Abstract
Historically, the human frontal pole (FP) has been considered as a single architectonic area. Brodmann’s area 10, in the frontal lobe with known contributions in the execution of various higher order cognitive processes. However, recent cytoarchitectural studies of the FP in humans have shown that this portion of cortex contains two distinct cytoarchitectonic regions. Since architectonic differences are accompanied by differential connectivity and functions, the frontal pole qualifies as a candidate region for exploratory parcellation into functionally discrete subregions. We investigated whether this functional heterogeneity is reflected in distinct segregations within cytoarchitectonically defined FP-areas using meta-analytic co-activation based parcellation (CBP). The CBP method examined the co-activation patterns of all voxels within the FP as reported in functional neuroimaging studies archived in the BrainMap database. Voxels within the FP were subsequently clustered into sub-regions based on the similarity of their respective meta-analytically derived co-activation maps. Performing this CBP analysis on the FP via k-means clustering produced a distinct 3-cluster parcellation for each hemisphere corresponding to previously identified cytoarchitectural differences. Post-hoc functional characterization of clusters via BrainMap metadata revealed that lateral regions of the FP mapped to memory and emotion domains, while the dorso- and ventromedial clusters were associated broadly with emotion and social cognition processes. Furthermore, the dorsomedial regions contain an emphasis on theory of mind and affective related paradigms whereas ventromedial regions couple with reward tasks. Results from this study support previous segregations of the FP and provide meta-analytic contributions to the ongoing discussion of elucidating functional architecture within human FP.
Keywords: frontal pole, meta-analysis, co-activations, BrainMap, connectivity-based parcellation, frontal lobe
Introduction
The frontal pole (FP) of the human brain, often referred to as BA 10, is situated in the most rostral curvature of the cerebral cortex. During hominid evolution, this region experienced a differential reorganization in apes and humans, and subsequently encompasses a significantly larger proportion of the cortex in humans than in other species (Öngür et al., 2003; Semendeferi et al., 2001; Semendeferi et al., 2011). This region continues to develop deep into adolescence in humans and has been shown to play a crucial role in a diverse range of higher order cognitive functions, including many adapted behaviors claimed to be “human-specific” (Duncan, 2010; Kovach et al., 2012; Ramnani & Owen, 2004; Waskom et al., 2014).
Anatomical definition of the FP was guided by a combination of post-mortem human and nonhuman primate histology and cytoarchitectural studies. Brodmann’s (1909) classic cytoarchitectural definition of BA 10 encompassed a wide area of 6-layer granular isocortex located on the rostral surface of the frontal lobe as well as the contiguous region along the medial wall of the hemisphere. Brodmann’s definition (as adopted by Talairach & Tournoux, 1988) has been widely employed in neuroimaging and neuropsychological research. However, treatment of the anatomically defined FP as a single homogenous area, without respect to its’ functional properties, likely masks a more detailed regional specificity within the rostral frontal cortex. Furthermore, functional boundaries of this region have been highly variable across studies, leading to inconsistencies in their resultant functional properties. Indeed, a recent cytoarchitectural study of the FP in humans (Bludau et al., 2014) showed that the frontopolar cortex contains two distinct cytoarchitectonic regions. This mapping study distinguished between a region on the rostral surface of the frontal lobe that they labeled area Fp1, and an area located along the mesial surface of the superior frontal gyrus that they labeled Fp2. Cytoarchitecturally, Fp1 shows higher cell density in layer II and in lower parts of layer III, and a broader layer IV than area Fp2. Thus, in a region that was once thought to be cytoarchitecturally homogeneous (Dumontheil et al., 2008), we now have evidence to the contrary, which suggests that there may be functionally discrete sub-regions of the FP.
In addition to using cytoarchitectural differences to subdivide a region, it is also possible to distinguish cortical areas based on their patterns of connectivity. For example, fiber tracing studies in the marmoset and the macaque monkey have indicated that areas within the FP possess different anatomical connection patterns (Burman et al., 2011; Petrides & Pandya, 2007). These connectional differences are further supported by diffusion tensor imaging (DTI) findings in humans that indicate that the FP can be divided into sub-regions based on connection patterns (Liu et al., 2013). Using a clustering procedure, Liu performed a connectivity-based parcellation and defined three subregions of the frontopolar cortex and neighboring transitional area of the extreme rostral orbitofrontal cortex.
It is also possible to parcellate regions based on differences in functional connectivity patterns. Connectivity-based parcellation techniques can be applied to resting-state fMRI data to identify sub-regions within an ROI based on differences in voxel-wise time-series correlations between the seed and the whole-brain. Most previous efforts to identify functional distinctions within subregions of the FP were carried out, however, before quantitative coordinate-based meta-analytic methods were made available (Christoff & Gabrieli, 2000; Gilbert et al., 2010; Gilbert et al., 2006). More recently, a robust and task-dependent approach for investigating connectivity between brain regions has emerged with the advent of meta-analytic connectivity modeling (MACM) (Eickhoff et al., 2010; Laird et al., 2009b; Robinson et al., 2010). This technique mines the co-activation patterns reported across hundreds of published neuroimaging studies archived in the BrainMap database (http://brainmap.org) in order to determine the task-based functional connectivity of brain regions. This data-driven parcellation technique provides a complementary approach toward the delineation of cortical modules (Muhle-Karbe et al., 2014). The methodology is motivated by the notion that the function of a brain region is ultimately constrained by its connections with other areas (Passingham et al., 2002) known from monkey and cat axonal tracing, which implies that functional units should be distinguishable based on the dissimilarity of their connections. Bludau et al. provided a preliminary MACM in which they tested whether FP areas defined by probabilistic locations of FP1 and FP2 showed different patterns of co-activation. Their results showed definite regional differences, however they did not test whether a parcellation based on task-based functional connectivity follows similar contours as their cytoarchitecturally defined areas.
Although structure and function are closely related in brain architecture, there is not necessarily a one-to-one relationship between them. Instead, it is possible for differential functional zones to exist even within an area that shares gross similarities in cytoarchitecture. This occurrence has been noted in previous studies examining the prefrontal cortex (Duncan & Owen, 2000), but has yet to be explicitly studied across a range of cognitive processes within the FP. To further investigate the task-based functional connectivity of the FP, we conducted co-activation based parcellation (Eickhoff et al., 2011; Johansen-Berg et al., 2004) in conjunction with MACM. This allowed us to test whether regional differences in the whole-brain functional co-activation patterns of the FP enable identification of discrete subdivisions of the region. These frontopolar sub-regions were then functionally characterized by means of forward and reverse inference to determine their behavioral profiles according to the BrainMap taxonomic classification system.
Methods
Region of Interest Definition
The region of interest (ROI) for each hemisphere encompassed the two cytoarchitectonic areas of BA 10; the lateral frontopolar area 1 (FP1) and the medial frontopolar area 2 (FP2) as defined by Bludau et al. (2014). A detailed description of the analyses carried out to identify the cytoarchitectonic organization of the FP can be found in (Bludau et al., 2014). In summary, observer-independent detection of cytoarchitectonic borders was performed via histological analysis of 10 post-mortem human brains. To this end, histological sections (thickness = 20μm) containing the frontal polar region were digitized with an in-plane resolution of 1.02μm per pixel. Gray-level index (GLI; Wree et al., 1982) images of these slices were then calculated, thus providing a means for identification of the cytoarchitectonic organization for the region (e.g. identification of the borders for each cellular layer within the cortex, volume fraction of cells within cellular layers). A sliding window procedure was used for border detection along the cortical ribbon, which compared adjacent groups of profiles against each other (Schleicher & Zilles, 1990; Schleicher et al., 1999; Schleicher et al., 2000;Schleicher et al., 2009; Schleicher et al., 2005).
The frontopolar areas were 3D-reconstructed using linear and non-linear transformation algorithms (Hömke, 2006), and normalized to the T1-weighted single-subject template of the MNI (Montreal Neurological Institute; (Evans, Janke, Collins, & Baillet, 2012; Evans et al., 1992). From there, a maximum probability map (MPM) of Fp1 and Fp2 was created that assigned the cytoarchitectonic area of each voxel with the highest probability in the reference space of the MNI template (Amunts et al., 2005; Eickhoff et al., 2006; Eickhoff et al., 2005). This allowed the inclusion of only those voxels into the ROI where the frontal polar fields had been more likely found than any other brain region in histological examination (Fig. 1A).
Figure 1.
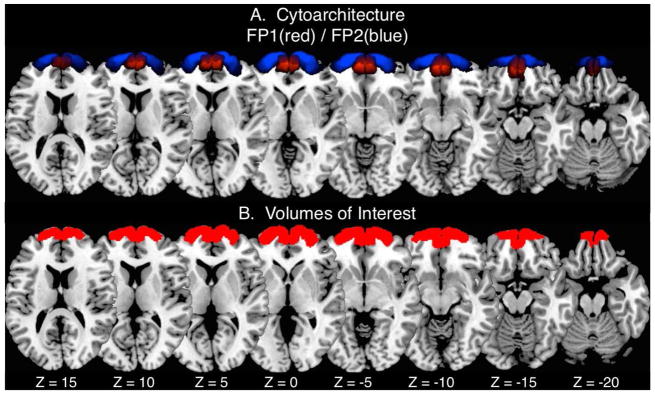
The volumes of interest used in the current CBP analysis were derived from A) the cytoarchitectonic borders of area frontopolaris one (FP 1 in red) and area frontopolaris two (FP 2 in blue) as identified in Bludau et al., (2014). B) The FP1 and FP2 within each hemisphere were joined to create a single binary mask for the right hemisphere and an additional binary mask for the left hemisphere for the CBP analysis.
Taking into consideration that the FP includes a midline region along the medial wall of the rostral frontal lobe, we separated the initial search region into two independent ROIs for the right and left hemisphere. This was done to ensure that resultant parcellation solutions would not contain cross-hemispheric clusters. The MPM of the right and left FP were thresholded and reformatted into two binary masks, where voxels within the ROI were assigned a value of 1 and all other voxels a value of zero. The resultant left hemisphere ROI consisted of 3020 voxels, while the resultant right hemisphere ROI consisted of 2777 voxels (voxel size: 2 × 2 × 2 mm3) (Fig. 1B).
Data Processing Outline
Once the boundaries of our ROIs (the right and left FP) were established, a meta-analytic connectivity map was created for each voxel within each ROI. These voxel-wise MACMs assigned the probability of co-activation of each remaining voxel in the brain with the seed-voxel based on the thousands of experiments archived in the BrainMap database. Next, voxels within the ROI were grouped together (via k-means clustering) based on the similarities of their MACM co-activation maps. The stability and consistency of k-means cluster solutions were assessed using a combination of different cluster stability metrics to identify an optimal parcellation solution.
A second MACM was performed using each cluster within the optimal parcellation solution as independent seed regions. This step in our analysis yielded a whole-brain co-activation map for each cluster within the right and left FP. Lastly, functional characterization of each cluster was assessed by testing for significant overrepresentation of taxonomic classes of the BrainMap database, which describe psychological and experimental information regarding all archived studies. The culmination of these analysis steps provides a data-driven framework to examine functional properties of the human frontal pole.
Meta-Analytic Connectivity Mapping
Whole-brain co-activation maps were computed for each voxel within each ROI (left and right FP, independently) using data archived in the BrainMap database (Laird et al., 2005; Laird et al., 2009a; Laird et al., 2011a). This type of analysis can be considered complementary to a seed-based correlation analysis of a single voxel from an fMRI time-series (Laird et al., 2013). However, instead of correlating the time-series of a seed-voxel, MACM derived voxel-wise co-activation profiles provide a measure of the co-activation probability of a given seed voxel with every other voxel in the brain using the ALE method (Eickhoff et al., 2009; Eickhoff et al., 2012). Of note, only fMRI and PET studies reporting activations in healthy subjects were included (i.e., no pharmacological or otherwise interventions, no between-group comparisons), yielding approximately 7,200 functional neuroimaging experiments at the time of the query.
When mapping voxel-wise co-activation profiles within a volume, one must take into consideration the variable and usually low number of foci reported for each seed voxel. To account for this variability, we calculated the co-activation profile for each voxel using a set of n experiments (determined by an inclusion filter) containing the closest activation foci for every voxel in each ROI (Bzdok et al., 2012; Cieslik et al., 2013; Clos et al., 2013). The inclusion filter (referred to in some papers as a spatial filter because of its impact on the spatial range of included foci) was applied to every voxel systematically to include the closest 20 – 200 experiments in steps of two (i.e., we selected the nearest 20, 22, 24… 200 experiments; 91 filter sizes in total) where the foci proximity was measured using Euclidian distances. For example, the co-activation profile for the voxel at coordinate x,y,z (where x,y,z occurs within the ROI) was measured using the 20 experiments containing an activation foci closest to x,y,z; this step was then repeated for the remaining 90 filter sizes (i.e. the closest 22, 24, …, 200 sets of experiment foci) and for each voxel within the ROI.
We examined co-activation profiles using a range of inclusion filters because the number of experiments associated with a seed voxel has a considerable influence on the ensuing whole-brain maps (Bzdok et al., 2015). With out this inclusion filtering, an unbalanced number of experiments associated with the voxels within an ROI could results in a biased cluster solution. In other words, ensuing cluster solutions derived from unbalanced voxel-wise co-activation profiles could be strongly driven by their unequal sizes of seed voxel’s experiment sets, rather than their actual whole-brain connectivity pattern. The present approach to inclusion filtering provides a more uniform “resolution”, or level of detail, across the whole-brain as compared to applying a single predetermined radius constraint on the selection of experiments contributing to voxel-wise MACMs. The danger of using a more simple radius constraint filtering approach in an analysis such as this is the resultant low level of detail, or poor quality, of co-activation in parts of the brain that are reported less frequently in BrainMap experiments (Behrens et al., 2013) which makes the current approach superior compared to other model-free methods such as independent component analysis (Beckmann, 2012) or principal component analysis.
Voxel-wise brain-wide co-activation maps were generated for each of the 91 filter sizes using the activation likelihood estimation (ALE) algorithm, a coordinate-based meta-analytic approach. In particular, meta-analysis was performed using the revised ALE method (Eickhoff et al., 2009; Turkeltaub et al., 2012). Here, foci within a set of experiments (i.e., a single filter size) were treated as smoothed centers of 3D Gaussian probability distributions where the spatial extent of those Gaussian probability distributions was based on empirical estimates of between-subject and between-template variance of neuroimaging foci (Eickhoff et al., 2009) that reflect the spatial uncertainty associated with neuroimaging results. Smoothed foci were subsequently combined to create a modeled activation map that identified the probability of activation for each voxel within a given experiment using a “nonadditive” approach that prevented local summation effects (Turkeltaub et al., 2012). The union of experiment-wise modeled activation maps was then used to calculate ALE scores describing the co-activation probability of that particular voxel location with the current seed voxel. Unthresholded ALE scores of all voxels within gray matter (based on 10% probability according to the ICBM maps within MNI template space) were recorded for each voxel within the right and left FP ROIs.
In summary, we performed a quantitative ALE meta-analysis for each voxel within the ROI at each inclusion filter size. This allowed us to measure how likely any voxel in the brain was to co-activate with a particular seed-voxel within the ROI and subsequently assess the variability of voxel-wise co-activation patterns across the range of filter sizes employed.
Co-Activation Based Parcellation
Brain-wide co-activation profiles containing the unthresholded MACMs for all seed voxels were combined into a NS x NT co-activation matrix, where NS denotes the number of seed voxels (3020 and 2777 voxels for the left and right ROIs respectively) and NT the number of target voxels in the gray matter of the reference brain volume (~211,000 voxels) at 2 × 2 × 2 mm3 resolution. This step was performed 91 times, once for each inclusion filter, resulting in 91 individual co-activation matrices, each representing the whole-brain connectivity of the seed voxels at a particular filter size. Parcellation of the ROI into K non-overlapping clusters was carried out using K-means clustering, as implemented in Matlab, with K = 2 – 4 using one minus the correlation between the connectivity patterns of seed voxels as a distance measure (i.e., correlation distance). This parcellation was performed for each of the 91 filter sizes independently, yielding 3 (K-means cluster solutions) × 91 (filter size) independent cluster solutions. K-means clustering is a non-hierarchical clustering method that uses an iterative algorithm to separate the seed region into a previously selected number of K non-overlapping clusters (Forgy, 1965; Hartigan & Wong, 1979). The K-means algorithm randomly selects K voxels within the ROI as representative centers-of-mass for its respective cluster in which remaining voxels are assigned thereby assigned to the closest cluster center. This randomization of selected center-of-mass voxels cause final assignments of seed voxels to particular clusters to slightly vary from one initialization to another. Therefore, we repeated this process 100 times, and recorded the best solution for each of the 6 (left and right hemisphere) × 91 parcellations.
Inclusion Filter Selection
The CBP procedure outlined above produced three unique parcellation solutions (K = 2–4) for each inclusion filter of both the right and left FP. One of the many issues in analyzing and interpreting large datasets is identifying the optimal solution for a myriad of situations. The process for selecting an appropriate range of inclusion filters for MACM-based CBP analyses has recently evolved. Previous studies averaged K-means cluster solutions across all filter sizes (Bzdok et al., 2012; Cieslik et al., 2013); this was most appropriate where there was a generally homogenous set of solutions across inclusion filter sizes. The current study, however, implemented the more refined and multifaceted process presented in Clos et al., (2013) that examined the properties of the various solutions to identify the most stable range of inclusion filter sizes, and then determined the optimal number of K clusters based on a series of metrics (Bzdok et al., 2015; 2013; Clos et al., 2013; Eickhoff et al., 2014; Muhle-Karbe et al., 2014; EIckhoff et al., in press). In other words, we assessed the consistency of the cluster assignment for individual voxels across all filter sizes collected and selected the inclusion filter range that produced solutions most similar to the ‘consensus solution’ (i.e., the cluster solution most representative of the 91 filter sizes). This ensured that subsequent steps in our analysis were carried out using the most stable range of filter sizes that produced the most consistent co-activation profiles.
The first step of this two-step procedure involved identifying the consistency of cluster assignment for individual voxels across different inclusion filters. The range of filter sizes that produced clustering solutions most similar to the consensus solution was selected based on the proportion of deviant voxels (i.e., the number of times a given voxel assignment disagreed with the consensus solution) per filter size across K. Inclusion filters with few deviant voxels were most desirable, thus we selected those whose number of deviants was at least half a standard-deviation below the average number of deviants across all filter sizes thereby restricting the analysis to filter sizes that reflected solutions most similar to the consensus solution. Previous CBP studies have commonly observed a central tendency in the selection of the optimal filter range (Muhle-Karbe et al., 2014), where the intermediate portion of the initial range is deemed optimal (given some variation among studies). Accordingly, further analysis steps were restricted to the parcellations based on co-activation as estimated from the nearest 92 to 168, and 94 to 158 experiments for the right and left FP respectively.
Optimal Parcellation Solution
The second step in determining an optimal parcellation solution involved selecting the most appropriate number of K-means clusters based on the output solutions from the optimal range of inclusion filter sizes identified in the previous step of this analysis. The cluster solution yielding the highest measure of improvement (as compared to the K−1 cluster solution, where K is the number of clusters) was interpreted as bearing the highest model fit of the clustering given the data. We used six different criteria describing information-theoretic, cluster separation, and topological properties to assess cluster solutions across K.
The first topological criterion assessed was the percentage of voxels not assigned to its parent (K−1) solution. This metric takes into account the hierarchy of solutions as K increases, and corresponds to the percentage of voxels in the current solution, K, that were assigned to a different cluster than its K−1 solution (Kahnt et al., 2012). For example, voxels in cluster 1 of solution K would be excluded if the majority of its voxels stemmed from a cluster not in the same topological area in its K−1 solution. This metric favors hierarchical consistency of voxel assignment, and therefore, a K cluster parcellation was considered a good solution for those in which the percentage of lost voxels was below the median across all clustering steps. We additionally utilized another topological criterion that measured the percentage of “misclassified” voxels. This metric addressed across-filter stability of voxel-to-cluster assignment and represented the percentage of voxels (computed within each filter size) that were assigned to a different cluster compared to the consensus solution across all filter sizes. Favorable solutions for this metric were indicated by percentages that were not significantly higher than the K−1 solution, in particular when followed by a significant increase in the K+1 solution. The final topological measures assessed were the mean and minimum number of consistent voxels per cluster. For these measures, solutions in which the ratio between the minimum and the average cluster size was more than 0.5 were considered acceptable.
As an information-theoretic criterion, we evaluated the similarity of cluster assignment between the current solution and neighboring K−1 and K+1 solutions within the range of optimal filter-sizes using the variation of information (VI) metric. This metric was previously established as a cluster criterion for determining the optimal K-means parcellation of a particular brain region (Kelly et al., 2010; Kahnt et al., 2012; Clos et al., 2013; Bzdok et al., 2015). The variation of information between two cluster solutions C and C′ was computed by
| (Equation 1) |
where H represents the amount of information (entropy) present in the cluster solutions C and C′, respectively, while I is the mutual information shared by the two cluster solutions C and C′ (Clos et al., 2013). Using this metric, stable solutions exhibit a significant increase from K to K+1 solutions, or a significant decrease from K−1 to K solutions.
Lastly, we employed two cluster separation criteria to assess the goodness of fit of resultant parcellation solutions: the change in intercluster to intracluster distance, and the average silhouette value. We calculated the average intercluster to intracluster distance ratio as presented in Chang et al., (2012) by
| (Equation 2) |
where N represents the number of voxels in the matrix and K reflects the number of clusters. We take x to be each voxel and zi to be the center of cluster Ci. The change in intercluster to intracluster distances was measured by taking the first derivative of the average intercluster to intracluster distance ratio (Equation 2). Solutions were considered stable if the subsequent step did not offer a significantly larger increase in the inter/intra-cluster distance. The average silhouette value ranges from −1 to 1 and is a general measure of how similar a given voxel is to other voxels in its own cluster compared to voxels in other clusters. A parcellation solution, K, was deemed acceptable when its silhouette value was significantly higher than the K−1 value or not significantly lower than that of K−1.
Task-Dependent Connectivity of Parcellation
Once the optimal parcellation solution was identified for the right and left FP, meta-analytic connectivity modeling was performed on the individual ensuing clusters to characterize their task-based functional connectivity (i.e., co-activation) patterns. In this context, the seed of each MACM corresponds to the collection of voxels within one of the clusters resulting from the optimal CBP solution. In other words, unlike in the initial MACM where individual co-activation maps were created for each voxel within a given ROI, we here created a single co-activation map representative of all voxels within the respective cluster of a particular K-means solution. The co-activation profiles of each cluster were obtained by first identifying all experiments in BrainMap (meeting the previously mentioned search criteria) containing an activation coordinated within the parcellated seed region. Considering that each individual seed region (e.g., each cluster from the optimal parcellation solution) contained more than 300 voxels (2 × 2 × 2 mm3), we did not search for the nearest x experiments as in the previous voxel-wise co-activation analysis. Here, all experiments meeting the search criteria were exported into an individual workspace for each of the six clusters for further analysis.
The ALE algorithm was applied to each set of experiments to identify their respective co-activation patterns. In this instance of MACM, additional statistics were assessed where ALE scores of a given cluster were compared to a null-distribution reflecting a random spatial association between experiments with a fixed within-experiment distribution of foci in order to determine significance (Eickhoff et al., 2009). This random-effects inference assesses above-chance convergence between experiments, rather than the clustering of foci within a particular experiment. The observed ALE scores from the actual meta-analysis of experiments activating within a particular cluster were then tested against ALE scores obtained under a null-distribution of random spatial association yielding a P value based on the proportion of equal or higher random values (Eickhoff et al., 2012). Resulting non-parametric P values were transformed into Z-scores and thresholded at a cluster-level corrected threshold of P < 0.05 (cluster-forming threshold at voxel-level P < 0.001) and output as ALE/MACM map in image format.
Functional Characterization of Cluster Parcellations
Finally, quantitative functional decoding was performed on the ensuing clusters via forward and reverse inference of MACM maps. Functional characterization was based on the metadata associated with experiments archived in the BrainMap database, specifically the taxonomic classifications of Behavioral Domain and Paradigm Class. Briefly, the behavioral domain field captures the cognitive process isolated by the experimental contrast, while the paradigm class field categorizes the task performed during each experiment (see http://brainmap.org/scribe for more information regarding BrainMap taxonomy). These two metadata fields were chosen because previous studies have identified them as most salient for network characterization (Laird et al., 2011b).
We performed a forward and reverse inference analysis on each sub-region of the right and left FP. In doing so, we identified taxonomic labels for which the probability of finding activation in the respective cluster was significantly higher than chance (forward inference), and the most likely behavioral domains and paradigms given activation in the particular cluster (reverse inference; (Poldrack, 2006; Yarkoni et al., 2011; Poldrack et al., 2012; Bzdok et al., 2012; Cieslik et al., 2013;). Significance for the forward inference analyses was established using a binomial test (P < 0.05) in which we tested whether the conditional probability of activation given a particular label [P(Activation|Task)] was higher than the baseline probability of activating the region in question per se [P(Activation)]. Significance for reverse inference analyses was assessed by means of a chi-square test (P < 0.05). This likelihood P(Task|Activation) was derived from P(Activation|Task) as well as P(Task) and P(Activation) using Bayes’ rule.
Results
Co-Activation Based Parcellation
Co-activation based parcellation produced three unique parcellation solutions (K = 2–4) for both the right and left FP. These parcellations were based on co-activation profiles from the nearest 92 to 168, and 94 to 158 experiments (inclusion filter) for the right and left FP respectively. Selection of the optimal solution for each hemisphere was determined using six different cluster validity criteria: the percentage of voxels not assigned to its parent, percentage of “misclassified” voxels, the mean vs. minimum number of consistent voxels per cluster, optimal filter-sizes using the variation of information (VI) metric, the change in intercluster to intracluster distance, and the average silhouette value. The cluster solution identified as desirable or acceptable in the most number of these six criteria was deemed as the “optimal solution”.
Regarding the left hemisphere parcellations (Table 1; Supplementary Fig. 1); the percentage of voxels not with parent indicated that the three-cluster solution was a good candidate solution because it fell below the median value across all clustering steps. The percent of misclassified voxels suggested that the three-cluster solution was stable because it was not significantly higher than the previous solution (K = 2), but was followed by a significant increase in the next solution (K = 4). The smallest cluster in each parcellation solution was greater than one half of the mean number of voxels, indicating that all solutions were plausible. The variation of information across filter sizes suggested that the three-cluster parcellation was best because there was the smallest change in values from K = 2 to K = 3 as compared to other solutions. Likewise, the change in inter/intra cluster distances metric supported the solution for K = 3. Lastly, the average silhouette value supported both K = 3 and K = 4 solutions.
Table 1.
Six metrics were evaluated describing information-theoretic, cluster separation, and topological properties to assess cluster solutions across K. Metrics were assessed for each hemisphere independently. Taking these metrics into consideration, CBP solutions indicate that the left and right hemispheres each parcellate optimally into three clusters.
| Left Hemisphere | Right Hemisphere | ||||||
|---|---|---|---|---|---|---|---|
| k = | 2 | 3 | 4 | 2 | 3 | 4 | |
| Metric Evaluated | Percent of Voxels not with Parent | ✓ | ✓ | ||||
| Percent of Missclassified Voxels | ✓ | ✓ | |||||
| Mean & Min # of Consistent Voxels/Cluster | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Variation of Information Across Filter Sizes | ✓ | ✓ | |||||
| Change in Inter/Intra Cluster Distance | ✓ | ✓ | |||||
| Average Silhouette Value | ✓ | ✓ | ✓ | ✓ | |||
When evaluating the right hemisphere parcellations (Table 1; Supplementary Fig. 2), the percentage of voxels not with parent indicated that the three-cluster solution was a good candidate solution because it fell below the median value across all clustering steps. The percent of misclassified voxels metric also supported the three-cluster solution, as it was the only parcellation that was not significantly greater than the previous solution, and was followed with a significant increase to the next solution. Comparing the mean vs. minimum number of voxels per cluster indicated that all solutions examined were plausible. The variation of information across filter sizes suggested that the three-cluster parcellation was best because there was the smallest change in values from K = 2 to K = 3. The change in inter/intra cluster distances metric supported the K = 3 solution. Lastly, the average silhouette value indicated that the three- and four-cluster solutions were considered as acceptable solutions.
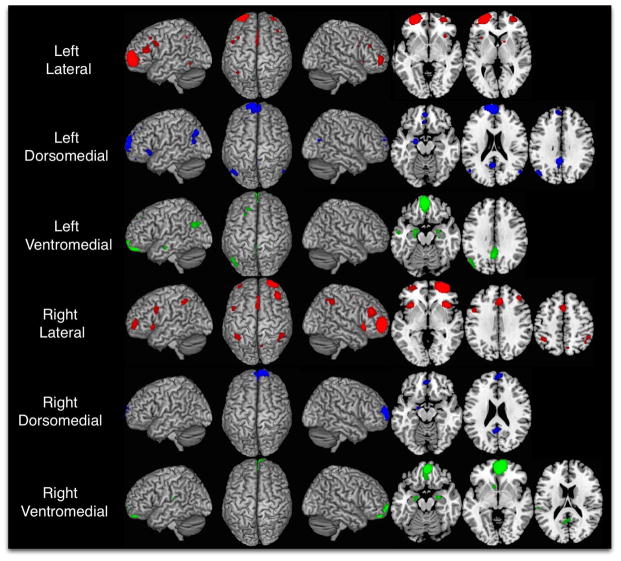
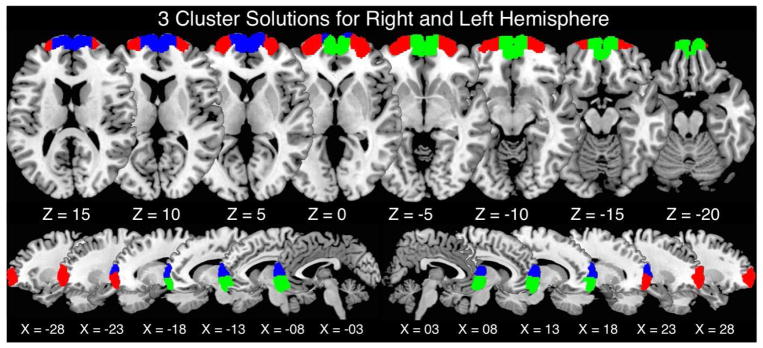
Taking these metrics into consideration, CBP solutions indicate that the left and right hemispheres parcellate optimally into three discrete sub-regions (Table 1; Fig. 2). These divisions were fairly symmetric across hemispheres, with both right and left frontopolar region yielding a single lateral cluster and two medial clusters (dorsal and ventral with the transition occurring approximately at z = 2). Right hemisphere clusters encompassed 7427 mm3, 5848 mm3, and 4748 mm3 in the lateral, ventromedial, and dorsomedial regions respectively. Left hemisphere clusters were comprised of 7527 mm3, 7018 mm3, and 5156 mm3 voxels in the lateral, ventromedial, and dorsomedial regions respectively.
Figure 2.
CBP was performed on the right and left FP independently. Optimal solutions contained a three-cluster solution for each hemisphere. Right and left dorsomedial, ventromedial, and lateral clusters are shown in blue, green, and red, respectively.
As can be seen in Figures 1 and 2, the CBP divisions (Fig. 2) follow a similar, although not identical, alignment with the cytoarchitecturally-defined boundaries of Fp1 and Fp2 (Fig. 1a). In both hemispheres, the lateral cluster of the CBP division was completely contained within the borders of FP 1. This lateral CBP derived cluster stops around x = +/− 16, whereas Fp1 extends to the anterior medial wall of the hemisphere. The CBP analysis indicates that the more medial section on the rostral surface of the frontal lobe is occupied by a cluster containing both the medial rostral surface of the FP and the area just posterior to it on the medial wall that would be equivalent to Fp2. Furthermore, the CBP analysis indicates that this medial cluster can be split into distinct dorsal and ventral regions at K = 3, whereas the cytoarchitectural divisions defined by Bludau et al. (2014) did not feature such a ventral-dorsal split in either Fp1 or Fp2 similar to the CBP results at K = 2.
Task-Dependent Functional Connectivity of Parcellated Regions
Meta-analytic connectivity modeling was performed on each cluster within the optimal parcellation for each hemisphere of the three-cluster solution. In doing so, the specific task-dependent functional connectivity patterns of the three clusters for each hemisphere was established and cluster-level corrected (Fig. 3, Table 2). In other words, we identified areas of the brain that were most likely to be activated, given activation of the seed region.
Figure 3.
Meta-analytic connectivity modeling (MACM) was performed on each of the ensuing clusters resulting from the preferred K-means clustering solution as indicated in Table 1. MACMs identify regions that commonly co-activate with the particular seed region. Right and left dorsomedial, ventromedial, and lateral clusters are shown in blue, green, and red, respectively. Cluster-level corrected threshold of P < 0.05 (cluster-forming threshold at voxel-level P < 0.001).
Table 2.
Peak locations of the overall co-activation pattern, via MACM, for each cluster. Coordinates correspond to MNI space, anatomical labels were provided by the Talairach Daemon (Lancaster et al., 1997; Lancaster et al., 2000). Cluster-level corrected threshold of P < 0.05 (cluster-forming threshold at voxel-level P < 0.001).
| Left Hemisphere | Right Hemisphere | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lateral | X | Y | Z | Size (mm) | Lobe | Gyrus | BA | X | Y | Z | Size (mm) | Lobe | Gyrus | BA |
| 1 | −30 | 56 | −2 | 14085 | Frontal | Middle Frontal | 10 | 32 | 58 | −2 | 13192 | Frontal | Middle Frontal | 10 |
| 2 | 0 | 28 | 34 | 3947 | Frontal | Cingulate | 32 | 2 | 28 | 38 | 5560 | Limbic | Cingulate | 32 |
| 3 | 30 | 56 | −2 | 3277 | Frontal | Middle Frontal | 10 | 34 | 22 | −4 | 3344 | Sub-lobar | Claustrum | |
| 4 | −44 | 26 | 18 | 1745 | Frontal | Middle Frontal | 46 | 42 | 36 | 28 | 2776 | Frontal | Middle Frontal | 9 |
| 5 | −48 | 6 | 30 | 1221 | Frontal | Inferior Frontal | 6 | −36 | 54 | 4 | 2416 | Frontal | Middle Frontal | 10 |
| 6 | −38 | −52 | 46 | 537 | Parietal | Inferior Parietal | 40 | −34 | 20 | −2 | 2032 | Sub-lobar | Claustrum | |
| 7 | 34 | 24 | −4 | 511 | Sub-lobar | Insula | −38 | −48 | 50 | 1384 | Parietal | Inferior Parietal | 40 | |
| 8 | 42 | 34 | 30 | 313 | Frontal | Middle Frontal | 9 | 50 | −44 | 48 | 1104 | Parietal | Inferior Parietal | 40 |
| 9 | −34 | 20 | 2 | 267 | Sub-lobar | Insula | 13 | −48 | 10 | 36 | 1048 | Frontal | Precentral | 6 |
| 10 | 14 | 10 | 6 | 261 | Sub-lobar | Caudate | 8 | −66 | 52 | 48 | Parietal | Precuneus | 7 | |
| 11 | −38 | 20 | 44 | 257 | Frontal | Middle Frontal | 6 | 46 | 10 | 24 | 40 | Frontal | Inferior Frontal | 9 |
| 12 | −50 | −60 | −8 | 211 | Temporal | Fusiform Gyrus | 37 | |||||||
| 13 | −26 | −60 | 50 | 165 | Parietal | Superior Parietal | 7 | |||||||
| 14 | 4 | 40 | −2 | 105 | Limbic | Anterior Cingulate | 24 | |||||||
| 15 | 48 | 10 | 30 | 75 | Frontal | Precentral Gyrus | 9 | |||||||
| 16 | −48 | 38 | −6 | 45 | Frontal | Inferior Frontal | 45 | |||||||
| 17 | 40 | 20 | 48 | 45 | Frontal | Middle Frontal | 6 | |||||||
| VM | ||||||||||||||
| 1 | −2 | 54 | −6 | 23986 | Limbic | Anterior Cingulate | 32 | 4 | 52 | −8 | 19880 | Limbic | Anterior Cingulate | 32 |
| 2 | −6 | −56 | 26 | 7761 | Limbic | Posterior Cingulate | 31 | −6 | −56 | 22 | 3232 | Limbic | Posterior Cingulate | 23 |
| 3 | −48 | −68 | 28 | 1925 | Temporal | Middle Temporal | 39 | 24 | −6 | −18 | 584 | Limbic | Parahippocampal | |
| 4 | −20 | −6 | −16 | 1597 | Limbic | Parahippocampal | −20 | −8 | −16 | 568 | Limbic | Parahippocampal | ||
| 5 | 24 | −8 | −20 | 1193 | Limbic | Parahippocampal | −8 | 16 | −6 | 456 | Sub-lobar | Caudate | ||
| 6 | −16 | 36 | 44 | 565 | Frontal | Superior Frontal | 8 | −60 | −28 | 18 | 64 | Temporal | Superior Temporal | 42 |
| 7 | −54 | −8 | −18 | 493 | Temporal | Middle Temporal | 21 | |||||||
| 8 | −8 | 16 | −6 | 433 | Sub-lobar | Caudate | ||||||||
| 9 | 28 | −20 | −12 | 359 | Limbic | Parahippocampal | 28 | |||||||
| 10 | −24 | 26 | 50 | 231 | Frontal | Middle Frontal | 6 | |||||||
| 11 | −4 | 60 | 24 | 63 | Frontal | Superior Frontal | 9 | |||||||
| DM | ||||||||||||||
| 1 | −4 | 60 | 8 | 22639 | Frontal | Medial Frontal | 9 | 6 | 60 | 6 | 15616 | Frontal | Medial Frontal | 9 |
| 2 | −6 | −48 | 32 | 5383 | Parietal | Precuneus | 31 | −2 | −52 | 22 | 1432 | Limbic | Posterior Cingulate | 23 |
| 3 | −46 | −72 | 36 | 2671 | Temporal | Angular Gyrus | 39 | −18 | −6 | −16 | 200 | Limbic | Parahippocampal | |
| 4 | −20 | −6 | −16 | 1299 | Limbic | Parahippocampal | 0 | 40 | 8 | 16 | Limbic | Anterior Cingulate | 24 | |
| 5 | −48 | 22 | −8 | 1191 | Frontal | Inferior Frontal | 47 | |||||||
| 6 | −2 | 32 | −18 | 513 | Limbic | Anterior Cingulate | 32 | |||||||
| 7 | 54 | −70 | 20 | 427 | Temporal | Middle Temporal | 39 | |||||||
| 8 | −20 | 40 | 44 | 63 | Frontal | Superior Frontal | 8 | |||||||
The right lateral cluster contained a relatively widespread co-activation pattern including portions of the anterior cingulate gyrus, bilateral middle frontal gyrus, insula, and inferior parietal lobe. Similar to the right lateral cluster, the left lateral cluster contained a relatively high level of co-activations, including the anterior cingulate, bilateral middle frontal gyrus extending into the left inferior frontal gyrus, bilateral mid frontal gyrus, bilateral insula, the left fusiform gyrus and right caudate. The right dorsomedial cluster revealed co-activation in the posterior cingulate, the left medial temporal gyrus, and the left globus pallidus in conjunction with the parahippocampus. Co-activation with the left dorsomedial cluster was highly left-lateralized, with the exception to bilateral co-activation in the middle temporal gyrus; this included the left middle and inferior frontal gyrus, left parietal, and left parahippocampus. The right ventromedial region co-activated with the anterior and posterior cingulate, the left superior temporal gyrus, and bilateral amygdala. In addition, the left ventromedial cluster co-activated with the left superior and inferior frontal gyrus, left caudate and lentiform nucleus, left superior temporal gyrus, and bilateral amygdala & anterior hippocampus. Of note, each of the three clusters for the left hemisphere shared a common co-activation in the posterior cingulate cortex, and the left angular gyrus.
Functional Characterization of Cluster Parcellations
After parcellating the right and left FP into discrete clusters and delineating the connectivity of the ensuing subdivisions, the functional profiles of each sub-region were determined based on cognitive terms from the BrainMap taxonomy. Specifically, we performed a forward and reverse inference analysis of the behavioral domains and paradigm classes associated with each subregion of the right and left FP, wherein forward inference derives co-activation from a psychological term, and reverse inference derives a psychological term from regional co-activation (Poldrack, 2006). In particular, these analyses demonstrate that although the FP may be activated in the context of a wide range of tasks, meta-analysis provides a rigorous methodology for examining which tasks consistently yield activation with a measure of statistical significance. Overall, functional decoding results indicated a high degree of agreement across significant metadata in the forward and reverse inference analyses, and bilateral clusters were typically associated with similar metadata results (i.e., the right and left lateral clusters presented with similar functional characterizations) (Fig. 4). Notably, the results of the forward and reverse inference analyses are inherently constrained by the taxonomic terms available in the BrainMap database.
Figure 4.
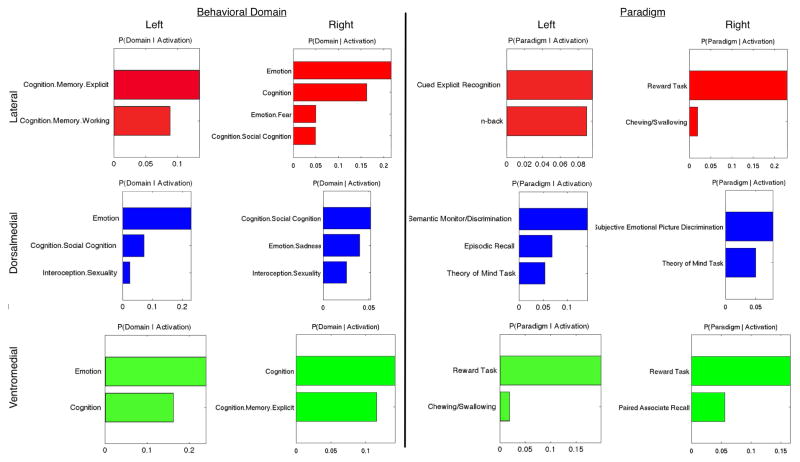
A forward and reverse inference analysis was performed on each cluster to identify behavioral domains and paradigm classes presented in the BrainMap database that were significantly associated with each cluster. Results from the reverse inference analyses are shown, however metadata fields meeting significance in the forward inference analyses correspond highly with those shown here. Metadata fields meeting significance (p < 0.05) are shown in blue for the left hemisphere and teal for the right hemisphere.
As expected, a majority of the behavioral domains significantly associated with FP clusters were related to cognition. More specifically, the left lateral cluster was associated with explicit and working memory behaviors, while the right cluster was more broadly associated with general cognition and emotion, as well as fear and social cognition. The left lateral cluster was also significantly associated with the paradigm classes of cued explicit recognition and the n-back task, while the right lateral cluster was significantly associated with reward tasks and chewing/swallowing tasks. Bilateral dorsomedial regions were significantly related to social cognition and sexuality; the left hemisphere was additionally related to general emotion, while the right hemisphere was more specifically associated with sadness. These dorsomedial regions were both linked to theory of mind tasks, with the left dorsomedial cluster additionally linked to episodic recall and semantic discrimination, while the right dorsomedial region was associated with subjective emotional picture discrimination. Lastly, bilateral ventromedial clusters were broadly related to general cognition behaviors with the left hemisphere additionally linked with general emotion, and right hemisphere with explicit memory. These ventromedial clusters were also related to paradigms including pared associate recall, chewing/swallowing, and reward tasks.
The ROIs generated from the parcellation solutions shown in Figure 4 will be provided online through the BrainMap database website at brainmap.org.
Discussion
Connectivity-based parcellation was performed on regions of the left and right FP using boundaries defined by cytoarchitectural analysis of human areas Fp1 and Fp2. An optimal cluster solution was determined using metrics relevant to information-theoretic criteria, cluster separation, and topological properties. Convergence of acceptable parcellation metrics was observed independently for the three-cluster solution in each hemisphere, resulting in identification of ventromedial, dorsomedial, and lateral sub-regions of the FP. The symmetry between the three-cluster solutions of the right and left FP were very high, with their borders in relative agreement to their cytoarchitecture. Meta-analytic connectivity modeling was performed for these FP sub-regions, yielding individual whole brain co-activation maps for each of the CBP clusters. Furthermore, performing functional decoding on the three sub-regions yielded a complex set of cognitive and affective behavioral profiles that suggest unique functional specialization across the FP. Functional characterization of the FP subregions revealed that the lateral portions of the FP mapped to memory and emotion domains, while medial clusters were associated broadly with emotion and social cognition processes. We further show that dorsomedial regions contain an emphasis on theory of mind and affective related paradigms whereas ventromedial regions couple with reward tasks.
Methodological Limitations
A few methodological limitations warrant attention in considering the present findings. Although we have applied several criteria for determining the optimal number of clusters, at present the field lacks a gold-standard criterion. As such, different sets of criteria might lead to further parcellation of the FP. Nevertheless, the three cluster solution appeared optimal by several different criteria. A second issue concerns the extent to which the results of the MACM technique may be biased by the types of studies in the neuroimaging literature. Peak activation coordinates of an individual fMRI study may reflect bias due to methodological issues in both functional connectivity and task-based activation results. Studies archived in BrainMap do not represent an even distribution of paradigms due to the nature that some tasks may be better suited to the MRI environment, used more frequently than others, or pertain to a cognitive domain of high interest. Furthermore, the functions probed in neuroimaging studies are almost certainly influenced by funding priorities of granting agencies, which in many cases place priority on functions relevant to understanding disease states. Among a set of tasks that engage the FP, those that are more frequently studied will likely have the most influence on task-related CBP. Similarly, methodological issues in studies utilized in the MACM could influence the results. Although the BrainMap database only includes “whole brain” studies, susceptibility related signal dropout may lead to an underrepresentation of co-activations within certain areas, thus reducing the impact of these areas on the CBP. While this cannot be ruled out as a potential source of bias, it is clear from prior MACM analyses that the technique successfully identifies patterns of co-activation even in difficult to image regions (e.g., Robinson et al., 2010; Zald et al., 2013). More specifically, a recent CBP analysis successfully segmented the human subiculum (Chase et al., 2015), a region also known to be susceptible to artifacts, and thus this issue should not substantially impact the results of the CBP as long as there are a sufficient number of studies with sufficient image quality, as is the case for the large BrainMap database. Finally, the specific pattern of connectivity identified with MACM is agnostic to dynamics in function connectivity, and the causal direction and type (i.e., feedback, feedforward, etc.) of connections, which are critical for understanding information flow within high-level cortical networks (Ray & Zald, 2012).
Agreement and disagreement with Cytoarchitectonic and DTI Parcellation
The FP consists of two cytoarchitectonically distinct areas: lateral frontopolar area 1 (Fp1) and medial frontopolar area 2 (Fp2; Bludau et al., 2014). Using regions of interest based on their cytoarchitectonic division, Bludau and colleagues provided further evidence for a broad lateral vs. medial FP distinction based on patterns of coactivation and differential engagement in specific functional domains. We were therefore particularly interested whether task-based CBP would produce a similar solution. Consistent with the prior work, the combined dorsomedial and ventromedial clusters identified via our task-based CBP share a large portion of voxels with Fp2, while the lateral cluster in the current CBP analysis overlapped with Fp1. Despite these similarities, there are two notable differences between the cytoarchitectural results of Bludau et al. (2014) and the results of CBP. Most importantly, in the CBP analysis, the medial aspects of the rostral surface of the FP clustered with medial wall regions equivalent to Fp2, rather than clustering with the rest of the rostral surface, as would have been expected based on the cytoarchitectural boundaries in Bludau et al. (2014). It is worth noting that individual differences in cytoarchitectural boundaries exist in this region. Whereas in Figure 1a we display Bludau et al.’s regions with a strict boundary, in reality there are individual differences in the boundary between Fp1 and Fp2 within this medial rostral zone, as can be seen in the probabilistic maps presented in Figure 11 from Bludau et al. (2014). As such, this region may be viewed as transitional when considered across subjects. Nevertheless, the greater similarity of this area connectionally to the medial wall than the rostral surface regions was unexpected.
The most recognizable difference between the CBP and the cytoarchitectural segregation of the frontopolar cortex into Fp1 and Fp2 involves the split of the medial region into dorsal and ventral areas. While this specific split was not predicted based on existing cytoarchitectural divisions, it is consistent with a frequently articulated, although not always precisely defined, split between ventromedial vs. dorsomedial prefrontal cortex (e.g., Northoff & Bermpohl, 2004; Roy et al., 2012). Although not specific to just medial areas of FP, data from neuroanatomical tract tracing studies in macaques support a difference between dorsal and ventral (orbital) portions of area 10, with more dorsal portions showing greater engagement of superior frontal regions, while ventral regions show preferential ventromedial connectivity (Petrides & Pandya, 2007). Nevertheless, this dorsal-ventral distinction appears more subtle than the lateral-medial distinction in that there is a significant overlap in the areas that co-activate with the dorsomedial and ventromedial FP, both within the medial FP itself and in more distal connections. For instance, within the medial FP zones there was a significant area of overlap in the co-activations suggesting the presence of a relatively broad transitional area between the dorsal and ventral sectors. Similarly, in posterior regions such as the retrosplenial cortex, there was significant overlap of voxels co-activating with both the dorso- and ventro - medial areas. Yet, there were substantial enough differences between the dorsal and ventral co-activation patterns to distinguish between the two regions indicating that differential functional zones can arise within a particular microstructural region. Functional differences also emerged in terms of the types of studies that engage the areas, providing support for a general distinction between dorso- and ventro- medial FP regions.
In summary, although there is broad agreement in distinguishing a lateral and medial FP region, the assignment of the most medial aspects of the rostral surface differs across analyses, as does the presence or absence of a dorsomedial vs. ventromedial distinction. We do not propose that the divisions that we describe based on functional connectivity should supplant cytoarchitectural definitions. Nevertheless, the present analyses do suggest that differences in functional connectivity allow distinctions that may not be apparent on the basis of cytoarchitecture alone. Cortex with nearly identical architectural features may share very similarly processing features, but due to differences in connections, they may be involved in distinguishable functions. Alternatively, a region that appears to be relatively homogenous on some cytoarchitectural features may nevertheless possess substantial differences in other anatomical features such as the types of interneurons, which could lead to distinct functional properties. Similarly, while an area may be treated as homogenous on architectural grounds because there is no clear qualitative boundary between areas, there may nevertheless be a gradient in anatomical characteristics that lead to distinct functional properties within the region even though the boundary between these functional zones remains imprecise. As such, the present discrepancy between parcellation using cytoarchitecture and task-related CBP should provoke further examination of the detailed quantitative features of FP structure and their relation to the functional properties of the FP.
The only prior connectivity based parcellation studies of the human FP utilized diffusion imaging of anatomical connection patterns. Much like the present study, Liu et al. (2013) identified a three-cluster parcellation scheme defined by a lateral, medial, and orbital region that best fit their DTI data. While the areas proposed by Liu and colleagues are not identical to the current suggested parcellation, the clusters share a majority of voxels with the dorsomedial, ventromedial and lateral clusters, respectively. Additional resting-state connectivity analyses presented by Liu et al. (2013) using the centers of mass of their DTI-defined clusters identified similar patterns as the MACMs presented in the current study. However, it must be noted that the topography of the FP region displayed by Liu et al. does not fully fit with the applied topographical and cytoarchitectural boundaries of the FP, in that their scheme suggests that this cortex extends much further laterally in the ventral sections of the FP than in more intermediate or dorsal sections. This stands in contrast to maps developed on the basis of cytoarchitectural features (Brodmann, 1909; Bludau et al., 2014; Sarkisov et al. 1949), in which the lateral extension of the FP is at least as broad in its intermediate planes (z = 0) as its inferior planes. Because of this, the area that we label as lateral FP extends substantially more laterally than what is characterized as lateral FP by Liu et al., and some of the more lateral aspects of the area that Liu et al. describe as orbital correspond more closely to our lateral area and cytoarchitectonic area Fp1. Recently, Moayedi et al., (2014) used DWI to create two- and three-cluster subdivisions of the FP, ultimately concluding that the structural connectivity of the two-cluster solution was more consistent at the population level. A visual inspection indicates that solutions provided by Moayedi and colleagues are topologically similar to those of the current study, yielding a medial and lateral division of the FP at K = 2 with further breakdown of the medial cluster into ventral and dorsal subregions at K = 3. Resting state analyses of FP clusters by Moayedi and colleagues provided only a two-cluster solution where functional connectivity in the lateral cluster contained a high correspondence to that of the lateral cluster in the current study, however comparisons of the medial portion of the FP was not possible given the difference in number of clusters across studies. Likewise, clustering and connectional differences between Moayedi et al., (2014) and the current study can be largely attributed to differences in the number of clusters, as well as the defined boundaries of the FP. More specifically, Moayedi et al., (2014) used a cortical surface parcellation of BA 10 provided by FreeSurfer (http://surfer.nmr.mgh.harvard.edu/).
Of note, Moayedi et al. (2014) identified a “lateral” and “rostral” anatomical parcellation solution at k = 4 that could be visually interpreted as a sub-division of the lateral portion of the FP. However, Moayedi et al. ultimately suggested the 2- and 3-cluster solutions as more optimal given their greater consistency across subjects. Liu et al., (2013) produced similar results in their DTI based parcellation, citing greater inconsistency for parcellations containing a larger number of clusters. Additional previous review of the localization of activations related to different functional tasks has suggested that there are either two or three sub-regions in the frontal polar region (Gilbert et al., 2006; 2010). Similarly, the lateral portion of the FP eventually decomposed into smaller sub-regions in preliminary CBP analyses at higher k-means cluster solutions. However, these higher k-means solutions were less optimal because they were less hierarchically consistent, contained a larger percent of misclassified voxels, contained a greater amount of variation (with regards to voxel-wise co-activation profiles) across filter sizes, and were less symmetric across hemispheres.
Task Based Functional Connectivity and Functional Differences
The specific pattern of co-activations revealed by the MACM analysis highlights a number of features of FP connectivity. First, there was marked divergence in the functional connectivity of the lateral and medial regions of the FP. This observation may seem circular given that the three FP regions were defined based on their divergent pattern of functional connectivity. Nevertheless, the extent of the divergence is remarkable, as there was little overlap between co-activations of the lateral and medial regions with exception for the posterior cingulate, left amygdala, and the FP itself. In accordance with general patterns of connections observed in tract tracing data from nonhuman primates (Barbas & Pandya, 1989; Carmichael & Price, 1996; Petrides & Pandya, 2007), lateral portions of the FP showed greater connectivity with more dorsolateral prefrontal regions, particularly in the middle frontal gyrus, while the medial FP regions showed greater association with areas along the superior frontal gyrus and the medial wall of the frontal lobe.
In general our MACM results show substantial similarities to the MACM analysis of cytoarchitecturally defined areas Fp1 and Fp2 reported in (Bludau et al., 2014). However, they differ in terms of some of the ventral prefrontal co-activations that have been articulated for Fp1. These ventral frontal co-activations appear to be strictly associated with ventromedial regions. Given their differential association across studies, it seems likely that these connections derive specifically from the ventral portions of the medial rostral surface that is part of Fp1, but whose task based functional connectivity is more similar to cortex along the ventromedial wall. The functional association of this ventromedial FP region to other areas along the ventromedial wall of the frontal lobe is consistent with the structural connectivity of this region in Macaque monkeys. Specifically, Carmichael and Price (1996) describe both medial and orbital portions of the FP as part of a larger medial frontal network based on their preferential pattern of connections with other medial regions.
The patterns of co-activations generally showed congruence with the differences in functional domains engaging the different FP sub-regions. For instance, the left lateral FP showed preferential engagement during working memory tasks, and both prefrontal and parietal areas that are active during working memory tasks showed functional connectivity with the lateral FP regions (Owen et al., 2005; Rottschy et al., 2012). On the other hand, despite a frequent engagement of the lateral FP during explicit memory tasks, the lateral FP failed to show co-activations with medial temporal lobe regions. To the extent that medial temporal lobe co-activations included the hippocampus, this was limited to medial FP, for which co-activations occurred contiguous with the amygdala. This discordance naturally raises a question as to what role the lateral FP may have in episodic memory if it is not engaged in conjunction with medial temporal regions. Although activated during memory retrieval, Koechlin and Hyafil (2007) argue that this engagement is unlikely to reflect the retrieval process itself, but that it instead reflects the coordination of multiple embedded subtasks necessary for the recurrent retrieval of relevant information and judgment of stimuli or representations necessary to complete the memory tasks. As such, these activations may be more reflective of the executive regulation of processes necessary for task performance as opposed to memory retrieval itself.
A distinguishing feature of the medial FP areas is their significant level of connectivity to limbic-paralimbic regions. Both medial FP areas showed significant and partially overlapping associations with the posterior cingulate/retrosplenial cortex, an area that is often associated with emotion and memory functions (Maddock, 1999; Vann et al., 2009). These connections are consistent with demonstrated efferents from area 10 to posterior cingulate cortices in monkeys (Petrides & Pandya, 2007). Similarly, both ventro- and dorsomedial FP showed functional connectivity with the amygdala, with a broader pattern of connection arising for the ventromedial FP. While the strength of direct projections between the amygdala and FP is relatively modest (Carmichael & Price, 1995; Ghashghaei et al., 2007), the FP nevertheless sends direct efferents to the basolateral amygdala in nonhuman primates (Petrides & Pandya, 2007). Ventromedial FP was additionally the only part of the FP that showed coactivation with the ventral striatum. Taken together, such connections are consistent with the frequent engagement of medial FP regions in emotional, reward-related and social processing tasks. This pattern is also consistent with the involvement of medial prefrontal networks in visceral functions (Price et al., 2006).
The presence of emotion and social processing as a core functional domain in the medial FP is consistent with past functional analyses of the medial FP (Bludau et al., 2014; Gilbert et al., 2006). Nevertheless, it is notable that there has sometimes been an implied or even explicit equation of the development of isocortical areas with the triumph of executive cognitive abilities over more “primitive” emotional processes (for example MacLean, 1990). Thus, the strong involvement of a portion of the FP in emotional processing may be viewed as a challenge to such a perspective. Yet, such emotional processing may be easily integrated into models of this portion of the brain. Koechlin and Hyafil (2007) posit that the FP implements cognitive branching and task selection on the basis of potential reward value. Other theorists have noted the FP’s potential role in evaluation of internal representations (Christoff & Gabrieli, 2000), including evaluations of the potential reward value of future actions (Boorman et al., 2009). Similarly, the ability to evaluate potential social responses figures heavily in action choice, and thus FP processing of current or predicted social consequences may directly contribute to these computational steps.
Conclusion
The current study presented a meta-analytic functional parcellation of the human FP based on co-activation patterns across thousands of neuroimaging studies archived in the BrainMap database. CBP analyses carried out independently for each hemisphere both support three-cluster solutions dividing the FP into dorsomedial, ventromedial, and lateral sub-regions. Results from these analyses suggest that differences in functional connectivity reflect aspects of brain organization that are overlapping, but not necessarily identical with distinctions based on cytoarchitecture (Bludau et al., 2014). Consequently, future development of carefully constructed multimodal mapping techniques may contribute to a more comprehensive understanding of cortical organization. The co-activation based parcellation method additionally provided the unique advantage of facilitating probes into the cognitive implications of a particular solution thus allowing us to further elucidate functional specificity within human FP.
Supplementary Material
Cluster criteria for selecting the best-fit K-means solution for the left frontal pole.
Cluster criteria for selecting the best-fit K-means solution for the right frontal pole.
Acknowledgments
This study was supported by the National Institutes of Mental Health (MH74457, MH084812, and MH097870). This project was made possible by a collaboration agreement allowing comprehensive access to the BrainMap database, a copyrighted electronic compilation owned by the University of Texas Board of Regents. The research leading to these results has received funding from the European Union Seventh Framework Programme (FP7/2007–2013) under grant agreement no. 604102 (Human Brain Project; SBE and KA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah NJ, et al. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anatomy and Embryology. 2005;210(5–6):343–352. doi: 10.1007/s00429-005-0025-5. [DOI] [PubMed] [Google Scholar]
- Barbas H, Pandya DN. Architecture and intrinsic connections of the prefrontal cortex in the rhesus monkey. The Journal of Comparative Neurology. 1989;286(3):353–375. doi: 10.1002/cne.902860306. [DOI] [PubMed] [Google Scholar]
- Beckmann CF. Modelling with independent components. Neuro Image. 2012;62(2):891–901. doi: 10.1016/j.neuroimage.2012.02.020. [DOI] [PubMed] [Google Scholar]
- Behrens TEJ, Fox PT, Laird AR, Smith SM. What is the most interesting part of the brain? Trends in Cognitive Sciences. 2013;17(1):2–4. doi: 10.1016/j.tics.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bludau S, Eickhoff SB, Mohlberg H, Caspers S, Laird AR, Fox PT, et al. Cytoarchitecture, probability maps and functions of the human frontal pole. Neuro Image. 2014;93(P2):260–275. doi: 10.1016/j.neuroimage.2013.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boorman ED, Behrens TEJ, Woolrich MW, Rushworth MFS. How green is the grass on the other side? Frontopolar cortex and the evidence in favor of alternative courses of action. Neuron. 2009;62(5):733–743. doi: 10.1016/j.neuron.2009.05.014. [DOI] [PubMed] [Google Scholar]
- Brodmann K. Vergleichende Lokalisationslehre der Groshirnrinde. Leipzig: Barth; 1909. [Google Scholar]
- Burman KJ, Reser DH, Yu HH, Rosa MGP. Cortical Input to the Frontal Pole of the Marmoset Monkey. Cerebral Cortex (New York, NY: 1991) 2011;21(8):1712–1737. doi: 10.1093/cercor/bhq239. [DOI] [PubMed] [Google Scholar]
- Bzdok D, Heeger A, langner R, Laird AR, Fox PT, Palomero-Gallagher N, et al. Subspecialization in the human posterior medial cortex. Neuro Image. 2015;106:55–71. doi: 10.1016/j.neuroimage.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bzdok D, Laird AR, Zilles K, Fox PT, Eickhoff SB. An investigation of the structural, connectional, and functional subspecialization in the human amygdala. Human Brain Mapping. 2012;34(12):3247–3266. doi: 10.1002/hbm.22138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bzdok D, langner R, schilbach L, Engemann DA, Laird AR, Fox PT, Eickhoff SB. Segregation of the human medial prefrontal cortex in social cognition. Frontiers in Human Neuroscience. 2013;7:1–17. doi: 10.3389/fnhum.2013.00232/abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. Journal of Comparative Neurology. 1995 doi: 10.1002/cne.903630408. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Connectional networks within the orbital and medial prefrontal cortex of macaque monkeys. The Journal of Comparative Neurology. 1996;371(2):179–207. doi: 10.1002/(SICI)1096-9861(19960722)371:2<179::AID-CNE1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Chang LJ, Yarkoni T, Khaw M, Sanfey AG. Decoding the role of the insula in human cognition: functional parcellation and large-scale reverse inference. Cerebral Cortex. 2012:bhs065. doi: 10.1093/cercor/bhs065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase HW, Clos M, Dibble S, Fox PT, Grace AA, Phillips ML, Eickhoff SB. Evidence for an anterior–posterior differentiation in the human hippocampal formation revealed by meta-analytic parcellation of fMRI coordinate maps: Focus on the subiculum. Neuro Image. 2015;113:44–60. doi: 10.1016/j.neuroimage.2015.02.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoff K, Gabrieli JDE. The frontopolar cortex and human cognition: Evidence for a rostrocaudal hierarchical organization within the human prefrontal cortex. Psychobiology. 2000;28(2):168–186. [Google Scholar]
- Cieslik EC, Zilles K, Caspers S, Roski C, Kellermann TS, Jakobs O, et al. Is There “One” DLPFC in Cognitive Action Control? Evidence for Heterogeneity From Co-Activation-Based Parcellation. Cerebral Cortex (New York, NY: 1991) 2013;23(11):2677–2689. doi: 10.1093/cercor/bhs256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clos M, Amunts K, Laird AR, Fox PT, Eickhoff SB. Tackling the multifunctional nature of Broca’s region meta-analytically: Co-activation-based parcellation of area 44. Neuro Image. 2013;83(C):174–188. doi: 10.1016/j.neuroimage.2013.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumontheil I, Burgess PW, Blakemore SJ. Development of rostral prefrontal cortex and cognitive and behavioural disorders. Developmental Medicine & Child Neurology. 2008;50(3):168–181. doi: 10.1111/j.1469-8749.2008.02026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J. The multiple-demand (MD) system of the primate brain: mental programs for intelligent behaviour. Trends in Cognitive Sciences. 2010;14(4):172–179. doi: 10.1016/j.tics.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends in Neurosciences. 2000;23(10):475–483. doi: 10.1016/S0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Fox PT, Bzdok D, Hensel L. Functional segregation of the human dorsomedial prefrontal cortex. Cerebral Cortex. doi: 10.1093/cercor/bhu250. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Bzdok D, Laird AR, Kurth F, Fox PT. Activation likelihood estimation meta-analysis revisited. Neuro Image. 2012;59(3):2349–2361. doi: 10.1016/j.neuroimage.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuro Image. 2005;25(4):1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Heim S, Zilles K, Amunts K. Testing anatomically specified hypotheses in functional imaging using cytoarchitectonic maps. Neuro Image. 2006;32(2):570–582. doi: 10.1016/j.neuroimage.2006.04.204. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: A random-effects approach based on empirical estimates of spatial uncertainty. Human Brain Mapping. 2009;30(9):2907–2926. doi: 10.1002/hbm.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Jbabdi S, Caspers S, Laird AR, Fox PT, Zilles K, Behrens TEJ. Anatomical and Functional Connectivity of Cytoarchitectonic Areas within the Human Parietal Operculum. Journal of Neuroscience. 2010;30(18):6409–6421. doi: 10.1523/JNEUROSCI.5664-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Bzdok D, Laird AR, Roski C, Caspers S, Zilles K, Fox PT. Co-activation patterns distinguish cortical modules, their connectivity and functional differentiation. Neuro Image. 2011;57(3):938–949. doi: 10.1016/j.neuroimage.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Fox PT, Bzdok D, Hensel L. Functional Segregation of the Human Dorsomedial Prefrontal Cortex. Cerebral Cortex. 2014 doi: 10.1093/cercor/bhu250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans AC, Marrett S, Neelin P, Collins L, Worsley K, Dai W, et al. Anatomical mapping of functional activation in stereotactic coordinate space. Neuro Image. 1992;1(1):43–53. doi: 10.1016/1053-8119(92)90006-9. [DOI] [PubMed] [Google Scholar]
- Evans AC, Janke AL, Collins DL, Baillet S. Brain templates and atlases. Neuro Image. 2012;62(2):911–922. doi: 10.1016/j.neuroimage.2012.01.024. [DOI] [PubMed] [Google Scholar]
- Forgy EW. Cluster analysis of multivariate data: efficiency versus interpretability of classifications. Biometrics. 1965;21:768–769. [Google Scholar]
- Ghashghaei HT, Hilgetag CC, Barbas H. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuro Image. 2007;34(3):905–923. doi: 10.1016/j.neuroimage.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert SJ, Spengler S, Simons JS, Steele JD, Lawrie SM, Frith CD, Burgess PW. Functional specialization within rostral prefrontal cortex (Area 10): A meta-analysis. Journal of Cognitive Neuroscience. 2006;18(6):932–948. doi: 10.1162/jocn.2006.18.6.932. [DOI] [PubMed] [Google Scholar]
- Gilbert SJ, Gonen-Yaacovi G, Benoit RG, Volle E, Burgess PW. Distinct functional connectivity associated with lateral versus medial rostral prefrontal cortex: A meta-analysis. Neuro Image. 2010;53(4):1359–1367. doi: 10.1016/j.neuroimage.2010.07.032. [DOI] [PubMed] [Google Scholar]
- Hartigan JA, Wong MA. Algorithm AS 136: A K-Means Clustering Algorithm. Applied Statistics. 1979;28(1):100. doi: 10.2307/2346830. [DOI] [Google Scholar]
- Hömke L. A multigrid method for anisotropic PDEs in elastic image registration. Numerical Linear Algebra with Applications 2006 [Google Scholar]
- Johansen-Berg H, Behrens TEJ, Robson MD, Drobnjak I, Rushworth MFS, Brady JM, et al. Changes in connectivity profiles define functionally distinct regions in human medial frontal cortex. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(36):13335–13340. doi: 10.1073/pnas.0403743101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahnt T, Chang LJ, Park SQ, Heinzle J, Haynes JD. Connectivity-Based Parcellation of the Human Orbitofrontal Cortex. Journal of Neuroscience. 2012;32(18):6240–6250. doi: 10.1523/JNEUROSCI.0257-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly C, Uddin LQ, Shehzad Z, Margulies DS, Castellanos FX, Milham MP, Petrides M. Broca’s region: linking human brain functional connectivity data and non-human primate tracing anatomy studies. The European Journal of Neuroscience. 2010;32(3):383–398. doi: 10.1111/j.1460-9568.2010.07279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koechlin E, Hyafil A. Anterior Prefrontal Function and the Limits of Human Decision-Making. Science. 2007;318(5850):594–598. doi: 10.1126/science.1142995. [DOI] [PubMed] [Google Scholar]
- Kovach CK, Daw ND, Rudrauf D, Tranel D, O’Doherty JP, Adolphs R. Anterior prefrontal cortex contributes to action selection through tracking of recent reward trends. Journal of Neuroscience. 2012;32(25):8434–8442. doi: 10.1523/JNEUROSCI.5468-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Lancaster JL, Fox PT. BrainMap: The social evolution of a functional neuroimaging database. Neuroinformatics. 2005;3:65–78. doi: 10.1385/ni:3:1:065. [DOI] [PubMed] [Google Scholar]
- Laird AR, Eickhoff SB, Florian K, Fox PM, Uecker AM, Turner JA, Robinson JL, Lancaster JL, Fox PT. ALE meta-analysis workflows via the BrainMap database: Progress towards a probabilistic functional brain atlas. Frontiers in Neuroinformatics. 2009a;3 doi: 10.3389/neuro.11.023.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Eickhoff SB, Li K, Robin DA, Glahn DC, Fox PT. Investigating the Functional Heterogeneity of the Default Mode Network Using Coordinate-Based Meta-Analytic Modeling. Journal of Neuroscience. 2009b;29(46):14496–14505. doi: 10.1523/JNEUROSCI.4004-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Eickhoff SB, Fox PM, Uecker AM, Ray KL, Saenz JJ, Jr, et al. The BrainMap strategy for standardization, sharing, and meta-analysis of neuroimaging data. BMC Research Notes. 2011a;4(1):349. doi: 10.1186/1756-0500-4-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Fox PM, Eickhoff SB, Turner JA, Ray KL, McKay DR, et al. Behavioral interpretations of intrinsic connectivity networks. Journal of Cognitive Neuroscience. 2011b;23(12):4022–4037. doi: 10.1162/jocn_a_00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Eickhoff SB, Rottschy C, Bzdok D, Ray KL, Fox PT. Networks of task co-activations. Neuro Image. 2013;80(C):505–514. doi: 10.1016/j.neuroimage.2013.04.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT. Automated Talairach Atlas labels for functional brain mapping. Human Brain Mapping. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Rainey LH, Summerlin JL, Freitas CS, Fox PT, Evans AC, Toga AW, Mazziotta JC. Automated labeling of the human brain: A preliminary report on the development and evaluation of a forward-transform method. Hum Brain Mapp. 1997;5:238–242. doi: 10.1002/(SICI)1097-0193(1997)5:4<238::AID-HBM6>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Qin W, Li W, Fan L, Wang J, Jiang T, Yu C. Connectivity-Based Parcellation of the Human Frontal Pole with Diffusion Tensor Imaging. Journal of Neuroscience. 2013;33(16):6782–6790. doi: 10.1523/JNEUROSCI.4882-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean PD. The triune brain in evolution: Role in paleocerebral functions. 1990 doi: 10.1126/science.250.4978.303-a. [DOI] [PubMed] [Google Scholar]
- Maddock RJ. The retrosplenial cortex and emotion: new insights from functional neuroimaging of the human brain. Trends in Neurosciences. 1999;22(7):310–316. doi: 10.1016/s0166-2236(98)01374-5. [DOI] [PubMed] [Google Scholar]
- Moayedi M, Salomons TV, Dunlop KAM, Downar J, Davis KD. Connectivity-based parcellation of the human frontal polar cortex. Brain Structure and Function. 2014 doi: 10.1007/s00429-014-0809-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhle-Karbe PS, Derrfuss J, Lynn MT, Neubert FX, Fox PT, Brass M, Eickhoff SB. Co-Activation-Based Parcellation of the Lateral Prefrontal Cortex Delineates the Inferior Frontal Junction Area. Cerebral Cortex (New York, NY: 1991) 2014 doi: 10.1093/cercor/bhu239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G, Bermpohl F. Cortical midline structures and the self. Trends in Cognitive Sciences. 2004 doi: 10.1016/j.tics.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore ET. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Human Brain Mapping. 2005;25(1):46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öngür D, Ferry AT, Price JL. Architectonic subdivision of the human orbital and medial prefrontal cortex. The Journal of Comparative Neurology. 2003;460(3):425–449. doi: 10.1002/cne.10609. [DOI] [PubMed] [Google Scholar]
- Passingham RE, Stephan KE, Kötter R. The anatomical basis of functional localization in the cortex. Nature Reviews Neuroscience. 2002;3(8):606–616. doi: 10.1038/nrn893. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Efferent Association Pathways from the Rostral Prefrontal Cortex in the Macaque Monkey. Journal of Neuroscience. 2007;27(43):11573–11586. doi: 10.1523/JNEUROSCI.2419-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA. Can cognitive processes be inferred from neuroimaging data? Trends in Cognitive Sciences. 2006;10(2):59–63. doi: 10.1016/j.tics.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Mumford JA, Schonberg T, Kalar D, Barman B, Yarkoni T. Discovering Relations Between Mind, Brain, and Mental Disorders Using Topic Mapping. PLoS Computational Biology. 2012;8(10):e1002707. doi: 10.1371/journal.pcbi.1002707.s002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, McCrory E, Noppeney U, Mechelli A, Moore CJ, Biggio N, Devlin JT. How reading differs from object naming at the neuronal level. Neuro Image. 2006;29(2):643–648. doi: 10.1016/j.neuroimage.2005.07.044. [DOI] [PubMed] [Google Scholar]
- Ramnani N, Owen AM. Anterior prefrontal cortex: insights into function from anatomy and neuroimaging. Nature Reviews Neuroscience. 2004;5(3):184–194. doi: 10.1038/nrn1343. [DOI] [PubMed] [Google Scholar]
- Ray RD, Zald DH. Anatomical insights into the interaction of emotion and cognition in the prefrontal cortex. Neuroscience and Biobehavioral Reviews. 2012;36(1):479–501. doi: 10.1016/j.neubiorev.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JL, Laird AR, Glahn DC, Lovallo WR, Fox PT. Metaanalytic connectivity modeling: Delineating the functional connectivity of the human amygdala. Human Brain Mapping. 2010;31(2):173–184. doi: 10.1002/hbm.20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottschy C, langner R, Dogan I, Reetz K, Laird AR, Schulz JB, et al. Modelling neural correlates of working memory: a coordinate-based meta-analysis. Neuro Image. 2012;60(1):830–846. doi: 10.1016/j.neuroimage.2011.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy M, Shohamy D, Wager TD. Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends in Cognitive Sciences. 2012;16(3):147–156. doi: 10.1016/j.tics.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkisov SA, Filimonoff IN, Preobrashenskaya NS. [Cytoarchitecture of the human cortex cerebri] Moscow: Medgiz; 1949. (in Russian) [Google Scholar]
- Schleicher A, Zilles K. A quantitative approach to cytoarchitectonics: analysis of structural inhomogeneities in nervous tissue using an image analyser. Journal of Microscopy. 1990;157(Pt 3):367–381. doi: 10.1111/j.1365-2818.1990.tb02971.x. [DOI] [PubMed] [Google Scholar]
- Schleicher A, Amunts K, Geyer S, Kowalski T, Schormann T, Palomero-Gallagher N, Zilles K. A stereological approach to human cortical architecture: identification and delineation of cortical areas. Journal of Chemical Neuroanatomy. 2000;20(1):31–47. doi: 10.1016/s0891-0618(00)00076-4. [DOI] [PubMed] [Google Scholar]
- Schleicher A, Amunts K, Geyer S, Morosan P, Zilles K. Observer-independent method for microstructural parcellation of cerebral cortex: A quantitative approach to cytoarchitectonics. Neuro Image. 1999;9(1):165–177. doi: 10.1006/nimg.1998.0385. [DOI] [PubMed] [Google Scholar]
- Schleicher A, Morosan P, Amunts K, Zilles K. Quantitative architectural analysis: a new approach to cortical mapping. Journal of Autism and Developmental Disorders. 2009;39(11):1568–1581. doi: 10.1007/s10803-009-0790-8. [DOI] [PubMed] [Google Scholar]
- Schleicher A, Palomero-Gallagher N, Morosan P, Eickhoff SB, Kowalski T, de Vos K, et al. Quantitative architectural analysis: a new approach to cortical mapping. Anatomy and Embryology. 2005;210(5–6):373–386. doi: 10.1007/s00429-005-0028-2. [DOI] [PubMed] [Google Scholar]
- Semendeferi K, Armstrong E, Schleicher A, Zilles K, Van Hoesen GW. Prefrontal cortex in humans and apes: A comparative study of area 10. American Journal of Physical Anthropology. 2001;114(3):224–241. doi: 10.1002/1096-8644(200103)114:3<224::AID-AJPA1022>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Semendeferi K, Teffer K, Buxhoeveden DP, Park MS, Bludau S, Amunts K, et al. Spatial Organization of Neurons in the Frontal Pole Sets Humans Apart from Great Apes. Cerebral Cortex (New York, NY: 1991) 2011;21(7):1485–1497. doi: 10.1093/cercor/bhq191. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. 3-Dimensional proportional system: an approach to cerebral imaging 1988 [Google Scholar]
- Turkeltaub PE, Eickhoff SB, Laird AR, Fox PM, Wiener M, Fox PT. Minimizing within-experiment and within-group effects in activation likelihood estimation meta-analyses. Human Brain Mapping. 2012;33(1):1–13. doi: 10.1002/hbm.21186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vann SD, Aggleton JP, Maguire EA. What does the retrosplenial cortex do? Nature Reviews Neuroscience. 2009 doi: 10.1038/nrn2733. [DOI] [PubMed] [Google Scholar]
- Waskom ML, Kumaran D, Gordon AM, Rissman J, Wagner AD. Frontoparietal Representations of Task Context Support the Flexible Control of Goal-Directed Cognition. Journal of Neuroscience. 2014;34(32):10743–10755. doi: 10.1523/JNEUROSCI.5282-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wree A, Schleicher A, Zilles K. Estimation of volume fractions in nervous tissue with an image analyzer. Journal of Neuroscience Methods. 1982;6(1–2):29–43. doi: 10.1016/0165-0270(82)90014-0. [DOI] [PubMed] [Google Scholar]
- Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD. Large-scale automated synthesis of human functional neuroimaging data. Nature Methods. 2011;8(8):665–670. doi: 10.1038/nmeth.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zald DH, McHugo M, Ray KL, Glahn DC, Eickhoff SB, Laird AR. Meta-analytic connectivity modeling reveals differential functional connectivity of the medial and lateral orbitofrontal cortex. Cerebral Cortex (New York, NY: 1991) 2013;24(1):232–248. doi: 10.1093/cercor/bhs308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilles K, Schleicher A, Palomero-Gallagher N, Amunts K. Quantitative analysis of cyto- and receptorarchitecture of the human brain. In: Toga A, Mazziotta J, editors. Brain mapping: the methods. 2. Academic Press; San Diego (CA): 2002. pp. 573–602. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cluster criteria for selecting the best-fit K-means solution for the left frontal pole.
Cluster criteria for selecting the best-fit K-means solution for the right frontal pole.