Abstract
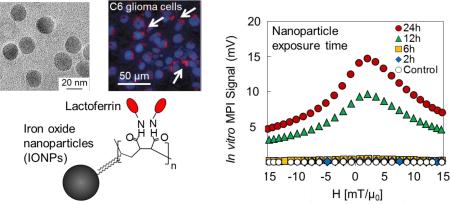
Magnetic Particle Imaging (MPI) is a new real-time imaging modality, which promises high tracer mass sensitivity and spatial resolution directly generated from iron oxide nanoparticles. In this study, monodisperse iron oxide nanoparticles with median core diameters ranging from 14 to 26 nm were synthesized and their surface was conjugated with lactoferrin to convert them into brain glioma targeting agents. The conjugation was confirmed with the increase of the hydrodynamic diameters, change of zeta potential, and Bradford assay. Magnetic particle spectrometry (MPS), performed to evaluate the MPI performance of these nanoparticles, showed no change in signal after lactoferrin conjugation to nanoparticles for all core diameters, suggesting that the MPI signal is dominated by Néel relaxation and thus independent of hydrodynamic size difference or presence of coating molecules before and after conjugations. For this range of core sizes (14-26 nm), both MPS signal intensity and spatial resolution improved with increasing core diameter of nanoparticles. The lactoferrin conjugated iron oxide nanoparticles (Lf-IONPs) showed specific cellular internalization into C6 cells with a 5-fold increase in MPS signal compared to IONPs without lactoferrin, both after 24h incubation. These results suggest that Lf-IONPs can be used as tracers for targeted brain glioma imaging using MPI.
Graphical Abstract
Monodisperse lactoferrin conjugated iron oxide nanoparticles were synthesized and evaluated for in vitro glioma imaging using Magnetic Particle Imaging (MPI).
Introduction
Magnetic nanoparticles, if suitably optimized and made biocompatible have translational potential in a variety of biomedical applications ranging from imaging to therapy.1 Due to their small size and large surface-to-volume ratio, nanoparticles are particularly effective as carriers for drug delivery, provided they are properly functionalized. They also have advantages such as increased efficiency for targeted chemotherapy and reduced side effects.2 Specifically, because of their magnetic properties and biocompatibility, superparamagnetic iron oxide nanoparticles have been used for various biomedical applications, such as drug and gene delivery,3-5 magnetic resonance imaging (MRI) contrast agents,3,6 biosensors,7 magnetic cell separation,8 hyperthermia treatment,9,10 and magnetic particle imaging (MPI).11-13 MPI is a new real-time imaging modality11 that can be potentially used for performing quantitative, 3D in vivo imaging of magnetic nanoparticles with high tracer mass sensitivity and spatial resolutions.12-14 While other established whole body imaging modalities such as computed tomography (CT) and MRI provide comparable spatial resolution, CT requires iodine contrast agents that have a high risk of contrast-induced nephropathy (CIN), particularly to patients with chronic kidney disease (CKD).15 MPI promises greater sensitivity compared to MRI as its signal is directly generated from magnetic nanoparticles with magnetization more than 107 times of the weak proton signal exploited in MRI. Furthermore, the tissues surrounding nanoparticles are diamagnetic and provide negligible background in MPI images, resulting in near-infinite contrasts and improving imaging specificity for molecular imaging applications.15 This combination of high sensitivity, no background signal interference, high contrast and a safe, biocompatible tracer, makes MPI a potentially strong candidate for molecular/targeted imaging applications. Recent reports also show that synergistic application of MPI with other whole-body imaging modalities such as MRI and near infra-red fluorescent (NIRF) imaging can be used for high sensitivity targeting and high resolution anatomical imaging.16,17
MPI exploits the nonlinear magnetization response of superparamagnetic iron oxide nanoparticles to an applied alternating magnetic field.18 During measurements nanoparticles are excited by an alternating magnetic field and the time-varying magnetization response induces an inductive signal in the pick-up coils. Signal localization of the received signal is achieved by an additional field gradient (selection field), which provides either a single field-free point (FFP) or field-free line (FFL) that is then scanned over the entire imaging volume. At any time, the magnetization of nanoparticles outside of FFP or FFL is saturated, thus no signal is induced. Only the nanoparticles within the FFP or FFL respond to the alternating magnetic field and induce a spatially-sensitive signal for image reconstruction.11
For potential clinical applications, MPI signal optimization is crucial to achieve high spatial resolutions and signal-to-noise ratios (SNR). Since the MPI signal is controlled by dynamic magnetization reversal of the nanoparticle tracers, it is important to tune the relaxation mechanism of nanoparticles by controlling their physical parameters such as core and hydrodynamic diameter, magnetization, and anisotropy. The MPI performance with various particle core and hydrodynamic diameters, size distributions, and under different environments has been studied previously.13,19,20,21 The nanoparticles investigated in those studies were coated with polymers that were designed for vascular imaging applications, optimized for maximum blood circulation and lacked targeting capability. However, for molecular imaging applications, it is necessary to functionalize nanoparticles with specific targeting ligands while ensuring that their optimized magnetic relaxation characteristics are not compromised. To achieve this functionalization, we first introduced carboxyl groups on the surface of nanoparticles using poly(maleic anhydride-alt-1-octadecene), which enables conjugation of various targeting ligands. Then, we conjugated lactoferrin as a ligand for brain tumor targeting.
Lactoferrin is a multifunctional glycoprotein, which belongs to the transferrin family, and possesses various biological functions such as antimicrobial, antibacterial, antiviral, antiparasitic, and antitumor activities.22 Furthermore, low-density lipoprotein receptor-related protein (LRP), a specific lactoferrin receptor with excellent selectivity, is highly expressed in glioma cells.23,24 Lactoferrin is also able to cross the blood–brain barrier (BBB) through the transcytosis processes within brain capillary endothelial cells.25 Because of their ability to target glioma cells and cross the blood-brain-barrier (BBB), lactoferrin-conjugated nanoparticles have great potential for brain tumor targeting.25,26 Previous studies also have shown that lactoferrin-conjugated nanoparticles have no effect on cell viability.23
In this paper, lactoferrin was conjugated to monodisperse iron oxide nanoparticles optimized as MPI tracers. We investigated the physical and magnetic properties of the nanoparticles before and after lactoferrin conjugation, the influence of lactoferrin conjugation on MPI performance and internalization into glioma cells. The capability of lactoferrin conjugated iron oxide nanoparticles (Lf-IONPs) as MPI tracer was assessed using in-vitro magnetic particle spectrometry (MPS). These results show that our monodisperse Lf-IONPs optimized for MPI can be used for brain tumor imaging, and this high sensitivity imaging has the potential to allow early diagnosis of brain tumors.
Experimental
Materials
Iron (III) chloride, anhydrous (98%) was purchased from Alfa-Aesar. Oleic acid (technical grade, 90%), 1-octadecene (technical grade, 90%), chloroform (HPLC, ≥99.8%), methanol (HPLC, ≥99.9%), poly(maleic anhydride-alt-1-octadecene) (Mn 30,000–50,000), N-(3-Dimethylaminopropyl)-N ′ - ethylcarbodiimide hydrochloride (commercial grade, powder), N-Hydroxysulfosuccinimide sodium salt (≥98%, HPLC), lactoferrin from human milk (≥85%, SDS-PAGE, lyophilized powder), paraformaldehyde powder (95%), potassium hexacyanoferrate (II) trihydrate, DAPI, alminum sulfate hydrate (98%), Dulbecco's phosphate buffered saline, and dimethyl sulphoxide (DMSO) Hybri-Max™ were purchased from Sigma-Aldrich. Sodium hydroxide solution 1N, hydrochloric acid 1N solution, and sodium bicarbonate were purchased from Fisher Scientific. Dulbecco's modified Eagle medium (DMEM) and 0.25% trypsin-EDTA were purchased from Gibco. Coomassie protein assay reagent was purchased from Thermo Scientific. All the reagents were used without further purification. Amicon® Ultra centrifugal filters ultracel®-50K was purchased from Merck Millipore. Cy5.5™ Mono NHS Ester was purchased from GE Health Care.
Synthesis of iron oxide nanoparticles
Iron oleate precursors were synthesized following previously published methods.27,28 Iron oxide nanoparticles were synthesized by the pyrolysis of the iron oleate precursor with excess oleic acid surfactant in 1-octadecene.10 Synthesized nanoparticles were purified with a mixture of chloroform and methanol three times by magnetic decantation. Purified nanoparticles were dried in vacuum for more than 30 min.
Phase transfer
250 mg of poly(maleic anhydride-alt-1-octadecene) (PMAO) was dissolved in 2.5 ml of chloroform, and 5 mg of iron oxide nanoparticles were dispersed into 2.5 ml of chloroform by sonication. PMAO solution was added to iron oxide nanoparticles and sonicated for 2h. Then, excess amount of 0.1M sodium hydroxide solution was added and sonicated for 1h. Chloroform was evaporated using rotary evaporator at 35°C, 100rpm for 90 min. Iron oxide nanoparticles transferred to sodium hydroxide solution were concentrated using centrifugal filter with the cut-off molecular weight of 50K. The solution was purified with deionized (DI) water by three times centrifugation at 14,100g for 1h. In order to improve the targeting uniformity and enhance the MPI signal intensity and resolution, aggregated nanoparticles were removed by centrifugation at 3,000g for 15 min, and supernatant was collected. The final nanoparticle solution was neutralized using PD-10 column balanced with DI water.
Lactoferrin conjugation
250μl of 10 mg/ml N-(3-Dimethylaminopropyl)-N ′ - ethylcarbodiimide hydrochloride (EDC), 250μl of 10 mg/ml NHydroxysulfosuccinimide sodium salt, and 7 μl of 1M sodium hydroxide solution were mixed. 0.5 mg of iron oxide nanoparticles were added and sonicated for 15 min to activate the carboxyl group on the surface. Excess EDC and sulfo-NHS were removed using PD-10 column balanced with 10 mM phosphate buffered-saline solution (PBS, pH 7.4). 250μl of 1 mg/ml lactoferrin was added to the activated iron oxide nanoparticles, and reacted overnight at room temperature. To purify the nanoparticles, they were centrifuged (12,000g, 1h), and redispersed in PBS for three times. The supernatant was collected for detecting the concentration of lactoferrin by Bradford assay. Resulting nanoparticles were centrifuged at 1000‒3000g for 15min and the supernatant was collected to remove aggregates.
Characterization of iron oxide nanoparticles
The morphology of iron oxide and lactoferrin conjugated iron oxide nanoparticles were characterized by Transmission Electron Microscope (TEM, FEI Tecnai™ G2 F20), equipped with a Gatan CCD camera operated at 200 KeV. The magnetization curves were measured using a vibrating sample magnetometer (VSM, Lakeshore) at room temperature. Saturation magnetization was determined by assuming density equal to stoichiometric magnetite (5180 kg/m3). The hydrodynamic diameters and zeta potentials of PMAO coated iron oxide nanoparticles (PMAO-IONPs) and lactoferrin conjugated iron oxide nanoparticles (Lf-IONPs) were measured by Zetasizer Nano-ZS (Malvern instruments, Worcestershire, UK). 20 μl of PMAO-IONPs and Lf-IONPs were dispersed in DI water and 10 mM phosphate buffered-saline solution (PBS, pH 7.4), respectively. Water was used for PMAO-IONPs due to their lack of long term stability in PBS. Iron concentration was determined using Inductively Couple Plasma Atomic Emission Spectrophotometer (ICP-AES, Jarrell Ash, US).
The lactoferrin concentration conjugated on the surface of the iron oxide nanoparticles was determined by Bradford assay. The concentration of the protein within the supernatant was measured, and the amount of lactoferrin on the nanoparticles was obtained by deducting the amount of lactoferrin in the supernatant from that added in the reaction. The experiments were performed in quadruplicate.
The binding affinity of lactoferrin conjugated to the surface of iron oxide nanoparticles was determined by the AssayMax Human Lactoferrin ELISA Kit (Assaypro) according to the manufacturer's protocols. A standard curve was prepared with free lactoferrin to compare the binding affinity of lactoferrin conjugated on iron oxide nanoparticles and free lactoferrin. The experiments were performed in quadruplicate.
Magnetic particle imaging
MPI performance was measured using a custom-built magnetic particle spectrometer (MPS) with the excitation field of 17 mT/μ0 at 26.0 kHz. The MPS excites magnetization of iron oxide nanoparticles using a transmit coil, and the time-varying magnetization (dm/dt) of nanoparticles induces a voltage signal in a receiver coil that will be used to generate the field-dependent differential susceptibility, (dm/dH), proportional to the MPI signal.20,29 As we have shown before, MPS can be used as a quick measurement to verify the magnetic relaxation regime of the nanoparticles, and its results match well with other conventional techniques such as ac susceptometry and magnetorelaxometry.30 200 μl of nanoparticle solutions at concentrations of 0.190–0.958 mgFe/ml were transferred to 0.6 ml microcentrifuge tubes and inserted into the system coil. Measurement was performed in triplicate. MPS data was analyzed using procedures published previously.29 The obtained signal was normalized by the amount of iron in each sample, which was measured using ICP-AES. AC hysteresis loops were calculated from the same data by integration of the MPS signal or the measured differential susceptibility.
Cell culture
C6 glioma cells were cultured in Dulbecco's modified Eagle medium (DMEM, GIBCO Life Technologies Co., Carlsbad, CA, USA) supplemented with 10 vol % bovine fetal calf serum (FCS, Hyclone Laboratories, Inc., Utah, USA) at 37 °C in humidified atmosphere of 5% CO2. The cells were trypsinized with 0.25% trypsin-EDTA, and re-suspended in DMEM medium for further experiments.
Cellular internalization
Prussian blue staining was performed to investigate the localization of lactoferrin iron oxide nanoparticles in C6 cells. Cells were seeded at 1 × 105 cells/well in 96-well plates (CELLSTAR, Greiner Bio-One, Monroe, US) and incubated for 24h at 37°C. The medium was exchanged with 50 μl of fresh medium containing 200 μg/ml of Lf-IONPs, and incubated for 24h. After incubation, the cells were washed with PBS three times, and fixed in 4% paraformaldehyde at room temperature. Then, the solution containing 5% Potassium hexa-cyanoferrate (II) trihydrate and 10 vol % hydrochloric acid was added to each well (100 μl/well). The cells were incubated at room temperature for 30 min, and rinsed with PBS. Image analysis was performed using VWR inverted microscope and cells were viewed through a high-aperture 20× objective lens. Images were acquired using a V5MP 5.0 MP USB 2.0 digital camera with the software Motic Images Plus 2.0 ML.
Confocal microscopy was used to investigate the internalization of lactoferrin conjugated iron oxide nanoparticles in C6 cells. For this study, 0.01 ml of 10 mg/ml Cy5.5-NHS dissolved in DMSO was added into 0.1 ml of 10 mg/ml lactoferrin dissolved in sodium bicarbonate (0.1M, pH 8.3). This mixture was incubated for 4h at room temperature, and purified by using centrifugal filter with the cut-off molecular weight of 50K. Conjugation of Cy5.5 labelled lactoferrin conjugated iron oxide nanoparticles was conducted with the same method as described in lactoferrin conjugation. Cells were seeded at 1 × 105 cells/well in Chamber Slide (Lab Tek II chamber slide system, Nalge Nunc International, Naperville, US) and incubated for 24h at 37°C. The medium was exchanged by 75 μl of fresh medium containing 200 μg/ml of Cy5.5 labelled Lf-IONPs. After incubation for 24h, the cells were washed with PBS three times, fixed in 4% PFA at room temperature for 30 min, stained with DAPI for 30min at room temperature, and mounted with mounting medium (VECTASHIELD HardSet Mounting Medium, Vector Laboratories, Burlingame, US), and stored at 4°C overnight. Cells were viewed through a Zeiss LSM 710 confocal microscope equipped with a high-aperture 63× objective lens. Images were taken with the ZEN software and analyzed with Image J.
In vitro MPI (magnetic particle imaging)
MPI performance of lactoferrin conjugated iron oxide nanoparticles internalized into C6 cells was measured using a custom-built magnetic particle spectrometer (MPS). Cells were seeded at 1 × 105 cells/well in 24 well plates (CELLSTAR, Greiner Bio-One, Monroe, US) and incubated for 24h at 37°C. The medium was exchanged by 200 μl of fresh medium containing 200 μg/ml of PMAO-IONPs or Lf-IONPs, and incubated for different time (4, 12, 24h). The cells were washed with PBS three times, and trypsinized with 0.25% Trypsin-EDTA. After centrifuge, the cell pellet was dissolved in 150 μl of PBS, transferred to 0.6 ml microcentrifuge tubes, and then inserted into the MPS system. MPI performance was measured with the excitation field of 17 mT/μ0 at 26.0 kHz. Measurement was performed in triplicate. MPS data was analyzed using procedures published previously.29 To measure the amount of Lf-IONPs internalized into C6 cells, the cells were lysed with concentrated hydrochloric acid. After an appropriate dilution of lysate with DI water, the amount of iron was measured by Inductively Couple Plasma Atomic Emission Spectrophotometer (ICP-AES, Jarrell Ash 955, US).
Results and discussion
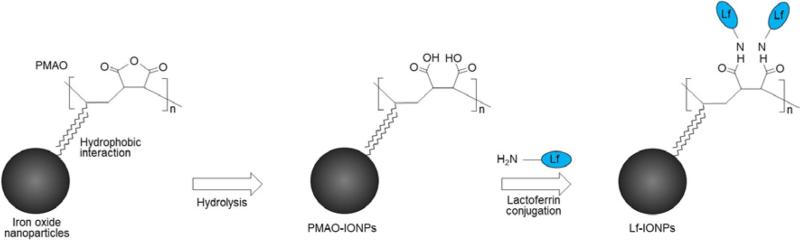
Our previous results show that synthesis of iron oxide nanoparticles with narrow core size distribution is crucial for enhanced MPI performance.13,20 Monodisperse iron oxide nanoparticles with core diameters of 14–26 nm were synthesized by thermal decomposition of iron oleate. These hydrophobic iron oxide nanoparticles were transferred to water using PMAO through hydrophobic interaction between the oleic acid and octadecene aliphatic chains of the PMAO.31 The maleic anhydride groups of PMAO were hydrolysed in sodium hydroxide solution, and carboxyl groups were introduced on the surface of the nanoparticles for further functionalization with lactoferrin. These carboxyl groups were activated using EDC/NHS, and lactoferrin was covalently attached to the activated nanoparticles (Figure 1).
Fig 1.
Schematic representation of phase transfer using PMAO and lactoferrin conjugation to iron oxide nanoparticles.
The conjugation of lactoferrin was evaluated by measuring the hydrodynamic diameter and zeta potential of the nanoparticles, and using Bradford assay. Physical characteristics of PMAO coated iron oxide nanoparticles (PMAO-IONPs) and lactoferrin conjugated iron oxide nanoparticles (Lf-IONPs) with core diameters of 14 nm (14nm-Lf-IONPs), 16 nm (16nm-Lf-IONPs), 20 nm (20nm-Lf-IONPs), 24 nm (24nm-Lf-IONPs), and 26 nm (26nm-Lf-IONPs) are shown in Table 1. The core diameters and their standard deviations were determined from TEM images, assuming a log-normal size distribution. The hydrodynamic diameters of PMAO-IONPs were 46–52 nm. Hydrodynamic diameter of the nanoparticles increased by 22–25 nm from the core diameter after conjugation of lactoferrin. Considering the size of lactoferrin molecules, ideally, we expect ~8nm increase in hydrodynamic size of the nanoparticles after lactoferrin conjugation. However, the final hydrodynamic sizes of the lactoferrin coated nanoparticles were slightly larger than these ideal sizes. This was due to presence of negligible traces of crosslinked nanoparticles in the samples. Zeta potential of the nanoparticles also changed after lactoferrin conjugation. The zeta potential of PMAO-IONPs dispersed in DI water was between –35 to –43 mV, which indicates the existence of deprotonated carboxyl groups on the surface of PMAO-IONPs.31 In contrast, Lf-IONPs dispersed in PBS, which is a commonly used biological medium, showed slightly negative zeta potential between –4 mV to –11 mV. The increase in hydrodynamic diameter and zeta potential of the nanoparticles confirmed the effective conjugation of lactoferrin to the surface of nanoparticles. Also, previous gel electrophoresis and Fourier transform infrared spectroscopy (FTIR) studies showed successful conjugation of lactoferrin to nanoparticles using EDC/NHS reactions.23 In addition, we used Bradford assay to quantify the number of lactoferrin molecules conjugated to the nanoparticles (Table 2). As we show in Fig. 4B, our nanoparticles had a fluorescent signal generated from Cy5.5-labelled lactoferrin molecules, which further supports presence of lactoferrin on nanoparticles.
Table 1.
Characterization data of PMAO coated iron oxide nanoparticles (PMAO-IONPs) and lactoferrin conjugated iron oxide nanoparticles (Lf-IONPs).
| Sample name | Core diameter | Magnetization Ms | Hydrodynamic diameter | Zeta potential |
|---|---|---|---|---|
| 14nm-PMAO-IONPs | 14 nm, σ=0.15 | 311 kA/m | 49 nm | −43 mV |
| 14 nm-Lf-IONPs | 306 kA/m | 74 nm | −7 mV | |
| 16nm-PMAO-IONPs | 16 nm, σ=0.10 | 306 kA/m | 48 nm | −43 mV |
| 16nm-Lf-IONPs | 306 kA/m | 70 nm | −8 mV | |
| 20nm-PMAO-IONPs | 20 nm, σ=0.14 | 337 kA/m | 52 nm | −41 mV |
| 20nm-Lf-IONPs | 326 kA/m | 75 nm | −6 mV | |
| 24nm-PMAO-IONPs | 24 nm, σ=0.10 | 342 kA/m | 50 nm | −41 mV |
| 24nm-Lf-IONPs | 322 kA/m | 72 nm | −11 mV | |
| 26nm-PMAO-IONPs | 26 nm, σ=0.16 | 322 kA/m | 46 nm | −35 mV |
| 26nm-Lf-IONPs | 342 kA/m | 71 nm | −4 mV |
Table 2.
The amount of lactoferrin conjugated to iron oxide nanoparticles and number of lactoferrin per iron oxide nanoparticle measured by Bradford assay.
| Sample name | Lactoferrin [ug/mgFe3O4] | Lactoferrin/ particle | % of lactoferrin conjugated |
|---|---|---|---|
| 14nm-Lf-IONPs | 447 | 22 | 89% |
| 17nm-Lf-IONPs | 471 | 35 | 94% |
| 20nm-Lf-IONPs | 475 | 69 | 95% |
| 24nm-Lf-IONPs | 401 | 101 | 80% |
| 26nm-Lf-IONPs | 434 | 138 | 87% |
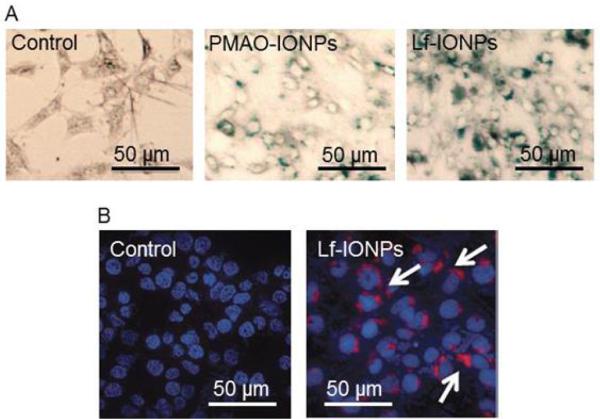
Fig. 4.
(A) Prussian blue staining after 24h incubation of C6 cells without nanoparticles (control), with PMAO-IONPs, and Lf-IONPs, showing higher uptake for Lf-IONPs as compared with PMAO-IONPs. Images were taken with inverted microscope at 20x. (B) Confocal images of C6 cells after 24h incubation without nanoparticles (control), and with Cy5.5 labelled Lf-IONPs (white arrow). Blue regions show the DAPI stained nucleus and red spots represent the Lf-IONPs labelled by Cy5.5.
The nanoparticles showed expected superparamagnetic and magnetization behaviours with variation consistent with their core sizes after PMAO coating or lactoferrin conjugation.
Bradford assay was conducted to quantify the amount of lactoferrin conjugated to nanoparticles with various core diameters. Between 401 and 475 μg of lactoferrin was added to 1 mg of PMAO-IONPs with core diameters of 14–26 nm (Table 2). The average number of lactoferrin molecules per single nanoparticle was calculated by assuming molecular weight of 90 kDa for lactoferrin and density of 5180 kg/m3 for iron oxide (Fe3O4) nanoparticles and using ICP for determination of iron concentration in each sample. Since 1 mg of IONPs contain 1×1014 particles for 14 nm IONPs and 2×1013 particles for 26 nm IONPs, and almost the same amount of lactoferrin molecules were conjugated to PMAO-IONPs regardless of their core sizes, the number of lactoferrin per particle increased as the core diameter increased from 14nm to 26nm (Table 2). The result of Bradford assay also indicates that 80–95% of lactoferrin added during the conjugation process was conjugated to the surface of nanoparticles.
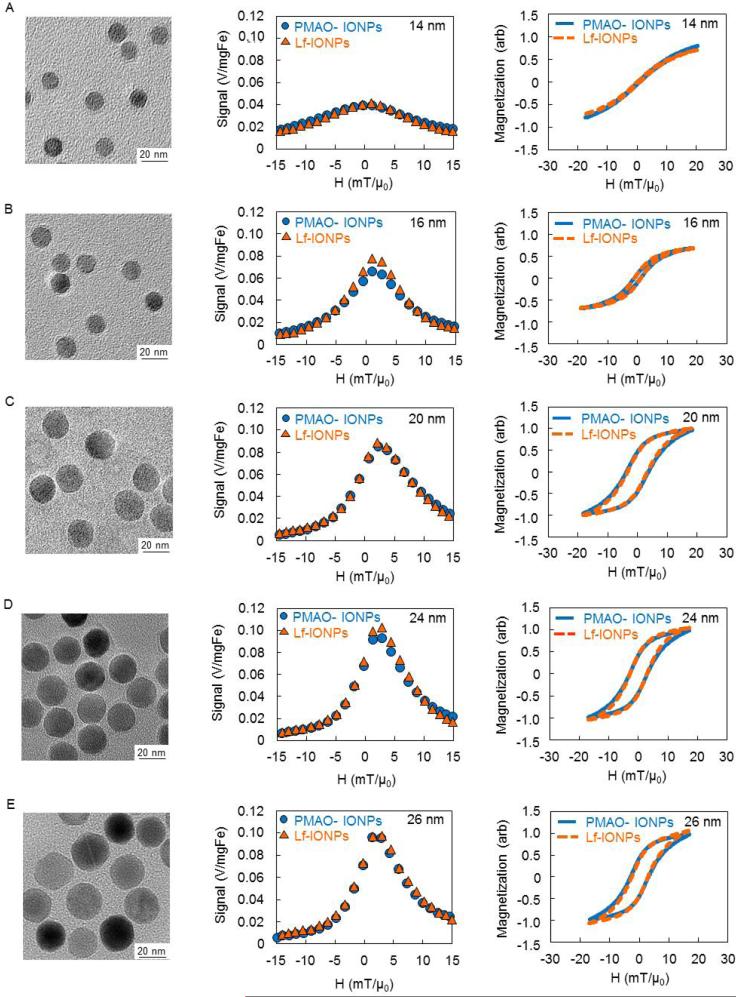
Figure 2 shows the TEM images of Lf-IONPs, MPS signals, and their calculated ac hysteresis loops for PMAO-IONPs and Lf-IONPs measured by our custom-built magnetic particle spectrometer (MPS).20,29 TEM images show spherical nanoparticles with uniform size distribution for all the core diameters, which agreed with our previous report.32 Comparing the MPS signals of nanoparticles before and after lactoferrin conjugation, PMAO-IONPs and Lf-IONPs showed similar signals. Higher signal intensities, narrower peaks, and a slight shift in the position of maximum intensities were observed with increasing core diameters of the PMAO-IONPs and Lf-IONPs. Under ac magnetic field at 26 kHz, the measured magnetic hysteresis loops differed from DC measurements by VSM (measured at room temperature for a measurement time of ~100s). Superparamagnetic behavior and slightly open hysteresis loops were observed for the nanoparticles with core diameters of 14 nm and 16 nm under ac magnetic field, respectively. In contrast, ferromagnetic behaviors were observed for the nanoparticles with the core diameters of 20-26 nm under ac magnetic field. In MPS, the time-varying magnetization of nanoparticles under ac magnetic field induces a signal in a receiver coil. Our MPS measures the nanoparticles magnetic response, and records it as a differential susceptibility, dm/dH or χdiff(H); note that an integration of the measured differential susceptibility gives the actual ac hysteresis loop. According to X-space MPI image reconstruction theory, the height and the FWHM of the measured response indicate the signal intensity and the spatial resolution of an equivalent MPI signal, respectively.33 Comparing the MPS signals of nanoparticles before and after lactoferrin conjugation, no significant change in χdiff(H) was observed for all the core diameters. This is because the MPS signal is dominated by Néel relaxation; in other words, the Brownian relaxation which is determined by the physical rotation of the nanoparticles, thus its hydrodynamic size, plays a negligible role. Higher intensity signals and smaller FWHMs were observed with increasing core diameter for both PMAOIONPs and Lf-IONPs. These results agree with previous reports, which revealed that MPS signal strongly depends on the median core diameter of the iron oxide nanoparticles.20,21 Although all the particles showed superparamagnetic behavior under a slowly-varying magnetic field in VSM measurements (typical measurement time ~100s), ferromagnetic behavior with an open hysteresis loop was observed for 16– 26nm nanoparticles under the MPS ac magnetic field at a frequency of 26 kHz (measurement time ~ 20 μs). Furthermore, the coercivity of the integrated ac hysteresis loops increased with the core diameter and shifted the maximum intensity (peak) in MPS to higher field values in agreement with our previous results.21 As is well known, superparamagnetic magnetite particles show zero coercivity and remanence in DC measurements, as the thermal energy is sufficient to overcome the anisotropy energy barrier and the magnetic moment of the particles fluctuates randomly within the measurement time. However under alternating magnetic field at 26 kHz, the measurement time is short enough for the magnetization to be blocked and it results in a ferromagnetic response1. Due to their adequate MPS signal and higher yield compared to 24 nm and 26 nm nanoparticles after phase transfer, we chose 20 nm nanoparticles for further characterization and in vitro study.
Fig. 2.
TEM images, MPS signals, and integrated ac magnetization curves of PMAO-IONPs and Lf-IONPs with core diameters of (A) 14 nm (14nm-Lf-IONPs), (B) 16 nm (16nm-Lf-IONPs), (C) 20 nm (20nm-Lf-IONPs), (D) 24 nm (24nm-Lf-IONPs), and (E) 26 nm (26nm-Lf-IONPs). The signals were divided by the amount of iron in each sample, and ac hysteresis loops were calculated by integration of the MPS signal (dm/dH). The MPS signals remained unchanged and independent of their coating compositions (PMAO or lactoferrin) for each core diameter.
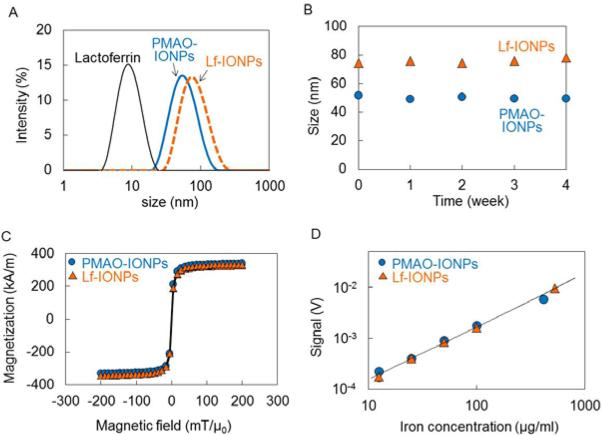
Figure 3A and 3B show the hydrodynamic size distribution and stability (up to 4 weeks) of PMAO coated IONPs (20nm-PMAO-IONPs) in water and lactoferrin conjugated IONPs (20nm-Lf-IONPs) in PBS, respectively. The hydrodynamic diameters for both of 20nm-PMAO-IONPs and 20nm-Lf-IONPs remained unchanged for at least up to 4 weeks, due to the electrostatic repulsion of negatively charged nanoparticles and stability of lactoferrin. Our studies show that PMAO or conjugates of PMAO with other molecules usually stabilize the nanoparticles in aqueous phases due to hydrophobic interaction. For example, we have shown the long-term stability in biological buffers, cell culture media, and mice blood for the iron oxide nanoparticles coated with a PAMO-PEG (polyethylene glycol) co-polymer.10,34,35
Fig. 3.
(A) Hydrodynamic size distribution and (B) stability over time of PMAO-IONPs and Lf-IONPs with core diameters of 20 nm. The average hydrodynamic diameters of lactoferrin, PMAO-IONPs, and Lf-IONPs were 8 nm, 52 nm, and 75 nm, respectively. These nanoparticles were stable for up to 4 weeks. (C) DC magnetization curves and (D) MPS signal of 20nm-PMAO-IONPs and 20nm-Lf-IONPs as a function of iron concentration. PMAO-IONPs and Lf-IONPs exhibited identical DC magnetization curves with superparamagnetic behaviors. Lf-IONPs showed linear signal variation with increasing nanoparticles concentration.
Figure 3C shows the DC magnetization curves of 20nm-PMAO-IONPs and 20nm-Lf-IONPs. Our VSM results showed identical magnetic behavior with superparamagnetic property for these nanoparticles. It demonstrates that lactoferrin conjugation had no influence on the DC magnetization of the nanoparticles. The saturation magnetization of 20nm-PMAO-IONPs and 20nm-Lf-IONPs were 71% and 70% of bulk saturation value for magnetite (92 Am2/kg),36 respectively.
Furthermore, it is important to determine the binding affinity of lactoferrin after their conjugation to the nanoparticles. Conformational changes of proteins after conjugation to nanoparticles have been reported before.37,38 These changes have influence on protein function, which might reduce their targeting ability. Lower binding affinity of antibody conjugated to the nanoparticles has also been reported due to the partial blocking of binding sites. 39 Therefore, we used ELISA assay to determine the binding affinity of lactoferrin after their conjugation to the nanoparticles using a polyclonal antibody specific for lactoferrin. Lactoferrin conjugated to iron oxide nanoparticles (20nm-PMAO-IONPs) exhibited binding affinity of 32% compared to that of the same amount of free lactoferrin, which was not conjugated to the nanoparticles. It indicates conformational change or partial blocking of their binding sites after conjugation to the nanoparticles.
Figure 3D shows the maximum intensity of the MPI signals of 20nm-PMAO-IONPs and 20nm-Lf-IONPs as a function of iron concentration in the biologically relevant range of 3–500 μg/ml. Both of the nanoparticles showed linear signal variations with the iron concentration in agreement with our previous reports.21 The MPS signals were linear up to 500 μgFe/ml, because of the increase of magnetization with the particle concentration. This linearity of the MPS signal with Fe concentration enables quantitative image interpretation using MPI.
We studied the internalization of Lf-IONPs into C6 cells by Prussian blue staining and confocal microscopy. Optical microscopy images in figure 4A show the Prussian blue staining of PMAO-IONPs and Lf-IONPs inside or on the surface of C6 cells after 24h incubation with nanoparticles. More blue color regions were observed for the C6 cells incubated with Lf-IONPs compared to those incubated with PMAO-IONPs. For further confirmation of the nanoparticles internalization, Lf-IONPs were labelled by a near infrared fluorescent agent (Cy5.5), and fluorescent images were taken by confocal microscopy. Figure 4B shows the confocal image of Cy5.5-Lf-IONPs internalized into C6 cells after 24h incubation with nanoparticles. Taken together, it is apparent that Lf-IONPs were internalized into most of the C6 cells after 24h incubation. Lactoferrin shows great potential as a ligand for brain tumor targeting because of the high expression of lactoferrin receptor (LRP) in glioma cells.23,24 LRP-mediated endocytosis is considered to be the main mechanism of cellular uptake of lactoferrin conjugated nanoparticles.40 Those nanoparticles taken up through endocytosis localize within endosomes which are the intracellular vesicles that fuse with lysosomes at later stages.41
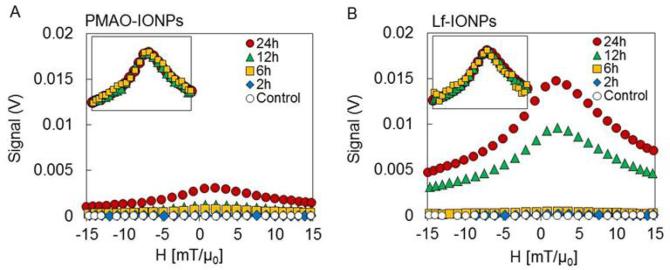
Figure 5 shows the MPS signals of the PMAO-IONPs and Lf-IONPs internalized into C6 cells after 2, 4, 6, 12 and 24h incubation. Higher signal intensities were observed for internalized Lf-IONPs in comparison with internalized PMAOIONPs at all time points while, as expected, no signal was observed for control C6 cells. Lf-IONPs internalized into C6 cells showed significant increase of MPS signal intensity after 12h incubation, and five-fold increase in the intensity compared to PMAO-IONPs after 24h incubation. Since MPS signal is generated only from iron oxide nanoparticles, these results suggest that significant amount of Lf-IONPs are internalized into C6 cells after 12h incubation, and Lf-IONPs was internalized more actively compared to PMAO-IONPs. Full width at half maximum (FWHM) of internalized Lf-IONPs remained the same between incubation time of 6 to 24h, promising a consistent spatial resolution in a real MPI scanner after nanoparticles internalization.
Fig. 5.
MPS signals and their normalized data (inset) of C6 cells in DMEM medium (control), (A) C6 cells after incubation with PMAO-IONPs for 2, 6, 12, and 24h, and (B) C6 cells after incubation with Lf-IONPs for 2, 6, 12, and 24h. Higher signal intensities were observed for C6 cells incubated with Lf-IONPs compared to those with PMAO-IONPs. FWHM remained the same between incubation time of 6 to 24h.
The concentration of Lf-IONPs internalized into C6 cells after 6h, 12h, and 24h incubation was calculated to be 26, 487, 738 μgFe/ml, respectively. These concentrations were calculated with comparing the maximum χdiff(H) of internalized Lf-IONPs (Figure 5B) and linear signal of χdiff(H) against Fe concentration in solutions (Figure 3D). We assumed that the relaxation behavior of Lf-IONPs inside the cells is the same as the Lf-IONPs in solution.
There is a broadening of the MPS signal (FWHM) and a shift in the maximum of χdiff(H) to higher fields, for the Lf-IONPs after internalization. MPS performance of nanoparticles depends on particle core and hydrodynamic diameters, size distributions and their environment.19,20,21 After internalization of the nanoparticles into the C6 cells, they accumulate in lysosomes and degrade in the low pH environment with the presence of chelating agents.42 These results are consistent with our previous reports showing that FWHM of MPS signal increases and the signal peak shifts to higher field values when iron oxide nanoparticles are partially dissolved and aggregated in a lysosome-like acidic buffer.43 Also, we have seen the same trends after internalization of the nanoparticles into the liver and spleen in mice models.17 Since MPI spatial resolution corresponds to FWHM of the MPS signals, development of surface modification approaches to prevent degradation and aggregation of the nanoparticles would improve the spatial resolution in MPI scanners. To our knowledge, this is the first report on evaluation of in vitro MPI performance of iron oxide nanoparticles with a tumor targeting ability. The results presented here indicate the potential of Lf-IONPs as tracers for brain tumor imaging in animal models using MPI which is a new and safe imaging technique.
Conclusions
Lactoferrin was successfully conjugated to monodisperse iron oxide nanoparticles with the median core diameters of 14 – 26 nm for brain glioma targeting; the larger (>20 nm) monodisperse nanoparticles are highly optimized as efficient MPI tracers and generate higher MPI signal intensity and better spatial resolution (i.e. narrower FWHM) under ac magnetic field. The binding affinity of the lactoferrin conjugated nanoparticles shows their targeting ability for brain glioma diagnosis, with the MPS signal remaining unchanged after lactoferrin conjugation. Lactoferrin-conjugated nanoparticles were successfully internalized into C6 glioma cells due to the binding affinity of lactoferrin to glioma cells. In-vitro MPS of our optimized Lf-IONPs demonstrated their potential use as tracers for brain glioma imaging using MPI.
Acknowledgements
This work was supported in part by NIH Grant Nos. 1RO1EB013689-01/NIBIB and NIH/NIBIB 2R42EB013520-02A1 Part of this work was conducted at the University of Washington NanoTech User Facility, a member of the NSF National Nanotechnology Infrastructure Network (NNIN). AT was also supported (2012-14) by the Japan Society for the Promotion of Science Postdoctoral Fellowship for Research Abroad.
References
- 1.Krishnan KM. IEEE Trans. Magn. 2010;46:2523–2558. doi: 10.1109/TMAG.2010.2046907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arruebo M, Fernandez-Pacheco R, Ibarra MR, Santamaria J. Nanotoday. 2007;2:22–32. [Google Scholar]
- 3.Jain TK, Richey Strand JM, Leslie-Pelecky DL, Flask CA, Labhasetwar V. Biomaterials. 2008;29:4012–4021. doi: 10.1016/j.biomaterials.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kami D, Takeda S, Itakura Y, Gojo S, Watanabe M, Toyoda M. Int. J. Mol. Sci. 2011;12:3705–3722. doi: 10.3390/ijms12063705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jayant RD, Atluri VSR, Agudelo M, Sagar V, Kaushik A, Nair M. Int. J. Nanomed. 2015;10:1077–1093. doi: 10.2147/IJN.S76517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chertok B, Moffat BA, David AE, Yu F, Bergemann C, Ross BD, Yang VC. Biomaterials. 2008;29:487–496. doi: 10.1016/j.biomaterials.2007.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang L, Ren X, Tang F, Zhang L. Biosen. Bioelectron. 2009;25:889–895. doi: 10.1016/j.bios.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 8.Wang D, He J, Rosenzweig N, Rosenzweig Z. Nano Lett. 2004;4:409–413. [Google Scholar]
- 9.Tomitaka A, Yamada T, Takemura Y. J. Nanomater. 2012;5:480626. [Google Scholar]
- 10.Khandhar AP, Ferguson RM, Simon JA, Krishnan KM. J. Biomed. Mater. Res. 2011;100A:728–737. doi: 10.1002/jbm.a.34011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gleich B, Weizenecker J. Nature. 2005;435:1214–1217. doi: 10.1038/nature03808. [DOI] [PubMed] [Google Scholar]
- 12.Weizenecker J, Gleich B, Rahmer J, Dahnke H, Borgert J. Phys. Med. Biol. 2009;54:L1–10. doi: 10.1088/0031-9155/54/5/L01. [DOI] [PubMed] [Google Scholar]
- 13.Ferguson MR, Khandhar AP AP, Kemp SJ, Arami H, Saritas EU, Croft LR, Konkle J, Goodwill PW, Halkola A, Rahmer J, Borgert J, Conolly SM, Krishnan KM. IEEE Med. Imaging. doi: 10.1109/TMI.2014.2375065. in press, doi: 10.1109/TMI.2014.2375065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bauer LM, Situ SF, Griswold MA, Samia ACS. J. Phys. Chem. Lett. 2015;6:2509–2517. doi: 10.1021/acs.jpclett.5b00610. [DOI] [PubMed] [Google Scholar]
- 15.Saritas EM, Goodwill PW, Croft LR, Konkle JJ, Lu K, Zheng B, Conolly SM. J. Magn. Res. 2013;229:116–126. doi: 10.1016/j.jmr.2012.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shin TH, Choi Y, Kim S, Cheon J. Chem. Soc. Rev. 2015;44:4501–4516. doi: 10.1039/c4cs00345d. [DOI] [PubMed] [Google Scholar]
- 17.Arami H, Khandhar AP, Tomitaka A, Yu E, Goodwill PW, Conolly SM, Krishnan KM. Biomaterials. 2015;52:251–261. doi: 10.1016/j.biomaterials.2015.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pablico-Lansigan MH, Situ SF, Samia ACS. Nanoscale. 2013;5:4040–4055. doi: 10.1039/c3nr00544e. [DOI] [PubMed] [Google Scholar]
- 19.Ferguson RM, Khandhar AP, Krishnan KM. J. Appl. Phys. 2012;111:07B318. doi: 10.1063/1.3676053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferguson RM, Minard KR, Khandhar AP, Krishnan KM KM. Med. Phys. 2011;38:1619–1626. doi: 10.1118/1.3554646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arami H, Ferguson RM, Khandhar AP, Krishnan KM. Med. Phys. 2013;40:071904. doi: 10.1118/1.4810962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adlerova L, Bartoskova A, Faldyna M. Vet. Med. 2008;53:457–468. [Google Scholar]
- 23.Xie H, Zhu Y, Jiang W, Zhou Q, Yang H, Gu N, Zhang Y, Xu H, Xu H, Yang X. Biomaterials. 2011;32:495–502. doi: 10.1016/j.biomaterials.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 24.Pang Z, Feng L, Hua R, Chen J, Gao H, Pan S, Jiang X, Zhang P. Mol. Pharm. 2010;7:1995–2005. doi: 10.1021/mp100277h. [DOI] [PubMed] [Google Scholar]
- 25.Huang R, Ke W, Han L, Liu Y, Shao K, Ye L, Lou J, Jiang C, Pei Y. J. Cer. Blood. Flow. Metab. 2009;29:1914–1923. doi: 10.1038/jcbfm.2009.104. [DOI] [PubMed] [Google Scholar]
- 26.Hu K, Li J, Shen Y, Lu W, Gao X, Zhang Q, Jiang X. J. Control. Release. 2009;134:55–61. doi: 10.1016/j.jconrel.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 27.Kalele S, Narain R, Krishnan KM. J. Magn. Magn. Mater. 2009;321:1377–1380. [Google Scholar]
- 28.Narain R, Gonzales M, Hoffman AS, Stayton PS, Krishnan KM. Langmuir. 2007;23:6299–6304. doi: 10.1021/la700268g. [DOI] [PubMed] [Google Scholar]
- 29.Ferguson RM, Khandhar A, Arami H, Hua L, Hovorka O, Krishnan KM. Biomed. Eng. (Biomedizinische Technik) 2013;58:1–15. doi: 10.1515/bmt-2012-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ludwig F, Remmer H, Kuhlmann C, Wawrzik T, Arami H, Ferguson RM, Krishnan KM. J. Magn. Magn. Mater. 2014;360:169–173. doi: 10.1016/j.jmmm.2014.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moros M, Pelaz B, López-Larrubia P, Garía-Martin ML, Grazú V, Fuente JM. Nanoscale. 2010;2:1746–1755. doi: 10.1039/c0nr00104j. [DOI] [PubMed] [Google Scholar]
- 32.Hufschmid R, Arami H, Ferguson RM, Gonzales M, Teeman E, Brush LN, Browning ND, Krishnan KM. Nanoscale. 2015;7:11142–11154. doi: 10.1039/c5nr01651g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goodwill P, Saritas EU, Croft LR, Kim TN, Krishnan KM, Schaffer DV, Conolly SM. Adv. Mater. 2012;24:3870–3877. doi: 10.1002/adma.201200221. [DOI] [PubMed] [Google Scholar]
- 34.Yu W, Chang E, Sayes CM, Drezek R, Colvin VL. Nanotechnology. 2006;17:4483–4487. [Google Scholar]
- 35.Khandhar AP, Ferguson RM, Arami H, Krishnan KM. Biomaterials. 2013;34:3837–3845. doi: 10.1016/j.biomaterials.2013.01.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sato T, Iijima T, Seki M, Inagaki N. J. Magn. Magn. Mater. 1987;65:252–256. [Google Scholar]
- 37.Lynch I, Dawson KA. Nanotoday. 2008;3:41–47. [Google Scholar]
- 38.Mahmoudi M, Shokrgozar MA, Sardari S, Moghadam MK, Vali H, Laurent S, Stroeve P. Nanoscale. 2011;3:1127–1138. doi: 10.1039/c0nr00733a. [DOI] [PubMed] [Google Scholar]
- 39.Wang S, Mamedova N, Kotov NA, Chen W, Studer J. Nano Lett. 2002;2:817–822. [Google Scholar]
- 40.Su Z, Xing L, Chen Y, Xu Y, Yang F, Zhang C, Ping Q, Xiao Y. Mol. Pharm. 2014;11:1823–1834. doi: 10.1021/mp500238m. [DOI] [PubMed] [Google Scholar]
- 41.Iversen TG, Skotland T, Sandvig K. Nano Today. 2011;6:176–185. [Google Scholar]
- 42.Tomitaka A, Jo J, Aoki I, Tabata Y. Inflammation Regeneration. 2014;34:45–55. [Google Scholar]
- 43.Arami H, Krishnan KM. J. Appl. Phys. 2014;115:17B306. doi: 10.1063/1.4867756. [DOI] [PMC free article] [PubMed] [Google Scholar]