Introduction
Mutations in TJP2 (also named ZO-2), encoding tight-junction protein 2 (TJP2), cause an autosomal-recessive form of progressive intrahepatic cholestasis.1 Association of TJP2 deficiency with hepatocellular carcinoma (HCC) in childhood has not been recognized.
Case Reports
Patient 1
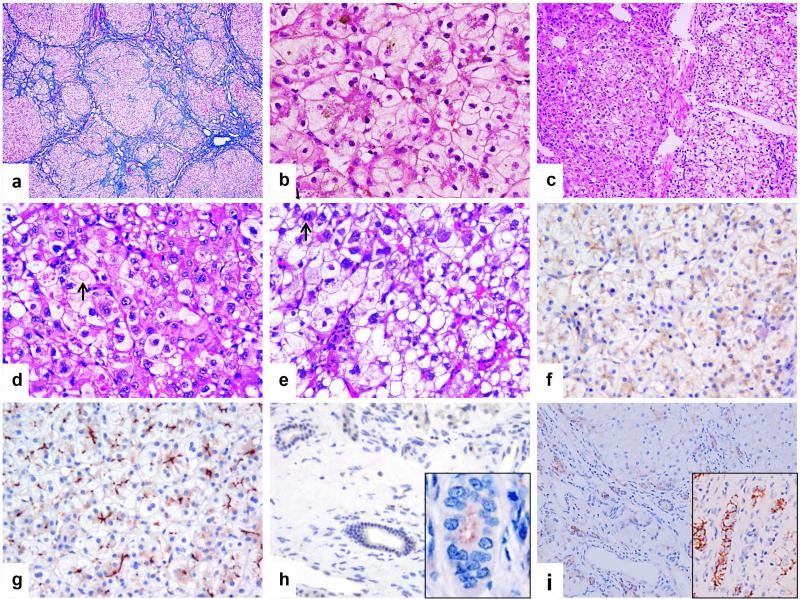
A 26-month-old Caucasian girl with intermittent jaundice of neonatal onset and unknown etiology, with normal-range serum gamma-glutamyl transferase activity (GGT), presented with liver failure. Computed tomography revealed innumerable well-defined hepatic masses. Serum alpha-fetoprotein (AFP) level was high (171,000 ng/ml). Liver biopsy (Fig. 1a-e) found moderately differentiated HCC (expressing glypican-3, p53, and AFP) in a background of chronic cholestatic hepatitis with cirrhosis. Mutational analysis detected no significant variants in ABCB11. Both bile salt export pump (BSEP) and multidrug resistance protein 3 (Fig. 1f-g) were well-expressed. Because these features may result from TJP2 deficiency, TJP2 was sequenced.1 Compound heterozygous mutations were found, with c.2668-1G>T /c.2438dupT (p.Asn814Glnfs) (NM_004817.3). TJP2 expression was not immunohistochemically demonstrable and claudin-1 expression was markedly decreased (Fig. 1h-i), supporting the diagnosis of TJP2 deficiency. The patient died 3 weeks after admission. Autopsy confirmed multifocal HCC (0.5 - 4.0 cm in greatest dimension).
Figure 1.
Patient 1. Non-neoplastic liver shows micronodular cirrhosis (a) and prominent rosetting with bile plugs in canalicular lumina (b). c-d. Low and high-magnification views of HCC. Tumor cells show variable cytoplasmic clearing and round to oval, sometimes pleomorphic nuclei with variably-sized nucleoli. Some cells contain distinct round to oval eosinophilic cytoplasmic proteinaceous inclusions (arrow in d). Macrosteatosis and mitosis (arrow in e) are also present. Bile salt export pump (f) and multiple drug resistance protein 3 (g) are well-expressed in nonneoplastic liver. h. Complete absence of tight junction protein 2 (TJP2) staining in bile ducts. i. Markedly decreased claudin-1 staining in both cholangiocytes and canalicular margins. “Control liver” shows normal TJP2 expression in bile duct (inset in h) and strong claudin-1 expression in both bile ducts and canaliculi (inset in i). (a. Trichrome stain; b-f. Hematoxylin-eosin stain; Original magnification: 40 × for a, 100 × for c, 200 × for b, d and e, 400 × for f-i)
Patient 2
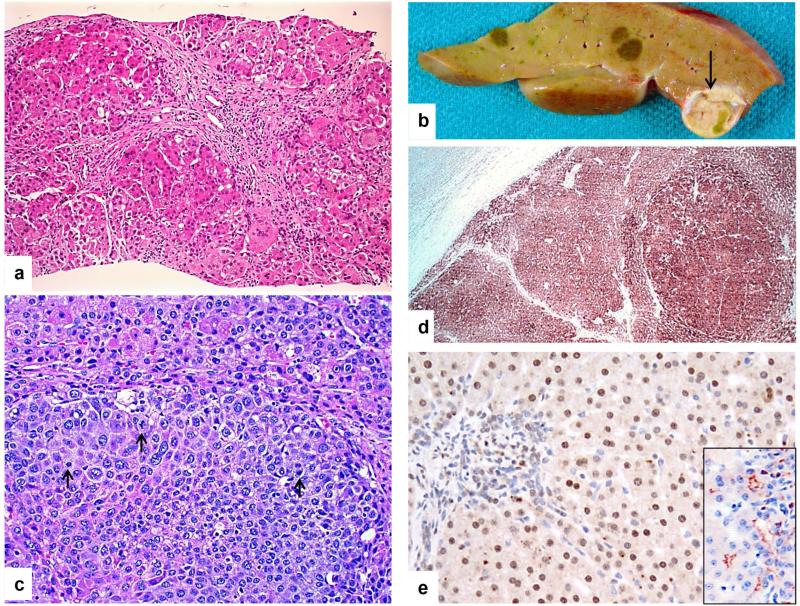
A 6-month-old Caucasian boy was referred for persistent cholestasis with near-normal GGT following hepatoportoenterostomy for presumed biliary atresia. His parents denied consanguinity. No etiology was defined; work-up included Sanger sequencing of ABCB11. Liver biopsy found cholestatic hepatitis (Fig.2a). Icterus resolved by age 19 months, but a growing lesion in the right liver lobe, with rising serum AFP, prompted liver transplantation at age 2 years. The explanted liver was cirrhotic, with multiple cholestatic nodules and a single, well-encapsulated 2-cm tumor that diffusely expressed AFP and glypican-3; a central region of well-differentiated HCC was found (Fig.2b-d). Blood and tumor whole-exome sequencing found homozygosity for c.817delG (p.A273fs) in TJP2, predicted to cause a frameshift in all transcripts of TJP2. Sanger sequencing validated the mutation, for which each parent was heterozygous. TJP2 expression was absent (Fig. 2e) and claudin-1 expression was markedly decreased in non-tumoral liver. The patient is well 2 years after transplantation.
Figure 2.
Patient 2. a. Liver biopsy at 6 months displays severe cholestasis, giant cell transformation, and micronodular cirrhosis. b. Macroscopic photograph of the explant reveals multiple cholestatic nodules, and one much larger and softer tan nodule with focal bile pigmentation (arrow) within which hepatocellular carcinoma (HCC) was found histologically. c. Within the large, soft nodule, a sharply delineated smaller aggregate of highly pleomorphic and mitotically active cells (arrows) is typical of HCC. d. Glypican-3 expression (brown staining) is seen in HCC and regenerative nodule but not in non-neoplastic hepatocytes (left upper). e. Tight junction protein 2 (TJP2) is not expressed at bile canaliculi of the patient's liver-biopsy specimen, whereas it is observed anomalously in hepatocyte nuclei. “Control liver” shows normal canalicular expression of TJP2 (inset). (a & c: Hematoxylin-eosin stain; Original magnification: 125 × for d, 200 × for a, c and e)
Discussion
Both patients had clinical and histological features similar to those of BSEP deficiency, which predisposes to HCC in childhood.2 However, neither ABCB11 mutation nor BSEP deficiency was demonstrable. Instead, biallelic TJP2 truncating mutations were found in both cases. TJP2 expression at cholangiocyte apices and bile-canaliculus margins was entirely absent. Like Sambrotta et al.,1 we found deficiency of canalicular claudin-1 expression. We therefore ascribe liver disease in both patients to severe TJP2 deficiency.
We infer that TJP2 deficiency predisposes to HCC development in early childhood. How it does so is unclear. While many toddlers have severe cholestatic liver disease, very few develop HCC. This makes an indirect and non-specific effect of TJP2 deficiency unlikely. Down-regulation of TJP2 function in various tumor types3 may suggest direct tumorigenesis. Such down-regulation is, however, perhaps secondary and non-specific.
HCC in children with BSEP deficiency may arise by several routes. A case in which mutations like those found in adult HCC underlay tumorigenesis is reported,4 as are several cases in which massive gene amplification was found.5 These mechanisms remain to be assessed in pediatric HCC with backgrounds of other cholestatic disorders, including TJP2 deficiency.
TJP2 mutation must be considered in patients with intrahepatic cholestasis and normal-range GGT. TJP2 deficiency may predispose to HCC in early childhood, warranting close monitoring and early liver transplantation.
Acknowledgments
Financial Support
This work was partially supported by a grant to SEP NHGRI/NCI U01HG006485.
References
- 1.Sambrotta M, Strautnieks S, Papouli E, Rushton P, Clark BE, Parry DA, et al. Mutations in TJP2 cause progressive cholestatic liver disease. Nature Genetics. 2014;46:326–328. doi: 10.1038/ng.2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knisely AS, Strautnieks SS, Meier Y, Stieger B, Byrne JA, Portmann BC, et al. Hepatocellular carcinoma in ten children under five years of age with bile salt export pump deficiency. Hepatology. 2006;44:478–486. doi: 10.1002/hep.21287. [DOI] [PubMed] [Google Scholar]
- 3.Gonzalez-Mariscal L, Lechuga S, Garay E. Role of tight junctions in cell proliferation and cancer. Progress in Histochemistry and Cytochemistry. 2007;42:1–57. doi: 10.1016/j.proghi.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Vilarinho S, Erson-Omay EZ, Harmanci AS, Morotti R, Carrion-Grant G, Baranoski J, et al. Paediatric hepatocellular carcinoma due to somatic CTNNB1 and NFE2L2 mutations in the setting of inherited bi-allelic ABCB11 mutations. Journal of Hepatology. 2014;61:1178–1183. doi: 10.1016/j.jhep.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Iannelli F, Collino A, Sinha S, Radaelli E, Nicoli P, D'Antiga L, et al. Massive gene amplification drives paediatric hepatocellular carcinoma caused by bile salt export pump deficiency. Nature Communications. 2014;5:3850. doi: 10.1038/ncomms4850. [DOI] [PubMed] [Google Scholar]