Abstract
Objectives
It would be quite convenient if daily dietary cadmium intake (Cd-D) can be estimated either from Cd in blood (Cd-B) or from Cd in urine (Cd-U). The aim of the study was to examine if Cd-D can be estimated from Cd-B or Cd-U.
Methods
The data available in a previous publication were employed for regression analyses between Cd-D and Cd-B, and between Cd-D and Cd-U. 30 sites in various prefectures throughout Japan were surveyed and 20 adult women/site on average provided food duplicate, peripheral blood, and second morning urine samples. Geometric means were taken as representative values and employed in regression analyses.
Results
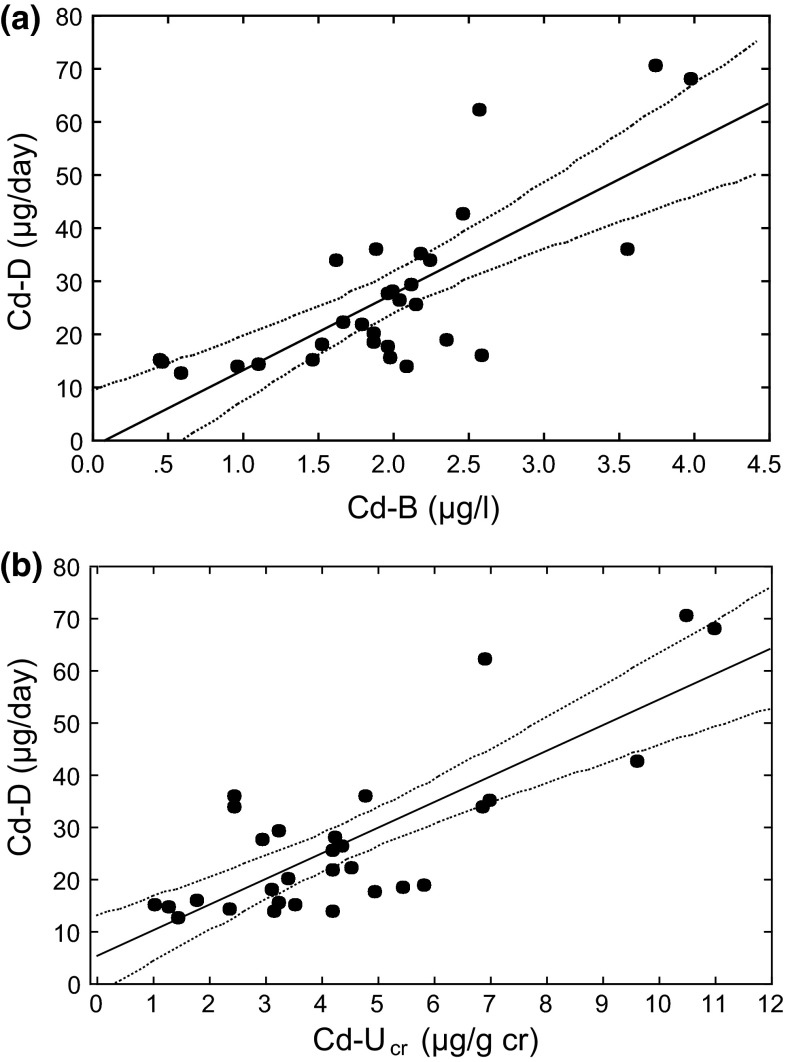
Cd-D, Cd-B, and Cd-Ucr [i.e., Cd-U after correction for creatinine (cr) concentration] distributed in ranges of 12.5–70.5 μg/day, 0.46–3.98 μg/l, and 1.16–11.02 μg/g cr, respectively. A close correlation was observed between Cd-D and Cd-B, and also between Cd-D and Cd-Ucr with r = 0.76 and r = 0.79 (p < 0.001 for both), respectively. Both regression lines passed close to the origins. Application of 1.23 μg Cd/l blood and 1.26 μg Cd/g cr in urine (average levels for adult Japanese women) to the regression equations gave 16.5 and 11.5 μg Cd/day.
Conclusions
The analyses suggested that it may be possible to estimate Cd-D from Cd-B or Cd-U. Cd-B-based estimation should be more respected. As variations in the estimation parameters and estimated values are inherent to field surveys, care should be taken in the application of the study results. Application on a group basis (and not on an individual basis) should be considered.
Keywords: Cadmium in blood, Cadmium in urine, Dietary cadmium intake, Estimation, Japan
Introduction
Cadmium (Cd) is a well-studied environmental pollutant with insidious toxicities on the kidney and then the bone [1, 2]. Elevated dietary Cd intake has been a long-standing environmental concern in Japan [3, 4], primarily because Cd contents in rice harvested in Japan is high [5, 6] as compared with the levels in rice from other countries, whereas boiled rice is a historical staple food for general populations [7]. It is noted that Cd-D has been gradually decreasing in the recent years [3, 8], e.g., >50 μg/day in late 1960s, <50 μg/day in 1970s, and about 30 μg/day in 1980s [3]. The other report indicated that Cd-D was 45–50 μg/day in late 1970s, and then decreased gradually to reach 20–25 μg/day in 2004 and 2005 [8]. High Cd-D, however, remains as a matter worthy of attention [8].
For determination of dietary Cd intake, food duplicate sample collection [9] followed by blending, wet-digestion, and graphite furnace atomic absorption spectrometry (or inductively coupled plasma mass spectrometry) is a reliable method [10], but the procedures are quite hand-consuming and nerve-taxing first of all to sample donors and also to the analytical chemists.
Based on the statistical analyses of existing data on Cd in whole-day food duplicate samples (Cd-D), Cd in urine after correction for urinary creatinine concentration (Cd-Ucr), and Cd in peripheral blood (Cd-B) [11], a successful trial was made in this study group to establish equations which allow estimation of Cd-D from Cd-B as well as from Cd-Ucr. The results were presented in this short communication.
Materials and methods
Data on Cd-D, Cd-B, and Cd-Ucr were cited from Ikeda et al. [11]. The data were based on the field surveys in 30 sites distributed throughout Japan from northern-most Hokkaido Island to the southern-most Okinawa Islands, and 20 adult women/site on average participated in the study by offering food duplicate, peripheral blood, and second morning urine samples. Geometric mean values (GMs) were taken to represent each study site. The GM values were in a range of 12.5–70.5 μg/day for Cd-D, 0.46–3.98 μg/l for Cd-B, and 1.1–11.02 μg/g cr for Cd-Ucr.
The GM values were subjected to linear regression analyses between Cd-D and Cd-B and between Cd-D and Cd-Ucr. Equations for regression lines and the 95 % upper and lower limit curves were calculated with a software ‘Excel Statistics’ [12]. Comparison of two regression lines in terms of slopes, intercepts, and correlation coefficients was conducted by analysis of covariance after Ichihara [13].
Results and discussion
The results of regression analyses were depicted in Fig. 1[(a) for Cd-B vs. Cd-D and (b) for Cd-Ucr vs. Cd-D] for visual understanding of close correlation between the paired parameters. Equations for regression lines (Eqs. 2, 5) and the 95 % upper and lower limit curves (Eqs. 1, 3, 4, and 6) were given in the top 2nd–7th lines (the 1991–1997 survey) in Table 1. It was clear that Cd-D increased as a linear function of the increase in Cd-B and also in Cd-Ucr. The correlation coefficients were >0.7 and p < 0.001 for both cases. However, variations around the regression lines were also noted.
Fig. 1.
Linear correlation of Cd in blood and Cd in urine (as corrected for creatinine concentration) with Cd in daily diet; the 1991–1997 survey [11]. The line in the middle is a calculated regression line. The two curves on both sides show the 95 % upper and lower limits. Each dot (n = 30) represent one survey site. a Cadmium in blood (Cd-B) versus cadmium in daily diet (Cd-D). b Cadmium in urine as corrected for creatinine concentration (Cd-Ucr) versus cadmium in daily diet (Cd-D)
Table 1.
Equations for regression lines and confidence ranges
| Survey | X | Y | Typea | Equation | ||
|---|---|---|---|---|---|---|
| The 1991–1997 survey (n = 30) | ||||||
| Cd-B | Cd-D | 95 % UL | Eq. 1 | |||
| (μg/l) | (μg/day) | RL | r = 0.76b | Eq. 2 | ||
| 95 %LL | Eq. 3 | |||||
| Cd-Ucr | Cd-D | 95 % UL | Eq. 4 | |||
| (μg/g cr) | (μg/day) | RL | r = 0.79b | Eq. 5 | ||
| 95 %LL | Eq. 6 | |||||
| The 1977–1981 survey (n = 18) | ||||||
| Cd-B | Cd-D | 95 % UL | ||||
| (μg/l) | (μg/day) | RL | r = 0.68c | |||
| 95 %LL | ||||||
aThe equations are regression line (RL) and 95 %upper (95 % UL) or lower limit (95 % LL)
b p < 0.001 for both cases
c p < 0.01
To the knowledge of the authors, this is the first successful trial to develop the equations for estimating dietary Cd intake from Cd levels in blood or urine for Japanese populations. As the procedures for food duplicate sampling (followed by instrumental analyses) are complex, the Cd-B- or Cd-U-based estimation of Cd-D will be a practical and convenient measure in Cd burden study, as Cd-B and Cd-U have been commonly measured [1, 2]. The present analyses are based on the results from surveys in adult Japanese women, but the equations in Table 1 may be also applicable to other rice-depending populations in East and South-East Asia. A preliminary analysis of Cd-D and Cd-Ucr data from rice-depending areas in Asia [14] gave a regression line between Cd-D and Cd-Ucr with statistically no significant (p < 0.05) difference from the regression line in Table 1 (data not shown). Analyses on the relationship between Cd-D and Cd-B (in addition to Cd-U) are envisaged. Further studies are also desirable on the applicability of the present results to rice non-depending populations who depend on, e.g., wheat and other cereals as staple food.
With regard to the choice of Cd-B or Cd-U, repeated measurement of Cd-B and Cd-U was conducted with participation of five adult women volunteers to compare stability of the two exposure parameters. Thus, urine samples were collected once a month for 12 times, and peripheral blood was sampled once every 3 months for four times. When GM values were compared (i.e., 12 GMs for Cd-Ucr and 4 GMs for Cd-B), the highest and the lowest GM for Cd-Ucr were 3.9 and 2.0 μg/g cr, respectively (with the highest/lowest ratio of 1.9), whereas 3.3 and 2.9 μg/l for Cd-B (the ratio: 1.1) [15], indicating better stability for Cd-B than for Cd-U. This observation may suggest that more care should be practiced in using Cd-Ucr (rather than Cd-B) to estimate Cd-D, as the estimate based on better stabilized parameter should be more respected.
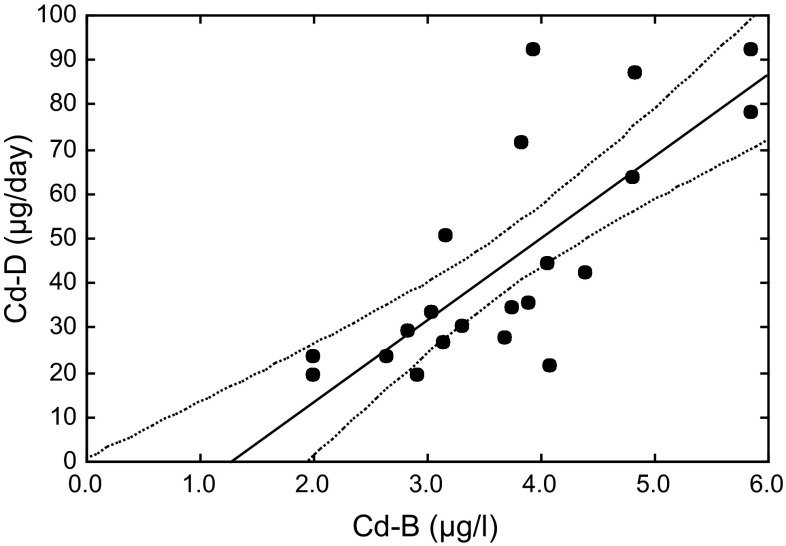
Retrieval through published literatures revealed that data on Cd-D coupled with Cd-B or Cd-U were very few possibly due to the complexity of the procedures for food duplicate collection and analyses, as described above. Thus, the data for validation of the proposed equations (top 7 lines in Table 1) are limited. This study group conducted field surveys on Cd exposure of adult women also in 1977–1981, and Watanabe et al. [16] published GM values for Cd-D in combination with Cd-B in 18 locations. It should be noted that the GM Cd-D, 37.0 μg/day [16] in 1977–1981, was substantially higher than the levels, 24.7 μg/day [11] in 1991–1997. For validation purpose, Cd-B and Cd-D correlation was analyzed in a manner similar to the present study. The results are presented in the bottom 4 lines (the 1977–1981 survey) in Table 1 and graphically presented in Fig. 2. Although the regression line for the 1977–1981 survey apparently had a steeper slope and a lower intercept than the line for the 1991–1997 survey, further statistical analysis showed that there was no significant difference (p > 0.05) between the pairs of the intercepts, the slopes, and the correlation coefficients, which may suggest reproducibility of the present study results.
Fig. 2.
Linear correlation of Cd in blood with Cd in daily diet; the 1977–1981 survey [16]. Notes are as under Fig. 1 except n = 18
Horiguchi et al. [4] conducted a mass analysis for Cd-B and Cd-Ucr in five locations in Japan, with 1380 participating women in total. For Cd-D, they measured Cd intake through boiled rice consumption (Cd-rice). From Cd-rice, Cd-D was estimated with two separate assumptions, i.e., assumption A was such that Cd-D was twice the Cd-rice [meaning that the same amount of Cd came from rice and from foods (and water) other than rice], and assumption B was such that 15 μg Cd was expected to come from foods (and water) other than rice (irrespective of individuals and survey sites). GM values for Cd-D with assumption A were in a range of 6.99–51.99 μg/day. With assumption B, the GM values distributed from 19.88 to 44.07 μg/day. GM Cd-B and GM Cd-Ucr were in ranges of 1.65–3.61 μg/l and 2.63–4.08 μg/g cr, respectively [4]. Application of the reported Cd-B and Cd-Ucr values to Eqs. 2 and 5 (Table 1) gave 34.8 ± 11.2 μg/day (AM ± ASD) in a range of 24.8–53.0 and 21.5 ± 2.6.2 μg/day (AM ± ASD) in a range of 18.2–25.3 μg/day, respectively. Statistical analysis by paired t test showed no significant difference (p > 0.10) between Cd-B-based Cd-D estimation and assumption A-based Cd-D, and also between Cd-B-based Cd-D estimation and assumption B-based Cd-D. It was also the case when Cd-Ucr-based Cd-D estimation was compared with assumption A-based Cd-D. However, a difference was significant (p < 0.05) between Cd-Ucr-based Cd-D estimation and assumption B-based Cd-D. In short, Cd-B-based estimation gave reliable estimate (better than Cd-Ucr-based one) [4], in agreement with the observation that Cd-B is a more stable parameter that Cd-U [15].
With regard to possible confounders, smoking cigarettes is a known non-occupational Cd source for general populations. It was observed that daily consumption of 6–10 cigarettes may induce 0.6 μg Cd/l urine [17] (which should be roughly equivalent to 0.6 μg/g cr, when creatinine concentration is assumed to be about 1 g/l in women [18]). Therefore, the effect on Cd-D versus Cd-Ucr relationship should be minimal.
Application of Cd-B of 1.23 μg/l (the average Cd-B for adult Japanese women population in 2003–2011 [13] ) to the Eq. 2 in Table 1 gave 16.5 μg/day as the Cd-B-based best estimate for Cd-D in recent years (i.e., around 2003–2011). It was also possible to estimate Cd-D based on Cd-Ucr (Eq. 5 in Table 1), taking the national average of 1.26 μg/g cr for adult women with no known specific Cd exposures [18]. The calculation gave 16.5 and 11.5 μg/day, respectively. Regarding variations when the same estimating parameter is used, the application of these Cd-B and Cd-Ucr values to Eqs. 1 and 3 or Eqs. 4 and 6 gave 95 % estimation ranges for Cd-D of 11.4–21.7 and 5.8–17.3 μg/day, respectively. There was a substantial over-up between the two ranges, but about 40 % difference was noted between the two estimates of 11.5 and 16.5 μg Cd/day. Nevertheless, the level of 11.5–16.5 μg Cd/day may suggest a gradual decrease in Cd-D as compared with the levels of 20–40 μg/day in 1980s–1990s [3].
There are several limitations in the present analysis. First of all, the database employed [11] was more than 10 years old. Literature survey revealed that no later data are available in which Cd-B or Cd-U was examined together with Cd-D as stated previously, although reliable and convenient methods for Cd-D estimation are ever requested.
The number of sites surveyed was limited to 30 locations and the numbers of participants were 20 subjects/site on average [11]. Possibly due to such limitation at least in part, variation around the regression lines was noted although the correlation between pairs of parameters (i.e., Cd-B vs. Cd-D and Cd-Ucr vs. Cd-D) was highly significant (p < 0.001). Thus, the variation range for estimated Cd-D was still substantial as discussed above.
Such variation in the estimating parameters as well as the estimated values may be inherent to field surveys, and care should be taken in the application of the study results. One of the counter measures would be the use of a representative value, e.g., GM in case of Cd-B and Cd-U (and a median would be close to GM). A recommended procedure therefore will be the application of the equation only on a group basis, e.g., application of geometric mean values, and not individual measures.
The present analyses were based on the observation from non-polluted areas where Cd-B was <4.0 μg/l or Cd-Ucr was <11 μg/g cr (Fig. 1). Applicability of the Eqs. 2 and 5 (Table 1) to the groups from polluted areas with higher Cd-D is an important question from a practical view point. Further studies are apparently necessary and no answer is currently available. With this regard, Hara et al. [19] conducted a survey in a Cd-polluted area and reported a very high Cd-D of 205.7 μg/day, whereas Saito et al. [20] reported that Cd-U for women was 8.6 μg/l. Both values were assumedly arithmetic means. Only 10 Cd-D measurements were conducted whereas 154 women offered urine samples, and therefore questions may remain on the representativeness of the Cd-D values for total participants. In addition, it was not clear whether the food duplicate samples were for men or for women; it is known that Cd-D may differ between men and women, and is greater for men than for women [21] possibly because men used to take more rice than women. Nevertheless, a large difference between the reported Cd-D (205.7 μg/day) and Eq. 5-based estimate of 47.5 μg/day suggests that the relation between Cd-U and Cd-D may be different when the dietary Cd exposure is very high and therefore Eq. 5 may not be applicable to the cases where Cd-D is high due to pollution with Cd. In 2008, the Food Safety Committee, Japan, reported a tolerable weekly intake (TWI) of 7 μg/kg body weight/week to the Minister of Health, Labour and Welfare [8]. In practice, the present study results may not be applicable to the populations for whom the GM Cd-D is in excess of the TWI. Assuming that the average body weight for adult Japanese population is about 60 kg [7], the TWI when calculated for daily intake basis would be about 60 μg/day. Application of this value to Eqs. 3 and 5 in Table 1 gives 4.3 μg/l for Cd-B and 11.2 μg/g cr for Cd-Ucr as possible critical values for exclusion. Nevertheless, further studies are apparently envisaged to validate the applicability of this TWI value as the exclusion criterion.
Acknowledgments
The authors are grateful to Dr. T. Kawai of Osaka Occupational Health Service Center, Osaka, Japan for his technical support to this study.
Conflict of interests
The authors declare that they have no conflicts of Interest.
References
- 1.International Programme on Chemical Safety (IPCS) Environmental health criteria I34. Cadmium. Gcneva: World Health Organization; 1992. [Google Scholar]
- 2.Internatlional Programme on Chemical Safety (IPCS) Envrronmenttal health criteria 135. Cadmium—environmental aspects. Geneva: World Health Organization; 1992. [Google Scholar]
- 3.Ikeda M, Ezaki T, Tsukahara T, Moriguchi J. Dietary cadmium intake in polluted and non-polluted areas in Japan in the past and in the present. Int Arch Occup Environ Health. 2004;77:227–234. doi: 10.1007/s00420-003-0499-5. [DOI] [PubMed] [Google Scholar]
- 4.Horiguchi H, Oguma E, Sasaki S, Okubo H, Murakami K, Miyamoto K, et al. Age-relevant renal effects of cadmium exposure through consumption of home-harvested rice in female Japanese farmers. Environ Int. 2013;56:1–9. doi: 10.1016/j.envint.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Watanabe T, Shimbo S, Moon C-S, Zhang Z-W, Ikeda M. Cadmium contents in rice samples from various areas in the world. Sci Total Environ. 1996;184:191–196. doi: 10.1016/0048-9697(96)05100-5. [DOI] [PubMed] [Google Scholar]
- 6.Shimbo S, Zhang Z-W, Watanabe T, Nakatsuka H, Matsuda-Inoguchi N, Higashikawa K, et al. Cadmium and lead contents in rice and other cereal products in Japan in 1998-2000. Sci Total Environ. 2001;281:165–175. doi: 10.1016/S0048-9697(01)00844-0. [DOI] [PubMed] [Google Scholar]
- 7.National Institute of Health and Nutrition, Japan . The National Health and Nutrition Survey, 2011. Tokyo: Daiichi Shuppann Publishers; 2015. pp. 68–71. [Google Scholar]
- 8.Food Safety Committee, Japan. Document on safety of dietary cadmium intake, attached to the report to the Minster of Health, Labour and Welfare, Japan. 2008.
- 9.Acheson KJ, Campbell IT, Edholm OG, Miller DS, Stock MJ. The measurement of food and energy intake in man—an evaluation of some techniques. Am J Clin Nutr. 1980;33:1147–1154. doi: 10.1093/ajcn/33.5.1147. [DOI] [PubMed] [Google Scholar]
- 10.Ikeda M, Ohashi F, Fukui Y, Sakuragi S, Moriguchi J. Cadmium, chromium, lead, manganese and nickel concentrations in blood of women in non-polluted areas in Japan, as determined by inductively coupled plasma-sector field-mass spectrometry. Int Arch Occup Environ Health. 2011;84:139–150. doi: 10.1007/s00420-010-0542-2. [DOI] [PubMed] [Google Scholar]
- 11.Ikeda M, Zhang Z-W, Moon C-S, Shimbo S, Watanabe T, Nakatsuka H, et al. Possible effects of environmental cadmium exposure on kidney function in the Japanese general population. Int Arch Occup Environ Health. 2000;73:15–25. doi: 10.1007/PL00007933. [DOI] [PubMed] [Google Scholar]
- 12.Esumi & Co. EXCEL STATISTICS. Tokyo (Japan).
- 13.Ichihara K. Statistics for Bioscience. Tokyo (Japan): Nankodo Publishers; 1990. [Google Scholar]
- 14.Ikeda M, Zhang Z-W, Shimbo S, Watanabe T, Nakatsuka H, Moon C-S, et al. Urban population exposure to lead and cadmium in east and south-east Asia. Sci Total Environ. 2000;249:373–384. doi: 10.1016/S0048-9697(99)00527-6. [DOI] [PubMed] [Google Scholar]
- 15.Yamagami T, Suna T, Fukui Y, Ohashi F, Takada S, Sakurai H, et al. Biological variations in α1-microglobulin, β2-microglobulin and N-acetyl-β-D-glucosaminidase in adult women in a non-polluted area. Int Arch Occup Environ Health. 2008;81:263–271. doi: 10.1007/s00420-007-0206-z. [DOI] [PubMed] [Google Scholar]
- 16.Watanabe T, Nakatsuka H, Shimbo S, Iwami O, Imai Y, Moon C-S, et al. Reduced cadmium and lead burden in Japan in the past 10 years. Int Arch Occup Environ Health. 1996;68:305–314. doi: 10.1007/BF00409415. [DOI] [PubMed] [Google Scholar]
- 17.Ikeda M, Moriguchi J, Ezaki T, Fukui Y, Ukai H, Okamoto S, et al. Smoking-induced increase in urinary cadmium levels among Japanese women. Int Arch Occup Environ Health. 2005;78:533–540. doi: 10.1007/s00420-005-0612-z. [DOI] [PubMed] [Google Scholar]
- 18.Ezaki T, Tsukahara T, Moriguchi J, Furuki K, Fukui Y, Ukai H, et al. No clear-cut evidence for cadmium-induced tubular dysfunction among over 10,000 women in the Japanese general population; a nationwide large-scale survey. Int Arch Occup Environ Health. 2003;76:186–196. doi: 10.1007/s00420-002-0389-2. [DOI] [PubMed] [Google Scholar]
- 19.Hara Y. Part 1. General view. In: Saito H, Tskebayashi S, Harada K, Hara Y, editors. Chronic cadmium poisoning. Nagasaki: Nagasaki University School of Medicine (the “n department of Medicine); 2013. p. 42. [Google Scholar]
- 20.Saito H. Part 2. Health survey on residents. In: Saito H, Tskebayashi S, Harada K, Hara Y, editors. Chronic cadmium poisoning. Nagasaki: Nagasaki University School of Medicine (the “n department of Medicine); 2013. p. 56. [Google Scholar]
- 21.Watanabe T, Koizumi A, Fujita H, Kumai M, Ikeda M. Dietary cadmium intakes of farmers in nonpolluted areas in Japan, and the relation with blood cadmium levels. Environ Res. 1985;37:33–43. doi: 10.1016/0013-9351(85)90047-7. [DOI] [PubMed] [Google Scholar]