard SJ, et al. Pharmacokinetics and pharmacodynamics of posaconazole for invasive pulmonary aspergillosis: clinical implications for antifungal therapy. J Infect Dis. 2011;203:1324–1332. doi: 10.1093/infdis/jir023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Slater JL, et al. Disseminated Candidiasis Caused by Candida albicans with Amino Acid Substitutions in Fks1 at Position Ser645 Cannot Be Successfully Treated with Micafungin. Antimicrobial Agents and Chemotherapy. 2011;55:3075–3083. doi: 10.1128/AAC.01686-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wiederhold NP, et al. Caspofungin dose escalation for invasive candidiasis due to resistant Candida albicans. Antimicrobial Agents and Chemotherapy. 2011;55:3254–3260. doi: 10.1128/AAC.01750-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Castanheira M, et al. Frequency of fks mutations among Candida glabrata isolates from a 10-year global collection of bloodstream infection isolates. Antimicrobial Agents and Chemotherapy. 2014;58:577–580. doi: 10.1128/AAC.01674-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Eng WK, et al. The yeast FKS1 gene encodes a novel membrane protein, mutations in which confer FK506 and cyclosporin A hypersensitivity and calcineurin-dependent growth. Gene. 1994;151:61–71. doi: 10.1016/0378-1119(94)90633-5. [DOI] [PubMed] [Google Scholar]
- 73.Jensen RH, Johansen HK, Arendrup MC. Stepwise development of a homozygous S80P substitution in Fks1p, conferring echinocandin resistance in Candida tropicalis. Antimicrobial Agents and Chemotherapy. 2013;57:614–617. doi: 10.1128/AAC.01193-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pasquale T, et al. Emergence of Candida tropicalis resistant to caspofungin. J Antimicrob Chemother. 2008;61:219. doi: 10.1093/jac/dkm453. [DOI] [PubMed] [Google Scholar]
- 75.Johnson ME, Edlind TD. Topological and mutational analysis of Saccharomyces cerevisiae Fks1. Eukaryotic Cell. 2012;11:952–960. doi: 10.1128/EC.00082-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pfaller MA, et al. In vitro susceptibility of invasive isolates of Candida spp. to anidulafungin, caspofungin, and micafungin: six years of global surveillance. J Clin Microbiol. 2008;46:150–156. doi: 10.1128/JCM.01901-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mora-Duarte J, et al. Comparison of caspofungin and amphotericin B for invasive candidiasis. N Engl J Med. 2002;347:2020–2029. doi: 10.1056/NEJMoa021585. [DOI] [PubMed] [Google Scholar]
- 78.Kale-Pradhan PB, et al. Comparative efficacy of echinocandins and nonechinocandins for the treatment of Candida parapsilosis Infections: a meta-analysis. Pharmacotherapy. 2010;30:1207–1213. doi: 10.1592/phco.30.12.1207. [DOI] [PubMed] [Google Scholar]
- 79.Colombo AL, et al. Global distribution and outcomes for Candida species causing invasive candidiasis: results from an international randomized double-blind study of caspofungin versus amphotericin B for the treatment of invasive candidiasis. Eur J Clin Microbiol Infect Dis. 2003;22:470–474. doi: 10.1007/s10096-003-0973-8. [DOI] [PubMed] [Google Scholar]
- 80.Ghannoum MA, et al. Differential in vitro activity of anidulafungin, caspofungin and micafungin against Candida parapsilosis isolates recovered from a burn unit. Clin Microbiol Infect. 2009;15:274–279. doi: 10.1111/j.1469-0691.2008.02660.x. [DOI] [PubMed] [Google Scholar]
- 81.Kabbara N, et al. Breakthrough C. parapsilosis and C. guilliermondii blood stream infections in allogeneic hematopoietic stem cell transplant recipients receiving long-term caspofungin therapy. Haematologica. 2008;93:639–640. doi: 10.3324/haematol.11149. [DOI] [PubMed] [Google Scholar]
- 82.Forrest GN, Weekes E, Johnson JK. Increasing incidence of Candida parapsilosis candidemia with caspofungin usage. J Infect. 2008;56:126–129. doi: 10.1016/j.jinf.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 83.Garcia-Effron G, et al. A naturally occurring proline-to-alanine amino acid change in Fks1p in Candida parapsilosis, Candida orthopsilosis, and Candida metapsilosis accounts for reduced echinocandin susceptibility. Antimicrob Agents Chemother. 2008;52:2305–2312. doi: 10.1128/AAC.00262-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Johnson ME, Katiyar SK, Edlind TD. New Fks hot spot for acquired echinocandin resistance in Saccharomyces cerevisiae and its contribution to intrinsic resistance of Scedosporium species. Antimicrobial Agents and Chemotherapy. 2011;55:3774–3781. doi: 10.1128/AAC.01811-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ben-Ami R, et al. The fitness and virulence cost of fks1 mutations causing echinocandin-resistance in Candida albicans. The Journal of Infectious Diseases. 2011 doi: 10.1093/infdis/jir351. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.d’Enfert C. Biofilms and their role in the resistance of pathogenic Candida to antifungal agents. Curr Drug Targets. 2006;7:465–470. doi: 10.2174/138945006776359458. [DOI] [PubMed] [Google Scholar]
- 87.Mitchell KF, et al. Role of matrix beta-1,3 glucan in antifungal resistance of non-albicans Candida biofilms. Antimicrobial agents and chemotherapy. 2013;57:1918–1920. doi: 10.1128/AAC.02378-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Desai JV, et al. Regulatory role of glycerol in Candida albicans biofilm formation. MBio. 2013;4:e00637–00612. doi: 10.1128/mBio.00637-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Walker LA, Gow NA, Munro CA. Fungal echinocandin resistance. Fungal Genetics and Biology. 2010;47:117–126. doi: 10.1016/j.fgb.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Reinoso-Martin C, et al. The yeast protein kinase C cell integrity pathway mediates tolerance to the antifungal drug caspofungin through activation of Slt2p mitogen-activated protein kinase signaling. Eukaryotic Cell. 2003;2:1200–1210. doi: 10.1128/EC.2.6.1200-1210.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Levin DE. Regulation of cell wall biogenesis in Saccharomyces cerevisiae: the cell wall integrity signaling pathway. Genetics. 2011;189:1145–1175. doi: 10.1534/genetics.111.128264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Munro CA, et al. The PKC, HOG and Ca2+ signalling pathways co-ordinately regulate chitin synthesis in Candida albicans. Molecular Microbiology. 2007;63:1399–1413. doi: 10.1111/j.1365-2958.2007.05588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Walker LA, et al. Stimulation of chitin synthesis rescues Candida albicans from echinocandins. PLoS Pathogens. 2008;4:e1000040. doi: 10.1371/journal.ppat.1000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.LaFayette SL, et al. PKC signaling regulates drug resistance of the fungal pathogen Candida albicans via circuitry comprised of Mkc1, calcineurin, and Hsp90. PLoS Pathogens. 2010;6:e1001069. doi: 10.1371/journal.ppat.1001069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Singh SD, et al. Hsp90 governs echinocandin resistance in the pathogenic yeast Candida albicans via calcineurin. PLoS Pathogens. 2009;5:e1000532. doi: 10.1371/journal.ppat.1000532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shapiro RS, et al. The Hsp90 co-chaperone Sgt1 governs Candida albicans morphogenesis and drug resistance. PLoS One. 2012;7:e44734. doi: 10.1371/journal.pone.0044734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Singh-Babak SD, et al. Global analysis of the evolution and mechanism of echinocandin resistance in Candida glabrata. PLoS Pathog. 2012;8:e1002718. doi: 10.1371/journal.ppat.1002718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cowen LE. Hsp90 orchestrates stress response signaling governing fungal drug resistance. PLoS Pathog. 2009;5:e1000471. doi: 10.1371/journal.ppat.1000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cowen LE, Lindquist S. Hsp90 potentiates the rapid evolution of new traits: drug resistance in diverse fungi. Science. 2005;309:2185–2189. doi: 10.1126/science.1118370. [DOI] [PubMed] [Google Scholar]
- 100.Lamoth F, et al. Transcriptional activation of heat shock protein 90 mediated via a proximal promoter region as trigger of caspofungin resistance in Aspergillus fumigatus. J Infect Dis. 2014;209:473–481. doi: 10.1093/infdis/jit530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cowen LE, et al. Harnessing Hsp90 function as a powerful, broadly effective therapeutic strategy for fungal infectious disease. Proc Natl Acad Sci USA. 2009;106:2818–2823. doi: 10.1073/pnas.0813394106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gow NA, et al. Immune recognition of Candida albicans beta-glucan by dectin-1. J Infect Dis. 2007;196:1565–1571. doi: 10.1086/523110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Plaine A, et al. Functional analysis of Candida albicans GPI-anchored proteins: roles in cell wall integrity and caspofungin sensitivity. Fungal Genet Biol. 2008;45:1404–1414. doi: 10.1016/j.fgb.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lee KK, et al. Elevated cell wall chitin in Candida albicans confers echinocandin resistance in vivo. Antimicrobial Agents and Chemotherapy. 2012;56:208–217. doi: 10.1128/AAC.00683-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Walker LA, Gow NA, Munro CA. Elevated chitin content reduces the susceptibility of Candida species to caspofungin. Antimicrobial Agents and Chemotherapy. 2013;57:146–154. doi: 10.1128/AAC.01486-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cota JM, et al. Increases in SLT2 expression and chitin content are associated with incomplete killing of Candida glabrata by caspofungin. Antimicrobial Agents and Chemotherapy. 2008;52:1144–1146. doi: 10.1128/AAC.01542-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Stevens DA, et al. Escape of Candida from caspofungin inhibition at concentrations above the MIC (paradoxical effect) accomplished by increased cell wall chitin; evidence for beta-1,6-glucan synthesis inhibition by caspofungin. Antimicrobial Agents and Chemotherapy. 2006;50:3160–3161. doi: 10.1128/AAC.00563-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Clemons KV, et al. Assessment of the paradoxical effect of caspofungin in therapy of candidiasis. Antimicrobial Agents and Chemotherapy. 2006;50:1293–1297. doi: 10.1128/AAC.50.4.1293-1297.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Stevens DA, Espiritu M, Parmar R. Paradoxical effect of caspofungin: reduced activity against Candida albicans at high drug concentrations. Antimicrobial Agents and Chemotherapy. 2004;48:3407–3411. doi: 10.1128/AAC.48.9.3407-3411.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Healey KR, et al. CRS-MIS in Candida glabrata: sphingolipids modulate echinocandin-Fks interaction. Molecular Microbiology. 2012;86:303–313. doi: 10.1111/j.1365-2958.2012.08194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Healey KR, et al. Candida glabrata mutants demonstrating paradoxical reduced caspofungin susceptibility but increased micafungin susceptibility. Antimicrobial Agents and Chemotherapy. 2011;55:3947–3949. doi: 10.1128/AAC.00044-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Reagan DR, et al. Characterization of the sequence of colonization and nosocomial candidemia using DNA fingerprinting and a DNA probe. Journal of Clinical Microbiology. 1990;28:2733–2738. doi: 10.1128/jcm.28.12.2733-2738.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Harriott MM, Noverr MC. Importance of Candida-bacterial polymicrobial biofilms in disease. Trends Microbiol. 2011;19:557–563. doi: 10.1016/j.tim.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cheng S, et al. Candida glabrata intra-abdominal candidiasis is characterized by persistence within the peritoneal cavity and abscesses. Infect Immun. 2014;82:3015–3022. doi: 10.1128/IAI.00062-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fekkar A, et al. Emergence of echinocandin-resistant Candida spp. in a hospital setting: a consequence of 10 years of increasing use of antifungal therapy? Eur J Clin Microbiol Infect Dis. 2014;33:1489–1496. doi: 10.1007/s10096-014-2096-9. [DOI] [PubMed] [Google Scholar]
- 116.Beyda ND, et al. FKS mutant Candida glabrata: risk factors and outcomes in patients with candidemia. Clin Infect Dis. 2014;59:819–825. doi: 10.1093/cid/ciu407. [DOI] [PubMed] [Google Scholar]
- 117.Blanchard E, et al. Prior caspofungin exposure in patients with hematological malignancies is a risk factor for subsequent fungemia due to decreased susceptibility in Candida spp.: a case-control study in Paris, France. Antimicrob Agents Chemother. 2011;55:5358–5361. doi: 10.1128/AAC.00690-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Scott LJ. Micafungin: a review of its use in the prophylaxis and treatment of invasive Candida infections. Drugs. 2012;72:2141–2165. doi: 10.2165/11209970-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 119.Hope WW, et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: prevention and management of invasive infections in neonates and children caused by Candida spp. Clin Microbiol Infect. 2012;18(Suppl 7):38–52. doi: 10.1111/1469-0691.12040. [DOI] [PubMed] [Google Scholar]
- 120.de la Torre P, Reboli AC. Micafungin: an evidence-based review of its place in therapy. Core Evid. 2014;9:27–39. doi: 10.2147/CE.S36304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.van Burik JA, et al. Micafungin versus fluconazole for prophylaxis against invasive fungal infections during neutropenia in patients undergoing hematopoietic stem cell transplantation. Clin Infect Dis. 2004;39:1407–1416. doi: 10.1086/422312. [DOI] [PubMed] [Google Scholar]
- 122.Chou LS, et al. Caspofungin as primary antifungal prophylaxis in stem cell transplant recipients. Pharmacotherapy. 2007;27:1644–1650. doi: 10.1592/phco.27.12.1644. [DOI] [PubMed] [Google Scholar]
- 123.Mattiuzzi GN, et al. Open-label, randomized comparison of itraconazole versus caspofungin for prophylaxis in patients with hematologic malignancies. Antimicrobial Agents and Chemotherapy. 2006;50:143–147. doi: 10.1128/AAC.50.1.143-147.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Doring M, et al. Caspofungin as antifungal prophylaxis in pediatric patients undergoing allogeneic hematopoietic stem cell transplantation: a retrospective analysis. BMC Infect Dis. 2012;12:151. doi: 10.1186/1471-2334-12-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Xu SX, et al. Newer antifungal agents for fungal infection prevention during hematopoietic cell transplantation: a meta-analysis. Transplant Proc. 2013;45:407–414. doi: 10.1016/j.transproceed.2012.07.149. [DOI] [PubMed] [Google Scholar]
- 126.Ziakas PD, Kourbeti IS, Mylonakis E. Systemic antifungal prophylaxis after hematopoietic stem cell transplantation: a meta-analysis. Clin Ther. 2014;36:292–306 e291. doi: 10.1016/j.clinthera.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 127.Bizerra FC, et al. Breakthrough candidemia due to multidrug-resistant Candida glabrata during prophylaxis with a low dose of micafungin. Antimicrobial Agents and Chemotherapy. 2014;58:2438–2440. doi: 10.1128/AAC.02189-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ruggero MA, Topal JE. Development of echinocandin-resistant Candida albicans candidemia following brief prophylactic exposure to micafungin therapy. Transpl Infect Dis. 2014;16:469–472. doi: 10.1111/tid.12230. [DOI] [PubMed] [Google Scholar]
- 129.Cowen LE, Steinbach WJ. Stress, drugs, and evolution: the role of cellular signaling in fungal drug resistance. Eukaryotic Cell. 2008;7:747–764. doi: 10.1128/EC.00041-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Harrison BD, et al. A tetraploid intermediate precedes aneuploid formation in yeasts exposed to fluconazole. PLoS Biology. 2014;12:e1001815. doi: 10.1371/journal.pbio.1001815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Galhardo RS, Hastings PJ, Rosenberg SM. Mutation as a stress response and the regulation of evolvability. Critical Reviews in Biochemistry and Molecular Biology. 2007;42:399–435. doi: 10.1080/10409230701648502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chen G, et al. Hsp90 stress potentiates rapid cellular adaptation through induction of aneuploidy. Nature. 2012;482:246–250. doi: 10.1038/nature10795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sheltzer JM, et al. Aneuploidy drives genomic instability in yeast. Science. 2011;333:1026–1030. doi: 10.1126/science.1206412. [DOI] [PMC free article] [PubMed] [Google Scholar]


