Abstract
Cyclooxygenase-2 expression by malignant tumors, including colonic adenocarcinoma, is associated with increased tumor aggression and poor prognosis. Nuclear factor kappa B is a key regulator of cyclooxygenase-2 and is regulated by two pathways, the ‘canonical’ and the ‘alternative’ pathway. The alternative pathway is triggered by members of the tumor necrosis factor cytokine family, including RelB and p52. This present study was undertaken to evaluate cyclooxygenase-2 and the alternative nuclear factor-kappa B signaling pathway in colonic adenocarcinoma. Formalin-fixed, paraffin-embedded tissue samples diagnosed with colonic adenocarcinoma and a human colonic adenocarcinoma cell line, LS174, were studied. The expression of cyclooxygenase-2, RelB and p52 were determined using immunohistochemistry, immunofluorescence, and Western blots. Quantitative analysis of mRNA by real-time reverse transcriptase polymerase chain reaction and chromatin immunoprecipitation were performed on the tissue and cell samples. To investigate nuclear factor kappa B gene regulation of the cyclooxygenase-2 gene, dual luciferase assays were performed, and LS174 cells were transfected with RelB or p100/p52 short interfering RNA. Upregulation of cyclooxygenase-2 was associated with activation of the alternative nuclear factor kappa B signaling pathway components RelB, and p52, in colonic adenocarcinoma cells in tissues and the cell line, LS174. Chromatin immunoprecipitation assay determined that cyclooxygenase-2 gene was associated with both RelB and p52. A luciferase reporter assay showed that the nuclear factor kappa B enhancer of cyclooxygenase-2 was sufficient to regulate the transcriptional activity of a heterologous promoter in LS174 cells. RNA interference-mediated knockdown of RelB or p52 resulted in significant inhibition of cyclooxygenase-2 at both mRNA and protein levels in LS174 cells. These findings support a potential role for inhibition of components of the alternative nuclear factor kappa B signaling pathway, RelB-p52-cyclooxygenase-2, as a possible therapeutic target in the treatment of adenocarcinoma of the colon. Further studies on the role of this pathway in this and other malignancies are recommended.
Keywords: Cyclooxygenase-2, colonic carcinoma, nuclear factor kappa B, immunohistochemistry, immunofluorescence, polymerase chain reaction
Introduction
The development and progression of colonic adenocarcinoma is a multi-stage process, involving mutations in oncogenes and tumor suppressor genes that lead to uncontrolled cell proliferation [1-3]. Cyclooxygenases (COXs) are important regulators of cell proliferation, by converting free arachidonic acid into prostaglandin-H2, a precursor of other prostaglandins and thromboxanes [4,5]. These compounds also play a role in immune function, inflammation, apoptosis and angiogenesis, all of which contribute to the development or progression of malignancy.
There are three members of the human COX family. COX-1 is found ubiquitously in human tissues and regulates physiologic homeostasis [4]. COX-3 is a product of an alternative RNA splicing and is associated with the regulation of pain and fever [6]. COX-2 is an inducible enzyme that has a role in the development of epithelial cell dysplasia, carcinoma, invasion and metastasis [7-9]. The cellular expression of COX-2 is induced by cytokines and growth factors [7-9]. Tumors with high levels of COX-2 are likely to be more aggressive and to have a worse prognosis [10]. Targeting the inhibition of COX-2 expression may help to control the progression of carcinoma, including colonic carcinoma.
Nuclear factor kappa B (NF-kB) is a ubiquitous and inducible cell transcription factor and a key regulator in the production of COX-2 [11-13]. NF-kB consists of a small family of closely-related protein dimers, which bind to a common sequence motif known as the kB site. NF-kB is composed of the Rel family members of DNA-binding proteins: p50/p105 (NF-kB1), p52/p100 (NF-kB2), RelA (p65), RelB and c-Rel, all of which contain an N-terminal Rel homology domain responsible for homo- and heterodimerization [14-17].
There are two regulatory pathways that have been described to control the activity of this family of NF-kB proteins. The first is the ‘canonical’ NF-kB pathway, which is triggered in response pro-inflammatory cytokines and to microbial and viral infections. The second pathway is the ‘alternative’ or ‘non-canonical’ pathway, which is triggered by members of the tumor necrosis factor (TNF) cytokine family. Nuclear factor RelA is a member of the canonical pathway, whereas RelB and p52 are members of the alternative pathway.
In the canonical NF-kB pathway, p65 predominantly heterodimerizes with p50 that is constitutively processed from p105 [14-17]. In the ‘alternative’ NF-kB pathway, the components RelB and p100 are triggered by a subset of the tumor necrosis factor (TNF) family members, requiring phosphorylation-induced p100 processing into p52 [15]. The activated NF-kB can mediate the expression of pro-inflammatory genes, and the targets of NF-kB are important regulators of cell proliferation [17-19]. The IkB kinase (IKK) is involved in downstream targeting of NF-kB and also has a role in regulating inflammation and cell proliferation [17-19]. The RelB/p52 complex has been shown to regulate transcription of COX-2 in a variety of tissue types [20].
This present study was undertaken to evaluate COX-2 and the alternative NF-kB signaling pathway in colonic adenocarcinoma. The approaches were, firstly, to study the regulatory mechanisms of COX-2, nuclear RelB, and p52 expression in human colonic carcinoma in tissue samples. Second, in vitro functional studies were done on the effects of RelB or p100/p52 on COX-2 levels in the human colonic adenocarcinoma cell line, LS174.
Methods and methods
Patients studied
Colonic adenocarcinoma tissue samples patients were studied who were treated at the Second Hospital of Jilin University, Changchun, China. These patients had histologically confirmed adenocarcinoma, diagnosed on biopsy and confirmed on surgical resection.
Tissue samples
Tissue samples had been fixed in buffered formalin for 24 h, dehydrated in 70% ethanol, paraffin-embedded, sectioned and stained for routine diagnostic histopathology using hematoxylin and eosin (H&E). Formalin-fixed, paraffin-embedded (FFPE) tissue blocks and sections were chosen which contained tumor and adjacent uninvolved colon. The extra FFPE sections that were taken for this study, following patient diagnosis and treatment.
Patient consents
Tissue samples were obtained as part of the patient diagnosis (biopsy or polypectomy) or surgical treatment (colectomy) with full, informed patient consent. This study was approved by the local Ethics Committee Jilin University, Changchun, China.
Cell line
Human colon adenocarcinoma LS174 cells (CL-188) were obtained from American Type Culture Collection (ATCC, VA, USA). LS174 cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) and supplemented with 10% fetal calf serum (FCS) at 37°C in 5% CO2.
Primary antibodies
The anti-COX-2 antibody was purchased from Thermo Fisher Scientific, Rockford, IL, USA. Polyclonal anti-RelB antibody was purchased from MyBioSource, Inc., San Diego, CA, USA. The polyclonal anti-p52 antibody was purchased from antibodies-online Inc., Atlanta, GA, USA. Horseradish peroxidase-conjugated, biotinylated secondary antibodies and fluorescein-conjugated secondary antibodies against either rabbit or mouse IgG were purchased from Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA. Mouse IgG1 isotype control for immunoprecipitation was purchased from eBioscience, Inc., San Diego, USA.
Immunohistochemistry
Tissue sections of colon cancer and adjacent non-tumor-containing tissues were evaluated using immunohistochemical staining. Tissue sections were de-waxed and incubated with the primary antibodies to COX-2, RelB or p52 at primary dilutions. Tissue sections were washed with Tris-buffered saline (TBS) and Tween 20 (TBST). Sections were incubated with biotinylated anti-rabbit or anti-mouse secondary antibody diluted in TBS. Sections were washed again with TBST, and secondary antibodies were detected using an avidin-biotin-complex (ABC) reagent, incubated for 30 min. The brown chromogenic substrate 3,3’-diaminobenzidine (DAB) was used for light microscopic visualization of antibody localization.
Immunofluorescent staining
LS174 cells were fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) and permeabilized for 10 minutes in 0.5% Triton X-100 in PBS at room temperature. Cells were washed with PBS and incubated with the primary antibodies to COX-2, RelB or p52 at room temperature for 1 hr. LS174 cells were washed with PBS containing 0.1% bovine serum albumen (BSA) and incubated with the fluorophore-conjugated, secondary antibodies, Alexa Fluor 488 goat anti-rabbit IgG (Invitrogen) for 1 h at room temperature. The cells were counterstained with 4’,6-diamidino-2-phenylindole (DAPI) (Invitrogen, USA) and visualized under a fluorescence microscope (Olympus, Japan).
Western blotting
Whole-cell lysates were used for Western blotting analysis. Antibodies used included those to COX-2, RelB, and p52.
RNA isolation and quantitative analysis of mRNA by real-time reverse transcriptase polymerase chain reaction (RT-PCR)
Total RNA was extracted from colonic tissues or the LS174 cells with the use of Trizol (Invitrogen). Reverse transcription of RNA was performed with 2.5 g of total RNA using the SuperScript® First-Strand Synthesis System for RT-PCR kit (Invitrogen, USA). The cDNA was subjected to real-time PCR using SYBR green dye (Qiagen, CA, USA) on an ABI 7300 real-time PCR machine (Applied Biosystem, CA, USA). The method used was as described by Suzuki and colleagues [21].
Chromatin immunoprecipitation (ChIP)
LS174 cells were fixed in 1% formaldehyde for 5 min and the reactions terminated by the addition of glycine. Cells were lysed in ChIP lysis buffer with freshly added 1X protease inhibitor cocktail (Roche Applied Science, CA). Cells were sonicated to shear the chromatin into 300-600 base-pair fragments. Chromatin was immunoprecipitated with appropriate ChIP grade antibodies. These antibodies DNA was purified using a phenol and chloroform extraction and an ethanol precipitation method. Real-time PCR was performed for COX-2 promoter region encompassing the nuclear factor kappa B (NF-kB) enhancers. The PCR products were quantified using SYBR green dye (Qiagen) and fold enrichment was determined by the method of cycle threshold (CT) using the formula, 2-ΔΔCT.
Dual luciferase assay
To investigate gene regulation in a heterologous context, four copies of the distal NF-kB enhancer of the COX-2 gene were placed upstream of the minimal promoter that drives gene expression of firefly luciferase. A mutant version was cloned as a baseline control. A plasmid containing a cytomegalovirus (CMV) promoter, which is not regulated by any NF-kB enhancer, was co-transfected as an internal reference. Transfection with pCOX-2-NF and pCOX-2-NFMut in LS174 cells were performed according to the method recently reported by He and colleagues [22]. Dual luciferase assays were performed on a Victor 3V (PerkinElmer, MA) according to the manufacturer’s protocol (Promega, WI).
Gene transfection
LS174 cells were transfected with 50 nM RelB or p100/p52 short interfering RNA (siRNA) (Invitrogen) or a non-targeting control using the transfection reagent Lipofectamine 2000 (Invitrogen). Cells were pre-incubated in serum-free DMEM/F-12 for 30 min at room temperature prior to transfection. Forty-eight hours after exposure to the siRNA or the non-silencing RNA, total RNA was isolated from the cells and analyzed by RT-PCR.
Statistical analysis
Each experiment was performed in triplicate. The Student’s t-test was used to compare two groups under a variety of conditions. P < 0.05 was considered statistically significant.
Results
COX-2, RelB, and p52 are upregulated in human colonic adenocarcinoma
Figure 1A shows the positive immunostaining of cyclooxygenase-2 (COX-2) in sections of colonic adenocarcinoma, compared with weak or negative immunostaining in adjacent, normal tissues. Western blot analysis and reverse transcription polymerase chain reaction (RT-PCR) showed that both the protein levels and mRNA levels of COX-2 were increased in colonic adenocarcinoma compared with normal tissues (Figure 1B). The immunolocalization of RelB and p52 in colonic adenocarcinoma cells were found to be nuclear (Figure 1C). These results suggest that activation of RelB and p52 may contribute to upregulation of COX-2 in colonic adenocarcinoma.
Figure 1.
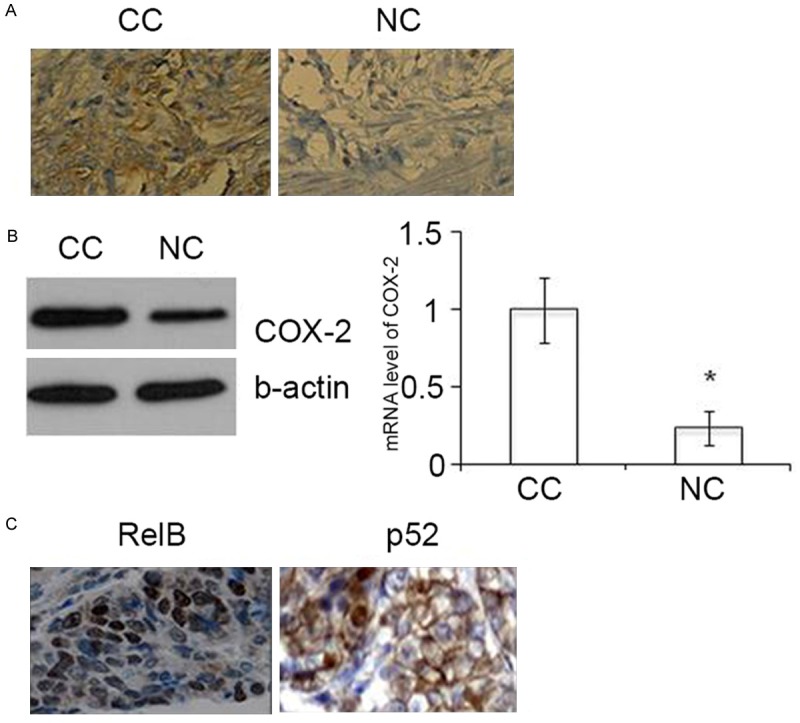
Upregulation of COX-2 and nuclear RelB-p52 in colonic adenocarcinoma tissue sections. A. Immunohistochemical staining of COX-2 in colonic cancer (CC) and adjacent non-cancerous tissues (NC). Original magnification, x200. B. Western blot (left panel) and RT-PCR assays (right panel) for COX-2 expression in colon cancer and adjacent non-cancerous tissue. * P < 0.01. C. Immunohistochemical staining of RelB and p52 in colonic cancer tissues. Original magnification, x200.
RelB and p52 are constitutively activated in LS174 cells
The analysis of RelB and p52 signaling pathway activation in LS174 was done using immunofluorescence staining. Figure 2 shows that both RelB and p52 were localized in the nuclei of LS174 colonic adenocarcinoma cells using immunofluorescence.
Figure 2.
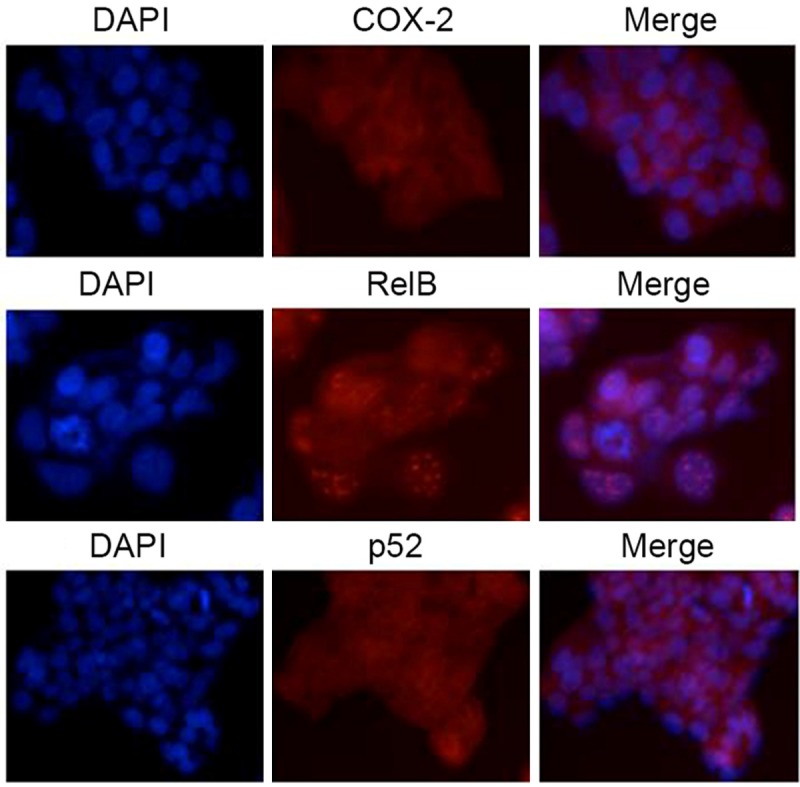
Activation of RelB and p52 seen in LS174 cells. Immunofluorescence staining was performed with the primary antibodies to COX-2, RelB and p52.
RelB and p52 interact with COX-2 at the distal NF-kB enhancer of COX-2
The COX-2 gene promoter contains two NF-kB enhancers: GGGATTCCCT (distal) and GGGACTACCC (proximal) (Figure 3A). Chromatin immunoprecipitation (ChIP) analysis was done to investigate whether the RelB-p52 dimer binds to these NF-kB enhancers. Figure 3B shows that both RelB and p52 were enriched at the distal NF-kB enhancer, compared with the proximal one. The RelB-p52 dimer is more likely to interact with COX-2 gene by binding the distal NF-kB enhancer in human colonic adenocarcinoma.
Figure 3.
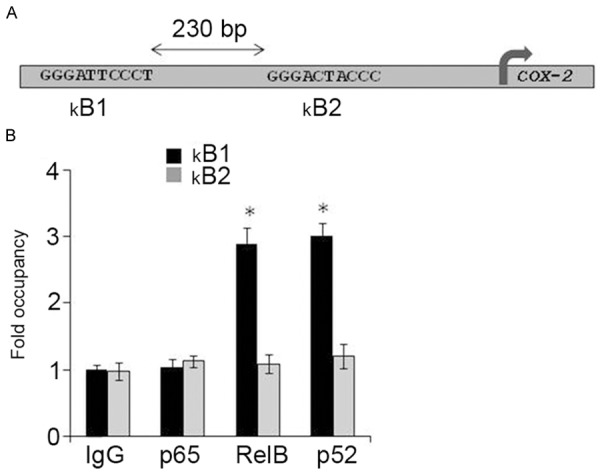
Interaction between RelB/p52 and COX-2 gene promoter in LS174 cells. A. A schematic representation of NF-κB enhancers in the COX-2 gene promoter. B1, distal; B2, proximal. B. ChIP assays were performed with the use of anti-RelB or anti-p52 antibodies to assess these interactions.
The distal NF-kB enhancer of COX-2 regulates the heterologous promoter in LS174 cells
Figure 4A shows the four copies of the distal NF-kB enhancer of the COX-2 gene upstream of the minimal promoter that drives gene expression of firefly luciferase (Figure 4A). As shown in Figure 4B, from three independent experiments, the distal NF-kB enhancer of COX-2 gene conferred NF-kB stimulation to this minimal promoter by more than 3-fold above the baseline. These results suggest that the distal NF-kB enhancer is sufficient for RelB- and p52-dependent regulation of the COX-2 gene in human colonic adenocarcinoma.
Figure 4.
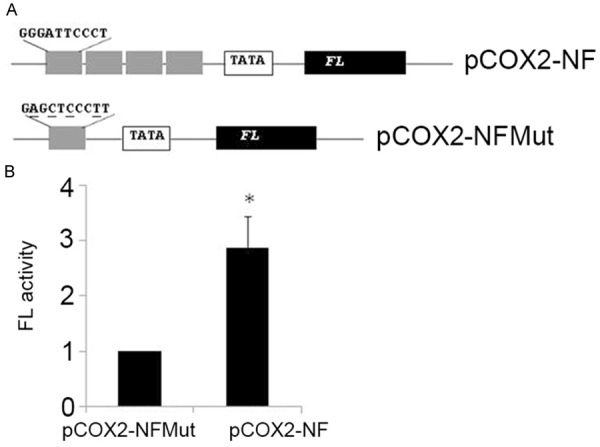
The distal NF-kB enhancer of COX-2 regulates transcription of a heterologous promoter. A. A schematic representation of the DNA constructs. FL, firefly luciferase reporter gene. B. Dual luciferase assays were performed to assess FL activity. *P < 0.01.
Depletion of RelB or p52 inhibits expression of COX-2
Following transfection of the LS174 cells with short interfering RNA (siRNA), knockdown of p52 (Figure 5A) or RelB (Figure 5B) inhibited the expression of COX-2. These results support the view that RelB and p52 dimers are regulators of COX-2 in human colonic adenocarcinoma.
Figure 5.
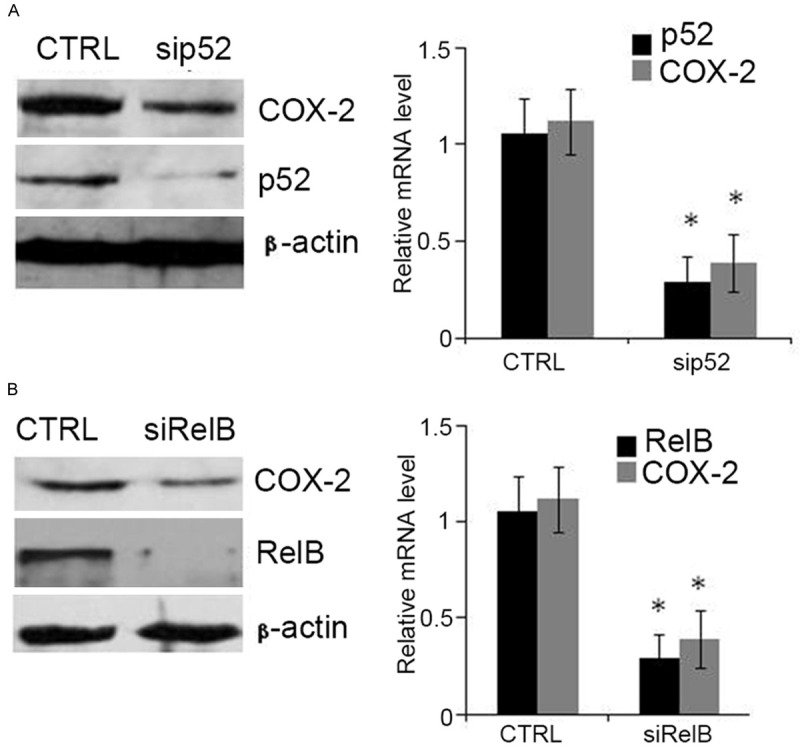
Inactivation of RelB or p52 down-regulates COX-2 in LS174 cells. The LS174 cells were treated siRNAs targeting p52 (A) or RelB (B) for 48 h. Western blot (left panel) or RT-PCR (right panel) were used to assess protein or mRNA levels, respectively. *P < 0.01.
Discussion
The inducible enzyme, cyclooxygenase-2 (COX-2) plays a role in inflammation, angiogenesis and cell proliferation in a variety of tissues [23,24]. This study has demonstrated upregulation of COX-2, RelB, and p52 in tissue samples of colonic adenocarcinoma and in vitro in LS174 cells. This study also showed that depletion of RelB and p52 associated with the COX-2 gene promoter at the distal nuclear factor kappa B (NF-kB) enhancer led to a significant down-regulation of COX-2. The results suggest that this alternative RelB-p52-COX-2 pathway may play a role in inflammation, cell proliferation, and progression in adenocarcinoma of the colon.
Overexpression of COX-2 has previously been reported in some tumors, including hematological malignancies and colorectal cancer [23,25,26]. Patients having elevated levels of COX-2 associated with their tumors have been shown to have a poorer prognosis [27]. The findings of this study support those of previous studies that have shown that tumor necrosis factor (TNF) can activate the RelB/p52 signaling pathway [15,17]. There is increasing evidence to support the view that COX-2 may play a role in the different steps in cancer progression [25]. Further studies on the roles of the COX-2 pathways would allow for the development of a targeted approach to treatment for a variety of tumor types, in a variety of locations and stages.
The inducible transcription factor, nuclear factor-kB (NF-kB), is regulated by an alternative pathway, triggered by certain members of the tumor necrosis factor (TNF) cytokine family, including RelB and p52. In its location in the cell nucleus, NF-kB can regulate several genes, including COX-2. In this study, we found that the increase of both RelB and p52 in the nuclei of colonic carcinoma cells was accompanied by increased COX-2 expression. Nuclear accumulation of RelA was not observed in this study. ChIP assays demonstrated a direct interaction between RelB/p52 and the COX-2 gene promoter. This study suggests that the transcription of COX-2 in colon cancer was promoted via the RelB/p52 complex. Further studies are recommended, including studies with fresh, unfixed tumor tissue, to examine the nuclear accumulation of RelB and p52 and to determine whether the findings have any prognostic significance.
The NF-kB family has Rel homology that handles protein-DNA interaction and hetero- or homodimerization [16]. NF-kB dimers share an intrinsic ability to bind to an NF-kB enhancer, defined as a family of DNA-binding sites of 9-11 bp in length that regulate target genes. The consensus NF-kB enhancer, which presents a major source of complexity in NF-kB system, is represented as 5’-GGGRNNYYCC-3’ (R represents purine, N is any nucleotide, and Y represents pyrimidine) [28]. Previously reported luciferase and gel shift assays have revealed two positive regulatory elements on the 5’-flanking region of mouse COX-2 gene: kB1 or the distal 5’-GGGATTCCC-3’, located at nucleotides -401 to -393 bp and kB2 or the proximal 5’-TTGCGCAAC-3’ at -138 to -130 bp [28,29]. In this study, neither RelB nor NF-kB localized to COX-2 at the kB2 site in LS174 cells, possibly because the binding of RelB/p52 heterodimer is dependent on a middle segment of a consecutive adenine (A) and thymine (T) in the NF-kB enhancer [29,30].
In conclusion, this study has demonstrated that upregulation of COX-2 is associated with activation of the alternative NF-kB signaling pathway in colonic adenocarcinoma cells in tissues and a cell line. These findings support a role for inhibition of components of the alternative NF-kB signaling pathway as a possible therapeutic target in the treatment of adenocarcinoma of the colon. Further studies on the role of this pathway in this and other malignancies are recommended.
Disclosure of conflict of interest
None.
References
- 1.Smith G, Carey FA, Beattie J, Wilkie MJ, Lightfoot TJ, Coxhead J, Garner RC, Steele RJ, Wolf CR. Mutations in APC, Kirsten-ras, and p53--alternative genetic pathways to colorectal cancer. Proc Natl Acad Sci U S A. 2002;99:9433–9438. doi: 10.1073/pnas.122612899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hsieh JS, Lin SR, Chang MY, Chen FM, Lu CY, Huang TJ, Huang YS, Huang CJ, Wang JY. APC, K-ras, and p53 gene mutations in colorectal cancer patients: correlation to clinicopathologic features and postoperative surveillance. Am Surg. 2005;71:336–343. [PubMed] [Google Scholar]
- 3.Calistri D, Rengucci C, Seymour I, Lattuneddu A, Polifemo AM, Monti F, Saragoni L, Amadori D. Mutation analysis of p53, K-ras, and BRAF genes in colorectal cancer progression. J Cell Physiol. 2005;204:484–488. doi: 10.1002/jcp.20310. [DOI] [PubMed] [Google Scholar]
- 4.Chandrasekharan NV, Simmons DL. The cyclooxygenases. Genome Biol. 2004;5:241. doi: 10.1186/gb-2004-5-9-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roelofs HM, Te Morsche RH, van Heumen BW, Nagengast FM, Peters WH. Over-expression of COX-2 mRNA in colorectal cancer. BMC Gastroenterol. 2014;14:1. doi: 10.1186/1471-230X-14-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chandrasekharan NV, Dai H, Roos KL, Evanson NK, Tomsik J, Elton TS, Simmons DL. COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: cloning, structure, and expression. Proc Natl Acad Sci U S A. 2002;99:13926–13931. doi: 10.1073/pnas.162468699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seibert K, Masferrer JL. Role of inducible cyclooxygenase (COX-2) in inflammation. Receptor. 1994;4:17–23. [PubMed] [Google Scholar]
- 8.Fosslien E. Biochemistry of cyclooxygenase (COX)-2 inhibitors and molecular pathology of COX-2 in neoplasia. Crit Rev Clin Lab Sci. 2000;37:431–502. doi: 10.1080/10408360091174286. [DOI] [PubMed] [Google Scholar]
- 9.Fosslien E. Molecular pathology of cyclooxygenase-2 in neoplasia. Ann Clin Lab Sci. 2000;30:3–21. [PubMed] [Google Scholar]
- 10.Greenhough A, Smartt HJ, Moore AE, Roberts HR, Williams AC, Paraskeva C, Kaidi A. The COX-2/PGE2 pathway: key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis. 2009;30:377–386. doi: 10.1093/carcin/bgp014. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto K, Arakawa T, Ueda N, Yamamoto S. Transcriptional roles of nuclear factor kappa B and nuclear factor-interleukin-6 in the tumor necrosis factor alpha-dependent induction of cyclooxygenase-2 in MC3T3-E1 cells. J Biol Chem. 1995;270:31315–31320. doi: 10.1074/jbc.270.52.31315. [DOI] [PubMed] [Google Scholar]
- 12.Ackerman WE 4th, Summerfield TL, Vandre DD, Robinson JM, Kniss DA. Nuclear factorkappa B regulates inducible prostaglandin E synthase expression in human amnion mesenchymal cells. Biol Reprod. 2008;78:68–76. doi: 10.1095/biolreprod.107.061663. [DOI] [PubMed] [Google Scholar]
- 13.Deng J, Miller SA, Wang HY, Xia W, Wen Y, Zhou BP, Li Y, Lin SY, Hung MC. beta-catenin interacts with and inhibits NF-kappa B in human colon and breast cancer. Cancer Cell. 2002;2:323–334. doi: 10.1016/s1535-6108(02)00154-x. [DOI] [PubMed] [Google Scholar]
- 14.Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 15.Sun SC. Non-canonical NF-kappaB signaling pathway. Cell Res. 2011;21:71–85. doi: 10.1038/cr.2010.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vallabhapurapu S, Karin M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu Rev Immunol. 2009;27:693–733. doi: 10.1146/annurev.immunol.021908.132641. [DOI] [PubMed] [Google Scholar]
- 17.Brantley DM, Chen CL, Muraoka RS, Bushdid PB, Bradberry JL, Kittrell F, Medina D, Matrisian LM, Kerr LD, Yull FE. Nuclear factor-kappaB (NF-kappaB) regulates proliferation and branching in mouse mammary epithelium. Mol Biol Cell. 2001;12:1445–1455. doi: 10.1091/mbc.12.5.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Plumpe J, Malek NP, Bock CT, Rakemann T, Manns MP, Trautwein C. NF-kappaB determines between apoptosis and proliferation in hepatocytes during liver regeneration. Am J Physiol Gastrointest Liver Physiol. 2000;278:G173–183. doi: 10.1152/ajpgi.2000.278.1.G173. [DOI] [PubMed] [Google Scholar]
- 19.O’Connell MA, Cleere R, Long A, O’Neill LA, Kelleher D. Cellular proliferation and activation of NF kappa B are induced by autocrine production of tumor necrosis factor alpha in the human T lymphoma line HuT 78. J Biol Chem. 1995;270:7399–7404. doi: 10.1074/jbc.270.13.7399. [DOI] [PubMed] [Google Scholar]
- 20.Wang B, Parobchak N, Rosen T. RelB/NFkappaB2 regulates corticotropin-releasing hormone in the human placenta. Mol Endocrinol. 2012;26:1356–1369. doi: 10.1210/me.2012-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suzuki S, Fukasawa H, Misaki T, Togawa A, Ohashi N, Kitagawa K, Kotake Y, Niida H, Hishida A, Yamamoto T, Kitagawa M. Upregulation of Cks1 and Skp2 with TNFalpha/NF-kappaB signaling in chronic progressive nephropathy. Genes Cells. 2011;16:1110–1120. doi: 10.1111/j.1365-2443.2011.01553.x. [DOI] [PubMed] [Google Scholar]
- 22.He D, Miao H, Xu Y, Xiong L, Wang Y, Xiang H, Zhang H, Zhang Z. MiR-371-5p facilitates pancreatic cancer cell proliferation and decreases patient survival. PLoS One. 2014;9:e112930. doi: 10.1371/journal.pone.0112930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jost PJ, Ruland J. Aberrant NF-kappaB signaling in lymphoma: mechanisms, consequences, and therapeutic implications. Blood. 2007;109:2700–2707. doi: 10.1182/blood-2006-07-025809. [DOI] [PubMed] [Google Scholar]
- 24.Morham SG, Langenbach R, Loftin CD, Tiano HF, Vouloumanos N, Jennette JC, Mahler JF, Kluckman KD, Ledford A, Lee CA, Smithies O. Prostaglandin synthase 2 gene disruption causes severe renal pathology in the mouse. Cell. 1995;83:473–482. doi: 10.1016/0092-8674(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 25.Sobolewski C, Cerella C, Dicato M, Ghibelli L, Diederich M. The role of cyclooxygenase-2 in cell proliferation and cell death in human malignancies. Int J Cell Biol. 2010;2010:215158. doi: 10.1155/2010/215158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joo YE, Kim HS, Min SW, Lee WS, Park CH, Park CS, Choi SK, Rew JS, Kim SJ. Expression of cyclooxygenase-2 protein in colorectal carcinomas. Int J Gastrointest Cancer. 2002;31:147–154. doi: 10.1385/IJGC:31:1-3:147. [DOI] [PubMed] [Google Scholar]
- 27.Peng L, Zhou Y, Wang Y, Mou H, Zhao Q. Prognostic significance of COX-2 immunohistochemical expression in colorectal cancer: a meta-analysis of the literature. PLoS One. 2013;8:e58891. doi: 10.1371/journal.pone.0058891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y, Dang J, Wang H, Allgayer H, Murrell GA, Boyd D. Identification of a novel nuclear factor-kappaB sequence involved in expression of urokinase-type plasminogen activator receptor. Eur J Biochem. 2000;267:3248–3254. doi: 10.1046/j.1432-1327.2000.01350.x. [DOI] [PubMed] [Google Scholar]
- 29.Fusco AJ, Huang DB, Miller D, Wang VY, Vu D, Ghosh G. NF-kappaB p52:RelB heterodimer recognizes two classes of kappaB sites with two distinct modes. EMBO Rep. 2009;10:152–159. doi: 10.1038/embor.2008.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sen R, Baltimore D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell. 1986;46:705–716. [PubMed] [Google Scholar]


