Abstract
Type 1 diabetes mellitus (T1D) is an autoimmune disease often diagnosed in childhood that results in pancreatic β-cell destruction and life-long insulin dependence. T1D susceptibility involves a complex interplay of genetic and environmental factors and has historically been attributed to adaptive immunity, though there is now increasing evidence for a role of innate inflammation. Here, we review studies that define a heightened age-dependent innate inflammatory state in T1D families that is paralleled with high fidelity by the T1D-susceptible BioBreeding rat. Innate inflammation may be driven by changes in interactions between the host and environment, such as through an altered microbiome, intestinal hyper-permeability, or viral exposures. Special focus is placed on the temporal measurement of plasma induced transcriptional signatures of recent onset T1D patients and their siblings as well as in the Biobreeding rat as it defines the natural history of innate inflammation. These sensitive and comprehensive analyses have also revealed that those who successfully managed T1D risk develop an age-dependent immunoregulatory state, providing a possible mechanism for the juvenile nature of T1D. Therapeutic targeting of innate inflammation has been proven effective in preventing and delaying T1D in rat models. Clinical trials of agents that suppress innate inflammation have had more modest success, but efficacy is improved by the addition of combinatorial approaches that target other aspects of T1D pathogenesis. An understanding of innate inflammation and mechanisms by which this susceptibility is both potentiated and mitigated offers important insight into T1D progression and avenues for therapeutic intervention.
Introduction
Type 1 diabetes mellitus (T1D) is an autoimmune disease in which the insulin-producing pancreatic β-cells are targeted and destroyed by infiltrating immunocytes, resulting in lifelong dependence on exogenous insulin. T1D is one of the most common chronic diseases of childhood with peak ages of onset at 5–7 years of age and again peri-pubertally (1). While residual β-cells have been detected in patients with even long-standing T1D, evidence supports that individuals present clinically after a significant loss of β-cell mass and function (2, 3), at which point glucose homeostasis can no longer be maintained. As such, hyperglycemia develops with classic symptoms of polyuria, polydipsia, and weight loss. Globally, the incidence of T1D has been increasing over the past several decades, with the number of new cases rising by approximately 3% per year in children and teens (4).
T1D is a complex disease involving an interaction of multiple genetic loci and environmental factors, perhaps best reflected by the study of T1D in monozygotic twins who exhibit < 100% concordance rates despite long-term follow-up (5). The largest genetic contribution to T1D risk is conveyed by the human leukocyte antigen (HLA) locus, with > 90% of patients possessing DR3 and/or DR4 HLA-DRB1 class II alleles compared to a carrier frequency of approximately 40% in Caucasians (5, 6). These high risk alleles appear to be evolutionarily selected for their ability to present a broad range of microbial peptides to T-cells, but are associated with many autoimmune diseases (7), likely because of their propensity to also present self-peptides to T-cells (8). In addition to the HLA locus, genome wide association studies have identified >40 additional loci that contribute lesser degrees of risk. Within these mapped regions reside disease promoting genetic variants; many of the most highly characterized candidate genes encode protein products related to immune function (e.g. PTPN22, CTLA4, IFIH1, IL2RA and SH2B3) (9). The environmental trigger(s) of T1D remain unknown, but the process of autoimmunity, once initiated, occurs over months to years (10, 11). During this time of declining β-cell function and mass, disease-specific but non-pathogenic autoantibodies (AA) against β-cell autoantigens appear in various titers and combinations. These serve as a marker of β-cell autoimmune responses and risk of disease progression, and are present in > 90% of newly diagnosed T1D patients (12). Notably, the risk of progressing to T1D increases with the number of detectable AAs, such that the presence of two or more AA indicates a greater than 80% likelihood of developing T1D within 15 years (13). However, there is considerable variability in the rate of progressing from seroconversion (development of AA) to clinical T1D onset, ranging from weeks to decades (14). The factors that govern this variability are not understood.
Historically, studies of T1D pathogenesis have focused on adaptive immunity, however, increasing attention is now being directed towards the role of innate immunity (reviewed in (15)). Studies by our group and others support the hypothesis that T1D pathogenesis involves elevated innate immune activity coupled with failures in central and peripheral tolerance mechanisms that allow for expansion of auto-reactive T-cells (16, 17). Several observations are driving an increased focus on innate immune processes in T1D pathogenesis: 1) Monotherapies that have aggressively targeted and suppressed adaptive immunity have failed to induce sustained disruption of the underlying disease process (18–20), whereas recent preclinical studies using combination therapies targeting both innate and adaptive immunity suggest greater efficacy (21, 22). 2) The increase in T1D incidence that has occurred in recent decades is too rapid to be due to solely genetic shifts; this is supported in part by the observation that those with “low risk” HLA genotypes now have the highest rate of increased T1D incidence (23, 24). These epidemiological changes suggest the presence of environmental changes that potentiate autoimmunity, perhaps through dysregulated innate immune processes.
A full understanding of the mechanisms underlying age-dependent diabetes susceptibility remains unclear. This review will focus on recent studies that have identified a heightened innate inflammatory state associated with diabetes susceptibility in T1D families and T1D rat models; proposed mechanisms for how this baseline innate inflammation progresses to T1D, such as through viral triggering and defective immunoregulation; and how therapeutically targeting innate inflammatory processes may reduce disease incidence.
Innate Immunity and Inflammation in T1D
The innate immune system plays a vital role as the first line of defense against microbial infection. Leukocytes of the innate immune system, which consist of natural killer cells, granulocytes (mast cells, eosinophils, basophils, neutrophils), macrophages, and dendritic cells, function within the immune system by recognizing common microbial ligands and initiating generalized inflammatory responses against the microbe or infected cells (reviewed in (25)). Innate pattern recognition receptors (PRRs), including toll-like receptors (TLRs), RIG-I-like helicases (immunoreceptors for viral RNA), and nucleotide-binding oligomerization domain–like receptors, are largely responsible for this host-environment interaction and, once stimulated, promote the synthesis and release of pro-inflammatory mediators (such as interleukin-1 (IL-1) and interferon-α (IFN-α)), that promote the development of adaptive T-cell mediated immunity. Overall shifts in baseline innate inflammatory activity may be mediated through the inheritance of potentiating genetic variants in immune pathways as well as through environmental changes that influence innate immune cells by altering the magnitude or types of microbial exposures.
Several theories, (reviewed in (26)) have been put forth to explain how genetic and environmental factors interact to promote T1D pathogenesis, and are applicable to the epidemiological changes being observed in T1D and other autoimmune diseases. The “hygiene hypothesis” postulates that decreasing infectious disease exposures and the subsequent paucity of innate immune stimulation in early life primes the adaptive immune system towards autoimmunity (27). It is supported, in part, by the inverse relationship between T1D incidence and infectious disease mortality rates between countries (28). The “fertile field hypothesis” posits that microbial infections induce an innate immunological state primed for expansion of auto-reactive T-cells given the right stimuli (29). The gastrointestinal (GI) tract represents a major site for host:environment interaction and the “perfect storm hypothesis” considers a role for interplay between the gut microbiome, genetic modifiers of immune function, and increased intestinal permeability (30). Notably, increased intestinal permeability has been reported in T1D patients, their unaffected family members, and in rat models of T1D (31–35). Furthermore, a growing body of evidence suggests that multiple environmental factors, including viral infections, dietary alterations, and the composition of the GI microbiome, can contribute to T1D development in genetically susceptible subjects, potentially through alterations in mucosal immunity and PRR ligand exposure (3, 26, 30, 36). Together the data suggest a complex interplay of multiple genetic and environmental factors in the pathogenesis of T1D.
Evidence of viral triggering of T1D in genetically susceptible hosts: Rat models and human studies
Investigations into the viral triggering of T1D have been greatly facilitated by the use of diabetes susceptible rat strains. As with humans, diabetes in the BioBreeding diabetes resistant (BBDR) and LEW1.WR1 rat models is T-cell mediated and dependent on a high risk major histocompatibility complex (MHC) class II haplotype (RT1u) (37, 38). Unlike their congenic BB diabetes prone (BBDP) littermates, BBDR rats are wild-type for Gimap5+/+ (39), a gene necessary for post-thymic T-cell survival (40–42). They are therefore neither lymphopenic nor deficient in regulatory T-cells (Tregs) (43, 44), and do not develop insulitis or spontaneous diabetes. While approximately 2% of LEW1.WR1 rats develop T1D spontaneously (45), higher rates of diabetes can be induced in both young LEW1.WR1 and BBDR rats by viruses that include Kilham’s rat virus (KRV) (45–49), a ubiquitous rat parvovirus (45, 50, 51). Very high rates of T1D penetrance (up to 100%) can be achieved in both BBDR and LEW1.WR1 rats after KRV infection if the animals are first primed with polyinosinic:polycytidylic acid (poly I:C), a TLR3 agonist known to activate innate inflammation (45, 48), highlighting the role of a background inflammatory profile in driving diabetogenesis.
In genetically susceptible hosts, a simple causal link between viral exposure and progression to diabetes cannot be made. Like KRV, rat cytomegalovirus was found to induce T1D in up to 60% of LEW1.WR1 rats, whereas H-1, vaccinia, and Coxsackie B4 viruses did not (45). In this same study, simultaneous inoculation of KRV and rat cytomegalovirus induced diabetes in 100% of animals. Overall, the data show that the potential of a given viral infection to induce T1D varies by the type of virus and is influenced by the sequence or combination of viral exposures (45). Importantly, the ability of KRV to induce T1D in LEW.1WR1 and BBDR rats declines with age (45, 49), and suggests that developmental changes in susceptible hosts are determinants of viral diabetogenicity. Relevant to the low rate of T1D progression among human carriers of high risk HLA haplotypes, such viral induction protocols do not induce T1D in MHC-matched Wistar-Furth rats, suggesting that factors beyond the MHC are necessary for viral induction of T1D (51, 52). The ability to induce diabetes within the context of high genetic risk in an age-dependent manner is a significant attribute of the BBDR and LEW1.WR1 rat models that may parallel human diabetogenesis.
Counter to rat models, conclusive proof of viral triggering of human T1D has remained elusive. However, epidemiologic studies have supported a role for viruses in human T1D as incidence varies with season and birth month (53, 54), and a multitude of studies have identified a higher rate of enterovirus infection among T1D patients than controls (55–61). A systematic meta-analysis concluded a significant association between enterovirus infection and T1D associated autoimmunity as defined as seroconversion or progression to T1D; specifically, the likelihood of finding a previous enterovirus infection was 9 times higher in T1D patients and 3 times higher in individuals with diabetes-related autoimmunity as compared to healthy controls (62). This analysis has been further supported by the recently reported association of Coxsackie B1 virus with an increased risk of β-cell autoimmunity (63), as well as immunohistochemical staining studies that detected presence of the enteroviral capsid protein VP1 at a higher frequency in islets of patients with new onset T1D compared to controls (64). Genetic studies have also implicated anti-viral IFN-α driven immune responses in diabetogenesis through the association of several candidate genes (IFIH1, TLR7/TLR8, FUT2, and GPR183) (65, 66) that encode proteins involved in responses to viral infection. Further, targeted and genome-wide transcriptomic analysis of two large longitudinal studies of at-risk infant cohorts, BABYDIET (67) and the Finnish Type 1 Diabetes Prediction and Prevention study (68), have identified a transient IFN driven-signature in individuals prior to seroconversion and overt diabetes. More specifically, the IFN signature was found to be temporally preceded by recent respiratory infections, suggesting some degree of causality (67). It is also possible, however, that at-risk and T1D subjects possess a heightened baseline innate inflammatory state and may tend towards a more vigorous response when challenged by infection.
Evidence of an underlying innate inflammatory state associated with T1D susceptibility
One means by which innate immune cells mediate their actions is through the liberation of soluble mediators. In T1D, the sites of inflammation, namely the pancreatic islets and draining lymph nodes, are largely inaccessible except at necropsy/autopsy and represent a minimal fraction of body mass. As such, inflammatory cytokines, chemokines, and other mediators (small nucleotides, lipids) may be at a locally high concentration at these sites but are often too dilute in the periphery for direct measurement. Further, measurement of a single or few cytokines may be uninformative due to important combinatorial effects.
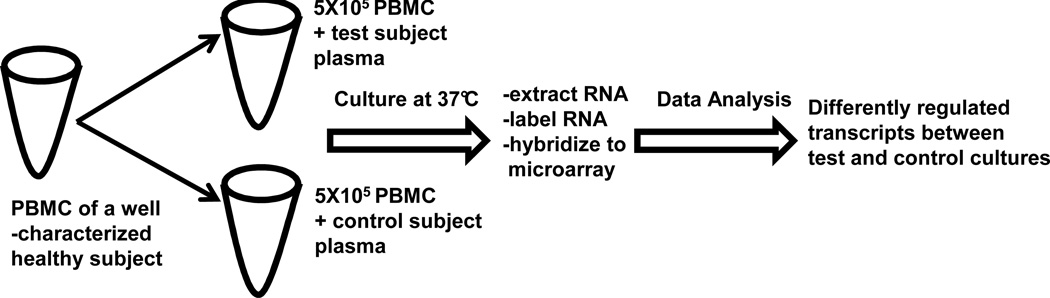
We have employed an alternative approach to assay the extracellular milieu associated with T1D susceptibility. This strategy, illustrated in Figure 1, uses patient plasma to induce transcriptional responses in a “reporter” cell population. With this indirect approach, peripheral blood mononuclear cells (PBMCs) of a well-controlled healthy blood donor are used as sensitive biosensors that transcriptionally respond to the dilute disease-associated factors in patient plasma. This response is then measured with a comprehensive genome-scale array (48, 69–71). After the application of pathway analyses, the array data can be quantitatively interpreted in terms of inflammatory and regulatory immune activities.
Figure 1. Assay Format.
Serum/plasma induced transcriptional bioassay. The optimized assay uses commercially supplied peripheral blood mononuclear cells (PBMC) of a single draw of a single well-characterized person representative of the normal population for human analyses and PBMC of Brown Norway rats for rat analyses. In a volume of 500ul, PBMC are cultured with patient serum or plasma (40% or 20% for human or rat analyses, respectively). Purified RNA (~100ng) from PBMC is labeled and hybridized to the appropriate Affymetrix GeneChip. The conditioned media are retained for other analyses. Signal intensities are normalized with Robust Multichip Analysis (RMA; www.bioconductor.org/) and complex analyses are conducted with other softwares.
Temporal Measurement of Plasma Induced Signatures in BB rats
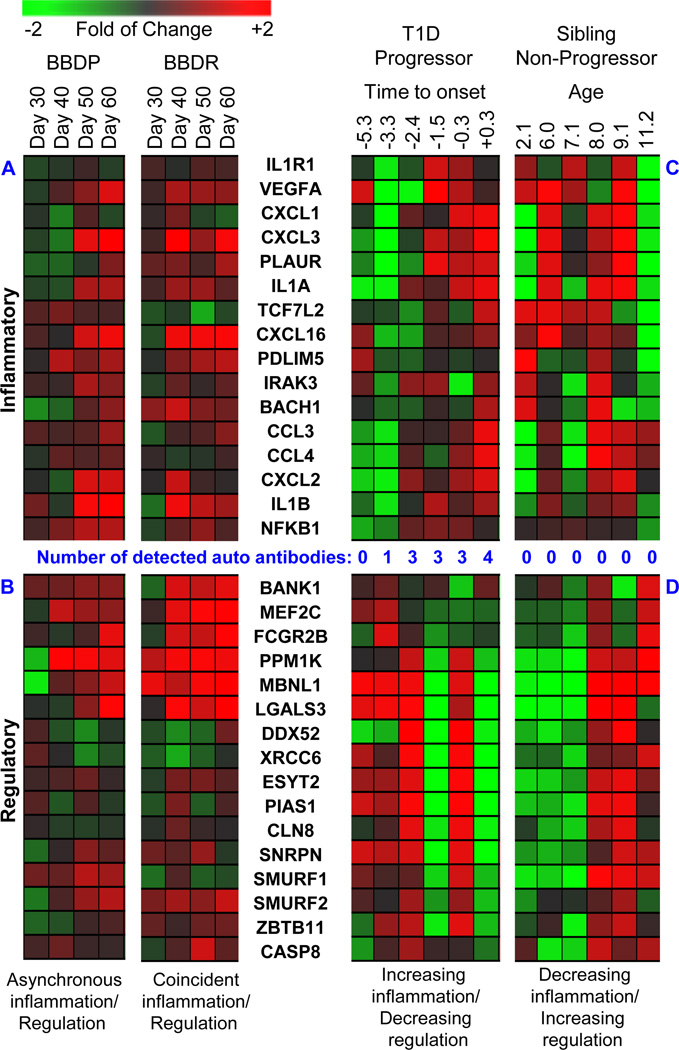
We applied this approach to plasma samples longitudinally collected from spontaneously diabetic BBDP rats and non-diabetic BBDR rats at 30, 40, 50 and 60 days of life, with the objective of studying the secretome associated with both T1D progression and non-progression in the context of high genetic susceptibility (48). In line with their shared susceptibility, plasma of both BB sub-strains induced a transcriptional response indicative of innate immune activation and consistent with PRR ligand exposure (Figure 2A). This signature included robust induction of genes associated with inflammatory IL-1 and nuclear factor-kappa B (NF-kB) signaling, such as Il1a, Il1b, Il1r1, Irak3, Ccl3, Ccl4, and Nfkb1. However, a key feature distinguishing BBDR from BBDP plasma was temporal induction of many interleukin-10 (IL-10) and transforming growth factor-β (TGFβ) dependent genes known to be involved in the regulation of inflammatory responses, consistent with the presence of Treg activity (Figure 2B). This included transcripts encoding Smurf1, Smurf2, Fcgr2b, Pias1, Casp8, and Lgals3. The longitudinal analysis also revealed that the dynamics and balance of the inflammatory and regulatory responses of the two BB sub-strains were highly distinct: BBDR rats exhibited simultaneous induction of transcription consistent with both pro-inflammatory and immunoregulatory processes by age 40 days. In contrast, longitudinal samples collected from BBDP rats exhibited asynchronous dynamics, with later induction of inflammatory transcription, consistent with their overall lymphopenia, as well as a weaker and even later induction of IL-10 mediated regulatory transcription, consistent with their deficiency in Tregs. During this time frame, BBDP rats exhibit histological evidence of low grade insulitis by 50 days of age, which progresses to profound β-cell destruction and T1D onset by ~60 days of age (72, 73).
Figure 2. Heatmap.
Dynamics of T1D progression and non-progression in BB rats and T1D families. Orthologues were identified and BB rat plasma induced transcriptional analyses were conducted as previously described (48). Human plasma induced transcriptional analyses were conducted as previously described (82). Respectively, Panel A and Panel B show inflammatory and regulatory transcripts induced by plasma temporally collected from spontaneously diabetic BBDP and diabetes inducible BBDR rats. Respectively, Panel C and Panel D show inflammatory and regulatory transcripts induced by plasma temporally collected from a human subject that developed T1D and a healthy sibling of a T1D patient that did not seroconvert to autoantibody positivity or progress to T1D. Expression levels illustrated in the heat maps are normalized against the mean of all time points.
Consistent with the temporal induction of the immunoregulation seen in the transcriptional analyses, BBDR rats exhibit an age-dependent decrease in circulating cytokine/chemokine levels when directly measured by ELISA. Specifically, cytokines and chemokines including Il-1α, Ccl2, Ccl3, and Cxcl1 were at their highest levels at 30–40 days and found to subsequently decline by 50–60 days of life (48). In contrast, significant temporal changes in circulating cytokine and chemokine levels were generally unmeasurable in BBDP rats, likely owing to their lymphopenia. Plasma levels of the Treg-derived regulatory cytokine IL-10 did not significantly differ between the strains over time. However, the highest IL-10 level was observed at day 40 in the BBDR rats, whereas in BBDP rats the highest levels were not measured until day 60, again indicating asynchronous induction of immunoregulatory processes between the two BB sub-strains. While the direct circulating cytokine measurements provided some degree of validation, the plasma induced signatures were highly informative, revealing a much more complex and dynamic process.
It has been known that susceptibility of LEW.1WR1 rats to KRV-induced T1D declines with age (45, 49), but it is not known why this occurs. Hence, we conducted studies to determine if the temporal immunoregulatory changes we observed in BBDR rats inversely correlated with an age-dependent decline in susceptibility of KRV-induced T1D in BBDR rats. Indeed, the penetrance of T1D induced by KRV with or without a priming dose of poly I:C significantly declined with increasing age in the BBDR rat (48). For example, 100% of weanling rats (< 30 days of age) developed T1D after poly I:C- and KRV-inoculation as compared to 0% of the rats older than 40 days of life. Thus, the age-dependent decline in T1D susceptibility following immunological perturbation in BBDR rats corresponds with both an age-dependent acquisition of an IL-10- and TGF-β-mediated immunoregulated state and a decline in measurable pro-inflammatory cytokine levels. Importantly, these observations offer mechanistic insight as to the juvenile nature of T1D susceptibility in the BBDR rat.
T1D susceptibility of the BB rat extends beyond that conveyed by the high-risk MHC (in BBDR and BBDP rats) and Gimap5 deficiency (in BBDP rats). This is reflected by the expression of the chemokine eotaxin (Ccl11), an attractant for mast cells, granulocytes and T-helper 2 lymphocytes, by the pancreatic β-cells of BB rats. Islet-specific eotaxin expression, which we interpret as a marker of islet duress, is histologically detected by 40 days of life in both insulitic BBDP rats and insulitis-free BBDR rats (73, 74). Interestingly, this phenotype is not observed in the MHC-matched Wistar-Furth rat or when the RT1u class II MHC and/or Gimap5 deficiency, the two largest genetic contributors to T1D risk in BBDP rats, are introgressed onto a Fischer (F344) control strain (unpublished results). Consistent with their lack of an underlying inflammatory state, in longitudinal studies, these new F344 derived control strains completely lack the inflammatory and regulatory dynamics exhibited by the plasma induced signatures of BBDP and BBDR rats (unpublished results) and do not develop insulitis or diabetes.
The reasons for the baseline elevated inflammatory states in BBDR and BBDP rats remains to be answered. Consistent with the existence of additional genetic modifiers of immune function is the recent report that BB rats possess a single nucleotide substitution in the protein tyrosine phosphatase non-receptor type 22 (PTPN22) gene (a gene also associated with human T1D susceptibility) that results in T-cell hyper-responsiveness (75). Another source of inflammation could be intestinal hyper-permeability, possibly mediated by genetic variation and/or the composition of the intestinal microbiome, which could promote translocation of bacteria and/or PRR ligands and a heightened systemic innate inflammatory state. Consistent with this possibility, we previously reported levels of lipopolysaccharide, a TLR agonist of bacterial origin, to be higher in BBDP and BBDR rat serum than in that of Brown Norway rats (70). Notably, administration of the probiotic Lactobacillus johnsonii N6.2 increased tight junction protein expression and reduced expression of proinflammatory cytokines in the ileum, and decreased the incidence of T1D in BBDP rats (76). A similar underlying pathomechanism, involving interactions between inherited susceptibility, gut microbiota and environmental triggers (for example, viruses) may also exist in the LEW1.WR1 rat, as antibiotic treatment has been determined to protect rats from KRV-induced insulitis and T1D (77).
Measurement of Plasma Induced Signatures in human T1D
We have extensively applied the same sensitive and comprehensive approach detailed in Figure 1 to human T1D. In our seminal report (17), similar to the BB rat, we observed that plasma of autoantibody positive (AA(+)) recent onset (RO) T1D subjects induced a partially IL-1 dependent signature consistent with immune activation and PRR ligand exposure relative to plasma of unrelated healthy controls (uHC). RO T1D subjects lacking titers for the commonly tested AA were not different from their AA(+) counterparts, indicating that the signature was independent of AA status (17). Signatures of subjects with long-standing T1D (>10 years post-diagnosis) were more similar to those of uHC (17), suggesting that 1) the RO T1D signature is related to active autoimmunity and 2) long-standing T1D is immunologically quiescent relative to the immediate post-onset period. Importantly, we analyzed longitudinal samples of subjects who subsequently progressed to T1D, and found that the signature associated with RO T1D preceded both the development of AA and the clinical diagnosis of T1D by as much as 5 years (17). To explore the disease-specificity of the approach, we have applied it to H1N1 influenza, bacterial pneumonia, and cystic fibrosis with airway Pseudomonas aeruginosa colonization (71) as well as multiple sclerosis and Crohn’s disease (unpublished results) and have found specific, mechanistically-informative signatures for each disease state. Other groups have used this approach to identify disease specific signatures associated with systemic onset juvenile idiopathic arthritis and sepsis (78, 79). Importantly, corroborating evidence of an innate inflammatory state associated with T1D has been generated through the application of global serum-based proteomic analysis of diabetes patients and unrelated healthy controls (80).
Our more recent efforts have focused on using plasma induced transcriptional bioassays to understand the immune states that exist within T1D families and how these change with progression and non-progression to T1D over time. Siblings of those affected with T1D have ~6% probability of developing T1D and most who progress to T1D possess a DR3 and/or DR4 HLA haplotype (5, 81). Therefore, we conducted a cross-sectional study of age-matched RO T1D patients, uHC (no family history of T1D), healthy AA(−) high HLA risk siblings (DR3 and/or DR4, termed HRS) and healthy AA(−) low HLA risk siblings (non-DR3/non-DR4, termed LRS) (82). While RO T1D plasma induced a unique signature relative to the uHC, HRS and LRS, the signatures associated with these 3 healthy control cohorts were also distinct from one another. Expectedly, the RO T1D and HRS groups were found most similar to one another as they share the most genetic susceptibility. Unexpectedly, however, the LRS and uHC cohorts possessed the most distinct signatures from one another. It was also surprising that the LRS signature exhibited the most robust induction of inflammatory transcripts and the lowest induction of transcripts associated with immunoregulatory activity. Pathway analyses revealed a continuum of immune states across the four cohorts: Relative to uHC, T1D family members exhibited an elevated, partially-IL-1 dependent inflammatory state. Among HRS, this familial inflammatory state was found more regulated with greater IL-10/TGF-β bias compared to LRS. RO T1D patients possessed signatures intermediate to those of LRS and HRS, such that inflammation decreased and regulation increased across the LRS→ROT1D→HRS→uHC continuum. These relationships were confirmed through in vitro studies whereby co-cultures were spiked with either cytokines or receptor-blocking antibodies (82).
Additional follow-up studies included 25-plex cytokine analyses of the four cohorts. Significant differences were not detected between the 3 T1D family cohorts. However, significantly elevated levels of inflammatory cytokines and chemokines (specifically IL-1α, IL-12p40, CCL2, CCL3 and CCL4) were detected in RO T1D patients and their healthy AA(−) siblings relative to uHC. The selected ELISA panel could not differentiate the four cross-sectional cohorts whereas the plasma-induced signature could, reflecting the bioassay’s ability to capture a net response to the sum of plasma-borne factors. Taken together, our studies support that an innate inflammatory state exists in T1D families independent of HLA haplotype, AA status, and disease progression. Further, in HRS where high-risk HLA haplotypes convey greater probability of an adaptive response and T1D progression, immunoregulatory mechanisms are more active.
We have also longitudinally studied siblings of those with T1D to capture the changes in immune response associated with either progression or non-progression to T1D (82). Among those who progress to T1D (Figure 2C), we consistently observe temporal increases in inflammatory activity (e.g. IL1A, IL1B, IL1R1, IRAK3, CCL3, CCl4, and NFKB1) with coincident reductions in regulatory activity. Perhaps expectedly, an increase in inflammatory bias is seen with, or just prior to, the detection of circulating AAs in HRS. The opposite is also observed, with regulatory bias being associated with decreases in the number of detected AAs. Consistent with the cross-sectional HRS cohort signature, longitudinal analysis of persistently AA(−) HRS subjects revealed temporal increases transcripts associated with immunoregulatory processes and suggested the possibility of increased Treg activity (Figure 2D). This included transcripts encoding SMURF1, SMURF2, FCGR2B, PIAS1, CASP8, and LGALS3.
Using the phenotyping protocol described by Miyara et al. (83), we longitudinally examined abundances of activated and resting Treg in banked peripheral blood mononuclear cell samples of our T1D family cohorts. Those subjects whose signatures showed increasing inflammatory bias over time (subjects who progressed to T1D and persistently AA(+) HRS) showed a flat or downward trend in the percentage of activated Tregs. Conversely, the percentage of activated Tregs increased as the inflammatory signature decreased over time in AA(−) HRS. Combined, these data suggest that the innate inflammatory state common to T1D family members is more actively regulated in an age-dependent manner in the presence of high risk HLA haplotypes, offering mechanistic insight as to why human T1D susceptibility declines with age (82).
The innate inflammatory state detailed above in T1D subjects and their family members is in part dependent on IL-1, a pro-inflammatory cytokine central to innate immunity that has been implicated in T1D risk. IL-1 is known to act directly on β-cells, by impairing insulin biosynthesis and release (84–86), and by promoting cytokine and hyperglycemia-induced β-cell death (84, 87, 88). These effects can be mitigated by IL-1 antagonism (89, 90). IL-1 also amplifies adaptive immunity by enhancing the expansion and survival of naïve and memory T-cells and by promoting T-helper-1 and T-helper 17 effector T cell differentiation and proliferation (91). Although the literature describing peripheral cytokine and chemokine levels in T1D are mixed, likely due to the use of healthy related subjects as controls, elevated peripheral levels of IL-1 has been previously reported in T1D subjects (92, 93). Furthermore, compared to controls, leukocytes of T1D subjects have been reported to express elevated innate transcriptional programs as well as exhibit elevated responsiveness as evidenced by the liberation of higher levels of IL-1 upon mitogen stimulation in vitro (93–97).
Studies have also reported evidence of immune hyper-responsiveness in unaffected family members of those with T1D compared to unrelated healthy controls. Both T1D subjects and their family members have quantitatively fewer peripheral dendritic cells than healthy controls; however, the peripheral dendritic cells of high-risk T1D relatives produce more IFNα in response to TLR9 stimulation than controls (98). Further, the PBMCs of children with T1D and their first degree relatives express higher spontaneous and mitogen-stimulated levels of TNF-α and IFN-γ, both β-cell toxic cytokines (93).
In line with our observations of temporally induced immunoregulation among those who do not progress to T1D, other evidence supports the existence of active homeostatic mechanisms that control autoimmunity. The most commonly recognized example is that many family members of T1D subjects never progress to clinical onset of T1D despite the development of autoantibodies. In addition, AA- first-degree relatives of T1D subjects have been reported to have a balance of inflammatory and regulatory T cells intermediate to that of recent onset T1D and uHC (99). The variable ability of a given subject to successfully manage the innate inflammatory state likely stems from varying combinations of genetic predisposition and environmental factors that encompass viruses as well as diet and the GI microbiome (3, 26, 30).
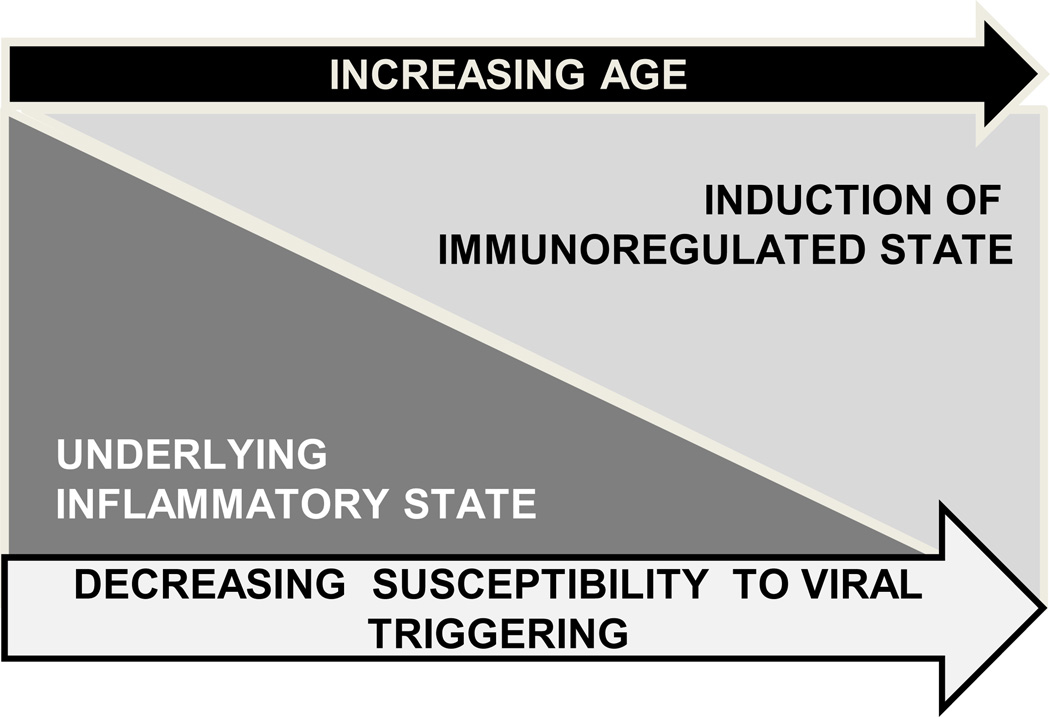
Our working model for age-dependent T1D susceptibility in humans and BB rats is illustrated in Figure 3. Our rodent and human studies have identified an elevated inflammatory state associated with T1D susceptibility that is consistent with hypersensitivity towards and/or increased exposure to PRR ligands; this perhaps represents the proposed “fertile field” (29). Increased sensitivity to PRR ligands could be mediated by genetics, while increased PRR ligand exposure could be mediated by increased intestinal permeability, a phenotype that may be genetically-controlled but environmentally influenced. Coincident with the rising incidence of T1D, antibiotic usage has become common, and there has been a shift in Western populations towards a high-fat, high-carbohydrate diet (100, 101); these environmental changes mediate alterations in the GI microbiome. Indeed, changes in the GI microbiome have been reported to result in increases in intestinal permeability and pro-inflammatory bias in human and animal studies (102–106). Moreover, altered intestinal ultra-structure and intestinal barrier dysfunction have been associated with T1D in humans (31–33, 35) and rodent models including the BB rat (107–110). Notably, zonulin, a protein that modulates intestinal permeability through mediation of intercellular tight junction disassembly, has been found elevated in T1D patients and their healthy first degree relatives (34) and blockade of the zonulin receptor reduces T1D incidence in BBDP rats (111). Furthermore, new studies have identified significant alterations in the GI microbiome associated with T1D progression that include reduced community diversity and increased inflammation-associated organisms between the time of seroconversion and diabetes onset (36).
Figure 3. Model.
Model mechanism for age-dependent decline in T1D susceptibility. Studies support the existence of an elevated innate inflammatory state associated with T1D susceptibility in human T1D families and diabetic rat models. In human T1D families, this state is independent of HLA, autoantibody status and disease progression. In BBDR rats, this state is independent of the MHC, insulitis and disease progression, but is associated with the ability of KRV to trigger disease progression. This inflammatory state may be the consequence of genetics, diet and intestinal microbiome. We further hypothesize that this heightened inflammatory state represents a “fertile field” in which the additive inflammation mediated through viral infection can lead to the breaking of immunological tolerance and the progression of autoimmunity in susceptible hosts. Our studies support that this heightened underlying inflammatory state is subsequently supplanted by induction of an immunoregulatory state over time. As these endogenous regulatory processes become more robust, the immune balance makes environmental triggering of T1D progression less likely.
Our studies have also identified age-dependent endogenous regulatory mechanisms, reflected in part by temporal increases in Treg percentages, that we hypothesize act to manage the underlying innate inflammatory state associated T1D susceptibility. Importantly, exposure of Tregs to inflammatory inputs, including endotoxin or cytokines, can impair their suppressive capacity (112, 113). Thus, in addition to genetic variation and the endogenous innate state, additional inflammatory signals such as those mediated by viral infection, particularly in younger subjects, may impair compensatory regulatory mechanisms and promote autoimmunity.
Therapeutic targeting of innate inflammation in Type 1 Diabetes
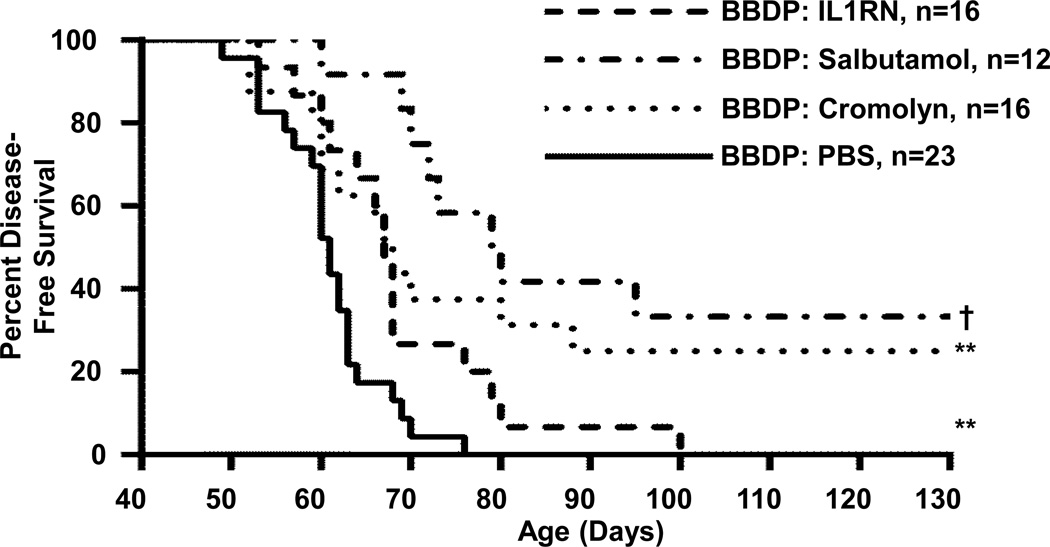
Therapeutic targeting of innate inflammatory processes can delay and/or prevent diabetes in rat models of T1D. We have treated spontaneously diabetic BBDP rats with agents that are known to inhibit degranulation of innate immune cells. Cromolyn (disodium cromoglycate) inhibits mast cell activation/degranulation by impairing phosphorylation of moesin, which is involved in signal transduction and regulating functional associations between the cell surface and cytoskeleton (114). Salbutamol is a β2-adrenergic agonist that inhibits the intermediate conductance of the calcium activated potassium channel expressed by mast cells (115), that has been shown to reduce the severity of disease in other autoimmune models (115, 116). As shown in Figure 4, both cromolyn (74) and salbutamol significantly delayed or prevented T1D onset in otherwise spontaneously diabetic BBDP rats. We have also targeted the IL-1 pathway with IL-1 receptor antagonist (IL-1RN, anakinra), which binds IL-1 receptor 1 thereby blocking the actions of both IL-1α and IL-1β. Treatment of BBDP rats with IL-1RN more modestly delayed diabetes onset in BBDP rats (70).
Figure 4. Survival Plots.
Therapeutic targeting of innate inflammation pathways in BBDP rats delays/prevents T1D. Survival plots of BBDP rats after intraperitoneal delivery of interleukin-1 receptor antagonist (IL1RN, 350µg/kg/day, dash line), salbutamol (17.5µg/g five days per week, dash-dot line) and cromolyn (200µg/g five days per week, dot line). Control BBDP rats were treated daily with phosphate buffered saline (PBS, solid line). All treatments were initiated by 30 days of age. T1D onset was defined as the first of two consecutive days with fasting blood glucose ≥ 250mg/dl. The median age of survival in the saline-treated control group was 61 days (n=23, range 49–76 days), for IL1RN it was 67 days (n=16, range 53–100 days), for salbutamol it was 79 days (n=12, range 60–130 days) and for cromolyn it was 68 days (n=16, range 52–130 days). Significance based on log rank test, ** p<0.01, † p<0.0001.
Therapeutic targeting of innate immunity can also reduce viral triggering of T1D in susceptible rat models. Pre-treatment with salicylate, a potent inhibitor of NFkB activity (117), successfully prevented KRV-induced diabetes in BBDR rats, and was associated with reduced serum levels of pro-inflammatory cytokines and the acute phase protein, haptoglobin (118). Further, LEW1.WR1 rats co-administered the anti-inflammatory histone deacetylase inhibitor ITF-2357 and KRV were protected from diabetes through modulation of innate inflammation (119). Co-administration of IL-1RN with KRV prevented viral triggering of T1D in LEW1.WR1 rats without interfering with the adaptive immunity responses necessary for clearing infection (120). Consistent with a role for the GI microbiome and mucosal immunity in potentiating the innate state of T1D susceptible rats is the finding that administration of antibiotics in the drinking water of LEW1.WR1 rats reduced viral-induced T1D (77).
It is important to point out that successful targeting of innate immunity in rat models has generally been initiated in young animals before significant progression of the adaptive immune response has occurred (i.e., near the time of weaning in the spontaneous BBDP model and before or coincident with viral triggering of diabetogenesis in the BBDR or LEW1.WR1 rats). This is in contrast to the immunotherapeutic trials that have been completed in human T1D, which have focused on patients after clinical onset, a time relatively late in the disease course and well after the establishment of targeted adaptive immune responses towards islet antigens. These differences likely contribute to why animal studies have been more successful than existing human trials in mitigating disease progression. Overall therapies targeting innate immunity in human subjects remains largely in its infancy. A small pilot study of the potent anti-inflammatory agent anti-TNF-α in newly diagnosed pediatric T1D subjects suggested some degree of β-cell preservation (121). Administration of alpha-1 antitrypsin (AAT) to a small group of T1D subjects led to reduced TLR-induced IL-1β response in monocytes and dendritic cells 12 months after treatment, but did not result in significant metabolic improvements (122). Circulating plasma AAT, an endogenous inhibitor of neutrophil serine proteases, is decreased in new onset T1D patients, thus representing a possible mechanism for enhanced innate inflammation (123). In vitro, AAT inhibits LPS-induced production of TNF-α and IL-1β (124, 125) while enhancing regulatory IL-10 expression (124). Vitamin D has also been identified as a potential therapeutic given its broad anti-inflammatory properties (126) and its ability to promote the generation of tolerogenic mature dendritic cells (127). However, small studies of vitamin D supplementation in recent onset T1D have only resulted in modest β-cell protection (128, 129).
On a larger scale, IL-1RN (Anakinra) and human monoclonal IL-1β antibody (canakinumab) were employed in two randomized, placebo-controlled trials in recent onset T1D subjects (the Anti-Interleukin-1 in Diabetes Action and TrialNet Canakinumab trials) (130). Unfortunately, while well-tolerated, neither IL-1RN nor canakinumab resulted in preserved β-cell function, as measured by stimulated c-peptide area under the curve. Despite this failure, using our plasma induced transcriptional bioassay, our group has shown reduced inflammatory bias and enhanced regulatory bias in these subjects; further, inflammation was inversely correlated with c-peptide response in IL-1RN-treated subjects, suggesting that in a subset of subjects exhibiting the greatest reduction in inflammation, there was improved β-cell function (131). However, IL-1RN and canakinumab normalized only a small portion of the probe sets identified in historical cross-sectional analyses of healthy controls (unpublished data), suggesting that the immune state associated with new onset T1D arises from a complex milieu of mediators that extends beyond an acute IL-1 mediated inflammatory signal; as such, targeting innate inflammation with monotherapies at diagnosis may be too late to be effective. Because of the complexity of diabetes pathogenesis, successful intervention may require combinatorial approaches targeting both innate and adaptive immunity. Indeed, preclinical studies suggest that IL-1 blockade in combination with other therapies may preserve β-cell function more effectively than using it alone (21, 22).
In sum, T1D susceptibility may be in part attributed to a baseline heightened innate inflammatory state, as has been documented in recently diagnosed T1D subjects and their family members. The exact reasons for this heightened innate inflammation remain largely unknown but altered host:environment interactions are likely involved. Our studies suggest that siblings of those with T1D who successfully mitigate this inflammation with an age-dependent increase in immunoregulatory processes do not progress to T1D. Conversely, failures of these processes to either initiate or fully develop results in seroconversion and/or progression to T1D. Acknowledgement of the important, temporal role of innate inflammation in T1D susceptibility allows not only for an understanding of why diabetes is largely a disease of juvenile onset but also offers the potential for new therapeutic approaches.
Acknowledgements
The work was supported by The Juvenile Diabetes Research Foundation International (grants 5-2012-220, 17-2012-621 to MJH); American Diabetes Association (grant 7-12-BS-075 to MJH); National Institutes of Health (grants R01AI078713 to MJH, DP3DK098161 to MJH, and the National Center for Advancing Translational Sciences, National Institutes of Health grant 8UL1TR000055); The Children’s Research Institute of Children’s Hospital of Wisconsin (grants FP6477 to SMC and FP7674 to MJH); and The Children’s Hospital of Wisconsin Foundation. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. All authors have read the journal’s authorship agreement the manuscript has been reviewed by and approved by all authors.
Abbreviations
- T1D
type 1 diabetes mellitus
- HLA
human leukocyte antigen
- AA
diabetes-specific autoantibodies
- PRR
pattern recognition receptors
- TLR
toll-like receptors
- IL-1
interleukin-1
- IFN-α
interferon-gamma
- GI
gastrointestinal
- BB
Biobreeding rat strain
- BBDR
Biobreeding diabetes resistant
- BBDP
Biobreeding diabetes prone
- MHC
major histocompatibility complex
- Treg(s)
regulatory T lymphocyte(s)
- KRV
Kilham’s rat virus
- Poly I:C
polyinosinic:polycytidylic acid
- PBMC(s)
peripheral blood mononuclear cell(s)
- IL-10
interleukin-10
- TGF-β
transforming growth factor-beta
- ELISA
enzyme-linked immunosorbent assay
- PTPN22
protein tyrosine phosphatase non-receptor type 22
- RO T1D
recent-onset type 1 diabetes
- uHC
unrelated healthy control (no family history of type 1 diabetes)
- HRS
high risk sibling (healthy, autoantibody negative, and possess DR3 and/or DR4 haplotype)
- LRS
low risk sibling (healthy, autoantibody negative, lacking either DR3 or DR4 haplotype)
- IL-1RN
interleukin-1 receptor antagonist (drug name: anakinra)
- AAT
alpha-1 antitrypsin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All authors have read and understand the policy on disclosure of conflicts of interest. The authors have no conflicts of interests to declare.
Contributor Information
Susanne M. Cabrera, Email: scabrera@mcw.edu.
Angela M. Henschel, Email: ahensche@mcw.edu.
Martin J. Hessner, Email: mhessner@mcw.edu.
References
- 1.Harjutsalo V, Sjoberg L, Tuomilehto J. Time trends in the incidence of type 1 diabetes in Finnish children: a cohort study. Lancet. 2008 May 24;371(9626):1777–1782. doi: 10.1016/S0140-6736(08)60765-5. [DOI] [PubMed] [Google Scholar]
- 2.Zheng S, Mathews CE. Metabolic abnormalities in the pathogenesis of type 1 diabetes. Current diabetes reports. 2014;14(9):519. doi: 10.1007/s11892-014-0519-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pugliese A. The multiple origins of Type 1 diabetes. Diabetic medicine : a journal of the British Diabetic Association. 2013 Feb;30(2):135–146. doi: 10.1111/dme.12081. [DOI] [PubMed] [Google Scholar]
- 4.Dabelea D. The accelerating epidemic of childhood diabetes. Lancet. 2009 Jun 13;373(9680):1999–2000. doi: 10.1016/S0140-6736(09)60874-6. [DOI] [PubMed] [Google Scholar]
- 5.Steck AK, Rewers MJ. Genetics of type 1 diabetes. Clinical chemistry. 2011 Feb;57(2):176–185. doi: 10.1373/clinchem.2010.148221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noble JA, Valdes AM, Varney MD, Carlson JA, Moonsamy P, Fear AL, et al. HLA class I and genetic susceptibility to type 1 diabetes: results from the Type 1 Diabetes Genetics Consortium. Diabetes. 2010 Nov;59(11):2972–2979. doi: 10.2337/db10-0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gough SC, Simmonds MJ. The HLA Region and Autoimmune Disease: Associations and Mechanisms of Action. Current genomics. 2007 Nov;8(7):453–465. doi: 10.2174/138920207783591690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mangalam AK, Taneja V, David CS. HLA class II molecules influence susceptibility versus protection in inflammatory diseases by determining the cytokine profile. Journal of immunology. 2013 Jan 15;190(2):513–518. doi: 10.4049/jimmunol.1201891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Concannon P, Rich SS, Nepom GT. Genetics of type 1A diabetes. The New England journal of medicine. 2009 Apr 16;360(16):1646–1654. doi: 10.1056/NEJMra0808284. [DOI] [PubMed] [Google Scholar]
- 10.Atkinson MA, Eisenbarth GS. Type 1 diabetes: new perspectives on disease pathogenesis and treatment. Lancet. 2001 Jul 21;358(9277):221–229. doi: 10.1016/S0140-6736(01)05415-0. [DOI] [PubMed] [Google Scholar]
- 11.Eisenbarth GS. Type I diabetes mellitus. A chronic autoimmune disease. The New England journal of medicine. 1986 May 22;314(21):1360–1368. doi: 10.1056/NEJM198605223142106. [DOI] [PubMed] [Google Scholar]
- 12.Bingley PJ. Clinical applications of diabetes antibody testing. The Journal of clinical endocrinology and metabolism. 2010 Jan;95(1):25–33. doi: 10.1210/jc.2009-1365. [DOI] [PubMed] [Google Scholar]
- 13.Ziegler AG, Rewers M, Simell O, Simell T, Lempainen J, Steck A, et al. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. Jama. 2013 Jun 19;309(23):2473–2479. doi: 10.1001/jama.2013.6285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knip M, Korhonen S, Kulmala P, Veijola R, Reunanen A, Raitakari OT, et al. Prediction of type 1 diabetes in the general population. Diabetes Care. 2010 Jun;33(6):1206–1212. doi: 10.2337/dc09-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zipris D. Innate immunity in type 1 diabetes. Diabetes Metab Res Rev. 2011 Nov;27(8):824–849. doi: 10.1002/dmrr.1256. [DOI] [PubMed] [Google Scholar]
- 16.Todd JA. Etiology of type 1 diabetes. Immunity. 2010 Apr 23;32(4):457–467. doi: 10.1016/j.immuni.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 17.Wang X, Jia S, Geoffrey R, Alemzadeh R, Ghosh S, Hessner MJ. Identification of a molecular signature in human type 1 diabetes mellitus using serum and functional genomics. Journal of immunology. 2008 Feb 1;180(3):1929–1937. doi: 10.4049/jimmunol.180.3.1929. [DOI] [PubMed] [Google Scholar]
- 18.Keymeulen B, Vandemeulebroucke E, Ziegler AG, Mathieu C, Kaufman L, Hale G, et al. Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. The New England journal of medicine. 2005 Jun 23;352(25):2598–2608. doi: 10.1056/NEJMoa043980. [DOI] [PubMed] [Google Scholar]
- 19.Pescovitz MD, Greenbaum CJ, Krause-Steinrauf H, Becker DJ, Gitelman SE, Goland R, et al. Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. The New England journal of medicine. 2009 Nov 26;361(22):2143–2152. doi: 10.1056/NEJMoa0904452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stiller CR, Dupre J, Gent M, Jenner MR, Keown PA, Laupacis A, et al. Effects of cyclosporine immunosuppression in insulin-dependent diabetes mellitus of recent onset. Science. 1984 Mar 30;223(4643):1362–1367. doi: 10.1126/science.6367043. [DOI] [PubMed] [Google Scholar]
- 21.Ablamunits V, Henegariu O, Hansen JB, Opare-Addo L, Preston-Hurlburt P, Santamaria P, et al. Synergistic reversal of type 1 diabetes in NOD mice with anti-CD3 and interleukin-1 blockade: evidence of improved immune regulation. Diabetes. 2012 Jan;61(1):145–154. doi: 10.2337/db11-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pagni PP, Bresson D, Rodriguez-Calvo T, Bel Hani A, Manenkova Y, Amirian N, et al. Combination therapy with an anti-IL-1beta antibody and GAD65 DNA vaccine can reverse recent-onset diabetes in the RIP-GP mouse model. Diabetes. 2014 Jun;63(6):2015–2025. doi: 10.2337/db13-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gillespie KM, Bain SC, Barnett AH, Bingley PJ, Christie MR, Gill GV, et al. The rising incidence of childhood type 1 diabetes and reduced contribution of high-risk HLA haplotypes. Lancet. 2004 Nov 6–12;364(9446):1699–1700. doi: 10.1016/S0140-6736(04)17357-1. [DOI] [PubMed] [Google Scholar]
- 24.Steck AK, Armstrong TK, Babu SR, Eisenbarth GS. Type 1 Diabetes Genetics C. Stepwise or linear decrease in penetrance of type 1 diabetes with lower-risk HLA genotypes over the past 40 years. Diabetes. 2011 Mar;60(3):1045–1049. doi: 10.2337/db10-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007 Oct 18;449(7164):819–826. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 26.Atkinson MA, Chervonsky A. Does the gut microbiota have a role in type 1 diabetes? Early evidence from humans and animal models of the disease. Diabetologia. 2012 Nov;55(11):2868–2877. doi: 10.1007/s00125-012-2672-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bach JF, Chatenoud L. The hygiene hypothesis: an explanation for the increased frequency of insulin-dependent diabetes. Cold Spring Harbor perspectives in medicine. 2012 Feb;2(2):a007799. doi: 10.1101/cshperspect.a007799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abela AG, Fava S. Association of incidence of type 1 diabetes with mortality from infectious disease and with antibiotic susceptibility at a country level. Acta diabetologica. 2013 Dec;50(6):859–865. doi: 10.1007/s00592-013-0464-z. [DOI] [PubMed] [Google Scholar]
- 29.von Herrath MG, Fujinami RS, Whitton JL. Microorganisms and autoimmunity: making the barren field fertile? Nature reviews Microbiology. 2003 Nov;1(2):151–157. doi: 10.1038/nrmicro754. [DOI] [PubMed] [Google Scholar]
- 30.Vaarala O, Atkinson MA, Neu J. The “perfect storm” for type 1 diabetes: the complex interplay between intestinal microbiota, gut permeability, and mucosal immunity. Diabetes. 2008 Oct;57(10):2555–2562. doi: 10.2337/db08-0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bosi E, Molteni L, Radaelli MG, Folini L, Fermo I, Bazzigaluppi E, et al. Increased intestinal permeability precedes clinical onset of type 1 diabetes. Diabetologia. 2006 Dec;49(12):2824–2827. doi: 10.1007/s00125-006-0465-3. [DOI] [PubMed] [Google Scholar]
- 32.Carratu R, Secondulfo M, de Magistris L, Iafusco D, Urio A, Carbone MG, et al. Altered intestinal permeability to mannitol in diabetes mellitus type I. Journal of pediatric gastroenterology and nutrition. 1999 Mar;28(3):264–269. doi: 10.1097/00005176-199903000-00010. [DOI] [PubMed] [Google Scholar]
- 33.Kuitunen M, Saukkonen T, Ilonen J, Akerblom HK, Savilahti E. Intestinal permeability to mannitol and lactulose in children with type 1 diabetes with the HLA-DQB1*02 allele. Autoimmunity. 2002 Aug;35(5):365–368. doi: 10.1080/0891693021000008526. [DOI] [PubMed] [Google Scholar]
- 34.Sapone A, de Magistris L, Pietzak M, Clemente MG, Tripathi A, Cucca F, et al. Zonulin upregulation is associated with increased gut permeability in subjects with type 1 diabetes and their relatives. Diabetes. 2006 May;55(5):1443–1449. doi: 10.2337/db05-1593. [DOI] [PubMed] [Google Scholar]
- 35.Secondulfo M, Iafusco D, Carratu R, deMagistris L, Sapone A, Generoso M, et al. Ultrastructural mucosal alterations and increased intestinal permeability in non-celiac, type I diabetic patients. Digestive and liver disease : official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver. 2004 Jan;36(1):35–45. doi: 10.1016/j.dld.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 36.Kostic AD, Gevers D, Siljander H, Vatanen T, Hyotylainen T, Hamalainen AM, et al. The Dynamics of the Human Infant Gut Microbiome in Development and in Progression toward Type 1 Diabetes. Cell host & microbe. 2015 Feb 11;17(2):260–273. doi: 10.1016/j.chom.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mordes JP, et al., editors. Animal Models of Autoimmune Diabetes. Philadelphia: LIppincott, Williams, and Wilkins; 2004. [Google Scholar]
- 38.Jacob HJ, Pettersson A, Wilson D, Mao Y, Lernmark A, Lander ES. Genetic dissection of autoimmune type I diabetes in the BB rat. Nat Genet. 1992 Sep;2(1):56–60. doi: 10.1038/ng0992-56. [DOI] [PubMed] [Google Scholar]
- 39.MacMurray AJ, Moralejo DH, Kwitek AE, Rutledge EA, Van Yserloo B, Gohlke P, et al. Lymphopenia in the BB rat model of type 1 diabetes is due to a mutation in a novel immune-associated nucleotide (Ian)-related gene. Genome research. 2002 Jul;12(7):1029–1039. doi: 10.1101/gr.412702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Daheron L, Zenz T, Siracusa LD, Brenner C, Calabretta B. Molecular cloning of Ian4: a BCR/ABL-induced gene that encodes an outer membrane mitochondrial protein with GTP-binding activity. Nucleic acids research. 2001 Mar 15;29(6):1308–1316. doi: 10.1093/nar/29.6.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pandarpurkar M, Wilson-Fritch L, Corvera S, Markholst H, Hornum L, Greiner DL, et al. Ian4 is required for mitochondrial integrity and T cell survival. Proceedings of the National Academy of Sciences of the United States of America. 2003 Sep 2;100(18):10382–10387. doi: 10.1073/pnas.1832170100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schulteis RD, Chu H, Dai X, Chen Y, Edwards B, Haribhai D, et al. Impaired survival of peripheral T cells, disrupted NK/NKT cell development, and liver failure in mice lacking Gimap5. Blood. 2008 Dec 15;112(13):4905–4914. doi: 10.1182/blood-2008-03-146555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lundsgaard D, Holm TL, Hornum L, Markholst H. In vivo control of diabetogenic T-cells by regulatory CD4+CD25+ T-cells expressing Foxp3. Diabetes. 2005 Apr;54(4):1040–1047. doi: 10.2337/diabetes.54.4.1040. [DOI] [PubMed] [Google Scholar]
- 44.Poussier P, Ning T, Murphy T, Dabrowski D, Ramanathan S. Impaired post-thymic development of regulatory CD4+25+ T cells contributes to diabetes pathogenesis in BB rats. Journal of immunology. 2005 Apr 1;174(7):4081–4089. doi: 10.4049/jimmunol.174.7.4081. [DOI] [PubMed] [Google Scholar]
- 45.Tirabassi RS, Guberski DL, Blankenhorn EP, Leif JH, Woda BA, Liu Z, et al. Infection with viruses from several families triggers autoimmune diabetes in LEW*1WR1 rats: prevention of diabetes by maternal immunization. Diabetes. 2010 Jan;59(1):110–118. doi: 10.2337/db09-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mordes JP, Bortell R, Doukas J, Rigby M, Whalen B, Zipris D, et al. The BB/Wor rat and the balance hypothesis of autoimmunity. Diabetes/metabolism reviews. 1996 Jul;12(2):103–109. doi: 10.1002/(SICI)1099-0895(199607)12:2<103::AID-DMR161>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 47.Zipris D, Hillebrands JL, Welsh RM, Rozing J, Xie JX, Mordes JP, et al. Infections that induce autoimmune diabetes in BBDR rats modulate CD4+CD25+ T cell populations. Journal of immunology. 2003 Apr 1;170(7):3592–3602. doi: 10.4049/jimmunol.170.7.3592. [DOI] [PubMed] [Google Scholar]
- 48.Chen YG, Mordes JP, Blankenhorn EP, Kashmiri H, Kaldunski ML, Jia S, et al. Temporal induction of immunoregulatory processes coincides with age-dependent resistance to viral-induced type 1 diabetes. Genes and immunity. 2013 Sep;14(6):387–400. doi: 10.1038/gene.2013.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ellerman KE, Richards CA, Guberski DL, Shek WR, Like AA. Kilham rat triggers T-cell-dependent autoimmune diabetes in multiple strains of rat. Diabetes. 1996 May;45(5):557–562. doi: 10.2337/diab.45.5.557. [DOI] [PubMed] [Google Scholar]
- 50.Mordes JP, Bortell R, Blankenhorn EP, Rossini AA, Greiner DL. Rat models of type 1 diabetes: genetics, environment, and autoimmunity. Ilar J. 2004;45(3):278–291. doi: 10.1093/ilar.45.3.278. [DOI] [PubMed] [Google Scholar]
- 51.Guberski DL, Thomas VA, Shek WR, Like AA, Handler ES, Rossini AA, et al. Induction of type I diabetes by Kilham's rat virus in diabetes-resistant BB/Wor rats. Science. 1991 Nov 15;254(5034):1010–1013. doi: 10.1126/science.1658938. [DOI] [PubMed] [Google Scholar]
- 52.Blankenhorn EP, Cort L, Greiner DL, Guberski DL, Mordes JP. Virus-induced autoimmune diabetes in the LEW.1WR1 rat requires Iddm14 and a genetic locus proximal to the major histocompatibility complex. Diabetes. 2009 Dec;58(12):2930–2938. doi: 10.2337/db09-0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kahn HS, Lawrence JM, Morgan TM, Marcovina SM, Case LD, Imperatore G, et al. Association of Type 1 Diabetes With Month of Birth Among US Youth The SEARCH for Diabetes in Youth Study. Diabetes Care. 2009 Nov;32(11):2010–2015. doi: 10.2337/dc09-0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moltchanova EV, Schreier N, Lammi N, Karvonen M. Seasonal variation of diagnosis of Type 1 diabetes mellitus in children worldwide. Diabetic Med. 2009 Jul;26(7):673–678. doi: 10.1111/j.1464-5491.2009.02743.x. [DOI] [PubMed] [Google Scholar]
- 55.Andreoletti L, Hober D, Hober-Vandenberghe C, Belaich S, Vantyghem MC, Lefebvre J, et al. Detection of coxsackie B virus RNA sequences in whole blood samples from adult patients at the onset of type I diabetes mellitus. Journal of medical virology. 1997 Jun;52(2):121–127. doi: 10.1002/(sici)1096-9071(199706)52:2<121::aid-jmv1>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 56.Craig ME, Howard NJ, Silink M, Rawlinson WD. Reduced frequency of HLA DRB1*03-DQB1*02 in children with type 1 diabetes associated with enterovirus RNA. The Journal of infectious diseases. 2003 May 15;187(10):1562–1570. doi: 10.1086/374742. [DOI] [PubMed] [Google Scholar]
- 57.Helfand RF, Gary HE, Jr, Freeman CY, Anderson LJ, Pallansch MA. Serologic evidence of an association between enteroviruses and the onset of type 1 diabetes mellitus. Pittsburgh Diabetes Research Group. The Journal of infectious diseases. 1995 Nov;172(5):1206–1211. doi: 10.1093/infdis/172.5.1206. [DOI] [PubMed] [Google Scholar]
- 58.Lonnrot M, Korpela K, Knip M, Ilonen J, Simell O, Korhonen S, et al. Enterovirus infection as a risk factor for beta-cell autoimmunity in a prospectively observed birth cohort: the Finnish Diabetes Prediction and Prevention Study. Diabetes. 2000 Aug;49(8):1314–1318. doi: 10.2337/diabetes.49.8.1314. [DOI] [PubMed] [Google Scholar]
- 59.Nairn C, Galbraith DN, Taylor KW, Clements GB. Enterovirus variants in the serum of children at the onset of Type 1 diabetes mellitus. Diabetic medicine : a journal of the British Diabetic Association. 1999 Jun;16(6):509–513. doi: 10.1046/j.1464-5491.1999.00098.x. [DOI] [PubMed] [Google Scholar]
- 60.Richardson SJ, Willcox A, Bone AJ, Foulis AK, Morgan NG. The prevalence of enteroviral capsid protein vp1 immunostaining in pancreatic islets in human type 1 diabetes. Diabetologia. 2009 Jun;52(6):1143–1151. doi: 10.1007/s00125-009-1276-0. [DOI] [PubMed] [Google Scholar]
- 61.Sarmiento L, Cabrera-Rode E, Lekuleni L, Cuba I, Molina G, Fonseca M, et al. Occurrence of enterovirus RNA in serum of children with newly diagnosed type 1 diabetes and islet cell autoantibody-positive subjects in a population with a low incidence of type 1 diabetes. Autoimmunity. 2007 Nov;40(7):540–545. doi: 10.1080/08916930701523429. [DOI] [PubMed] [Google Scholar]
- 62.Yeung WC, Rawlinson WD, Craig ME. Enterovirus infection and type 1 diabetes mellitus: systematic review and meta-analysis of observational molecular studies. Bmj. 2011;342:d35. doi: 10.1136/bmj.d35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Laitinen OH, Honkanen H, Pakkanen O, Oikarinen S, Hankaniemi MM, Huhtala H, et al. Coxsackievirus B1 is associated with induction of beta-cell autoimmunity that portends type 1 diabetes. Diabetes. 2014 Feb;63(2):446–455. doi: 10.2337/db13-0619. [DOI] [PubMed] [Google Scholar]
- 64.Richardson SJ, Leete P, Bone AJ, Foulis AK, Morgan NG. Expression of the enteroviral capsid protein VP1 in the islet cells of patients with type 1 diabetes is associated with induction of protein kinase R and downregulation of Mcl-1. Diabetologia. 2013 Jan;56(1):185–193. doi: 10.1007/s00125-012-2745-4. [DOI] [PubMed] [Google Scholar]
- 65.Barrett JC, Clayton DG, Concannon P, Akolkar B, Cooper JD, Erlich HA, et al. Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nature genetics. 2009 Jun;41(6):703–707. doi: 10.1038/ng.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Virgin HW, Todd JA. Metagenomics and personalized medicine. Cell. 2011 Sep 30;147(1):44–56. doi: 10.1016/j.cell.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ferreira RC, Guo H, Coulson RM, Smyth DJ, Pekalski ML, Burren OS, et al. A type I interferon transcriptional signature precedes autoimmunity in children genetically at risk for type 1 diabetes. Diabetes. 2014 Jul;63(7):2538–2550. doi: 10.2337/db13-1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kallionpaa H, Elo LL, Laajala E, Mykkanen J, Ricano-Ponce I, Vaarma M, et al. Innate immune activity is detected prior to seroconversion in children with HLA-conferred type 1 diabetes susceptibility. Diabetes. 2014 Jul;63(7):2402–2414. doi: 10.2337/db13-1775. [DOI] [PubMed] [Google Scholar]
- 69.Jia S, Kaldunski M, Jailwala P, Geoffrey R, Kramer J, Wang X, et al. Use of transcriptional signatures induced in lymphoid and myeloid cell lines as an inflammatory biomarker in Type 1 diabetes. Physiological genomics. 2011 Jun 15;43(11):697–709. doi: 10.1152/physiolgenomics.00235.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kaldunski M, Jia S, Geoffrey R, Basken J, Prosser S, Kansra S, et al. Identification of a serum-induced transcriptional signature associated with type 1 diabetes in the BioBreeding rat. Diabetes. 2010 Oct;59(10):2375–2385. doi: 10.2337/db10-0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Levy H, Wang X, Kaldunski M, Jia S, Kramer J, Pavletich SJ, et al. Transcriptional signatures as a disease-specific and predictive inflammatory biomarker for type 1 diabetes. Genes and immunity. 2012 Dec;13(8):593–604. doi: 10.1038/gene.2012.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bieg S, Simonson W, Ellefsen K, Lernmark A. Rel B is an early marker of autoimmune islet inflammation in the biobreeding (BB) rat. Pancreas. 2000 Jan;20(1):47–54. doi: 10.1097/00006676-200001000-00007. [DOI] [PubMed] [Google Scholar]
- 73.Hessner MJ, Wang X, Meyer L, Geoffrey R, Jia S, Fuller J, et al. Involvement of eotaxin, eosinophils, and pancreatic predisposition in development of type 1 diabetes mellitus in the BioBreeding rat. Journal of immunology. 2004 Dec 1;173(11):6993–7002. doi: 10.4049/jimmunol.173.11.6993. [DOI] [PubMed] [Google Scholar]
- 74.Geoffrey R, Jia S, Kwitek AE, Woodliff J, Ghosh S, Lernmark A, et al. Evidence of a functional role for mast cells in the development of type 1 diabetes mellitus in the BioBreeding rat. Journal of immunology. 2006 Nov 15;177(10):7275–7286. doi: 10.4049/jimmunol.177.10.7275. [DOI] [PubMed] [Google Scholar]
- 75.Sarmiento J, Wallis RH, Ning T, Marandi L, Chao G, Veillette A, et al. A Functional Polymorphism of Ptpn22 Is Associated with Type 1 Diabetes in the BioBreeding Rat. Journal of immunology. 2015 Jan 15;194(2):615–629. doi: 10.4049/jimmunol.1302689. [DOI] [PubMed] [Google Scholar]
- 76.Valladares R, Sankar D, Li N, Williams E, Lai KK, Abdelgeliel AS, et al. Lactobacillus johnsonii N6.2 mitigates the development of type 1 diabetes in BB-DP rats. PloS one. 2010;5(5):e10507. doi: 10.1371/journal.pone.0010507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hara N, Alkanani AK, Ir D, Robertson CE, Wagner BD, Frank DN, et al. Prevention of virus-induced type 1 diabetes with antibiotic therapy. Journal of immunology. 2012 Oct 15;189(8):3805–3814. doi: 10.4049/jimmunol.1201257. [DOI] [PubMed] [Google Scholar]
- 78.Allantaz F, Chaussabel D, Stichweh D, Bennett L, Allman W, Mejias A, et al. Blood leukocyte microarrays to diagnose systemic onset juvenile idiopathic arthritis and follow the response to IL-1 blockade. The Journal of experimental medicine. 2007 Sep 3;204(9):2131–2144. doi: 10.1084/jem.20070070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Khaenam P, Rinchai D, Altman MC, Chiche L, Buddhisa S, Kewcharoenwong C, et al. A transcriptomic reporter assay employing neutrophils to measure immunogenic activity of septic patients' plasma. Journal of translational medicine. 2014;12:65. doi: 10.1186/1479-5876-12-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang Q, Fillmore TL, Schepmoes AA, Clauss TR, Gritsenko MA, Mueller PW, et al. Serum proteomics reveals systemic dysregulation of innate immunity in type 1 diabetes. The Journal of experimental medicine. 2013 Jan 14;210(1):191–203. doi: 10.1084/jem.20111843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Aly TA, Ide A, Humphrey K, Barker JM, Steck A, Erlich HA, et al. Genetic prediction of autoimmunity: initial oligogenic prediction of anti-islet autoimmunity amongst DR3/DR4-DQ8 relatives of patients with type 1A diabetes. Journal of autoimmunity. 2005;25(Suppl):40–45. doi: 10.1016/j.jaut.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 82.Chen YG, Cabrera SM, Jia S, Kaldunski ML, Kramer J, Cheong S, et al. Molecular signatures differentiate immune states in type 1 diabetic families. Diabetes. 2014 Nov;63(11):3960–3973. doi: 10.2337/db14-0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009 Jun 19;30(6):899–911. doi: 10.1016/j.immuni.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 84.Mandrup-Poulsen T, Bendtzen K, Nerup J, Dinarello CA, Svenson M, Nielsen JH. Affinity-purified human interleukin I is cytotoxic to isolated islets of Langerhans. Diabetologia. 1986 Jan;29(1):63–67. doi: 10.1007/BF02427283. [DOI] [PubMed] [Google Scholar]
- 85.Sandler S, Andersson A, Hellerstrom C. Inhibitory effects of interleukin 1 on insulin secretion, insulin biosynthesis, and oxidative metabolism of isolated rat pancreatic islets. Endocrinology. 1987 Oct;121(4):1424–1431. doi: 10.1210/endo-121-4-1424. [DOI] [PubMed] [Google Scholar]
- 86.Spinas GA, Hansen BS, Linde S, Kastern W, Molvig J, Mandrup-Poulsen T, et al. Interleukin 1 dose-dependently affects the biosynthesis of (pro)insulin in isolated rat islets of Langerhans. Diabetologia. 1987 Jul;30(7):474–480. doi: 10.1007/BF00279615. [DOI] [PubMed] [Google Scholar]
- 87.Maedler K, Sergeev P, Ris F, Oberholzer J, Joller-Jemelka HI, Spinas GA, et al. Glucose-induced beta cell production of IL-1beta contributes to glucotoxicity in human pancreatic islets. The Journal of clinical investigation. 2002 Sep;110(6):851–860. doi: 10.1172/JCI15318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yamada K, Takane-Gyotoku N, Yuan X, Ichikawa F, Inada C, Nonaka K. Mouse islet cell lysis mediated by interleukin-1-induced Fas. Diabetologia. 1996 Nov;39(11):1306–1312. doi: 10.1007/s001250050574. [DOI] [PubMed] [Google Scholar]
- 89.Dayer-Metroz MD, Wollheim CB, Seckinger P, Dayer JM. A natural interleukin 1 (IL-1) inhibitor counteracts the inhibitory effect of IL-1 on insulin production in cultured rat pancreatic islets. Journal of autoimmunity. 1989 Apr;2(2):163–171. doi: 10.1016/0896-8411(89)90152-2. [DOI] [PubMed] [Google Scholar]
- 90.Zumsteg U, Reimers JI, Pociot F, Morch L, Helqvist S, Brendel M, et al. Differential interleukin-1 receptor antagonism on pancreatic beta and alpha cells. Studies in rodent and human islets and in normal rats. Diabetologia. 1993 Aug;36(8):759–766. doi: 10.1007/BF00401148. [DOI] [PubMed] [Google Scholar]
- 91.Dinarello CA, Simon A, van der Meer JW. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nature reviews Drug discovery. 2012 Aug;11(8):633–652. doi: 10.1038/nrd3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dogan Y, Akarsu S, Ustundag B, Yilmaz E, Gurgoze MK. Serum IL-1beta, IL-2, and IL-6 in insulin-dependent diabetic children. Mediators of inflammation. 2006;2006(1):59206. doi: 10.1155/MI/2006/59206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hussain MJ, Maher J, Warnock T, Vats A, Peakman M, Vergani D. Cytokine overproduction in healthy first degree relatives of patients with IDDM. Diabetologia. 1998 Mar;41(3):343–349. doi: 10.1007/s001250050913. [DOI] [PubMed] [Google Scholar]
- 94.Bradshaw EM, Raddassi K, Elyaman W, Orban T, Gottlieb PA, Kent SC, et al. Monocytes from patients with type 1 diabetes spontaneously secrete proinflammatory cytokines inducing Th17 cells. Journal of immunology. 2009 Oct 1;183(7):4432–4439. doi: 10.4049/jimmunol.0900576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kaizer EC, Glaser CL, Chaussabel D, Banchereau J, Pascual V, White PC. Gene expression in peripheral blood mononuclear cells from children with diabetes. The Journal of clinical endocrinology and metabolism. 2007 Sep;92(9):3705–3711. doi: 10.1210/jc.2007-0979. [DOI] [PubMed] [Google Scholar]
- 96.Meyers AJ, Shah RR, Gottlieb PA, Zipris D. Altered Toll-like receptor signaling pathways in human type 1 diabetes. Journal of molecular medicine. 2010 Dec;88(12):1221–1231. doi: 10.1007/s00109-010-0666-6. [DOI] [PubMed] [Google Scholar]
- 97.Padmos RC, Schloot NC, Beyan H, Ruwhof C, Staal FJ, de Ridder D, et al. Distinct monocyte gene-expression profiles in autoimmune diabetes. Diabetes. 2008 Oct;57(10):2768–2773. doi: 10.2337/db08-0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kayserova J, Vcelakova J, Stechova K, Dudkova E, Hromadkova H, Sumnik Z, et al. Decreased dendritic cell numbers but increased TLR9-mediated interferon-alpha production in first degree relatives of type 1 diabetes patients. Clinical immunology. 2014 Jul;153(1):49–55. doi: 10.1016/j.clim.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 99.Petrich de Marquesini LG, Fu J, Connor KJ, Bishop AJ, McLintock NE, Pope C, et al. IFN-gamma and IL-10 islet-antigen-specific T cell responses in autoantibody-negative first-degree relatives of patients with type 1 diabetes. Diabetologia. 2010 Jul;53(7):1451–1460. doi: 10.1007/s00125-010-1739-3. [DOI] [PubMed] [Google Scholar]
- 100.Cordain L, Eaton SB, Sebastian A, Mann N, Lindeberg S, Watkins BA, et al. Origins and evolution of the Western diet: health implications for the 21st century. The American journal of clinical nutrition. 2005 Feb;81(2):341–354. doi: 10.1093/ajcn.81.2.341. [DOI] [PubMed] [Google Scholar]
- 101.de Souza RJ, Swain JF, Appel LJ, Sacks FM. Alternatives for macronutrient intake and chronic disease: a comparison of the OmniHeart diets with popular diets and with dietary recommendations. The American journal of clinical nutrition. 2008 Jul;88(1):1–11. doi: 10.1093/ajcn/88.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008 Jun;57(6):1470–1481. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 103.Hildebrandt MA, Hoffmann C, Sherrill-Mix SA, Keilbaugh SA, Hamady M, Chen YY, et al. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology. 2009 Nov;137(5):1716–1724. e1–e2. doi: 10.1053/j.gastro.2009.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Turnbaugh PJ, Backhed F, Fulton L, Gordon JI. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell host & microbe. 2008 Apr 17;3(4):213–223. doi: 10.1016/j.chom.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R, Gordon JI. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Science translational medicine. 2009 Nov 11;1(6):6ra14. doi: 10.1126/scitranslmed.3000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Walter J, Ley R. The human gut microbiome: ecology and recent evolutionary changes. Annual review of microbiology. 2011;65:411–429. doi: 10.1146/annurev-micro-090110-102830. [DOI] [PubMed] [Google Scholar]
- 107.Neu J, Reverte CM, Mackey AD, Liboni K, Tuhacek-Tenace LM, Hatch M, et al. Changes in intestinal morphology and permeability in the biobreeding rat before the onset of type 1 diabetes. Journal of pediatric gastroenterology and nutrition. 2005 May;40(5):589–595. doi: 10.1097/01.mpg.0000159636.19346.c1. [DOI] [PubMed] [Google Scholar]
- 108.Patrick C, Wang GS, Lefebvre DE, Crookshank JA, Sonier B, Eberhard C, et al. Promotion of autoimmune diabetes by cereal diet in the presence or absence of microbes associated with gut immune activation, regulatory imbalance, and altered cathelicidin antimicrobial Peptide. Diabetes. 2013 Jun;62(6):2036–2047. doi: 10.2337/db12-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Visser JT, Bos NA, Harthoorn LF, Stellaard F, Beijer-Liefers S, Rozing J, et al. Potential mechanisms explaining why hydrolyzed casein-based diets outclass single amino acid-based diets in the prevention of autoimmune diabetes in diabetes-prone BB rats. Diabetes Metab Res Rev. 2012 Sep;28(6):505–513. doi: 10.1002/dmrr.2311. [DOI] [PubMed] [Google Scholar]
- 110.Visser JT, Lammers K, Hoogendijk A, Boer MW, Brugman S, Beijer-Liefers S, et al. Restoration of impaired intestinal barrier function by the hydrolysed casein diet contributes to the prevention of type 1 diabetes in the diabetes-prone BioBreeding rat. Diabetologia. 2010 Dec;53(12):2621–2628. doi: 10.1007/s00125-010-1903-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Watts T, Berti I, Sapone A, Gerarduzzi T, Not T, Zielke R, et al. Role of the intestinal tight junction modulator zonulin in the pathogenesis of type I diabetes in BB diabetic-prone rats. Proceedings of the National Academy of Sciences of the United States of America. 2005 Feb 22;102(8):2916–2921. doi: 10.1073/pnas.0500178102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chen Z, Barbi J, Bu S, Yang HY, Li Z, Gao Y, et al. The ubiquitin ligase Stub1 negatively modulates regulatory T cell suppressive activity by promoting degradation of the transcription factor Foxp3. Immunity. 2013 Aug 22;39(2):272–285. doi: 10.1016/j.immuni.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.van Loosdregt J, Fleskens V, Fu J, Brenkman AB, Bekker CP, Pals CE, et al. Stabilization of the transcription factor Foxp3 by the deubiquitinase USP7 increases Treg-cell-suppressive capacity. Immunity. 2013 Aug 22;39(2):259–271. doi: 10.1016/j.immuni.2013.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Theoharides TC, Wang L, Pang X, Letourneau R, Culm KE, Basu S, et al. Cloning and cellular localization of the rat mast cell 78-kDa protein phosphorylated in response to the mast cell “stabilizer” cromolyn. The Journal of pharmacology and experimental therapeutics. 2000 Sep;294(3):810–821. [PubMed] [Google Scholar]
- 115.Malfait AM, Malik AS, Marinova-Mutafchieva L, Butler DM, Maini RN, Feldmann M. The beta2-adrenergic agonist salbutamol is a potent suppressor of established collagen-induced arthritis: mechanisms of action. Journal of immunology. 1999 May 15;162(10):6278–6283. [PubMed] [Google Scholar]
- 116.Nishii M, Inomata T, Niwano H, Takehana H, Takeuchi I, Nakano H, et al. Beta2-Adrenergic agonists suppress rat autoimmune myocarditis: potential role of beta2-adrenergic stimulants as new therapeutic agents for myocarditis. Circulation. 2006 Aug 29;114(9):936–944. doi: 10.1161/CIRCULATIONAHA.105.607903. [DOI] [PubMed] [Google Scholar]
- 117.Kopp E, Ghosh S. Inhibition of NF-kappa B by sodium salicylate and aspirin. Science. 1994 Aug 12;265(5174):956–959. doi: 10.1126/science.8052854. [DOI] [PubMed] [Google Scholar]
- 118.Yang C, Jurczyk A, Diiorio P, Norowski E, Brehm MA, Grant CW, et al. Salicylate Prevents Virus-Induced Type 1 Diabetes in the BBDR Rat. PLoS ONE. 2013;8(10):e78050. doi: 10.1371/journal.pone.0078050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hara N, Alkanani AK, Dinarello CA, Zipris D. Histone deacetylase inhibitor suppresses virus-induced proinflammatory responses and type 1 diabetes. J Mol Med (Berl) 2014 Jan;92(1):93–102. doi: 10.1007/s00109-013-1078-1. [DOI] [PubMed] [Google Scholar]
- 120.Hara N, Alkanani AK, Dinarello CA, Zipris D. Modulation of virus-induced innate immunity and type 1 diabetes by IL-1 blockade. Innate Immun. 2013 Sep 23;20(6):574–584. doi: 10.1177/1753425913502242. [DOI] [PubMed] [Google Scholar]
- 121.Mastrandrea L, Yu J, Behrens T, Buchlis J, Albini C, Fourtner S, et al. Etanercept Treatment in Children With New-Onset Type 1 Diabetes Pilot randomized, placebo-controlled, double-blind study. Diabetes Care. 2009 Jul;32(7):1244–1249. doi: 10.2337/dc09-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gottlieb PA, Alkanani AK, Michels AW, Lewis EC, Shapiro L, Dinarello CA, et al. alpha1-Antitrypsin therapy downregulates toll-like receptor-induced IL-1beta responses in monocytes and myeloid dendritic cells and may improve islet function in recently diagnosed patients with type 1 diabetes. The Journal of clinical endocrinology and metabolism. 2014 Aug;99(8):E1418–E1426. doi: 10.1210/jc.2013-3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wang Y, Xiao Y, Zhong L, Ye D, Zhang J, Tu Y, et al. Increased neutrophil elastase and proteinase 3 and augmented NETosis are closely associated with beta-cell autoimmunity in patients with type 1 diabetes. Diabetes. 2014 Dec;63(12):4239–4248. doi: 10.2337/db14-0480. [DOI] [PubMed] [Google Scholar]
- 124.Janciauskiene S, Larsson S, Larsson P, Virtala R, Jansson L, Stevens T. Inhibition of lipopolysaccharide-mediated human monocyte activation, in vitro, by alpha1-antitrypsin. Biochemical and biophysical research communications. 2004 Aug 27;321(3):592–600. doi: 10.1016/j.bbrc.2004.06.123. [DOI] [PubMed] [Google Scholar]
- 125.Pott GB, Chan ED, Dinarello CA, Shapiro L. Alpha-1-antitrypsin is an endogenous inhibitor of proinflammatory cytokine production in whole blood. Journal of leukocyte biology. 2009 May;85(5):886–895. doi: 10.1189/jlb.0208145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Badenhoop K, Kahles H, Penna-Martinez M. Vitamin D, immune tolerance, and prevention of type 1 diabetes. Current diabetes reports. 2012 Dec;12(6):635–642. doi: 10.1007/s11892-012-0322-3. [DOI] [PubMed] [Google Scholar]
- 127.Ferreira GB, Gysemans CA, Demengeot J, da Cunha JP, Vanherwegen AS, Overbergh L, et al. 1,25-Dihydroxyvitamin D3 promotes tolerogenic dendritic cells with functional migratory properties in NOD mice. Journal of immunology. 2014 May 1;192(9):4210–4220. doi: 10.4049/jimmunol.1302350. [DOI] [PubMed] [Google Scholar]
- 128.Ataie-Jafari A, Loke SC, Rahmat AB, Larijani B, Abbasi F, Leow MK, et al. A randomized placebo-controlled trial of alphacalcidol on the preservation of beta cell function in children with recent onset type 1 diabetes. Clinical nutrition. 2013 Dec;32(6):911–917. doi: 10.1016/j.clnu.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 129.Gabbay MA, Sato MN, Finazzo C, Duarte AJ, Dib SA. Effect of cholecalciferol as adjunctive therapy with insulin on protective immunologic profile and decline of residual beta-cell function in new-onset type 1 diabetes mellitus. Archives of pediatrics & adolescent medicine. 2012 Jul 1;166(7):601–607. doi: 10.1001/archpediatrics.2012.164. [DOI] [PubMed] [Google Scholar]
- 130.Moran A, Bundy B, Becker DJ, DiMeglio LA, Gitelman SE, Goland R, et al. Interleukin-1 antagonism in type 1 diabetes of recent onset: two multicentre, randomised, double-blind, placebo-controlled trials. Lancet. 2013 Jun 1;381(9881):1905–1915. doi: 10.1016/S0140-6736(13)60023-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hessner MJ, editor. Serum signature analysis of participants of the Anti-Interleukin-1 in Diabetes Action (AIDA) Trial. Abstracts of American Diabetes Association's 73th Annual Meeting; Chicago, IL. 2013. [Google Scholar]