Abstract
Purpose
To identify successful recruitment strategies, challenges and best practices for researchers to engage African American communities in clinical studies taken into consideration target participants’ culture and context.
Methods
We reviewed 50 studies conducted from 2001 to 2012 at an inner‐city research center to determine the type, duration, anticipated enrollments and actual enrollments. Survey was sent to study coordinators to obtain data on recruitment and retention strategies, challenges and dropout rates. We also interviewed 25 study coordinators on challenges and strategies.
Results
Of the 50 studies, 24 had completed recruitment at the time of this report. The completed studies achieved a median recruitment rate of 88% (range: 50–110). Successful recruitment and retention strategies included: field‐based strategy and snowballing. Major barriers were: distrust, compensation, education disadvantage, lack of interest, and inability to have study partner. Strategies to reduce barriers included providing informational sessions, disseminating newsletters about study outcomes. Best practices include being culturally sensitive including demonstrating a caring attitude and being responsive to participants needs.
Conclusions
Cultural competence is critical in order to design and implement successful recruitment strategies in this population. Research teams should consist of multiethnic staff, involve the community, demonstrate trust and deliver concise education of the research endeavor.
Keywords: minorities, cultural competency, recruitment and retention strategies, African Americans, underrepresented minorities, clinical trials
Introduction
Past medical experimentation and poor medical treatment have served to fortify African Americans’ and other racial and ethnic minorities mistrust in the medical system thus, they are often hesitant to participate in research activities.1, 2, 3, 4, 5, 6 Major barriers to African Americans’ participation in clinical trials include a lack of awareness about trials, low socioeconomic status, mistrust, poor communication skills, and a lack of disease education.1, 6, 7, 8 We believe that most of these barriers can be overcome by using appropriate and culturally sensitive strategies with regard to recruitment. In addition, adequate minority representation in clinical trials is important if differential effects among diverse groups are to be assessed and for the results to be generalized.9, 10
A review of the literature revealed that many researchers have examined minority participation in clinical research; however, the primary focus of these studies has been on the lack of minority participation and the challenge of recruiting minorities into clinical trials.1, 11 The literature also suggested the need to explore more culturally competent strategies to enhance recruitment successes with African Americans and other minorities from diverse cultural backgrounds.
Significance of the study
Thus, there is a need to conduct research on culturally competent recruitment strategies for African Americans in order to better understand ways that can help researchers improve current strategies to increase minority participation in clinical trials. A research design and consent process that educates participants and includes a high level of commitment by study staff must be integral part of this process. It is an ethical obligation to ensure that potential participants fully understand the purpose of the study, procedures and associated risks for participating in a study as well as perceived benefits of the study to them, their community and/or the scientific community at large.
This retrospective study examined (1) both successful recruitment strategies and challenges in working with African American populations in general, and (2) successful recruitment strategies utilized at an inner‐city medical campus of a historical black university. The successful strategies discussed can also be applied in the recruitment of other minorities from diverse cultural backgrounds.
Underrepresentation of minorities in clinical trials
Historically, African Americans and other minority groups were largely excluded from clinical trials until the National Institutes of Health (NIH) mandated the inclusion women and minorities in studies9, 12 yet African Americans and other minority groups continue to be underrepresented in trials.13 Some of the major reasons cited for excluding minorities, as noted by12 include, “the difficulty of recruitment and retention, a general belief that racial populations are essentially monolithic, without significant differences, and the desire and need to focus on optimizing internal validity” (p. 1345).12
It is also well documented that minority populations do not trust researchers and the research establishment, many expressing fear of being used as guinea pigs.2, 3, 4, 8, 14 Some researchers8, 9, 15 found that not only do African Americans have distrust of the medical and scientific community, but this distrust is compounded by a lack of knowledge about clinical trials, the language of research and cultural barriers. Coakley et al.1 noted similar findings. A related issue is fear that information will not be kept confidential.16
In a study of perception of participation in cancer trials among African Americans, Brown et al.11 reported that most participants’ refusal to participate was due to fear of additional burdens and that many patients and family members did not understand the trial well. This fear seems to be compounded by the assumption that they may be harmed and/or exploited.2, 3, 12, 17 Similarly, Corbie‐Smith et al.4 analyzed data from a national telephone survey on participation in clinical research, using a 7‐item index of distrust, and reported that African Americans had a significantly higher mean distrust index score than whites. African Americans were also found to be more likely than whites not to trust that their physicians would fully explain the nature of research. In support of the findings above, Herring et al.17 conducted 15 key‐informant interviews and 6 focus groups and reported that blacks had a general mistrust of the medical/scientific community and a perceived lack of cultural sensitivity in the recruitment and with the conduct of study.
Since there continues to be an underlying element of mistrust between minorities and majority research establishments, researches argues that African Americans and other minorities need more of their own physicians practicing and in their communities.3 Kennedy further stresses that patients and physicians who belong to the same race or ethnic group are more likely to share cultural beliefs, values, and experiences in society which makes them more comfortable with each other. In support of the above argument, Chalela et al.9 reported that 86% of patients participating in their study responded that their own physician told them about the study. Chalela et al.9 thus suggested that a trusting relationship between the clinician and the patients would aide participation in clinical trials.
While there are many social and cultural factors that influence participation of minority populations in clinical trials,14, 18 it has been argued that a lack of cultural competence by health‐care providers and researchers is one of the most dynamic factors relative to mistrust of the health care system by African Americans and other minority populations.9, 19 The Office of Minority Health20 defines culture as an “integrated pattern of human behavior evident in shared values, beliefs, norms and perceptions of individuals, to social groups, and institutions”; further, cultural competence is defined as “having the capacity to function effectively as an individual or an organization within the context of the cultural beliefs, behaviors, and needs presented by consumers and their communities.” Cultural competence, therefore, by definition, is seen as an important part of building trust between those in the health system and minority community of interest, thus cultural competence is an important part of research process.
Recruitment issues with African American and other minority populations
The success of a clinical trial and the achievement of desired outcomes depend on the ability to effectively recruit and retain minority populations in clinical research.12 The use of mainstream media has not been very effective in the recruitment of this population.21, 22 It is clear that use of nontraditional strategies are warranted. In support of this argument23, 24 maintain that use of culturally sensitive recruitment approaches are important when conducting research with African Americans. They state that research information should be clear, simple, and the format should be appealing. Adderley‐Kelly and Green19 stress that to be successful in conducting research with African Americans, researchers need to work with communities with a spirit of openness and collaboration. Based on their findings, the authors concluded that cultural competency, establishing trust and building relationships are important factors that should be taken into consideration when working with minority populations.
Similarly, Calamaro23 notes that when researchers respect and understand the cultural norms of their target ethnic group, they can develop strategies that can increase study participation. Furthermore, some research from the University of Maryland25 have indicated that having a team member with similar cultural background, an extensive understanding and/or experience in working with the target ethnic group can provide the opportunity to establish greater trust with the community. Relatively, Coakley et al.1 found that minority patients seek physicians of their own race or those of similar culturally backgrounds. Since physicians are the gateway to patients (in many cases), training and having enough minority physicians involved with conducting clinical trials would be beneficial.
In addition, health literacy and minority populations is an ongoing area of concerned and debate.26, 27 In agreement with this debate, Taylor12 argues that participant literacy level is a critical problem that can impact on recruiting, retention, and study instrument completion. Thus, he recommends assessing literacy before clinical trial implementation and suggest the Woodcock‐McGrew Mini‐Battery of Achievement to this end.28 Relatively, Ejiogu et al.29 argue that researchers should be able to concisely articulate purposes of research, provide clear explanation of direct benefits (if any) with the understanding that many studies may have none.
Study objectives
Given the paucity of research identifying culturally component strategies for successful recruiting and maintaining African American populations into clinical trials, a study was undertaken. The primary purpose of this study was to examine recruitment practices utilized to recruit African American populations in clinical trials at an inner‐city medical institution located on the campus of a historically black university. The study sought to answer two primary questions:
Over time, what have been the specific strategies used to successfully recruitment African American participants in clinical trials?
What have been the major barriers to successful recruitment and retention of African American participants in clinical trials?
The findings from this study can yield important insights and best practices for recruiting and engaging in research with African American populations.
Methods and Procedures
This study was conducted in two phases. Phase I consisted of a review of all studies conducted at the Howard University Clinical Research Unit from 2001 to 2012 and entered in the WebCAMP, a data management software tool for clinical research. At total of 50 studies met the criteria. These studies were examined in terms of their foci, duration, target versus actual enrollment, participant demographics, and eligibility criteria. Phase II of the present investigation included primary data collection from current study coordinators at the Howard University CRU. In particular, a survey (see Table 1) was forwarded to current study coordinators (N = 25) to inquire about recruitment and retention strategies, challenges faced while conducting the studies, the number of dropouts in studies, reasons participants provided for dropping out of studies, and overcoming barriers. In addition to the survey, face‐to‐face interviews were conducted with all 25 study coordinators in order to further probe and clarify information collected on the surveys relating to challenges occurring during the recruitment stage and strategies used to retain participants in studies.
Table 1.
Qualitative items on study coordinators’ survey
| 1. What recruitment strategies were used to enroll participants in the study? |
| 2. What recruitment strategies were the most effective? |
| 3. What were some of the barriers you encountered during the recruitment process? |
| 4. What strategies were used to reduce these barriers? |
| 5. What were some of the reasons participants dropped out of the study? |
| 6. What retention strategies were used to keep participants enrolled in the study? |
Data Analysis
Data were entered in a Microsoft Excel (Microsoft, Redmond, WA, USA) spreadsheet for screening and analysis. Quantitative and qualitative data analysis methods were used for the analysis of survey and interview data.
Quantitative data analysis
Quantitative and semiquantitative measures were analyzed using appropriate summary statistics such as mean, median, counts, and percentages while dispersion was measured using range.
Qualitative data analysis
A thematic content analysis to analyze qualitative survey responses
Qualitative responses from the interviews were transcribed in Microsoft Word. The files were then given to two researchers for data analysis. The researchers organized the files by renaming them according to the interviewee and name of study. The files were then merged into one file in Microsoft Excel to create a master file. Separate worksheets were created for each interview question and each participant was given an identification number to identify their response on each worksheet. Two separate Microsoft Excel spreadsheets, one per coder, were then created for each researcher to individually code data. The researchers first performed an initial read through of the data to become familiar with the data and to develop a priori codes. The researchers then met to discuss codes that each research developed and generated a preliminary list of codes. Next, the researchers proceeded to code all participants’ responses. After all responses were coded, the researchers met to review their codes and to resolve any discrepancies. A codebook was created with the final list of codes, code definitions, and the frequency of each code.
Results
Quantitative results
Table 2 below provides a summary of the characteristics of the 50 studies reviewed. As indicated in the table, the study coordinators were from diverse cultural backgrounds, with a majority being African American (n = 19, 79.2%). Between 2001 and 2012, there were total 12 unique Principal Investigators (PIs), of which 9 were African American, who brought their studies to the CRU. On average, the study coordinators had been working in their related areas with the same PI for about 4 years.
Table 2.
Results from review of 50 clinical trials at Howard University CRU: 2001–2012
| Number of studies (percent) | |
|---|---|
| Funding source | |
| Industry | 14 (28%) |
| Federal | 36 (72%) |
| Types of study | |
| Nonmedication | 32 (64% |
| Medication use | 18 (36%) |
| Type of design | |
| Cross‐sectional | 38 (76%) |
| Prospective | 12 (24%) |
| Study coordinators | |
| Black/African American | 19 (79.2%) |
| Others | 5 (20.8%) |
| Race/Ethnicity of PI | |
| Black/African American | 9 (75%) |
| Others | 3(25%) |
In terms of study type, 8 (16%) were in Aging and/or Alzheimer's disease, 10 (20%) in sickle cell disease, 4 (8%) in HIV, 4 (8%) in posttraumatic stress disorder (PTSD), and 4 (8%) in genetic‐epidemiology studies. Others involve: diabetes, hypertension, cancer, stress, substance use, and alcohol. Thirty‐eight (76%) studies were cross‐sectional and 12 (24%) were prospective. Thirty‐six (72%) studies were government funded and 14 (28%) received industry funding.
The anticipated enrollment of these studies ranged from 2 to 900, and actual enrollment ranged from 2 to 908. The small enrollment numbers are due to the subject matter being studies, such as rare diseases including sickle cell diseases. The prospective studies had follow‐up periods from 3 months to 5 years. The median follow‐up period was 34 months (range: 6 months to 5 years). Twenty‐four of the 50 studies were completed, and achieved median recruitment rates of 88% (range: 50–110), which is quite good for this population.
Eight of the 12 prospective studies had dropped out, with an average rate of 23.3%. One study involving PTSD patients had 7% drop‐out rate. The length of study for these prospective trials ranged from 6 to 60 months. Three (12.5%) of the 8 studies with dropouts involved elderly participants in an Alzheimer's study which ranged from 10% to 33%. Seven (14%) of the 50 studies involved children. Eighteen (36%) of the total number of studies involved the use of study related medication and were considered to have more than minimum risk, with median recruitment rate of 72% (range: 27%–82%). There were 10 (22%) studies that were still enrolling participants at the time of this study.
Qualitative results
Study coordinators were asked six open‐ended questions (see Table 1) about recruitment strategies they utilize, barriers to recruitment, barrier reduction strategies, reasons for participant dropouts, and retention strategies they employed with the studies. The top codes for each open‐ended question are presented in Figures 1– 6.
Figure 1.
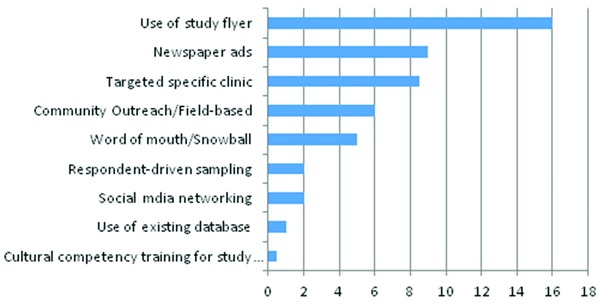
Recruitment strategies data indicate number of response.
Figure 6.
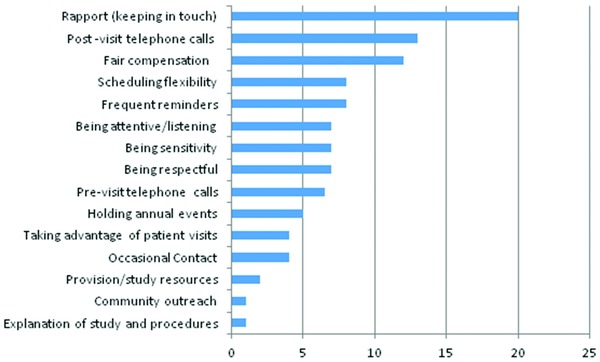
Retention strategies data are number of responses.
Commonly used recruitment strategies
Recruitment strategies are presented in Figure 1 below. The most commonly used recruitment strategies respectively were: (1) distribution of flyers at different locations, such as; university bulletin boards, outpatient clinics, communities with low‐income residential housing, flyers on local buses, shopping centers, community recreational centers, metro buses stops, and metro trains stops; (2) advertisements in local newspapers that their target populations were likely to read; (3) targeting patients on their regular clinics visits; (4) field‐based recruitment strategies involving; community outreach, talking to individuals in target communities, talking to individuals associated with community based organizations to create relationships and establish trust with the target population through individuals acting as liaisons; and (5) a snowball approach in which current participants would tell potential participants about the study.
Most effective recruitment strategies
The most effective recruitment strategies are presented on Figure 2. Study coordinators reported that a field‐based approach was found to be the single most effective recruitment strategy when working with African American populations. This approach involves going out into communities, working with people in communities, and establishing relationships with hopes to build trust among those who might be potential study participants. Results from our analysis showed that going into the community to engage potential participants and educating and explaining the study in person to potential participants are the most effective ways to recruit African American participants into studies. Also, placing study advertisements in the newspapers (i.e., advertising the study in newspapers that circulate in the specific study target population) and snowball samplings were the next successful strategies.
Figure 2.
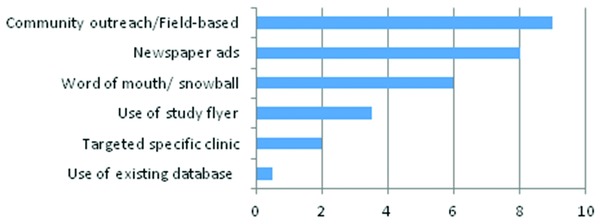
Most effective recruitment strategies data indicate number of responses.
Barriers to recruitment
The top five barriers to recruitment of African American participants into clinical trials, according to the study coordinators in this study included: (1) African American's distrust or uneasiness and feelings of discomfort with participation in the trial. (2) Low compensation: It is not clear why African Americans often feel they are not adequately compensated for the time put into participating in clinical trials than Caucasians. The perceived low compensation could be reflected in the long‐term potential return of the time investment. (3) Participants’ inability to have a study partner, especially in the elderly African American population. For example, for most of Alzheimer's disease related studies, involvement of a partner is a requirement; (4) participants’ with low level of education; (5) participants not having adequate means of transportation to get to the clinical trial site; and (6) participants’ lack of interest in the study. Descriptors of the common barriers encountered during the recruitment process are presented in Figure 3. Other reported barriers were confidentiality and privacy concerns.
Figure 3.

Barriers data indicate number of responses.
Barrier reduction strategies
Study coordinators reported a variety of strategies used to reduce barriers to African Americans’ participation in clinical trials. The most cited strategies were: (1) treating persons with respect (dignity); (2) creating and disseminating newsletters that share information about the studies (awareness); (3) allowing time for question and answer sessions after information sessions and during the informed consent process to address participants’ concerns and concerns for family members (engagement); (4) prestudy information sessions given to the general public in various community settings and target participant groups (education); and (5) demonstrating to participants that they care (compassion). Thus, the study coordinators report that educating the African American community about research in general and clinical trial, in particular, along with engagement activities, awareness sessions and treating potential participants with dignity and concern for their welfare were the most effective strategies they found to participant recruitment. Other culturally competent strategies used to reduce barriers included addressing the issue of compensation. To address the issues with low compensation, many of these studies provide in addition to the monetary compensation, parking validation tickets, transportation fare and lunch coupons. In addition, issues related with privacy and confidentiality were addressed. A complete list of strategies used is provided in Figure 4.
Figure 4.
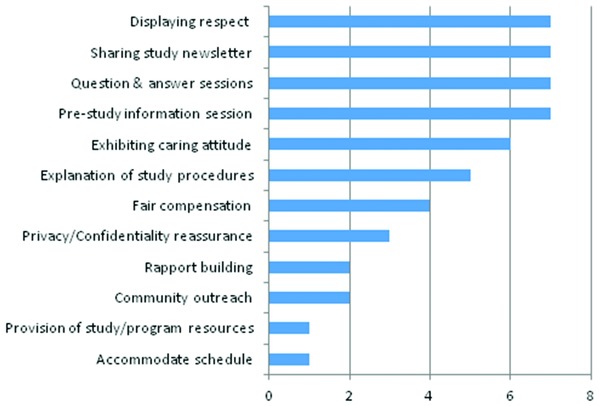
Barriers reduction strategies data are number of responses.
Reasons for dropout
The most common reported reason for dropping out of the study was participants relocating out of the study area (moving). For example, two of the eight studies involved exclusively college students and several of the participants moved out upon graduation; this may further explain the high number of dropouts. Other reasons for participants dropping out of studies were: (1) personal obligations; (2) loss to follow‐up; (3) noncompliance with study requirements (i.e., study doctor's decision); and (4) monetary or other incentives for the study being too low (see Figure 5).
Figure 5.
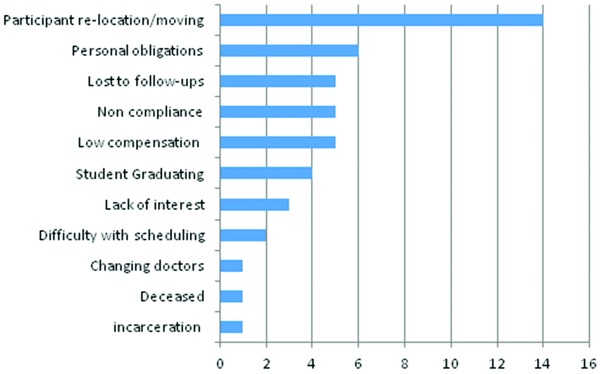
Participant dropout reasons data indicate number of responses.
Retention strategies
Study coordinators reported a number of strategies employed in an effort to retain participants. Figure 6 below present the most cited retention strategies used as indicated by the coordinators. These include (1) principal investigators and study coordinators cultivating rapport with participants; (2) postvisit telephone calls to inquire about participants’ well‐being, how they feel about the studies and their concerns; (3) providing a additional incentives to participants per follow‐up visits (particularly with regard to follow‐up studies) such as parking validation tickets and lunch coupons; (4) being flexible about accommodating participants’ schedules, including after‐hours appointments and weekends; (5) sending reminders to participants about their upcoming appointments via telephone or sending post cards; (6) listening to participants concerns about the study or any other personal issues; (7) study staff being sensitive to participants’ needs; and (8) the study team being respectful. These are not only culturally competent strategies for retaining African American participants, but also good strategies for retaining study participants in other ethnicity.
Discussion
It can be stated that it is of paramount importance to demonstrate cultural competence when it comes to recruiting and retaining minorities in clinical trials. Our study findings indicate that, in order for a clinical trial to be successful in recruitment and retention, establishing a personal contact with minority or ethnic populations seem to be extremely important. This finding is consistent with those of other studies.9, 19 While issues pertaining to trust or participants’ feelings of uneasiness about participating in clinical trials are associated with the historical experiences of many racial and ethnic groups relative to medical research and treatment, strategies to reduce barriers to recruitment and retain participants need to be culturally sensitive. Findings from this study indicate that use of direct contact or community‐based outreach approaches (i.e., field‐based strategy) is the most effective strategy for recruiting and retaining African American participants in clinical trials.
Our observation revealed that studies were successful (whether the principal investigator was the clinician of care or not) when the recruitment strategies applied a field‐based recruitment strategy (i.e., reaching out to the target population in their communities, use of recruiters in the effort to build trust in the community, and having contact persons in the target population community). It was also noted that a majority of the research teams had members who understood the culture of the target population. Some of these team members had worked with the target communities over an extended period time. Hence, having an opportunity to establish ongoing relationship with these communities also played a key role in recruitment and retention successes.
Barriers cited with regard to African Americans participation in clinical trials include the lack of awareness about trials and communication issues. It can be argued that one way to build trust with minority population is through informational sessions to include a question and answer session. Our findings show that this is a powerful strategy for engaging this population. This finding is supported by existing literature. Coakley et al.1 found that information empowers individuals and family members and allow for informed decision makings. Brown et al.30 reported that ethnic‐specific information about health risk associated with recipients’ health condition increased recruitment of minority women into a clinical trial. Further, Brown et al.11 reported that many patients and family members misunderstood trial information and that many felt that a question prompt lists and decision aids would assist in decision making.
In addition, our study results show that the best strategies to reduce related barriers and retain participants are associated with the ability to keep constant contact with participants. Further, being respectful and showing a caring attitude are the important factors in this population. This is a demonstration to the participants that the researchers value and appreciate them. This finding may not be specific to African America population but can apply to other racial populations regardless but may be more so in the African American population because of their past historical experiences with medical experimentation. Privacy and confidential of data seem to be associated with the issue of trust. It is, therefore, important to inform potential participants how these issues will be handled. Thus, privacy and confidential of data needs to be part of recruitment efforts.16 Moreover, it can be argued that success in engaging African Americans in clinical trials in this study may be due, in large part, to the fact that most of the investigators were of the same ethnic background as participants. The conclusion is in agreement with existing findings. Hence, training more minority investigators to be engaged in clinical trials can increase the participation of minorities in clinical trials.1 A limitation of our report is that the study was conducted in African Americans with minimal proficiency of the English language as an eligibility criteria. Further studies can be conducted to generalize the findings to include other black immigrants with no or limited English proficiency.
In conclusion, it is recommended that successful recruitment and retention of African American participants in clinical trials would be aided by clinical and translational research teams who are culturally competent. This can be done by utilizing multiethnic investigators and coordinators who actively engage with target minority communities, demonstrating trust and respect to participants and potential participants, and delivering culturally relevant education of the research endeavor.
Acknowledgments
This project has been funded in whole or in part with Federal funds (Grant # UL1TR000101 previously UL1RR031975 from the National Center for Advancing Translational Sciences [NCATS], National Institutes of Health [NIH], through the Clinical and Translational Science Awards Program [CTSA], a trademark of DHHS part of the Roadmap Initiative, “Re‐Engineering the Clinical Research Enterprise,” and by the National Institute on Minority Health and Health Disparities/NIH Award Number G12MD007597). We also acknowledge the following study personnel: Altaee, Duaa; Akintilo, Abiodun; Diaz, Sharmin; Evans, Mariah; Godoy, Adrian; Houston, Patricia E; Johnson, Megan S; Johnson, Steven; Jarret, Desheema; Jones, Gwendolyn; Kalu, Nnenna; Ketete, Muluemebet; Lavela, Joseph; Mbaba, Mary; Ogunlana, Oludolapo O; Ordor, Debra A; Reed, Caroline K; Scott, Denise; Settles‐Reaves; Beverlyn D; Tirmazi, Taqi M; Ukaegbu, Chidiebera; Wilkins, Terry; Wise, Sandra; Wolday, Saba; Yorrick, Ralson.
References
- 1. Coakley M, Fadiran EO, Parrish LJ, Griffith RA, Weiss E, Carter C. Dialogues on diversifying clinical trials: successful strategies for engaging women and minorities in clinical trials. J Women's Health. 2012; 21(7): 713–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Braunstein, JB , Sherber NS, Schulman SP, Ding EL, Powe NR. Race, medical research distrust, perceived harm, and willingness to participate in cardiovascular prevention trials. Medicine. 2008; 87(1): 1–9. [DOI] [PubMed] [Google Scholar]
- 3. Kennedy BR, Mathis CC, Woods AK. African Americans and their distrust of the health care system: healthcare for diverse populations. J Cult Divers. 2007; 4(2): 56–60. [PubMed] [Google Scholar]
- 4. Corbie‐Smith G, Thomas SB, St. George DMM. Distrust, race, and research. Arch Int Med. 2002; 162(21): 2458–2463. [DOI] [PubMed] [Google Scholar]
- 5. Corbie‐Smith G, Thomas SB, Williams MV, Moody‐Ayers S. Attitudes and beliefs of African Americans toward participation in medical research. J Gen Int Med. 1999; 14(9): 537–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Harris Y, Gorelick PP, Samuels P, Bempong I. Why African Americans may not be participating in clinical trials. J Natl Med Assoc. 1996; 88(10): 630–634. [PMC free article] [PubMed] [Google Scholar]
- 7. Harris Y, Burnett B, Bonecutter FJ. The recruitment triangle: reasons why African Americans enroll refuse to enroll, or voluntarily withdraw from a clinical trial. An interim report from the African‐American Antiplatelet Stroke Prevention Study (AAASPS). J Natl Med Assoc. 1998; 90(3): 141–145. [PMC free article] [PubMed] [Google Scholar]
- 8. Shavers‐Hornaday VL, Lynch CF, Burmeister LF, Torner JC. Why are African Americans under‐represented in medical research studies? Impediments to participation. Ethn Health. 1997; 2(1–2): 31–45. [DOI] [PubMed] [Google Scholar]
- 9. Chalela P, Suarez L, Munoz E, Gallion KJ, Pollock BH, Weitman SD, Karnad A, Ramirez AG. Promoting factors and barriers to participation in early phase clinical trials: patients’ perspectives. J Commun Med Health Educ. 2014; 4(14): 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ford E, Jenkins V, Fallowfield L, Stuart N, Farewell D, Farewell V. Clinicians’ attitudes towards clinical trials of cancer therapy. Br J Cancer. 2011; 104(10): 1535–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brown RF, Cadet DL, Houlihan RH, Thomson MD, Pratt EC, Sullivan A, Siminoff LA. Perception of Participation in a Phase I, II, or III clinical trial among African American patients with cancer: What do refusers say? J Oncol Pract. 2013; 9(6): 287–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Taylor RE. Pharmacological and cultural considerations in alcohol treatment clinical trials: issues in clinical research related to race and ethnicity. ACER. 2003; 27(8): 1345–1348. [DOI] [PubMed] [Google Scholar]
- 13. Institute of Medicine . A national cancer clinical trials system for the 21st century: Reinvigorating the NCI cooperative group program. Washington, DC: The National Academies Press, 2010. [PubMed] [Google Scholar]
- 14. Ford JG, Howerton MW, Lai GY, Gary TL, Bolen S, Gibbons MC, Tilburt J, Baffi C, Tanpitkupongse TP, Wilson RF, et al. Recruiting underrepresented populations to cancer clinical trials: a systematic review. Cancer. 2008; 112(2): 228–242. [DOI] [PubMed] [Google Scholar]
- 15. Catt S, Langridge C, Fallowfield L, Talbot DC, Jenkins VA. Reasons given by patients for participating, or not, in Phase 1 cancer trials. Eur J Cancer. 2011; 47(10): 1490–1497. [DOI] [PubMed] [Google Scholar]
- 16. Bonham VL. Ethical, legal, and social implications: a community view Paper presented at: Symposium on Genomic Medicine, National Medical Association, National Human Genome Institute; 2002; Bethesda, MD. [Google Scholar]
- 17. Herring P, Montgomery S, Yancey AK, Williams D, Fraser G. Understanding the challenges in recruiting blacks to a longitudinal cohort study: the Adventist health study. Ethn Dis. 2004; 14(3): 423–430. [PubMed] [Google Scholar]
- 18. Cox K, McGarry J. Why patients don't take part in cancer clinical trials: an overview of the literature. Eur J Cancer Care (Eng). 2003; 12(2): 114–122. [DOI] [PubMed] [Google Scholar]
- 19. Adderley‐Kelly B, Green PM. Strategies for successful conduct of research with low‐income African American populations. Nursing Outlook. 2005; 53(3): 147–152. [DOI] [PubMed] [Google Scholar]
- 20. Office of Minority Health . National standards for culturally and linguistically appropriated services in health care. Rockville, MD: NIMHD, NIH Publication, 2001. [Google Scholar]
- 21. Kressin NR, Meterko M, Wilson NJ. Racial disparities in participation in biomedical research. J Natl Med Assoc. 2000; 92(2): 62–68. [PMC free article] [PubMed] [Google Scholar]
- 22. Crawley LM. African American participation in clinical trials: situating trust and trustworthiness. J Natl Med Assoc. 2001; 93(12): 14S–17S. [PMC free article] [PubMed] [Google Scholar]
- 23. Calamaro CJ. Culture competence in researcher: research design and subject recruitment. J Pediatr Health Care. 2008; 22(5): 329–332. [DOI] [PubMed] [Google Scholar]
- 24. Sector‐Turner M, Sieving R, Garwick A, Spratt R, Duke N. Culturally sensitive community engaged research with African American young women: lessons learned. J Community Health Nurs. 2010; 27(3): 160–172. [DOI] [PubMed] [Google Scholar]
- 25. The University of Maryland . Building trust between minorities and researchers. http://www.buildingtrustumd.org/about‐project
- 26. The National Academy of Sciences . Informed consent and health literacy: a workshop. http://www.iom.edu/Activities/PublicHealth/HealthLiteracy/2014‐JUL‐28/Panel%201/1Welcome.aspx.
- 27. Institute of Medicine . Roundtable on health literacy: Health literacy past, present and future: A workshop. Washington, DC: 2014. [PubMed] [Google Scholar]
- 28. Woodcock RW, McGrew KS, Werder JK. Woodcock‐McGrew‐Werder Mini‐Battery of Achievement. Chicago: Riverside Publishing Company; 1994. [Google Scholar]
- 29. Ejiogu N, Norbeck JH, Mason MA, Cromwell BA, Zonderman AB, Evans MK. Recruitment and retention strategies for minority or poor clinical research participants: lessons from the healthy aging in neighborhoods of diversity across the life span study. Gerontologist. 2011; 51(1): S33–S45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brown SD, Lee K, Schoffman DE, King AC, Crawley LM, Kiernan M. Minority recruitment into clinical trials: experimental findings and practical implications. Contemp Clin Trials. 2012; 33(4): 620–623. [DOI] [PMC free article] [PubMed] [Google Scholar]


