Abstract
Background
Bamboo leaf extract solution (BLES) and sodium copper chlorophyllin solution (SCCS) are known for their anti-oxidant activities. Oral malodor is often related with periodontal pathogens. The present study was undertaken to investigate the anti-bacterial effect of both BLES and SCCS on anaerobic periodontal bacteria producing oral malodorous volatile sulfur compounds (VSC).
Methods
Porphyromonas gingivalis W83 (PG), Prevotella intermidai TDC19B (PI), Fusobacterium nucleatum ATCC25586 (FN) and Prevotella nigrescence ATCC33563 (PN) were investigated as oral isolated bacteria. VSC production ability of the oral strains was investigated by gas chromatography. With serial dilution of BLES or SCCS, the strains PG, PI, FN or PN were cultured anaerobically with AnaeroPack at 37 ℃ for 3 days. For the determination of anti-bacterial action of BLES or SCCS, the inoculum was cultured with original concentrations of BLES 0.16% (w/v) or SCCS 0.25% (w/v).
Results
Gas chromatography exhibited that all strains, PG, PI, FN and PN were responsible for producing a high range of H2S and a moderate range of CH3SH. Anti-bacterial effect of BLES or SCCS on the strains was observed. Inhibition of BLES or SCCS on the strains was revealed as concentration dependent. BLES or SCCS inhibited bacterial proliferation at higher concentrations (PG; 0.04% BLES or 0.03% SCCS, PI; 0.002% BLES or 0.03% SCCS, FN; 0.005% BLES or 0.01% SCCS, PN; 0.01% BLES or 0.015% SCCS). No viable bacterial colony observed at original concentration of BLES 0.16% or SCCS 0.25%. Strain growth was eliminated from inhibition at lower concentrations (PG; 0.02% BLES or 0.015% SCCS, PI; 0.001% BLES or 0.015% SCCS, FN; 0.002% BLES or 0.007% SCCS, PN; 0.005% BLES or 0.007% SCCS).
Conclusion
High concentrations of both BLES (0.16%) and SCCS (0.25%) show superior inhibiting capability on all four oral malodor associated periodontal anaerobes during testing, suggesting that these compounds might have a beneficial effect on oral health care.
Keywords: anti-bacterial effect, bamboo leaf extract solution, oral malodor, sodium copper chlorophyllin solution, volatile sulfur compounds
For hundreds of years, plants have proven to be a valuable source for biologically active compounds. Indeed, from ancient history some plant extracts have been used to cure various diseases. In this paper, we focus on two of these extracts, bamboo leaf extract solution (BLES) and sodium copper chlorophyllin solution (SCCS), which are literally eco-friendly as well as biodegradable.
BLES has been used particularly in Asian countries as an herbal medicine. Scientists believe various BLES compounds possess numerous pharmacological capabilities because of their anti-oxidant properties.1, 2 Years of research indicate that BLES possesses qualities that fight against microbes, diabetes, cancers, inflammation, obesity, fatigue, and lipidomics, while helping to prevent cardiovascular disease.3 In traditional systems of medicine, bamboo has been used from ancient times in various therapeutic applications for treating stomach heat, epilepsy, cough and phlegm, fainting and loss of consciousness in feverish diseases and a variety of mental disorders that develop with aging.4 In Japan, the traditional kumazasa bamboo leaf has been used to protect food from bacterial attack, such as sheets (called “chimaki”) to wrap sushi in or for packing rice balls. This is apart from its use for treating halitosis, body odor, stomatitis, hemorrhoids and healing wounds.5
On the other hand, SCCS can be extracted from different grass species as well as from bamboo leaves.6 SCCS also has a long history of traditional use in the medicinal arena, particularly in wound healing and odor control.7 Based on available literature, SCCS possesses potential anti-oxidative and anti-bacterial activity.8, 9 According to Goldberg,10 a water soluble chlorophyll solution was able to suppress malodor in a Vincent’s stomatitis patient after spraying it in the mouth and between the teeth since this anti-odor ability is apparently due to its anti-oxidizing properties. Carballo et al.11 suggested that SCCS can be effective as a gelatin film or coating to prevent food from microbial contamination. In recent times both BLES and SCCS have become commercially available and consumers use them for various purposes, especially as dietary supplements and food preservatives.12, 13 The consumption of chlorophyllin has long been associated with potential health benefits and even now, you can find many advertisements for many chlorophyll containing products, mostly dietary supplements. Considering that these benefits have already been researched, now the interest on SCCS and BLES is spreading to dentistry.
To date, neither solution has ever been tested for effectiveness against oral malodor associated anaerobic periodontal bacteria. Because of this, both of these compounds caught our attention as interesting enough to implement a study to test their properties against oral malodor associated anaerobic periodontal bacteria. We felt sure that we had a strong basis for a good study as the existence of a correlation between oral malodor and periodontal bacteria has been well known for a long time.14
Oral malodor, often called “bad breath,” defined simply as a foul odor coming out of the mouth, is one of the most common problems throughout the world. It has become one of the major complaints made by patients when they visit the dentist after dental caries and periodontal disease. The main etiology of oral malodor is oral and non-oral in origin. However, approximately 80 to 90% of cases originate orally and are usually associated with bacterial overgrowth on the dorsum of the tongue (stemming from poor oral hygiene), accumulation of food debris, bacterial plaque, gingivitis, periodontosis, deep carious lesions, exposed necrotic tooth pulp, pericoronitis, mucosal ulcerations, imperfect dental restorations, unclean dentures and factors causing decreased salivary flow. Oral malodor is thought to be caused by volatile sulfur compounds (VSC) including H2S, CH3SH and (CH3)2S produced by anaerobic periodontal bacteria.15 VSC are a family of gases which are primarily responsible for halitosis, a condition in which objectionable odor is present in the mouth; two members of this family, H2S and CH3SH are strongly co-related with intra-oral halitosis.16 Evidence suggests that anaerobic bacteria present in periodontal pockets of periodontosis are usually the primary cause of this condition.14
To find out possible etiological factors, multiple approaches require special diagnosis and investigation. The present study, however, was undertaken to assess the inhibiting capability of BLES and SCCS against oral malodor associated anaerobic periodontal bacterium and expected to have a major impact on suppressing oral malodor.
MATERIALS AND METHODS
Obligatory anaerobic periodontal bacteria
All four of the following strains—Porphyromonas gingivalis W83 (PG), Prevotella intermidai TDC19B (PI), Fusobacterium nucleatum ATCC25586 (FN), Prevotella nigrescence ATCC33563 (PN)—were purchased from Professor Kazuyuki Ishihara (Tokyo Dental College, Tokyo, Japan).
Gas chromatography
Gas chromatography is usually applied to measurement of VSC of air in the mouth. This test is an efficient and highly reproducible method for analyzing VSC and can be measured selectively, sensitively and quantitatively. Therefore, VSC production by strains PG, PI, FN and PN were examined by gas chromatography.17 The system of VSC analysis includes oral chroma CHM-1 (Abimedical, Kawasaki, Japan), gas volume: 0.5 mL, measurement time: 8 min, detection unit: ng/10 mL, operating temperature limit: 10 to 30 ℃, and storage temperature limit: –20 to 60 ℃. A small pore on an agar plate cap was first made and sealed with plastic tape before culturing the strains. After achieving lawns of confluent growth on the plate incubated at 37 ℃ for 3 days with AnaeroPack (Mitsubishi Gas Chemical, Tokyo, Japan), the air from the agar plate was aspirated through the sealed pore with a 1 mL gas-tight syringe. From the syringe, 0.5 mL air was discarded and 0.5 mL air directly injected into the sample hole of the gas chromatograph. After 8 min, results could be obtained. This procedure was repeated five times for each bacterial strain.
Anti-bacterial agents
We used 0.16% (w/v) BLES and 0.25% (w/v) SCCS. Both anti-bacterial solutions were provided by Tama Biochemical (Tokyo). Components in BLES and SCCS are shown in Table 1.
Table 1.
The amounts of major inorganic and/or organic components in BLES and SCCS*
| BLES (per 100g) | SCCS (per 100 g) | ||
| Na | 260 mg | Na | 220 mg |
| Cu | 72 mg | Cu | 72 mg |
| K | 50 mg | K | 1 mg |
| Beta-carotene | 400 μg | Mg | 1.5 mg |
| Alpha-tocopherol | 1.4 mg | Fe | 0.55 mg |
| Beta-tocopherol | < 0.1 mg | Zn | 0.18 mg |
| Gamma-tocopherol | < 0.2 mg | Mn | 0.017 mg |
| Delta-tocopherol | 0.1 mg | ||
| Vitamin C | < 1 mg | ||
BLES, bamboo leaf extract solution; SCCS, sodium copper chlorophyllin solution.
*As for SCCS, components other than sodium copper chlorophyllin.
Culture media
Anaerobic culture media, BHK RS, PEA BHK and BBE, were purchased from Kyokuto Pharmaceutical Industrial (Tokyo). Prior to the anti-bacterial test selection of appropriate culture medium was done on the basis of providing satisfactory bacterial growth, where BHK RS showed the capability of producing good growth for all bacterium compare to others medium, therefore BHK RS medium was used throughout the experiments to assist all 4 strains anaerobic to grow well. The ingredients of BHK RS are follows: Polypepton 10.0 g, casein pepton 10.0 g, yeast extract 5.0 g, glucose 1.0 g, sodium sulfite 5.0 g, sodium chloride 5.0 g, vitamin K1 0.01 g, hemin 0.01 g, sodium pyruvate 1.0 g, arginine 1.0 g, cysteine hydrochloride 0.3 g, agar 15.0 g, hemolytic rabbit blood, defibrinated sheep blood, PH 7.0.
Anaerobic culture
Three days of incubation at 37 ℃ with the AnaeroPack was performed.
Dilution liquid medium
Tryptic soy broth used as dilution liquid medium.
Anti-bacterial assay
To determine the anti-bacterial activity of BLES and SCCS, the experiment was carried out by the serial two-fold dilution method.18 Before testing, each bacterial suspension was prepared by 2 mL tryptic soy broth to give a starting concentration of approximately 1.5 × 105 CFU/mL.
Two hundred μL of 0.16% BLES in tryptic soy broth was followed by serial two-fold dilution with 200 μL suspension each. Finally, 200 μL of BLES 0.16%, 0.08%, 0.04%, 0.02%, 0.01%, 0.005%, 0.002% and 0.001% were obtained, respectively. Two hundred μL of 0.25% SCCS was also diluted by tryptic soy broth and the same diluting procedure as BLES was done. The final series of dilution was as follows: 200 μL of SCCS 0.25%, 0.12%, 0.06%, 0.03%, 0.015%, 0.007%, 0.003% and 0.001%. After mixing with 100 μL of each bacteria, the suspension was uniformly inoculated onto the agar surface and incubated at 37 ℃ for 3 days with the AnaeroPack. The experiment was repeated five times for each strain.
Culture for anti-bacterial assay (direct application)
At the beginning, bacterial suspensions corresponding to 1.5 × 105 CFU/mL were first inoculated on the agar plate and incubated for 1 day. After initial incubation, the number of colonies were counted followed by 400 μL original concentration of BLES 0.16% or SCCS 0.25%, poured onto the 1 day incubated bacterial surface and then again incubated at 37 ℃ for 3 days. Finally, colonies were counted. The experiment was performed in duplicate for each strain. In concentration dependent test, inoculates were mainly mixed with several concentrations of BLES or SCCS and then cultured, therefore the intention behind this particular experiment was to see how bacterium react, when original concentration of BLES or SCCS directly applied on them after 1 day incubation. Furthermore, to determine the anti-bacterial action, the inhibited non-colony zone from PN was inoculated on the agar plate with the help of needle inoculators and again incubated at 37 ℃ for 3 days.
Statistical analysis
The data was presented as the mean ± SD after counting the colony number. Statistical analysis was attained by two-tailed paired-sample t-test with IBM SPSS software version 21 (IBM, Armonk, NY).
RESULTS
Gas chromatography
Gas-chromatographic evaluation is shown in Fig. 1. All 4 strains are capable of producing a high range of H2S, particularly in strains PG, PI, and PN, where the amount of H2S exceeds 2 ng/mL, except for FN which is less than 2 ng/mL. Moderate release of CH3SH was detected in all strains. The range of CH3SH in strains PG, PI, and PN remained beyond 0.7 ng/mL to 1 ng/mL but for FN which was below 0.5 ng/mL. (CH3)2S was only detected in PI. However, this was a trace amount compared to H2S and CH3SH. Regarding the gas-chromatographic test in this study, strains PG, PI, FN and PN were revealed as strong candidates for producing oral malodor.
Fig. 1.
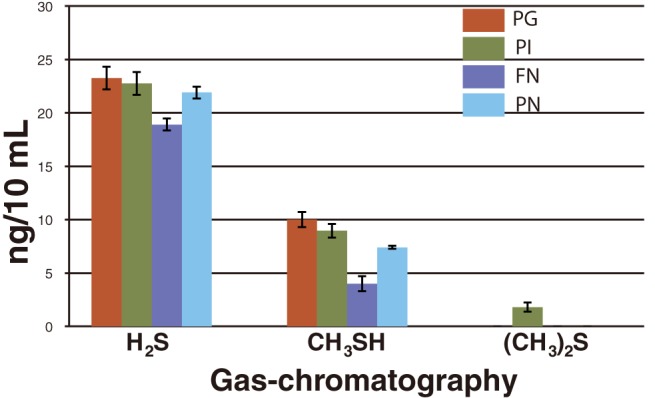
Investigation of VSC producing capability of four oral strains (PG, PI, FN and PN), performed by gas chromatography. Error bars represent mean ± SD for 5 time experiments.
FN, Fusobacterium nucleatum ATCC25586; PG, Porphyromonas gingivalis W83; PI, Prevotella intermidai TDC19B; PN, Prevotella nigrescence ATCC33563.
Anti-bacterial effect of BLES and SCCS
Porphyromonas gingivalis W83
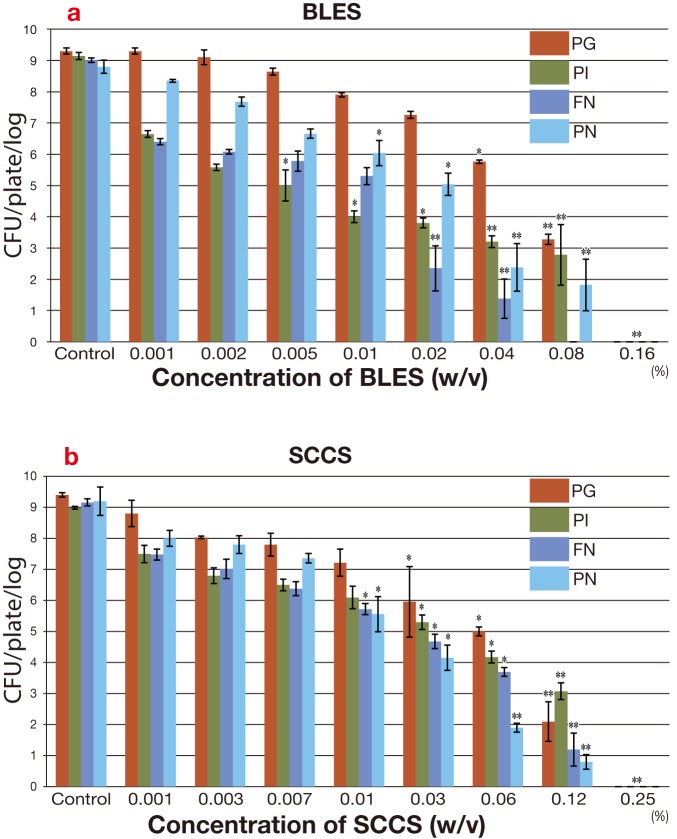
The anti-bacterial activity of BLES on PG is shown in Fig. 2a. PG growth was not inhibited in very low concentrations of BLES, 0.001% to 0.02% but gradually inhibited as BLES was increased in a concentration dependent manner. Statistically significant differences of BLES with PG was revealed: P < 0.01 at 0.04%. High inhibition of BLES with PG was revealed: P < 0.001 at 0.08% and 0.16%.
Fig. 2.
Survival rate of PG, PI, FN and PN with gradual concentrations of BLES or SCCS. The bar charts represent the CFU/plate/log. on, mean ± SD from 5 independent experiments. Control means of (1.5 × 105 CFU/plate/mL). The values **P < 0.001 and *P < 0.01 are shown with controls. a: Survival rate of PG, PI, FN and PN with BLES. b: Survival rate of PG, PI, FN and PN with SCCS.
BLES, bamboo leaf extract solution; CFU, colony forming unit; FN, Fusobacterium nucleatum ATCC25586; PG, Porphyromonas gingivalis W83; PI, Prevotella intermidai TDC19B; PN, Prevotella nigrescence ATCC33563.
The anti-bacterial activity of SCCS on PG is shown in Fig. 2b, where SCCS inhibited PG in a similar manner as BLES in Fig. 2a. The inhibitory activities of SCCS with PG were weak in very low concentrations of SCCS, 0.001% to 0.01%. PG growth started to decrease at 0.03%. Statistically significant differences of SCCS with PG were revealed: P < 0.01 at 0.03% and 0.06% and P < 0.001 at 0.12%. No bacterial growth was observed in the original concentration P < 0.001. The anti-bacterial activity of SCCS on PG is slightly higher than that of BLES.
Prevotella intermidai TDC19B
The anti-bacterial activity of BLES on PI is shown in Fig. 2a. Formation of PI colony started to decrease with a lower concentration of BLES, 0.001%, and no PI growth in the original concentration of 0.16% was observed (P < 0.001). Therefore, inhibition of PI growth in a concentration dependent manner was clearly observed.
The anti-bacterial activity of SCCS on PI shows in Fig. 2b. SCCS was weakly affected by PI growth inhibition than BLES. In lower SCCS concentrations, 0.001% to 0.01%, PI growth was moderately inhibited
SCCS inhibited PI growth (P < 0.01) in concentrations of 0.03% and 0.06%. SCCS were strongly inhibitory to PI (P < 0.001) in concentrations of 0.12% and 0.25%. The anti-bacterial activity of SCCS on PI is lower than that of BLES.
Fusobacterium nucleatum ATCC25586
The anti-bacterial activity of BLES on FN shows in Fig. 2a. BLES greatly inhibited FN growth. The complete inhibitory concentration of BLES on FN was higher at 0.08% (P < 0.001), which was the only activity as far as we could see. Statistically significant values were found P < 0.01 at 0.005% and 0.01%, and P < 0.001 at 0.02% to 0.16%.
The anti-bacterial activity of SCCS on FN is shown in Fig. 2b. FN growth was gradually inhibited by increasing SCCS from 0.001%. Statistically significant values were obtained at P < 0.01 in concentrations of 0.01% to 0.06%. It was highly inhibitory of SCCS on FN in 0.12% and 0.25% P < 0.001. The anti-bacterial activity of SCCS on FN is lower than that of BLES.
Prevotella nigrescence ATCC33563
The anti-bacterial effect of BLES on PN is shown in Fig. 2a. PN growth in lower concentrations of BLES, 0.001% to 0.005% were slightly inhibited. PN growth gradually decreased in higher concentrations of BLES. Statistically significant values were obtained: P < 0.01 at 0.01% and 0.02%, and P < 0.001 at 0.04% to 0.16%.
The anti-bacterial effect of SCCS on PN is shown in Fig. 2b. PN growth in lower concentrations of SCCS, 0.001% to 0.007% were slightly inhibited. Statistically significant values were obtained: P < 0.01 at 0.01% and 0.03%, and P < 0.001 at 0.06% to 0.25%. The anti-bacterial activity of SCCS on PN is similar to that of BLES.
Anti-bacterial activity of SCCS with PG is higher than that of BLES while it is lower with PI and FN. However, anti-bacterial activity of SCCS with PN is similar to that of BLES.
Culture for anti-bacterial assay (direct application)
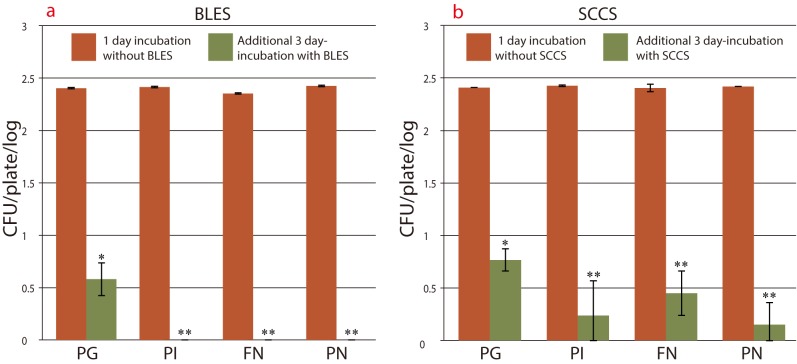
BLES (original concentration) inhibition on the 4 strains is shown in Fig. 3a. After 1 day incubation, all four strains showed similar growth and then after 3 days of incubation with BLES 0.16%, PI, FN and PN were completely inhibited at the original concentration (P < 0.001); however, PG survived with a fewer number of colonies (P < 0.01).
Fig. 3.
Growth rate of four strains after 1 day incubation, followed by additional 3 days incubation with 0.16% BLES or 0.25% SCCS. Error bars represent mean ± SD from duplicate experiments. The values **P < 0.001 and *P < 0.01 are shown with 1 day incubation. a: Growth rate of 4 strains with or without 0.16% BLES. b: Growth rate of 4 strains with or without 0.25% SCCS.
BLES, bamboo leaf extract solution; CFU, colony forming unit; FN, Fusobacterium nucleatum ATCC25586; PG, Porphyromonas gingivalis W83; PI, Prevotella intermidai TDC19B; PN, Prevotella nigrescence ATCC33563; SCCS, sodium copper chlorophyllin solution.
SCCS (original concentration) inhibition on the 4 strains is shown in Fig. 3b. All strains were inhibited at 0.25% SCCS compared with 1 day of incubated bacteria. Statistically significant values were obtained (P < 0.001) for PI, FN and PN, (P < 0.01) for PG. It was noted that BLES showed a relatively strong anti-bacterial effect compare to SCCS against all strains. Additionally, when inhibited non-colony zone from PN was cultured, the bacterial strain showed further proliferation (data not shown).
DISCUSSION
Oral malodor emerging from the degradation of organic substrates such as glucose, mucin, peptides and proteins present in saliva, crevicular fluid, oral soft tissues and retained debris and some microbial degradation products are volatile sulfur compounds (VSC).15 Among VSC, H2S and CH3SH are the prominent elements of oral malodor associated with chronic periodontosis.19 Regarding our study, after evaluation of the gas from PG, PI, FN and PN by gas chromatography, results indicated all four species are capable of producing a high range of H2S (around 2 ng/mL) and a moderate range of CH3SH (around 1 ng/mL) except FN (below 0.5 ng/mL). Yaegaki et al.17 demonstrated after evaluation of mouth air from periodontosis patients indicated that H2S level remains below 1 ng/mL and CH3SH remains around 4 ng/mL compare with healthy subjects. This is an opposite to what we found, but the difference could depend on both bacterial characteristics and the environment in which they were grown. However, the mechanism of VSC production in periodontal disease is complicated and may also involve other clinical factors.17 Studies found in the literature suggested that for the formation of H2S and CH3SH, anaerobic bacterium PG, PI, FN and PN are not only highly co-related but other anaerobic bacterium such as Actinobacillus actinomycetemcomitans, Tannerella forsythia and Treponema denticola also play a vital role.20, 21 Our gas-chromatographic results showed that among the four bacteria only PI can produce (CH3)2S. Another study agrees with this, showing that (CH3)2S in the oral breath has a positive correlation with PI.21 Not only this, but VSC, especially H2S and CH3SH, are referred to as highly toxic and not only responsible for oral malodor, but may also contribute to the pathogenesis of periodontosis.14
We have showed the anti-bacterial activity of BLES and SCCS against anaerobic periodontal bacteria for the first time in this study, demonstrated in a concentration dependent manner, where all 4 strains were highly susceptible to the original concentration of the solution. The anti-bacterial activity of BLES and SCCS has been shown to be dependent on both solution concentrations and bacterial species or strains against which they are tested. It is evident that the inhibitory action of BLES and SCCS were enhanced by their components, namely; Na, Cu and K, which are contained in both BLES and SCCS. Among them, Na in the form of NaClO has been used effectively as an anti-bacterial agent in endodontic irrigation solutions and has a strong anti-bacterial effect against oral anaerobes PG, PI and PN.22 Besides that, Cu also has an anti-bacterial effect against few multidrug-resistant bacteria,23 and KI has been used in treatments to clean root canals of mandibular molars because of its anti-microbial activity.24 In addition, when BLES and SCCS are introduced into an oral anaerobe environment, these solutions create an unfavorable environment for the anaerobes and may act in living cells to break down carbon dioxide and free oxygen that prevents anaerobic bacterial respiration due to their anti-oxidant ability.
In this study, BLES exhibited a relatively higher anti-bacterial activity compared to SCCS against PI and FN but SCCS showed a stronger effect against PG, and PN which resulted in both solutions having a similar effectiveness. We believe these phenomena of difference and similarity may be defined by some component differences among the solutions and individual bacterial characteristics, while Na, Cu and K are contained in both. SCCS contains additional components of, Mg, Fe, Zn and Mn, all of these having anti-bacterial activity.25–28 Fe combined with Cu can delay anaerobic bacterial growth and Zn citrate also can decrease anaerobic bacteria from oral cavity.26, 27 Consequently, it is difficult to determine which of the identified compounds might be among the most active constituents for BLES, which is highly effective. Bamboo leaf have components like beta carotene, alpha tocopherol, gamma tocopherol, all having strong anti-bacterial activity,29 moreover phenolic compounds that possessed by bamboo leaf itself also proven to have strong bactericidal action.30 Therefore we hypothesized that these compounds may be the reason for BLES more effective.
In this study, when either BLES or SCCS (original concentration) was poured directly on a 1 day incubated bacterial surface, it inhibited bacterial growth. However, further culturing of non-colony zone inoculates revealed that bacterial strains can further proliferate, which might indicate that both BLES and SCCS possess bacteriostatic ability. Highest concentration of BLES and SCCS, eventually been able to inhibit four bacterial growth and proliferation entirely in concentration dependent assay, therefore we used highest concentrations of BLES and SCCS in direct application assay to see whether they can inhibit bacterial growth entirely like concentration dependent assay. After the original anaerobic-culture of PN was treated with SCCS for 3 days, no growth zone on the SCCS-containing agar were touched by a loop and were incubated on a BHK RS plate without SCCS where abundant colonies appeared for another 3 day anaerobic culture (data not shown). This particular experiment was mainly undertaken to find out whether the anti-bacterial activity of BLES and SCCS are bactericidal or bacteriostatic.
To emphasize BLES and SCCS as an anti-bacterial agent, resistance to several antimicrobial agents in anaerobic bacteria has been more frequently increased. Particularly genera prevotella and porphyromonas have become increasingly resistant to many anti-anaerobic agents such as penicillins and cephalosporins. Antimicrobial agents commonly used in the treatment of anaerobic infections are beta-lactam antibiotics (carbapenems), metronidazole and beta-lactam compounds (ampicillin, amoxicillin, ticarcillin and piperacillin), the broad-spectrum quinolones moxifloxacin and gatifloxacin have potential to treat mixed aerobic and anaerobic infections, however resistance to these agents seems to increase among several genera of anaerobic bacteria.31 In addition both BLES and SCCS can be regarded as safe and non-toxic, study showed that maximum tolerated dose of bamboo leaf in both rats and mice was greater than 10 g/kg body weight and did not shown any significant hematological, clinical, chemical and histopathological changes, literally no adverse reaction was found when leucopenia patients treated with SCCS made tablet with 40 mg dose.32, 33
In treating oral malodor, the first line of defense should be to identify the cause and eliminate it, so it is obvious that a reduction or inhibition of bacterial growth on the dorsum of the tongue is most important. Suppressing oral malodor could be done through both a mechanical and therapeutic approach including properly continued oral hygiene along with tooth brushing, tongue scraping, and flossing combined with the use of an anti-microbial mouth cleaning products, a number of products being commercially available including toothpastes and tongue gels, and mouthwashes which contain antimicrobial agents to reduce the number of bacteria. However, many of these don’t have extended effectivity.34 Chlorhexidine is considered the most effective anti-plaque and anti-gingivitis agent, although it has some side effects such as brownish discoloration of the pellicle covering the teeth and tongue, alteration in taste, increased desquamation of oral mucosa and increased calculus formation.34 Regarding anti-bacterial effects, few chemicals might be beneficial to neutralizing oral malodor, although these may include zinc chloride and sodium chloride.35 Products containing natural substances, usually classified as cosmetics, may improve the patient compliance; probiotic micro-organisms in chewing gums or lozenges might restrain the re-growth of bacteria associated with oral malodor by preemptively colonizing the oral cavity. To date, there are not so many clinical studies investigating the clinical efficacy of oral products containing probiotics.36
In conclusion, considering these results, BLES and SCCS certainly have inhibiting effects on growth and proliferation of oral anaerobic bacteria PG, PI, FN and PN, particularly against high concentrations, all bacterium showing superior susceptibility. In this context, the anti-bacterial activity of BLES and SCCS makes them strong candidates for the development of a new anti-bacterial agent against bacteria causing oral malodor and associated oral diseases.
Acknowledgments
Ackonwlegments:We would like to thank Dr. Masumi Ueda, former professor at the Tottori University College of Medical Care Technology, for his precious advice and support in this study and Professor Seiji Kageyama and Professor Shuhei Tomita for their invaluable suggestions and critical review of this research.
The authors declare no conflict of interest.
REFERENCES
- 1. Hu C, Zhang Y, Kitts DD. Evaluation of antioxidant and prooxidant activities of bamboo phyllostachys nigra var. Henosis leaf extract in vitro. J Agric Food Chem. 2000; 48: 3170-6. . [DOI] [PubMed] [Google Scholar]
- 2. Wang J, Yue YD, Tang F, Sun J. TLC screening for antioxidant activity of extracts from fifteen bamboo species and identification of antioxidant flavone glycosides from leaves of Bambusa. textilis McClure. Molecules. 2012; 17: 12297-311. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Goyal AK, Brahma BK. Antioxidant and nutraceutical potential of bamboo: an overview. Int J Fund Appl Sci. 2014; 3: 2-10. [Google Scholar]
- 4. Tripathi YC, Khawlhring L, Vasu NK. Traditional and contemporary medicinal applications of bamboos. 1st ed Nath S, Singh S, Sinha A, Das R, Krishnamurthy R editors. Ranchi (India): Institute of Forest Productivity; 2015. p.224-32. DOI: 10.13140/2.1.2553.1209. [DOI] [Google Scholar]
- 5. Shirotake S, Nakamura J, Kaneko A, Anabuki E, Shimizu N. creening bactericidal action of cytoplasm extract from kumazasa bamboo (Sasa veitchii) leaf against antibiotics-resistant pathogens such as MRSA and VRE Strains. J Bioequiv Availab. 2009; 1: 80-5. DOI: 10.4172/jbb.1000012. [DOI] [Google Scholar]
- 6. Ping LL, Rong ZS, Ying DD. Technological study on making sodium copper chlorophyllin from bamboo-leaves. Journal of Chongqing University (Natural Science Edition). 2000; 23: 109-112. Chinese with English Abstract. [Google Scholar]
- 7. Brett DW. Chlorophyllin--A healer? A Hypothesis for its activity. Wounds. 2005; 17: 190-5. [Google Scholar]
- 8. Marquez UML, Barros RMC, Sinnecker P, et al. Antioxidant activity of chlorophylls and their derivatives. Food Res Intern. 2005; 38: 885-91. DOI: 10.1016/j.foodres.2005.02.012. [DOI] [Google Scholar]
- 9. Mowbray S. The antibacterial activity of chlorophyll. Br Med J. 1957; 2: 268-70. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Goldberg SL. The use of water soluble chlorophyll in oral sepsis: An experimental study of 300 cases. Am J Surg. 1943; 62: 117-23. DOI: 10.1016/S0002-9610(43)90301-0. [DOI] [Google Scholar]
- 11. Lopez-Carballo G, Hernandez-munoz P, Gavara R, Ocio MJ. Photoactivated chlorophyllin-based gelatin films and coatings to prevent microbial contamination of food products. Int J Food Microbiol. 2008; 15: 65-70. . [DOI] [PubMed] [Google Scholar]
- 12. Wang J, Yue YD, Jiang H, Tang F. Rapid screening of flavone C-glycosides in the leaves of different species of bamboo and simultaneous quantitation of four marker compounds by HPLC-UV/DAD. Int J Anal Chem. 2012; 2012: 20511. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ferruzzi MG, Schwartz SJ. Thermal degradation of commercial grade sodium copper chlorophyllin. J Agric Food Chem. 2005; 53: 7098-102. . [DOI] [PubMed] [Google Scholar]
- 14. Nakano Y, Yoshimura M, Koga T. Correlation between oral malodor and periodontal bacteria. Microbes Infect. 2002; 4: 679-83. . [DOI] [PubMed] [Google Scholar]
- 15. Van den Broek T, Feenstra L, de Baat A.-L. A review of the current literature on aetiology and measurement methods of halitosis. J Dent. 2007; 35: 627-35. . [DOI] [PubMed] [Google Scholar]
- 16. Tangerman A, Winkel EG. Intra-and extra-oral halitosis: finding of a new form of extra-oral blood-borne halitosis caused by dimethyl sulphide. J Clin Periodontol. 2007; 34: 748-55. . [DOI] [PubMed] [Google Scholar]
- 17. Yaegaki K, Sanada K. Volatile sulfur compounds in mouth air from clinically healthy subjects and patients with periodontal disease. J Periodontal Res. 1992; 27: 233-8. . [DOI] [PubMed] [Google Scholar]
- 18. Jorgensen JH, Ferraro MJ. Antimicrobial susceptibility testing: a review of general principles and contemporary practices. Clin Infect Dis. 2009. December 1; 49: 1749-55. . [DOI] [PubMed] [Google Scholar]
- 19. Tsai CC, Chou HH, Wu TL, Yang YH, Ho KY, Wu YM, et al. The levels of volatile sulfur compounds in mouth air from patients with chronic periodontitis. J Periodontal Res. 2008; 43: 186-93. . [DOI] [PubMed] [Google Scholar]
- 20. Tanaka M, Yamamoto Y, Kuboniwa M, Nonaka A, Nishida N, Maeda K, et al. Contribution of periodontal pathogens on the tongue dorsa analyzed with real-time PCR to oral malodor. Microbes Infect. 2004; 6: 1078-83. . [DOI] [PubMed] [Google Scholar]
- 21. Kuroshita R, Amano S, Takahashi S, Sakagami H, Ohmori Y, Watanabe S. Insulin pump therapy is perceived as liberating, but to many it can imply a sense of the diabetes made visible. European Diabetes Nursing. 2010; 20: 57-64. DOI: 10.1016/S0917-2394(10)70193-2 [DOI] [Google Scholar]
- 22. Siqueira JF Jr, Batista MM, Faraga RC, de Uzeda M. Antibacterial effects of endodontic irrigants on black-pigmented gram-negative anaerobes and facultative bacteria. J Endod. 1998; 24: 414-6. . [DOI] [PubMed] [Google Scholar]
- 23. Steindl G, Heuberger S, Springer B. Antimicrobial effect of copper on multidrug-resistant bacteria. Veterinary Medicine Austria. 2012; 99: 38-43. [Google Scholar]
- 24. Tello-Barbaran J, Nakata HM, Salcedo-Moncada D, Bramante CM, Ordinola-Zapata R. The antimicrobial effect of iodine-potassium iodide after cleaning and shaping procedures in mesial root canals of mandibular molars. Acta Odontol Latinoam. 2010; 23: 244-7. . [PubMed] [Google Scholar]
- 25. Robinson DA, Griffith RW, Shechtman D, Evans RB, Conzemius MG. In vitro antibacterial properties of magnesium metal against Escherichia coli, pseudomonas aeruginosa and staphylococcus aureus. Acta Biomater. 2010; 6: 1869-77. . [DOI] [PubMed] [Google Scholar]
- 26. Bird LJ, Coleman ML, Newman DK. Iron and copper act synergistically to delay anaerobic growth of bacteria. Appl Environ Microbiol. 2013; 79: 3619-27. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hu D, Sreenivasan PK, Zhang YP, De Vizio W. The effects of a zinc citrate dentifrice on bacteria found on oral surfaces. Oral Health Prev Dent. 2010; 8: 47-53. . [PubMed] [Google Scholar]
- 28. Rekha K, Nirmala M, Nair MG, Anukaliani A. Structural, optical, photocatalytic and antibacterial activity of zinc oxide and manganese doped zinc oxide nanoparticles. Physica B. 2010; 405: 3180-5. DOI: 10.1016/j.physb.2010.04.042 [DOI] [Google Scholar]
- 29. Ulusoy S, Bosqelmez-Tinaz G, Secilmis-Canbay H. Tocopherol, carotene, phenolic contents and antibacterial properties of rose essential oil, hydrosol and absolute. Curr Microbial. 2009; 59: 554-8. . [DOI] [PubMed] [Google Scholar]
- 30. Zhang J, Gong J, Ding Y, Lu B, Wu X, Zhang Y. Antibacterial activity of water-phase extracts from bamboo shavings against food spoilage microorganism. Afr. J. Biotechnol. 2010; 9: 7710-7. DOI: 10.5897/AJB10.932 [DOI] [Google Scholar]
- 31. Yu VL Weber R Raoult D Rex JH Seenivasan MH Antimicrobial therapy and vaccines. 2nd ed New York: Apple Tree Productions; 2002. p.55-62. [Google Scholar]
- 32. Lu B, Wu X, Tie X, Zhang Y, Zhang Y. Toxicology and safety of anti-oxidant of bamboo leaves. Part 1: Acute and subchronic toxicity studies on anti-oxidant of bamboo leaves. Food Chem Toxicol. 2005; 43: 783-92. . [DOI] [PubMed] [Google Scholar]
- 33. Gao F, Hu XF. Analusis of the therapeutic effect of sodium copper chlorophyllin tablet in treating 60 cases of leucopenia. Chin J Integr Med. 2005; 11: 279-82. . [DOI] [PubMed] [Google Scholar]
- 34. Quirynen M, Zhao H, van Steenberghe D. Review of the treatment strategies for oral malodour. Clin oral investig. 2002; 6: 1-10. . [DOI] [PubMed] [Google Scholar]
- 35. Tuzun B, Firatli S, Tuzun Y, Firatli E, Wolf R. Oral therapeutics and oral cosmetics. Clin Dermatol. 2001; 19: 449-51. . [DOI] [PubMed] [Google Scholar]
- 36. Teughels W, Van Essche M, Sliepen I, Qiurynen M. Probiotics and oral healthcare. Periodontol 2000. 2008; 48: 111-47. . [DOI] [PubMed] [Google Scholar]