Abstract
Eplerenone is a competitive antagonist of the aldosterone receptor with an additional PI3K-Akt activity. The existing cram has been intended to explore, whether eplerenone treatment attenuates the expansion of myocardial infarction in isoproterenol treated rats by restoring hemodynamic, biochemical, and histopathological changes. Isoproterenol induced cardiotoxicity was evidenced by marked ST elevation, decrease in systolic, diastolic, mean arterial pressures. Maximal positive rate of developed left ventricular pressure (+LVdP/dt max, a indicator of myocardial contraction), maximal negative rate of developed left ventricular pressure (-LVdP/dt max, a meter of myocardial relaxation) and an increase in left ventricular end-diastolic pressure (LVEDP, a marker of pre-load) were also shown. In addition, a significant reduction in activities of myocardial creatine kinase-MB isoenzyme, lactate dehydrogenase, superoxide dismutase, catalase, and reduced glutathione level along with increase in malondialdehyde content were observed. Oral pre-treatment with eplerenone (50, 100 and 150 mg/kg) daily for a period of 14 days, constructively modulated the studied parameters in isoproterenol-induced myocardial injury. The protective role of eplerenone on isoproterenolinduced myocardial damage was further confirmed by histopathological examinations. Eplerenone at doses of 100 mg/kg and 150 mg/kg produced more pronounced protective effects than 50 mg/kg body weight. Together, our study provides evidence for protective effects of eplerenone on myocardium in experimentally induced myocardial infarction.
Keywords: Eplerenone, isoproterenol, myocardial infarction, ST elevation, LVEDP
Introduction
Myocardial infarction (MI) is a fatal ischemic disease frightening round about all the countries with towering rate of morbidity and mortality [1] MI is manifested as an imbalance between the blood supply to demand in myocardium resulting in ischemia and irreversible necrosis of tissue [2]. It is apparent that oxidative stress augmentation is a key aspect in development of myocardial infarction and also in other cardiovascular diseases. In preclinical setups, drugs which are hypothesised to have beneficial effects in MI are evaluated using isoproterenol (ISO) induced myocardial infarction model in rats ever since it was introduced in the early 1960’s. Subcutaneous administration of isoproterenol, a beta-adrenergic agonist results in acute phase of myocardial infarction by altering the blood pressure, heart rate and ECG along with Left ventricular dysfunction, oxidative stress augmentation and histopathological changes similar to those seen in myocardial infracted patients [3]. Eplerenone, a selective aldosterone antagonist, is a well-known drug used to treat heart failure. However, the validity of its therapeutic benefits in ISO induced changes in myocardial infarction has not been done. Animal studies suggest that aldosterone can have an adverse effect on the heart, independent of Ang II, including a vascular inflammatory response, myocytes necrosis, fibrosis, and hypertrophy. Since, β-adrenergic stimulation induces renin release and therefore increases the plasma levels of angiotensin II and aldosterone, part of the fibrotic process induced by β-adrenergic stimulation could be related to the overproduction of aldosterone and angiotensin II [4]. Previously aldosterone antagonists such as spironolactone and potassium can renovate have shown potency in inhibiting the beta adrenergic action of isoproterenol and prevented cardiac fibrosis suggesting that blockade of aldosterone receptors partly inhibits the effects of β-adrenergic stimulation on the induction of cardiac hypertrophy and fibrosis. Early studies have also reported that aldosterone blockade resulted in reduced oxidative stress and improved endothelial function in experimental atherosclerosis [5], also eplerenone administration resulted in significant improvement of cardiac function as well as remodelling in failing rat hearts [6]. Apart from this, eplerenone also attenuated collagen synthesis induced by corticosterone exerting its protection against cardiac fibrosis [7]. Moreover, recent studies revealed that activating the PI3K/Akt signalling pathway is a scorching new-fangled target to treat heart failure, cardiac dysfunctions and cardiac hypertrophy. Owing to this, Eplerenone, which is an activator of PI3K-AKT pathway [8] is studied in isoproterenol induced myocardial infarction which might exert cardioprotection through this mechanism. However, till date the effect of eplerenone on cardiac function, cardiac injury markers, endogenous antioxidant and tissue architecture has not been studied so far. Therefore, present study was designed to assess the impact of aldosterone receptor blockade or activating the signalling of PI3K-AKT pathway by eplerenone on hemodynamic, biochemical and histopathological variables in cardiac fibrosis induced by β-adrenergic stimulation by ISO in rats.
Materials and methods
Animals
Male Wistar rats (150-200 g) were obtained from the central animal house facility of R. C. Patel Institute of Pharmaceutical Education and Research, Shirpur, India. The study protocol was reviewed and approved by the Institutional Animal Ethics Committee (Protocol approval no. IAEC/RCPIPER/2014/25/09). Animals were kept in the departmental animal house under controlled conditions of temperature at 25±2°C, relative humidity of 60±5% and light-dark cycle of 12:12 h. They were fed with standard food pellets (India) and water ad libitum.
Drugs and chemicals
Eplerenone was obtained as a gift sample from Glenmark laboratories, Nashik, India. Isoproterenol was brought from Sigma Aldrich, USA, and it was dissolved in 0.9% saline. CK-MB and LDH kits (Span diagnostics, Mumbai, India) were purchased. All chemicals used in this study were of analytical grade and purchased from Sigma Aldrich, USA.
Experimental protocol for myocardial infarction
ISO (100 mg/kg) was injected subcutaneously to rats daily for two consecutive days i.e. on days 13th and 14th respectively with 24 h interval to induce experimental myocardial infarction [9,10].
Experimental groups
A total of 40 animals were used for this study. They were randomly divided into five groups, with 8 rats in each group.
Group 1 (Vehicle treated)
Rats were treated with water (1 ml/kg/day p.o.) using intragastric tube for 14 days and on the day 13th and 14th they were treated with 0.3 ml of saline, s.c. at the interval of 24 h.
Group 2 (ISO)
Rats were administered water orally (1 ml/kg/day) for 14 days along with concurrent administration of ISO (100 mg/kg, s.c. at the interval of 24 h) on the day 13th and 14th.
Groups 3 to 5 (EPL+ISO)
Animals were treated with EPL (50, 100 and 150 mg/kg/day) orally for a period of 14 days along with concurrent administration of ISO (100 mg/kg, s.c. at the interval of 24 h) on the day 13th and 14th.
Surgical procedures for recording hemodynamic parameters
ECG monitoring
Animals were placed in supine position on a board and ECG was continuously recorded with standard 3 lead skin electrodes, with 2 electrodes towards the heart on right and left forelimbs and the neutral 3rd electrode on the hind limb facing the heart. The electrodes connected to the data acquisition system power lab (AD Instruments, Australia) and ECG were recorded.
Blood pressure and LVEDP monitoring
Surgical procedure as described by Teerlink, et al. [11] was followed for recording of hemodynamic parameters. On the day 15th i.e. after the last injection of ISO, rats were anaesthetized with urethane (1000 mg/kg, i.p.). The body temperature of animals was maintained at 37°C throughout the experimental protocol. The neck was opened with a ventral midline incision to perform tracheotomy. The right carotid artery was cannulated with polyethylene tube (internal diameter 0.30 mm; outer diameter 0.40 mm) attached to a three way cannula. The cannula filled with heparinized saline was connected to the data acquisition system, (AD Instruments, Australia) using a pressure transducer for the measurement of systolic arterial pressure, diastolic arterial pressure, mean arterial pressure and heart rate.
For the measurement of left ventricular hemodynamic variables, the cannula is advanced from the right carotid artery into the left ventricle. The left ventricular pressure dynamics were recorded on a Lab chart Pro. The left ventricular pressures (left ventricular end-diastolic pressure (LVEDP) and maximum rate of rise and fall of left ventricular pressure (peak +LVdP/dt max and peak -LVdP/dt max) curve were recorded [12,13].
After recording of the hemodynamic parameters, animals of all groups were sacrificed with an overdose of anaesthesia (urethane 2000 mg/kg, i.p.); their hearts were excised and processed for biochemical and histopathological studies. For biochemical analysis, hearts were removed and stored in liquid nitrogen, whereas form icroscopic examinations they were fixed in 10% buffered formalin solution.
Estimation of biochemical parameters
A 10% homogenate of myocardial tissue was prepared in ice chilled phosphate buffer (50 mM, pH 7.4), and The homogenate was centrifuged at 2000 g for 20 min at 4°C, and the aliquot of the chilled homogenate was used to estimate the content of malondialdehyde (MDA) [14] and reduced glutathione (GSH) [15], Catalase [16] and SOD [17], Total protein [18], The amounts of CK-MB isoenzyme (CK-MB), and Lactate dehydrogenase (LDH).
Histopathology
Tissues fixed in buffer formalin were embedded in paraffin and serial sections (3 µm thick) were cut using microtome (Leica RM 2125, Germany). Each section was stained with hematoxylin and eosin (H&E). The sections were examined under the light microscope and photographs were taken at 4× and 10× magnification. At least four hearts from each group were assessed for light microscopic studies.
Statistical analysis
The data is presented as mean ± SEM, One-way ANOVA followed by Dunnet Post-hoc test was applied for significance of hemodynamic and biochemical data of different groups. The value of P<0.05 was considered as a significant.
Results
Mortality
An overall mortality of 5 % was observed during the study protocol. The loss of animals was due to bleeding or improper cannulation of blood vessel while performing the surgery.
Effect of EPL on ST elevation of ECG
Table 1 and Figure 1 showed the effect of EPL on the ECG. ISO treated rats showed a significant elevation of the ST segment from 0.028±0.020 mV to 0.088±0.013 mV when compared to normal rats (P<0.001). A dose dependant significant decrease in ST elevation was seen with all three doses of EPL among which 150 mg/kg showed potent decrease in ST elevation from 0.088±0.013 mV to 0.032±0.0044 mV compared to ISO control (P<0.001).
Table 1.
Effect of EPL on hemodynamic parameters
| Treatment groups | SAP (mmHg) | DAP (mmHg) | MAP (mmHg) | HR (BPM) | ST elevation (mV) |
|---|---|---|---|---|---|
| Normal | 112.3±13.0 | 87.67±06.40 | 88.17±05.80 | 324.6±61.90 | 0.028±0.02 |
| ISO | 78.87±11.3## | 51.33±20.10### | 50.67±19.00### | 295.00±29.10 | 0.088±0.01### |
| EPL (50) | 89.00±09.60 | 67.67±16.50* | 71.50±16.20* | 381.2±73.90 | 0.050±0.02* |
| EPL (100) | 105.20±10.80** | 84.83±17.20*** | 84.50±16.90** | 366.5±19.20 | 0.038±0.01*** |
| EPL (150) | 111.3±7.50*** | 85.67±17.40*** | 86.77±17.40*** | 328.4±53.90 | 0.032±0.004*** |
Data was expressed as mean ± SEM
p<0.01 as compared to normal;
p<0.001 as compared to normal;
p<0.05 as compared to ISO, Significance was determined by One-Way ANOVA followed by Dunnet Post-hoc test;
p<0.01 as compared to ISO, Significance was determined by One-Way ANOVA followed by Dunnet Post-hoc test;
p<0.001 as compared to ISO, Significance was determined by One-Way ANOVA followed by Dunnet Post-hoc test.
SBP: systolic blood pressure, DBP: diastolic blood pressure, MBP: mean blood pressure, HR: heart rate. ISO: isoproterenol; EPL: eplerenone. The figure in the parenthesis indicates the dose in mg/kg.
Figure 1.
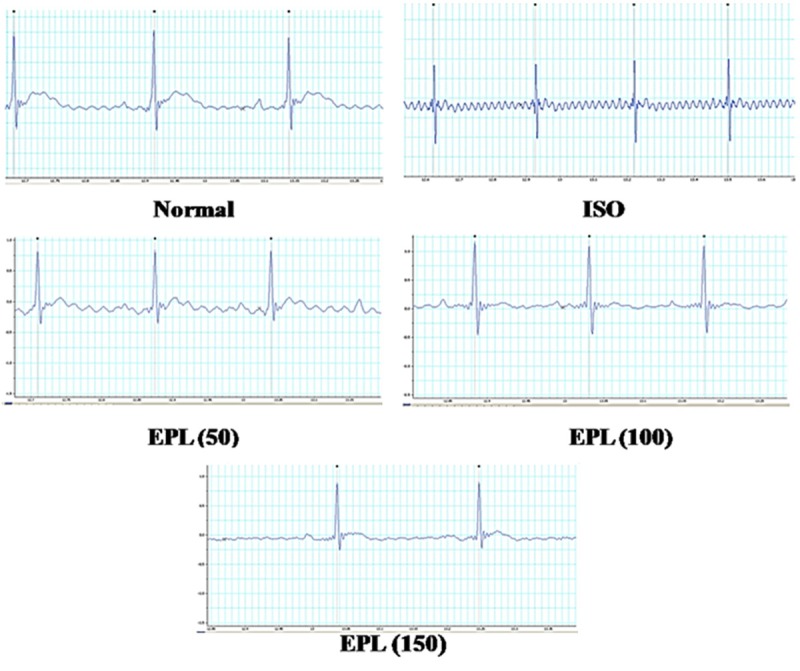
Effect of EPL (50, 100 and 150 mg/kg) on ECG in ISO induced myocardial infarction in rats.
Effect of EPL on cardiac function
Table 1 shows the effects of EPL on arterial blood pressure. ISO control rats showed a significant (P<0.001) decrease in systolic arterial pressure from 112.3±13.03 mmHg to 78.0±11.37 mmHg, diastolic arterial pressure from 87.67±6.4 mmHg to 51.33±20.1 mmHg, and mean arterial pressure from 87.67±6.4 mmHg to 51.33±20.1 mmHg respectively as compared to normal or vehicle treated group. Although ISO treatment led to an increase in heart rate per min, but the increase was not found to be statistically significant. Fourteen days EPL treatment at doses of 50, 100 and 150 mg/kg attenuated systolic, diastolic and mean arterial pressure as compared to vehicle treated group except EPL (50) does not showed any effect. Heart rate did not significantly differ between any drug treatment and the control.
Figure 2 exhibits the deleterious effect of isoproterenol on LVEDP, +LVdP/dt max, -LVdP/dt min respectively and their restoration towards the control values by Eplerenone. Isoproterenol administration resulted in ventricular dysfunction as indicated by increased LVEDP from 5.61±1.988 to 9.82±0.54 mmHg (P<0.001), decreased +LVdP/dt max from 2382±788 to 1146±243.2 mmHg/s (P<0.001) and -LVdP/dt min from 2136±506.3 to 868.8±159 mmHg/s (P<0.05) as compared to normal or vehicle treated rats. EPL dose-dependently prevented rise in LVEDP and improved +LVdP/dt max and -LVdP/dt min in comparison to ISO control group. EPL at the doses of 100 and 150 mg/kg significantly prevented the increase in LVEDP from 9.82±0.54 to 5.2±1.3 (P<0.001), from 9.82±0.54 to 4.8±1.6 mmHg (P<0.001) and from 9.82±0.54 to 5.24±1.83 mmHg (P<0.001) respectively as compared to ISO control group. Similarly, the dose of 150 mg/kg significantly increased +LVdP/dt max from 1146±243.2 to 2443±652.5 mmHg/s (P<0.001) and -LVdP/dt min from 868.8±159 to 1878±723.4 mmHg/s (P<0.001) was observed as compared to ISO treated rats.
Figure 2.
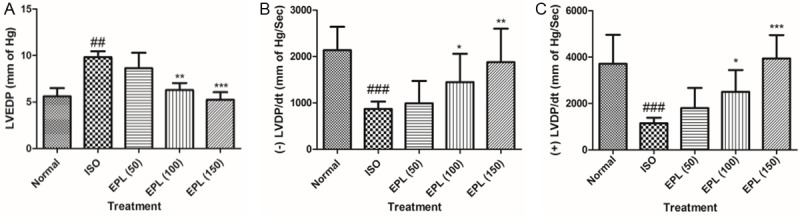
Effect of EPL (50, 100 and 150 mg/kg) on left ventricular end-diastolic pressure, maximum positive and maximum negative rates of left ventricular pressure development in ISO induced myocardial infarction in rats. A. LVEDP; B. (+) LVEDP; C. (-) LVEDP; LVEDP: left ventricular end-diastolic pressure, ISO: isoproterenol, EPL: eplerenone. Data was analysed by using one way analysis of variance (ANOVA) followed by Dunnett’s post hoc test. ###p<0.001 as compared to normal, ***p<0.001 as compared to ISO. The figure in the parenthesis indicates the dose in mg/kg.
Effect of EPL on cardiac injury markers
A significant decline in myocardial injury marker enzymes, CK-MB and LDH were observed in the myocardium of ISO treated group (P>0.001) as compared to normal group (Figure 3). ISO treatment at all doses (100, 150 mg/kg) significantly (P>0.001) attenuated the depletion of myocardial enzymes except EPL (50); CK-MB and LDH in as compared to ISO control group.
Figure 3.
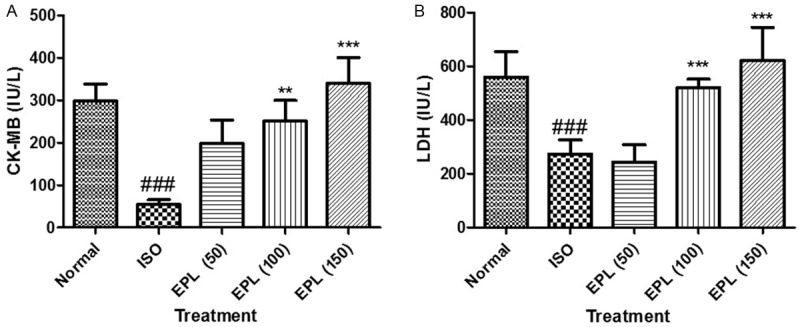
Effect of EPL (50, 100 and 150 mg/kg) on level of CK-MB and LDH in isoproterenol induced myocardial infarction in rats. Data was analysed by using one way analysis of variance (ANOVA) followed by Dunnett’s post hoc test. ###p<0.001 as compared to normal, ***p<0.001 as compared to ISO. ISO: isoproterenol; EPL: eplerenone. The figure in the parenthesis indicates the dose in mg/kg.
Effect of EPL on malondialdehyde content
Figure 4 represents the level of MDA in the heart of normal and experimental animals. Rats challenged with ISO showed a significant (P<0.001) increase in level of MDA from 139.9±17.10 to 246.5±66.3 µg/mg of protein, when compared to normal or vehicle treated rats. Treatment with EPL at a dose of 100 and 150 mg/kg showed maximal protection of myocardium and attenuated the level of MDA (P<0.001) when compared with isoproterenol-control rats.
Figure 4.
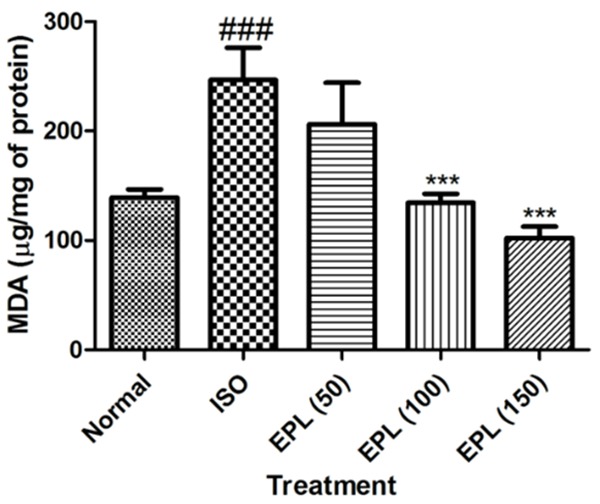
Effect of EPL (50, 100 and 150 mg/kg) on level of malondialdehyde (MDA) in isoproterenol induced myocardial infarction in rats. MDA: malondialdehyde, ISO: isoproterenol. Data was analysed by using one way analysis of variance (ANOVA) followed by Dunnett’s post hoc test. ###p<0.001 as compared to normal, ***p<0.001 as compared to ISO. ISO: isoproterenol; EPL: eplerenone. The figure in the parenthesis indicates the dose in mg/kg.
Effect of EPL on the activities of catalase, SOD and GSH content
Cardiotoxicity-induced with ISO exhibited a significant (P<0.05) decrease in activities of catalase from 33.02±7.84 to 16.4±3.3 U/mg protein, SOD from 64.77±7.7 to 23.11±8.9 U/mg protein and GSH from 65.27±24.31 to 25.9±11.4 U/mg protein as compared to vehicle control rats.
EPL at all doses counteracted the deleterious effect of ISO by increasing the content of these antioxidants except EPL (50). However, significant increase in the levels of SOD, GSH and catalase (P<0.05) was observed with 100 and 150 mg/kg (Table 2).
Table 2.
Effect of EPL on anti-oxidant parameters of isoproterenol induced myocardial infarction in rats
| Treatment groups | Catalase (U/mg protein) | SOD (U/mg protein) | GSH (µg/mg protein) |
|---|---|---|---|
| Normal | 33.02±7.84 | 64.77±7.71 | 65.27±24.31 |
| ISO | 16.45±3.70### | 23.11±8.92### | 25.99±11.49### |
| EPL (50) | 21.13±2.12 | 30.84±9.87 | 36.61±16.46* |
| EPL (100) | 30.06±4.40** | 73.08±12.83*** | 63.94±24.71*** |
| EPL (150) | 30.1±4.99* | 60.35±8.022** | 73.50±17.07*** |
Data was expressed as mean ± SEM;
p<0.001 as compared to normal;
p<0.05 as compared to ISO, Significance was determined by One-Way ANOVA followed by Dunnet Post-hoc test;
p<0.01 as compared to ISO, Significance was determined by One-Way ANOVA followed by Dunnet Post-hoc test;
p<0.001 as compared to ISO, Significance was determined by One-Way ANOVA followed by Dunnet Post-hoc test.
ISO: isoproterenol; EPL: eplerenone. The figure in the parenthesis indicates the dose in mg/kg.
Histopathological alterations of cardiac tissues
Figure 5A shows the light micrograph of normal heart showing normal architecture without any fraying or infarction. Light micrograph of ISO control group shows focal confluent necrosis of muscle fibers with inflammatory cell infiltration (Figure 5B). The degree of myocardial damage in EPL 50 mg/kg in ISO treated rats was similar to that of ISO control group with similar morphological changes (Figure 5C). EPL (100 mg/kg) treated group showed myonecrosis with less edema and inflammatory cells (Figure 5D). EPL at 150 mg/kg treated rat heart shows mild edema with significant reduction in infarction, showing normal myocardial architecture (Figure 5E).
Figure 5.
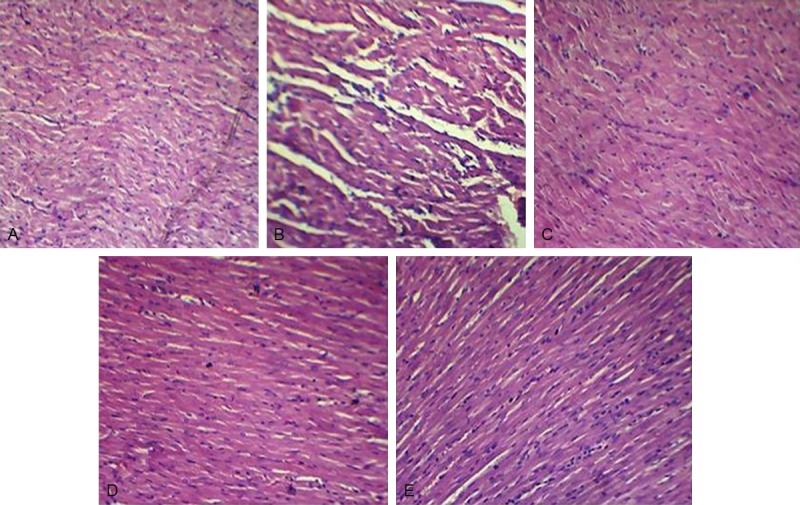
Effect of EPL (50, 100 and 150 mg/kg) on histopathology in isoproterenol induced myocardial infarction in rats. (A) Light micrograph of vehicle treated rat heart showing normal architecture of myocytes (H&E, 40×), (B) light micrographs of isoproterenol (100 mg/kg/day, s.c) alone and (C-E) light micrograph of eplerenone (50, 100, 150 mg/kg respectively) treated isoproterenol induced myocardial infracted rat hearts (H&E, 40×). ISO: isoproterenol; EPL: eplerenone. The figure in the parenthesis indicates the dose in mg/kg.
Discussion
Isoproterenol dispute in rats causes several physiological and efficient changes in the heart, producing acute myocardial infarction which mimics the resemblance to human MI [19], hence this model has been extensively in employment to walk around the mechanisms and treatment of myocardial necrosis. ISO is also known to produce MI by various mechanisms such as myocardial hyper function due to increased chronotropism, inotropism, hypotension, increased calcium overload in myocytes and oxidative stress due to the formation of adrenochromes through its auto oxidation, which are responsible for generation of highly toxic oxygen derived free radicals [20]. In our experiment, we found that EPL protected myocardium from ISO induced myocardial functional and structural injury via improved hemodynamic, biochemical and histopathological parameters suggesting its cardioprotective action. Acute administration of some mineralocorticoid receptor blockers such as spironolactone and potassium caronoate is known to act by inhibiting aldosterone which is synthesised by the stimulation of β adreno receptors by isoproterenol [21]. Moreover, reduced oxidative stress and improved endothelial function also observed in experimental atherosclerosis and early after myocardial infarction [22]. The present study showed that, fourteen days pre-treatment with EPL in isoproterenol-treated rat’s exhibit dose-dependent reduction in ST elevation which reflects the potential difference in the boundary between ischemic and non-ischemic zones and the loss of cell membrane function. Apart from this, systolic, diastolic and mean arterial pressures were normalised as compared to vehicle treated group. However, the results were significant at EPL 100 and 150 mg/kg in ISO challenged rats as compared to vehicle treated. As per heart rate is concerned, no statistically significant changes were observed among groups of EPL treatment in ISO treated rats. Further, it is interesting to recall that administration of EPL resulted in significant improvement of cardiac function and remodelling in failing rat hearts [23]. Chronic administration of EPL potentially reduced left ventricular dysfunction [24]. Present study showed that EPL improved left ventricular end-diastolic function by increasing inotropic (+LVdP/dt max, marker of myocardial contraction) and lusitropic (-LVdP/dt max, marker of myocardial relaxation) states of the heart. It also ameliorated ISO induced increase in LVEDP, a marker of pre-load that again reflects an improvement of left ventricular function. Besides the blood pressure reduction property of eplerenone, recent reports document the blood pressure-independent beneficial effects of EPL on myocardium of rat models [25]. Recent studies have demonstrated that EPL stimulates eNOS through the Akt/protein kinase B pathway, reduces iNOS via NF-B after the development of the oxidative stress-LOX-1 pathway, and inhibits MAP kinase and its downstream effector p70S6 kinase through the PKCII-c-Srcpathway; thereby exerting cardioprotective effects. Taken together, our result suggests that EPL has protective effect against ISO induced cardiac changes through its bifunctional effects that are, a class effect of aldosterone antagonism and by PI3K-Akt activation. Apart from hemodynamic and ventricular functions, several diagnostic marker enzymes like CK-MB isoenzyme and lactate dehydrogenase are present in myocardium and have been used as a predictor for pathological changes. These enzymes are released into the extracellular fluid during myocardial injury [26]. In the present study, we observed a decrease in activities of these enzymes in the hearts of ISO control rats which indicates ISO induced necrotic damage of the myocardial membrane. We found that administration of EPL significantly prevented the loss in activities of CK-MB isoenzyme and LDH in the heart tissue during ISO insult in rats. The cardioprotection offered by EPL might be due to reduction of myocardial damage by preservation of cell membrane integrity and stability and thus restricting the leakage of CK-MB isoenzyme and LDH. Lipid peroxidation is an important pathogenic event in myocardial necrosis and accumulation of lipid end products reflects damage of the cardiac constituents [27]. The increased levels of MDA, a lipid peroxidation end-product, observed in our study following ISO administration might be due to free radical mediated membrane damage. Findings of the present study show that, pre-treatment with EPL significantly decreased MDA contents near normal level. Isoproterenol treatment is known to produce free radical moieties via its quinine metabolites that react with oxygen to produce superoxide anions and other reactive oxygen species and thus develops oxidative stress in rat myocardium. Free radical scavenging antioxidants such as SOD, catalase and GSH are the first line of cellular defence against oxidative injury. The observed decrease in the levels of these antioxidants in the heart following isoproterenol administration in our study confirms the excessive generation of reactive oxygen species, such as superoxide and hydrogen peroxide, which in turns leads to consumption of these endogenous antioxidants. It has been well documented that isoproterenol causes increased oxidative stress in rat heart as evidenced by reduction in myocardial SOD and catalase activities and reduced GSH level [28]. In the present study, we observed that the decreased activities of SOD, catalase and GSH level in ISO injected rats were significantly ameliorated by EPL treatment. These finding are in accordance with studies reporting that EPL has antioxidant effect in mouse model of atherosclerosis. These findings suggested that blood pressure-independent cardioprotective mechanisms of EPL might be related to reduction in oxidative stress and/or improvement in cellular antioxidative defence mechanism against oxidative stress. Therefore, one of the possibilities exists that the cardioprotection offered by EPL might be due to its antioxidant activity, which could exert a beneficial effect against pathological alterations caused by free radicals in isoproterenol-induced myocardial necrosis.
To further investigate the cardioprotective action of EPL on ISO induced myocardial necrosis, light microscopic study was performed (Table 3). Histopathological findings of the heart pre-treated with EPL (100 and 150 mg/kg) showed a well preserved normal morphology of cardiac muscle with no evidence of necrosis when compared to isoproterenol induced myocardial infracted heart and further confirmed the protective action of EPL.
Table 3.
Effect of eplerenone on histopathological changes in different experimental groups
| Treatment groups | Myonecrosis | Inflammatory cells | Edema |
|---|---|---|---|
| Normal | - | - | - |
| ISO | +++ | +++ | +++ |
| EPL (50) | ++ | + | ++ |
| EPL (100) | ++ | + | + |
| EPL (150) | + | - | + |
Severe (+++), moderate (++), mild (+) and nil (–).
In the light of these findings, present study supports the hypothesis that eplerenone improved cardiac function, decreased oxidative stress, cardiac injury and lipid peroxidation process. In addition, eplerenone normalizes histopathological changes caused by isoproterenol administration. These cardioprotective actions of eplerenone might be due to anti-oxidative mechanism and PI3K activation. However, additional data may help to know the exact mechanism of action of eplerenone in isoproterenol-induced myocardial infarction.
Acknowledgements
The authors gratefully acknowledge the financial support received under Young Scientist Research Scheme (File no. SB/YS/LS-114/2013) of Science and Engineering Research Board (SERB), Department of Science and Technology, New Delhi, India. The authors also gratefully acknowledge the financial support from College of Medicine and Health Sciences, United Arab Emirates University, United Arab Emirates.
Disclosure of conflict of interest
None.
References
- 1.Taylor B, Kilpatrick R, Song X, Muntner P. Effect of LDL-C on risk of recurrent myocardial infarction, unstable angina, and ischemic stroke in a high risk, secondary prevention patient population. J Am Coll Cardiol. 2015;65:10S. [Google Scholar]
- 2.Yang XM, Cui L, White J, Kuck J, Ruchko MV, Wilson GL, Alexeyev M, Gillespie MN, Downey JM, Cohen MV. Mitochondrially targeted Endonuclease III has a powerful anti-infarct effect in an in vivo rat model of myocardial ischemia/reperfusion. Basic Res Cardiol. 2015;110:1–13. doi: 10.1007/s00395-014-0459-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lobo Filho HG, Ferreira NL, Sousa RB, Carvalho ER, Lobo PL, Lobo Filho JG. Experimental model of myocardial infarction induced by isoproterenol in rats. Rev Bras Cir Cardiovasc. 2011;26:469–476. doi: 10.5935/1678-9741.20110024. [DOI] [PubMed] [Google Scholar]
- 4.Schafer A, Vogt C, Fraccarollo D, Widde J, Flierl U, Hildemann SK, Ertl G, Bauersachs J. Eplerenone improves vascular function and reduces platelet activation in diabetic rats. J Physiol Pharmacol. 2010;61:45–52. [PubMed] [Google Scholar]
- 5.Sartório CL, Fraccarollo D, Galuppo P, Leutke M, Ertl G, Stefanon I, Bauersachs J. Mineralocorticoid receptor blockade improves vasomotor dysfunction and vascular oxidative stress early after myocardial infarction. Hypertension. 2007;50:919–925. doi: 10.1161/HYPERTENSIONAHA.107.093450. [DOI] [PubMed] [Google Scholar]
- 6.Kobayashi N, Yoshida K, Nakano S, Ohno T, Honda T, Tsubokou Y, Matsuoka H. Cardioprotective mechanisms of eplerenone on cardiac performance and remodeling in failing rat hearts. Hypertension. 2006;47:671–679. doi: 10.1161/01.HYP.0000203148.42892.7a. [DOI] [PubMed] [Google Scholar]
- 7.Omori Y, Mano T, Ohtani T, Sakata Y, Takeda Y, Tamaki S, Tsukamoto Y, Miwa T, Yamamoto K, Komuro I. Glucocorticoids induce cardiac fibrosis via mineralocorticoid receptor in oxidative stress: contribution of elongation factor eleven-nineteen lysine-rich leukemia (ELL) Yonago Acta Med. 2014;57:109–115. [PMC free article] [PubMed] [Google Scholar]
- 8.Goyal SN, Haiderali S, Reddy N, Arya DS, Patil CR. Prediabetes: grounds of pitfall signalling alteration for cardiovascular disease. RSC Advances. 2014;4:58272–58279. [Google Scholar]
- 9.Hassan MQ, Akhtar MS, Akhtar M, Ali J, Haque SE, Najmi AK. Edaravone protects rats against oxidative stress and apoptosis in experimentally induced myocardial infarction: Biochemical and ultrastructural evidence. Redox Rep. 2015 doi: 10.1179/1351000215Y.0000000011. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anandan R, Chatterjee NS, Sivakumar R, Mathew S, Asha KK, Ganesan B. Dietary Chitosan Supplementation Ameliorates Isoproterenol-Induced Aberrations in Membrane-Bound ATPases and Mineral Status of Rat Myocardium. Biol Trace Elem Res. 2015;167:103–109. doi: 10.1007/s12011-015-0289-4. [DOI] [PubMed] [Google Scholar]
- 11.Teerlink JR, Löffler B, Hess P, Maire JP, Clozel M, Clozel JP. Role of endothelin in the maintenance of blood pressure in conscious rats with chronic heart failure. Acute effects of the endothelin receptor antagonist Ro 47-0203 (bosentan) Circulation. 1994;90:2510–2518. doi: 10.1161/01.cir.90.5.2510. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y, Zhong N, Gia J, Zhou Z. Effects of chronic intermittent hypoxia on the hemodynamics of systemic circulation in rats. Jpn J Physiol. 2004;54:171–174. doi: 10.2170/jjphysiol.54.171. [DOI] [PubMed] [Google Scholar]
- 13.AlAhmari L, AlAhmari T, Lee L, Jue J, Levy R, Swiston J, Brunner N. Utility Of Echocardiographic Estimates Of Left Ventricular Filling Pressures In A Population Referred For Pulmonary Hypertension. Am J Respir Crit Care Med. 2015;191:A4853–A4859. [Google Scholar]
- 14.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 15.Moron MS, Depierre JW, Mannervik B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta. 1979;582:67–78. doi: 10.1016/0304-4165(79)90289-7. [DOI] [PubMed] [Google Scholar]
- 16.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 17.Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 18.Bradford M. A rapid and Sensitive method for the quantitation Ofmicrograrn quantities Of protein utilizing the principle Of protein-dye binding. Anal Biochem. 1976;2:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 19.Shukla SK, Sharma SB. β-Adrenoreceptor Agonist Isoproterenol Alters Oxidative Status, Inflammatory Signaling, Injury Markers and Apoptotic Cell Death in Myocardium of Rats. Indian J Clin Biochem. 2015;30:27–34. doi: 10.1007/s12291-013-0401-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aswar U, Mahajan U, Nerurkar G, Aswar M. Amelioration of cardiac hypertrophy induced by abdominal aortic banding in ferulic acid treated rats. Biomedicine & Aging Pathology. 2013;3:209–217. [Google Scholar]
- 21.Klein J. Aldosterone inhibits uncoupling protein-1, induces insulin resistance, and stimulates proinflammatory adipokines in adipocytes. Horm Metab Res. 2005;37:455–459. doi: 10.1055/s-2005-870240. [DOI] [PubMed] [Google Scholar]
- 22.George J, Carr E, Davies J, Belch J, Struthers A. High-dose allopurinol improves endothelial function by profoundly reducing vascular oxidative stress and not by lowering uric acid. Circulation. 2006;114:2508–2516. doi: 10.1161/CIRCULATIONAHA.106.651117. [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi N, Hara K, Tojo A, Onozato ML, Honda T, Yoshida K, Mita Si, Nakano S, Tsubokou Y, Matsuoka H. Eplerenone shows renoprotective effect by reducing LOX-1-mediated adhesion molecule, PKCepsilon-MAPK-p90RSK, and Rho-kinase pathway. Hypertension. 2005;45:538–544. doi: 10.1161/01.HYP.0000157408.43807.5a. [DOI] [PubMed] [Google Scholar]
- 24.Rockman HA, Chien KR, Choi DJ, Iaccarino G, Hunter JJ, Ross J, Lefkowitz RJ, Koch WJ. Expression of a β-adrenergic receptor kinase 1 inhibitor prevents the development of myocardial failure in gene-targeted mice. Proc Natl Acad Sci U S A. 1998;95:7000–7005. doi: 10.1073/pnas.95.12.7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pocock SJ, Pitt B. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364:11–21. [Google Scholar]
- 26.Mustafa GH, Gorea R, Rahman S, Khan MS. Diagnostic efficacy of cardiac troponin in post-mortem examination of acute myocardial infarction. Int J Ethics, Trauma & Victimol. 2015:1. [Google Scholar]
- 27.Mahmood T, Siddiqui HH, Dixit R, Bagga P, Hussain S. Protective Effect of Bombyx mori L Cocoon (Abresham) and its Formulations against Isoproterenol-Induced Cardiac Damage. Tropical Journal of Pharmaceutical Research. 2015;14:63–72. [Google Scholar]
- 28.Ojha S, Azimullah S, Mohanraj R, Sharma C, Yasin J, Arya DS, Adem A. Thymoquinone Protects against Myocardial Ischemic Injury by Mitigating Oxidative Stress and Inflammation. Evid Based Complement Alternat Med. 2015;501:143629. doi: 10.1155/2015/143629. [DOI] [PMC free article] [PubMed] [Google Scholar]


