Abstract
Bone marrow stroma plays a critical role in the bone metastasis of breast cancer. Bone marrow-derived mesenchymal stem cells (BMSC) are critical to facilitate cancer progression. Human bone morphogenetic protein 9 (BMP9) is the most potent osteogenic factor and one of bone-stored growth factors involved in both promotion and inhibition of different cancers. However, it is unclear whether BMP9 correlates with the bone metastasis of breast cancer. This study was to evaluate the role of BMP9 in the interaction between BMSC and breast cancer cells (BCC). To determine whether BMP9 is able to block the tumor promoting effect of BMSC, an in vitro model was developed using breast cancer MDA-MB-231 cells co-cultured with bone marrow-derived mesenchymal stem cells HS-5 with-BMP9 overexpression. The expressions of metastasis-related genes were detected to identify important factors mediating the role of BMP9 in breast cancer cells. Results showed BMP9 could inhibit invasion and promote apoptosis of MDA-MB-231 cells. The expressions of interleukin-6 (IL-6), matrix metalloproteinase-2 (MMP-2) and monocyte chemoattratctant protein-1 (MCP-1) decreased in the MDA-MB-231 cells of BMP9 over-expression group, and the expressions of epithelial-mesenchymal transition (EMT)-related molecules was also reduced. On the other hand, the expression of stromal cell derived factor-1 (SDF-1) decreased in HS-5 cells of BMP9 over-expression group. Taken together, BMP9 is able to inhibit the migration and promote the apoptosis of breast cancer by regulating the interaction between MDA-MB-231 cells and HS-5 cells in which SDF-1/CXCR4-PI3K pathway and EMT are involved.
Keywords: Bone morphogenetic protein 9, breast cancer, bone metastasis, SDF-1/CXCR4-PI3K pathway, epithelial-mesenchymal transition
Introduction
Bone is a common site of distant metastasis of many cancers including breast cancer [1-3]. Nearly all the patients with advanced breast cancer suffer from bone metastasis. The metastatic bone lesions are usually osteolytic and may cause pathological fracture, intractable bone pain, nerve compression and hypercalcemia [4,5]. Bone marrow-derived mesenchymal stem cells (BMSCs) in the breast cancer tissues provide a favorable microenvironment for the cancer cell growth [6]. The interaction between BMSCs and breast cancer cells (BCCs) has been found to be an important process in the bone metastasis and bone osteolysis in breast cancer. A better understanding of this interaction and the potential regulatory mechanism may be helpful to develop novel strategies for the cancer treatment.
There are a variety of growth factors stored in the bone including bone morphogenetic proteins (BMPs), transforming growth factor-β (TGFβ), fibroblast growth factors (FGFs), platelet-derived growth factors (PDGFs) and insulin-like growth factors (IGFs) [7]. These factor are also known as bone-stored growth factors or bone-derived growth factors. Among them, the contribution of TGF-β to breast cancer bone metastasis has been well understood. TGF-β elevate the parathyroid hormone-related protein (PTHrP) production in the breast cancer cells through the Smad signaling pathway. Then, PTHrP enhances osteoclastogenesis and bone destruction through up-regulating osteoclast-activating factors [8]. However, the functions and molecular mechanisms of other bone-stored growth factors are poorly understood.
Bone morphogenetic protein 9 (BMP9), a bone-stored growth factor, has been confirmed as the most effective BMP in the bone formation. BMP9 has been implicated in the proliferation and metastasis of both prostate and ovarian cancer cells [9,10]. In our previous studies, results showed that BMP9 was able to inhibit the proliferation and metastasis of breast cancer cells [11]. However, the effects of BMP9 on the breast cancers cells in bone microenvironment remain unclear.
The present study aimed to investigate the role of BMP9 in the regulation of interaction between BMSCs and BCCs. Tumor microenvironment was simulated by co-culturing MDA-MB-231 cells (BCCs) and HS-5 cells (BMSCs) in vitro, and then overexpressed BMP9 by AdBMP9 in this co-culture system. Our results showed that BMP9 was able to inhibit the invasion of MDA-MB-231 cells though blocking the SDF-1/CXCR4-PI3K axis and reversing the epithelial-mesenchymal transition (EMT). Metastasis related factors including interleukin-6 (IL-6), monocyte chemotactic protein-1 (MCP-1) and matrix metalloproteinases 9 (MMP9) also decreased in BMP9 over-expression group. These findings suggest that BMP9 may serve as a therapeutic target for treatment of breast cancer bone metastasis.
Materials and methods
Cell culture and preparation of recombinant adenovirus
Human breast cancer MDA-MB-231 cells and bone marrow-derived mesenchymal stem cells HS-5 were purchased from the China Center for Type Culture Collection (CCTCC) and the American Type Culture Collection (ATCC), respectively. MDA-MB-231 cells and HS-5 cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal bovine serum (GIBCO, USA) and 1% penicillin-streptomycin solution at 37°C in a humidified atmosphere containing 5% CO2. Recombinant adenoviruses expressing green fluorescent protein and BMP9 (AdGFP, AdBMP9), were kindly provided by Professor TC He (Chicago University, USA), and all the recombinant adenoviruses were amplified in HEK293 (CCTCC) cells before use [12].
Indirect co-culture
MDA-MB-231 cells and HS-5 cells were co-cultured in a transwell culture system (0.4-μm pore size; Millipore). MDA-MB-231 cells (1.3×105 cells/well) and HS-5 cells (1×105 cells/chamber) were seeded into six-well culture plates and culture chamber, respectively. On the following day, MDA-MB-231 cells were infected with AdBMP9 or AdGFP. In Neu-BMP9 group, Neu-BMP9 was added simultaneously. Eight hours later, the medium was refreshed with serum-free DMEM. Then, the inserts were transferred into six-well plates and two kinds of cells were co-cultured [13].
Wound healing assay
On day 0, a wound was created at the center of monolayer MDA-MB-231 cells using a pipette tip followed by co-culturing. Wound healing was observed under a microscope, and images were captured at 0, 24 and 48 h after the wound was made. The wound-healing rate was calculated as: (width at 0 h-width at 48 h)/ width at 0 h ×100%.
Transwell invasion assays
Transwell invasion assay was conducted as previously described [14]. MDA-MB-231 cells (4×104 cells/0.4 ml) were added to the upper chamber, and DMEM from the co-culture system with 10% FBS was added to the lower chamber as a chemoattractant. After 24 h, cells were dried for 5 min, fixed in dehydrated alcohol, and stained with crystal violet solution. Finally, the transmembrane cells were counted under a microscope at 200×. Means were obtained from five randomly selected fields in each well. The experiment was conducted at least thrice.
Detection of apoptosis by flow cytometry
Briefly, MDA-MB-231 cells were collected after 3 days of co-culture. Cells were washed twice with ice-cold phosphate buffered saline (PBS; pH 7.4) and re-suspended. Apoptotic cells were detected by flow cytometry. Each experiment was performed at least thrice.
RNA extraction and semi-quantitative RT-PCR
Total RNA was independently extracted from MDA-MB-231 cells and HS-5 cells using TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. First-strand cDNA was synthesized using the Reverse Transcriptase M-MLV (RNase H-) kit with random hexamer primers. β-actin was used as an internal control. Real-time RT-PCR was performed using the CFX Connect™ Real-Time System (Bio-Rad) and the SYBR Prime-Script RT-PCR kit (Perfect Real Time), according to the manufacturers’ instructions. Data were analyzed with the 2-∆∆Ct method. Primers used for PCR are shown in Table 1.
Table 1.
Sequences of primers and product length
| Gene | Sequences of primers | Product length/bp | |
|---|---|---|---|
| β-actin | Forward | 5’-CACCACACCTTCTACAATGAGC-3’ | 700 |
| Reverse | 5’-GTGATCTCCTTCTGCATCCTGT-3’ | ||
| GAPDH | Forward | 5’-CAGCGACACCCACTCCTC-3’ | 120 |
| Reverse | 5’-TGAGGTCCACCACCCTGT-3’ | ||
| BMP9 | Forward | 5’-CTGCCCTTCTTTGTTGTCTT-3’ | 322 |
| Reverse | 5’-CCTTACACTCGTAGGCTTCATA-3’ | ||
| SDF-1 | Forward | 5’-TGAGCTACAGATGCCCATGC-3’ | 178 |
| Reverse | 5’-TTCTCCAGGTACTCCTGAATCC-3’ | ||
| IL-6 | Forward | 5’-TAGTGAGGAACAAGCCAGAG-3’ | 234 |
| Reverse | 5’-TACATTTGCCGAAGAGCC-3’ | ||
| MCP-1 | Forward | 5’-ATGAAAGTCTCTGCCGCCCTT-3’ | 300 |
| Reverse | 5’-TCAAGTCTTCGGAGTTTGGGT-3’ | ||
Western blot assay
After 3 days of co-culture, proteins were independently extracted from MDA-MB-231 cells and HS-5 cells. Briefly, cells were collected, lysed in RAPI buffer and then centrifuged at 500×g for 15 min at 4°C. The supernatant was collected and protein concentration was determined with BCA (bicinchoninic acid) assay. Proteins of the lysate were separated in 10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred onto PVDF membranes. Membranes were blocked with 5% bovine serum albumin at room temperature for 2 h and incubated with primary antibodies: mouse anti-β-actin monoclonal antibody (Santa Cruz, sc47778, 1:1000), rabbit anti-BMP9 polyclonal antibody (Santa Cruz, sc-130703, 1:1000), rabbit anti-CXCR4 monoclonal (Abcam, #3108-1, 1:1000), rabbit anti-SDF-1 polyclonal antibody (Santa Cruz, sc-28876, 1:1000), rabbit anti-Akt monoclonal (CST, #4691, 1:1000), rabbit Anti-P-Akt monoclonal (CST, #9271, 1:1000), MMP2 (Bioworld, BS1236, 1:1000), MMP9 (Immunoway, YT1892, 1:1000), Vimentin (Bioworld, BS1491, 1:1000), Fibronectin (Bioworld, BS1644, 1:1000) at 4°C overnight. After washing thrice with TBST, the membranes were incubated with secondary antibody (IgG) for 1 h. Then, proteins of interest were visualized by enhanced chemoluminescence (Millipore Corporation, Billerica, MA, USA) in substrate using the Quantity One Software (BIO-RAD, USA).
Gelatin zymography
The activities of MMPs in the conditioned medium were determined by gelatin zymography as previously described [15]. Briefly, the conditioned medium of co-culture system was collected and centrifuged at 1000 rpm for 15 min at 4°C. Protein concentration was determined by the BCA assay, and then 30 μg of total protein was mixed with SDS buffer without β-mercaptoethanol. Proteins were separated by 10% SDS-polyacrylamide gel electrophoresis. The membranes were washed in 2.5% Triton X-100, incubated for 36 h at 37°C in 200 mM NaCl containing 40 mM Tris-HCl and 10 mM CaCl2 (pH 7.6) and finally stained with Coomassie Blue R-250 for 30 min followed by rinsing repeatedly. The gelatinolytic or fibrinolytic activities were determined by measuring the optical density.
Immunofluorescence staining
After 3 days of co-culture in chamber coverslips, MDA-MB-231 cells were fixed with 4% paraformaldehyde for 15 min. Immunohistochemistry was performed using antibodies as previously described: CXCR4 (1:100), vimentin (1:100) and fibronectin (1:100). Images were captured under a fluorescence microscope at 200×.
In vivo tumorigenesis assays
All experiments were approved by the Institutional Animal Care and Use Committee of the Chongqing Medical University and regional authorities. Four-week-old female nude mice (Balb/c, Beijing, China) were housed under specific pathogen-free conditions. MDA-MB-231 cells were infected with AdBMP9 or control vectors in vitro. Mice in each group were implanted subcutaneously with MDA-MB-231 cells (1×107 cells) mixed with HS-5 cells (1×107 cells). After 2 weeks, when tumors were observable, the tumor diameters were measured once every 3 days with a caliper. Mice were sacrificed after 1 month, tumors were collected, paraffin-embedded, and stained with hematoxylin-eosin (H-E).
Immunohistochemistry
Paraffin-embedded tumor sections were prepared following conventional methods. The expressions of CXCR4, SDF, vimentin and fibronectin were detected by immunohistochemistry (IHC). In brief, sections were re-hydrated, heat-treated in citric acid buffer for antigen retrieval and then incubated with primary antibodies at 4°C overnight. On the following day, sections were incubated with secondary antibody and visualized with 3,3-diaminobenzidine tetrachloride (DAB). The sections were finally counterstained with Gill’s hematoxylin, mounted and coverslipped.
TUNEL staining procedure
Apoptosis cells in the tumors were determined by TUNEL-staining (400×). Paraffin-embedded sections were processed as in immunohistochemistry, incubated with proteinase K Sol for 15 min, and then rinsed thrice in PBS. These sections were incubated with the TUNEL reaction mix (5 µl of enzyme solution: 45 µl of labeling solution per section) for 1 h at 37°C and rinsed thrice in PBS. Then, POD was added, followed by incubation for 30 min at 37°C. After rinsing thrice in PBS and visualization with diaminobenzidine, these sections were finally counterstained with Gill’s hematoxylin, mounted, and coverslipped. Sections in positive control group were treated with DNase I, and those in negative control group treated as described above except replacing TUNEL reaction mixture with Fluorescein-12-dUTP. The apoptosis index (AI) was calculated as follow: (TUNEL positive cells/total tumor cells)×100%.
Statistical analysis
Data are expressed as means ± standard deviation (SD). All the statistical analyses were performed with SPSS 17.0 by using the independent sample Student’s t–test between two groups. A value of P<0.05 was considered statistically significant.
Results
Effects of BMP9 on breast cancer cells and the metastasis related factors of both cells in the co-culture system
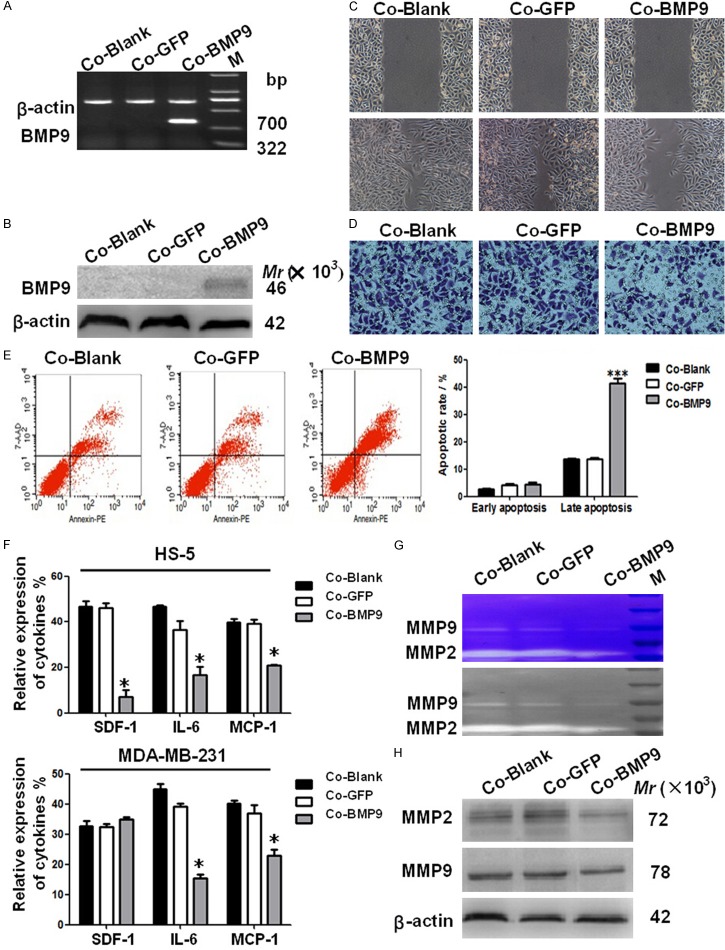
BMP9 is the most effective one for the bone formation. In the present study, MDA-MB-231 cells with BMP9 over-expression were co-cultured with HS-5. Then, the roles of BMP9 were investigated in breast cancer which frequently causes osteolytic metastasis. The migration, invasion and apoptosis of MDA-MB-231 cells with BMP9 over-expression were determined in the co-culture system. These biological behaviors of breast cancer cells are known to play significant roles in the osteolytic metastasis [16]. RT-PCR and western blot assay indicated that BMP9 mRNA and protein expression increased significantly in MDA-MB-231 cells after trasnfection (Figure 1A, 1B). BMP-9 over-expression significantly decreased the wound healing rate from 84.67±4.67% to 46.33±2.87% (P<0.05) and the invasive cells from 191.33±3.21 to 112.0±3.0 (P<0.05) (Figure 1C, 1D). BMP9 over-expression also increased of the AI MDA-MB-231 cells (P<0.001) (Figure 1E). However, there were no significant differences in above parameters between AdGFP group and blank control group. These results suggest that BMP9 is able to inhibit the migration and increase the apoptosis of MDA-MB-231 cells in the co-culture system.
Figure 1.
Effects of BMP9 over-expression on the biobehaviors of breast cancer cells and the metastasis related proteins in the co-culture system. A, B. BMP9 mRNA and protein expressions in MDA-MB-231 cells transfected with AdBMP9. BMP9 expressions were normalized to those of β-actin. C. Cell migration was evaluated by the wound healing assay in MDA-MB-231 cells (×100; P<0.05 vs control groups). D. The migration of MDA-MB-231 cells was evaluated by the transwell migration assay (×200; P<0.05 vs control groups). E. Apoptosis rate of MDA-MB-231 cells in the co-culture system was determined by flow cytometry. The number of late apoptotic cells in the presence of BMP9 over-expression significantly increased when compared with control groups (**P<0.01). F. mRNA expressions of SDF-1, IL-6 and MCP-1 were detected in MDA-MB-231 cells and HS-5 cells. The expressions of IL-6 and MCP-1 decreased in both MDA-MB-231 cells with BMP-9 over-expression and HS-5 cells, but the SDF-1 expression decreased in only HS-5 cells (*P<0.05 vs control groups). G. MMPs activity in the conditioned medium collected from the co-culture system was determined by zymographic analysis. H. MMP-2 and MMP-9 protein expressions were detected by Western blot assay. (*P<0.05, vs control groups). Co-Blank: MDA-MB-231+HS-5; Co-GFP: MDA-MB-231/GFP+HS-5; Co-BMP9: MDA-MB-231/BMP9+HS-5.
Metastasis related factors are critical for the osteolytic metastasis of breast cancer [17]. The expressions of metastasis related proteins such as IL-6, IL-8, bone sialoprotein (BSP), stromal cell derived factor-1 (SDF-1), C-X-C chemokine 4 (CXCR4), MCP-1 and MMPs were also measured. RT-PCR showed that BMP-9 over-expression in the co-culture system decreased IL-6 and MCP-1 expressions in HS-5 cells and MDA-MB-231 cells. While, SDF-1 expression decreased only in HS-5 cells (Figure 1F). No significant differences were observed in the expressions of IL-8, BSP, and CXCR4 (data not shown). Cell metastasis is highly related to the proteolytic activity of MMPs, which may regulate the extracellular matrix (ECM)-cell and intercellular interactions during the invasion [18]. To determine the effects of BMP9 on the MMP activity, the enzymatic activity of MMPs was measured by zymographic analysis. Results showed MMP-9 and MMP-2 activities decreased in BMP-9 over-expression group (Figure 1G). BMP9 over-expression also markedly reduced the protein expressions of MMP-2 and MMP9 (Figure 1H). These results indicate that BMP9 in the co-culture system can down-regulate the expressions of metastasis related factors including SDF-1, IL-6 and MCP-1. Meanwhile, the protein expressions and activities of MMP2/MMP9 also reduced in BMP-9 over-expression group.
EMT markers were decreased and SDF-1/CXCR4-PI3K pathway was blocked following BMP9 over-expression in the co-culture system
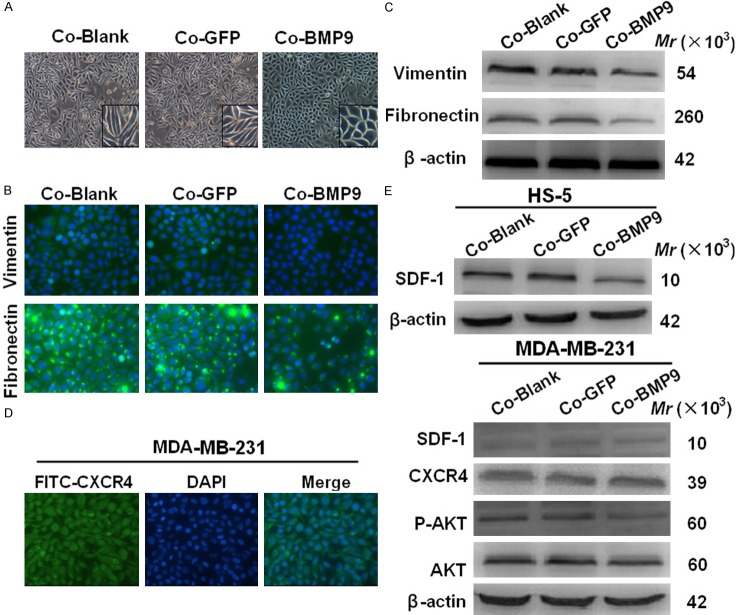
Epithelial-mesenchymal transition (EMT) has been regarded as a mechanism underlying the malignant progression of breast cancer. MDA-MB-231 cells have been found as in a state of “partial EMT” with invasive behavior [19]. Interestingly, our results showed BMP9 reversed the “partial EMT” of MDA-MB-231 cells. MDA-MB-231 cells in BMP9 over-expression group were cuboidal, a typical characteristic of epithelium, but cells in control group were fibroblast-like (Figure 2A). Immunofluorescence staining further revealed that the expressions of mesenchymal markers (vimentin and fibronectin) reduced in the presence of BMP-9 over-expression in the co-culture system (Figure 2B). Western blot assay showed vimentin and fibronectin expressions also significantly decreased in BMP9 over-expression group (Figure 2C). These results suggest that BMP-9 over-expression incompletely restores the epithelial characteristics of MDA-MB-231 cells in the co-culture system.
Figure 2.
EMT markers were decreased and SDF-1/CXCR4-PI3K pathway was blocked following BMP9 over-expression in the co-culture system. A. MDA-MB-231 cells of BMP9 over-expression group morphological changes compared with the control group (×100). B. BMP-9 over-expression reduced vimentin and fibronectin expressions in MDA-MB-231 cells in the co-culture system (immunofluorescence staining; ×400). C. Western blot assay showed BMP-9 decreased vimentin and fibronectin expressions in cultured MDA-MB-231 cells (*P<0.05, vs control groups). D. The expression of chemokine receptor CXCR4 in breast cancer MDA-MB-231 cells was detected by immunofluorescence staining (400×). E. Key molecules of SDF-1/CXCR4-PI3K signaling pathway. BMP-9 blocked SDF-1/CXCR4-PI3K pathway by down-regulating the ligand, SDF-1, of CXCR4 and its downstream molecule p-Akt (*P<0.05, vs control group). Co-Blank: MDA-MB-231+HS-5; Co-GFP: MDA-MB-231/GFP+HS-5; Co-BMP9: MDA-MB-231/BMP9+HS-5.
To explore the mechanism by which BMP9 inhibits the biobehaviors of breast cancer cells, the SDF-1/CXCR4 axis and EMT, factors responsible for the tumor progression, were investigated. Immunofluorescence staining showed CXCR4 expression was detectable in MDA-MB-231 cells (Figure 2D), but CXCR4 protein expression remained unchanged as shown in western blot assay (Figure 2E). Then, the effects of BMP9 on the SDF-1/CXCR4-PI3K pathway were studied, and results indicated that the expressions of SDF-1 in HS-5 cells, and its downstream molecules (Akt and p-Akt) in MDA-MB-231 cells markedly decreased in BMP9 over-expression group (Figure 2E).
BMP9 inhibited tumor behavior through blocking the SDF-1/CXCR4-PI3K pathway and incompletely reversing the EMT of breast cancer cells in the co-culture system
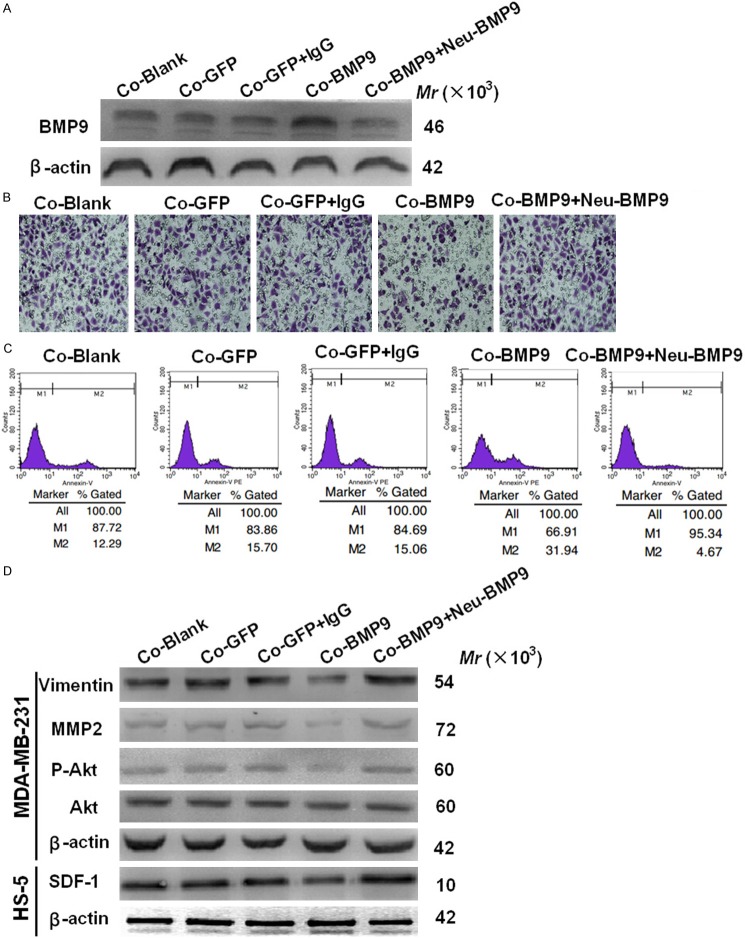
The BMP9 was neutralized to further verify our findings. Firstly, the transfection efficiency of AdBMP9 and Neu-BMP9 was confirmed by western blot assay (Figure 3A). Transwell invasion assays showed cell invasiveness was restored and the apoptosis of MDA-MB-231 cells decreased after BMP-9 neutralization. (Figure 3B, 3C). Akt is a down-stream molecule of SDF-1/CXCR4-PI3K signaling pathway and widely recognized as a migration-promoting factor. Western blot assay showed BMP9 suppressed the SDF-1 expression and the p-Akt activation, whereas BMP9 neutralization increased p-Akt activation (Figure 3D). Western blot assay showed the expressions of MMP2 (a metastasis related factor) and vimentin (an EMT marker) increased in BMP9 neutralization group (Figure 3D).
Figure 3.
Effects of BMP9 neutralization on the invasion, apoptosis and expressions of metastasis-associated molecules in MDA-MB-231 cells. A. BMP9 protein expression in the medium was detected by western blot assay. B. The invasiveness of MDA-MB-231 cells was examined after incubation for 24 h. (*P<0.05 vs control cells; ×400). C. Apoptosis rate of MDA-MB-231 cells in the co-culture system was detected by flow cytometry. The number of apoptotic cells decreased in BMP9 neutralization group when compared with control groups (*P<0.05). D. Effects of BMP9 on MMP2, key molecules of EMT, and PI3K-Akt signaling pathway (*P<0.05 vs control cells). Co-Blank: MDA-MB-231+HS-5; Co-GFP: MDA-MB-231/GFP+HS-5; Co-GFP: MDA-MB-231/GFP+HS-5+IgG; Co-BMP9: MDA-MB-231/BMP9+HS-5; Co-BMP9: MDA-MB-231/BMP9+HS-5+Neu-BMP9.
BMP9 promoted apoptosis and reduced the expression of metastasis related protein in vivo
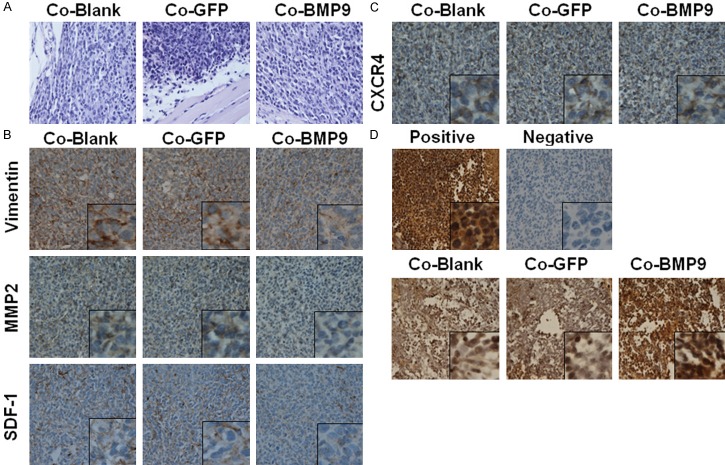
To investigate the effects of BMP9 in vivo, MDA-MB-231 cells were transfected with AdBMP9 and control vector AdGFP. Then, these cells were mixed with HS-5 cells at a ratio of 1:1, and subcutaneously implanted into nude mice. Mice were sacrificed 1 month later. Hematoxylin-eosin staining of tumor sections showed that the cell morphology was similar among groups (Figure 4A). Immunohistochemistry revealed that vimentin, MMP2 and SDF-1 showed a weak expression in BMP9 over-expression group (Figure 4B), and there was no significant difference in the CXCR4 expression among groups (Figure 4C). The effect of BMP9 on the apoptosis of cancer cells was investigated by TUNEL staining (Figure 4D). The AI in BMP9 over-expression group increased from 25.34±3.83% to 37.0±5.53% (P<0.05). These results indicate that the SDF-1/CXCR4 axis and EMT may be involved in the BMP9 induced inhibition of MDA-MB-231 cells metastasis in the co-culture system.
Figure 4.
Effects of BMP9 on MDA-MB-231 cells in vivo. A. Hematoxylin-eosin staining in different groups. There were no significant differences among groups. B, C. The expressions of metastasis-associated molecules (vimentin, MMP2, SDF-1 and CXCR4) in MDA-MB-231 cells transfected with AdBMP9 were determined by immunohistochemistry. D. TUNEL staining of different groups. The apoptosis index increased in MDA-MB-231 cells with BMP-9 over-expression (×400; *P<0.05 vs control cells). Co-Blank: MDA-MB-231+HS-5; Co-GFP: MDA-MB-231/GFP+HS-5; Co-BMP9: MDA-MB-231/BMP9+HS-5.
Discussion
Cancers including breast cancer are not self-sustaining entities and may constantly interact with their microenvironment [20]. As an important component of the tumor microenvironment, BMSCs can interact with BCCs in both primary and bone metastatic cancers. BCCs may recruit BMSCs to the primary site in the early stage of breast cancer [6]. Growing evidence shows that the interaction between BMSCs and cancer cells plays a critical role in the breast cancer metastasis [21]. Their interaction is crucial for the progress of the tumor in the early stage of breast cancer and may facilitate the bone damage in the late stage of breast cancer [22,23]. Thus, microenvironmental interactions are potential targets for the treatment of breast cancer.
BMP9, also known as growth differentiation factor 2 or GDF-2, has been implicated in the growth, adhesion, invasion, and migration of many types of cancers including breast cancer. BMP9 may promote the growth of ovarian cancer cells through the BMP/SMAD pathway, while inhibit the growth and enhance the apoptosis of prostate cancer PC-3 cells through the non-classical BMP/SMAD pathway [9,10]. Our previous studies demonstrated that BMP9 inhibited invasiveness of MDA-MB-231 breast cancer cells [11]. BMP9, the most potent osteogenic factor, may represent a more effective strategy for the augmentation of bone regeneration than other BMPs currently used in clinical practice [24]. As a secretary protein, BMP9 is a key bone associated molecule in the bone marrow. However, the role of BMP9 in the bone metastasis of breast cancer remains unclear. In our previous studies, results showed that BMP9 reduced the RANKL secretion of HS-5 cells to inhibit the invasiveness of MDA-MB-231 cells by blocking the AKT signaling pathway.
In the present study, BMP9 over-expression was introduced in a tumor microenvironment simulated by co-culture of MDA-MB-231 cells (BCC) and HS-5 cells (BMSC). Results demonstrated that BMP9 exerted significant inhibitory effects on the migration and invasion of MDA-MB-231 cells. Moreover, the apoptotic cells in BMP9 over-expression group also increased. Metastasis related proteins including IL-6, MCP-1, SDF-1 and MMPs significantly decreased. Studies have shown that IL-6 is closely related to the activation of osteoclasts in breast cancer [25], and MCP-1 can recruit breast cancer cells to the bone [26]. Therefore, the reduced IL-6 and MCP-1 expression following BMP9 over-expression attenuates bone damage. However, the mechanism underlying the BMP9 mediated regulation of these metastasis related factors is required to be further investigated. The responses to BMP9 vary between cancers. Studies have reported the opposite roles of BMP9 in the estrogen-related ovarian cancer and prostate cancer [9,10]. Our results indicated that BMP9 exerted inhibitory effects on the breast cancer, another estrogen-related malignancy. The inhibitory effect of BMP9 was also confirmed in the breast cancer MDA-MB-231 cells and SK-BR-3 cells [11,27]. The tumorigenic and anti-tumorigenic roles of BMP9 reflect its complicated interactions in tumor developmental processes. Thus, the biological responses of cancer cells to BMP9 depend not only on the dosage, cell type and tumor microenvironment, but other factors not yet defined.
It is well known that EMT is crucial for the breast cancer metastasis. More recently, the hanced expression of BMP family members has been implicated in the induction of EMT [28,29]. Our results showed MDA-MB-231 cells with BMP9 over-expression presented changed cell morphology. The “partial EMT” cells became typically cuboidal. In addition, BMP9 down-regulated the expressions of EMT markers (vimentin and fibronectin) in MDA-MB-231 cells, and the opposite findings were observed in neu-BMP9 group. However, there was no significant difference in the E-cadherin expression (data not shown). These findings were consistent with previous results on BMP2 [30]. Taken together, these results demonstrate that BMP9 over-expression can incompletely reverse EMT in a co-culture system.
SDF-1, also known as CXCL12, is highly expressed in the bone marrow stromal cells. Different from normal mammary epithelial cells, BCCs express the unique receptor, CXCR4, of SDF-1. Then, the effect of BMP9 on the SDF-1/CXCR4 axis was further investigated. BMP9 decreased SDF-1 secretion in HS-5 and had no effect on the CXCR4 expression in MDA-MB-231 cells. The expression of its down-stream molecule p-Akt also reduced in MDA-MB-231 cells. However, SDF-1 and p-Akt expressions increased after BMP9 silencing. Thus, these findings indicate that BMP9 may block the SDF-1/CXCR4-PI3K pathway to inhibit the migration of cancer cells in a co-culture system. The roles of BMP9 in the co-culture system (blocking SDF-1/CXCR4-PI3K pathway and reversing EMT of breast cancer cells) are illustrated in Figure 5.
Figure 5.
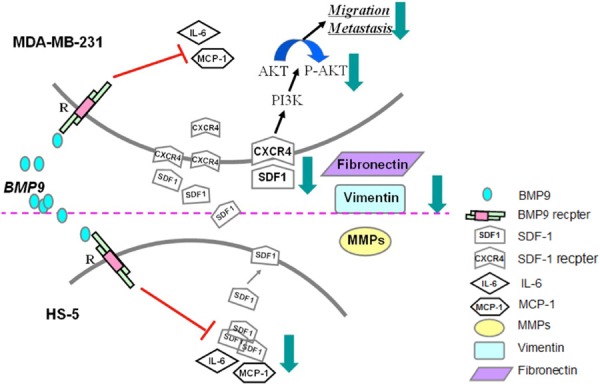
Diagram of BMP9’s effects in the co-culture system.
In vivo experiments showed BMP9 reduced the expressions of key molecules of EMT (vimentin) and SDF-1/CXCR4 axis (SDF-1). TUNEL staining indicated BMP9 induced the apoptosis of MDA-MB-231 cells, which was consistent with findings from in vitro experiments. While, there was no significant difference between BMP9 silencing group and control group. This may be related to the low endogenous expression of BMP9 in MDA-MB-231 cells.
In conclusion, our findings suggest that BMP9 can inhibit the migration and invasion, as well as promote the apoptosis of breast cancer cells in the simulated tumor microenvironment. The expressions of metastasis related factors (SDF-1, MMPs, vimentin, fibronectin, IL-6, MCP-1) decrease following BMP9 over-expression, while BMP9 neutralization exerts opposite effects. SDF-1/CXCR4-PI3K pathway and EMT may be involved in this process. The inhibitory effects of BMP9 are also confirmed in vivo. On the basis of our findings, BMP9 may serve as novel strategy for the therapy of breast cancer, but more studies are required to confirm our findings in clinical trials.
Disclosure of conflict of interest
None.
References
- 1.Weilbaecher KN, Guise TA, McCauley LK. Cancer to bone: a fatal attraction. Nat Rev Cancer. 2011;11:411–425. doi: 10.1038/nrc3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roodman GD. Mechanisms of bone metastasis. N Engl J Med. 2004;350:1655–1664. doi: 10.1056/NEJMra030831. [DOI] [PubMed] [Google Scholar]
- 3.Yoneda T, Hiraga T. Crosstalk between cancer cells and bone microenvironment in bone metastasis. Biochem Biophys Res Commun. 2005;328:679–687. doi: 10.1016/j.bbrc.2004.11.070. [DOI] [PubMed] [Google Scholar]
- 4.Mundy GR. Mechanisms of bone metastasis. Cancer. 1997;80:1546–1556. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1546::aid-cncr4>3.3.co;2-r. [DOI] [PubMed] [Google Scholar]
- 5.Coleman RE. Skeletal complications of malignancy. Cancer. 1997;80:1588–1594. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1588::aid-cncr9>3.3.co;2-z. [DOI] [PubMed] [Google Scholar]
- 6.Yan XL, Fu CJ, Chen L, Qin JH, Zeng Q, Yuan HF, Nan X, Chen HX, Zhou JN, Lin YL, Zhang XM, Yu CZ, Yue W, Pei XT. Mesenchymal stem cells from primary breast cancer tissue promote cancer proliferation and enhance mammosphere formation partially via EGF/EGFR/Akt pathway. Breast Cancer Res Treat. 2012;132:153–164. doi: 10.1007/s10549-011-1577-0. [DOI] [PubMed] [Google Scholar]
- 7.Yoneda T, Sasaki A, Mundy GR. Osteolytic bone metastasis in breast cancer. Breast Cancer Res Treat. 1994;32:73–84. doi: 10.1007/BF00666208. [DOI] [PubMed] [Google Scholar]
- 8.Yin JJ, Selander K, Chirgwin JM, Dallas M, Grubbs BG, Wieser R, Massague J, Mundy GR, Guise TA. TGF-beta signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development. J Clin Invest. 1999;103:197–206. doi: 10.1172/JCI3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ye L, Kynaston H, Jiang WG. Bone morphogenetic protein-9 induces apoptosis in prostate cancer cells, the role of prostate apoptosis response-4. Mol Cancer Res. 2008;6:1594–1606. doi: 10.1158/1541-7786.MCR-08-0171. [DOI] [PubMed] [Google Scholar]
- 10.Herrera B, van Dinther M, Ten Dijke P, Inman GJ. Autocrine bone morphogenetic protein-9 signals through activin receptor-like kinase-2/Smad1/Smad4 to promote ovarian cancer cell proliferation. Cancer Res. 2009;69:9254–9262. doi: 10.1158/0008-5472.CAN-09-2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang K, Feng H, Ren W, Sun X, Luo J, Tang M, Zhou L, Weng Y, He TC, Zhang Y. BMP9 inhibits the proliferation and invasiveness of breast cancer cells MDA-MB-231. J Cancer Res Clin Oncol. 2011;137:1687–1696. doi: 10.1007/s00432-011-1047-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci U S A. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Connor JC, Farach-Carson MC, Schneider CJ, Carson DD. Coculture with prostate cancer cells alters endoglin expression and attenuates transforming growth factor-beta signaling in reactive bone marrow stromal cells. Mol Cancer Res. 2007;5:585–603. doi: 10.1158/1541-7786.MCR-06-0408. [DOI] [PubMed] [Google Scholar]
- 14.Dirat B, Bochet L, Dabek M, Daviaud D, Dauvillier S, Majed B, Wang YY, Meulle A, Salles B, Le Gonidec S, Garrido I, Escourrou G, Valet P, Muller C. Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Res. 2011;71:2455–2465. doi: 10.1158/0008-5472.CAN-10-3323. [DOI] [PubMed] [Google Scholar]
- 15.Lv Z, Yang D, Li J, Hu M, Luo M, Zhan X, Song P, Liu C, Bai H, Li B, Yang Y, Chen Y, Shi Q, Weng Y. Bone morphogenetic protein 9 overexpression reduces osteosarcoma cell migration and invasion. Mol Cells. 2013;36:119–126. doi: 10.1007/s10059-013-0043-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin L, Zhang Y, Li H, Yao L, Fu D, Yao X, Xu LX, Hu X, Hu G. Differential secretome analysis reveals CST6 as a suppressor of breast cancer bone metastasis. Cell Res. 2012;22:1356–1373. doi: 10.1038/cr.2012.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, Barrera JL, Mohar A, Verastegui E, Zlotnik A. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 18.Bauvois B. New facets of matrix metalloproteinases MMP-2 and MMP-9 as cell surface transducers: outside-in signaling and relationship to tumor progression. Biochim Biophys Acta. 2012;1825:29–36. doi: 10.1016/j.bbcan.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 19.Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R, Weinberg RA. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 20.Bhowmick NA, Moses HL. Tumor-stroma interactions. Curr Opin Genet Dev. 2005;15:97–101. doi: 10.1016/j.gde.2004.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kidd S, Spaeth E, Dembinski JL, Dietrich M, Watson K, Klopp A, Battula VL, Weil M, Andreeff M, Marini FC. Direct evidence of mesenchymal stem cell tropism for tumor and wounding microenvironments using in vivo bioluminescent imaging. Stem Cells. 2009;27:2614–2623. doi: 10.1002/stem.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reddy BY, Lim PK, Silverio K, Patel SA, Won BW, Rameshwar P. The Microenvironmental Effect in the Progression, Metastasis, and Dormancy of Breast Cancer: A Model System within Bone Marrow. Int J Breast Cancer. 2012;2012:721659. doi: 10.1155/2012/721659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lamplot JD, Qin J, Nan G, Wang J, Liu X, Yin L, Tomal J, Li R, Shui W, Zhang H, Kim SH, Zhang W, Zhang J, Kong Y, Denduluri S, Rogers MR, Pratt A, Haydon RC, Luu HH, Angeles J, Shi LL, He TC. BMP9 signaling in stem cell differentiation and osteogenesis. Am J Stem Cells. 2013;2:1–21. [PMC free article] [PubMed] [Google Scholar]
- 25.Kinder M, Chislock E, Bussard KM, Shuman L, Mastro AM. Metastatic breast cancer induces an osteoblast inflammatory response. Exp Cell Res. 2008;314:173–183. doi: 10.1016/j.yexcr.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 26.Lu Y, Cai Z, Xiao G, Keller ET, Mizokami A, Yao Z, Roodman GD, Zhang J. Monocyte chemotactic protein-1 mediates prostate cancer-induced bone resorption. Cancer Res. 2007;67:3646–3653. doi: 10.1158/0008-5472.CAN-06-1210. [DOI] [PubMed] [Google Scholar]
- 27.Ren W, Liu Y, Wan S, Fei C, Wang W, Chen Y, Zhang Z, Wang T, Wang J, Zhou L, Weng Y, He T, Zhang Y. BMP9 inhibits proliferation and metastasis of HER2-positive SK-BR-3 breast cancer cells through ERK1/2 and PI3K/AKT pathways. PLoS One. 2014;9:e96816. doi: 10.1371/journal.pone.0096816. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 29.Kang MH, Kim JS, Seo JE, Oh SC, Yoo YA. BMP2 accelerates the motility and invasiveness of gastric cancer cells via activation of the phosphatidylinositol 3-kinase (PI3K)/Akt pathway. Exp Cell Res. 2010;316:24–37. doi: 10.1016/j.yexcr.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 30.Yang YL, Ju HZ, Liu SF, Lee TC, Shih YW, Chuang LY, Guh JY, Yang YY, Liao TN, Hung TJ, Hung MY. BMP-2 suppresses renal interstitial fibrosis by regulating epithelial-mesenchymal transition. J Cell Biochem. 2011;112:2558–2565. doi: 10.1002/jcb.23180. [DOI] [PubMed] [Google Scholar]