Abstract
Purpose
We report the biodistribution and radiation dosimetry of an integrin αvβ3 specific PET tracer 18F-AlF-NOTA-E[PEG4-c(RGDfk)]2) (denoted as 18F-Alfatide II). We also assessed the value of 18F-Alfatide II in patients with brain metastases.
Methods
Series of torso (from the skull to the thigh) static images were acquired in 5 healthy volunteers (3 M, 2 F) at 5, 10, 15, 30, 45 and 60 min after injection of 18F-Alfatide II (257 ± 48 MBq). Regions of interest (ROIs) were drawn manually, and the time-activity curves (TACs) were obtained for major organs. Nine patients with brain metastases were examined by static PET imaging with 18F-FDG (5.55 MBq/kg) and 18F-Alfatide II.
Results
Injection of 18F-Alfatide II was well tolerated in all healthy volunteers, with no serious tracer-related adverse events found. 18F-Alfatide II showed rapid clearance from the blood pool and kidneys. The total effective dose equivalent (EDE) and effective dose (ED) were 0.0277 ± 0.003 mSv/MBq and 0.0198 ± 0.002 mSv/MBq, respectively. The organs with the highest absorbed dose were the kidneys and the spleen. Nine patients with 20 brain metastatic lesions identified by MRI and/or CT were enrolled in this study. All 20 brain lesions were visualized by 18F-Alfatide II PET, while only 10 were visualized by 18F-FDG, and 13 by CT.
Conclusion
18F-Alfatide II is a safe PET tracer with a favorable dosimetry profile. The observed ED suggests that 18F-Alfatide II is feasible for human studies. 18F-Alfatide II has potential value in finding brain metastases of different cancer as a biomarker of angiogenesis.
Keywords: RGD peptide, Alfatide II, integrin, PET/CT, brain metastasis
Introduction
Angiogenesis is a key process in tumor growth and metastasis [1]. Targeted imaging of angiogenesis in vivo is an attractive strategy that represents a novel approach to noninvasively monitor angiogenesis and to assess the efficacy of anti-angiogenic therapies. Integrin αvβ3 has been identified as a favorable target for imaging angiogenesis, and it plays a key role in the regulation of cellular activation, survival, and migration [2]. Various cyclic arginine-glycine-aspartic acid (RGD)-based peptide probes have been developed to probe integrin expression [3, 4].
Positron emission tomography (PET) imaging allows non-invasive visualization and quantification of biological targets. A number of radiolabeled RGD peptides have been labeled with 18F [5], 64Cu [6], 68Ga [7] and 89Zr [8] for integrin αvβ3-targeted PET imaging. 18F is a medical cyclotron-produced positron isotope, and its half-life of 109.8 min matches well with the biological course of peptides. Therefore, 18F is a good choice for labeling cyclic RGD peptides. Several 18F-labeled RGD peptide tracers have been tested in oncologic patients, such as 18F-galacto-RGD [9] and 18F-AH111585 [10]. We recently successfully prepared 18F-AlF-NOTA-PRGD2 (denoted as 18F-Alfatide) [11]. It was shown that 18F-Alfatide has promising imaging properties and pharmacokinetics that are comparable to those of 18F-FPPRGD2 [12].
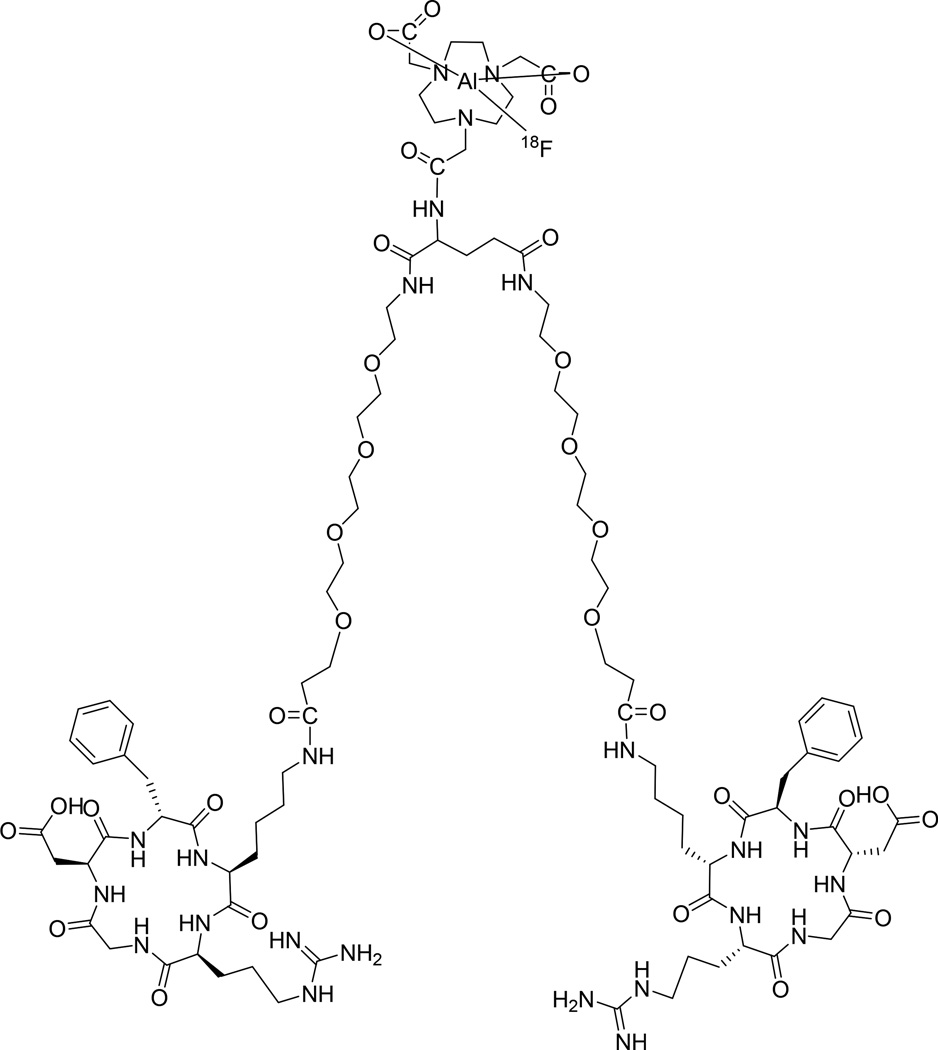
However, the glutamic acid linked dimeric peptide with a free α-amine is labile due to the neighboring amine participation in the hydrolysis. Liquid chromatograph-mass spectrometry (LC-MS) analysis determined that the instability of thiourea derivatives formed from α-amine was caused by participation of thiol group derived from thiourea, which is virtually the same as Edman degradation [13] except that the thiourea linkage is on the α-amine of glutamic acid instead of N-terminal amine group [14]. We thus developed a much more stable new tracer 18F-NOTA-E[PEG4-c(RGDfk)]2 (denoted as Alfatide II, Figure 1) for further biological evaluations [15, 16]. In the present study, the safety of 18F-Alfatide II was evaluated, and the absorbed dose of 18F-alfatide was estimated by analyzing the biodistribution data in healthy volunteers. We also evaluated the value of 18F-Alfatide II in patients with brain metastases.
Figure 1.
Schematic structure of 18F-NOTA-E[PEG4-c(RGDfk)]2 (denoted as [18F]Alfatide II).
Materials and Methods
Radiopharmaceutical preparation
Synthesis of 18F-alfatide II has been described previously [14].
Safety
Safety data were collected before and after injection of 18F-Alfatide II. Safety data included vital signs (blood pressure, respiratory rate, heart rate, and body temperature); physical examination; ECG; laboratory parameters (blood routine, liver function and kidney function) and adverse events.
Healthy volunteers
Five healthy volunteers (3 M, 2 F; mean year ± SD, 35.0 ± 3.5, age range, 33–39 y; mean weight ± SD, 74.0 ± 11.5, weight range, 62–85 kg) were included in the study. Each participant provided written informed consent using a form approved by the ethics committee of Wuxi No. 4 People’s Hospital. Participants were generally healthy based on a physical examination and routine laboratory tests. Exclusion criteria consisted of conditions of mental illness, severe liver or kidney disease, and cardiovascular disease. Participants were also excluded if they were suffering from infectious diseases or were pregnant.
Patients
The study protocol was approved by the ethics committee of Wuxi No. 4 People’s Hospital, and each patient gave written informed consent before the study. Nine patients (5 M, 4 F; age range, 27–71 y, mean age, 55 ± 15 y) with cerebral metastases were included in this study. The brain metastases were confirmed by MRI or CT. The patient information is summarized in Table 1. This study was registered at www.clinicaltrial.gov (NCT02441972).
Table 1.
Patient 1 demographic
| Patient no. | Age (y) | Sex | Histology |
|---|---|---|---|
| 1 | 48 | M | Left lung cancer bone metastasis, brain metastases |
| 2 | 54 | F | Ovarian cancer, brain metastasis |
| 3 | 54 | M | Postoperative left lung cancer, brain metastasis |
| 4 | 68 | F | Postoperative endometrial cancer, brain metastases |
| 5 | 66 | M | Left lung cancer, brain metastases |
| 6 | 71 | F | Left lung cancer, brain metastases |
| 7 | 27 | F | Gastric cancer, brain metastases |
| 8 | 69 | M | Right lung cancer, brain metastases |
| 9 | 39 | M | Unknown primary cancer, many metastases |
PET/CT acquisition and PET image analysis
No specific subject preparation, such as fasting, was requested on the day of 18F-Alfatide II imaging. PET/CT was performed on a Biograph 64 PET/CT scanner (Siemens Medical Solutions, Nurenberg, Germany). Healthy volunteers were positioned in the supine position on the scanner bed after injection of 18F-Alfatide II (257 ± 48 MBq, range, 203–296 MBq). Patients were positioned supine with their arms raised as did with the standard CT practice, with the inferior border set to the thigh and the superior border set to the cranial bone. A continuous low-dose CT scan for attenuation correction was acquired in spiral mode using 120 kV, 170 mAs, slice thickness 2 mm, and pitch 0.8. No intravenous or oral contrast agent was administered. PET was performed immediately after the CT scan with the patient in the same position covering an area identical to that covered by the CT scan with 2 min per bed position (five to six bed positions depending on the size of the patient). The acquired time points include 40 s/bed position at 5, 10, and 15 min post-injection; 2 min/bed position at 30, 45, and 60 min post-injection. PET image data were reconstructed on a 168 × 168 matrix using the ordered subset expectation maximization (OSEM) algorithm (3 iterations, 21 subsets) and corrected for attenuation using the CT data. The PET/CT images (half-body-attenuated and non-attenuated PET, CT and fused images) were transferred to a multimodal work station (Syngo (TrueD); Siemens Medical Solutions) for analysis. Regions of interest (ROIs) were drawn manually using 3D ellipsoid isocontour on each image with the assistance of the corresponding CT images.
For FDG PET, all 9 patients were fasted for at least 6 h before intravenous injection of 18F-FDG (5.55 MBq/kg). Scanning was performed at 60 min after tracer administration. Images were taken from thigh to cranial bone with the Siemens PET/CT scanner. Low dose CT was performed for attenuation correction and lesion localization. Image acquisition was performed with 1.5 min per bed position. Brain image was acquired separately. 18F-Alfatide II PET/CT was performed on the next day. No specific preparation was needed. The acquisition procedures were the same as those with FDG. ROIs were drawn manually on the site of brain metastases with the assistance of corresponding CT images by two experienced nuclear medical physicians. The results were expressed as maximum standardized uptake value (SUVmax).
Absorbed dose calculation
The dosimetry calculation was performed according to the EANM Dosimetry Guidance [17]. Based on the SUV of each organ, the decay uncorrected time-activity curve (TAC) was generated. The SUV values were converted to MBq/MBq based on organ weight from the adult male phantom (73.7 kg body weight) and the adult female phantom (56.9 kg body weight) provided by the OLINDA/EXM (version 1.1, Vanderbilt University, USA) [18, 19]. The time-integrated activity coefficient (residence time) of each organ was determined by fitting the data using a bi-phase exponential model provided by the software. The urine bladder volume was determined by reconstructed PET/CT images and the residence time was calculated by the trapezoidal method using Graphpad Prism (Version 4.0, GraphPad Software, Inc.). The void time was set as 60 min. The “remainder of body” was calculated for each time point as the decayed value of the original injected activity minus activity present in the identified source organs. Then the absorbed doses were calculated by entering the time-integrated activity coefficient for all source organs into OLINDA/EXM for either 73.7 kg adult male or 56.9 kg adult female.
Results
Safety
Healthy volunteers did not report any subjective effects following the injection of 18F-Alfatide II. No adverse events or serious adverse events occurred following 18F-Alfatide II injection, and no obvious changes in vital signs or clinical laboratory tests were found before and after the injection of 18F-Alfatide II.
Biodistribution
18F-Alfatide II was quickly cleared out through the urinary system, indicated by the high radioactivity accumulation in the kidneys and bladder. Besides, the liver and spleen also showed moderate uptake, while the other organs had low level of radioactivity distribution (Figure 2). The TACs of 18F-Alfatide II in various major organs of the five healthy volunteers are presented in Figure 3. The highest SUV in solid organs at 60 min after injection were found in the kidneys (6.32 ± 1.09) and the spleen (6.26 ± 0.68). Low activities in the brain, lung and muscle were found with SUV of 0.07 ± 0.05, 0.27 ± 0.05 and 0.82 ± 0.17, respectively.
Figure 2.
Representative torso maximum intensity projection (MIP) images of a healthy volunteer at different time points after intravenous injection of 18F-Alfatide II. Main regions with prominent 18F-Alfatide II uptake are kidneys, spleen, liver, urinary ducts and bladder.
Figure 3.
Decay corrected-time activity curves of major organs between 5 and 60 min after injection of 18F-Alfatide II in healthy volunteers (n = 5, 3 M and 2 F).
Absorbed dose calculation from PET/CT in healthy volunteers
The estimated absorbed dose of 18F-Alfatide II for each organ derived from PET/CT images of healthy volunteers is shown in Table 2. Since the bladder accumulated high amount of radioactivity due to renal clearance of 18F-Alfatide II, the urinary bladder wall had the highest absorbed dose (0.1129 ± 0.0629 mSv/MBq), which was followed by the liver (0.0670 ± 0.0392 mSv/MBq), spleen (0.0669 ± 0.0121 mSv/MBq) and kidneys (0.0535 ± 0.0135 mSv/MBq). The total effective dose equivalent (EDE) and effective dose (ED) were 0.0260 ± 0.0016 mSv/MBq and 0.0170 ± 0.0007 mSv/MBq, respectively.
Table 2.
Estimated absorbed dose after intravenous administration of 18F-Alfatide 1 II (mSv/MBq, n = 5, males and 2 females)
| Target organ | Mean | SD |
|---|---|---|
| Adrenals | 0.0119 | 0.0047 |
| Brain | 0.0014 | 0.0006 |
| Breasts | 0.0084 | 0.0091 |
| Gallbladder Wall | 0.0160 | 0.0065 |
| LLI Wall | 0.0075 | 0.0005 |
| Small Intestine | 0.0070 | 0.0018 |
| Stomach Wall | 0.0076 | 0.0027 |
| ULI Wall | 0.0073 | 0.0022 |
| Heart Wall | 0.0176 | 0.0058 |
| Kidneys | 0.0535 | 0.0135 |
| Liver | 0.0670 | 0.0392 |
| Lungs | 0.0078 | 0.0035 |
| Muscle | 0.0109 | 0.0053 |
| Ovaries | 0.0086 | N/A |
| Pancreas | 0.0263 | 0.0111 |
| Red Marrow | 0.0084 | 0.0031 |
| Osteogenic Cells | 0.0070 | 0.0032 |
| Skin | 0.0032 | 0.0011 |
| Spleen | 0.0669 | 0.0121 |
| Testes | 0.0052 | 0.0004 |
| Thymus | 0.0048 | 0.0021 |
| Thyroid | 0.0219 | 0.0053 |
| Urinary Bladder Wall | 0.1129 | 0.0629 |
| Uterus | 0.0195 | N/A |
| Total Body | 0.0092 | 0.0033 |
| Effective Dose Equivalent | 0.0260 | 0.0016 |
| Effective Dose | 0.0170 | 0.0007 |
Preliminary diagnostic value of 18F-Alfatide II in brain metastases
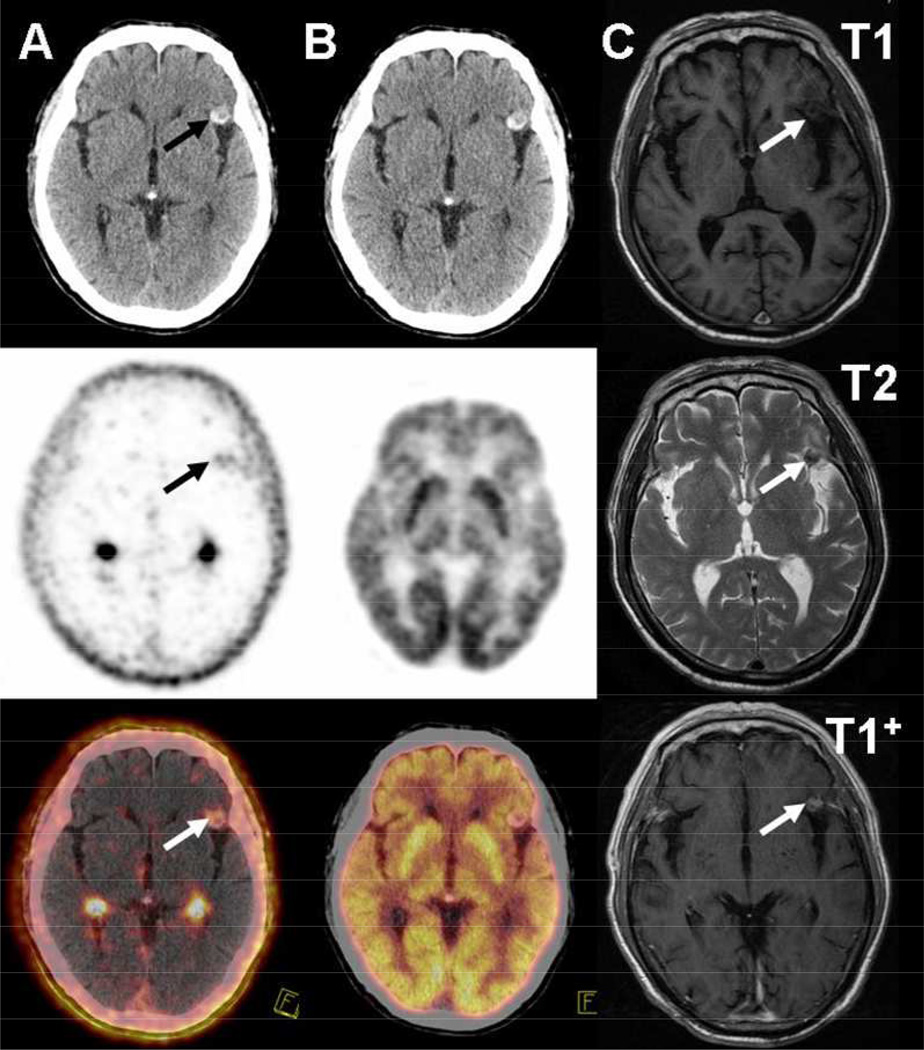
Nine patients with a total of 20 metastatic brain tumors were enrolled in this study. Sixteen brain metastases were diagnosed by MRI. Four brain metastases were diagnosed by morphological imaging (CT or PET/CT) for patients without MRI. All the brain lesions were visualized by 18F-Alfatide II, while only 10 by 18F-FDG, and 13 by CT. Of the brain lesions detected by 18F-FDG or CT, all are visible on 18F-alfatide II (Figs. 4–6). The SUVmax of brain metastases were calculated to be 1.8 ± 1.1 and 10.0 ± 5.7 on 18F-Alfatide II and 18F-FDG, respectively. Despite the overall higher uptake of 18F-FDG, 18F-Alfatide II showed much better tumor/background ratio (18.9 ± 14.1) over 18F-FDG (1.5 ± 0.5).
Figure 4.
18F-Alfatide II (A) and 18F-FDG (B) CT, PET and corresponding PET/CT image of a lung cancer patient with left frontal lobe metastasis (patient 1 in Table 1). In the left frontal lobe metastatic lesion, 18F-Alfatide II shows a high tumor/background ratio of 9.45, but not 18F-FDG. (C) T1-, T2- and enhanced T1-weighted magnetic resonance (MR) images of the left frontal lobe metastasis. Arrows point to brain metastatic lesion.
Figure 6.
18F-FDG (A) and 18F-Alfatide II (B) CT, PET and corresponding PET/CT images of a left lung cancer patient with right temporal lobe metastasis (patient 6 in Table 1). CT image shows high density with peripheral edema. The right temporal metastasis shows intense 18F-Alfatide II uptake with a tumor/background ratio of 13 and 18F-FDG PET shows no increased uptake.
Discussion
Although several 18F-labeled RGD tracers have entered clinical studies, the radiochemical syntheses are complex, and automation of these processes is challenging, which limit the widespread use of these traces in clinical settings [10, 20]. For example, the whole synthesis process of 18F-Galacto-RGD takes around 200 min and that of 18F-FPPRGD2 is about 120 min [21, 22]. With C-18 cartridge purification, 18F-Alfatide II can be produced with good radiochemical yield and high radiochemical purity within 20–30 min [23]. The fast-labeling process of 18F-alfatide II makes it very attractive for clinical translation.
18F-Alfatide II was found to be safe and well tolerated in all five healthy volunteers. No adverse events occurred following 18F-Alfatide II injection, and no obvious changes in vital signs or clinical laboratory tests were found before and after injection of 18F-alfatide II. The dosimetry calculations revealed an effective dose of less than 20 µSv/MBq. For a typical 255 MBq injected activity of 18F-alfatide II, the whole body effective dose is 4.335 mSv, which is within the limit of 30 mSv specified by the FDA, for a single administration of radioactive material for research use. The absorbed dose is similar to other RGD-based PET imaging probes including 18F-galacto-RGD (0.0187 ± 0.0024 mSv/MBq) [24], 18F-RGD-K5 (0.0150 ± 0.0010 mSv/MBq) [25] and 18F-AH111585 (0.0260 ± 0.0040 mSv/MBq) [26]. All these data acquired in the healthy volunteers demonstrated the safety of 18F-Alfatide II for further clinical applications.
Due to the excretion pathway and endogenous integrin expression, distribution study of 18F-alfatide II revealed relatively high radioactivity accumulation in the kidneys, spleen and liver. Minimal radiotracer accumulation was found in the lung region, which justified several successful applications of RGD based tracers including 18F-Alfatide in patients with lung cancer [27–29]. Due to the low background, high tumor/non-tumor ratio can be achieved, which facilitates lesion detection. For example, in our previous study with a small population of patients with lung cancer, all lesions can be visualized on 18F-Alfatide PET [27]. Besides, 18F-Alfatide PET may provide additional information for screening tumor-induced inflammation and scrofula lesions.
Although 18F-FDG PET plays a significant role in clinical oncology due to increased glucose metabolism in cancer cells, its sensitivity and specificity in the assessment of brain tumors is limited by high physiologic glucose uptake in normal brain tissue [30]. Consequently, other radiotracers for brain tumor detection have been intensively investigated for differential diagnosis, therapy response monitoring and prognosis prediction [31]. In several preclinical studies, RGD based tracers demonstrated very high imaging quality in visualization of xenografted or orthotopic brain tumors [32, 33]. In healthy volunteers, we observed very low tracer uptake in normal brain with a SUV of 0.068 ± 0.048 at 60 min after tracer injection, indicating that 18F-alfatide II does not penetrate the blood-brain barrier (BBB). Thus we hypothesized that 18F-alfatide II PET may allow clear diagnosis and evaluation of tumor metastases in the brain. Another rationale for this study is that angiogenesis, the target of 18F-Alfatide II, is one of the key pathophysiologic processes in brain metastases [34]. Consequently, all 20 metastasic brain lesions were clearly visualized by 18F-Alfatide II PET due to the extremely low background from the surrounding tissue. By contrast, only 10 lesions were detectable by 18F-FDG PET with a much lower tumor/background ratio. These data demonstrated the great value of 18F-Alfatide II in metastatic brain tumor detection.
The increased uptake of 18F-alfatide II may be a result of increased angiogenesis and integrin expression. We noticed the big variance of the values of SUVmax, which may reflect the difference of integrin expression in lesions of different origins and indicate great inter- and intra-individual variation of αvβ3 expression in cancer patients. Due to the lack of histological data in this study, we were not able to correlate the imaging results of 18F-Alfatide II PET with the expression level of integrin αvβ3.
One limitation of this study is the limited number of healthy volunteers and cancer patients. In heathy volunteers, most of RGD based tracers showed similar pharmacokinetic pattern as to the biodistribution of the radioactivity in major organs [24–26]. By taking the previously published results of the other RGD tracers and the data in this study from the healthy volunteers into consideration, we believe the 18F-Alfatide II is a safe PET tracer. Based on our patient data, the accurate diagnosis and prognosis value of 18F-Alfatide II PET also warrant further larger scale clinical investigations.
Conclusion
18F-Alfatide II is a safe integrin αvβ3 PET tracer. The biodistribution and radiation dosimetry of 18F-Alfatide II are similar to other 18F-radiotracers used for PET imaging of integrin αvβ3. The observed effective dose suggests that 18F-alfatide II is feasible for human studies. 18F-Alfatide II has a potential value in finding brain metastases as a biomarker of angiogenesis.
Figure 5.
18F-Alfatide II (A) and 18F-FDG (B) CT, PET and corresponding PET/CT images of a patient with unknown primary cancer and right parietal lobe metastasis (patient 9 in Table 1). CT image shows normal density compared with the contralateral lobe. In the brain metastatic region 18F-Alfatide II shows a high tumor/background ratio of 22.8, but no tumor contrast is identified with 18F-FDG PET.
Acknowledgment
This work was supported, in part, by the National Basic Research Program of China (973 program, 2013CB733802, 2014CB744503), National Natural Science Foundation (81028009, 81171399, 51473071, 81472749, 81401450, 81471691), National Significant New Drugs Creation Program (2012ZX09505-001-001), Jiangsu Province Foundation (BE2012622, BL2012031, BM2012066, BE2014609), Outstanding Professional Fund of Health Ministry in Jiangsu Province (RC2011095, Q201406), Wuxi Social Development Project (CSZON1320), Wuxi Hospital Management Center Project (YGZXL1316), and Intramural Research Program of the National Institute of Biomedical Imaging and Bioengineering (NIBIB), National Institutes of Health (NIH).
Footnotes
Compliance with ethical standards
Conflicts of interest
None.
Statement of human rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
References
- 1.Cai H, Conti PS. RGD-based PET tracers for imaging receptor integrin αvβ3 expression. J Labelled Compd Radiopharm. 2013;56:264–279. doi: 10.1002/jlcr.2999. [DOI] [PubMed] [Google Scholar]
- 2.Niu G, Chen X. Why integrin as a primary target for imaging and therapy. Theranostics. 2011;1:30–47. doi: 10.7150/thno/v01p0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaertner FC, Schwaiger M, Beer AJ. Molecular imaging of αvβ3 expression in cancer patients. Q J Nucl Med Mol Imaging. 2010;54:309–326. [PubMed] [Google Scholar]
- 4.Guo N, Lang L, Gao H, Niu G, Kiesewetter DO, Xie Q, et al. Quantitative analysis and parametric imaging of 18F-labeled monomeric and dimeric RGD peptides using compartment model. Mol Imaging Biol. 2012;14:743–752. doi: 10.1007/s11307-012-0541-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen X, Park R, Shahinian AH, Tohme M, Khankaldyyan V, Bozorgzadeh MH, et al. 18F-labeled RGD peptide: initial evaluation for imaging brain tumor angiogenesis. Nucl Med Biol. 2004;31:179–189. doi: 10.1016/j.nucmedbio.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Jackson AB, Nanda PK, Rold TL, Sieckman GL, Szczodroski AF, Hoffman TJ, et al. 64Cu-NO2A-RGD-Glu-6-Ahx-BBN(7–14)NH2: a heterodimeric targeting vector for positron emission tomography imaging of prostate cancer. Nucl Med Biol. 2012;39:377–387. doi: 10.1016/j.nucmedbio.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Israel I, Richter D, Stritzker J, van Ooschot M, Donat U, Buck AK, et al. PET imaging with [68Ga]NOTA-RGD for prostate cancer: a comparative study with [18F]fluorodeoxyglucose and [18F]fluoroethylcholine. Curr Cancer Drug Targets. 2014;14:371–379. doi: 10.2174/1568009614666140403123452. [DOI] [PubMed] [Google Scholar]
- 8.Jacobson O, Zhu L, Niu G, Weiss ID, Szajek LP, Ma Y, et al. MicroPET imaging of integrin alphavbeta3 expressing tumors using 89Zr-RGD peptides. Mol Imaging Biol. 2011;13:1224–1233. doi: 10.1007/s11307-010-0458-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beer AJ, Grosu AL, Carlsen J, Kolk A, Sarbia M, Stangier I, et al. [18F]galacto-RGD positron emission tomography for imaging of αvβ3 expression on the neovasculature in patients with squamous cell carcinoma of the head and neck. Clin Cancer Res. 2007;13:6610–6616. doi: 10.1158/1078-0432.CCR-07-0528. [DOI] [PubMed] [Google Scholar]
- 10.Kenny LM, Coombes RC, Oulie I, Contractor KB, Miller M, Spinks TJ, et al. Phase I trial of the positron-emitting Arg-Gly-Asp (RGD) peptide radioligand 18F-AH111585 in breast cancer patients. J Nucl Med. 2008;49:879–886. doi: 10.2967/jnumed.107.049452. [DOI] [PubMed] [Google Scholar]
- 11.Lang L, Li W, Guo N, Ma Y, Zhu L, Kiesewetter DO, et al. Comparison study of [18F]FAl-NOTA-PRGD2, [18F]FPPRGD2, and [68Ga]Ga-NOTA-PRGD2 for PET imaging of U87MG tumors in mice. Bioconjugate Chem. 2011;22:2415–2422. doi: 10.1021/bc200197h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wan W, Guo N, Pan D, Yu C, Weng Y, Luo S, et al. First experience of 18F-alfatide in lung cancer patients using a new lyophilized kit for rapid radiofluorination. J Nucl Med. 2013;54:691–698. doi: 10.2967/jnumed.112.113563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edman P. Method for determination of the amino acid sequence in peptides. Acta Chem Scand. 1950;4:283–293. [Google Scholar]
- 14.Lang L, Ma Y, Kiesewetter DO, Chen X. Stability analysis of glutamic acid linked peptides coupled to NOTA through different chemical linkages. Mol Pharm. 2014;11:3867–3874. doi: 10.1021/mp400706q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu C, Yue X, Lang L, Kiesewetter DO, Li F, Zhu Z, et al. Longitudinal PET imaging of muscular inflammation using 18F-DPA-714 and 18F-Alfatide II and differentiation with tumors. Theranostics. 2014;4:546–555. doi: 10.7150/thno.8159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo J, Guo N, Lang L, Kiesewetter DO, Xie Q, Li Q, et al. 18F-Alfatide II and 18F-FDG dual-tracer dynamic PET for parametric, early prediction of tumor response to therapy. J Nucl Med. 2014;55:154–160. doi: 10.2967/jnumed.113.122069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lassmann M, Chiesa C, Flux G, Bardies M. EANM Dosimetry Committee guidance document: good practice of clinical dosimetry reporting. Eur J Nucl Med Mol Imaging. 2011;38:192–200. doi: 10.1007/s00259-010-1549-3. [DOI] [PubMed] [Google Scholar]
- 18.Roivainen A, Kahkonen E, Luoto P, Borkowski S, Hofmann B, Jambor I, et al. Plasma pharmacokinetics, whole-body distribution, metabolism, and radiation dosimetry of 68Ga bombesin antagonist BAY 86-7548 in healthy men. J Nucl Med. 2013;54:867–872. doi: 10.2967/jnumed.112.114082. [DOI] [PubMed] [Google Scholar]
- 19.Mittra ES, Goris ML, Iagaru AH, Kardan A, Burton L, Berganos R, et al. Pilot pharmacokinetic and dosimetric studies of 18F-FPPRGD2: a PET radiopharmaceutical agent for imaging αvβ3 integrin levels. Radiology. 2011;260:182–191. doi: 10.1148/radiol.11101139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beer AJ, Schwaiger M. PET imaging of αvβ3 expression in cancer patients. Methods Mol Biol. 2011;680:183–200. doi: 10.1007/978-1-60761-901-7_13. [DOI] [PubMed] [Google Scholar]
- 21.Beer AJ, Haubner R, Goebel M, Luderschmidt S, Spilker ME, Wester HJ, et al. Biodistribution and pharmacokinetics of the αvβ3-selective tracer 18F-galacto-RGD in cancer patients. J Nucl Med. 2005;46:1333–1341. [PubMed] [Google Scholar]
- 22.Iagaru A, Mosci C, Mittra E, Zaharchuk G, Fischbein N, Harsh G, et al. Glioblastoma multiforme recurrence: an exploratory study of 18F-FPPRGD2 PET/CT. Radiology. 2015 May 12; doi: 10.1148/radiol.2015141550. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 23.Lang L, Li W, Guo N, Ma Y, Zhu L, Kiesewetter DO, et al. Comparison study of [18F]FAl-NOTA-PRGD2, [18F]FPPRGD2, and [68Ga]Ga-NOTA-PRGD2 for PET imaging of U87MG tumors in mice. Bioconjug Chem. 2011;22:2415–2422. doi: 10.1021/bc200197h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beer AJ, Haubner R, Wolf I, Goebel M, Luderschmidt S, Niemeyer M, et al. PET-based human dosimetry of 18F-galacto-RGD, a new radiotracer for imaging αvβ3 expression. J Nucl Med. 2006;47:763–769. [PubMed] [Google Scholar]
- 25.Doss M, Kolb HC, Zhang JJ, Belanger MJ, Stubbs JB, Stabin MG, et al. Biodistribution and radiation dosimetry of the integrin marker 18F-RGD-K5 determined from whole-body PET/CT in monkeys and humans. J Nucl Med. 2012;53:787–795. doi: 10.2967/jnumed.111.088955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McParland BJ, Miller MP, Spinks TJ, Kenny LM, Osman S, Khela MK, et al. The biodistribution and radiation dosimetry of the Arg-Gly-Asp peptide 18F-AH111585 in healthy volunteers. J Nucl Med. 2008;49:1664–1667. doi: 10.2967/jnumed.108.052126. [DOI] [PubMed] [Google Scholar]
- 27.Wan W, Guo N, Pan D, Yu C, Weng Y, Luo S, et al. First experience of 18F-alfatide in lung cancer patients using a new lyophilized kit for rapid radiofluorination. J Nucl Med. 2013;54:691–698. doi: 10.2967/jnumed.112.113563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma Q, Ji B, Jia B, Gao S, Ji T, Wang X, et al. Differential diagnosis of solitary pulmonary nodules using 99mTc-3P4-RGD2 scintigraphy. Eur J Nucl Med Mol Imaging. 2011;38:2145–2152. doi: 10.1007/s00259-011-1901-2. [DOI] [PubMed] [Google Scholar]
- 29.Beer AJ, Lorenzen S, Metz S, Herrmann K, Watzlowik P, Wester HJ, et al. Comparison of integrin αvβ3 expression and glucose metabolism in primary and metastatic lesions in cancer patients: a PET study using 18F-galacto-RGD and 18F-FDG. J Nucl Med. 2008;49:22–29. doi: 10.2967/jnumed.107.045864. [DOI] [PubMed] [Google Scholar]
- 30.Peet AC, Arvanitis TN, Leach MO, Waldman AD. Functional imaging in adult and paediatric brain tumours. Nat Rev Clin Oncol. 2012;9:700–711. doi: 10.1038/nrclinonc.2012.187. [DOI] [PubMed] [Google Scholar]
- 31.Gulyas B, Halldin C. New PET radiopharmaceuticals beyond FDG for brain tumor imaging. Q J Nucl Med Mol Imaging. 2012;56:173–190. [PubMed] [Google Scholar]
- 32.Dearling JL, Barnes JW, Panigrahy D, Zimmerman RE, Fahey F, Treves ST, et al. Specific uptake of 99mTc-NC100692, an αvβ3-targeted imaging probe, in subcutaneous and orthotopic tumors. Nucl Med Biol. 2013;40:788–794. doi: 10.1016/j.nucmedbio.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen X, Hou Y, Tohme M, Park R, Khankaldyyan V, Gonzales-Gomez I, et al. Pegylated Arg-Gly-Asp peptide: 64Cu labeling and PET imaging of brain tumor αvβ3-integrin expression. J Nucl Med. 2004;45:1776–1783. [PubMed] [Google Scholar]
- 34.Zadeh G, Guha A. Molecular regulators of angiogenesis in the developing nervous system and adult brain tumors (review) Int J Oncol. 2003;23:557–565. [PubMed] [Google Scholar]