Abstract
WNT1 inducible signaling pathway protein 1 (WISP-1) is a member of the CCN family of growth factors and reported to possess an important role in tumorigenesis by triggering downstream events via integrin signaling. However, the exact role of WISP-1 in cancer remains unclear. In this study, we examined the expression pattern of WISP-1 at both mRNA and protein levels and evaluated the prognostic value of WISP-1 in pancreatic ductal adenocarcinoma (PDA). Expression of WISP-1 at mRNA level was upregulated in 17/24 tumor tissues compared to the matched adjacent non-tumor tissues and the result was confirmed by western blotting at protein level. Immunohistochemical staining of 194 pairs of PDA specimens suggested that high expression of WISP-1 is strongly correlated with clinical stage (P=0.003), T classification (P=0.008) and liver metastasis (P=0.012). Consistently, Kaplan-Meier survival curves indicated that patients with high expression of WISP-1 had a shorter survival time independent of clinical stage and lymphatic metastasis status. Moreover, univariate and multivariate analysis confirmed WISP-1 expression, age, classification and liver metastasis as independent prognostic factors for overall survival of PDA patients. Taken together, these results suggest that WISP-1 may serve as a potential prognostic biomarker for PDA.
Keywords: WISP-1, pancreatic ductal adenocarcinoma, prognosis
Introduction
Pancreatic ductal adenocarcinoma (PDA) is considered to be one of the most lethal malignancy, ranking the fourth leading cause of cancer death in the United States. Despite the development of surgical techniques and systemic treatment, the 5-year survival for pancreatic cancer increased slightly from 3% during the mid-1970s to 7% during 2004 to 2010 for all stage combined. The only potential hope for cure is surgical resection. However, approximately 53% of the patients are diagnosed at a distant stage with a 5-year survival of only 2% [1]. Therefore, new insights into the biology and genetics of pancreatic cancer are urgently needed and it is necessary to identify more biological markers to accurately predict the patient prognosis and to develop novel treatments.
WNT1 inducible signaling pathway protein 1 (WISP-1) also known as CCN4/ELM-1 is cysteine-rich, secreted matricellular protein [2]. WISP-1 is induced by Wnt-1 protein and reported to be a downstream target of Wnt-1 and β-catenin [3]. What is more, WISP-1 is a member of the CCN family of growth factors. The name of CCN is introduced from the acronym of the first three members of the family: cysteine-rich protein 61 (cyr61), connective tissue growth factor (CTGF) and nephroblastoma overexpressed gene (NOV). The CCN proteins share properties including stimulation of cell proliferation, migration, adhesion, and extracellular matrix formation as well as angiogenesis and tumorigenesis [4-6]. Deregulation of WISP-1 protein may result in various pathologies including osteoarthritis, fibrosis and cancer, while the exact role of WISP-1 in tumor genesis is controversial [7-9].
Since no direct evidence of WISP-1 in PDA has been reported, we try to determine the relationship between WISP-1 and PDA prognosis by exploring WISP-1 expression pattern and its potential clinical significance in PDA. In this retrospective study, we found that the high expression of WISP-1 is correlates with poor prognosis in PDA, providing a clue of the possible function of WISP-1 in the tumor malignancy, progression and prognosis.
Materials and methods
Patients and tissue microarray
Human PDA tissue microarrays are consist of 194 cases of tumor and matched adjacent tissue. All the specimens were obtained from the patients diagnosed with PDA, who had undergone surgical resection or biopsy between January 2002 and June 2014 in the Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, and China. An additional 24 paired freshly frozen PDA tissues and corresponding adjacent tissues were also obtained from Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, China. The histology and clinical stages were classified according to the seventh edition of the American Joint Committee on Cancer (AJCC) staging system. The follow-up time was calculated from the date of surgery to the date of death, or the last known follow-up. The cases of PDA were selected in this study only if clinical data were available. All the patients survived longer than 1 month after the surgery. None of recruited patients had received radiotherapy, chemotherapy, hormone therapy or other related anti-tumor therapies before surgery.
The study was approved by the Medical Ethics Committees of Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University and written consent was obtained from all donors. All methods were carried out in accordance with the approved guidelines of School of Medical graduate Shanghai Jiao tong University.
Immunohistochemical staining
The human PDA tissue microarrays were deparaffinized and rehydrated. The slides were incubated with 0.3% hydrogen peroxide for 30 minutes and then blocked with 10% BSA (Sangon, Shanghai, China). Slides were incubated by the antibody for WISP-1 (Proteintech, US) at 4°C overnight with optimal dilution, followed by labeled with HRP (rabbit) second antibody (Thermo Scientific, US) at room temperature for 1 hour, treated with DAB (3, 3’-diaminobenzidine solution) substrate liquid (Gene Tech, Shanghai), and then counterstained by hematoxylin. All the slides were observed and photographed with a microscope (Carl Zeiss, Germany).
Evaluation of immunohistochemical staining
To evaluate the expression of WISP-1 protein, we adopted a reproducible semiquantitative method that takes both the ratio of positive cells and staining intensity into consideration and scored as follows: 0, negative; +, weak homogenous cytoplasmic staining; ++, strong staining in <30% of tumor cells; +++, strong staining in >30% of tumor cells. 0 and + were defined as low expression of WISP-1; ++ and +++ as high expression of WISP-1 [10]. All of the slides were independently evaluated by two senior pathologists.
RNA extraction and real-time quantitative PCR
Total RNA from primary tumor and adjacent tissue samples was extracted using Trizol reagent (Takara, Japan), and reversely transcribed using a PrimeScript RT-PCR kit (Takara, Japan) according to the manufacturer’s instructions. Quantitative real-time PCR was performed using a 7500 Real-time PCR system (Applied Biosystems, Inc. USA). Primer sequences used for WISP-1 detection were as follows, forward: 5’-TGCTGTAAGATGTGCGCTCAG-3’; reverse: 5’-ACACTCCTATTGCGTACCTCG-3’. Expression data was normalized to the geometric mean of an 18S housekeeping gene. The 2-∆Ct method was used to quantify the relative WISP-1 expression levels and normalized using the β-actin expression.
Western blotting analysis
Western blotting was performed as previously described [11]. The WISP-1 antibody was purchased from Proteintech Inc. and species-specific secondary antibody was purchased from Cell Signaling, Beverly, MA. Bound secondary antibodies were detected by Odyssey imaging system (LI-COR Biosciences, Lincoln, NE).
Statistical analysis
Statistical analyses and graphical representations were performed by SPSS 16.0 (SPSS Inc.; Chicago, IL, USA) and GraphPad Prism 6 (San Diego, CA) software. We use Pearson’s Chi-square (χ2) test to analyze the correlations between WISP-1 expression and clinicopathologic parameters of patients with PDA. Kaplan-Meier method and log-rank test were used to evaluate survival differences. Cox proportional hazards regression model was performed to examine univariate and multivariate hazard ratios for the study variables that were dichotomized. Only significantly different variables in univariate analysis including WISP-1 expression level, Clinical stage, Age, N classification, Liver metastasis were entered into the next multivariate analysis. All statistical tests were two-sided and a P<0.05 was considered statistically significant.
Results
Expression levels of WISP-1 mRNA in PDA tissues
The transcriptional levels of WISP-1 were examined byreal-time quantitative PCR in 24 pairs of resected specimens (tumor tissues and patient-matched adjacent non-tumor tissues) from PDA patients. The result suggests that mRNA level of WISP-1 upregulated in the tumor tissues compared to the matched adjacent non-tumor tissues (P=0.0082) in 17/24 (70.83%) of the cases. And quantification of 8 pairs of representative tumor tissues and matched adjacent non-tumor tissues was shown in Figure 1.
Figure 1.
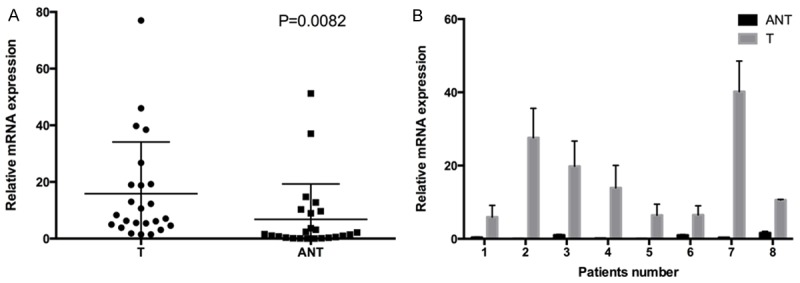
Expression of WISP-1 is increased in PDA tumors compared with adjacent non-tumor tissues at mRNA level. A. Increased WISP-1 mRNA expression in 24 matched tumor (T) and non-tumor tissue (ANT) was detected by RT-qPCR. B. Comparison of WISP-1 mRNA expression levels in eight representative pairs of PDA tissues (T) and matched adjacent non-tumorous tissues (ANT).
Expression levels of WISP-1 protein in PDA
WISP-1 protein levels were evaluated using Western blot and immunohistochemical staining (IHC). To verify the result of RT-qPCR, we detected the protein level of WISP-1 in 4 pairs of resected specimens with western blot and find that 4 PDA tissues displayed an increase in WISP-1 expression compared with the matched adjacent non-tumor tissues (Figure 2B and 2C).
Figure 2.
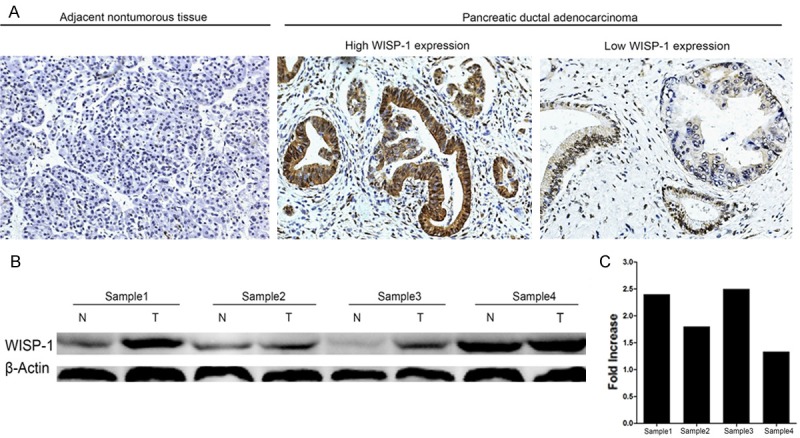
Expression of WISP-1 is elevated in PDA tumors compared with adjacent non-tumor tissues at protein level. A. Representative images of WISP-1 expression in PDA tumors and adjacent non-tumor tissues; B. The Western blots of WISP-1 expression in four pairs of PDA patients; C. Quantification of the western blots in four pairs of PDA patients.
Furthermore, WISP-1 expression in protein level was also detected in 194 cases of paired PDA samples by IHC. High expression of WISP-1 (++ or +++) was detected in 98/194 (50.52%) of tumor tissues, while only 63/194 (32.47%) in adjacent non-tumor tissues (Figure 2). The high expression rate of WISP-1 protein was significantly higher in tumor samples than that in non-tumor samples (P=0.000, χ2 test).
Relationship between WISP-1 expression and clinical parameters
In order to evaluate the clinical significance of WISP-1 expression in PDA, we used the Chi-square test to assess the correlations between levels of IHC staining and clinicopathologic parameters (age, gender, tumor size, tumor location, clinical stage, T classification, N classification, liver metastasis, vascular invasion and histological differentiation). We find that the IHC staining levels of WISP-1 in PDA tissues are strongly correlated with clinical stage (P=0.003), T classification (P=0.008) and liver metastasis (P=0.012). Whereas no significant association was observed between WISP-1 expression and other clinicopathologic parameters (Table 1).
Table 1.
Correlations between WISP-1 expression and clinicopathologic features in patients with pancreatic ductal adenocarcinoma (PDA)
| Clinicopathologic feature | Total 194 | Expression of WISP-1 | P value (χ2 test) | |
|---|---|---|---|---|
|
| ||||
| Low (n=95, 49.0%) | High (n=99, 51.0%) | |||
| Age (years) | ||||
| ≤65 | 103 | 48 (46.6) | 55 (53.4) | 0.483 |
| >65 | 91 | 47 (51.6) | 44 (48.4) | |
| Gender | ||||
| Male | 111 | 54 (48.6) | 57 (51.4) | 0.918 |
| Female | 83 | 41 (49.4) | 42 (50.6) | |
| Clinical stage (AJCC) | ||||
| I | 22 | 12 (54.5) | 10 (45.5) | 0.003 |
| II | 127 | 71 (55.9) | 56 (44.1) | |
| III | 33 | 11 (33.3) | 22 (66.7) | |
| IV | 12 | 1 (8.3) | 11 (91.7) | |
| Size | ||||
| ≤ 2 cm | 27 | 13 (48.1) | 14 (51.9) | 0.927 |
| >2 cm | 167 | 82 (49.1) | 85 (50.9) | |
| T classification | ||||
| T1 | 3 | 0 (0.0) | 3 (100.0) | 0.008 |
| T2 | 22 | 15 (68.2) | 7 (31.8) | |
| T3 | 130 | 68 (52.3) | 62 (47.7) | |
| T4 | 39 | 12 (30.8) | 27 (69.2) | |
| N classification | ||||
| Absent | 133 | 67 (50.4) | 66 (49.6) | 0.563 |
| Present | 61 | 28 (45.9) | 33 (54.1) | |
| Liver metastasis | ||||
| Absent | 181 | 93 (51.4) | 88 (48.6) | 0.012 |
| Present | 13 | 2 (15.4) | 11 (84.6) | |
| Vascular invasion | ||||
| Absent | 169 | 79 (46.7) | 90 (53.3) | 0.107 |
| Present | 25 | 16 (64.0) | 9 (36.0) | |
| Tumor location | ||||
| Head | 131 | 68 (51.9) | 63 (48.1) | 0.238 |
| Body/tail | 63 | 27 (42.9) | 36 (57.1) | |
| Histological differentiation | ||||
| Well | 11 | 6 (54.5) | 5 (45.5) | 0.703 |
| Moderate/poor | 183 | 89 (48.6) | 94 (51.4) | |
Values in parentheses indicate percentage values. The bold number represents the P-values with significant differences. AJCC staging is according to the 7th edition of the American Joint Committee on Cancer (AJCC) staging system.
Prognostic significance of WISP-1 in PDA patients
Patient survival analysis by Kaplan-Meier method and log-rank test demonstrates a clear correlation between WISP-1 protein expression level and the overall survival time of PDA patients (P=0.0013) (Figure 3). Patients with low expression of WISP-1 have a median survival of 19.03 months, while patients with high expression of WISP-1 only have a median survival of 9.67 months. We also evaluate the correlationship between WISP-1 expression and overall survival in PDA patients independent of clinical stages and status of lymphatic metastasis. All groups of Kaplan-Meier analysis indicate that the patients with low WISP-1 expression have a significantly longer overall survival time (Figure 4).
Figure 3.
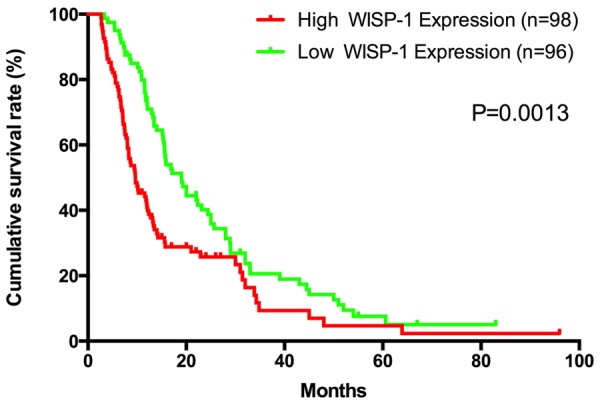
WISP-1 expression is associated with overall survival rate in PDA patients. Kaplan-Meier survival curves show high expression of WISP-1 was significantly correlated with poor survival of PDA. P-values were calculated by log-rank test.
Figure 4.
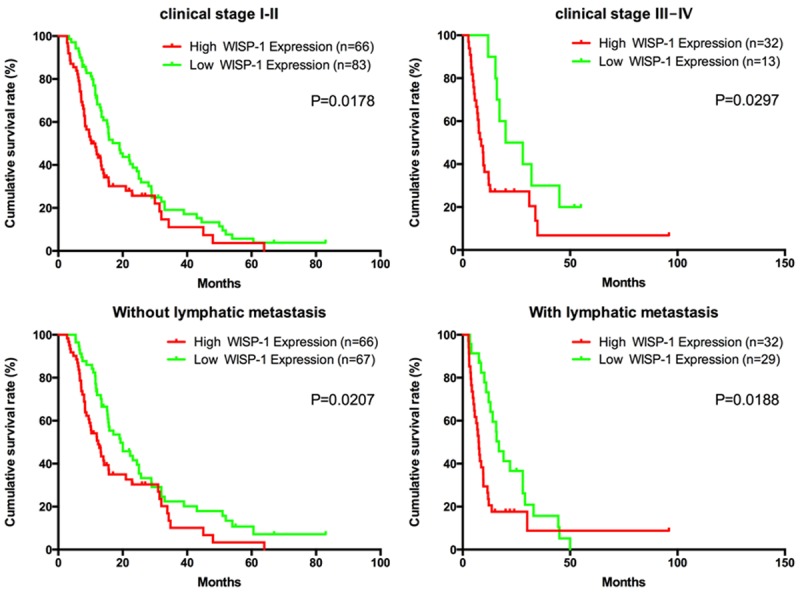
Correlation between WISP-1 expression and overall survival rate in PDA patients is independent of clinical stage and status of lymphatic metastasis. Patients with high expression of WISP-1 had a shorter survival time independent of clinical stage and status of lymphatic metastasis. P-values were calculated by log-rank test.
Furthermore, the univariate and multivariate analyses were performed to evaluate the impact of WISP-1 expression and other clinicopathologic parameters on prognosis in the 194PDA cases. The results demonstrate that the age (P=0.030), clinical stage (P=0.023), N classification (P=0.017), Liver metastasis (P=0.000) and WISP-1 expression (P=0.008) are significantly associated with overall survival. And then, results of the multivariate Cox regression analysis confirmed that age, classification, liver metastasis and WISP-1 expression are independent predictors of the overall survival in patients with PDA (Table 2).
Table 2.
Univariate and multivariate survival analysis of prognostic parameters in 194 cases of pancreatic ductal adenocarcinoma (PDA)
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Prognostic parameter | HR | 95% CI | P value | HR | 95% CI | P value |
| Expression of WISP-1 (low vs. high) | 1.563 | 1.125-2.171 | 0.008 | 1.594 | 1.133-2.242 | 0.007 |
| Age (≤65 vs. >65) | 1.437 | 1.037-1.991 | 0.030 | 1.754 | 1.242-2.478 | 0.001 |
| Gender (male vs. female) | 0.743 | 0.530-1.041 | 0.085 | - | - | - |
| Clinical stage (I vs. II vs. III vs. IV) | 1.329 | 1.040-1.700 | 0.023 | 1.005 | 0.750-1.348 | 0.973 |
| Size (≤2 cm vs. >2 cm) | 1.529 | 0.909-2.571 | 0.110 | - | - | - |
| T classification (T1 vs. T2 vs. T3 vs. T4) | 1.142 | 0.893-1.460 | 0.291 | - | - | - |
| N classification (absent vs. present) | 0.659 | 0.468-0.929 | 0.017 | 0.664 | 0.457-0.964 | 0.032 |
| Liver metastasis (absent vs. present) | 0.236 | 0.124-0.448 | 0.000 | 0.289 | 0.125-0.670 | 0.004 |
| Vascular invasion (absent vs. present) | 0.676 | 0.423-1.078 | 0.100 | - | - | - |
| Tumor location (head vs. body/tail) | 0.983 | 0.694-1.392 | 0.923 | - | - | - |
| Histology (well vs. moderate/poor) | 2.009 | 0.886-4.557 | 0.095 | - | - | - |
HR: Hazard ratio; CI: Confidence interval. The bold number represents the P-values with significant differences.
Discussion
Since the poor prognosis of PDA, great effort has been made on the investigation of genetic and molecular mechanism of tumorigenesis and tumor progression. It is accepted that PDA is characterized by gene alteration in 12 pathways, and Wnt/β-catenin signaling pathway act as a key determinant of tumor fate within the pancreas [12,13]. As a downstream target of Wnt-1 and β-catenin, WISP-1 can also activate Wnt/β-catenin pathway by phosphorylation and inactivation of GSK3β through activation of the Akt kinase, which eventuated in a strong positive feedback loop of WISP1 expression and Wnt/β-catenin signaling pathway [14]. Previous studies suggest that WISP-1 may possess an important role in tumorigenesis by triggering downstream events via integrin signaling.
High expression of WISP-1 is found in variety of cancers [9], our study demonstrated that WISP-1 expression upregulated in PDA tissues at both mRNA and protein level. Firstly we showed that WISP-1 was significantly elevated in 17/24 specimens at mRNA level in PDA tissues compared with their matched adjacent non-tumor tissues. Then, the overexpression of WISP-1 at protein level was confirmed by western blotting in 4 pairs of resected tissues. Finally, we found that tumor samples exhibited a higher expression pattern of WISP-1 by analyzing PDA tissue microarrays with IHC. Together, these data suggest that WISP-1 expression increased significantly in PDA tissues and may act as an oncogene.
Previous study has shown that prominent expression of WISP-1 is associated with an advanced stage of breast cancer at diagnosis [15]. By evaluating the relationship between WISP-1 expression and clinicopathologic parameters, we find that high expression of WISP-1 was strongly correlated with clinical stage, T classification and liver metastasis. Similar results have also been reported in colorectal cancer, lung cancer, esophageal cancer and cholangio carcinoma [16-19].
Our works further demonstrated that patients with higher expression of WISP-1 had a shorter survival time and confirmed that WISP-1 expression is an independent predictor of the overall survival in patients with PDA. The results consist with previous study, which recognized WISP-1 as an oncogene and demonstrated that WISP-1 attenuates p53-regulated apoptotic pathway in response to DNA damage [20]. However, Soon et al. demonstrated a negatively regulated pathway by WISP-1 involving integrins and Rac in the down-regulation of invasion [21]. Although, the exact role of WISP-1 is controversial and precise molecular mechanisms remain unclear, our results above imply that WISP-1 act as an oncogene in certain way and can be used as a novel prognostic marker for PDA patients.
In summary, we demonstrated that WISP-1 expression was increased in PDA and for the first time exhibited that high expression of this protein was correlated with poor prognosis in PDA patients. Based on these findings, we suggest that WISP-1 may be a potential prognostic biomarker for PDA.
Acknowledgements
This work was supprted by grant from science and technology funds of School of Medicine, Shanghai Jiao Tong University (14xJ10022).
Disclosure of conflict of interest
None.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Pennica D, Swanson TA, Welsh JW, Roy MA, Lawrence DA, Lee J, Brush J, Taneyhill LA, Deuel B, Lew M, Watanabe C, Cohen RL, Melhem MF, Finley GG, Quirke P, Goddard AD, Hillan KJ, Gurney AL, Botstein D, Levine AJ. WISP genes are members of the connective tissue growth factor family that are up-regulated in wnt-1-transformed cells and aberrantly expressed in human colon tumors. Proc Natl Acad Sci U S A. 1998;95:14717–14722. doi: 10.1073/pnas.95.25.14717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu L, Corcoran RB, Welsh JW, Pennica D, Levine AJ. WISP-1 is a Wnt-1- and beta-cateninresponsive oncogene. Genes Dev. 2000;14:585–595. [PMC free article] [PubMed] [Google Scholar]
- 4.Brigstock DR. The connective tissue growth factor/cysteine-rich 61/nephroblastoma overexpressed (CCN) family. Endocr Rev. 1999;20:189–206. doi: 10.1210/edrv.20.2.0360. [DOI] [PubMed] [Google Scholar]
- 5.Lau LF, Lam SC. The CCN family of angiogenic regulators: the integrin connection. Exp Cell Res. 1999;248:44–57. doi: 10.1006/excr.1999.4456. [DOI] [PubMed] [Google Scholar]
- 6.Perbal B. The CCN family of genes: a brief history. Mol Pathol. 2001;54:103–104. doi: 10.1136/mp.54.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perbal B, Brigstock D, Lau L. Report on the second international workshop on the CCN family of genes. Molecular Pathology. 2003;56:80. doi: 10.1136/mp.56.2.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stephens S, Palmer J, Konstantinova I, Pearce A, Jarai G, Day E. A functional analysis of Wnt inducible signalling pathway protein-1 (WISP-1/CCN4) J Cell Commun Signal. 2015:63–72. doi: 10.1007/s12079-015-0267-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gurbuz I, Chiquet-Ehrismann R. CCN4/WISP1 (WNT1 inducible signaling pathway protein 1): A focus on its role in cancer. The international journal of biochemistry & cell biology. 2015;62:142–146. doi: 10.1016/j.biocel.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 10.Nagai Y, Watanabe M, Ishikawa S, Karashima R, Kurashige J, Iwagami S, Iwatsuki M, Baba Y, Imamura Y, Hayashi N. Clinical significance of Wnt-induced secreted protein-1 (WISP-1/CCN4) in esophageal squamous cell carcinoma. Anticancer Res. 2011;31:991–997. [PubMed] [Google Scholar]
- 11.Li J, Yang XM, Wang YH, Feng MX, Liu XJ, Zhang YL, Huang S, Wu Z, Xue F, Qin WX, Gu JR, Xia Q, Zhang ZG. Monoamine oxidase A suppresses hepatocellular carcinoma metastasis by inhibiting the adrenergic system and its transactivation of EGFR signaling. J Hepatol. 2014;60:1225–1234. doi: 10.1016/j.jhep.2014.02.025. [DOI] [PubMed] [Google Scholar]
- 12.Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Kamiyama H, Jimeno A, Hong SM, Fu B, Lin MT, Calhoun ES, Kamiyama M, Walter K, Nikolskaya T, Nikolsky Y, Hartigan J, Smith DR, Hidalgo M, Leach SD, Klein AP, Jaffee EM, Goggins M, Maitra A, Iacobuzio-Donahue C, Eshleman JR, Kern SE, Hruban RH, Karchin R, Papadopoulos N, Parmigiani G, Vogelstein B, Velculescu VE, Kinzler KW. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801–1806. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heiser PW, Cano DA, Landsman L, Kim GE, Kench JG, Klimstra DS, Taketo MM, Biankin AV, Hebrok M. Stabilization of beta-catenin induces pancreas tumor formation. Gastroenterology. 2008;135:1288–1300. doi: 10.1053/j.gastro.2008.06.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang H, Luo H, Hu Z, Peng J, Jiang Z, Song T, Wu B, Yue J, Zhou R, Xie R, Chen T, Wu S. Targeting WISP1 to sensitize esophageal squamous cell carcinoma to irradiation. Oncotarget. 2015;6:6218–6234. doi: 10.18632/oncotarget.3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xie D, Nakachi K, Wang H, Elashoff R, Koeffler HP. Elevated levels of connective tissue growth factor, WISP-1, and CYR61 in primary breast cancers associated with more advanced features. Cancer Res. 2001;61:8917–8923. [PubMed] [Google Scholar]
- 16.Davies SR, Davies ML, Sanders A, Parr C, Torkington J, Jiang WG. Differential expression of the CCN family member WISP-1, WISP-2 and WISP-3 in human colorectal cancer and the prognostic implications. Int J Oncol. 2010;36:1129–1136. doi: 10.3892/ijo_00000595. [DOI] [PubMed] [Google Scholar]
- 17.Chen PP, Li WJ, Wang Y, Zhao S, Li DY, Feng LY, Shi XL, Koeffler HP, Tong XJ, Xie D. Expression of Cyr61, CTGF, and WISP-1 correlates with clinical features of lung cancer. PLoS One. 2007;2:e534. doi: 10.1371/journal.pone.0000534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagai Y, Watanabe M, Ishikawa S, Karashima R, Kurashige J, Iwagami S, Iwatsuki M, Baba Y, Imamura Y, Hayashi N, Baba H. Clinical significance of Wnt-induced secreted protein-1 (WISP-1/CCN4) in esophageal squamous cell carcinoma. Anticancer Res. 2011;31:991–997. [PubMed] [Google Scholar]
- 19.Tanaka S, Sugimachi K, Kameyama T, Maehara S, Shirabe K, Shimada M, Wands JR, Maehara Y. Human WISP1v, a member of the CCN family, is associated with invasive cholangiocarcinoma. Hepatology. 2003;37:1122–1129. doi: 10.1053/jhep.2003.50187. [DOI] [PubMed] [Google Scholar]
- 20.Su F, Overholtzer M, Besser D, Levine AJ. WISP-1 attenuates p53-mediated apoptosis in response to DNA damage through activation of the Akt kinase. Genes Dev. 2002;16:46–57. doi: 10.1101/gad.942902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soon LL, Yie TA, Shvarts A, Levine AJ, Su F, Tchou-Wong KM. Overexpression of WISP-1 down-regulated motility and invasion of lung cancer cells through inhibition of Rac activation. J Biol Chem. 2003;278:11465–11470. doi: 10.1074/jbc.M210945200. [DOI] [PubMed] [Google Scholar]


