Abstract
The effect of body asymmetry on anticipatory and compensatory postural adjustments was studied. Ten healthy subjects stood on the force platform and held an object in one hand which induced body asymmetry. Subjects were exposed to external perturbations applied to their shoulders while standing with either normal or narrow base of support. Bilateral electromyographic activity (EMG) of dorsal and ventral trunk and leg muscles and center of pressure displacements were recorded. Data was analyzed within the intervals typical for anticipatory (APA) and compensatory (CPA) postural adjustments. Integrals of EMG activity and co-contraction (C) and reciprocal (R) activation of muscles were calculated and analyzed. Reciprocal activation of muscles on the target side and co-contraction of muscles on the contralateral side was seen when standing in asymmetrical stance and being subjected to external perturbations. Decreased magnitudes of co-contraction and reciprocal activation of muscles were seen in the APA phase while standing asymmetrically with narrow base of support. The findings highlight the importance of investigating the role of body asymmetry in maintaining control of vertical posture. The outcome of the study provides a foundation for future studies focusing on improvement of postural control in individuals with body asymmetry due to unilateral weakness.
Keywords: postural control, anticipatory, compensatory, body asymmetry, co-contraction, reciprocal activation
1. Introduction
Humans perform many daily activities while standing asymmetrically. Holding a cup of coffee or talking on the phone are common activities associated with asymmetry of posture. Body perturbation is commonly experienced while maintaining asymmetrical posture (e.g. standing or walking in a crowded space with an object in one hand and getting bumped).
To maintain and restore balance in the case of external perturbation, the Central Nervous System (CNS) uses two main types of adjustments in the activity of the trunk and leg muscles. Anticipatory postural adjustments (APAs) control the position of the center of mass (COM) by activating the trunk and leg muscles prior to a forthcoming body perturbation, minimizing the danger of losing equilibrium [reviewed in (Massion 1992)]. Compensatory postural adjustments (CPAs) are initiated based on sensory feedback and serve as a mechanism to restore the COM position after a perturbation has already occurred (Alexandrov et al. 2005). Changes in both, APAs and CPAs are affected by direction and magnitude of the forthcoming perturbation (Aruin and Latash 1995; Aruin and Latash 1996), body stability (Aruin et al. 1998), and dimensions of the base of support (BOS) (Aruin et al. 1998; Dimitrova et al. 2004). In addition, the characteristics of a motor action that induce a perturbation (Aruin et al. 2003), body configuration (Aruin 2003), task requirement (Santos and Aruin 2009), and fear of falling (Adkin et al. 2002) influence APAs. It was also shown that when APAs are not generated, CPAs are the only mechanism used by the CNS to restore balance. Conversely, the corresponding CPA responses are minimized by generating and utilizing strong APAs (Santos et al. 2010b; Santos et al. 2010a).
In general, while maintaining equilibrium, the CNS employs two main patterns of muscle activation: reciprocal activation and co-contraction (Mochizuki et al. 2004). Reciprocal activation involves sequential activation of the anterior and posterior muscles. Thus, reciprocal activation of the hamstrings/quadriceps and erector spinae/rectus abdominis muscle pairs has been reported during rapid elbow flexion movements (Friedli et al. 1984) and reciprocal activation of the hamstrings/quadriceps muscles during unilateral arm movements (van der Fits et al. 1998). Reciprocal activation of muscles is considered an efficient energy saving strategy to control vertical posture (Latash et al. 1995). Co-contraction of muscles, on the other hand, increases joint stiffness and provides better body stability (Latash et al. 1995; Aruin and Almeida 1997; Massion et al. 1999). Co-contraction of the ventral and dorsal trunk and leg muscles has been reported during bilateral arm flexion movements in individuals with Parkinson’s disease (Aruin et al. 1997) and Down syndrome (Aruin and Almeida 1997). It has also been found that reciprocal patterns of muscle activation are utilized for postural control in cases of minor body instability, while co-contraction is the preferable postural control strategy in conditions with higher body instability (Santos and Aruin 2009).
The contribution of APAs and CPAs in the control of symmetrical vertical posture is well documented (see for example (Friedli et al. 1984; Massion 1992; Park et al. 2004; Alexandrov et al. 2005)). Similarly, in cases of body asymmetry induced by external rotation of a leg, significant asymmetrical APA patterns were reported in the right and left distal muscles during a release of the load from extended arms (Aruin 2006). In addition, when standing in asymmetrical posture (with one foot in front of another) and pushing an object with two hands, larger anticipatory and compensatory activity has been reported in the backward leg; when standing symmetrically and pushing with one hand, larger trunk muscle activity has been reported on the contralateral side (Lee and Aruin 2014). Moreover, larger anticipatory muscle activity on the intact side of the body has been described in individuals with lower leg amputation performing fast bilateral shoulder movements and releases or catches of a load (Aruin et al. 1997). It was also described in the literature that maintenance of upright posture is modified in asymmetrical positions associated with unipedal (Vernazza-Martin et al. 1999) or bipedal (Kazennikov et al. 2013) stances with asymmetrical load on the legs. Moreover, the anticipatory center of pressure (COP) displacement during arm lifts performed while standing asymmetrically depended on the mobility of the support under the leg and on loading of the leg (Kazennikov et al. 2015). It was suggested that asymmetry of posture induced by shifting body weight to one leg is associated with different involvement of the lower extremities in the maintenance of vertical posture: the loaded leg is responsible for posture regulation in the sagittal plane and the unloaded leg controls the posture in the frontal plane (Kazennikov et al. 2013).
Nevertheless, the available information on how asymmetrical posture affects APAs and CPAs is incomplete. While holding an object in one hand induces asymmetry of vertical posture, prior studies of grasping at the same time as standing (Bateni et al. 2004; Haddad et al. 2011) mainly did not specifically focus on investigation of postural control in terms of APA and CPA. As such, it is not entirely clear how body asymmetry induced by holding an object in one hand affects generation of APAs and CPAs utilized to maintain vertical posture. Additionally, little research has examined the role of APAs and CPAs during an asymmetrical standing task when exposed to external perturbations. Therefore the first purpose of the study was to investigate changes in the anticipatory and compensatory postural adjustments in the presence of body asymmetry induced by holding an object in one hand while being exposed to an external symmetrical perturbation. It is known that the CNS uses either reciprocal activation or co-contraction of the trunk and leg muscles to control vertical posture (Mochizuki et al. 2004). However, it is not clear how the CNS activates muscles in the presence of posture asymmetry induced by holding an object while being exposed to a perturbation. We hypothesized that in the presence of body asymmetry, there would be reciprocal activation of the ventral and dorsal trunk and leg muscles on the side of the handheld object, and co-contraction of the ventral and dorsal muscles on the contralateral body side in the APA phase. The second purpose of the study was to examine the effect of changes in the base of support and weight of the handheld object on the anticipatory and compensatory postural adjustments. It is known that while standing symmetrically, the level of anticipatory activation of muscles is dependent on the amount of body instability associated with the reduced base of support (Santos and Aruin 2009). Moreover, APA activity of dorsal and ventral muscles is smaller in conditions involving body instability (Aruin et al. 1998). As such, we hypothesized that decreased magnitudes of anticipatory muscle activity and increased COP displacements after the perturbation will be seen when standing asymmetrically with narrow base of support.
2. Method
2.1 Participants
Ten, young, right-handed volunteers (5 males, 5 females, age = 28.2±3.55 years, weight = 67.1±20.27kg, height = 1.66±0.08m) participated in the experiment. All subjects were free from any neuromuscular disorder that could affect their control of posture or holding an object. The project was approved by the University of Illinois at Chicago Institutional Review Board, and all participants provided written informed consent.
2.2 Experimental procedure
The participants were instructed to stand barefoot on the force platform with their feet parallel and shoulder width apart. This foot position was marked on top of the platform and reproduced across the trials. Participants were instructed to maintain a standing posture while being subjected to external perturbations that were induced at the shoulders by an aluminum pendulum. The pendulum was attached to the ceiling and consisted of a height adjustable central rod with the distal end designed as two padded pieces positioned shoulder width apart and projected towards the participant (Fig. 1). The length of the central rod was adjusted to each individual’s shoulder height, and the width of the padded surface was adjusted to match the subject’s shoulder width. A load (5% of the individual’s body weight) was attached to the pendulum next to its distal end. The pendulum was positioned at an initial angle of 30 degrees to the vertical (distance of 0.8 m from the body) and released by an experimenter. The subjects were able to see the pendulum at all times. Perturbations consisted of unidirectional forces applied by the pendulum on the shoulders of the subjects.
Fig. 1.
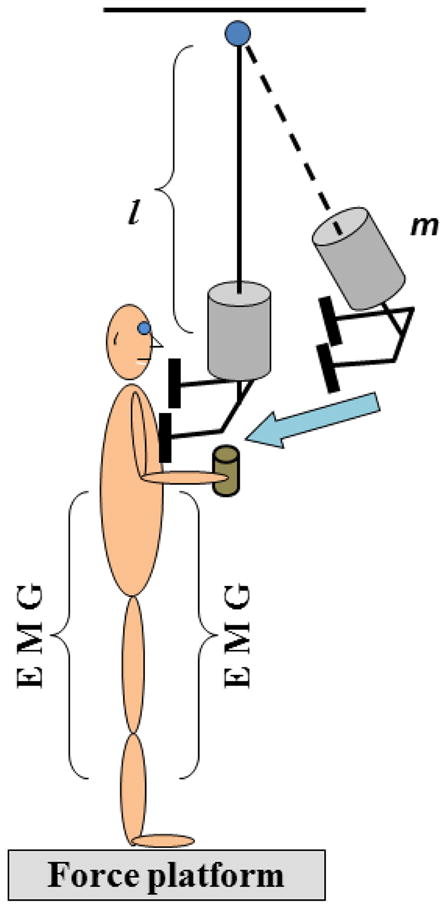
Schematic presentation of the experimental setup. The subjects received a perturbation at the shoulder level by the extended arm of the pendulum. l is the length of the pendulum and m is an extra weight.
The subjects were asked to perform the task while standing on the force platform with different bases of support: (1) regular (feet shoulder width apart) and (2) narrow (feet together). In each BOS condition, subjects stood with their right elbow at 90-degrees flexion while either (1) holding a 435g object (this condition will be referred to as “object”) or (2) with no object (0g) in the right hand (we will refer to this condition as “no object”). Five trials, each of 5s in duration, were collected in each of four experimental conditions. The order of experimental conditions was randomized.
2.3 Data collection
Electromyography (EMG) was recorded from the right and left lower limbs and trunk muscles. Since the subjects used their right hand to hold the object, we will refer this side as the “target” side and the left side as the “contralateral” side. Symbols “ts” and “cls” will be used respectively while referring to a particular muscle. After the skin was cleaned with alcohol wipes, a pair of disposable electrodes (3M Red Dot, 3M, USA) was attached to the muscle belly of tibialis anterior (TA), medial gastrocnemius (MG), rectus femoris (RF), biceps femoris (BF), rectus abdominis (RA), and erector spinae (ES). The distance between electrodes was 25mm. The ground electrode was positioned on the lateral mallelolus. EMG signals were band-pass filtered (10–500 Hz) and amplified (gain 2000) using the EMG system (Myopac, RUN Technologies, USA).
Ground reaction forces and moments of forces were recorded by a force platform (model OR-5, AMTI, USA). An accelerometer (model 208CO3, PCB Piezotronics Inc, USA) was attached the left sternoclavicular joint to determine the moment of pendulum impact (T0).
The forces, moments of forces, EMG, and accelerometer signals were digitized with a 16-bit A/D card at 1000Hz using LabVIEW 8.6.1 software (National Instruments, USA).
2.4 Data processing and analysis
MATLAB (MathWorks, USA) was used for data processing. The moment of the pendulum impact (body perturbation, T0) was derived from the acceleration signal using the Teager-Kaiser (TKE) onset time detection method (Li et al. 2007). EMG signals were high-pass filtered at 20 Hz, full-wave rectified, and low-pass filtered at 2 Hz (2nd order Butterworth). Estimation of the muscle onset was derived from the filtered EMG signal using the TKE onset time detection method (Li et al. 2007). Two integrals of EMG activity were calculated for each muscle in relation to T0: (1) from −150ms to +49ms (anticipatory postural adjustment, ∫APA) and (2) from +50ms to +249ms (compensatory postural adjustment, ∫CPA). In addition, integrals of the baseline activity, ∫ baseline, were calculated during the 200ms time window (−1000 to −800ms). The integrals of EMG activity during the APA and CPA phases were calculated using the equations:
Then the ∫APA and ∫CPA for each muscle was normalized by the maximum magnitude of the integral in each phase across all experimental conditions. Consequently, all of the ∫APA and ∫CPA were converted into −1 to 1 (inhibition and activation respectively) and presented as a ratio to maximum magnitude for further comparisons (Lee and Aruin 2013).
Based on prior literature suggesting that the CNS controls muscles as task-specific structural units (Bernshtein 1967; Slijper and Latash 2000; Slijper and Latash 2004), the sums (C) and differences (R) between normalized ∫EMG values were calculated. According to the framework of the equilibrium-point hypothesis (Feldman 1986), C indexes describe co-contraction and R indexes describe reciprocal changes in the activity of agonist–antagonist muscle pairs at a joint level (Slijper and Latash 2004). C and R indexes were calculated separately for the right and left side of the body and for the APA and CPA phases of postural control:
The calculation was applied to 3 body segments (trunk, thigh and shank). The C and R values were obtained for the ventral and dorsal muscles of each segment and for a combination of ventral muscles (RA, RF and TA) and dorsal muscles (ES, BF and MG). The C and R values were calculated using EMG integrals for target (ts) and contralateral (cls) sides separately. In the current study, we define each agonist-antagonist muscle pair used for the C and R calculation as one muscle coupling, for example RA and ES.
COP displacements in the anterior-posterior direction were calculated using equations described in the literature (Winter et al. 1996) as
where Mx is the moment in the sagittal plane, Fz and Fy are the vertical and the anterior-posterior components of the ground reaction force, and dz is the distance from the origin of the platform to the surface (0.038m).
Additionally, we calculated anticipatory COP displacements (COPAPA) as the COP magnitude at T0 and compensatory COP displacements (COPCPA) as the peak magnitudes after T0.
2.5 Statistical analysis
Multiple Student’s t-tests were first employed for analyzing baseline activity of the target and contralateral side muscles as well as for the determination of the status of muscles serving each body segment (co-contraction or reciprocal activation). Then, two-way repeated measures ANOVAs were performed with two factors: BOS (2 levels: regular and narrow) and object (2 levels: object and no object) for C or R values separately. If a muscle coupling had higher C value than R value, the ANOVA was used to evaluate the C rather than R, and vice versa. A separate two-way repeated measure, ANOVA, was used to analyze COPAPA and COPCPA displacements. Post hoc analyses were done using the Dunn-Sidak correction for multiple comparison adjustments. Critical value was set at α=0.05.
3. Results
3.1 EMG integrals
In general, the magnitudes of EMG integrals were affected by changes in the experimental conditions (object, no object, regular or small BOS). The findings are described below, using RF as an example. While subjects stood with a regular BOS, integrals of RF on the target side (∫RFts) during the APA phase were 0.26±0.04 and 0.23±0.04 for the no object and object conditions respectively. When subjects stood with a narrow BOS, ∫RFts decreased, reaching 0.12±0.02 and 0.12±0.02 for the no object and object conditions respectively. While standing with the regular BOS, anticipatory integrals of EMG of RF on the contralateral side (∫RFcls) were 0.26±0.05 and 0.21±0.05 for the no object and object conditions respectively. When standing with narrow BOS, the ∫RFts became smaller: 0.15±0.03 and 0.15±0.03 for no object and object conditions respectively.
The ∫RFts calculated during the CPA phase and regular BOS were 0.64±0.07 and 0.63±0.05 for no object and object conditions respectively. When subjects stood with a narrow BOS, ∫RFts on the target side were 0.42±0.06 and 0.40±0.05 for no object and holding object conditions respectively. At the same time, the ∫RFcls were 0.59±0.07 (no object) and 0.56±0.08 (holding object) in the regular BOS condition and they were 0.42±0.06 (no object) and 0.46±0.06 (holding object) in the narrow BOS condition, respectively. Notably, an object effect trend was observed in TAcls (F(1,9)=4.42, P=0.06). ∫TAcls were 0.28±0.05 when the participants stood with no object compared to 0.24±0.04 when they were asked to hold the 435g object.
The grand mean EMG integrals for all muscles on the target and contralateral sides of the body are shown in Table 1 separately for the APA and CPA phases.
Table 1.
Grand mean (mean of combination of conditions) of normalized EMG integrals for both APA and CPA phases.
| Muscles | Phase | Contralateral side (cls) | Target side (ts) |
|---|---|---|---|
| TA | APA | 0.263±0.048* | 0.215±0.035 |
| CPA | 0.601±0.042 | 0.602±0.053 | |
| MG | APA | −0.189±0.050ǂ | 0.177±0.066 |
| CPA | −0.102±0.069 | −0.071±0.051 | |
| RF | APA | 0.193±0.036Ɨ | 0.182±0.021Ɨ |
| CPA | 0.505±0.060Ɨ | 0.52±0.051Ɨ | |
| BF | APA | −0.050±0.057 | −0.076±0.069 |
| CPA | 0.112±0.077ǂ | 0.088±0.082 | |
| RA | APA | 0.133±0.021ǂ | 0.080±0.028 |
| CPA | 0.533±0.043ǂ | 0.386±0.062ǂ | |
| ES | APA | −0.218±0.073Ɨ | −0.071±0.057 |
| CPA | −0.092±0.070Ɨ | 0.119±0.048ǂ |
indicates significant effect of object;
indicates significant effect of BOS;
indicates significant effect of interaction.
3.2 Co-contraction and reciprocal activation of muscles
Two C and R based models were used to describe co-contraction and reciprocal activation of the sum of a group of body segments involving trunk, thigh, and shank (combination model), or muscles serving each body segment (individual model).
3.2.1 Three segments combination model
According to this model, C and R values were calculated using the sum of the trunk, thigh, and shank body segments. Fig. 2 shows C and R values computed during the APA and CPA phases for both the target and contralateral sides. The difference between the magnitudes of the C and R values is noticeable during the APA phase; in all four conditions, R values for the target side are larger than C values, suggesting that muscles were activated reciprocally. At the same time, C values are larger than R values for the muscles on the contralateral side, reflecting their co-contraction. During the compensatory phase, C values are larger than R values on both the contralateral and target sides, which reflect predominant co-contraction of muscles.
Fig. 2.
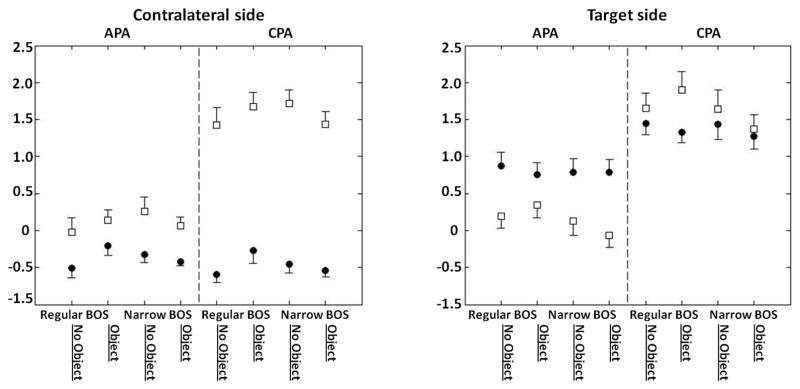
C and R values calculated for both, contralateral and target sides of the combination model (Cclswhole, Rclswhole, Ctswhole,, and Rtswhole,) during the APA and CPA phase. Circles represent R values and squares represent C values.
Two-way repeated measure ANOVA shows that during the APA phase, there is no condition effect and no interaction of the R value on the target side and C value on the contralateral side. However, during the CPA phase, there is a significant interaction (F(1,9)=4.88, P=0.05) between BOS and Object for the C value on the contralateral side. Within the No object condition, the contralateral side has a larger C value in the narrow BOS (2.84±0.2) than in the regular BOS (2.68±0.22), while in the Object condition, C value on the contralateral side was smaller in the narrow BOS condition (2.69±0.16) compared to the regular BOS condition (2.93±0.20).
3.2.2 Individual segment model
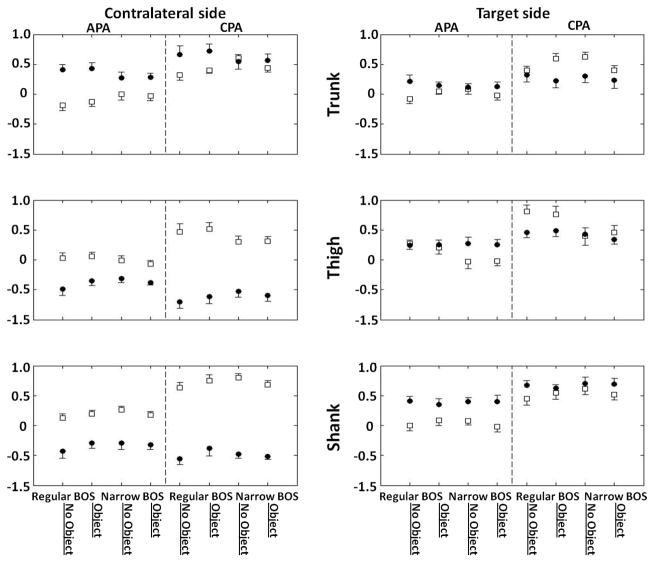
Fig. 3 shows the mean C and R values for each individual muscle coupling calculated for both the APA and CPA phases segment by segment for each experimental condition. In the APA phase, most of the target side muscle couplings were higher in R values compared to C values through all conditions except for the target thigh segment in conditions with the regular BOS and no Object (P=0.8). For the contralateral side muscles, shank and thigh, C values were higher than R values, however, R value for the trunk muscle was higher than C value.
Fig. 3.
C and R values calculated for both, contralateral and target sides of each segment, including trunk, thigh and shank. Circles represent R values and squares represent C values.
In the CPA phase on the target side, R value obtained from the shank muscles was higher than C value, while C values were higher than R values in most of the thigh and trunk muscles, except the target side thigh segment in the narrow BOS no Object condition (P=0.9). For the contralateral side, C values were higher than R values in both the shank and thigh muscles. At the same time, contralateral trunk segment muscles had higher R value compared to C value, except for the narrow BOS no Object condition.
Further examinations of co-contraction and reciprocal activation of muscles reveal that BOS affects target thigh segment R values (RTts, R value for target thigh muscles) in both the APA and CPA phases. Thus, the R values of the target side thigh muscles in the APA phase were 0.08±0.05 (F(1,9)=5.04, P=0.05) in the regular BOS condition, while they were 0.014±0.041 in the narrow BOS condition. The R value of the target side thigh muscles during the CPA phase was 0.26±0.07 in the regular BOS condition and it decreased to 0.10±0.05 in the narrow BOS condition (F(1,9)=10.40, P=0.01).
Significant effects on the BOS were observed for the contralateral thigh segment (CTcls, C value for thigh) during both the APA and CPA phases as well. In the APA phase, the C values of the target side thigh muscles were 0.41±0.07 and 0.33±0.07 (F(1,9)=6.68, P=0.03), in the regular BOS and narrow BOS conditions, respectively. In the CPA phase, the C value was 0.87±0.11 in the regular BOS condition while it decreased to 0.69±0.07 in the condition with narrow BOS (F(1,9)=8.27, P=0.01). There was also an Object and BOS interaction for the C value in the contralateral shank segment. While standing with regular BOS, C value was 0.99±0.08 in the no object condition, and it was 1.06±0.07 when holding the object. In conditions of standing with narrow BOS, C value was 1.11±0.07 and 1.02±0.06 for no object and object conditions respectively (interaction BOS*OBJECT F(1,9)=5.00, p=0.05).
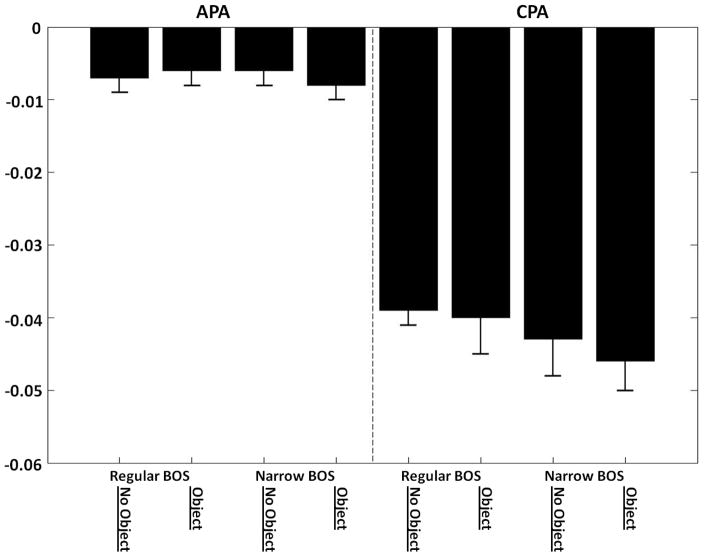
3.3 COP displacement
The COP displacements are shown in Fig. 4. No condition effects were observed in the COPAPA displacement in either the BOS (F(1,9)= 1.06, P=0.33) or object (F(1,9)=0.05, P=0.83) conditions. However, significant differences were found between the peaks of the COP displacement during the compensatory phase of postural control. Thus, in the regular BOS condition, the COPCPA displacement was −0.039±0.005m while it was −0.044±0.004m in the Narrow BOS condition (F(1,9)=6.68, P=0.029). At the same time, there was no significant difference in the COP CPA displacement between object conditions (F(1,9)=1.39, P=0.27).
Fig. 4.
The COPAPA and COPCPA displacement in the anterior-posterior direction.
4. Discussion
The current study investigated the effects of postural asymmetry on the anticipatory and compensatory postural adjustments that occurred when exposed to symmetrical external perturbations. Asymmetry of posture was induced by the subjects holding an object with the extended right arm (target side), and a perturbation was applied to the shoulder area. In addition, width of the base of support was manipulated as the subjects stood with different distances between their feet. Anticipatory and compensatory activity of the trunk and leg muscles was observed in all experimental conditions in both the target and contralateral muscles when subjects encountered a predictable symmetrical perturbation. Standing in an asymmetrical posture was mainly associated with reciprocal activation of muscles on the target side and co-contraction of muscles on the contralateral side of the body in the APA phase. As such, our first hypothesis that there would be reciprocal activation of the ventral and dorsal muscles on the target side and co-contraction of the ventral and dorsal muscles on the contralateral side in the APA phase was supported. Moreover, the decreased magnitudes of co-contraction and reciprocal muscles activation during the APA phase were seen while standing with a narrow base of support compared to standing with a normal, shoulder width base of support. This study outcome supports the second hypothesis that decreased magnitudes of anticipatory muscle activity and increased COP displacements after the perturbation would be seen when standing asymmetrically with a narrow base of support. However, weight of the hand-held object did not appear to significantly affect EMG activity and COP displacements as expected.
It is important to mention that subjects were exposed to similar perturbations in all experimental conditions. As such, the observed changes in the muscle activity and COP displacements are attributed solely to the effects of the induced body asymmetry and variations in the BOS.
4.1. Role of body asymmetry in anticipatory and compensatory postural adjustments
As described in the literature, APA asymmetries depended upon the side in which the body asymmetry was induced. Reduced APAs were observed in the leg muscles on the side of leg rotation, while increased APAs were seen in the muscles on the contralateral side (Aruin 2006). Similarly, smaller anticipatory EMG activity was seen in the muscles on the target side (the side the subjects were holding an object) in the current study. Moreover, we observed asymmetry-related change in the patterns of anticipatory activation in the ventral and dorsal muscles. Thus, reciprocal activation was seen in the muscles of the target side while co-contraction of muscles was observed on the contralateral side. It is known that healthy young subjects use reciprocal activation of muscles (efficient but more challenging strategy (Aruin and Latash 1996)) prior to the self-initiated or externally induced perturbations. At the same time, co-contraction of muscles is a common strategy used by aging adults or individuals with impairments to increase the stiffness of the joints and subsequent body stability (Manchester et al. 1989; Aruin and Almeida 1997; Hortobagyi and Devita 2006).
Asymmetry-related changes in the CPA phase were described during the performance of bilateral forward-reach tasks or pushing. Thus, asymmetry induced by standing on the non-dominant leg resulted in an increase in the activation of the soleus, tibialis anterior, and semitendinosus during the CPA phase of postural control (Mezaour et al. 2009). Moreover, when pushing an object while standing with one foot forward, compensatory activity in the ventral thigh muscles of the backward leg (gluteus medius and rectus femoris) was larger than in the forward leg (Lee and Aruin 2014).
Taken together, the previous and current findings suggest that in the presence of additional constraints associated with body asymmetry, the CNS can modify muscle activation strategies employing reciprocal activation of muscles on the target side and co-contraction of muscles on the contralateral side during both APA and CPA phases of postural control. Using such a strategy provides some advantages. Co-contracting muscles on the contralateral side (increasing the stiffness of the joints) allows for better body stabilization and provides a foundation for the stability needed to perform tasks using the target arm. Using reciprocal activation of muscles on the target side provides an important efficacy and flexibility in maintaining the position of the upper extremity. On the other hand, using co-contraction strategies on both sides might diminish the efficiency of postural control while being exposed to external perturbation. Nevertheless, both contralateral and target side muscles show co-contraction during the CPA phase of postural control indicating increased joint stiffness of the whole body. The CNS deliberately uses anticipatory co-contraction and reciprocal activation of muscles on different sides of the body and compensatory co-contraction of muscles on both sides. A more complex strategy used by CNS was revealed by segments analysis. Consistent with the combination model, when the subjects encountered symmetrical perturbations, the three segments (shankts, thights and trunkts) of the target side were in reciprocal activation. However, different patterns were observed on the contralateral side: the shank and thigh segment muscles showed co-contraction while the trunk segment muscles showed reciprocal activation. In conditions of holding an object while being perturbed, the CNS has to deal with these two tasks by leaning the trunk forward in anticipation of the perturbation impact and simultaneously turning lower extremities muscles to accommodate for the induced body asymmetry. Previous findings of the existence of the distal-to-proximal APA sequence in anticipation of a predicted perturbation (Santos et al. 2010b) and asymmetry-specific anticipatory activation of both proximal and distal muscles to compensate for additional mechanical constraint induced by asymmetrical posture (Aruin 2006) speak for the existence of such a dual tactic. This assumption is in line with the reported reorganization of joint stiffness during a pointing task that allows the trunk and the leg to play decisive roles in maintaining postural stability when the stance pattern changes from bilateral to unilateral (Hwang et al. 2006).
4.2. Role of BOS
Smaller anticipatory muscle activity was seen on the target side (the side the subjects were holding an object) while standing with their feet together as compared to when standing with their feet shoulder width apart. The observed changes in the muscle activity could be associated with changes in postural stability. Indeed, it was reported previously that under the condition of diminished body stability, the CNS suppresses the APAs to avoid additional disturbance of the equilibrium caused by APAs (Aruin et al. 1998; Santos and Aruin 2009). It was also reported that postural control during the performance of the pointing task was affected by changes in the BOS induced by bilateral and unilateral stances (Hwang et al. 2006). In addition, the upper limb movement strategies varied significantly with the adopted stance pattern (Hwang et al. 2006), suggesting the importance of BOS not only for balance maintenance but also for performance of the movement involving a target arm. The observed stance-related modifications seen as co-contraction of muscles are in line with the reported increase in stiffness in the musculoskeletal system, which has been shown to contribute to equilibrium control (Rietdyk et al. 1999). Moreover, maintaining bipedal stance under the two different stance conditions during horizontal surface translations was also associated with modulation of stiffness of muscles (Henry et al. 2001). While different patterns of activation of the trunk and leg muscles were utilized in conditions with different BOS, there was no BOS effect on the magnitudes of the COP displacement during the APA phase. The CNS deliberately modulated activity of the trunk and leg muscles to minimize COP displacements prior to the expected body perturbation. At the same time, the peaks of COP displacement (measured during the CPA phase of postural control) were different between conditions with different BOS. It was previously demonstrated that generation and utilization of strong APAs can result in smaller COP and COM displacements during the balance restoration (CPA) phase (Santos et al. 2010b; Santos et al. 2010a). Together, these results suggest the important role of muscle stiffness modulation in control of vertical posture in cases of asymmetry of stance and BOS variations.
5. Conclusion
Reciprocal activation of muscles on the target side and co-contraction of muscles on the contralateral side was seen when standing in asymmetrical stance and being subjected to external perturbation. Decreased magnitudes of co-contraction and reciprocal muscle activation were seen while standing in asymmetrical stance with a narrow base of support. The findings highlight the importance of investigating the role of body asymmetry in control of vertical posture. The outcome of the study provides a foundation for future studies focusing on an improvement of postural control in individuals with body asymmetry due to unilateral weakness.
Acknowledgments
This work was supported in part by the NIH grant # HD064838. We thank Charlie Ma for his assistance in data collection.
References
- Adkin AL, Frank JS, Carpenter MG, Peysar GW. Fear of falling modifies anticipatory postural control. Exp Brain Res. 2002;143:160–170. doi: 10.1007/s00221-001-0974-8. [DOI] [PubMed] [Google Scholar]
- Alexandrov AV, Frolov AA, Horak FB, Carlson-Kuhta P, Park S. Feedback equilibrium control during human standing. Biol Cybern. 2005;93:309–322. doi: 10.1007/s00422-005-0004-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aruin A, Almeida GL. A coactivation strategy in anticipatory postural adjustments in persons with Down syndrome. Motor Control. 1997;1:178–191. [Google Scholar]
- Aruin A, Mayka M, Shiratori T. Could a motor action that has no direct relation to expected perturbation be associated with anticipatory postural adjustments in humans? Neurosci Lett. 2003;341:21–24. doi: 10.1016/s0304-3940(03)00080-6. [DOI] [PubMed] [Google Scholar]
- Aruin AS. The effect of changes in the body configuration on anticipatory postural adjustments. Motor Control. 2003;7:264–277. doi: 10.1123/mcj.7.3.264. [DOI] [PubMed] [Google Scholar]
- Aruin AS. The effect of asymmetry of posture on anticipatory postural adjustments. Neurosci Lett. 2006;401:150–153. doi: 10.1016/j.neulet.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Aruin AS, Forrest WR, Latash ML. Anticipatory postural adjustments in conditions of postural instability. Electroencephalogr Clin Neurophysiol. 1998;109:350–359. doi: 10.1016/s0924-980x(98)00029-0. [DOI] [PubMed] [Google Scholar]
- Aruin AS, Latash ML. The role of motor action in anticipatory postural adjustments studied with self-induced and externally triggered perturbations. Exp Brain Res. 1995;106:291–300. doi: 10.1007/BF00241125. [DOI] [PubMed] [Google Scholar]
- Aruin AS, Latash ML. Anticipatory postural adjustments during self-initiated perturbations of different magnitude triggered by a standard motor action. Electroencephalogr Clin Neurophysiol. 1996;101:497–503. doi: 10.1016/s0013-4694(96)95219-4. [DOI] [PubMed] [Google Scholar]
- Aruin AS, Nicholas JJ, Latash ML. Anticipatory postural adjustments during standing in below-the-knee amputees. Clin Biomech (Bristol, Avon) 1997;12:52–59. doi: 10.1016/s0268-0033(96)00053-8. [DOI] [PubMed] [Google Scholar]
- Bateni H, Zecevic A, McIlroy WE, Maki BE. Resolving conflicts in task demands during balance recovery: does holding an object inhibit compensatory grasping? Exp Brain Res. 2004;157:49–58. doi: 10.1007/s00221-003-1815-8. [DOI] [PubMed] [Google Scholar]
- Bernshtein NA. The co-ordination and regulation of movements. Pergamon Press; Oxford, New York: 1967. [Google Scholar]
- Dimitrova D, Horak FB, Nutt JG. Postural muscle responses to multidirectional translations in patients with Parkinson’s disease. J Neurophysiol. 2004;91:489–501. doi: 10.1152/jn.00094.2003. [DOI] [PubMed] [Google Scholar]
- Feldman AG. Once more on the equilibrium-point hypothesis (lambda model) for motor control. J Mot Behav. 1986;18:17–54. doi: 10.1080/00222895.1986.10735369. [DOI] [PubMed] [Google Scholar]
- Friedli WG, Hallett M, Simon SR. Postural adjustments associated with rapid voluntary arm movements 1. Electromyographic data. J Neurol Neurosurg Psychiatry. 1984;47:611–622. doi: 10.1136/jnnp.47.6.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad JM, Rietdyk S, Ryu JH, Seaman JM, Silver TA, Kalish JA, Hughes CM. Postural asymmetries in response to holding evenly and unevenly distributed loads during self-selected stance. J Mot Behav. 2011;43:345–355. doi: 10.1080/00222895.2011.596169. [DOI] [PubMed] [Google Scholar]
- Henry SM, Fung J, Horak FB. Effect of stance width on multidirectional postural responses. J Neurophysiol. 2001;85:559–570. doi: 10.1152/jn.2001.85.2.559. [DOI] [PubMed] [Google Scholar]
- Hortobagyi T, Devita P. Mechanisms responsible for the age-associated increase in coactivation of antagonist muscles. Exerc Sport Sci Rev. 2006;34:29–35. doi: 10.1097/00003677-200601000-00007. [DOI] [PubMed] [Google Scholar]
- Hwang IS, Huang CT, Cherng RJ, Huang CC. Postural fluctuations during pointing from a unilateral or bilateral stance. Hum Mov Sci. 2006;25:275–291. doi: 10.1016/j.humov.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Kazennikov OV, Kireeva TB, Shlykov V. Characteristics of vertical posture maintenance during standing with asymmetrical legs loading. Fiziol Cheloveka. 2013;39:65–73. [PubMed] [Google Scholar]
- Kazennikov OV, Kireeva TB, Shlykov V. The influence of the leg load and the support mobility under leg on the anticipatory postural adjustment. Fiziol Cheloveka. 2015;41:57–64. [PubMed] [Google Scholar]
- Latash ML, Aruin AS, Neyman I, Nicholas JJ. Anticipatory postural adjustments during self inflicted and predictable perturbations in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1995;58:326–334. doi: 10.1136/jnnp.58.3.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YJ, Aruin AS. Three components of postural control associated with pushing in symmetrical and asymmetrical stance. Exp Brain Res. 2013;228:341–351. doi: 10.1007/s00221-013-3567-4. [DOI] [PubMed] [Google Scholar]
- Lee YJ, Aruin AS. Isolated and combined effects of asymmetric stance and pushing movement on the anticipatory and compensatory postural control. Clin Neurophysiol. 2014;125:768–776. doi: 10.1016/j.clinph.2013.09.036. [DOI] [PubMed] [Google Scholar]
- Li X, Zhou P, Aruin AS. Teager-Kaiser energy operation of surface EMG improves muscle activity onset detection. Ann Biomed Eng. 2007;35:1532–1538. doi: 10.1007/s10439-007-9320-z. [DOI] [PubMed] [Google Scholar]
- Manchester D, Woollacott M, Zederbauer-Hylton N, Marin O. Visual, vestibular and somatosensory contributions to balance control in the older adult. J Gerontol. 1989;44:M118–127. doi: 10.1093/geronj/44.4.m118. [DOI] [PubMed] [Google Scholar]
- Massion J. Movement, posture and equilibrium: interaction and coordination. Prog Neurobiol. 1992;38:35–56. doi: 10.1016/0301-0082(92)90034-c. [DOI] [PubMed] [Google Scholar]
- Massion J, Ioffe M, Schmitz C, Viallet F, Gantcheva R. Acquisition of anticipatory postural adjustments in a bimanual load-lifting task: normal and pathological aspects. Exp Brain Res. 1999;128:229–235. doi: 10.1007/s002210050842. [DOI] [PubMed] [Google Scholar]
- Mezaour M, Yiou E, Le Bozec S. Does symmetrical upper limb task involve symmetrical postural adjustments? Gait Posture. 2009;30:239–244. doi: 10.1016/j.gaitpost.2009.05.007. [DOI] [PubMed] [Google Scholar]
- Mochizuki G, Ivanova TD, Garland SJ. Postural muscle activity during bilateral and unilateral arm movements at different speeds. Exp Brain Res. 2004;155:352–361. doi: 10.1007/s00221-003-1732-x. [DOI] [PubMed] [Google Scholar]
- Park S, Horak FB, Kuo AD. Postural feedback responses scale with biomechanical constraints in human standing. Exp Brain Res. 2004;154:417–427. doi: 10.1007/s00221-003-1674-3. [DOI] [PubMed] [Google Scholar]
- Rietdyk S, Patla AE, Winter DA, Ishac MG, Little CE. NACOB presentation CSB New Investigator Award. Balance recovery from medio-lateral perturbations of the upper body during standing. North American Congress on Biomechanics. J Biomech. 1999;32:1149–1158. doi: 10.1016/s0021-9290(99)00116-5. [DOI] [PubMed] [Google Scholar]
- Santos MJ, Aruin AS. Effects of lateral perturbations and changing stance conditions on anticipatory postural adjustment. J Electromyogr Kinesiol. 2009;19:532–541. doi: 10.1016/j.jelekin.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Santos MJ, Kanekar N, Aruin AS. The role of anticipatory postural adjustments in compensatory control of posture: 1. Electromyographic analysis. J Electromyogr Kinesiol. 2010a;20:388–397. doi: 10.1016/j.jelekin.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Santos MJ, Kanekar N, Aruin AS. The role of anticipatory postural adjustments in compensatory control of posture: 2. Biomechanical analysis. J Electromyogr Kinesiol. 2010b;20:398–405. doi: 10.1016/j.jelekin.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slijper H, Latash M. The effects of instability and additional hand support on anticipatory postural adjustments in leg, trunk, and arm muscles during standing. Exp Brain Res. 2000;135:81–93. doi: 10.1007/s002210000492. [DOI] [PubMed] [Google Scholar]
- Slijper H, Latash ML. The effects of muscle vibration on anticipatory postural adjustments. Brain Res. 2004;1015:57–72. doi: 10.1016/j.brainres.2004.04.054. [DOI] [PubMed] [Google Scholar]
- van der Fits IB, Klip AW, van Eykern LA, Hadders-Algra M. Postural adjustments accompanying fast pointing movements in standing, sitting and lying adults. Exp Brain Res. 1998;120:202–216. doi: 10.1007/s002210050394. [DOI] [PubMed] [Google Scholar]
- Vernazza-Martin S, Martin N, Cincera M, Pedotti A, Massion J. Arm raising in humans under loaded vs. unloaded and bipedal vs. unipedal conditions. Brain Res. 1999;846:12–22. doi: 10.1016/s0006-8993(99)01846-6. [DOI] [PubMed] [Google Scholar]
- Winter DA, Prince F, Frank JS, Powell C, Zabjek KF. Unified theory regarding A/P and M/L balance in quiet stance. J Neurophysiol. 1996;75:2334–2343. doi: 10.1152/jn.1996.75.6.2334. [DOI] [PubMed] [Google Scholar]