Abstract
Objective
As dopamine neurotransmission impacts cognition, we hypothesized variants in the linked dopamine D2 receptor (DRD2) and ankyrin repeat and kinase domain (ANKK1) genes might account for some individual variability in cognitive recovery post-TBI.
Participants
Prospective cohort of 108 survivors of severe TBI, recruited consecutively from a level 1 trauma center.
Design
We examined relationships between DRD2 genetic variation and functional recovery at 6 and 12 months post-TBI.
Main Measures
Cognitive performance was evaluated using 8 neuropsychological tests targeting different cognitive domains. An overall cognitive composite was developed based on normative data. We also assessed functional cognition, depression status, and global outcome. Subjects were genotyped for 6 DRD2 tagging single nucleotide polymorphisms and Taq1A within ANKK1.
Results
ANKK1 Taq1A heterozygotes performed better than homozygotes across several cognitive domains at both time-points post-injury. When adjusting for age, GCS, and education, the Taq1A (ANKK1) and rs6279 (DRD2) variants were associated with overall composite scores at 6 months post-TBI (p=0.0468, 0.0430, respectively). At 12 months, only Taq1A remained a significant genetic predictor of cognition (p=0.0128). Following multiple comparisons correction, there were no significant associations between examined genetic variants and functional cognition, depression status, and global outcome.
Conclusion
These data suggest genetic variation within DRD2 influences cognitive recovery post-TBI. Understanding genetic influences on dopaminergic systems post-TBI may impact current treatment paradigms.
Keywords: Traumatic Brain Injury, Cognitive Deficits, DRD2, Dopamine, ANKK1, Genetic Variation, Rehabilomics
INTRODUCTION
Among the 1.7 million people treated for a traumatic brain injury (TBI) in the United States each year1, the majority of those individuals will experience persistent cognitive deficits following their TBI. Cognitive impairment post-TBI can impact return to work and community reintegration for patients2. While the resultant cognitive impairments have been well-studied3, identifying individual patterns in recovery is still difficult. The recent international guidelines for cognitive rehabilitation post-TBI4 emphasize the need for individualized cognitive rehabilitation paradigms. Genetic variation in systems known to affect cognition in healthy individuals may explain some variance across recovery trajectories5. This study approaches the issue of poor cognitive outcomes and prognostication following TBI from a Rehabilomics6,7 perspective. The Rehabilomics approach embodies a range of study designs intended to link individual variation in personal biology to the international classification of functioning framework for multidimensional outcomes. The framework can be used to assess prognosis, biological risk for complication and conditions, and to stratify treatment and/or assess treatment effects. In this approach, we aimed to understand individual variation in cognitive recovery by assessing genetic variation, in addition to other common individual factors that contribute to cognitive performance (i.e. age, sex, injury severity), with a goal to inform future studies assessing personalized rehabilitation management approaches.
In healthy populations, dopamine (DA) neurotransmission in the brain modulates attention, processing speed, executive functioning, and working memory8. Frontal lobe regions associated with these functions have dense projections to the DA-rich striatum. In fact, positron emission tomography (PET) studies characterizing striatal D2 receptor binding demonstrate that D2 binding is correlated to several aspects of cognition, such as working memory in healthy populations9. Dysfunctional DAergic signaling may explain many of the persistent cognitive deficits observed with TBI (see review, Bales et al10). Decreased DA transporter (DAT) levels in the prefrontal cortex and striatum11 have been confirmed in a rat model after TBI12, suggesting compensatory action by DA neurons to increase DA signaling post-TBI. In severe levels of injury associated with the controlled cortical impact (CCI) model of experimental TBI, tyrosine hydroxylase (TH), a rate-limiting enzyme in the synthesis of DA is upregulated in presynaptic terminals in the frontal cortex13 and in the striatum10,14. These changes in DA synthesis proteins also suggest compensatory mechanisms in presynaptic DA neurons to increase DA neurotransmission post-TBI. Interestingly, real time neurotransmission studies using fast scan voltammetry with stimulated DA release in the striatum demonstrate reductions in evoked DA overflow and altered DA clearance kinetics15 that can be restored with daily treatment with the neurostimulant methylphenidate14. In clinical TBI populations, both PET16 and single photon emission tomography17 studies show reduced striatal binding of DAT. Also, DAergic pharmacological treatments have shown promise in cognitive restoration post-TBI18,19. Together, these studies suggest that a dysfunctional dopaminergic state post-TBI could influence individual cognitive recovery.
Genetic variation that affect dopaminergic signaling genes may elucidate some of the individual variation in cognitive recovery post-TBI. Our previous work provides mounting evidence of genetic variation on DAergic signaling post-TBI. Variation in the DAT gene (DAT1) moderates cerebrospinal fluid (CSF) levels of DA20. We have also explored DAT1 and genetic variation associated with D2 function via the Taq1A variant in the ankyrin repeat and kinase domain (ANKK1) gene, just upstream from the DRD2 gene, with PET striatal binding using DA-associated ligands for D2 and DAT16. This study provided evidence that DAT1 genotype modulates DAT binding post-TBI and that both DAT1 and Taq1A genotype interact with injury status to influence DAT binding16. Another study in mild TBI demonstrated that Taq1A genotype moderates performance on a measure of memory recognition at 1 month post-injury21. Genetic variation within the enzyme that breaks down DA in the prefrontal cortex, catechol-O-methyltransferase (COMT), also can moderate cognitive recovery post-TBI22,23, as can pharmacological interventions24. Thus, there are several lines of evidence to support our hypothesis that genetic variation in DAergic genes may influence cognitive recovery post-TBI.
Genetic variability related to the D2 receptor has been investigated by assessing both variation within the DRD2 gene itself and in evaluating the ANNK1 gene immediately upstream of DRD2. The DRD2 associated polymorphism rs1800497 Taq1A within the ANNK1 gene has been studied extensively in psychiatric disorders and in DA-related endophenotypes, especially addictive behaviors25. Also, rs1800497 has been studied in response latency and memory recovery following mild TBI21,26. The Taq1A genotype influences striatal D2 receptor density27 and may impact autoreceptor mediated inhibition of DA synthesis28. We examined 6 single nucleotide polymorphisms (SNPs) within the DRD2 gene, including rs6279. Rs6279 is in linkage disequilibrium (LD) with rs6277, where LD refers to the degree of non-random association between alleles at two genetic loci, with higher LD suggesting two alleles are more likely to be present together in the population. Rs6277 is a synonymous mutation of C957T, which does not affect amino acid sequence, but reportedly alters mRNA stability and regulation of DA-induced D2 expression29. Given the functional implications of this region, this locus is a likely target for associations with cognitive function. We hypothesized that genetic variation within ANKK1 and DRD2 influences individual cognitive recovery. The aim of this study was to investigate variation involved in DA function as it modulates cognitive recovery post-TBI, specifically utilizing a cognitive composite score to assess multiple cognitive performance domains. In addition, we sought to address the specificity of these associations by examining relationships between these genes and other outcome measures of depression and global outcome.
METHODS
Participants
This study was approved by the University of Pittsburgh's Institutional Review Board and consisted of 108 consecutively recruited participants receiving care at inpatient and/or outpatient clinics within the University of Pittsburgh Medical Center (UPMC). Enrollment criteria included a non-penetrating TBI, with evidence of intracranial injury on Computed Tomography (CT), an admission GCS score ≤8 indicating severe TBI, and age, ≥16 and <75 years. Subjects were excluded for documented prolonged hypoxia prior to admission. Additionally, subjects were not excluded for psychiatric history, substance abuse, or learning disabilities. All subjects survived at least 1 year post-injury and were cognitively able to perform the neuropsychological battery. There was a 35.4% attrition rate for this study; a number of factors (subject willingness to participate, missed visits, etc.) contributed to this rate. Subjects were a subset of a larger study investigating possible genetic factors related to individual recovery following TBI.
While an admission GCS ≤8 was taken as evidence of severe TBI, we used the best GCS obtained within 24 hours post-injury for analysis, as the best GCS in 24hrs shows better sensitivity in discriminating cognitive outcomes30,31. Best GCS in 24hrs scores ranged from 3-15 (mean GCS, 8.02 ± 3.083, median=7). Demographic information, including age, sex, and education, was collected by chart review and subject or caregiver interviews. Subjects were aged 17-71 (mean age 34.19 ± 13.75 years) and 18.5% (n=20) were women.
Sample Collection and Genotyping
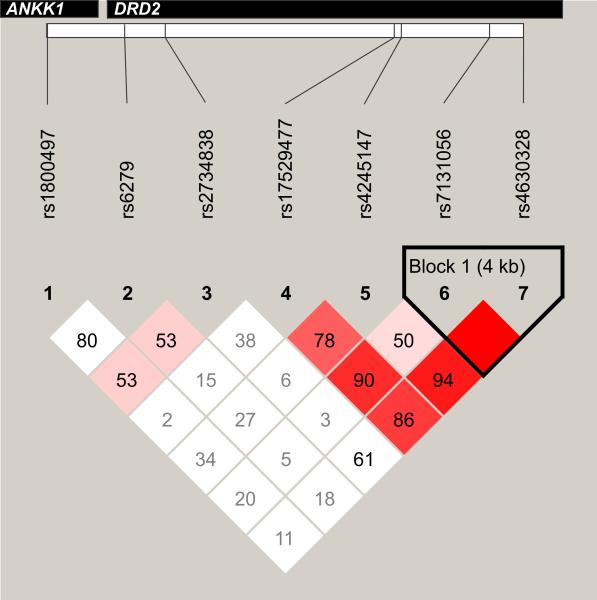
Subjects were genotyped for the dopamine receptor D2 (DRD2) associated polymorphism Taq1A in the ANNK1 gene (rs1800497) and 6 tagging SNPs in the DRD2 gene (rs6279, rs2734838, rs17529477, rs4245147, rs7131056, rs4630328). Haploview32 (version 4.2) was used to determine the degree of LD between these 7 SNPs within this population (see Figure 1). DNA was isolated from blood, using a simple salting out procedure, or from CSF, using the Qiamp protocol from Qiagen (Valencia, CA, USA). For Taq1A (rs1800497) genotyping, amplified DNA underwent 30 cycles of denaturation at 95°C for 1min., annealing at 58°C for 30s, and extension at 72°C for 1min., to amplify the 459bp product, which was then exposed to TaqI restriction endonuclease to perform restriction fragment length polymorphism (RFLP) analysis. Digested products were electrophoresed on a 3% agarose gel, stained with ethidium bromide for DNA band detection, and assigned a genotype based on presence/absence of original or cut DNA fragments. Primers used were 5’-CCGTCGACCCTTCCTGAGTGTCATCA-3’ and 5’-CCGTCGACGGCTGGCCAAGTTGTCTA-3’. In addition to SNPs selected for known functionality (ANKK1), tagging SNPs (tSNPs) covering DRD2, and 1kb flanking DNA 5’ and 3’, were selected using HapMap (Release 28); tSNP selection criteria was set at r2=0.80 and minor allele frequency (minor allele frequency, MAF) ≥0.20 to allow for robust evaluation of heterozygote status33 and potential associations with the outcomes of interest. rs2734838 was genotyped using Taqman allele discrimination and an ABI7000 (Applied Biosystems, Carlsbad, CA, USA), and the remainder were genotyped using iPLEX MassArray (Sequenom, San Diego, CA). Technical replicates were used across plates, and genotypes were double blind called for quality control.
Figure 1.
Targeted single nucleotide polymorphisms (SNPs) within ANKK1 and DRD2 shown here, lie within their respective genes as marked in the gray rectangles, and consecutively along the genome as mapped on the white rectangle. Linkage disequilibrium (LD) between the 7 SNPs examined (calculated LDs using Haploview v.4.2) is represented as the numbers in each square between each pair of SNPs (D’). Red squares indicate high LD and white squares indicate low LD based on algorithms calculated within Haploview. The bold black line around Block 1 indicates a haploblock that contains rs7131056 and rs4630328. In our sample, there is relatively low LD between rs1800497 and SNPs in DRD2.
Some participants were not successfully genotyped for each SNP (rs1800497, 1; rs6279, 2; rs2734838, 2; rs17529477, 2; rs4245147, 4; rs7131056, 1; rs4630328, 2). As this was a genetic association study, there were concerns about potential stratification effects34, where differences in allelic frequencies can influence genetic relationships with outcomes of interest. Given the different rs1800497 allelic distributions by race35 that could confound association studies, all reported associations were analyzed in a Caucasian sub-population (n=99) to avoid a false positive association between a genetic variant and cognitive recovery that could be due to underlying allelic distributions by race. Of note, all results were also conducted in the full population showing consistent findings (data not shown). Among Caucasians, allele frequencies were in Hardy-Weinberg equilibrium. Genetic variant frequencies did not differ by demographics or clinical variables, except for associations with race (data not shown).
Outcome Measurements
General outcome was assessed with the Glasgow Outcome Scale (GOS) (3=severe disability, 4=moderate disability, 5=good recovery) at 6 and 12 months36. Research-trained neuropsychometrists, blinded to genetic information, collected information used to generate GOS scores. Additionally, subjects in this cohort were evaluated, using the Functional Independence Measure subscale for Cognitive Function (FIM-Cog)37, at both 6 and 12 months. FIM-Cog is comprised of five component scales: expression, comprehension, social interaction, problem solving, and memory. Each scale is rated from one to seven, and the sum of these five components was considered the FIM-Cog Score.
Cognitive Composite Score
Cognitive performance was measured at both 6 and 12 months post-injury using a battery of 8 neuropsychological tests targeting 4 domains of cognition (attention, language fluency, memory, and executive function). Trail Making Tests A and B, a test where subjects draw lines between consecutive numbers (Part A) and then between alternating letters and numbers (Part B), was used to measure psychomotor processing speed and cognitive flexibility/task-switching, respectively38. Digit span, a sub-test from the Wechsler Adult Intelligence Scale-R, measures attention and memory by asking subjects to repeat a sequence of numbers forward and backwards39. Rey-Osterreith Complex Figure Test assesses visuo-spatial episodic memory by asking subjects to copy an abstract line drawing from memory40. The California Verbal Learning Test-II (CVLTII41) is a list learning paradigm, with subtests measuring learning, immediate recall, interference, and recognition. Different forms of the CVLT were used at 6 and 12 months to minimize practice effects from repeated administration. The Controlled Oral Word Association42 and Delis-Kaplan Executive Function Systems (DKEFS) Verbal Fluency assess verbal fluency. In both, participants are asked to name words beginning with a letter (phonemic) or within a subject category (semantic); a third condition in the DKEFS assesses ability to switch between two semantic categories. The Stroop Task43 examines selective attention and cognitive flexibility by asking subjects to name the color of ink a word is printed in, suppressing a habitual response (reading the word) to produce a more effortful response (naming ink color).
A cognitive composite score was developed based on normed t-scores for each test (considering age, sex, race, and education where applicable) to determine a measure of overall cognitive recovery. Based on a number of previous studies in TBI44–46, the development of a composite to evaluate general cognitive performance composite can improve consistency through aggregation of multiple tests47–49. Attention was measured using the Trail Making Test A, and the combined score of the forward and backward digit span tests. Memory was evaluated using the Rey-Osterreith Complex Figure Test (delay copy) and the Long Delay Free Recall Subsection of the California Verbal Learning Test. Language Fluency domain scores were calculated using the Controlled Oral Word Association Animals Subsection and the Delis-Kaplan Executive Function Systems Verbal Fluency Letter Fluency subsection. Lastly, executive function was measured using the Trail Making Test B and the Stroop Task Interference Sub-score. These tests were selected as the most representative measures for their associated domains. Raw scores from each test were converted into T scores using appropriate metrics (i.e education, age, sex, race) based on norms indicated by the test manufacturer. T-scores were averaged within each domain to create a domain sub-score. To calculate a cognitive composite score, subjects had to complete at least one test in each domain. At 6 months, there were 99 individuals with a cognitive composite, and the percentage of individual test completion ranged from 71% to 97%. At the 12 month time-point, there were 64 individuals with a cognitive composite; the percentage of test completion ≥92%. Mean values were calculated across domain sub-scores, and this mean was considered the overall cognitive composite score.
Post-TBI Depression (PTD)
Depression symptoms were evaluated at 6 and 12 months post-injury using the Patient Health Questionnaire-9 (PHQ9), a brief self-report inventory of depressive symptoms based on DSM-IV diagnostic criteria for Major Depressive Disorder. The PHQ-9 has been validated for assessing depression following TBI50,51. The PHQ-9 requires subjects to rate, on a scale between 0 (None) and 3 (Nearly Every Day), how often they experience each symptom over a two week period. A higher total score reflects a greater number of and/or greater severity of depressive symptoms, with a maximum score of 27. Subjects with TBI were grouped as “depressed, PTD” vs. “non-depressed, no PTD” using the DSM diagnostic criteria as they map to specific PHQ-9 questions51. Current DSM criteria require individuals to report at least 5 symptoms, with at least one being a cardinal symptom (anhedonia or depressive mood). In our study, subjects were categorized as depressed if they endorsed (score>0) at least five questions on the PHQ-9, with specific endorsement of either anhedonia, depressed mood, or both. This method is validated in populations with TBI (sensitivity, 93%; specificity, 89%) compared to the Structured Clinical Interview for DSM Diagnosis, a measure modeled after DSM diagnostic criteria51.
Statistical Analysis
Statistical analyses were completed using SAS (Cary, NC; version 9.3). Descriptive analyses included mean (with standard deviation or standard error of the mean) and/or median for continuous and ordinal variables including age, GCS, and education. Frequencies were calculated for categorical variables. Demographic and relevant clinical information was compared between genotype groups using Student's t-tests or Mann-Whitney U to compare means and Chi-Square or Fisher's Exact to compare frequencies.
Each SNP was screened for associations with each outcome (GOS, PTD, FIM-Cog, and overall cognitive composite) at each time-point. Due to the exploratory nature of analysis with tagging SNPs, three possible groupings were explored based on genotype (e.g. T/T vs. C/T vs. C/C), allele carrier status (e.g. C/C and C/T vs. T/T; T/T and C/T vs. C/C), or by heterozygote vs homozygote status (C/C and T/T vs. C/T), then correcting for multiple comparisons using false discovery rate (FDR)52. The lowest p-value for each SNP grouping (genotype, allele carrier status, or heterozygote vs homozygote) is reported. Of the SNPs screened for associations with outcomes, each significant association (p<0.05 following FDR) with a specific outcome was further evaluated for effects on subcomponents of the associated outcome scale. Similarly, those same SNPs were then included in multivariable regression models examining the associated outcome in order to control for demographic/clinical or injury severity characteristics, and potential confounders. If a SNP association with an outcome was significant following FDR at only 1 time-point, it was explored in multivariable models at both time-points in order to understand the clinical implications of that SNP across recovery. Variance explained by the model (r2) was examined with and without the SNP variables in the total models.
RESULTS
Genotype associations with demographics, functional cognition, and global outcome
Demographic information by genotype is presented in Table 1. There was a trend for significantly different distributions based on sex within DRD2 rs2734838 genotypes (χ2=5.6940; p=0.058). None of the other examined demographic variables were significantly different by any of the genotypes examined. Table 2 shows a summary of associations between each SNP and outcomes measured. There was a significant difference in PTD incidence by DRD2 rs2734838 genotype at 12 months post-TBI (χ2= 7.589; p=0.023), but it did not survive correction for multiple comparisons (p=0.338). There were significant associations between ANKK1 rs1800497 and GOS at 6 months, where a larger percentage of rs1800497 heterozygotes were categorized as a GOS=5 (45.0% compared to 20.3% for homozygotes, χ2=7.428, p=0.024, uncorrected) but this did not survive correction (p=0.281, FDR corrected). There was no difference between GOS score and rs1800497 at 12 months. FIM-Cog scores significantly differed by both rs1800497 and rs6279 at 6 months post-TBI. Both rs1800497 heterozygotes (p=0.028, uncorrected) and rs6279 C-homozygotes (p=0.021, uncorrected) had higher FIM-Cog scores at 6 months, but neither of these findings survived FDR correction (p=0.195 and p=0.194, respectively). At 12 months, rs2734838 A-carriers had lower FIM-Cog scores (p=0.040) but this comparison did not survive FDR correction (p=0.593). There were no other significant associations between genotype and FIM-Cog at 12 months.
Table 1.
Demographic and clinical variables by genotype.
| Variant | Genotype | Age1 | Sex2 | GCS3 | Education1 |
|---|---|---|---|---|---|
| rs1800497 | CC (n=55) | 35.4 ± 13.8 | 85.5% (47) | 7 | 12.9 ± 1.9 |
| CT (n=40) | 34.0 ± 14.8 | 75.0% (30) | 7 | 12.8 ± 2.0 | |
| TT (n=4) | 25.5 ± 7.3 | 75.0% (3) | 8 | 13.8 ± 2.1 | |
| p value | 0.315 | 0.425 | 0.714 | 0.519 | |
| rs6279 | CC (n=61) | 34.4 ± 14.5 | 83.6% (51) | 7 | 13.1 ± 2.0 |
| CG (n=30) | 33.5 ± 14.0 | 76.7% (23) | 7 | 12.6 ± 1.3 | |
| GG (n=6) | 37.8 ± 12.1 | 83.3% (5) | 6 | 13.2 ± 3.1 | |
| p value | 0.659 | 0.758 | 0.368 | 0.417 | |
| rs2734838 | AA (n=9) | 31.4 ± 14.0 | 66.7% (6) | 7 | 12.1 ± 0.8 |
| AG (n=45) | 34.3 ± 13.1 | 73.3% (33) | 7 | 12.7 ± 1.9 | |
| GG (n=43) | 34.6 ± 14.5 | 90.7% (39) | 7 | 13.3 ± 2.1 | |
| p value | 0.707 | 0.058 | 0.935 | 0.162 | |
| rs17529477 | AA (n=10) | 32.6 ± 15.2 | 80.0% (8) | 7.5 | 13.2 ± 2.9 |
| AG (n=47) | 34.6 ± 15.1 | 80.9% (38) | 7 | 12.9 ± 1.7 | |
| GG (n=40) | 34.5 ± 13.1 | 80.0% (32) | 7 | 12.6 ± 1.9 | |
| p value | 0.815 | 0.994 | 0.945 | 0.804 | |
| rs4245147 | CC (n=21) | 34.9 ± 15.2 | 85.7% (18) | 8 | 12.6 ± 1.8 |
| CT (n=49) | 32.9 ± 13.4 | 77.6% (38) | 7 | 13.2 ± 1.9 | |
| TT (n=25) | 37.4 ± 15.2 | 80.0% (20) | 7 | 12.6 ± 1.9 | |
| p value | 0.485 | 0.725 | 0.380 | 0.436 | |
| rs7131056 | AA (n=14) | 38.5 ± 13.2 | 92.9% (13) | 7 | 12.1 ± 1.6 |
| AC (n=55) | 32.2 ± 13.8 | 80.0% (44) | 7 | 12.9 ± 1.7 | |
| CC (n=29) | 36.4 ± 14.8 | 75.9% (22) | 8 | 13.0 ± 2.4 | |
| p value | 0.186 | 0.352 | 0.975 | 0.532 | |
| rs4630328 | AA (n=11) | 34.2 ± 15.9 | 81.8% (9) | 9 | 13.1 ± 2.7 |
| AG (n=52) | 34.0 ± 14.3 | 78.9% (41) | 7 | 13.1 ± 1.9 | |
| GG (n=34) | 35.2 ± 13.6 | 85.3% (29) | 7 | 12.3 ± 1.7 | |
| p value | 0.872 | 0.748 | 0.368 | 0.244 | |
Age and education reported mean ± standard deviation.
Percent men reported, (# of men).
Glasgow Coma Scale (GCS) reported as median.
Table 2.
SNP Associations with measured outcomes at 6 months and 12 months post-TBI.
| GOS | PTD | FIM-Cog | Cognitive Composite | |||||
|---|---|---|---|---|---|---|---|---|
| Variant | Raw | Corrected | Raw | Corrected | Raw | Corrected | Raw | Corrected |
| 6 Months | ||||||||
| rs1800497 | 0.02441 | 0.2807 | 0.1369 | 0.9231 | 0.0278 | 0.1946 | 0.0080 | 0.0427 |
| rs6279 | 0.1486 | 0.5201 | 0.7087 | 0.9670 | 0.0207 | 0.1946 | 0.0008 | 0.0128 |
| rs2734838 | 0.9109 | 0.9928 | 0.1837 | 0.9231 | 0.2390 | 0.3718 | 0.0547 | 0.1250 |
| rs17529477 | 0.8104 | 0.9928 | 0.6635 | 0.9670 | 0.2314 | 0.3718 | 0.1288 | 0.2290 |
| rs4245147 | 0.8906 | 0.9928 | 0.6695 | 0.9670 | 0.2236 | 0.3718 | 0.0673 | 0.1346 |
| rs7131056 | 0.8312 | 0.9928 | 0.8979 | 0.9670 | 0.1570 | 0.3663 | 0.4483 | 0.7086 |
| rs4630328 | 0.6704 | 0.9928 | 0.7497 | 0.9670 | 0.5629 | 0.6970 | 0.1624 | 0.2362 |
| 12 Months | ||||||||
| rs1800497 | 0.7892 | 0.9359 | 0.3290 | 0.9746 | 0.4218 | 0.6327 | 0.0294 | 0.2464 |
| rs6279 | 0.1088 | 0.5698 | 0.3220 | 0.9746 | 0.1772 | 0.5697 | 0.0308 | 0.2464 |
| rs2734838 | 0.1311 | 0.5698 | 0.0225 | 0.3375 | 0.0395 | 0.5697 | 0.1654 | 0.3828 |
| rs17529477 | 0.2255 | 0.5698 | 0.4696 | 0.9746 | 0.3426 | 0.6172 | 0.3577 | 0.5723 |
| rs4245147 | 0.3139 | 0.5886 | 0.9316 | 0.9746 | 0.0908 | 0.5697 | 0.2122 | 0.3828 |
| rs7131056 | 0.2659 | 0.5698 | 0.8967 | 0.9746 | 0.2472 | 0.6172 | 0.2100 | 0.3828 |
| rs4630328 | 0.2587 | 0.5698 | 0.6384 | 0.9746 | 0.3703 | 0.6172 | 0.2153 | 0.3828 |
All variants were examined for genotype, carrier, or heterozygotes vs homozygote associations with each outcome.
The raw p value reported here is the most significant association for each variant. Corrected p values are corrected for False Discovery Rate within each outcome measure. Bolded p values, p<0.1. Bolded and italic p values, p<0.05. (GOS, Glasgow Outcome Scale; PTD, Post-traumatic depression; FIM-Cog, Functional Independence Measure Cognition subscale)
Genotype associations with cognitive deficits
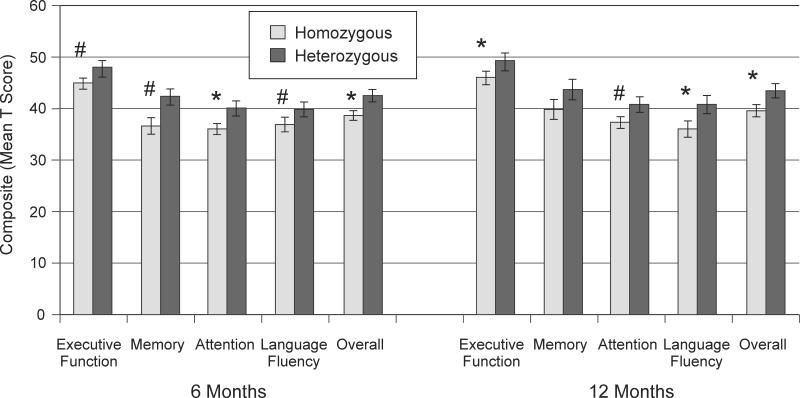
The two polymorphisms had significant associations with overall cognitive composite performance that survived correction for at least one time-point (see Table 2), and these polymorphisms were then evaluated further for effects on subcomponents of the cognitive composite. The ANKK1 rs1800497 polymorphism had the strongest relationship to overall cognitive composite scores when comparing homozygotes vs heterozygotes (see Table 2). In further evaluation, Rs1800497 heterozygotes performed better than homozygotes within some of the cognitive domains at both 6 and 12 months post-injury (Figure 2). Rs1800497 heterozygotes performed better in attention (p=0.009) at 6 months post-injury and with executive function (p=0.048) and language fluency (p=0.041) at 12 months, with trends on other domains noted. In Table 3, individual neuropsychological tests were examined by rs1800497 group. At 6 months, rs1800497 heterozygotes performed significantly better on Trails A, Trails B, and the CVLT Long Delay Free Recall. At 12 months, rs1800497 heterozygotes performed significantly better on Trails B and the COWA Animals category.
Figure 2.
Overall and domain specific cognitive composite scores at 6 and 12 months post-injury show rs1800497 (Taq1A) heterozygotes exhibit better cognitive recovery compared to homozygotes. Error bars represent SEM. *p<0.05, #p<0.10.
Table 3.
T scores on neuropsychological tests utilized at 6 and 12 months post-injury.
| Cognitive Test | 6 Months | 12 Months | ||||
|---|---|---|---|---|---|---|
| rs1800497 | CC, TT (n=59) | CT (n=40) | p value* | CC, TT (n=37) | CT (n=26) | p value |
| Rey | 41.31±11.60 | 43.78±10.57 | 0.140 | 43.72±9.47 | 44.23±11.78 | 0.368 |
| Digit Span | 41.38±6.98 | 42.12±7.43 | 0.221 | 40.58±7.27 | 42.38±8.02 | 0.182 |
| DKEFS | 40.71±10.30 | 42.57±10.54 | 0.156 | 40.97±8.99 | 44.83±11.12 | 0.067 |
| Trails A | 33.12±13.18 | 39.21±11.94 | 0.003 | 34.89±13.30 | 40.71±12.37 | 0.077 |
| Trails B | 36.98±13.23 | 43.28±14.14 | 0.006 | 37.43±13.83 | 44.23±12.41 | 0.045 |
| CVLT Long Delay Free Recall | 32.94±16.55 | 39.74±14.80 | 0.036 | 37.05±16.30 | 41.96±16.79 | 0.210 |
| Stroop | 54.11±8.13 | 52.93±7.93 | 0.182 | 54.38±7.06 | 53.68±7.70 | 0.293 |
| COWA Animals | 32.82±12.93 | 34.69±12.71 | 0.291 | 30.86±12.25 | 37.04±12.35 | 0.035 |
| rs6279 | CC (n=61) | CG, GG (n=36) | p value | CC (n=39) | CG, GG (n=25) | p value |
|---|---|---|---|---|---|---|
| Rey | 43.03±10.91 | 41.06±11.98 | 0.167 | 44.73±9.52 | 42.76±11.71 | 0.283 |
| Digit Span | 43.27±6.66 | 39.34±7.16 | 0.008 | 42.54±7.86 | 39.56±6.91 | 0.072 |
| DKEFS | 43.41±10.84 | 38.33±9.01 | 0.009 | 44.33±10.33 | 39.57±8.80 | 0.037 |
| Trails A | 38.51±12.14 | 30.91±13.30 | 0.002 | 39.51±12.01 | 33.17±14.18 | 0.045 |
| Trails B | 42.12±12.80 | 34.79±15.05 | 0.001 | 42.19±12.68 | 36.48±14.58 | 0.045 |
| CVLT Long Delay Free Recall | 37.85±16.43 | 32.63±14.96 | 0.074 | 41.03±17.23 | 35.83±15.16 | 0.137 |
| Stroop | 54.41±7.42 | 52.56±9.01 | 0.317 | 55.03±7.22 | 52.72±7.26 | 0.082 |
| COWA Animals | 36.07±12.69 | 29.79±12.20 | 0.017 | 34.12±12.49 | 32.36±12.92 | 0.362 |
Bolded p values, p<0.1. Bolded and italic p values, p<0.05.
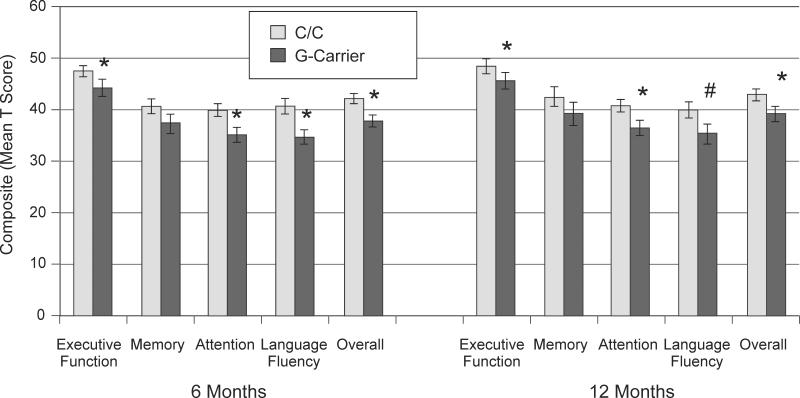
The DRD2 rs6279 polymorphism was also significantly related to cognitive performance. At 6 months post-injury, rs6279 C-homozygotes had higher overall composite scores (Table 2); at 12 months, rs6279 C-homozygotes performed significantly better overall (p=0.031) but this association did not survive correction for multiple comparison (p=0.246). Figure 3 shows that rs6279 C-homozygotes performed better in executive function (p=0.013), attention (p=0.001), and language fluency (p=0.003) domains at 6 months post-injury, and subjects this group performed better with executive function (p=0.022) and attention (p=0.039) domains at 12 months. In Table 3, individual tests were examined by rs6279 G-carrier status. At 6 months, rs6279 C-homozygotes performed significantly better on Digit Span, DKEFS, Trails A, Trails B, and COWA Animals. At 12 months, rs6279 C-homozygotes performed significantly better on DKEFS, Trails A and Trails B.
Figure 3.
Overall and domain specific cognitive composite scores at 6 and 12 months post-injury show rs6279 C-homozygotes exhibit better cognitive recovery compared to G-carriers. Error bars represent SEM. *p<0.05, #p<0.10.
To evaluate these polymorphisms in overall cognitive performance, both SNPs were tested in multivariable linear regression models predicting overall cognitive composite scores at 6 and 12 months. At 6 months, both rs1800497 and rs6279 contributed significantly to overall cognitive composite score prediction, even after adjusting for age, GCS, and education (Table 4). At 12 months, only rs1800497 remained significant in the final model. The addition of rs1800497 and rs6279 significantly improved each model (6 months, Δr2=0.084, F=2.84, p<0.05; 12 months, Δr2=0.099, F=4.15, p<0.05).
Table 4.
Multivariable model of overall cognitive composite scores at 6 and 12 Months.
| Variable | Parameter Estimate | Standard Error | t value | p value* | 95% Confidence Interval |
|---|---|---|---|---|---|
|
6 Months - Overall Composite (n=99), R2 = 0.301
| |||||
| Age | −0.12861 | 0.05222 | −2.46 | 0.0157 | (−0.23239 - −0.02484) |
| GCS | 0.81936 | 0.2586 | 3.17 | 0.0021 | (0.30545 - 1.33327) |
| Education | 1.20842 | 0.39974 | 3.02 | 0.0033 | (0.41403 - 2.00281) |
| Sex | 0.74451 | 1.99352 | 0.37 | 0.7097 | (−3.21720 - 4.70622) |
| rs1800497 Heterozygotes | 3.06912 | 1.52202 | 2.02 | 0.0468 | (0.04443 - 6.09382) |
| rs6279 G-carriers | −3.14798 | 1.53321 | −2.05 | 0.0430 | (−6.19491 - −0.10104) |
|
12 Months - Overall Composite (n=64), R2 = 0.347 | |||||
| Age | −0.11634 | 0.06158 | −1.89 | 0.0641 | (−0.23974 - 0.00706) |
| GCS | 0.80359 | 0.32919 | 2.44 | 0.0179 | (0.14387 - 1.46330) |
| Education | 1.63697 | 0.53028 | 3.09 | 0.0032 | (0.57426 - 2.69967) |
| Sex | 1.85685 | 2.10617 | 0.88 | 0.3818 | (−2.36400 - 6.07770) |
| rs1800497 Heterozygotes | 4.61380 | 1.79217 | 2.57 | 0.0128 | (1.02221 - 8.20539) |
| rs6279 G-carriers | −1.09666 | 1.78954 | −0.61 | 0.5425 | (−4.68299 - 2.48966) |
Bolded p values, p<0.1. Bolded and italic p values, p<0.05.
DISCUSSION
While cognitive function restoration is one of the most important aspects in recovery post-TBI, there is considerable heterogeneity in cognitive recovery patterns. In this study, we utilize a Rehabilomics framework6,7 to examine individual recovery patterns using genetic, clinical, and demographic factors that influence cognitive recovery, with the notion that genetics may influence other downstream outcomes like participation and quality of life. In this study, we showed that genetic polymorphisms in the ANKK1 and DRD2 gene were associated with cognitive recovery post-TBI.
While the DRD2 gene codes for D2 receptor expression, most studies have focused on the Taq1A polymorphism in ANKK1 as a potential functional moderator of D2 expression/function. Previously, it was believed that the relationship between rs1800497 and D2 receptors was due to high LD with variation in DRD2. rs1800497 is in high LD with SNPs within DRD2 that are linked to alternative splice variants capable of moderating neuronal activity during a working memory task53. Now, it is accepted that rs1800497 lies within theANKK1 gene54, calling into question the direct genetic link to D2 function. ANKK1 appears to code for a serine/threonine kinase that may interact with D2 receptor signal transduction. Also, rs1800497 induces an amino acid change (Glu713Lys) within a substrate binding domain; thus it is possible ANKK1 interacts with D2 or other DAergic signaling to impact cognitive recovery post-TBI, but more basic research into ANKK1 is needed to elucidate this mechanism.
Even with this superficial understanding of ANKK1's potential function, several studies have identified differences in DAergic function based on rs1800497 genotype. As D2 autoreceptors are important for regulation of striatal DA synthesis, PET studies utilizing [18F]fluorodopa as a measure of DA synthesis suggest T-carriers have increased DA levels and reduced D2 receptor expression28. Increased DA synthesis capacity, and/or basal DA levels, could be beneficial to recovery given the functional hypo-dopaminergic state post-TBI that is postulated based on our experimental14 and clinical16 work. Similarly, if ANKK1 affects DAergic signaling, heterozygotes may express both kinase structures, exhibiting dopamine levels in an optimal range for recovery, consistent with the ‘inverted U’ hypothesis for DAergic function55.
Many studies have examined rs1800497 Taq1A genotypes in ANKK1 grouped by T-carrier status. We grouped rs1800497 Taq1A based on genotype, allelic carrier status, and heterozygotes vs homozygotes. Using this approach, heterozygotes for rs1800487 had the best cognitive performance post-TBI. Consistent with this finding, multiple studies have evaluated heterozygosity for rs1800497 and found that heterozygote status is a biologically relevant comparison to explore, revealing a possible heterozygote advantage either due to an “inverted U” optimal level of expression or the broader range of expression in heterozygotes compared to homozygotes33. Consistent with much of the literature56, T-homozygotes are less frequent in our population (n=4). Thus, the comparison of rs1800497 heterozygotes (C/T, n=40) vs homozygotes (C/C, n=55, and T/T, n=4) is similar to a T-carrier approach (T/T and C/T compared to C/C). With mean cognitive composite comparisons, T-homozygotes were more similar to C-homozygotes than C/T heterozygotes, supporting a homozygotes/heterozygote comparison. However, utilizing a T-carrier approach showed similar findings; T-carriers (91.6% of which are heterozygotes) had better cognitive composite scores at 6 and 12 months (data not shown). Our study was designed a priori with a MAF criteria of >0.2 to provide robust heterozygosity within our sample size, maximizing our ability to evaluate heterozygosity with outcome prediction post-TBI. Examining heterozygosity across functional and tagging SNPs was an important study goal due to D2 receptor biology, particularly in relation to D2 receptor trafficking in and out of the cell membrane and its ability to dimerize with other key membrane receptors, including adenosine receptors, as a part of normal function57. Depending on SNP function, a mixture of different translated peptides, due to genetic heterozygosity associated with this receptor, could alter dimerization characteristics and affect DA signaling. A similar argument can be made regarding rs6279 genotypic groups, where heterozygosity could play a role in D2 signaling as well; thus, future studies will need to corroborate the findings reported here.
ANKK1 rs1800497 heterozygotes (T/C) exhibited better cognitive performance in this study. Clinically relevant, in some domains, homozygotes could be considered impaired with T scores <40, where heterozygotes are, by comparison, not significantly impaired. Previous work from our lab utilizing PET imaging indicates that T-carriers (A1-carriers, with the majority being heterozygotes) have higher striatal dopamine transporter (DAT) binding following TBI. Yet, in this same study, there were no significant differences in D2 binding by rs1800497 genotype. This lack of association between rs1800497 and D2 binding post-TBI, combined with another report of higher striatal DAT binding in A1-carriers,58 suggests there may be important distinctions in trafficking striatal DAT based on rs1800497 genotype. It is also possible protein-protein interactions between DAT and D259 may significantly differ by rs1800497 genotype.
Previous studies in mild TBI show that T-carriers performed worse on the CVLT recognition measure at 1 month post-injury21. Our data demonstrate heterozygotes (C/T) perform better on other neuropsychological measures in our severe TBI population. These apparent differences in gene x cognition associations may be due to several factors. In mild TBI, McAllister et al21,26 demonstrated gene-risk relationships that mirror findings in healthy populations. Importantly, our study examines a larger, more severely injured population, and cognition was examined farther out from injury. Thus, rs1800497 Taq1A may have a stratified injury severity interaction with cognitive recovery. Also, several factors (socioeconomic, access to care, spontaneous recovery) can influence cognitive recovery trajectories60,61. Outcome measure instruments also differed across these studies, suggesting this polymorphism may have domain-specific relationships to cognition. However, our study examines multiple cognitive domains, demonstrating that variation within ANKK1 and DRD2 influences multiple areas of cognition.
This study also examined 6 SNPs covering variation in the DRD2 gene. Rs6279 was significantly associated, across domains, with cognitive recovery at 6 months post-TBI; the association was less strong with cognition at 12 months. McAllister and colleagues21 found a relationship between rs6279 and CVLT recognition task performance in subjects with TBI, but no relationship in healthy controls. Rs6279 has not been well studied, but it is in high linkage disequilibrium with rs6277, a synonymous mutation C957T, that results in impaired stabilization of DRD2 mRNA29. This mutation may impair the ability of DA to stabilize DRD2 mRNA, which then may lead to reduced translation and D2 expression in response to increased DA concentrations. Rs6277 is implicated in risk for schizophrenia62 and posttraumatic stress disorder63. As there is no known functional impact of rs6279, our findings with rs6279 may actually be reflective of its high LD with rs6277.
Interestingly, multivariable models including rs6279 and rs1800497 showed that both SNPs have independent effects at 6 months post-injury. While only rs1800497 was significantly associated with overall composites in our 12 month model, it is possible that in a larger sample size, there may be a significant influence of rs6279, in addition to rs1800497, on cognition. However, it is also possible the mechanism of rs6279's impact on cognition post-TBI may be diminished at 12 months. The temporal dynamics of these DRD2 associations is consistent with other studies from our group that demonstrate transient genetic outcome associations across recovery post-TBI64,65. Domain specific analysis supports slightly different roles for each SNP in overall cognitive recovery. For example, at 6 months post-TBI, rs6279 shows stronger associations to executive function and language fluency compared to rs1800497. Additionally, the significant change in R2 following the addition of rs6279 and rs1800497 suggests the important role of these variants on cognitive recovery.
Our multivariable models also suggest there are specific injury-age interactions following TBI that differ from control populations. Our cognitive composites are adjusted for performance differences known to occur in the general population for age, sex, and education, based on standardized norms for each neuropsychological measure. Yet even after adjusting for these effects found in the normed data, age and education were still significant predictors of cognitive composite score in our multivariable models, suggesting these demographic variables have an amplified injury-specific effect on cognitive performance. Education may be a correlate for post-TBI cognitive reserve that could likely impact an individual's cognitive recovery trajectory post-TBI49,66. Given the detrimental effects of age on other TBI pathology/recovery domains67–70, age enhanced vulnerability to cognitive dysfunction post-TBI likely also occurs. Additionally, our inclusion of the best GCS in first 24 hours post-injury confirms other studies findings where best GCS in 24 hours predicts long-term outcome30,31.
This study also examined ANKK1 and DRD2 variation in post-TBI depression (PTD), FIM-Cog, and GOS. Despite some associative trends, there were no relationships between any of the variants examined and PTD following FDR correction. While there is emerging evidence of DAergic signaling involvement in depressive symptoms71, this study may be underpowered to examine DAergic relationships to PTD. Rs1800497 and rs6279 were associated with GOS and FIM-Cog, though none survived correction for multiple corrections. However, consistent with the Rehabilomics Model6,7, the data suggest a need for larger studies to examine how genetic relationships associated cognitive performance and may moderate downstream effects on other outcomes dimensions reflected with GOS and FIM-Cog.
One important caveat in this study is the reported findings were conducted in a population of Caucasians-only. DRD2 SNP distributions were significantly different by race (data not shown), highlighting the need to limit our analysis to Caucasians to avoid any potential stratification effects. This study was not powered to examine genotypic associations by race in post-TBI cognitive outcomes, and future studies are needed to examine more racially-diverse populations. While this study is limited by its overall sample size, it is one of the larger studies examining genetic factors in cognitive recovery post-TBI, similar to our previous studies72. Due to the loss to follow-up in this study, future studies will need to validate these findings in larger groups at 12 months, and to validate the observed genetic effects for consistency across recovery.
Investigating DA signaling effects of DA specific gene variants post-TBI may inform relationships to cognitive recovery. This report is one of the first studies to utilize cognitive composite scores to evaluate DA genetic modulators of TBI cognitive recovery72. After adjusting our neuropsychological data, using available normative data, our results show injury specific effects of age and education on cognitive performance after severe TBI. Our cognitive composite score was designed to be a proxy for overall cognitive test performance collapsed across multiple domains. Importantly, this composite has not been examined in terms of its predictive or ecological validity (i.e., how it might predict cognitive functioning in real life) and future studies are needed to determine this relationship.
Furthermore, this work may suggest a need to examine personalized approaches to treatments like cognitive rehabilitation strategies and neurostimulant use. For example, genetic associations with specific cognitive performance domains may help discriminate individuals more likely to require or benefit from treatment. Given recent findings of genetic differences in D2 receptor expression (based on PET) among individuals with severe TBI16, and our reported findings of genetic differences in cognitive performance after TBI, it stands to reason that treatments known to affect cognition via dopamine systems, such as neurostimulants like methylphenidate, may have differential effects on cognition as a function of D2 receptor genotype. Understanding how these genetic variants impacts DAergic pharmacological intervention73 post-TBI may allow for future personalization with regard to neurostimulants like methylphenidate. The temporal dynamics of our findings also suggest there may be specific timelines of therapeutic advantage for DAergic pharmacological intervention. Similarly, genetic variation, along with other personal factors, may help predict who is likely to have adverse side effects or may help guide treatment dosing to minimize side effects and optimize treatment effects. This work suggests a need for future evaluation, within a comprehensive Rehabilomics framework, of combinatorial treatment paradigms in cognitive rehabilitation post-TBI.
Acknowledgments
Research/Grant Support:
This research was supported by DOD W81XWH-071-0701, NIH R01 HD048162, NIDRR H133A120087, NIH R01NR013342.
Footnotes
Conflict of Interest Statement:
The authors declare no conflicts of interest.
REFERENCES
- 1.CDC - TBI - TBI in the US Report. at > http://www.cdc.gov/traumaticbraininjury/tbi_ed.html<.
- 2.Fleming Jennifer, Tooth Leigh, Mary Prediction of community integration and vocational outcome 2-5 years after traumatic brain injury rehabilitation in Australia. Brain Inj. 1999;13:417–431. doi: 10.1080/026990599121476. [DOI] [PubMed] [Google Scholar]
- 3.Levin H, Benton A, Grossman RG. Neurobehavioral Consequences of Closed Head Injury. Oxford University Press; 1982. [Google Scholar]
- 4.Bayley MT, et al. INCOG Guidelines for Cognitive Rehabilitation Following Traumatic Brain Injury: Methods and Overview. J. Head Trauma Rehabil. 2014;29:290–306. doi: 10.1097/HTR.0000000000000070. [DOI] [PubMed] [Google Scholar]
- 5.McAllister TW. Polymorphisms in Genes Modulating the Dopamine System: Do They Influence Outcome and Response to Medication After Traumatic Brain Injury? J. Head Trauma Rehabil. 2009;24:65–68. doi: 10.1097/HTR.0b013e3181996e6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wagner AK. TBI translational rehabilitation research in the 21st Century: exploring a Rehabilomics research model. Eur. J. Phys. Rehabil. Med. 2010;46:549–556. [PubMed] [Google Scholar]
- 7.Wagner AK, Zitelli KT. A Rehabilomics focused perspective on molecular mechanisms underlying neurological injury, complications, and recovery after severe TBI. Pathophysiol. Off. J. Int. Soc. Pathophysiol. ISP. 2012 doi: 10.1016/j.pathophys.2012.02.007. doi:10.1016/j.pathophys.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 8.Braver TS, Cohen JD. On the control of control: The role of dopamine in regulating prefrontal function and working memory. Control Cogn. Process. Atten. Perform. 2000;XVIII:713–737. [Google Scholar]
- 9.Aalto S, Brück A, Laine M, Nägren K, Rinne JO. Frontal and Temporal Dopamine Release during Working Memory and Attention Tasks in Healthy Humans: a Positron Emission Tomography Study Using the High-Affinity Dopamine D2 Receptor Ligand [11C]FLB 457. J. Neurosci. 2005;25:2471–2477. doi: 10.1523/JNEUROSCI.2097-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bales JW, Wagner AK, Kline AE, Dixon CE. Persistent cognitive dysfunction after traumatic brain injury: A dopamine hypothesis. Neurosci. Biobehav. Rev. 2009;33:981–1003. doi: 10.1016/j.neubiorev.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shimada R, Abe K, Furutani R, Kibayashi K. Changes in dopamine transporter expression in the midbrain following traumatic brain injury: an immunohistochemical and in situ hybridization study in a mouse model. Neurol. Res. 2014;36:239–246. doi: 10.1179/1743132813Y.0000000289. [DOI] [PubMed] [Google Scholar]
- 12.Yan HQ, Kline AE, Ma X, Li Y, Dixon CE. Traumatic brain injury reduces dopamine transporter protein expression in the rat frontal cortex. Neuroreport. 2002;13:1899. doi: 10.1097/00001756-200210280-00013. [DOI] [PubMed] [Google Scholar]
- 13.Yan HQ, et al. Tyrosine hydroxylase, but not dopamine beta-hydroxylase, is increased in rat frontal cortex after traumatic brain injury. Neuroreport. 2001;12:2323. doi: 10.1097/00001756-200108080-00009. [DOI] [PubMed] [Google Scholar]
- 14.Wagner AK, et al. Chronic methylphenidate treatment enhances striatal dopamine neurotransmission after experimental traumatic brain injury. J. Neurochem. 2009;108:986–997. doi: 10.1111/j.1471-4159.2008.05840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wagner AK, et al. Gender and environmental enrichment impact dopamine transporter expression after experimental traumatic brain injury. Exp. Neurol. 2005;195:475–483. doi: 10.1016/j.expneurol.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 16.Wagner AK, et al. The influence of genetic variants on striatal dopamine transporter and D2 receptor binding after TBI. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2014 doi: 10.1038/jcbfm.2014.87. doi:10.1038/jcbfm.2014.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donnemiller E, et al. Impaired dopaminergic neurotransmission in patients with traumatic brain injury: a SPET study using 123 I-ss-CIT and 123 I-IBZM. Eur. J. Nucl. Med. Mol. Imaging. 2000;27:1410–1414. doi: 10.1007/s002590000308. [DOI] [PubMed] [Google Scholar]
- 18.Whyte J, et al. Effects of methylphenidate on attention deficits after traumatic brain injury: a multidimensional, randomized, controlled trial. Am. J. Phys. Med. Rehabil. Assoc. Acad. Physiatr. 2004;83:401–420. doi: 10.1097/01.phm.0000128789.75375.d3. [DOI] [PubMed] [Google Scholar]
- 19.Bleiberg J, Garmoe W, Cederquist J, Reeves D. Effect of dexedrine on performance consistency following brain injury: A double-blind placebo crossover case study. Neuropsychiatry. Neuropsychol. Behav. Neurol. 1993 at > http://psycnet.apa.org/psycinfo/1994-22746-001<.
- 20.Wagner AK, et al. Sex and genetic associations with cerebrospinal fluid dopamine and metabolite production after severe traumatic brain injury. J. Neurosurg. 2007;106:538–547. doi: 10.3171/jns.2007.106.4.538. [DOI] [PubMed] [Google Scholar]
- 21.McAllister TW, et al. Single nucleotide polymorphisms in ANKK1 and the dopamine D2 receptor gene affect cognitive outcome shortly after traumatic brain injury: a replication and extension study. Brain Inj. BI. 2008;22:705–714. doi: 10.1080/02699050802263019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Willmott C, Withiel T, Ponsford J, Burke R. COMT Val158Met and Cognitive and Functional Outcomes after Traumatic Brain Injury. J. Neurotrauma. 2014 doi: 10.1089/neu.2013.3308. doi:10.1089/neu.2013.3308. [DOI] [PubMed] [Google Scholar]
- 23.Lipsky RH, et al. Association of COMT Val158Met Genotype With Executive Functioning Following Traumatic Brain Injury. J. Neuropsychiatry Clin. Neurosci. 2005;17:465–471. doi: 10.1176/jnp.17.4.465. [DOI] [PubMed] [Google Scholar]
- 24.Willmott C, Ponsford J, McAllister TW, Burke R. Effect of COMT Val158Met genotype on attention and response to methylphenidate following traumatic brain injury. Brain Inj. 2013;27:1281–1286. doi: 10.3109/02699052.2013.809553. [DOI] [PubMed] [Google Scholar]
- 25.Ponce G, et al. The ANKK1 kinase gene and psychiatric disorders. Neurotox. Res. 2009;16:50–59. doi: 10.1007/s12640-009-9046-9. [DOI] [PubMed] [Google Scholar]
- 26.McAllister TW, et al. Effect of the dopamine D2 receptor T allele on response latency after mild traumatic brain injury. Am. J. Psychiatry. 2005;162:1749–1751. doi: 10.1176/appi.ajp.162.9.1749. [DOI] [PubMed] [Google Scholar]
- 27.Thompson J, et al. D2 dopamine receptor gene (DRD2) Taq1 A polymorphism: reduced dopamine D2 receptor binding in the human striatum associated with the A1 allele. Pharmacogenetics. 1997;7:479–484. doi: 10.1097/00008571-199712000-00006. [DOI] [PubMed] [Google Scholar]
- 28.Laakso A, et al. The A1 allele of the human D2 dopamine receptor gene is associated with increased activity of striatal L-amino acid decarboxylase in healthy subjects. Pharmacogenet. Genomics. 2005;15:387–391. doi: 10.1097/01213011-200506000-00003. [DOI] [PubMed] [Google Scholar]
- 29.Duan J, et al. Synonymous mutations in the human dopamine receptor D2 (DRD2) affect mRNA stability and synthesis of the receptor. Hum. Mol. Genet. 2003;12:205–216. doi: 10.1093/hmg/ddg055. [DOI] [PubMed] [Google Scholar]
- 30.Udekwu P, Kromhout-Schiro S, Vaslef S, Baker C, Oller D. Glasgow Coma Scale score, mortality, and functional outcome in head-injured patients. J. Trauma. 2004;56:1084–1089. doi: 10.1097/01.ta.0000124283.02605.a5. [DOI] [PubMed] [Google Scholar]
- 31.Cifu DX, et al. Acute predictors of successful return to work 1 year after traumatic brain injury: a multicenter analysis. Arch. Phys. Med. Rehabil. 1997;78:125–131. doi: 10.1016/s0003-9993(97)90252-5. [DOI] [PubMed] [Google Scholar]
- 32.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 33.Comings DE, MacMurray JP. Molecular heterosis: a review. Mol. Genet. Metab. 2000;71:19–31. doi: 10.1006/mgme.2000.3015. [DOI] [PubMed] [Google Scholar]
- 34.Freedman ML, et al. Assessing the impact of population stratification on genetic association studies. Nat. Genet. 2004;36:388–393. doi: 10.1038/ng1333. [DOI] [PubMed] [Google Scholar]
- 35.Goldman D, et al. DRD2 dopamine receptor genotype, linkage disequilibrium, and alcoholism in American Indians and other populations. Alcohol. Clin. Exp. Res. 1993;17:199–204. doi: 10.1111/j.1530-0277.1993.tb00749.x. [DOI] [PubMed] [Google Scholar]
- 36.Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975;1:480–484. doi: 10.1016/s0140-6736(75)92830-5. [DOI] [PubMed] [Google Scholar]
- 37.Dodds TA, Martin DP, Stolov WC, Deyo RA. A validation of the functional independence measurement and its performance among rehabilitation inpatients. Arch. Phys. Med. Rehabil. 1993;74:531–536. doi: 10.1016/0003-9993(93)90119-u. [DOI] [PubMed] [Google Scholar]
- 38.Reitan R, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery: Theory and Clinical Interpretation. Neuropsychology Press; 1985. [Google Scholar]
- 39.Larrabee GJ, Curtiss G. Construct validity of various verbal and visual memory tests. J. Clin. Exp. Neuropsychol. 1995;17:536–547. doi: 10.1080/01688639508405144. [DOI] [PubMed] [Google Scholar]
- 40.Osterrieth P. The Complex Figure Copy Test. Arch. Psychol. 1944;30:206–356. [Google Scholar]
- 41.Delis DC, et al. The California Verbal Learning Test. The Psychological Corporation; 2000. [Google Scholar]
- 42.Borkowski J, Benton A, Spreen O. Word fluency and brain damage. Neuropsychologia. 1967;5:135–140. [Google Scholar]
- 43.Stroop JR. Studies of interference in serial verbal reactions. J. Exp. Psychol. Exp. Psychol. 1935;18:643–662. [Google Scholar]
- 44.Hart T, Whyte J, Kim J, Vaccaro M. Executive function and self-awareness of ‘real-world’ behavior and attention deficits following traumatic brain injury. J. Head Trauma Rehabil. 2005;20:333–347. doi: 10.1097/00001199-200507000-00005. [DOI] [PubMed] [Google Scholar]
- 45.Hartikainen KM, et al. Persistent symptoms in mild to moderate traumatic brain injury associated with executive dysfunction. J. Clin. Exp. Neuropsychol. 2010;32:767–774. doi: 10.1080/13803390903521000. [DOI] [PubMed] [Google Scholar]
- 46.Wagner AK, et al. Persistent hypogonadism influences estradiol synthesis, cognition and outcome in males after severe TBI. Brain Inj. BI. 2012 doi: 10.3109/02699052.2012.667594. doi:10.3109/02699052.2012.667594. [DOI] [PubMed] [Google Scholar]
- 47.Van Veelen NMJ, et al. Short term neurocognitive effects of treatment with ziprasidone and olanzapine in recent onset schizophrenia. Schizophr. Res. 2010;120:191–198. doi: 10.1016/j.schres.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 48.Dawson JD, Uc EY, Anderson SW, Johnson AM, Rizzo M. Neuropsychological predictors of driving errors in older adults. J. Am. Geriatr. Soc. 2010;58:1090–1096. doi: 10.1111/j.1532-5415.2010.02872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Green RE, et al. Examining moderators of cognitive recovery trajectories after moderate to severe traumatic brain injury. Arch. Phys. Med. Rehabil. 2008;89:S16–24. doi: 10.1016/j.apmr.2008.09.551. [DOI] [PubMed] [Google Scholar]
- 50.Cook KF, et al. Do Somatic and Cognitive Symptoms of Traumatic Brain Injury Confound Depression Screening? Arch. Phys. Med. Rehabil. 2011;92:818–823. doi: 10.1016/j.apmr.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fann JR, et al. Validity of the Patient Health Questionnaire-9 in assessing depression following traumatic brain injury. J. Head Trauma Rehabil. 2005;20:501–511. doi: 10.1097/00001199-200511000-00003. [DOI] [PubMed] [Google Scholar]
- 52.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B Methodol. 1995;57:289–300. [Google Scholar]
- 53.Zhang Y, et al. Polymorphisms in human dopamine D2 receptor gene affect gene expression, splicing, and neuronal activity during working memory. Proc. Natl. Acad. Sci. 2007;104:20552–20557. doi: 10.1073/pnas.0707106104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Neville MJ, Johnstone EC, Walton RT. Identification and characterization of ANKK1: a novel kinase gene closely linked to DRD2 on chromosome band 11q23.1. Hum. Mutat. 2004;23:540–545. doi: 10.1002/humu.20039. [DOI] [PubMed] [Google Scholar]
- 55.Cools R, D'Esposito M. Inverted-U-shaped dopamine actions on human working memory and cognitive control. Biol. Psychiatry. 2011;69:e113–125. doi: 10.1016/j.biopsych.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Munafó MR, Matheson IJ, Flint J. Association of the DRD2 gene Taq1A polymorphism and alcoholism: a meta-analysis of case–control studies and evidence of publication bias. Mol. Psychiatry. 2007;12:454–461. doi: 10.1038/sj.mp.4001938. [DOI] [PubMed] [Google Scholar]
- 57.Zawarynski P, et al. Dopamine D2 receptor dimers in human and rat brain. FEBS Lett. 1998;441:383–386. doi: 10.1016/s0014-5793(98)01588-9. [DOI] [PubMed] [Google Scholar]
- 58.Laine TP, et al. The A1 allele of the D2 dopamine receptor gene is associated with high dopamine transporter density in detoxified alcoholics. Alcohol Alcohol. Oxf. Oxfs. 2001;36:262–265. doi: 10.1093/alcalc/36.3.262. [DOI] [PubMed] [Google Scholar]
- 59.Lee FJ, et al. Dopamine transporter cell surface localization facilitated by a direct interaction with the dopamine D2 receptor. EMBO J. 2007;26:2127–2136. doi: 10.1038/sj.emboj.7601656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hoofien D, Vakil E, Gilboa A, Donovick PJ, Barak O. Comparison of the predictive power of socio-economic variables, severity of injury and age on long-term outcome of traumatic brain injury: sample-specific variables versus factors as predictors. Brain Inj. BI. 2002;16:9–27. doi: 10.1080/02699050110088227. [DOI] [PubMed] [Google Scholar]
- 61.León-Carrión J, Machuca-Murga F, Solís-Marcos I, León-Domínguez U, Domínguez-Morales MDR. The sooner patients begin neurorehabilitation, the better their functional outcome. Brain Inj. BI. 2013;27:1119–1123. doi: 10.3109/02699052.2013.804204. [DOI] [PubMed] [Google Scholar]
- 62.Monakhov M, Golimbet V, Abramova L, Kaleda V, Karpov V. Association study of three polymorphisms in the dopamine D2 receptor gene and schizophrenia in the Russian population. Schizophr. Res. 2008;100:302–307. doi: 10.1016/j.schres.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 63.Voisey J, et al. The DRD2 gene 957C<T polymorphism is associated with posttraumatic stress disorder in war veterans. Depress. Anxiety. 2009;26:28–33. doi: 10.1002/da.20517. [DOI] [PubMed] [Google Scholar]
- 64.Failla MD, et al. Variants of SLC6A4 in depression risk following severe TBI. Brain Inj. BI. 2013;27:696–706. doi: 10.3109/02699052.2013.775481. [DOI] [PubMed] [Google Scholar]
- 65.Failla MD, et al. Variation in the BDNF Gene Interacts With Age to Predict Mortality in a Prospective, Longitudinal Cohort with Severe TBI. Neurorehabil. Neural Repair. 2014 doi: 10.1177/1545968314542617. doi:10.1177/1545968314542617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schneider EB, et al. Functional recovery after moderate/severe traumatic brain injury A role for cognitive reserve? Neurology. 2014;82:1636–1642. doi: 10.1212/WNL.0000000000000379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wagner AK, et al. Relationships between cerebrospinal fluid markers of excitotoxicity, ischemia, and oxidative damage after severe TBI: the impact of gender, age, and hypothermia. J. Neurotrauma. 2004;21:125–136. doi: 10.1089/089771504322778596. [DOI] [PubMed] [Google Scholar]
- 68.Susman M, et al. Traumatic brain injury in the elderly: increased mortality and worse functional outcome at discharge despite lower injury severity. J. Trauma. 2002;53:219–223. doi: 10.1097/00005373-200208000-00004. discussion 223–224. [DOI] [PubMed] [Google Scholar]
- 69.Onyszchuk G, He Y-Y, Berman NEJ, Brooks WM. Detrimental effects of aging on outcome from traumatic brain injury: a behavioral, magnetic resonance imaging, and histological study in mice. J. Neurotrauma. 2008;25:153–171. doi: 10.1089/neu.2007.0430. [DOI] [PubMed] [Google Scholar]
- 70.Kumar A, et al. Traumatic brain injury in aged animals increases lesion size and chronically alters microglial/macrophage classical and alternative activation states. Neurobiol. Aging. 2013;34:1397–1411. doi: 10.1016/j.neurobiolaging.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dunlop BW, Nemeroff CB. The Role of Dopamine in the Pathophysiology of Depression. Arch Gen Psychiatry. 2007;64:327–337. doi: 10.1001/archpsyc.64.3.327. [DOI] [PubMed] [Google Scholar]
- 72.Wagner AK, et al. Association of KIBRA rs17070145 polymorphism and episodic memory in individuals with severe TBI. Brain Inj. BI. 2012 doi: 10.3109/02699052.2012.700089. doi:10.3109/02699052.2012.700089. [DOI] [PubMed] [Google Scholar]
- 73.Mi H, et al. PharmGKB summary: dopamine receptor D2. Pharmacogenet. Genomics. 2011;21:350–356. doi: 10.1097/FPC.0b013e32833ee605. [DOI] [PMC free article] [PubMed] [Google Scholar]