Abstract
HIV-1-associated disruption of intestinal homeostasis is a major factor contributing to chronic immune activation and inflammation. Dendritic cells (DCs) are crucial in maintaining intestinal homeostasis, but the impact of HIV-1 infection on intestinal DC number and function has not been extensively studied. We compared the frequency and activation/maturation status of colonic myeloid DC (mDC) subsets (CD1c+ and CD1cneg) and plasmacytoid DCs in untreated HIV-1-infected subjects with uninfected controls. Colonic mDCs in HIV-1-infected subjects had increased CD40 but decreased CD83 expression, and CD40 expression on CD1c+ mDCs positively correlated with mucosal HIV-1 viral load, with mucosal and systemic cytokine production, and with frequencies of activated colon and blood T cells. Percent of CD83+CD1c+ mDCs negatively correlated with frequencies of IFN-γ-producing colon CD4+ and CD8+ T cells. CD40 expression on CD1c+ mDCs positively associated with abundance of high prevalence mucosal Prevotella copri and P. stercorea, but negatively associated with a number of low prevalence mucosal species including Rumminococcus bromii. CD1c+ mDC cytokine production was greater in response to in vitro stimulation with Prevotella species relative to R. bromii. These findings suggest that during HIV infection, colonic mDCs become activated upon exposure to mucosal pathobiont bacteria leading to mucosal and systemic immune activation.
Keywords: Human intestinal dendritic cells, HIV-1 pathogenesis, microbiome, mucosal immunology
INTRODUCTION
A hallmark of HIV-1 disease is a gradual decline in peripheral blood CD4 T cells associated with chronic immune activation, defined by increased levels of pro-inflammatory cytokines, innate and adaptive immune cell activation, and soluble markers of inflammation.1 T cell activation, and in particular CD8+ T cell activation, has been shown to be a strong predictor of disease progression2–4. In the era of combination anti-retroviral therapy (cART), low levels of T cell activation and inflammation persist in many individuals despite controlled viral replication and have been linked to poor immune reconstitution and adverse clinical outcomes.1 Thus, understanding the mechanisms that drive chronic immune activation and the attendant inflammation in the setting of HIV-1 infection is important in order to develop therapeutic approaches to prevent inflammation-associated morbidity and mortality.
Although multiple factors likely contribute to chronic immune activation during HIV-1 infection, microbial translocation (MT) – the movement of bacteria or bacteria products from the gut lumen into the lamina propria (LP) and systemic circulation – has recently been implicated as a major driving force.5 Plasma bacterial lipopolysaccharides (LPS) levels have been associated with systemic T cell activation, and LPS levels in the first years of chronic HIV-1 infection were found to predict HIV-1 disease progression.6,7 In addition to LPS, other indicators of systemic MT such as sCD14, intestinal fatty acid binding protein (iFABP) and zonulin have also been associated with disease progression in untreated, and with mortality in treated, HIV-1-infected subjects.8,9
Increased MT occurs as a result of HIV-1-associated immunological and structural damage to the gastrointestinal (GI) tract. Within days of infection, irrespective of the route of transmission, HIV-1 replication results in the severe and rapid depletion of intestinal memory CD4 T cells including preferential depletion of T helper (Th)17 and Th22 cells, T cell subsets involved in normal mucosal defense and epithelial barrier maintenance.10 In addition, increased activated CD8+ T cell frequencies,11–13 increased pro-inflammatory cytokines,14 and alterations in the composition of microbial communities have been observed in the GI tract of HIV-1-infected subjects.10,15 We recently identified an altered colonic mucosal microbiome in untreated, HIV-infected subjects that was associated with plasma LPS levels and mucosal and systemic T cell activation.16 Furthermore, these altered microbial communities were associated with increased expression of the activation marker, CD40, on intestinal myeloid dendritic cells (mDCs).
Intestinal DCs sample luminal microbes and their products and are critical in mediating the delicate balance between immunogenic and tolerogenic intestinal immune responses,17 yet few studies have directly addressed the contribution of intestinal DCs to HIV-1-associated mucosal pathogenesis. We previously identified a subset of resident mDCs present in the LP of normal small and large bowel that were capable of producing pro-inflammatory cytokines (including IL-23) in response to in vitro stimulation with a viral Toll-like Receptor (TLR) ligand that mimicked innate signaling by HIV-1.18 Moreover, levels of pro-inflammatory IL-23 were synergistically increased when mDC were stimulated by a combination of bacterial and viral TLR ligands, suggesting that during HIV-1 infection, concurrent exposure to both virus and translocating enteric bacteria and bacterial products could result in enhanced production of pro-inflammatory cytokines by intestinal mDCs in vivo. Further, we showed that exposure to certain commensal bacteria enhanced HIV-1 infection of intestinal CD4 T cells in vitro, and this process was dependent upon the presence of mDCs.19 Based on these findings and the likelihood that LP DCs would be exposed to translocating mucosa-associated bacteria, we hypothesized that intestinal DCs would play a critical role in mediating viral and bacterial signals during HIV-1 infection in vivo.
RESULTS
CD40 expression is increased and CD83 expression decreased on colonic mDCs in HIV-1-infected subjects
Twenty-four HIV-1-infected individuals and 14 age- and sex-matched HIV-1 uninfected controls were enrolled into a cross-sectional study from whom rectosigmoid biopsies, peripheral blood, and stool samples were collected. Based on study entry criteria, HIV-1-infected subjects were ART-treatment naïve or had not been on treatment for more than 7 days in the preceding 6 months. Of the 24 HIV-1-infected subjects, 5 subjects reported in a study questionnaire that they had taken ART at some point during their course of HIV-1 infection. Of these, 3 had stopped ART at least 8 years (range 8–14yrs) prior to the study, 1 subject stopped 3 years prior, and the remaining subject stopped 13 months prior to inclusion in our study. Additional exclusion criteria are detailed in Supplementary Materials and Methods. Subject characteristics are provided in Table 1.
Table 1.
Subject Characteristics
| Uninfected subjects | HIV-1-infected subjects | |
|---|---|---|
| Number of subjects | 14 | 24 |
| Age (yrs) | 31 (23–54) | 33.5 (22–58) |
| Male/Female Ratio | 9/5 | 18/6 |
| CD4 count (cells/µl) | 724 (468–1071) | 445 (221–1248)* |
| Plasma Viral Load (HIV-1 RNA copies/ml) | - | 51350 (2880 – 207000) |
| Years since first HIV-1 seropositive test | - | 3.25 (0.17–15) |
| Ethnicity:n/s | ||
| Non-Hispanic | 11 (78.6%) | 19 (79.2%) |
| Hispanic | 3 (21.4%) | 5 (20.8%) |
| Race:n/s | ||
| White/Caucasian | 10 (71.4%) | 17 (70.8%) |
| Black/African American | 2 (14.2%) | 6 (25.0%) |
| Asian | 2 (14.2%) | 1 (4.2%) |
Values are shown as median (range) except for Ethnicity and Race which are shown as the number and percentage of each cohort. Statistical analysis was performed using the Mann-Whitney test for comparisons between uninfected and HIV-1-infected subjects and the Fisher Exact test or Chi-square test for comparison of categorical data.
p=0.001.
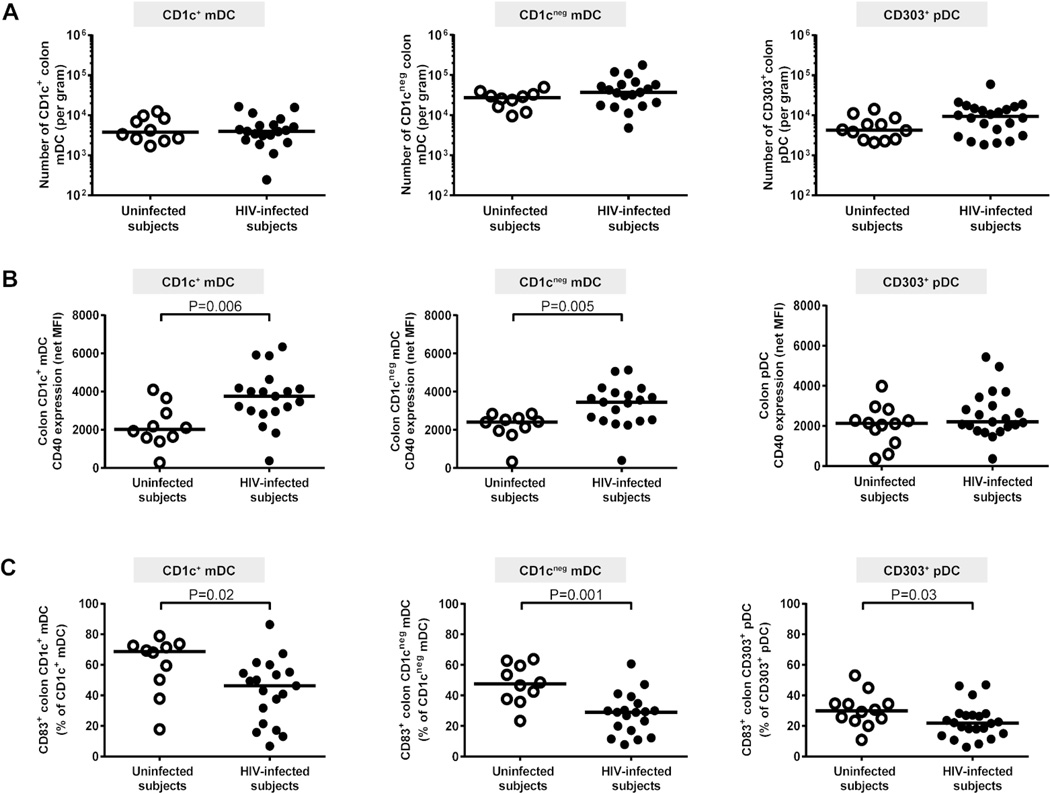
In initial studies, two phenotypically distinct colonic LP mDC subsets were identified, both of which expressed HLA-DR and CD11c but were delineated by expression of CD1c, (Supplementary Results, Supplementary Figure 1). Similar frequencies of both CD1c+ and CD1cneg mDCs were observed in uninfected and HIV-1-infected subjects when enumerated as either a percent of viable, CD45+ cells (Supplementary Table S1) or as an absolute number of DCs per gram of mucosal tissue (Figure 1a). Histological techniques were also utilized to enumerate CD11c+ DCs and HAM56+ tissue macrophages in colonic tissue sections obtained from a subset of HIV-1-infected (n=6) and uninfected (n=6) subjects. A similar number of CD11c+ DCs were enumerated in both cohorts (HIV-1-infected: median 12.1 CD11c+ cells/mm2, range 3.8–19.1; uninfected controls: 12.4 CD11c+ cells/mm2, 7.2–23.7; p=0.75), but a higher number of HAM56+ cells per mm2 of tissue were found in HIV-1-infected subjects (15.7 HAM56+ cells/mm2, 8.7–20.3) compared to uninfected controls (3.9 HAM56+ cells/mm2, 2.3–13.0; p=0.02). Increased frequencies of macrophages have also recently been reported in the duodenal mucosa of treatment naïve HIV-1-infected subjects.20
Figure 1. Colon dendritic cells (DCs) from HIV-1-infected subjects have an altered activation profile.
Multi-color flow cytometry techniques were used to determine frequencies and activation/maturation states of colon CD1c+ myeloid DCs (mDCs), CD1cneg mDCs and CD303+ plasmacytoid DCs (pDC) in uninfected (open circles) and HIV-1-infected (HIV-infected; closed circles) subjects. (A) Frequencies of CD1c+ mDCs, CD1cneg mDCs (uninfected n=10; HIV-infected n=19) and CD303+ pDCs (uninfected n=12, HIV-infected n=22) were evaluated as a percent of viable, CD45+ leucocytes and converted into a total number of DC per gram of tissue. (B) CD40 expression levels (Mean Fluorescence Intensity; MFI) and (C) percent of CD83+ DCs were assessed on CD1c+ mDCs, CD1cneg mDCs (uninfected n=10; HIV-infected n=19) and CD303+ pDCs (uninfected n=12, HIV-infected n=22). Appropriate isotype controls were removed to control for background staining (net). Lines represent median values and statistical analysis was performed using the Mann-Whitney test.
Colon CD303+ pDCs, normally found at very low frequencies,18 were next assessed for frequency and activation status. We did not observe any statistical difference in the frequencies of colonic pDCs (Figure 1a, Supplementary Table S1) although a trend towards higher numbers of pDCs in HIV-1-infected subjects (median: 9354 pDC/gram, 1835–59658) compared to uninfected subjects (4243, 2104–14155; p=0.09) was noted.
Colonic CD1c+ mDC and CD1cneg mDC activation based on CD40 expression was significantly higher in HIV-1-infected subjects compared to uninfected controls (Figure 1b). Conversely, CD40 expression on pDC was not statistically different between the two subject cohorts (Figure 1b). However, the absolute number of CD40+ pDCs was statistically greater in HIV-1-infected subjects (9047 CD40+ pDC/gram, 561–56192; n=21) compared to uninfected subjects (4380, 2212–11500; n=21; p<0.05). CD1c+ mDC activation levels significantly correlated with the number of CD40+ colonic pDCs (r=0.61, p=0.007; n=18). The percent of CD1c+ mDCs, CD1cneg mDCs and CD303+ pDCs expressing the DC maturation marker CD83 were all lower in HIV-1-infected subjects (Figure 1c).
Colonic CD1c+ mDC activation is associated with mucosal HIV-1 viral load
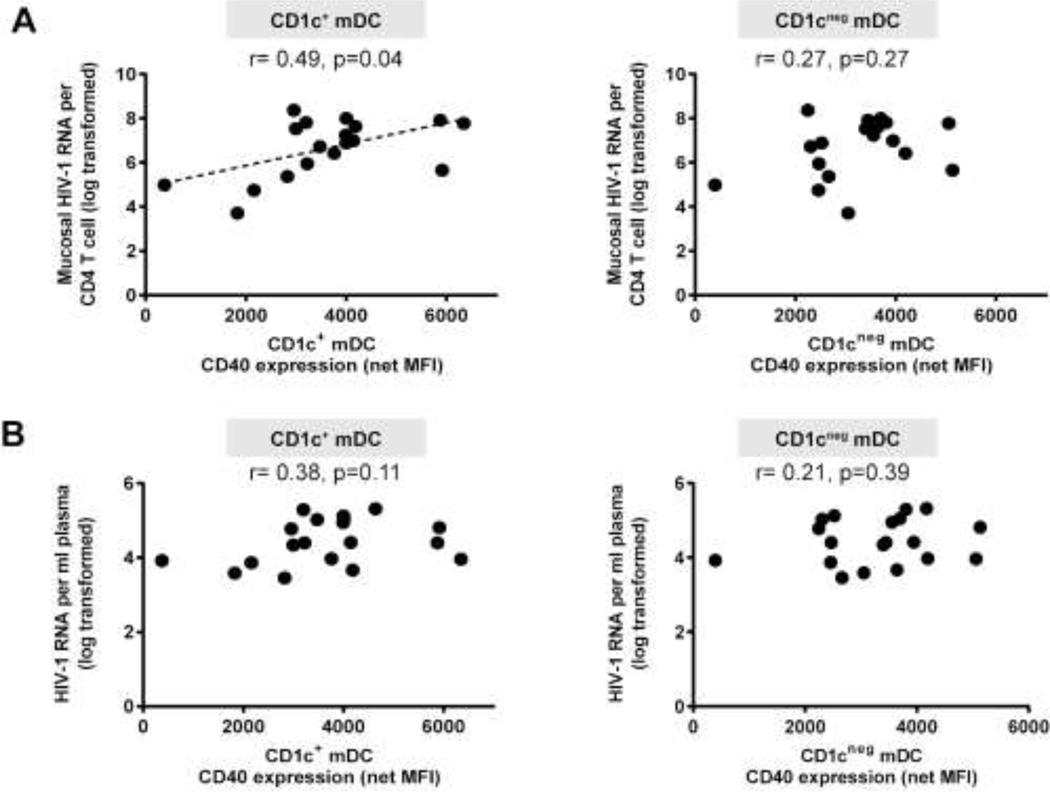
CD40 expression on CD1c+ mDCs positively associated with mucosal HIV-1 viral load whereas CD1cneg mDC CD40 expression did not (Figure 2a). Unlike our previous observations of activated blood DCs,21 CD40 expression on colon CD1c+ mDCs and CD1cneg mDCs did not correlate with either plasma viral load (Figure 2b) or with peripheral CD4 count (CD1c+ mDCs: r=−0.12, p=0.60; CD1cneg mDCs: r=−0.28, p=0.25). Although pDCs are known to be directly activated by HIV-1,22 no direct associations were observed between the number of CD40+ pDCs and either mucosal (r=0.16, p=0.50) or plasma viral load (r=0.03, p=0.88), or with peripheral CD4 count (r=0.09, p=0.69).
Figure 2. Activated colon CD1c+ myeloid dendritic cells (mDCs) correlate with mucosal HIV-1 viral load.
Correlations between CD40 expression levels (mean fluorescence intensity; MFI) on CD1c+ and CD1cneg mDCs (shown with background isotype values removed; net MFI) with (A) mucosal HIV-1 viral load (n= 18) and (B) plasma HIV-1 viral load (n=19). Statistical analysis was performed using the Spearman test. Dotted line is a visual representation of the significant association.
Colonic and systemic T cell activation correlate with colonic mDC activation
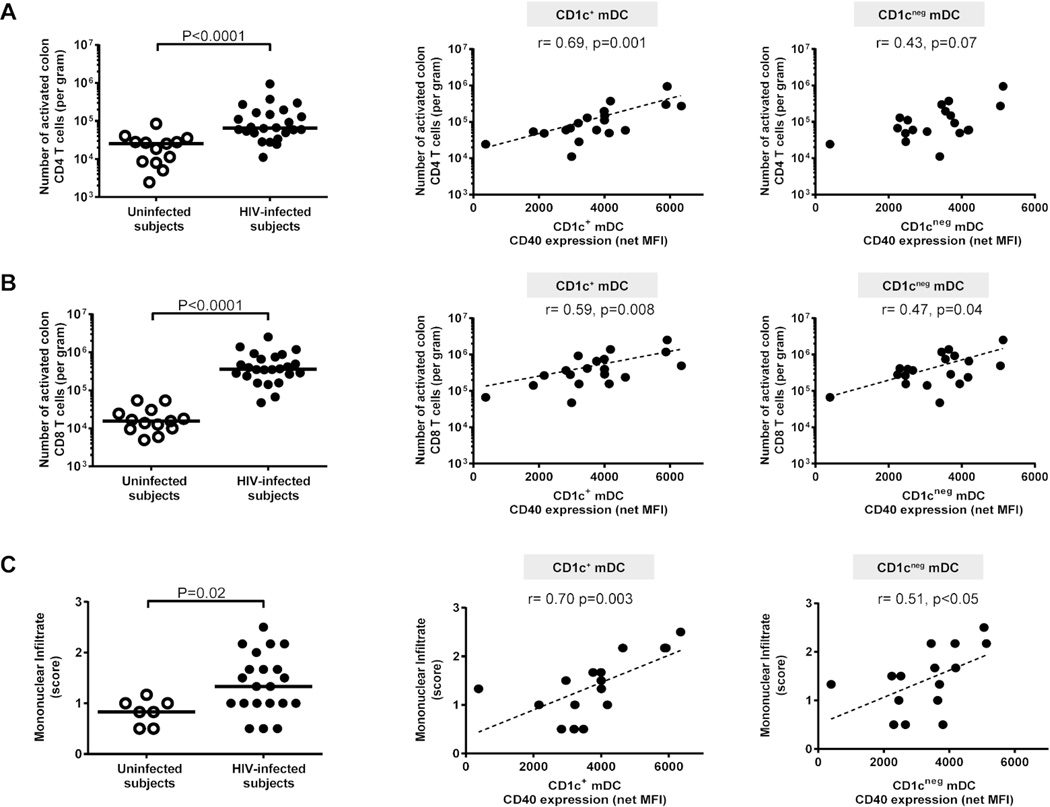
Activated colon CD4+ and CD8+ T cell frequencies were increased in HIV-1-infected subjects (Figure 3a, b, Supplementary Table S2), and CD40 expression levels on CD1c+ mDCs strongly associated with the number of activated colonic CD4+ and CD8+ T cells. Similar, but weaker associations were noted between CD1cneg mDC activation and activated colonic T cells (Figure 3a, b).
Figure 3. Activated colon CD1c+ myeloid dendritic cells (mDCs) correlate with mucosal T cell activation and mononuclear infiltration.
Multi-color flow cytometry techniques were used to determine frequencies of activated (percent CD38+ HLA-DR+) colonic mucosal CD4 and CD8 T cells and H&E staining to evaluate lamina propria (LP) infiltration of mononuclear cells in uninfected (open circles) and HIV-1-infected (HIV-infected; closed circles). Frequencies of colonic mucosal (A) CD38+HLA-DR+ CD4 T cells and (B) CD38+HLA-DR+ CD8 T cells (uninfected n=13; HIV-infected n=24) were evaluated (with background isotype values removed) as a percent of viable, CD45+ leucocytes and converted into a total number of activated CD4 or CD8 T cells per gram of tissue. Lines represent median values and statistical analysis was performed using the Mann-Whitney test. Correlations between CD40 expression levels (mean fluorescence intensity; MFI) on CD1c+ and CD1cneg mDCs (shown with background isotype values removed; net MFI) and activated (A) CD4 T cells or (B) CD8 T cells (shown with background isotype values removed) in HIV-infected subjects (n= 19) were performed using the Spearman test. Dotted line is a visual representation of the significant associations. (C) Mononuclear infiltrate assessed as the relative cellularity of the LP infiltrate consisting of lymphocytes, plasma cells, eosinophils and occasional neutrophils and scored on a scale of 0 = Not present, Minimal = 0.5, Mild = 1, Moderate = 2, and Severe = 3. Values are shown as the average score of 3 sections of colon biopsy from uninfected (open circles, n=7) and HIV-1-infected (HIV-infected; n=21) subjects. Lines represent median values and statistical analysis was performed using the Mann-Whitney test. Correlations between CD40 expression levels (mean fluorescence intensity; MFI) on CD1c+ and CD1cneg mDCs (shown with background isotype values removed; net MFI) and mononuclear infiltrate scores in HIV-infected subjects (n= 16) were performed using the Spearman test. Dotted line is a visual representation of the significant associations.
A larger infiltrate of mononuclear cells was measured by histology in the colonic LP of HIV-1-infected subjects relative to control subjects, and CD40 expression levels on both CD1c+ and CD1cneg mDCs were positively associated with the degree of mononuclear cell infiltration (Figure 3c).
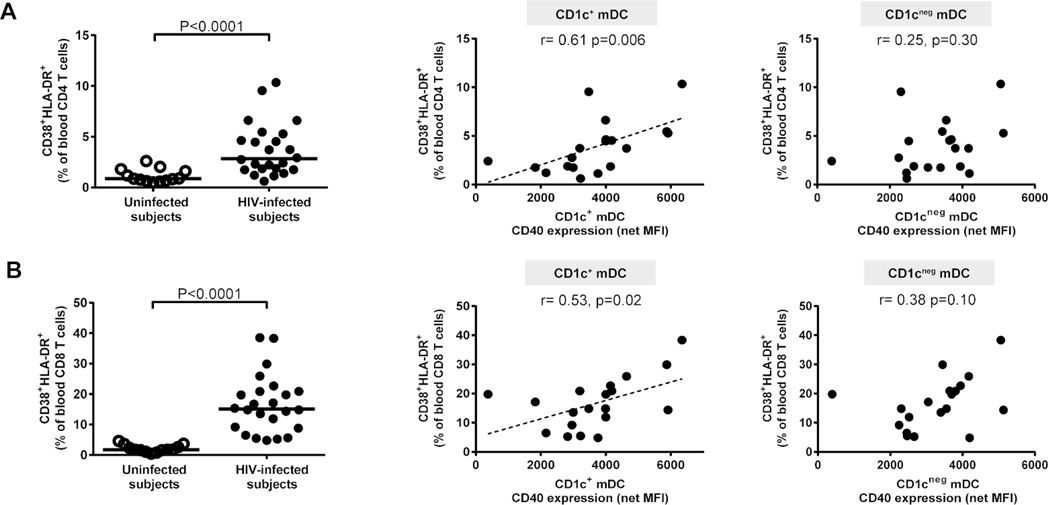
Significantly higher percentages of activated blood CD4 and CD8 T cells were found in HIV-1-infected subjects relative to uninfected controls, as expected (Figure 4a, b, Supplementary Table S2). Blood T cell activation frequencies were positively associated with activation levels of CD1c+ but not of CD1cneg mDCs (Figure 4a,b).
Figure 4. Activated colon CD1c+ myeloid dendritic cells (mDCs) correlate with systemic T cell activation.
Multi-color flow cytometry techniques were used to determine frequencies of activated (percent CD38+ HLA-DR+) blood CD4 and CD8 T cells in uninfected (open circles; n=13) and HIV-1-infected (HIV-infected; n=24; closed circles). Percentages of (A) CD38+HLA-DR+ blood CD4 T cells and (B) CD38+HLA-DR+ blood CD8 T cells as a fraction of blood CD4 or CD8 T cells (shown with background isotype values removed). Lines represent median values and statistical analysis was performed using the Mann-Whitney test. Correlations between CD40 expression levels (mean fluorescence intensity; MFI) on CD1c+ and CD1cneg mDCs (shown with background isotype values removed; net MFI) and activated blood (A) CD4 and (B) CD8 T cells (shown with background isotype values removed) in HIV-infected subjects (n= 19) were performed using the Spearman test. Dotted line is a visual representation of the significant associations.
Significant decreases in the frequencies of colonic Th1, Th17 and Th22 cells in conjunction with increased frequencies of IFN-γ+ CD8 T cells were observed in HIV-1-infected subjects compared to controls (Supplementary Table S3). However, no significant associations were found between levels of mDC activation and frequencies of cytokine-producing CD4 or CD8 T cells (data not shown).
Colonic mDC activation is associated with mucosal and plasma cytokine production
CD40 expression on CD1c+ mDC strongly associated with levels of a number of inflammatory mucosal cytokines including IL-23, IL-1β, IL-6 and TNF-α as well as with IL-10 levels in HIV-1-infected subjects (Table 2). Moreover, CD1c+ mDC activation positively associated with both mucosal IFN-γ and IL-17 production. Weaker but significant associations were observed between CD1cneg mDC activation levels and mucosal levels of IL-23 and IFN-γ.
Table 2.
Activated CD1c+ myeloid dendritic cell (mDC) or CD1cneg mDC associations with constitutive mucosal cytokines in HIV-1-infected subjects
| Cytokine (pg/ml) (median, range)* |
CD40 expression levels (MFI) on CD1c+ mDC (n=15) |
CD40 expression levels (MFI) on CD1cneg mDC (n=15) |
|
|---|---|---|---|
| IL-23 | 47.7, 0–1264 | r=0.67, p=0.008 | r=0.55, p=0.03 |
| IL-1β | 67.8, 7.2–2730 | r=0.72, p=0.003 | r=0.36, p=0.19 |
| IL-6 | 513.1, 0–8711 | r=0.68, p=0.006 | r=0.38, p=0.16 |
| TNF-α | 48.9, 0–2301 | r=0.66, p=0.009 | r=0.40, p=0.14 |
| IL-10 | 26.6, 7.4–759.9 | r=0.54, p=0.04 | r=0.36, p=0.19 |
| IFN-γ | 18.8, 0–5921 | r=0.71, p=0.004 | r=0.54, p=0.04 |
| IL-17 | 11.6, 0–125.5 | r=0.56, p=0.03 | r=0.27, p=0.33 |
Statistical analysis was performed using the Spearman test.
N=19 HIV-1-infected subjects. Bold values highlight statistically significant correlations. MFI: mean fluorescence intensity.
Plasma IL-6 levels were significantly increased in HIV-1-infected subjects (1.43pg/ml, 0.43–5.09; n=24) compared to controls (0.70pg/ml, 0.19–2.16; n=14; p=0.002); however, no significant correlation between mDC activation and IL-6 levels was observed in HIV-1-infected subjects (r=0.08, p=0.76). Plasma levels of other cytokines were evaluated in a subset of HIV-1-infected subjects (n=18). TNF-α levels strongly associated with plasma HIV-1 viral load (r=0.62, p=0.006) and with plasma IL-6 (r=0.64, p=0.004) and sCD14 levels (r=0.79, p=0.001). IL-10 levels also associated with plasma HIV-1 viral load (r=0.69, p=0.001) and with sCD14 (r=0.73, p=0.0007). TNF-α and IL-10 levels strongly correlated with each other (r=0.87, p<0.0001). CD1c+ mDC activation was positively associated with plasma levels of TNF-α (r=0.63, p=0.02; n=14) and IL-10 (r=0.76, p=0.002; n=14), whereas CD1cneg mDC activation was associated with plasma IFN-γ (r=0.59, p=0.03; n=14) and weakly with IL-10 (r=0.54, p=0.05; n=14).
Colonic mDCs are identified in association with tissue LPS to a greater extent than LTA
Levels of gram-negative bacterial LPS in the plasma of HIV-1-infected subjects were increased relative to controls (Figure 5a). In agreement with the early studies,6 plasma LPS levels in HIV-1-infected subjects correlated with blood CD4 (r=0.62, p=0.002) and CD8 (r=0.41, p=0.058) T cell activation. Moreover, plasma LPS levels significantly associated with levels of mucosal IL-1β (r= 0.58, p=0.02), IL-6 (r=0.54, p=0.03) and weakly with mucosal TNF-α (r=0.47, p=0.058). Increased plasma levels of the gram-positive cell wall component lipoteichoic acid (LTA) were also observed in HIV-1-infected subjects compared to controls (Figure 5a). However, LTA levels correlated only with blood CD4 T cell activation (r=0.47, p=0.03). No significant associations between these indicators of systemic MT and activated colonic CD1c+ mDCs (LTA (n=16): r=−0.16, p=0.55; LPS (n=17): r=0.22, p=0.39) or CD1cneg mDCs (LTA: r=−0.31, p=0.23; LPS: r=−0.03, p=0.91) were observed in HIV-1-infected subjects.
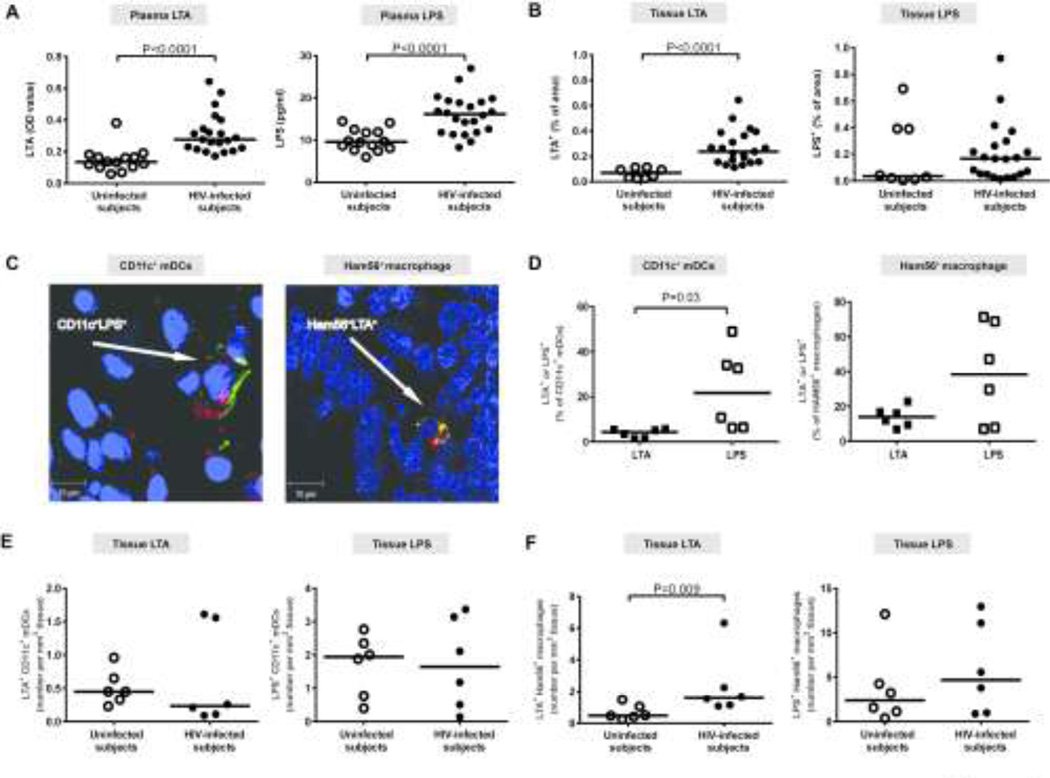
Figure 5. Colonic tissue and systemic levels of microbial products are increased in HIV-1-infected subjects and colonic myeloid dendritic cells (mDCs) associate with tissue LPS to a greater extent than LTA.
Levels of LTA and LPS were evaluated in the (A) plasma of uninfected (n=14) and HIV-1-infected (HIV-infected; LTA n=21; LPS n=22) subjects and in the (B) colonic lamina propria (LP) of uninfected (n=8) and HIV-1-infected (HIV-infected, n=21) subjects. Lines represent median values and statistical analysis was performed using the Mann-Whitney test. (C) Representative images demonstrating localization of mDCs (CD11c/green) or macrophages (Ham56/yellow) with either LTA or LPS (red) in formalin-fixed, paraffin-embedded colon biopsy tissue of an HIV-1-infected subject. (D) Comparisons between percentages of LTA+ or LPS+ CD11c+ mDCs and HAM56+ macrophages in HIV-infected subjects (n=6). Lines represent median values and statistical analysis was performed using the Wilcoxon matched-pairs signed rank test. (E, F) Number of LTA+ or LPS+ (E) CD11c+ mDCs and (F) Ham56+ macrophages per mm2 of tissue in uninfected (n=6) and HIV-infected subjects (n=6). Lines represent median values and statistical analysis was performed using the Mann-Whitney test.
Tissue LTA and LPS levels were both higher in HIV-1-infected subjects compared to control subjects, but only LTA levels reached statistical significance (Figure 5b). In HIV-1-infected subjects, no significant associations were found between tissue LTA or LPS levels and CD1c+ mDC (LTA (n=14): r=−0.14, p=0.63; LPS (n=14): r=−0.09, p=0.76) or CD1cneg mDC (LTA: r=0.21, p=0.47; LPS: r=15, p=0.61) activation.
In HIV-1-infected subjects, a greater fraction of CD11c+ mDCs and HAM56+ macrophages were associated with LPS than with LTA, although this did not reach statistical significance for macrophages (Figure 5c, d). Similar trends were observed in uninfected subjects (Figure 5e, f). Both LTA and LPS were more frequently associated with macrophages than with mDCs. When the numbers of LTA+ or LPS+ mDCs were compared between HIV-1-infected and uninfected subjects, no significant differences were observed (p=0.46, p=0.90 respectively) (Figure 5e). More LTA+ macrophages were observed in the LP of HIV-1-infected subjects than in uninfected subjects, whereas the number of LPS+ macrophages was similar between the two cohorts (Figure 5f).
Percent of CD83-expressing colonic mDCs is negatively associated with IFN-γ-producing colonic T cells
In HIV-1-infected subjects, the percent of CD83+ CD1c+ mDCs negatively correlated with the number of IFN-γ-producing colonic CD4 and CD8 T cells, whereas the percent of CD83+ CD1cneg mDCs negatively associated only with IFN-γ+ CD4 T cells (Figure 6a, b). A significant correlation between the percent of CD83+ pDCs and the percent of IFN-γ-producing CD8+ T cells (r=0.53, p=0.02; n=20) was noted; however, no association with the absolute number of IFN-γ+ CD8+ T cells or with any other immunological or virological parameters were observed.
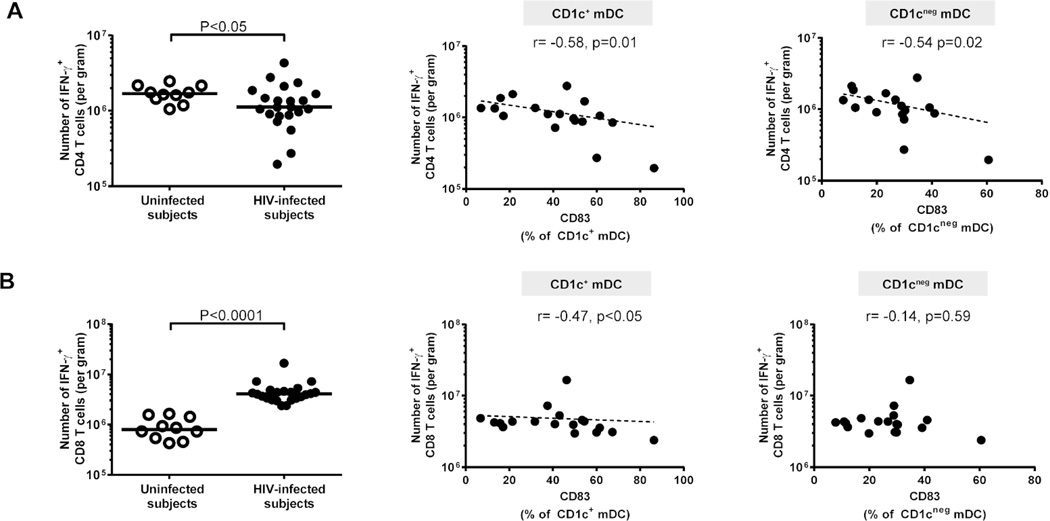
Figure 6. CD83+ myeloid dendritic cells (mDCs) negatively correlate with colonic IFN-γ-producing CD4 and CD8 T cells.
Multi-color flow cytometry techniques were used to determine frequencies of IFN-γ-producing colonic CD4 and CD8 T cells following mitogenic stimulation in uninfected (open circles; n=10) and HIV-1-infected (HIV-infected; n=22) subjects. Frequencies of colonic (A) IFN-γ+ CD4 T cells and (B) IFN-γ+ CD8 T cells were evaluated (background isotype values removed) as a percent of viable, CD45+ leucocytes and converted into a total number of activated CD4 or CD8 T cells per gram of tissue. Lines represent median values and statistical analysis was performed using the Mann-Whitney test. Correlations between percent of CD83+ CD1c+ and CD1cneg mDCs (shown with background isotype values removed) and number of IFN-γ+ (A) CD4 T cells or (B) CD8 T cells (shown with background isotype values removed) in HIV-infected subjects (n= 18) were performed using the Spearman test. Dotted line is a visual representation of the significant associations.
Abundances of altered mucosal bacterial species are associated with colonic mDC activation
We previously evaluated mucosal and fecal microbiomes to the genus level in a subset of study subjects16 and have now identified 21 mucosa-associated bacterial species, based on 99% identity to sequences in the SILVA database23 that are significantly over (6) or under (15) represented in HIV-1-infected subjects, termed “HIV-altered mucosal bacteria” (HAMB) species (Supplementary Table S4). Similar to results in our previous study,16 greater abundance of the two Proteobacteria spp. was noted only in the mucosa, whereas Prevotella species abundance was significantly greater in both mucosa and stool of HIV-1-infected subjects compared to controls.
Levels of CD40 on CD1c+ mDCs trended (p<0.1) toward positive associations with high abundance Prevotella copri and P. stercorea and toward negative associations with low abundance Bacteroides acidifaciens, Blautia schinikii and Rumminococcus bromii (Table 3). CD1cneg mDC activation also trended towards a negative association with R. bromii, but no clear associations with any other HAMB species were noted (Table 3).
Table 3.
Activated CD1c+ myeloid dendritic cell (mDC) or CD1cneg mDC associations with HIV-altered mucosal bacteria (HAMB) species
| HAMB species | Prevalence* | ↑ or ↓in HIV-1 infected subjects# |
CD40 expression levels (MFI) on CD1c+ mDC ¥ |
CD40 expression levels (MFI) on CD1cneg mDC |
|---|---|---|---|---|
| Prevotella copri | 100% | ↑ (p=0.02) | r= 0.55, p=0.0525 | r=0.16, p=0.60 |
| Prevotella stercorea | 88.2% | ↑ (p=0.01) | r= 0.48, p=0.0997 | r=0.01, p=0.97 |
| Bacteroides acidifaciens | 58.8% | ↓ (p=0.02) | r= −0.51, p=0.0592 | r= −0.29, p=0.28 |
| Blautia schinikii | 52.9% | ↓ (p=0.005) | r= −0.45, p=0.0863 | r= −0.21, p=0.38 |
| Rumminococcus bromii | 82.3% | ↓ (p=0.03) | r= −0.55, p=0.0542 | r= −0.50, p=0.0798 |
Percent of HIV-1-infected subjects with each species: statistical analysis to determine associations between activated colonic mDC Given the small samples size, these associations were only assessed when each species was detected in greater than 50% of HIV-1-infected subjects;
Increased (↑) or decreased (↓) abundance of each bacteria in HIV-1-infected subjects (n=17) compared to uninfected controls (n=14) determined using the Mann-Whitney test.
Associations between activated colonic CD1c+ mDC (n=13) or CD1cneg mDC (n=13) and abundance of HAMB were determined using the Spearman test. MFI: mean fluorescence intensity.
High abundance HAMB species induce greater cytokine+ CD1c+ mDC frequencies in vitro compared to low abundance HAMB species
Production of IL-23, IL-1β and IL-10 by CD1c+ mDC following stimulation of total LP mononuclear cells (LPMC) with P. copri and P. stercorea (high abundance HAMB) and R. bromii (low abundance HAMB) was assessed. These cytokines and HAMB were specifically chosen based on their in vivo associations with CD1c+ activation.
Exposure to each of the HAMB induced significant frequencies of IL-23-, IL-1β- and IL-10-producing CD1c+ mDCs, indicating that all three HAMB species activate colonic CD1c+ mDCs in vitro to some degree (Supplementary Table S5). P. copri and P. stercorea induced a higher percentage of IL-23+ CD1c+ mDCs compared to R. bromii, with this reaching statistical significance for P. stercorea (Figure 7a). P. copri induced the highest fraction of IL-1β+ CD1c+ mDCs, and this difference was highly significant (p<0.01) when compared to R. bromii (Figure 7b).
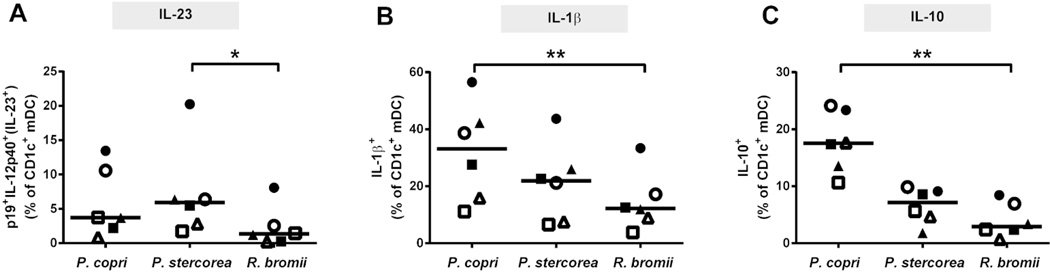
Figure 7. CD1c+ myeloid dendritic cell (mDC) cytokine production in response to in vitro stimulation with HIV-altered mucosal bacteria (HAMB) species.
Colonic LPMC (n=7 samples) were exposed to Prevotella copri P. stercorea or Ruminococcus bromii for 18–20hrs and multi-color intracellular cytokine flow cytometry techniques used to enumerate IL-23+ (IL-12p40+p19+), IL-1β+ and IL-10+ CD1c+ mDCs. Appropriate isotype controls were removed to control for background staining. Values are shown as HAMB-specific cytokine+ mDCs determined by removing the percent of cytokine+ CD1c+ mDCs detected in unstimulated cultures. Each symbol is a unique donor. Lines represent median values and statistical analysis was performed using the Friedman test for matched-paired comparisons across multiple groups, with a Dunn’s multiple comparison test performed when the overall p value was <0.05. *p<0.05, **p<0.01.
P. copri induced the highest percentage of IL-10+ CD1c+ mDCs, which was, on average, 3.3x and 7.7x that induced by P. stercorea and R. bromii, respectively, and reached statistical significance (p<0.01) relative to R. bromii (Figure 7c). In response to P. copri stimulation, a small fraction of IL-10+ CD1c+ mDC co-produced IL-23 (mean 8.2% ± 3.9% (SEM)), whereas a higher percentage of IL-10+ CD1c+ mDC co-produced IL-1β+ (62% ± 6.1%), suggesting that IL-23-producing CD1c+ mDC are a separate population of cells to those producing IL-10.
DISCUSSION
To our knowledge, this is the first study to address whether colonic mDC phenotype and function is altered in chronic untreated HIV-1 infection. In agreement with a number of previous studies in pathogenic SIV infection,24–27 no significant differences were observed in the frequency of colon CD1c+ or CD1cneg mDCs in HIV-1-infected subjects compared to uninfected controls. However, HIV-1 infection induced an activated, but dysregulated intestinal mDC phenotype characterized by increased levels of CD40 expression and decreased CD83 expression, similar to what we previously described for lymph node DCs during HIV infection.21
We show for the first time that specific Prevotella species, increased in the stool and colonic mucosa of HIV-1-infected subjects, correlated in abundance with colonic mDC activation levels in vivo and also had the capability of inducing strong pro-inflammatory cytokine production by colonic mDCs in vitro. These findings expand upon our previous observations that in HIV-1-infected subjects, mucosal abundance of Prevotella spp. (genus level) was associated both with CD1c+ mDC activation and with colon CD4 and CD8 T cell activation.16 Taken together, these results suggest that increased abundance of these pathobiont bacterial species in the intestinal mucosa may contribute to HIV-associated mucosal inflammation and immune activation, supporting previous studies that demonstrated the ‘pathogenic potential’ of Prevotella species in periodontal disease, 28 ulcerative colitis, 29 and arthritis.30
Although typically indicative of “maturation”, the precise role for DC expression of CD83 in directing immune responses is not well understood. Down-regulation of membrane-bound CD83 by RNA interference31,32 or by viruses such as HCMV33 and HSV-134 on human blood DCs resulted in decreased T cell stimulatory capacity. However, fewer CD83+ cells were detected in the inflamed areas of colonic and ileal Crohn’s disease samples compared to control and uninflamed areas,35 suggesting that in the intestinal mucosa, CD83 may have regulatory effects. This concept of CD83-mediated mucosal regulation is further supported by our observation that in HIV-1-infected subjects, frequencies of colonic CD83+ mDCs were inversely associated with IFN-γ-producing colonic T cells. However, further studies are warranted to determine the mechanistic relationship between CD83-expressing mucosal mDC and IFN-γ-producing T cells and to evaluate if this is an mDC-mediated process or, conversely, IFN-γ-producing T cells play a role in modulating intestinal mDC activation during HIV-1 infection.
A potential ‘central role’ for activated colonic mDC in HIV-associated pathogenesis is further highlighted by our observations that CD40 expression levels on CD1c+ mDCs positively correlated with colonic CD4 and CD8 T cell activation. Further, CD1c+ mDC activation also associated with blood CD4 and CD8 T cells activation, thereby linking colon mDC activation to a marker of HIV-1 disease progression.2,3. Moreover, activated CD1c+ mDCs in HIV-1-infected subjects was associated with numerous mucosal cytokines, including IL-23 and IL-1β. Within the mucosa, increased levels of IL-23 and IL-1β have been implicated in intestinal inflammation mediated, in part, through the promotion of T cell-associated IFN-γ and IL-17 production.36,37 In our study, levels of CD40 expression on colonic mDCs were also associated with mucosal levels of IFN-γ and IL-17, suggesting an intricate relationship between mDC activation, mucosal T cell activation, and cytokine-production in the setting of HIV-1 infection. These in vivo observations expand on our previous in vitro study that demonstrated a requirement for LP mDCs in the in vitro expansion and enhanced infection of Th1 and Th17 cells in response to exposure to commensal bacteria and HIV-1.19 Although we did not see direct correlations between mDC activation levels and absolute Th1 or Th17 frequencies, this finding may be due to the fact that these mucosal Th subsets are depleted early in the course of HIV infection38 and thus absolute Th cell numbers might not be expected to reflect ongoing mucosal inflammation during chronic disease.
Intriguingly, HIV-1-associated colonic mDC activation levels positively associated with mucosal and systemic IL-10 production, a cytokine with well described immuno-regulatory functions.39 Increased levels of IL-10, in conjunction with increased levels of pro-inflammatory cytokines, have been reported in both acute and chronic HIV-1 infection.40,41 Systemically administered IL-10 stimulated the production of IFN-γ during human endotoxemia,42 suggesting that IL-10 can have pro-inflammatory effects, especially concurrent with exposure to microbial products. IL-10 regulates production of IL-23 by human blood mDCs in response to commensal bacteria in vitro43 and a similar negative feedback mechanism to compensate for increased production of pro-inflammatory IL-23 by intestinal mDCs in response to translocating commensal bacteria may be at play in the colon of HIV-1-infected subjects. Indeed, we observed production of IL-10 by CD1c+ mDC in response to in vitro exposure to HAMB, and these DCs were a different population to those producing IL-23. Although our current in vivo and in vitro observations suggest a role for intestinal mDCs in IL-10 production, the exact nature of the immune-regulatory versus pro-inflammatory effects of IL-10 in the setting of HIV-1 infection requires further investigation.
Estes et. al. utilized quantitative image analysis to directly demonstrate translocation of LPS and E. coli in the colon of chronically SIV-infected rhesus macaques, and increased levels of MT were due, in part, to ineffective phagocytosis by intestinal macrophages.44 To our knowledge, no studies have quantitated levels of microbial products in the human colonic LP during chronic untreated HIV-1 infection nor evaluated the co-localization of microbial products with resident LP mDCs and macrophages. In our study, HIV-1-infected subjects had heightened tissue levels of both LTA and LPS, although neither appeared to directly correlate with mucosal mDC activation. These results suggest that both gram-negative and gram-positive bacteria and their products are translocating even though only gram-negative bacteria were increased in abundance in the mucosa. A greater fraction of LTA and LPS was associated with macrophages than with mDCs, in keeping with the reported robust phagocytic ability of tissue macrophages.45 Despite an increase in LTA and LPS tissue levels and in the number of LP macrophages in HIV-1-infected subjects, we only observed an increase in the number of LTA+ LP macrophages in this cohort, suggesting a defect in macrophage function in the context of bacterial uptake of LPS or gram-negative bacteria in chronic HIV-1 infection. Indeed, the ability of LP macrophages and mDCs to limit MT in the LP of both LTA and LPS must still be somewhat ineffective given the increased levels of these bacterial products in the plasma of HIV-1-infected subjects.
In a previous study, stimulation of LPMC with a synthetic TLR7/8 ligand that mimics HIV-1 ssRNA, in conjunction with a gram-negative bacterial TLR4 ligand (LPS), resulted in a synergistic increase in IL-23 production by intestinal mDC in vitro.18 In this clinical study, levels of CD1c+ mDC activation correlated with mucosal HIV-1 viral load, suggesting that HIV-1 itself may play a role in intestinal mDC activation. Moreover, in both uninfected and HIV-1-infected subjects, the fraction of mDCs found in association with LPS was higher than that with LTA, suggesting an increased likelihood of gram-negative bacteria directly activating colonic mDC. This finding is in keeping with our observations that gram-negative Prevotella species abundance correlated with colonic mDC activation in vivo, and that Prevotella species induced higher frequencies of cytokine-expressing mDCs in vitro than did gram-positive R. bromii. These observations raise the possibility that HIV-1 and translocating gram-negative bacteria act in concert to induce intestinal mDC activation in vivo and thereby potentiate mucosal inflammation. Further studies will be needed to understand whether HIV-1-associated activation is mediated by direct HIV-1/mDC interactions or in a bystander fashion via effects of HIV-1 on colonic pDCs 46 or other cells, as well as to identify the exact viral and bacterial determinants responsible for intestinal DC activation and cytokine production.
As part of this study, we also investigated the impact of HIV-1 infection on colonic pDC frequency and activation state. A trend towards increased frequencies of intestinal pDCs, a significant increase in numbers of CD40+ pDCs, and a decrease in percentages of CD83-expressing pDCs were observed in HIV-1-infected subjects compared to uninfected controls. Further, numbers of activated pDCs were directly associated with levels of CD1c+ mDC activation, suggesting that common factors might be driving activation in both DC subsets. These observations are in keeping with recent studies demonstrating increased pDC frequencies in the ileum of chronically HIV-infected subjects47 as well as increased pDC frequencies with poly-functional cytokine phenotypes in pathogenic SIV infection models25,26,48. Unlike mDCs, pDCs are rarely found in intestinal tissue under steady state conditions, 18,22 thus accumulation of activated pDCs during chronic HIV-1 infection is likely due to increased migration from the blood to the colon.25,26,47
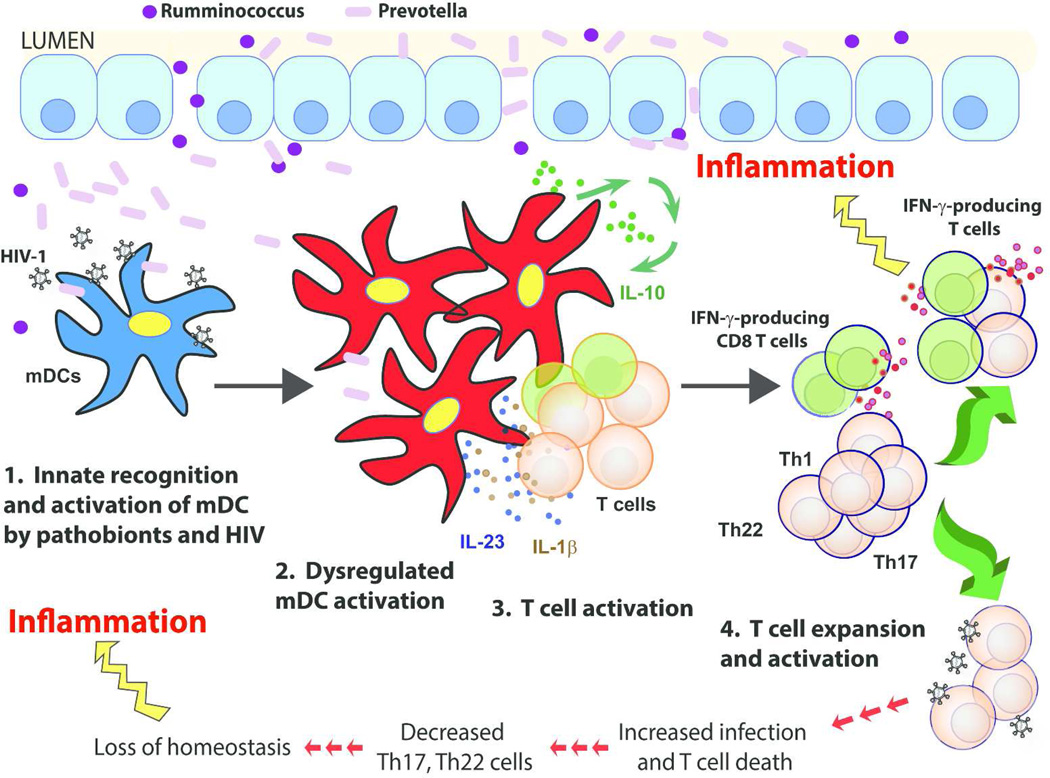
In conclusion, we propose a model whereby colonic mDCs drive mucosal immune activation and inflammation during chronic untreated HIV-1 infection (Figure 8). Increased translocation of gram-negative Prevotella into the LP synergizes with HIV to induce intestinal mDC activation. Activated mDC subsequently stimulate bacteria-specific CD4 T cells19,49 through cell-cell contact and production of inflammatory cytokines (IL-23, IL-1β), with additional non-specific T cell activation potentially occurring via CD83-mediated loss of mucosal T cell regulation. This process leads to expansion of Th1, Th17 and Th22 as well as inflammatory IFN-γ-producing CD8 T cells. Activated Th1/17/22 cells are targets for viral replication19 which ultimately results in their infection and depletion.49 Increased mucosal inflammation, a loss of DC-mediated regulation, and a lack of “protective” Th17 and Th22 cells further contribute to epithelial barrier breakdown and MT, thereby potentiating a vicious cycle that ultimately leads to systemic immune activation and its attendant comorbidities. The clinical implications of our in vitro findings are currently speculative and additional in vivo clinical studies that block microbial translocation50–52 and those that alter the microbiome composition and bacteria-associated metabolic pathways are required to provide further evidence that interactions between the microbiome and mDC contribute to intestinal inflammation during HIV-1 infection. These types of studies would also be invaluable in furthering our understanding of the factors contributing to HIV-1-associated mucosal pathogenesis by determining if the microbiome is altered due to ongoing mDC-mediated mucosal inflammation or if the increased abundances of pathobionts induce mDC activation, or if both processes are involved.
Figure 8. Proposed model illustrating colonic myeloid dendritic cells (mDCs) driving mucosal immune activation and inflammation during chronic untreated HIV-1 infection.
HIV replication in the lamina propria (LP) results in epithelial barrier disruption, leading to the 1) increased translocation of gram-negative Prevotella into the LP which synergizes with HIV-1 to induce 2) a dysregulated mDC activation profile characterized by increased levels of CD40 and decreased CD83 expression. Activated mDCs subsequently induce 3) increased T cell activation via stimulation of bacteria-specific CD4 T cells19,49 through cell-cell contact (e.g. CD40/CD40L), production of inflammatory cytokines (IL-23, IL-1β) and potentially via CD83-mediated loss of T cell regulation.35 mDCs produce IL-10 to compensate for increased pro-inflammatory cytokine production;43 however, this may also exacerbate IFN-γ production.42 In total, this culminates in 4) increased T cell activation and expansion of T helper (Th)1, Th17 and Th22 and IFN-γ-producing CD8 T cells. Activated Th1/17/22 cells are targets for viral replication19 which ultimately results in their infection and depletion. Increased mucosal mDC and T cell activation and inflammation, a loss of mDC-mediated regulation, and a lack of “protective” Th17 and Th22 cells further contribute to epithelial barrier breakdown and microbial translocation, thereby potentiating a vicious cycle that ultimately leads to systemic inflammation and immune activation and their attendant comorbidities.
MATERIALS AND METHODS
Study participants and study design
Twenty-four HIV-1-infected adult subjects and 14 HIV-1-seronegative (uninfected) adult control subjects were enrolled in this cross-sectional study at the University of Colorado Anschutz Medical Campus. Efforts were made to enroll control subjects who were matched for age and sex to the HIV-1-infected subjects. Clinical characteristics for study subjects are detailed in Table 1. Based on study entry criteria, HIV-1-infected subjects were cART-naïve or had not been on treatment for greater than 7 days in the preceding 6 months. Exclusion criteria are extensively described in a previous publication16 and detailed in Supplementary Materials. All subjects voluntarily gave written, informed consent. This study was approved by the Colorado Multiple Institutional Review Board (COMIRB) at the University of Colorado Anschutz Medical Campus.
Collection, storage and processing of clinical samples
Collection, storage and processing of rectal swabs, colon biopsies and peripheral blood mononuclear cells (PBMC) are detailed in Supplementary Materials.
Enumeration of colonic mucosal HIV-1 viral load
Colonic mucosal HIV-1 viral load was determined as previously described.16 To account for variation in the number of CD4+ T cells in different samples, HIV RNA copy numbers were normalized per CD4 T cell within each biopsy.
Determination of mucosa-associated bacterial species
Laboratory and analytic methods used to profile the intestinal microbiomes of study participants were described previously.16 Species-level taxonomic classification of 16S rRNA sequence datasets was obtained via BLAST53 of subject sequences against a database built from Silva23 bacterial sequences marked as type strains, cultivars, or genomes. A species name was assigned when a sequence overlapped the Silva database sequence by at least 95% sequence length with at least 99% sequence identity and the taxonomy of the database hit matched the taxonomy returned by SINA54 as determined previously.16
Plasma LTA, LPS and sCD14 measurements
Serum LTA levels were assessed using a custom ELISA.16 LPS levels were measured in EDTA plasma samples using the Limulus Amebocyte Lysate (LAL) assay (Lonza, Switzerland) following the manufacturer’s protocol as previously detailed.16 sCD14 levels were measure in heparin plasma using a commercially available ELISA (R&D Systems, Minneapolis, MN).16
Mucosal and plasma cytokine measurements
A Custom Q-plex Array (Quansys Biosciences, Logan UT) was used to measure mucosal cytokine levels in culture supernatants and measurement of plasma cytokine levels were performed using the Human Cytokine High Sensitivity Screen as detailed in Supplementary Materials. Levels of plasma IL-6 were evaluated in EDTA plasma samples using a commercially available ELISA (R&D Systems).16
Histological staining and analysis of colonic biopsies
Assessment of microbial product levels, CD11c+ mDCs, and HAM56+ macrophages in colonic LP is detailed extensively in Supplementary Materials. Using Zeiss Zen Software (Jena, Germany), the total area and the area that stained with LTA/LPS was calculated within the LP. To analyze whether microbial products preferentially associated with mDCs or macrophages the total number of mDCs and macrophages that either did or did not associate with microbial products (LTA/LPS) were enumerated per square millimeter of LP using Image J Software (NIH Bethesda, MD)
Assessment of mononuclear infiltration is detailed in Supplementary Materials. Evaluation was performed by a gastrointestinal pathologist who was blinded to the HIV-1 status of each patient. The degree of mononuclear infiltration was quantified on a scale of 0 = Not present, Minimal = 0.5, Mild = 1, Moderate = 2, and Severe = 3.
In vitro stimulations
In vitro mitogenic stimulation of single-cell colon biopsy preparations: Evaluation of frequencies of colonic CD4 T cells capable of producing IFN-γ (Th1), IL-17 (Th17) or IL-22 (Th22) and frequencies of IFN-γ-producing CD8 T cells from isolated colon cells following mitogenic stimulation are detailed in Supplementary Materials. In vitro exposure of LPMC to commensal bacteria: Cytokine responses by LP CD1c+ mDCs to P. copri, P. stercorea and R. bromii were assessed utilizing an ex vivo tissue culture model consisting of isolated colon LPMC from normal tissue18,19,49,55 and is detailed in Supplementary Materials.
Commensal bacteria stocks
Expansion of P. copri (DSM# 18205, DSMZ, Braunschweig, Germany), P. stercorea (DSM# 18206) and R. bromii (ATCC# 27255, ATCC Manassas, VA) was performed at 37°C under anaerobic conditions per manufacturer’s protocols as described in Supplementary Materials.
Surface and intracellular flow cytometry staining assays, acquisition and analysis
DC and T cell frequencies, activation and cytokine production from colon cells isolated from biopsies and from PBMC: Multi-color flow cytometry protocols to evaluate colon and blood DC and T cell frequencies and activation status and to determine T cell cytokine frequencies are detailed in Supplementary Materials. For enumeration of colonic DC and T cell frequencies, the percentage of DCs or T cells within viable, CD45+ cells was converted to an absolute number per gram based on the frequency within viable, CD45+ cells, initial cell counts and biopsy weights. Similarly, the percent of activated colon CD4 and CD8 T cells as well as the percent of cytokine+ CD4 and CD8 T cells were also converted to a total number per gram of mucosal tissue.
Phenotypic and functional characterization of mDC subsets in LPMC from normal colon tissue: Multi-color flow cytometry staining protocols used to characterize LP CD1c+ mDCs and CD1cneg mDCs and enumerate cytokine+ CD1c+ mDCs following in vitro stimulation of LPMC in normal colon tissue are detailed in Supplementary Materials.
Flow cytometry acquisition: All flow cytometry data were acquired on an LSRII Flow Cytometer (BD Biosciences). Routine quality control using the Cytometer Setup & Tracking feature within the BD FACSDiva software version 6.1.2 (BD Biosciences) was performed daily as previously detailed19.
Statistical analysis
Non-parametric statistics were performed with no adjustments for multiple comparisons due to the exploratory nature of this study. Analysis and graphing were performed using GraphPad Prism Version 6 for Windows (GraphPad Software, San Diego, CA). Comparisons between independent groups were made using the Mann–Whitney test and the Friedman test with a multiple Dunn comparison test for matched–paired comparisons across multiple groups. To determine the differences between groups of matched paired data, the Wilcoxon matched-pairs signed-rank test was performed. Correlations between variables were assessed using the Spearman test. Fisher exact tests and Chi-squared tests were used for comparisons of categorical data. A p value of <0.05 was considered significant.
Supplementary Material
ACKNOWLEDGEMENTS
We wish to express our sincere gratitude to all the study participants as well as the physicians and staff at the University of Colorado Infectious Disease Group Practice Clinic. We thank the staff at the Clinical and Translational Research Center (CTRC) and the University Hospital endoscopy clinic for their assistance with our clinical study. We also acknowledge the staff at University of Colorado Hospital CTRC Core Lab for performing the IL-6 assay and the San Diego CFAR Translational Virology Core for performing viral RNA measurements on colon tissue.
This study was supported by National Institutes of Health Grants RO1 DK088663, R01 AI108404. AI36214 and, in part, by NIH/NCATS Colorado CTSI Grant Number UL1 TR000154.
Footnotes
DISCLOSURE
The authors declare no conflict of interest.
REFERENCES
- 1.Hunt PW. HIV and inflammation: mechanisms and consequences. Current HIV/AIDS reports. 2012;9:139–147. doi: 10.1007/s11904-012-0118-8. [DOI] [PubMed] [Google Scholar]
- 2.Deeks SG, et al. Immune activation set point during early HIV infection predicts subsequent CD4+ T-cell changes independent of viral load. Blood. 2004;104:942–947. doi: 10.1182/blood-2003-09-3333. [DOI] [PubMed] [Google Scholar]
- 3.Giorgi JV, et al. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. The Journal of infectious diseases. 1999;179:859–870. doi: 10.1086/314660. [DOI] [PubMed] [Google Scholar]
- 4.Giorgi JV, et al. Elevated levels of CD38+ CD8+ T cells in HIV infection add to the prognostic value of low CD4+ T cell levels: results of 6 years of follow-up. The Los Angeles Center, Multicenter AIDS Cohort Study. J Acquir Immune Defic Syndr. 1993;6:904–912. [PubMed] [Google Scholar]
- 5.Marchetti G, Tincati C, Silvestri G. Microbial translocation in the pathogenesis of HIV infection and AIDS. Clinical microbiology reviews. 2013;26:2–18. doi: 10.1128/CMR.00050-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brenchley JM, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nature medicine. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 7.Marchetti G, et al. Microbial translocation predicts disease progression of HIV-infected antiretroviral-naive patients with high CD4+ cell count. AIDS. 2011;25:1385–1394. doi: 10.1097/QAD.0b013e3283471d10. [DOI] [PubMed] [Google Scholar]
- 8.Hunt PW, et al. Gut epithelial barrier dysfunction and innate immune activation predict mortality in treated HIV infection. The Journal of infectious diseases. 2014;210:1228–1238. doi: 10.1093/infdis/jiu238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sandler NG, et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. The Journal of infectious diseases. 2011;203:780–790. doi: 10.1093/infdis/jiq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.George MD, Asmuth DM. Mucosal immunity in HIV infection: what can be done to restore gastrointestinal-associated lymphoid tissue function? Current opinion in infectious diseases. 2014;27:275–281. doi: 10.1097/QCO.0000000000000059. [DOI] [PubMed] [Google Scholar]
- 11.Hayes TL, et al. Impact of highly active antiretroviral therapy initiation on CD4(+) T-cell repopulation in duodenal and rectal mucosa. AIDS. 2013;27:867–877. doi: 10.1097/QAD.0b013e32835d85b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kok A, et al. Early initiation of combined antiretroviral therapy preserves immune function in the gut of HIV-infected patients. Mucosal immunology. 2015;8:127–140. doi: 10.1038/mi.2014.50. [DOI] [PubMed] [Google Scholar]
- 13.Schneider T, et al. Abnormalities in subset distribution, activation, and differentiation of T cells isolated from large intestine biopsies in HIV infection. The Berlin Diarrhoea/Wasting Syndrome Study Group. Clinical and experimental immunology. 1994;95:430–435. doi: 10.1111/j.1365-2249.1994.tb07014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGowan I, et al. Increased HIV-1 mucosal replication is associated with generalized mucosal cytokine activation. J Acquir Immune Defic Syndr. 2004;37:1228–1236. doi: 10.1097/01.qai.0000131846.12453.29. [DOI] [PubMed] [Google Scholar]
- 15.Mutlu EA, et al. A compositional look at the human gastrointestinal microbiome and immune activation parameters in HIV infected subjects. PLoS pathogens. 2014;10:e1003829. doi: 10.1371/journal.ppat.1003829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dillon SM, et al. An altered intestinal mucosal microbiome in HIV-1 infection is associated with mucosal and systemic immune activation and endotoxemia. Mucosal immunology. 2014;7:983–994. doi: 10.1038/mi.2013.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chirdo FG, Millington OR, Beacock-Sharp H, Mowat AM. Immunomodulatory dendritic cells in intestinal lamina propria. European journal of immunology. 2005;35:1831–1840. doi: 10.1002/eji.200425882. [DOI] [PubMed] [Google Scholar]
- 18.Dillon SM, et al. Human intestinal lamina propria CD1c+ dendritic cells display an activated phenotype at steady state and produce IL-23 in response to TLR7/8 stimulation. J Immunol. 2010;184:6612–6621. doi: 10.4049/jimmunol.1000041. [DOI] [PubMed] [Google Scholar]
- 19.Dillon SM, et al. HIV-1 infection of human intestinal lamina propria CD4+ T cells in vitro is enhanced by exposure to commensal Escherichia coli. J Immunol. 2012;189:885–896. doi: 10.4049/jimmunol.1200681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Allers K, et al. Macrophages accumulate in the gut mucosa of untreated HIV-infected patients. The Journal of infectious diseases. 2014;209:739–748. doi: 10.1093/infdis/jit547. [DOI] [PubMed] [Google Scholar]
- 21.Dillon SM, et al. Plasmacytoid and myeloid dendritic cells with a partial activation phenotype accumulate in lymphoid tissue during asymptomatic chronic HIV-1 infection. J Acquir Immune Defic Syndr. 2008;48:1–12. doi: 10.1097/QAI.0b013e3181664b60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Brien M, Manches O, Bhardwaj N. Plasmacytoid dendritic cells in HIV infection. Advances in experimental medicine and biology. 2013;762:71–107. doi: 10.1007/978-1-4614-4433-6_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quast C, et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic acids research. 2013;41:D590–596. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klatt NR, et al. Loss of mucosal CD103+ DCs and IL-17+ and IL-22+ lymphocytes is associated with mucosal damage in SIV infection. Mucosal immunology. 2012;5:646–657. doi: 10.1038/mi.2012.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kwa S, et al. Plasmacytoid dendritic cells are recruited to the colorectum and contribute to immune activation during pathogenic SIV infection in rhesus macaques. Blood. 2011;118:2763–2773. doi: 10.1182/blood-2011-02-339515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reeves RK, et al. SIV infection induces accumulation of plasmacytoid dendritic cells in the gut mucosa. The Journal of infectious diseases. 2012;206:1462–1468. doi: 10.1093/infdis/jis408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wijewardana V, et al. Kinetics of myeloid dendritic cell trafficking and activation: impact on progressive, nonprogressive and controlled SIV infections. PLoS pathogens. 2013;9:e1003600. doi: 10.1371/journal.ppat.1003600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar PS, et al. New bacterial species associated with chronic periodontitis. Journal of dental research. 2003;82:338–344. doi: 10.1177/154405910308200503. [DOI] [PubMed] [Google Scholar]
- 29.Lucke K, Miehlke S, Jacobs E, Schuppler M. Prevalence of Bacteroides and Prevotella spp. in ulcerative colitis. Journal of medical microbiology. 2006;55:617–624. doi: 10.1099/jmm.0.46198-0. [DOI] [PubMed] [Google Scholar]
- 30.Scher JU, et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. eLife. 2013;2:e01202. doi: 10.7554/eLife.01202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aerts-Toegaert C, et al. CD83 expression on dendritic cells and T cells: correlation with effective immune responses. European journal of immunology. 2007;37:686–695. doi: 10.1002/eji.200636535. [DOI] [PubMed] [Google Scholar]
- 32.Prechtel AT, Turza NM, Theodoridis AA, Steinkasserer A. CD83 knockdown in monocyte-derived dendritic cells by small interfering RNA leads to a diminished T cell stimulation. J Immunol. 2007;178:5454–5464. doi: 10.4049/jimmunol.178.9.5454. [DOI] [PubMed] [Google Scholar]
- 33.Arrode G, Boccaccio C, Abastado JP, Davrinche C. Cross-presentation of human cytomegalovirus pp65 (UL83) to CD8+ T cells is regulated by virus-induced, soluble-mediator-dependent maturation of dendritic cells. Journal of virology. 2002;76:142–150. doi: 10.1128/JVI.76.1.142-150.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kruse M, et al. Mature dendritic cells infected with herpes simplex virus type 1 exhibit inhibited T-cell stimulatory capacity. Journal of virology. 2000;74:7127–7136. doi: 10.1128/jvi.74.15.7127-7136.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Silva MA, et al. Dendritic cells and toll-like receptors 2 and 4 in the ileum of Crohn's disease patients. Digestive diseases and sciences. 2008;53:1917–1928. doi: 10.1007/s10620-007-0105-x. [DOI] [PubMed] [Google Scholar]
- 36.Ahern PP, Izcue A, Maloy KJ, Powrie F. The interleukin-23 axis in intestinal inflammation. Immunological reviews. 2008;226:147–159. doi: 10.1111/j.1600-065X.2008.00705.x. [DOI] [PubMed] [Google Scholar]
- 37.Santarlasci V, Cosmi L, Maggi L, Liotta F, Annunziato F. IL-1 and T Helper Immune Responses. Frontiers in immunology. 2013;4:182. doi: 10.3389/fimmu.2013.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brenchley JM, et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. The Journal of experimental medicine. 2004;200:749–759. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maynard CL, Weaver CT. Diversity in the contribution of interleukin-10 to T-cell-mediated immune regulation. Immunological reviews. 2008;226:219–233. doi: 10.1111/j.1600-065X.2008.00711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Norris PJ, et al. Elevations in IL-10, TNF-alpha, and IFN-gamma from the earliest point of HIV Type 1 infection. AIDS research and human retroviruses. 2006;22:757–762. doi: 10.1089/aid.2006.22.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stacey AR, et al. Induction of a striking systemic cytokine cascade prior to peak viremia in acute human immunodeficiency virus type 1 infection, in contrast to more modest and delayed responses in acute hepatitis B and C virus infections. Journal of virology. 2009;83:3719–3733. doi: 10.1128/JVI.01844-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lauw FN, et al. Proinflammatory effects of IL-10 during human endotoxemia. J Immunol. 2000;165:2783–2789. doi: 10.4049/jimmunol.165.5.2783. [DOI] [PubMed] [Google Scholar]
- 43.Manuzak J, Dillon S, Wilson C. Differential interleukin-10 (IL-10) and IL-23 production by human blood monocytes and dendritic cells in response to commensal enteric bacteria. Clinical and vaccine immunology: CVI. 2012;19:1207–1217. doi: 10.1128/CVI.00282-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Estes JD, et al. Damaged intestinal epithelial integrity linked to microbial translocation in pathogenic simian immunodeficiency virus infections. PLoS pathogens. 2010;6:e1001052. doi: 10.1371/journal.ppat.1001052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smythies LE, et al. Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bacteriocidal activity. The Journal of clinical investigation. 2005;115:66–75. doi: 10.1172/JCI19229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fonteneau JF, et al. Human immunodeficiency virus type 1 activates plasmacytoid dendritic cells and concomitantly induces the bystander maturation of myeloid dendritic cells. Journal of virology. 2004;78:5223–5232. doi: 10.1128/JVI.78.10.5223-5232.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lehmann C, et al. Longitudinal analysis of distribution and function of plasmacytoid dendritic cells in peripheral blood and gut mucosa of HIV infected patients. The Journal of infectious diseases. 2014;209:940–949. doi: 10.1093/infdis/jit612. [DOI] [PubMed] [Google Scholar]
- 48.Li H, Gillis J, Johnson RP, Reeves RK. Multi-functional plasmacytoid dendritic cells redistribute to gut tissues during simian immunodeficiency virus infection. Immunology. 2013;140:244–249. doi: 10.1111/imm.12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Steele AK, et al. Microbial exposure alters HIV-1-induced mucosal CD4+ T cell death pathways Ex vivo. Retrovirology. 2014;11:14. doi: 10.1186/1742-4690-11-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kristoff J, et al. Early microbial translocation blockade reduces SIV-mediated inflammation and viral replication. The Journal of clinical investigation. 2014;124:2802–2806. doi: 10.1172/JCI75090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sandler NG, et al. Sevelamer does not decrease lipopolysaccharide or soluble CD14 levels but decreases soluble tissue factor, low-density lipoprotein (LDL) cholesterol, and oxidized LDL cholesterol levels in individuals with untreated HIV infection. The Journal of infectious diseases. 2014;210:1549–1554. doi: 10.1093/infdis/jiu305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tenorio AR, et al. Rifaximin has a Marginal Impact on Microbial Translocation, T-cell Activation and Inflammation in HIV-Positive Immune Non-responders to Antiretroviral Therapy - ACTG A5286. The Journal of infectious diseases. 2015;211:780–790. doi: 10.1093/infdis/jiu515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. Journal of molecular biology. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 54.Pruesse E, Peplies J, Glockner FO. SINA: accurate high-throughput multiple sequence alignment of ribosomal RNA genes. Bioinformatics. 2012;28:1823–1829. doi: 10.1093/bioinformatics/bts252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Howe R, et al. Evidence for dendritic cell-dependent CD4(+) T helper-1 type responses to commensal bacteria in normal human intestinal lamina propria. Clin Immunol. 2009;131:317–332. doi: 10.1016/j.clim.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.