Abstract
We previously showed that paternal cocaine exposure reduced the reinforcing efficacy of cocaine in male offspring. Here, we sought to determine whether paternal cocaine experience could also influence anxiety levels in offspring. Male rats were allowed to self-administer cocaine (controls received saline passively) for 60 days and then were bred with naïve females. Measures of anxiety and cocaine-induced anxiogenic effects were assessed in the adult offspring. Cocaine-sired male offspring exhibited increased anxiety-like behaviors, as measured using the novelty-induced hypophagia and defensive burying tasks, relative to saline-sired males. In contrast, sire cocaine experience had no effect on anxiety-like behaviors in female offspring. When challenged with an anxiogenic (but not anorectic) dose of cocaine (2.5 mg/kg, i.p.), anxiety-like behavior was enhanced in all animals to an equal degree regardless of sire drug experience. Since anxiety and depression are often comorbid, we also assessed measures of depressive-like behavior. Sire cocaine experience had no effect on depression-like behaviors, as measured by the forced swim task, among male offspring. In a separate group of naïve littermates, select neuronal correlates of anxiety were measured. Male offspring of cocaine-experienced sires showed increased mRNA and protein expression of corticotropin-releasing factor (CRF) receptor 2 in the hippocampus. Together, these results indicate that cocaine-experienced sires produce male progeny that have increased baseline anxiety, which is unaltered by subsequent cocaine exposure.
Introduction
Environmental challenges can be passed on to the next generation via epigenetic mechanisms - changes to DNA or nucleosomes in the absence of alterations in the DNA sequence. These inherited epigenetic alterations can have profound influences on gene expression, physiology and behavior in the offspring (Bale et al., 2010; Feil and Fraga, 2011; Morgan and Whitelaw, 2008; Radley et al., 2011; Vassoler and Sadri-Vakili, 2013). For example, male, but not female, offspring of cocaine-exposed sires exhibited delayed acquisition and reduced maintenance of cocaine self-administration (Vassoler et al., 2013). This effect was due at least in part to increased brain-derived neurotrophic factor (BDNF) in the medial prefrontal cortex (mPFC) (Vassoler et al., 2013), which was previously shown to decrease the reinforcing efficacy of cocaine (Berglind et al., 2007; Sadri-Vakili et al., 2010). Acetylation at BDNF promoters in the sperm of sires that self-administered cocaine was increased, suggesting that cocaine-induced reprogramming of the germline is the likely mechanism by which cocaine resistance was transmitted to the male progeny.
It is well documented that cocaine produces both appetitive and aversive effects. This is demonstrated by the fact that rodents trained to voluntarily travel down a runway into a chamber to receive cocaine infusions exhibit avoidance and retreating behaviors at the entrance to the chamber (Ettenberg and Geist, 1991). The avoidance and retreating behaviors are reduced following administration of anxiolytic agents (Ettenberg, 2009). Consistent with these findings, rats with higher anxiety levels show reduced cocaine taking on a progressive ratio reinforcement schedule (Bush and Vaccarino, 2007). The bi-directional effects of cocaine are time-dependent: initially, cocaine is rewarding, but following a delay the effects are aversive and include agitation and anxiety (Ettenberg and Bernardi, 2007). Rats exposed to a specific environment immediately following cocaine administration exhibit conditioned place preference for this context. In contrast, animals that are exposed to a distinct context 15 minutes after cocaine administration will actively avoid that environment (Ettenberg et al., 1999). The aversive effects of cocaine in this model were attenuated by anxiolytic administration (Ettenberg and Bernardi, 2007). Together, these results indicate that cocaine is initially reinforcing but quickly transitions to producing anxiety, which may partially explain instances of resilience to cocaine addiction in humans and rodents (Kreek et al., 2009; Majewska, 2002; Simmons et al., 2012). In our previous work, the delayed acquisition and decreased maintenance of cocaine self-administration among the male offspring of cocaine-experienced sires appeared to be primarily due to a decrease in the reinforcing effects of cocaine (Vassoler et al., 2013). However, it is possible that an enhancement of cocaine's aversive effects could also reduce cocaine intake in male cocaine-sired rats.
The current experiments were designed to examine whether paternal cocaine experience influences anxiety-like behavior in progeny. We assessed measures of anxiety using the novelty induced hypophagia (NIH) and defensive burying paradigms. We also examined depressive-like behavior using a forced swim test (FST). Western blot and RT-PCR were used to examine neuronal correlates of these behaviors in the hippocampus.
Materials and Methods
Animals and housing
Male and female Sprague-Dawley rats were obtained from Taconic Laboratories. Animals were maintained on a 12/12 hr light/dark cycle with food and water available ad libitum; all experimental procedures occurred during the light cycle. Surgeries, cocaine self-administration and breeding were conducted as previously described (Vassoler et al., 2013). All animal care and experiments were approved by the Institutional Animal Care and Use Committee of the Perelman School of Medicine at the University of Pennsylvania and conducted in accordance with the National Institute of Health guidelines.
Novelty Induced Hypophagia (NIH)
NIH was conducted as previously described with minor adaptations (Carr et al., 2011). Food and water were removed from the home cage 90 min prior to training and testing. Rats were habituated to the testing room in their home cages for 30 minutes. During 8 days of training and subsequent testing on days 9 and 11, animals had access to peanut butter chips (Reese's Peanut Butter Chips, 2.257 g) for 15 minutes in their home cages. On day 10, animals were given access to the peanut butter chips in a novel environment located in the same room. The novel environment was a brightly lit (1380 lux) polycarbonate cage of the same dimensions as the home cage (48cm L × 26cm W × 20 cm H) with a wire mesh floor and white walls. The novel arena was cleaned with 70 % ethanol between trials. Across training and testing, the latency to consume the peanut butter chips was recorded. Latency was determined as the time to start eating peanut butter chips by an observer blinded to siring. To determine the anxiogenic effects of cocaine, separate animals (naïve, saline-sired, or cocaine-sired) were randomly assigned to receive acute injections of saline, 2.5 mg/kg, or 5.0 mg/kg cocaine (i.p.) 30 min prior to the three test sessions. Animals were habituated to the injection on the last 3 days of training using a sham i.p. injection.
Defensive Burying (DB)
The shock-probe defensive burying test was conducted as previously described (Carr et al., 2011). Rats were placed into a clear polycarbonate cage 48 cm L × 26 cm W × 20 cm H, with a 1-cm diameter hole located 7 cm from the base of one end of the cage to accommodate the shock-probe. Fresh bedding lined the cage to a depth of 5 cm and lighting was set to 150 lx. The shock-probe extended 6 cm into the cage. The probe was attached to a shock generator (SGS-003, BRS-LVE, Laurel, MD) set to deliver 5mA of current when the probe was contacted. At the beginning of each test, animals were placed on the end opposite the shock probe, facing away from the probe. Rats typically approached the probe to investigate within 10-15 seconds, making contact with their paw or snout. The 15-minute test period began when the rat contacted the probe and received a shock. The probe remained electrified for the duration of the session. All sessions were videotaped and time spent burying was measured by an investigator blind to treatment conditions.
Forced Swim Task
This task was conducted as previously described with minor adaptations (Carr et al., 2011). Male rats from multiple saline-sired or cocaine-sired litters were selected (n=10/group). Rats underwent two swim sessions on consecutive days. The first session lasted 15 min, the second swim was 5 min in duration and administered 24 h after the first swim. The swim tests were conducted in glass cylinders (20 cm diameter) filled with water (25± 1°C). The 5-min test was videotaped and scored for frequency of climbing, swimming, and immobility behaviors using a time-sampling method by an observer blinded to sire.
Western Blot
Whole-cell hippocampal tissue was processed for Western blot as described previously (Anderson et al., 2008). Equal amounts of protein (20 μg) were loaded and separated in 10% Tris-Glycine gels (Invitrogen) and transferred to nitrocellulose membranes using the i-Blot dry transfer system (Invitrogen). Membranes were incubated overnight at 4°C with selective antibodies to CRFR2 (1:1000, Santa Cruz), or GAPDH (1:2000, Millipore). Membranes were then incubated with fluorescent secondary antibodies (1:5000, IR-dye 680 or IR-dye 800 Licor Biosciences), before being imaged on an Odyssey fluorescent scanner (Licor Biosciences). GAPDH was used as a loading control.
RT-PCR
Reactions were prepared in 96-well optical reaction plates (ABI) with optical adhesive covers (ABI) using SYBR Green PCR Master Mix (ABI). Three technical replicates were used for each animal. Reactions were carried out in the Step One Plus with an initial incubation at 95° C for 10 min, and 40 subsequent cycles of 95° C for 15 s, 60° C for 30 s. Primer sequences can be found in Table 1. Fold change was calculated as 2exp(−ΔΔCt). ΔCt values were corrected using housekeeping gene expression levels for each sample. For each sample, the ΔCt was calculated against the mean for that gene's sample set. Next, these ΔCt values were normalized to the ΔCt from housekeeping genes for each sample to account for variability in cDNA input. Because Ct values are on a logarithmic scale, fold change is equal to two raised to the difference between experimental and control ΔCt values. The data presented is the calculated mean for the biological replicates with n being equal to the number of biological replicates (that is, the number of rats examined).
Table 1. Male offspring mRNA expression levels in the hippocampus.
Primer sequences used to measure mRNA transcripts from the hippocampus of saline-sired and cocaine-sired male offspring. Fold change of mRNA expression levels.
| Gene | Primer Sequences | Fold change (± sem) | P value | |
|---|---|---|---|---|
| Forward | Reverse | |||
| Crh | CGATTCTGATCCGCATGGGT | CAGCAACACGCGGAAAAAGT | 0.84 (± 0.09) | 0.5149 |
| Crhr1 | CTGAACCCTGTGTCCACCTC | CACTCCCGGTAGCCATTGTT | 1.05 (± 0.15) | 0.8434 |
| Crhr2 | CAAGTACAACACGACCCGGA | TGATGATGAGGGCGATTCGG | 2.40 (± 0.68) | 0.0268* |
| Crhbp | CAGGTGCCTGTTGGAAATGC | CATGAAACGGTGAGTGCTGC | 0.82 (± 0.03) | 0.3737 |
| Nr3c2 | GGCCATCTCCAATGGTGTGA | AAAGAGGCGCCTGAACATGA | 0.89 (± 0.08) | 0.4381 |
| Nr3c1 | CTGAGGGGAGGAGCTACAGT | GCCCAAGTCATTCCCCATCA | 0.78 (± 0.06) | 0.2638 |
| Hprt | GTCAAGCAGTACAGCCCCAA | TGGCCACATCAACAGGACTC | housekeeper | |
| Tuba4a | AGGCTCGAGAGGATATGGCT | AACACAGTGAACAGGGCTCC | housekeeper | |
| Gapdh | AAGATTGTCAGCAATGCATCC | ACTGTGGTCATGAGCCCTTC | housekeeper | |
Statistical Analyses
Two-way repeated measures ANOVAs were used to compare latency to feed in cocaine- and saline-sired rats, and after cocaine treatment. Burying time was compared using two-tailed t-tests. Two-way ANOVAs were used to compare time spent immobile, swimming and climbing in saline-sired rats and cocaine-sired rats. For Western blots, quantification was performed by normalizing the intensity of all bands with protein-specific antibodies to the GAPDH intensity, followed by normalizing that value to the saline control (saline-sired male) values. Immunoblot analyses for change due to sire drug exposure were performed using a t-test (unpaired, one-way, p<0.05). For quantitative real-time RT–PCR experiments, t-tests were used to compare fold change values for each gene between cocaine- and saline-sired rats. Two-tailed P values are reported in Table 1.
Results
Enhanced novelty-induced hypophagia and defensive burying in male, but not female, cocaine-sired rats
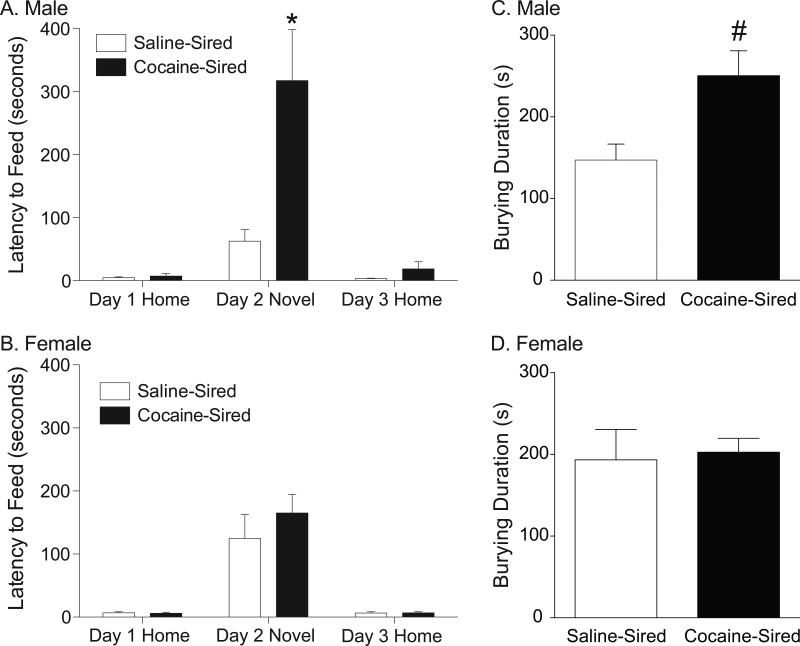
In order to assess measures of anxiety in cocaine-sired and saline-sired offspring, latency to feed in familiar (home cage) and novel environments was determined. Mixed models ANOVAs (within subject factor = environment; between factor = sire) revealed a significant main effect of sire (F1,17=8.44, p<0.0099), a significant main effect of environment (F2,34=18.18, p<0.0001), and a significant sire by environment interaction (F2,34=8.33, p<0.0011). Post hoc analyses indicated a significant increase in latency to feed in the novel environment in cocaine-sired relative to saline-sired males (Bonferroni, p<0.001, Figure 1A). Analysis of the NIH data from female offspring demonstrated a significant main effect of environment (F2,34=34.02, p<0.0001), but no significant effect of sire (p<0.424) and no significant sire by environment interaction (p<0.496, Figure 1B). Thus, novelty-induced hypophagia was observed in all groups. Siring condition had no influence on NIH in females, but cocaine-sired males displayed a significantly enhanced latency to feed in a novel environment relative to saline-sired males. These results indicate that male offspring of cocaine-experienced sires are more anxious than control animals. Defensive burying was also measured to further assess anxiety-like behavior in the offspring of cocaine-exposed and control sires. Male offspring of cocaine-experienced sires spent more time burying than control animals (t20=2.725, p=0.0131, Figure 1C), suggesting increased anxiety-like behavior. Female offspring spent equal time burying regardless of siring (t17=0.2351, p=0.8170, Figure 1D).
Figure 1. Cocaine-sired male offspring have increased anxiety-like behavior.
Data are expressed as latency (s, mean ± SEM) to consume peanut butter chips in a familiar or novel environment during a 15-minute test session. A) Cocaine-sired male offspring (n=10 animals, selected from 8 litters) show increased latency to feed compared to saline-sired male offspring (n=9 rats, selected from 9 litters). B) There is no difference between cocaine-sired (n=10 rats, 9 litters) and saline-sired (n=9 rats, 9 litters) female offspring latency to feed in the novel environment. * p<0.001 for cocaine-sired vs. saline-sired latency in the novel environment. C) Cocaine-sired male offspring (n=12 animals, 9 litters) spend more time burying compared to saline-sired male offspring (n=10 animals, 9 litters). D) There is no difference in burying time between cocaine-sired (n=10 rats, 9 litters) and saline-sired (n=9 rats, 7 litters) female offspring. # p<0.05 comparing siring.
Cocaine increased novelty-induced hypophagia equally in all siring conditions
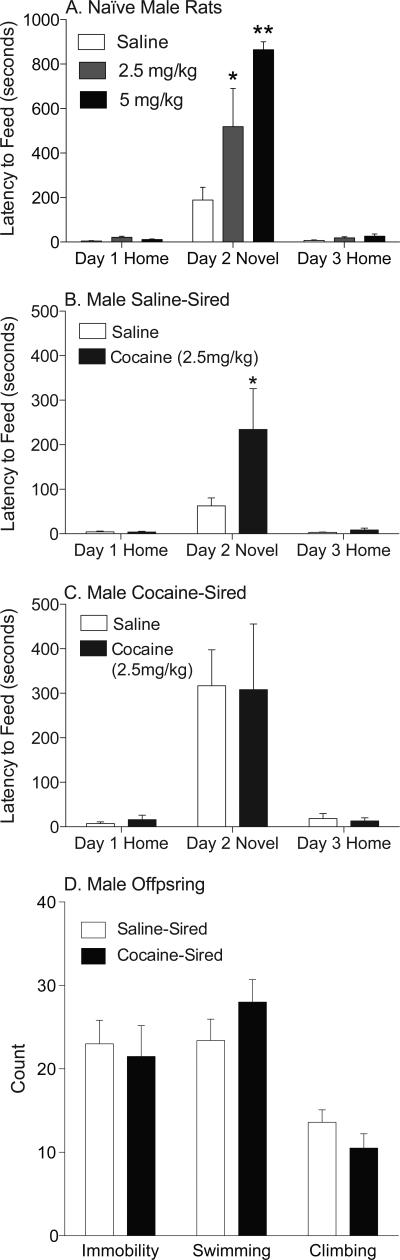
We next examined cocaine-induced anxiety using the NIH task. To address the potential confound of cocaine-induced appetite suppression in this paradigm, we tested the influence of two doses of cocaine on feeding latency in drug-naïve male rats. Consistent with previous reports, injections of low cocaine doses (2.5 and 5.0 mg/kg i.p.) had no influence on feeding behavior in the home cage (Cooper and van der Hoek, 1993), but dose-dependently increased latency to feed in a novel environment (Figure 2A). Mixed models ANOVA (within factor = environment; between factor = dose) revealed a significant main effect of drug (F2,9=9.64, p<0.0058), a significant main effect of environment (F2,9=72.75, p<0.0001), and a significant drug by environment interaction (F4,18=10.30, p<0.0002). Post hoc tests showed a significant increase in latency to feed in the novel environment at 2.5 mg/kg and 5 mg/kg cocaine relative to saline (Bonferroni, p<0.01 at 2.5 mg/kg, p<0.001 at 5 mg/kg).
Figure 2. Acute cocaine increases latency to feed in a novel environment.
A) Animals received 0 (n=4), 2.5 mg/kg (n=4), or 5 mg/kg (n=4) cocaine (i.p.) 30 minutes prior to all test sessions. Cocaine dose-dependently increased latency to feed in the novel environment, but not in the homecage. B) Animals received the 2.5 mg/kg dose of cocaine (i.p.) 30 minutes prior to all testing sessions. Cocaine (n=6 animals, selected from 5 litters) significantly increased latency to feed in saline-sired male offspring relative to saline injection (n=9 rats, 8 litters). C) Pre-exposure to 2.5 mg/kg cocaine (n=6 animals, selected from 6 litters) did not enhance latency to feed compared to cocaine-sired male offspring treated with saline (n=10 animals, 9 litters). D) Paternal cocaine experience did not influence immobility, swimming or climbing in the male offspring during a forced swim test. Data are represented as counts (mean ± SEM) of immobility, swimming, or climbing behaviors recorded every 5 seconds during a 5 minute forced swim (n=10 rats, 6 litters for each group). * p<0.01, ** p<0.001.
These results indicate that both doses of cocaine would be appropriate to assess the anxiogenic effects of cocaine. We used the 2.5 mg/kg dose of cocaine to measure the anxiogenic properties of cocaine in male progeny of cocaine-experienced rats. For male offspring of saline-exposed sires, mixed models ANOVA (within factor = environment; between factor = treatment) showed a significant main effect of drug treatment (F1,13=4.782, p<0.0476), a significant main effect of environment (F2,26=14.63, p<0.0001), and a significant treatment by environment interaction (F2,26=5.097, p<0.0136). Post hoc tests revealed a significant difference between groups when the injections occurred in the novel environment (Bonferroni, p<0.01) indicating that cocaine increased NIH in saline-sired rats (Figure 2B). For male progeny of cocaine-experienced sires, a separate ANOVA showed a significant effect of environment (F2,28=16.20, p<0.0001), but no significant effect of drug treatment (p<0.9737) and no significant treatment by environment interaction (p<0.9878). These results indicate that cocaine did not further enhance NIH in cocaine-sired rats (Figure 2C). Note that enhanced NIH among the male offspring of cocaine-experienced sires was replicated in this experiment. In rats that received saline only, latency to feed in the novel environment was significantly greater in cocaine-sired relative to saline sired rats (t17=2.918, p<0.0096). Taken together, these results indicate that cocaine produces similar levels of anxiety in male offspring of cocaine-experienced sires and in control progeny. Because anxiety and depression are often comorbid, we also assessed behavior in a forced swim test. Siring had no impact on swimming, climbing or immobility during the forced swim test (F1254=1.227, p=0.3013, Figure 2D), suggesting that paternal cocaine exposure does not alter depression-like phenotypes in male offspring.
Increased mRNA and protein expression of corticotropin releasing factor receptor 2 in the hippocampus of male cocaine-sired rats
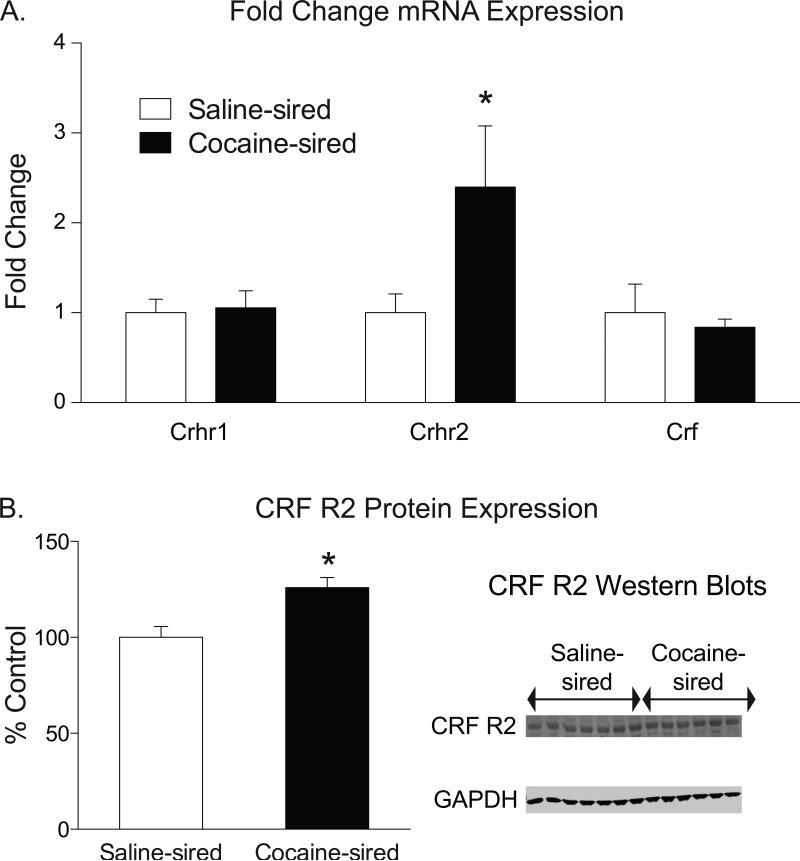
The hippocampus modulates the expression of anxiety and performance in the NIH paradigm (Gundersen and Blendy, 2009; McEwen et al., 2012; Onksen et al., 2012). CRF signaling as well as glucocorticoid and mineralocorticoid receptors are known to influence anxiety-related behavior. We used quantitative RT-PCR to assess hippocampal mRNA expression of Crf (encodes CRF), Crhr1 (CRF-R1), Crhr2 (CRF-R2) Nr3c2 (Mineralocorticoid Receptor 1), and Nr3c1 (Glucocorticoid Nuclear Receptor 1) in male progeny of saline- and cocaine-experienced rats. Hippocampal Crhr2 expression was increased in cocaine-sired rats relative to saline-sired controls (t11=2.553, p<0.05, Figure 3A). Crf, Crhr1, Nr3c2, and Nr3c1 gene expression was similar in saline-sired compared to cocaine-sired male offspring (Table 1). We also measured hippocampal protein levels in male saline- and cocaine-sired offspring using Western blot. CRF receptor 2 (CRF-R2) protein expression was increased in male offspring of cocaine-experienced sires relative to control (t12=3.285, p<0.01, Figure 3B). Taken together, these findings indicate that gene and protein expression of CRF-R2 is increased in the hippocampus of cocaine-sired males.
Figure 3. Cocaine-sired male offspring have increased hippocampal expression of CRFR2.
A) Cocaine-sired male (n=5 rats, 5 litters) offspring showed increased expression of Crhr2 transcript compared to saline-sired male offspring (n=4 animals, 4 litters). There was no effect of sire on Crf, Crhr1, Nr3c2, or Nr3c1 expression levels. B) Cocaine-sired male offspring (n=6, 5 litters) showed increased levels of CRFR2 protein compared to saline-sired male (n=4 rats, 4 litters). Western blots for CRFR2 and GAPDH are shown on the right. Data are shown as mean ± SEM, * p<0.05.
Discussion
We previously showed that sire cocaine experience reduced the acquisition and maintenance of cocaine self-administration in male, but not female, offspring (Vassoler et al., 2013). The NIH and defensive burying tests were chosen to measure anxiety-like behavior because both tasks have outstanding predictive validity for the anxiolytic effects of therapeutics (De Boer and Koolhaas, 2003; Dulawa and Hen, 2005). We found that male, but not female offspring of cocaine-experienced sires have increased anxiety. Because both phenotypes are sex-specific, we investigated whether increased anxiety could contribute to reduced cocaine taking in male offspring of sires that self-administered cocaine. Previous reports indicate animals with higher baseline anxiety levels self-administer less cocaine on a progressive ratio schedule (Bush and Vaccarino, 2007). However, it is unclear whether cocaine taking further increases anxiety-like behavior in that study. Hence, rats with higher anxiety could also be more sensitive to the anxiogenic properties of cocaine, which may contribute to the reported correlation between higher anxiety and lower cocaine taking (Bush and Vaccarino, 2007). Here, we found that the anxiogenic effect of cocaine was not increased in male offspring of cocaine-experienced sires, suggesting that cocaine-induced anxiety does not contribute to the reduction of cocaine taking that we previously observed (Vassoler et al., 2013).
Anxiety and depression are often co-morbid disorders and commonly associated with drug abuse (Swendsen and Merikangas, 2000). In our model of the inter-generational effects of paternal cocaine exposure, male offspring of cocaine-experienced sires had similar performance as controls in a forced swim test. The FST paradigm is commonly used to study depressive-like behaviors in rodents and has been shown to have terrific predictive validity (Castagne et al., 2011). Taken together, our findings indicate that paternal cocaine taking selectively increases baseline anxiety levels in male offspring without causing depressive-like phenotypes.
Parental Drug Use and Offspring Emotional Regulation
A growing body of evidence suggests that parental drug exposure enhances anxiety-like behaviors in offspring. Prenatal cocaine exposure in utero in rats increases anxiety-like behaviors in both male and female offspring (Sithisarn et al., 2011), with at least one study showing a more substantial anxiogenic phenotype in male progeny (Salas-Ramirez et al., 2010). In mice, paternal experimenter-delivered cocaine injections produced offspring with increased immobility in the tail suspension test, a model of depression, but no evidence of increased anxiety-like behavior in the elevated plus maze (Killinger et al., 2012). The discrepancy between these findings and our current results could be due to the differences in species (mice versus rats) and to methodological considerations (non-contingent cocaine injections versus cocaine self-administration). We chose to use the self-administration paradigm because it is highly homologous to cocaine taking in humans. One interesting possibility is that the influence of paternal cocaine exposure on progeny depends on the way cocaine is delivered. Further studies would be needed to investigate this possibility.
Cocaine Exposure Correlates of Anxiety
Corticotropin releasing factor (CRF) is an integral part of the hypothalamic-pituitary-adrenal (HPA) axis. Chronic cocaine exposure results in HPA axis dysregulation (Corominas et al., 2010; Hahn et al., 2009; Zorrilla et al., 2001), and changes in CRF levels are thought to contribute to anxiety-like behaviors associated with cocaine withdrawal (Ambrosio et al., 1997; Basso et al., 1999; Sarnyai et al., 1995; Sarnyai et al., 2001). We found increased hippocampal CRF-R2 expression at the transcript and protein levels, which could contribute to the development of anxiety-like behavior that we observed in these animals. Reports regarding the influence of CRF2 receptors on anxiety-like behaviors are mixed with some studies indicating that activation of CRF-R2 is anxiogenic (Bakshi et al., 2002; Takahashi et al., 2001), while other work suggests that CRF-R2 stimulation is anxiolytic (Bale et al., 2000; Kishimoto et al., 2000; Valdez et al., 2002). Future studies could examine whether alterations in CRF-R2 signaling in limbic regions contribute to the development of anxiety in offspring of animals with a history of cocaine intake. Altered CRF signaling in the hippocampus could also have profound consequences on hippocampal function (Blank et al., 2002; Chen et al., 2010; Rebaudo et al., 2001) and hippocampus-dependent processes such as memory formation.
Translational Implications
A number of studies indicate that the children of parents dependent on alcohol and other drugs of abuse display increased prevalence of psychopathologies including attention deficit disorder, conduct disorder, depression and, notably, anxiety (Clark et al., 1997; Earls et al., 1988; Hill and Muka, 1996). Focusing specifically on paternal drug addiction, children whose fathers met the criteria for substance abuse dependence had higher levels of anxiety and other pathologies (Moss et al., 2001; Moss et al., 2002). Gender differences in these studies were not clear, although substantially more male children were included than females (Moss et al., 2002). Although compelling, these results should be interpreted with caution given that substance dependent fathers often have histories of other psychopathologies (Clark et al., 2004). Moreover, the parents in these studies were generally poly-drug abusers and the influence of specific drugs on offspring behavior was not assessed. Nonetheless, the present results in rats are consistent with clinical observations of increased incidences of anxiety disorders among the children of fathers with substance use disorders. Thus, we have developed an animal model that can be used to further assess the neurobiological changes resulting in enhanced anxiety in the offspring of cocaine-dependent fathers, which could lead to the development of more specific and potentially novel treatments for this particular anxiety disorder.
Summary and Conclusions
Our findings indicate that paternal cocaine self-administration caused increased anxiety-like behaviors in male, but not in female offspring. Cocaine-sired males did not exhibit depressive-like behavior, relative to saline-sired male offspring, in the FST indicating that paternal cocaine exposure may elicit anxiety-like behaviors without co-morbid depressive-like phenotypes. Notably, acute exposure to cocaine followed by a 30-minute delay enhanced latency to feed in saline-sired, but not cocaine-sired males. We observed increases in mRNA and protein expression of CRF-R2 in the hippocampus of cocaine-sired males, which may contribute to the development of heightened anxiety that we observed. Thus, sire cocaine experience promotes a sex-specific, anxiety-like phenotype in offspring, potentially via modifications to proteins in the hippocampus known to influence mood regulation.
Acknowledgements
This work was supported by grants from the US National Institutes of Health (R01 DA33641, K02 DA18678, T32 DA28874, F31 DA31535, K01 DA30445). We thank, B. Kimmey, A. Arreola, S. Friedman, and K. Ige for technical assistance. We thank I. Lucki, G. Carr, and L. Briand for advice on experimental design.
Footnotes
Authors Contributions: SLW, FMV, HDS and RCP designed the study. SLW, FMV and MEW acquired and analyzed data. RCP contributed to data analysis and interpretation of findings. MEW drafted the manuscript. SLW, FMV, HDS and RCP provided revision of the manuscript. All authors reviewed and approved the final version of this manuscript.
The authors declare no competing financial interests.
References
- Ambrosio E, Sharpe LG, Pilotte NS. Regional binding to corticotropin releasing factor receptors in brain of rats exposed to chronic cocaine and cocaine withdrawal. Synapse. 1997;25:272–276. doi: 10.1002/(SICI)1098-2396(199703)25:3<272::AID-SYN6>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Anderson SM, Famous KR, Sadri-Vakili G, Kumaresan V, Schmidt HD, Bass CE, Terwilliger EF, Cha JH, Pierce RC. CaMKII: a biochemical bridge linking accumbens dopamine and glutamate systems in cocaine seeking. Nat Neurosci. 2008;11:344–353. doi: 10.1038/nn2054. [DOI] [PubMed] [Google Scholar]
- Bakshi VP, Smith-Roe S, Newman SM, Grigoriadis DE, Kalin NH. Reduction of stress-induced behavior by antagonism of corticotropin-releasing hormone 2 (CRH2) receptors in lateral septum or CRH1 receptors in amygdala. J Neurosci. 2002;22:2926–2935. doi: 10.1523/JNEUROSCI.22-07-02926.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL, Baram TZ, Brown AS, Goldstein JM, Insel TR, McCarthy MM, Nemeroff CB, Reyes TM, Simerly RB, Susser ES, Nestler EJ. Early life programming and neurodevelopmental disorders. Biol Psychiatry. 2010;68:314–319. doi: 10.1016/j.biopsych.2010.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL, Contarino A, Smith GW, Chan R, Gold LH, Sawchenko PE, Koob GF, Vale WW, Lee KF. Mice deficient for corticotropin-releasing hormone receptor-2 display anxiety-like behaviour and are hypersensitive to stress. Nat Genet. 2000;24:410–414. doi: 10.1038/74263. [DOI] [PubMed] [Google Scholar]
- Basso AM, Spina M, Rivier J, Vale W, Koob GF. Corticotropin-releasing factor antagonist attenuates the “anxiogenic-like” effect in the defensive burying paradigm but not in the elevated plus-maze following chronic cocaine in rats. Psychopharmacology (Berl) 1999;145:21–30. doi: 10.1007/s002130051028. [DOI] [PubMed] [Google Scholar]
- Berglind WJ, See RE, Fuchs RA, Ghee SM, Whitfield TW, Jr., Miller SW, McGinty JF. A BDNF infusion into the medial prefrontal cortex suppresses cocaine seeking in rats. Eur J Neurosci. 2007;26:757–766. doi: 10.1111/j.1460-9568.2007.05692.x. [DOI] [PubMed] [Google Scholar]
- Blank T, Nijholt I, Eckart K, Spiess J. Priming of long-term potentiation in mouse hippocampus by corticotropin-releasing factor and acute stress: implications for hippocampus-dependent learning. J Neurosci. 2002;22:3788–3794. doi: 10.1523/JNEUROSCI.22-09-03788.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush DE, Vaccarino FJ. Individual differences in elevated plus-maze exploration predicted progressive-ratio cocaine self-administration break points in Wistar rats. Psychopharmacology (Berl) 2007;194:211–219. doi: 10.1007/s00213-007-0835-7. [DOI] [PubMed] [Google Scholar]
- Carr GV, Schechter LE, Lucki I. Antidepressant and anxiolytic effects of selective 5-HT6 receptor agonists in rats. Psychopharmacology (Berl) 2011;213:499–507. doi: 10.1007/s00213-010-1798-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castagne V, Moser P, Roux S, Porsolt RD. Rodent models of depression: forced swim and tail suspension behavioral despair tests in rats and mice. Curr Protoc Neurosci. 2011 doi: 10.1002/0471142301.ns0810as55. Chapter 8:Unit 8 10A. [DOI] [PubMed] [Google Scholar]
- Chen Y, Rex CS, Rice CJ, Dube CM, Gall CM, Lynch G, Baram TZ. Correlated memory defects and hippocampal dendritic spine loss after acute stress involve corticotropin-releasing hormone signaling. Proc Natl Acad Sci USA. 2010;107:13123–13128. doi: 10.1073/pnas.1003825107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DB, Cornelius J, Wood DS, Vanyukov M. Psychopathology risk transmission in children of parents with substance use disorders. Am J Psychiatry. 2004;161:685–691. doi: 10.1176/appi.ajp.161.4.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DB, Moss HB, Kirisci L, Mezzich AC, Miles R, Ott P. Psychopathology in preadolescent sons of fathers with substance use disorders. J Am Acad Child Adolesc Psychiatry. 1997;36:495–502. doi: 10.1097/00004583-199704000-00012. [DOI] [PubMed] [Google Scholar]
- Cooper SJ, van der Hoek GA. Cocaine: a microstructural analysis of its effects on feeding and associated behaviour in the rat. Brain Res. 1993;608:45–51. doi: 10.1016/0006-8993(93)90772-f. [DOI] [PubMed] [Google Scholar]
- Corominas M, Roncero C, Casas M. Corticotropin releasing factor and neuroplasticity in cocaine addiction. Life Sci. 2010;86:1–9. doi: 10.1016/j.lfs.2009.11.005. [DOI] [PubMed] [Google Scholar]
- De Boer SF, Koolhaas JM. Defensive burying in rodents: ethology, neurobiology and psychopharmacology. Eur J Pharmacol. 2003;463:145–161. doi: 10.1016/s0014-2999(03)01278-0. [DOI] [PubMed] [Google Scholar]
- Dulawa SC, Hen R. Recent advances in animal models of chronic antidepressant effects: the novelty-induced hypophagia test. Neurosci Biobehav Rev. 2005;29:771–783. doi: 10.1016/j.neubiorev.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Earls F, Reich W, Jung KG, Cloninger CR. Psychopathology in children of alcoholic and antisocial parents. Alcohol Clin Exp Res. 1988;12:481–487. doi: 10.1111/j.1530-0277.1988.tb00230.x. [DOI] [PubMed] [Google Scholar]
- Ettenberg A. The runway model of drug self-administration. Pharmacol Biochem Behav. 2009;91:271–277. doi: 10.1016/j.pbb.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettenberg A, Bernardi RE. Effects of buspirone on the immediate positive and delayed negative properties of intravenous cocaine as measured in the conditioned place preference test. Pharmacol Biochem Behav. 2007;87:171–178. doi: 10.1016/j.pbb.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettenberg A, Geist TD. Animal model for investigating the anxiogenic effects of self-administered cocaine. Psychopharmacology (Berl) 1991;103:455–461. doi: 10.1007/BF02244244. [DOI] [PubMed] [Google Scholar]
- Ettenberg A, Raven MA, Danluck DA, Necessary BD. Evidence for opponent-process actions of intravenous cocaine. Pharmacol Biochem Behav. 1999;64:507–512. doi: 10.1016/s0091-3057(99)00109-4. [DOI] [PubMed] [Google Scholar]
- Feil R, Fraga MF. Epigenetics and the environment: emerging patterns and implications. Nat Rev Genet. 2011;13:97–109. doi: 10.1038/nrg3142. [DOI] [PubMed] [Google Scholar]
- Gundersen BB, Blendy JA. Effects of the histone deacetylase inhibitor sodium butyrate in models of depression and anxiety. Neuropharmacology. 2009;57:67–74. doi: 10.1016/j.neuropharm.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn J, Hopf FW, Bonci A. Chronic cocaine enhances corticotropin-releasing factor-dependent potentiation of excitatory transmission in ventral tegmental area dopamine neurons. J Neurosci. 2009;29:6535–6544. doi: 10.1523/JNEUROSCI.4773-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Muka D. Childhood psychopathology in children from families of alcoholic female probands. J Am Acad Child Adolesc Psychiatry. 1996;35:725–733. doi: 10.1097/00004583-199606000-00012. [DOI] [PubMed] [Google Scholar]
- Killinger CE, Robinson S, Stanwood GD. Subtle biobehavioral effects produced by paternal cocaine exposure. Synapse. 2012;66:902–908. doi: 10.1002/syn.21582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto T, Radulovic J, Radulovic M, Lin CR, Schrick C, Hooshmand F, Hermanson O, Rosenfeld MG, Spiess J. Deletion of crhr2 reveals an anxiolytic role for corticotropin-releasing hormone receptor-2. Nat Genet. 2000;24:415–419. doi: 10.1038/74271. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Schlussman SD, Reed B, Zhang Y, Nielsen DA, Levran O, Zhou Y, Butelman ER. Bidirectional translational research: Progress in understanding addictive diseases. Neuropharmacology 56 Suppl. 2009;1:32–43. doi: 10.1016/j.neuropharm.2008.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majewska MD. HPA axis and stimulant dependence: an enigmatic relationship. Psychoneuroendocrinology. 2002;27:5–12. doi: 10.1016/s0306-4530(01)00033-6. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Eiland L, Hunter RG, Miller MM. Stress and anxiety: structural plasticity and epigenetic regulation as a consequence of stress. Neuropharmacology. 2012;62:3–12. doi: 10.1016/j.neuropharm.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan DK, Whitelaw E. The case for transgenerational epigenetic inheritance in humans. Mamm Genome. 2008;19:394–397. doi: 10.1007/s00335-008-9124-y. [DOI] [PubMed] [Google Scholar]
- Moss HB, Baron DA, Hardie TL, Vanyukov MM. Preadolescent children of substance-dependent fathers with antisocial personality disorder: psychiatric disorders and problem behaviors. Am J Addict. 2001;10:269–278. doi: 10.1080/105504901750532157. [DOI] [PubMed] [Google Scholar]
- Moss HB, Lynch KG, Hardie TL, Baron DA. Family functioning and peer affiliation in children of fathers with antisocial personality disorder and substance dependence: associations with problem behaviors. Am J Psychiatry. 2002;159:607–614. doi: 10.1176/appi.ajp.159.4.607. [DOI] [PubMed] [Google Scholar]
- Onksen JL, Briand LA, Galante RJ, Pack AI, Blendy JA. Running-induced anxiety is dependent on increases in hippocampal neurogenesis. Genes Brain Behav. 2012;11:529–538. doi: 10.1111/j.1601-183X.2012.00788.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Kabbaj M, Jacobson L, Heydendael W, Yehuda R, Herman JP. Stress risk factors and stress-related pathology: neuroplasticity, epigenetics and endophenotypes. Stress. 2011;14:481–497. doi: 10.3109/10253890.2011.604751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebaudo R, Melani R, Balestrino M, Izvarina N. Electrophysiological effects of sustained delivery of CRF and its receptor agonists in hippocampal slices. Brain Res. 2001;922:112–117. doi: 10.1016/s0006-8993(01)03160-2. [DOI] [PubMed] [Google Scholar]
- Sadri-Vakili G, Kumaresan V, Schmidt HD, Famous KR, Chawla P, Vassoler FM, Overland RP, Xia E, Bass CE, Terwilliger EF, Pierce RC, Cha JH. Cocaine-induced chromatin remodeling increases brain-derived neurotrophic factor transcription in the rat medial prefrontal cortex, which alters the reinforcing efficacy of cocaine. J Neurosci. 2010;30:11735–11744. doi: 10.1523/JNEUROSCI.2328-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas-Ramirez KY, Frankfurt M, Alexander A, Luine VN, Friedman E. Prenatal cocaine exposure increases anxiety, impairs cognitive function and increases dendritic spine density in adult rats: influence of sex. Neuroscience. 2010;169:1287–1295. doi: 10.1016/j.neuroscience.2010.04.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnyai Z, Biro E, Gardi J, Vecsernyes M, Julesz J, Telegdy G. Brain corticotropin-releasing factor mediates ‘anxiety-like’ behavior induced by cocaine withdrawal in rats. Brain Res. 1995;675:89–97. doi: 10.1016/0006-8993(95)00043-p. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Shaham Y, Heinrichs SC. The role of corticotropin-releasing factor in drug addiction. Pharmacol Rev. 2001;53:209–243. [PubMed] [Google Scholar]
- Simmons RK, Howard JL, Simpson DN, Akil H, Clinton SM. DNA methylation in the developing hippocampus and amygdala of anxiety-prone versus risk-taking rats. Dev Neurosci. 2012;34:58–67. doi: 10.1159/000336641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sithisarn T, Bada HS, Dai H, Randall DC, Legan SJ. Effects of perinatal cocaine exposure on open field behavior and the response to corticotropin releasing hormone (CRH) in rat offspring. Brain Res. 2011;1370:136–144. doi: 10.1016/j.brainres.2010.11.024. [DOI] [PubMed] [Google Scholar]
- Swendsen JD, Merikangas KR. The comorbidity of depression and substance use disorders. Clin Psychol Rev. 2000;20:173–189. doi: 10.1016/s0272-7358(99)00026-4. [DOI] [PubMed] [Google Scholar]
- Takahashi LK, Ho SP, Livanov V, Graciani N, Arneric SP. Antagonism of CRF(2) receptors produces anxiolytic behavior in animal models of anxiety. Brain Res. 2001;902:135–142. doi: 10.1016/s0006-8993(01)02405-2. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Inoue K, Koob GF, Rivier J, Vale W, Zorrilla EP. Human urocortin II: mild locomotor suppressive and delayed anxiolytic-like effects of a novel corticotropin-releasing factor related peptide. Brain Res. 2002;943:142–150. doi: 10.1016/s0006-8993(02)02707-5. [DOI] [PubMed] [Google Scholar]
- Vassoler FM, Sadri-Vakili G. Mechanisms of transgenerational inheritance of addictive-like behaviors. Neuroscience. 2013;264:198–206. doi: 10.1016/j.neuroscience.2013.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassoler FM, White SL, Schmidt HD, Sadri-Vakili G, Pierce RC. Epigenetic inheritance of a cocaine-resistance phenotype. Nature Neuroscience. 2013;16:42–47. doi: 10.1038/nn.3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla EP, Valdez GR, Weiss F. Changes in levels of regional CRF-like-immunoreactivity and plasma corticosterone during protracted drug withdrawal in dependent rats. Psychopharmacology (Berl) 2001;158:374–381. doi: 10.1007/s002130100773. [DOI] [PubMed] [Google Scholar]