Abstract
Background
Osteolysis resulting from wear debris production from the bearing surfaces is a major factor limiting long-term survival of hip implants. Oxidized zirconium head on crosslinked polyethylene (XLPE) is a modern bearing coupling. However, midterm in vivo wear data of this coupling are not known.
Questions/purposes
The purpose of this study was to investigate in vivo whether the combination of an oxidized zirconium femoral head on XLPE produces less wear than a ceramic head on XLPE or a ceramic head on conventional polyethylene (CPE) couplings and whether any of these bearing combinations results in higher hip scores.
Methods
Between 2003 and 2007, we performed 356 total hip arthroplasties in 288 patients; of those, 199 (69.1%) patients (199 hips) were enrolled in what began as a randomized trial. Unfortunately, after the 57th patient, the randomization process was halted because of patients’ preference for the oxidized zirconium bearing instead of the ceramic after (as they were informed by the consent form), and after that, alternate allocation to the study groups was performed. Hips were allocated into four groups: in Group A, a 28-mm ceramic head on CPE was used; in Group B, a 28-mm ceramic head on XLPE; in Group C, a 28-mm Oxinium head on XLPE; and in Group D, a 32-mm Oxinium head on XLPE. The authors prospectively collected in vivo wear data (linear wear, linear wear rate, volumetric wear, and volumetric wear rate) using PolyWare software. Preoperative and postoperative clinical data, including Harris and Oxford hip scores, were also collected at regular intervals. Of those patients enrolled, 188 (95%) were available for final followup at a minimum of 7 years (mean, 9 years; range, 7–12 years).
Results
All bearing surfaces showed a varying high bedding-in effect (plastic deformation of the liner) up to the second postoperative year. At 5 years both oxidized zirconium on XLPE groups showed lower (p < 0.01) volumetric wear (mean ± SD mm3) and volumetric wear rates (mean ± SD mm3/year) (Group C: 310 ± 55–206 ± 55 mm3/year, Group D: 320 ± 58–205 ± 61 mm3/year) when compared with ceramic on CPE (Group A: 791 ± 124–306 ± 85 mm3/year) and ceramic on XLPE (Group B: 1420 ± 223–366 ± 88 mm3/year) groups. For those patients who had completed 10 years of followup (20 patients [44.5%] of Group A, 21 [45.7%] of Group B, 23 [47.9%] of Group C, and 22 [44.9%] of Group D), at 10 years, both oxidized zirconium on XLPE groups also showed lower (p < 0.01) volumetric wear (mean ± SD mm3) and volumetric wear rates (mean ± SD mm3/year) (Group C: 356 ± 64 to 215 ± 54 mm3/year, Group D: 354 ± 50 to 210 ± 64 mm3/year) when compared with ceramic on CPE (Group A: 895 ± 131 to 380 ± 80 mm3/year) and ceramic on XLPE (Group B: 1625 ± 253 to 480 ± 101 mm3/year) groups. When wear rates of both oxidized zirconium groups were compared, no differences were found at any time interval with the numbers available. Two hips (one from Group A and one from Group B) are scheduled for revision as a result of wear and osteolysis. There were no differences in hip scores among the groups with the numbers available.
Conclusions
In this study, in vivo wear parameters were lower when the combination of an oxidized zirconium head on XLPE liner was used at an average of 9 years (range, 7–12 years) followup. Further larger-scale clinical studies should confirm these findings and evaluate osteolysis and revision rates in association with the use of this bearing coupling.
Level of Evidence
Level II, therapeutic study.
Introduction
Total hip arthroplasty (THA) provides reliable pain-free function to patients with end-stage hip pathology [36, 37]. It is accepted that long-term survival of a THA is a multifactorial issue with the implant, the diagnosis, the patient, the surgeon, and surgical technique being equally important. Wear debris production from the bearing surfaces leads to the development of interface osteolysis, which, these days, is thought to be the main factor limiting long-term survival of the artificial joint [8, 30]. Alternative bearing surfaces have thus been developed to reduce the amount of wear particles produced [8, 30].
Cobalt-chrome or ceramic heads on polyethylene liners have been used in the majority of patients and the technique is preferred by many surgeons, but it is associated with wear and osteolysis [18, 45, 46]. Metallic femoral heads on polyethylene, introduced by Sir John Charnley, remain the gold standard; however, ceramic heads on polyethylene (introduced in the early 1970s) demonstrated, in hip simulators, up to 20 times less wear than Charnley’s technique [38, 42], and used this way, the ceramic heads show a relatively low risk of fracture. Polyethylene, moreover, has undergone many improvements, from the development of ultrahigh-molecular-weight (CPE) to crosslinked (XLPE) polyethylene. These improved polyethylenes have shown better wear characteristics in combination with mainly metallic heads in vitro [31, 32, 38] and in vivo [4, 8, 14, 26, 30].
Hard-on-hard bearings (such as ceramic-on-ceramic or metal-on-metal) may represent attractive options because of reduced wear particle production, but they may not be suitable for all patients. Ceramic-on-ceramic combinations have been found to squeak and break [25, 39] and require optimal placement to avoid the risk of neck-to-socket impingement [41, 43]. Metal-on-metal combinations have been associated with pseudotumor formation [2, 21] and increased metal ion release, which may lead to cell apoptosis and to DNA changes [22]. Oxidized zirconium head technology has been proposed as an alternative bearing surface based on the theoretical assumption that the wear characteristics of a ceramic surface and the metallic substrate can be combined in one head [1, 5, 16, 17]. The combination of oxidized zirconium on XLPE has been tested in vitro and shown satisfactory wear characteristics. However, mid-term in vivo wear data related to the two available sizes of this bearing surface are lacking. As soon as this coupling was released for clinical use, we planned and conducted this study to evaluate its in vivo wear characteristics and to compare them with those of the most commonly used bearing couplings of this period of time (28-mm ceramic head on either CPE or XLPE).
In this study we prospectively evaluated, at 7- to 12-year followup, the in vivo wear characteristics of 28- and 32-mm oxidized zirconium heads on XLPE couplings and compared them with 28-mm ceramic-on-CPE and ceramic-on-XLPE bearing surfaces and whether any of these bearing combinations results in higher hip scores.
Materials and Methods
In January 2003 a prospective randomized study was initiated in our department to evaluate the clinical and in vivo wear performance of an oxidized zirconium head on XLPE (Oxinium; Smith & Nephew, Memphis, TN, USA) [5, 13, 15] in THA compared with other conventional and hard-on-hard bearing surfaces. Written informed consent was obtained from all patients for participation in this prospective study. The study was also approved by the National and Hospital Ethical Committees. The plan was to randomize patients into four different groups: Group A, 28-mm ceramic head (Biolox® forte; Ceramtec, Plochingen, Germany) on a CPE liner; Group B, 28-mm ceramic head on XLPE; Group C, 28-mm oxidized zirconium head on XLPE; and Group D, 32-mm oxidized zirconium head on XLPE. After inclusion of the 57th hip, randomization was discontinued because many patients demanded the new bearing surface rather than the conventional one (having realized that this was available after reading the consent form). Thus, the process was completed with alternate allocation of hips into the different groups with the last allocation performed in October 2007. During this period of time, 356 THAs in 288 patients (237 [82%] patients having osteoarthritis of the hip) were performed by the senior surgeon (TK). Of those, 199 patients (199 hips) who underwent THA for hip osteoarthritis (84% of the patients with that diagnosis) were allocated into the four different groups (Group A: 47 hips, Group B: 48 hips, Group C: 51 hips, and Group D: 53 hips). Inclusion criteria were end-stage osteoarthritis of the hip, age between 65 and 75 years, and one major joint involvement. Exclusion criteria were bone metabolic disease, avascular necrosis, and inflammatory and posttraumatic arthritis. There were 118 female and 81 male patients with 105 right and 94 left hips. Despite the alternate allocation procedure, patients appeared to be well matched for age, sex, side, and body mass index (Table 1). All operations were performed by the same surgeon (TK). In all patients the Synergy cementless femoral stem (Smith & Nephew) and the Reflexion cementless acetabular cup (Smith & Nephew) with the mentioned bearing coupling combinations were used (Fig. 1).
Table 1.
Patients’ demographics
| Study groups | Group A | Group B | Group C | Group D | Differences |
|---|---|---|---|---|---|
| Number of hips included | 47 | 48 | 51 | 53 | |
| Age (years; range) | 71 (65–75) | 72 (66–75) | 72 (67–75) | 70 (65–73) | p > 0.05 |
| Gender | |||||
| Male | 19 | 21 | 22 | 19 | p > 0.05 |
| Female | 30 | 28 | 28 | 32 | p > 0.05 |
| Side | |||||
| Left | 23 | 22 | 25 | 24 | p > 0.05 |
| Right | 27 | 24 | 29 | 25 | p > 0.05 |
| Body mass index (kg/m2; range) | 33 (27–36) | 32 (26–36) | 33 (25–37) | 32 (24–37) | p > 0.05 |
Fig. 1.
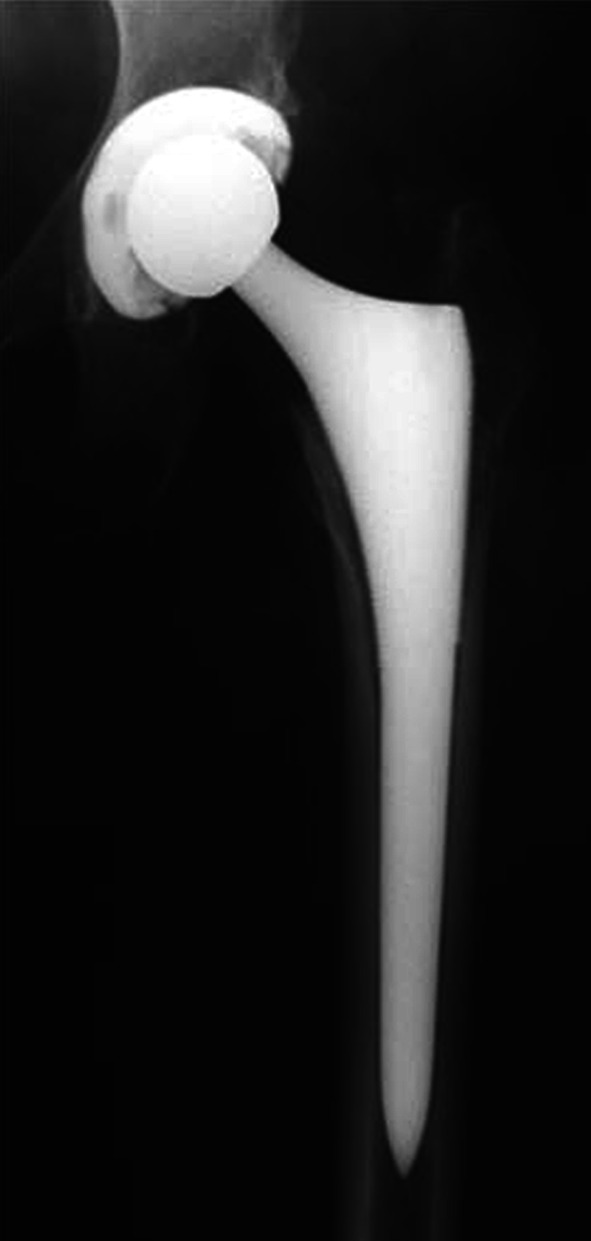
Satisfactory clinical and radiological outcome of a Synergy-Reflexion (32-mm oxidized zirconium on XLPE) cementless THA at 10 years followup is shown. No signs of osteolysis are detected.
At the latest followup (mean, 9 years; range, 7–12 years), there were 188 (188 hips) patients available for assessment (Table 2). Four (2%) patients died for reasons unrelated to surgery and seven (3.5%) were lost to followup. Twenty patients (44.5%) of Group A, 21 (45.7%) of Group B, 23 (47.9%) of Group C, and 22 (44.9%) of Group D had completed 10 years followup.
Table 2.
Patients’ data
| Study groups | Group A 28-mm ceramic on CPE |
Group B 28-mm ceramic on XLPE |
Group C 28-mm oxidized zirconium on XLPE |
Group D 28-mm oxidized zirconium on XLPE |
|---|---|---|---|---|
| Number of hips available | 45 | 46 | 48 | 49 |
| Clinical rating scores | Preoperative | Preoperative | Preoperative | Preoperative |
| Postoperative | Postoperative | Postoperative | Postoperative | |
| HHS (mean; range) | 45 (28–60) | 48 (30–57) | 51 (26–64) | 46 (25–55) |
| 92 (62–100) | 91 (58–100) | 94 (65–100) | 90 (55–100) | |
| Oxford Hip Score | 17 ± 8 | 17 ± 8 | 18 ± 8 | 18 ± 8 |
| 37 ± 8 | 38 ± 8 | 38 ± 8 | 38 ± 8 | |
| Linear wear (mean ± SD) (μm) | Cup inclination | |||
| 40°–50° | 40°–50° | 40°–50° | 40°–50° | |
| > 50° | > 50° | > 50° | > 50° | |
| 2 years | 1051 ± 260 | 985 ± 210 | 733 ± 174 | 700 ± 168 |
| 1068 ± 478 | 1010 ± 380 | 831 ± 198 | 828 ± 174 | |
| 5 years | 1478 ± 180 | 2356 ± 285 | 635 ± 155 | 650 ± 164 |
| 1723 ± 433 | 2489 ± 410 | 844 ± 190 | 891 ± 201 | |
| 10 years | 1700 ± 305 | 2750 ± 345 | 700 ± 162 | 695 ± 162 |
| 2322 ± 485 | 3111 ± 399 | 880 ± 210 | 899 ± 188 | |
| Linear wear rate (μm/year) | ||||
| 2 years | 522 ± 140 | 475 ± 120 | 348 ± 087 | 341 ± 85 |
| 530 ± 231 | 495 ± 285 | 403 ± 101 | 406 ± 97 | |
| 5 years | 260 ± 58 | 382 ± 78 | 122 ± 38 | 124 ± 34 |
| 341 ± 82 | 470 ± 277 | 163 ± 45 | 177 ± 52 | |
| 10 years | 158 ± 52 | 243 ± 64 | 68 ± 22 | 65 ± 23 |
| 210 ± 64 | 299 ± 287 | 85 ± 37 | 82 ± 41 | |
| Volumetric wear (mm3) | ||||
| 2 years | 53.6 ± 11.5 | 72.3 ± 12.8 | 34.2 ± 5.9 | 31.1 ± 6.5 |
| 5 years | 79.1 ± 12.4 | 142.0 ± 22.3 | 31.0 ± 5.5 | 32.0 ± 5.8 |
| 10 years | 89.5 ± 13.1 | 162.5 ± 25.3 | 35.6 ± 6.4 | 35.4 ± 5.0 |
| Volumetric wear rate (mm3/year) | ||||
| 2 years | 40.5 ± 11.5 | 37.9 ± 9.0 | 19.1 ± 4.5 | 24.4 ± 6.7 |
| 5 years | 30.6 ± 8.5 | 36.6 ± 8.8 | 20.6 ± 5.5 | 20.5 ± 6.1 |
| 10 years | 38.0 ± 8.0 | 48.0 ± 10.1 | 21.5 ± 5.4 | 21.0 ± 6.4 |
CPE = conventional polyethylene; XLPE = crosslinked polyethylene; HHS = Harris hip score.
Preoperative and postoperative hip scores (Harris hip score and Oxford hip score) were prospectively collected at regular intervals (preoperative, 3 weeks, 6 weeks, 3 months, 6 months, 1 year, and every year thereafter) and stored in the Orthowave database (Aria Ltd, Lyon, France). Reoperations and revisions were recorded.
For the in vivo wear study, PolyWare Auto Revision 6 computer software (Draftware Inc, Vevay, IN, USA) was used [9, 10, 27]. Every radiograph (AP and lateral) was digitized with a scanner and analyzed by the software automatically, detecting the circles that correspond with the femoral head and acetabular shell and thus their centers (dual-circle technique) (Fig. 2). The single point where an observer intervenes is to accept or reject the analysis based on contact or lack of contact of the circles with the periphery of the acetabular shell and femoral head. After acceptance of the analysis, the software extracts the results in a text file containing linear wear in millimeters, linear wear rate (mm/year), volumetric wear (mm3), and volumetric wear rate (mm3/year) (Table 2). Acetabular cup tilt and anteversion are also recorded. In the early stages, during the introduction of the method, a pilot study (20 patients) revealed satisfactory inter- and intraobserver agreement (values higher than 90%).
Fig. 2.
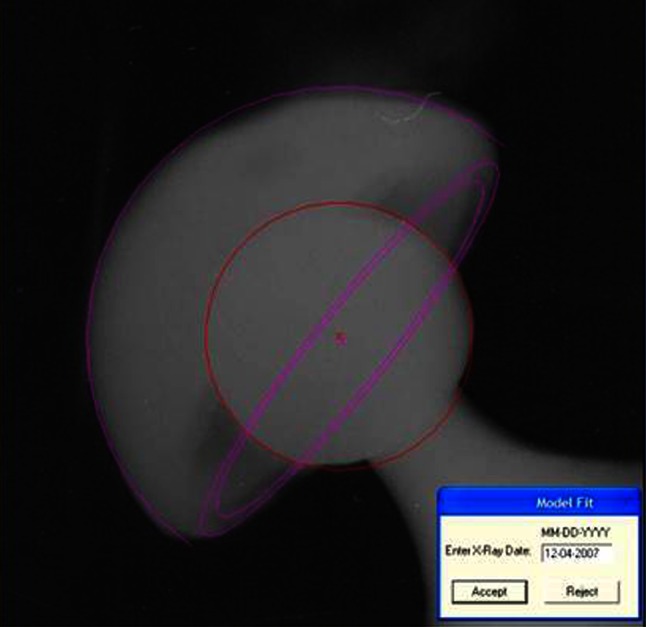
The dual-cycle PolyWare software wear analysis method is shown.
In 174 (86%) hips the acetabular cup was placed within 40° to 50° of abduction. Wear data for all cups and for those implanted with abduction greater than 50° are also shown (Table 2). As a result of the small numbers of cups with abduction greater than 50°, we did not statistically correlate cup abduction and wear rate [19].
For statistical analysis, Student’s t-test and Mann Whitney tests were used to evaluate possible statistical differences of values within and between groups of patients at different time intervals. All statistical analyses were performed using SPSS Version 12.0 (SPSS, Chicago, IL, USA) at the biostatistics department of our university. A p value ≤ 0.05 was considered to be significant.
Results
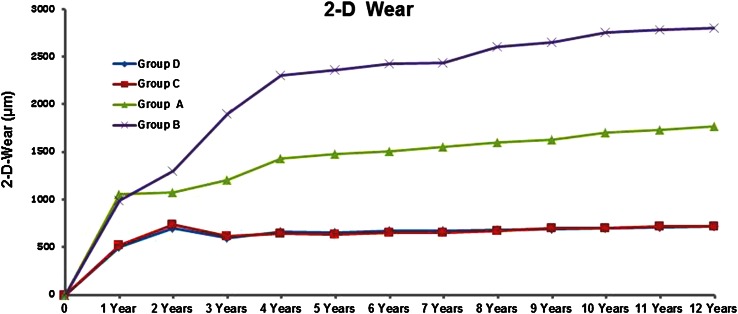
At 2, 5, and 10 years (with patients available) followup, Groups C and D (oxidized zirconium on XLPE) showed less linear wear (mean value ± SD mm) (Group C; 2 years: 733 ± 174, 5 years: 635 ± 155, 10 years: 700 ± 162, Group D; 2 years: 700 ± 168, 5 years: 650 ± 164, 10 years: 695 ± 162) when compared with Groups A (ceramic on CPE) and B (ceramic on XLPE) (Group A; 2 years: 1051 ± 260, 5 years: 1478 ± 180, 10 years: 1700 ± 305, Group B; 2 years: 985 ± 210, 5 years: 2356 ± 285, 10 years: 2750 ± 345; all p values < 0.01) (Fig. 3). At 5 and 10 years, Group A showed less linear wear when compared with Group B (all p values < 0.05). No differences with the numbers available (all p values > 0.05) were detected between Groups C and D at any of the time intervals. In all groups an initial (up to 2 years), a bedding-in effect (plastic deformation of the liner) was observed.
Fig. 3.
Two-dimensional (2-D) linear wear values in the four groups are shown. Both oxidized zirconium on XPLE groups showed lower linear wear compared with the other groups.
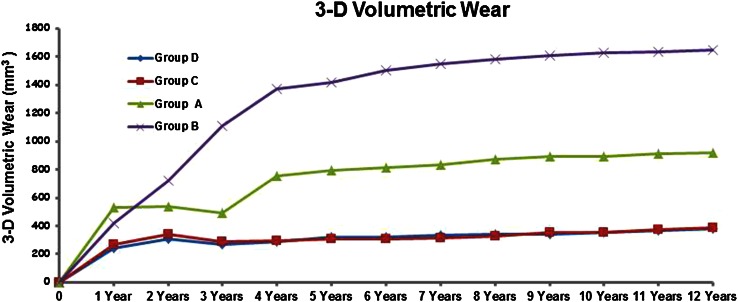
At 2, 5, and 10 years (with patients available) followup, Groups C and D (oxidized zirconium on XLPE) showed less volumetric wear (mean value ± SD mm3) (Group C; 2 years: 34.2 ± 5.9, 5 years: 31.0 ± 5.5, 10 years: 35.6 ± 6.4, Group D; 2 years: 31.1 ± 6.5, 5 years: 32.0 ± 5.8, 10 years: 35.4 ± 5.0) when compared with Groups A (ceramic on CPE) and B (ceramic on XLPE) (Group A; 2 years: 53.6 ± 11.5, 5 years: 79.1 ± 12.4, 10 years: 89.5 ± 13.1, Group B; 2 years: 72.3 ± 12.8, 5 years: 142.0 ± 22.3, 10 years: 162.5 ± 25.3; all p values < 0.05) (Fig. 4). At 5 and 10 years, Group A showed less volumetric wear when compared with Group B (all p values < 0.05). No differences with the numbers available (all p values > 0.05) were detected between Groups C and D at any of the time intervals. In all groups an initial (up to 2 years) bedding-in effect (plastic deformation) was observed.
Fig. 4.
Three-dimensional (3-D) volumetric wear values in the four groups are shown. Both oxidized zirconium on XPLE groups showed lower volumetric wear compared with the other groups.
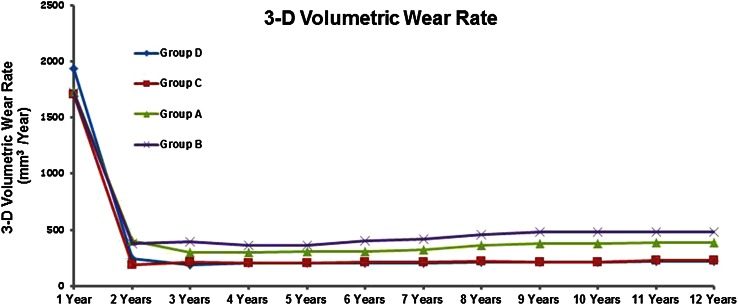
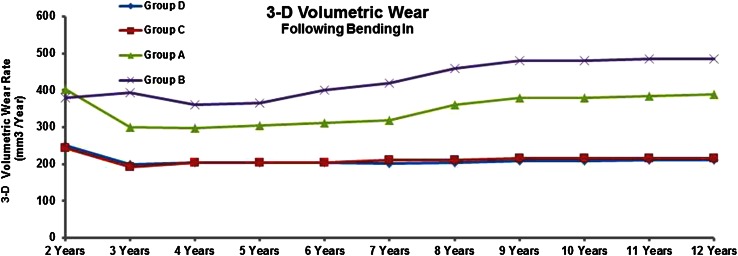
At 2, 5, and 10 years followup, Groups C and D (oxidized Oxinium on XLPE) showed less volumetric wear rate (mean values ± SD) (Group C; 2 years: 19.1 ± 4.5, 5 years: 20.6 ± 5.5, 10 years: 21.5 ± 5.4, Group D; 2 years: 24.4 ± 6.7, 5 years: 20.5 ± 6.1, 10 years: 21.0 ± 6.4) when compared with Groups A (ceramic on CPE) and B (ceramic on XLPE) (Group A; 2 years: 40.5 ± 11.5, 5 years: 30.6 ± 8.5, 10 years: 38.0 ± 8.0, Group B; 2 years: 37.9 ± 9.0, 5 years: 36.6 ± 8.8, 10 years: 48.0 ± 10.1; all p values < 0.05) (Fig. 5). At 5 and 10 years, Group A showed less volumetric wear rate when compared with Group B (all p values < 0.05). No differences (all p values > 0.05) were detected between Groups C and D at any of the time intervals with the numbers available. Volumetric wear rate values after the removal of the initial 2 years are shown (Fig. 6). In all groups, an initial (up to 2 years) bedding-in effect (plastic deformation of the liner) was observed.
Fig. 5.
Three-dimensional (3-D) volumetric wear rate values in the four groups are shown. Both oxidized zirconium on XPLE groups showed lower volumetric wear rate compared with the other groups.
Fig. 6.
Three-dimensional (3-D) wear rate values after the second postoperative year (removal of the initial bedding-in phase) in the four groups are shown. Both oxidized zirconium on XPLE groups showed lower volumetric wear rate compared with the other groups.
No differences with the numbers available between groups concerning Harris hip score and Oxford hip score were detected at final followup (all p values > 0.05; Table 2). One THA (0.41%) (Group C) was revised as a result of infection at 2 years followup. One THA (0.41%) (Group D) was revised as a result of dislocation at 4 years followup and two THAs (0.82%) (Groups A and C) were revised as a result of leg length discrepancy both at 2 years followup. In two (1%) (both Group B) asymptomatic THAs, radiological signs of wear (both cups had PolyWare linear wear rate greater than 500 μm/year at 5 years) and osteolysis were detected at 8 and 10 years followup (Fig. 7). Although both patients were advised to undergo revision surgery, these procedures had not been performed at the time of this writing.
Fig. 7.

Radiological signs of osteolysis in an asymptomatic female patient with a Synergy-Reflexion (28-mm ceramic on XLPE) cementless THA at 10 years followup are shown.
Discussion
Wear debris production and the biological response to the particles generated are perhaps the most important factors limiting the long-term survival of THA. Crosslinked polyethylene has been developed with superior wear properties to CPE [31, 32, 38]. It has been suggested that the reduction in wear debris will limit osteolysis and increase the longevity of the implants [4, 14, 26]. In addition, oxidized zirconium femoral heads have been developed; the design rationale of this surface was the combination of ceramic wear characteristics and the mechanical properties of the metallic substrate [1, 5, 16, 17]. In clinical terms, XLPE has been combined with cobalt-chrome, ceramic, and oxidized zirconium femoral heads [12, 29, 33–35]. However, the in vivo midterm wear performance of the two existing sizes [3, 7] of oxidized zirconium femoral heads has not been compared with that of ceramic heads. The objective of this study was to compare, in vivo, four wear parameters (linear wear, volumetric wear, and linear and volumetric wear rate) with four bearing couplings (made by the same manufacturer) in THA: ceramic on CPE, ceramic on XLPE, 28-mm oxidized zirconium on XLPE, and 32-mm oxidized zirconium on XLPE. We found that both 28-mm and 32-mm oxidized zirconium femoral heads on XLPE acetabular cup liners showed less linear wear, volumetric wear, and volumetric wear rate than the two other couplings at a mean of 9 years followup. No differences were found at any of the time intervals when both sizes of oxidized zirconium femoral head were compared, and no differences in hip scores were observed among the bearing couples tested.
This study is a retrospective review of prospectively collected data and therefore has a number of limitations. It cannot be considered as a prospective randomized trial despite the fact that it has been designed as such. Alternate allocation rather than true randomization renders a study susceptible to subversion of randomization, which could result in inflated treatment effect sizes of the new treatment(s), but still is considered a more robust study design than a historically controlled study or a simple case series. However, 199 (84%) patients out of the 234 patients with osteoarthritis who underwent surgery during this period of time were enrolled in the study, limiting its effect. The number of patients is relatively small and a group of patients with the bearing surface coupling of metallic (cobalt-chrome) femoral head on XLPE was not included in the study because this combination was not our practice during the years of the initiation of the study. Patients aged 65 to 75 years old were included in the study because, in our opinion, they qualify for alternative bearing surfaces. For patients older than 75 years conventional bearings and in patients younger than 65 years we consider that ceramic-on-ceramic bearing surfaces are indicated. To minimize the effect of diagnosis, only patients with osteoarthritis were included. Thus, the results of this study should apply mainly to patients with osteoarthritis whose ages are between 65 and 75 years. Another shortcoming of the study is the method of in vivo wear measurement. Despite the fact that PolyWare computer software is a clinically validated tool and superior to conventional methods in terms of accuracy and reliability, other methods such as einbildroentgenanalyse and roentgen stereophotogrammetric analysis are perhaps more accurate [6, 9–11, 20, 24, 27]. The PolyWare software was chosen because it is easier and less expensive to apply to a larger number of patients in the everyday clinical setting. Another limitation is the XLPE liners used. These were the liners provided by the Reflexion acetabular cup manufacturer (Smith & Nephew). It is now known that not all XLPE liners are the same in terms of the manufacturing process and that the clinical performance of each should be critically considered [30]. In vitro and in vivo wear rates presented by one type of XLPE liner are not necessarily reproduced in other studies. Additionally, it has been observed that in vitro wear data do not always correspond to long-term in vivo wear behavior. In this study in vivo wear rates of the conventional PE on ceramic head coupling were superior to XLPE on a ceramic head. In our opinion, this is a manufacturer-specific finding and should be considered with caution. For the Reflexion XLPE liner used in our study, a different crosslinking protocol with 10 Mrad gamma radiation at room temperature and a melting annealing step were introduced and final sterilization was performed in gas, either VHP or EtO. The effect of acetabular cup abduction and anteversion on the wear performance of the bearing surfaces has been shown by others [19]. In this study, relevant data are provided but further analysis was not performed as a result of the small numbers of outliers (Table 2). As a single-surgeon series, it was consistent in many ways, but it may not generalize to the practices of others.
A number of high-quality studies, both clinical and radiological, support the statement that XLPE liners show superior mid- and long-term clinical performance when compared with CPE liners [8, 12, 14, 26, 30, 40]. There is also strong evidence derived from head-to-head clinical comparisons of XLPE liners made by different manufacturers [44]. In most of these studies a cobalt-chrome metallic femoral head was used. Oxidized zirconium femoral heads on XLPE are associated with considerably less wear on hip wear simulators compared with cobalt-chrome femoral heads articulating to either CPE or XLPE liners even when damaged [5, 17]. Long-term clinical data related to oxidized zirconium on XLPE coupling are lacking with only a few studies showing satisfactory performance in early and midterm stages [13, 15, 29]. To the best of our knowledge, there are no studies comparing oxidized zirconium head couplings with ceramic head couplings. Comparison of the results of this study with others is also difficult as a result of the different methods and parameters used for evaluating in vivo wear. Our study started soon after the clinical introduction of oxidized zirconium technology and, to the best of our knowledge, is the in vivo wear study with the longest followup; however, because osteolysis and failures related to it generally would be expected to occur in the second decade and beyond, longer followup is called for.
Midterm data related to clinical outcome scoring systems did not show differences compared with other similar published studies, as we expected [23, 27, 28]. However, we continue to record clinical outcomes to early detect possible future adverse clinical effects of these bearing couplings.
We found that oxidized zirconium on XLPE showed the lowest wear rate and demonstrated satisfactory in vivo wear and clinical performance at a mean of 9 years. The ceramic on CPE coupling, as expected, showed higher wear than all other couplings we evaluated, all of which used XLPE. Longer followup with XLPE will be needed to ensure that no second decade complications arise with that material, but preliminary results in our study and others favor XLPE over CPE for its wear performance [30]. In our department we continue to use oxidized zirconium on XLPE bearing surfaces as a result of their satisfactory in vivo wear performance and the lack of both local and systematic biologic adverse reactions and mechanical failures. However, one has to realize that second decade clinical and in vivo wear data are needed to confirm our findings. Moreover, comparable studies including groups with a metallic head on XPLE and cost-effectiveness data are also needed.
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research ® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Allain J, Roudot-Thorval F, Delecrin J, Anract P, Migaud H, Goutallier D. Revision total hip arthroplasty performed after fracture of a ceramic femoral head: a multicenter survivorship study. J Bone Joint Surg Am. 2003;85:825–830. doi: 10.2106/00004623-200305000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Almousa SA, Greidanus NV, Masri BA, Duncan CP, Garbuz DS. The natural history of inflammatory pseudotumors in asymptomatic patients after metal-on-metal hip arthroplasty. Clin Orthop Relat Res. 2013;471:3814–3821. doi: 10.1007/s11999-013-2944-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amlie E, Hovik O, Reikeras O. Dislocation after total hip arthroplasty with 28 and 32-mm femoral head. J Orthop Traumatol. 2010;11:111–115. doi: 10.1007/s10195-010-0097-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beksac B, Salas A, Della Valle AG, Salvati E. Wear is reduced in THA performed with highly cross-linked polyethylene. Clin Orthop Relat Res. 2009;467:1765–1772. doi: 10.1007/s11999-008-0661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benezra V, Mangin S, Treska M. Microstructural investigation of the oxide scale on Zr-2.5 Nb and its interface with the alloy substrate. Mater Res Soc Symp Proc. 1999;550:337–342. doi: 10.1557/PROC-550-337. [DOI] [Google Scholar]
- 6.Borlin N, Thien T, Karrholm J. The precision of radiostereometric measurements. Manual vs digital measurements. J Biomech. 2002;35:69–79. doi: 10.1016/S0021-9290(01)00162-2. [DOI] [PubMed] [Google Scholar]
- 7.Burroughs BR, Hallstrom B, Golladay GJ, Hoeffel D, Harris WH. Range of motion and stability in total hip arthroplasty with 28, 32, 38, and 44 mm femoral head sizes: an in vitro study. J Arthroplasty. 2005;20:11–19. doi: 10.1016/j.arth.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 8.Calvert GT, Devane PA, Fielden J, Adams K, Horne JG. A double-blind, prospective, randomized controlled trial comparing highly cross-linked and conventional polyethylene in primary total hip arthroplasty. J Arthroplasty. 2009;24:505–510. doi: 10.1016/j.arth.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 9.Devane PA, Bourne RB, Rorabeck CH, Hardie RM, Horne JG. Measurement of polyethylene wear in metal-backed acetabular cups. I. Three-dimensional technique. Clin Orthop Relat Res. 1995;319:303–316. [PubMed] [Google Scholar]
- 10.Devane PA, Horne JG. Assessment of polyethylene wear in total hip replacement. Clin Orthop Relat Res. 1999;369:59–72. doi: 10.1097/00003086-199912000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Ebramzadeh E, Sangiorgio SN, Lattuada F, Kang JS, Chiesa R, McKellop HA, Dorr LD. Accuracy of measurement of polyethylene wear with use of radiographs of total hip replacements. J Bone Joint Surg Am. 2003;85:2378–2384. doi: 10.2106/00004623-200312000-00016. [DOI] [PubMed] [Google Scholar]
- 12.Fukui K, Kaneuji A, Sugimori T, Ichiseki T, Kitamura K, Matsumoto T. Wear comparison between a highly cross-linked polyethylene and conventional polyethylene against a zirconia femoral head: minimum 5-year follow-up. J Arthroplasty. 2011;26:45–49. doi: 10.1016/j.arth.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 13.Garvin KL, Hartman CW, Mangla J, Murdoch N, Martell JM. Wear analysis in THA utilizing oxidized zirconium and crosslinked polyethylene. Clin Orthop Relat Res. 2009;467:141–145. doi: 10.1007/s11999-008-0544-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glyn-Jones S, Isaac S, Hauptfleisch J, McLardy-Smith P, Murray DW, Gill HS. Does highly cross-linked polyethylene wear less than conventional polyethylene in total hip arthroplasty? A double blind, randomized, and controlled trial using roentgen stereophotogrammetric analysis. J Arthroplasty. 2008;23:337–343. doi: 10.1016/j.arth.2006.12.117. [DOI] [PubMed] [Google Scholar]
- 15.Good V, Ries M, Barrack RL, Widding K, Hunter G, Heuer D. Reduced wear with oxidized zirconium femoral heads. J Bone Joint Surg Am. 2003;85(Suppl 4):105–110. doi: 10.2106/00004623-200300004-00013. [DOI] [PubMed] [Google Scholar]
- 16.Hasegawa M, Sudo A, Uchida A. Cobalt-chromium head wear following revision hip arthroplasty performed after ceramic fracture. A case report. Acta Orthop Scand. 2006;77:833–835. doi: 10.1080/17453670610013088. [DOI] [PubMed] [Google Scholar]
- 17.Heuer D, Good V, Widding K. Wear performance of damaged oxidized Zr-2.5 Nb modular femoral heads. Soc Biomat. 2003;366:7–10. [Google Scholar]
- 18.Jasty MJ, Floyd WE, Schiller AL, Goldring SR, Harris WH. Localized osteolysis in stable, non-septic total hip replacement. J Bone Joint Surg Am. 1986;68:912–919. [PubMed] [Google Scholar]
- 19.Kadar T, Furnes O, Aamodt A, Indrekvam K, Havelin LI, Haugan K, Espehaug B, Hallan G. The influence of acetabular inclination angle on the penetration of polyethylene and migration of the acetabular component: a prospective, radiostereometric study on cemented acetabular components. J Bone Joint Surg Br. 2012;94:302–307. doi: 10.1302/0301-620X.94B3.27460. [DOI] [PubMed] [Google Scholar]
- 20.Karachalios T, Hartofilakidis G, Zacharakis N, Tsekoura M. A 12- to 18- year radiographic follow-up study of Charnley low-friction arthroplasty. Clin Orthop Relat Res. 1993;296:140–147. [PubMed] [Google Scholar]
- 21.Kwon YM, Ostlere SJ, McLardy-Smith P, Athanasou NA, Gill HS, Murray DW. Asymptomatic pseudotumors after metal-on-metal hip resurfacing arthroplasty prevalence and metal ion study. J Arthroplasty. 2011;26:511–518. doi: 10.1016/j.arth.2010.05.030. [DOI] [PubMed] [Google Scholar]
- 22.Ladon D, Doherty A, Newson R, Turner J, Bhamra M, Case CP. Changes in metal levels and chromosome aberrations in the peripheral blood of patients after metal-on-metal hip arthroplasty. J Arthroplasty. 2004;19(Suppl 3):78–83. doi: 10.1016/j.arth.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 23.Lewis PM, Moore CA, Olsen M, Schemitsch EH, Waddell JP. Comparison of mid-term clinical outcomes after primary total hip arthroplasty with Oxinium vs cobalt chrome femoral heads. J Arthroplasty. 2008;31(Suppl 2):114–122. [PubMed] [Google Scholar]
- 24.Livermore J, Ilstrup D, Morrey B. Effect of femoral head size on wear of the polyethylene acetabular component. J Bone Joint Surg Am. 1990;72:518–528. [PubMed] [Google Scholar]
- 25.Mai K, Verioti C, Ezzet KA, Copp SN, Walker RH, Colwell CW., Jr Incidence of ‘squeaking’ after ceramic-on-ceramic total hip arthroplasty. Clin Orthop Relat Res. 2010;468:413–417. doi: 10.1007/s11999-009-1083-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manning DW, Chiang PP, Martell JM, Galante JO, Harris WH. In vivo comparative wear study of traditional and highly crosslinked polyethylene in total hip arthroplasty. J Arthroplasty. 2005;20:880–886. doi: 10.1016/j.arth.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 27.Martell JM, Berdia S. Determination of polyethylene wear in total hip replacements with use of digital radiographs. J Bone Joint Surg Am. 1997;79:1635–1641. doi: 10.2106/00004623-199711000-00004. [DOI] [PubMed] [Google Scholar]
- 28.McCalden RW, MacDonald SJ, Rorabeck CH, Bourne RB, Chess DG, Charron KD. Wear rate of highly cross-linked polyethylene in total hip arthroplasty. A randomized controlled trial. J Bone Joint Surg Am. 2009;91:773–782. doi: 10.2106/JBJS.H.00244. [DOI] [PubMed] [Google Scholar]
- 29.Morison ZA, Patil S, Khan HA, Bogoch ER, Schemitsch EH, Waddell JP. A randomized controlled trial comparing Oxinium and cobalt-chrome on standard and cross-linked polyethylene. J Arthroplasty. 2014;29(Suppl):164–168. doi: 10.1016/j.arth.2014.04.046. [DOI] [PubMed] [Google Scholar]
- 30.Mu Z, Tian J, Wu T, Yang J, Pei F. A systematic review of radiological outcomes of highly cross-linked polyethylene versus conventional polyethylene in total hip arthroplasty. Int Orthop. 2008;33:599–604. doi: 10.1007/s00264-008-0716-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muratoglu OK, Greenbaum ES, Bragdon CR, Jasty M, Freiberg AA, Harris WH. Surface analysis of early retrieved acetabular polyethylene liners: a comparison of conventional and highly crosslinked polyethylenes. J Arthroplasty. 2004;19:68–77. doi: 10.1016/j.arth.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 32.Muratoglu OK, Merrill EW, Bragdon CR, O’Connor D, Hoeffel D, Burroughs B, Jasty M, Harris WH. Effect of radiation, heat, and aging on in vitro wear resistance of polyethylene. Clin Orthop Relat Res. 2003;417:253–262. doi: 10.1097/01.blo.0000093004.90435.d1. [DOI] [PubMed] [Google Scholar]
- 33.Mutimer J, Devane PA, Adams K, Horne JG. Highly crosslinked polyethylene reduces wear in total hip arthroplasty at 5 years. Clin Orthop Relat Res. 2010;468:3228–3233. doi: 10.1007/s11999-010-1379-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakahara I, Nakamura N, Nishii T, Miki H, Sakai T, Sugano N. Minimum five-year follow-up wear measurement of Longevity highly cross-linked polyethylene cup against cobalt-chromium or zirconia heads. J Arthroplasty. 2010;8:1182–1187. doi: 10.1016/j.arth.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 35.Rissanen P, Aro S, Slatis P, Sintonen H, Paavolainen P. Health and quality of life before and after hip or knee arthroplasty. J Arthroplasty. 1995;10:169–175. doi: 10.1016/S0883-5403(05)80123-8. [DOI] [PubMed] [Google Scholar]
- 36.Ritter MA, Albohm MJ, Keating EM, Faris PM, Meding JB. Comparative outcomes of total joint arthroplasty. J Arthroplasty. 1995;10:737–741. doi: 10.1016/S0883-5403(05)80068-3. [DOI] [PubMed] [Google Scholar]
- 37.Santavirta S, Boehler M, Harris WH, Konttinen YT, Lappalainen R, Muratoglu O, Rieker C, Salzer M. Alternative materials to improve total hip replacement tribology. Acta Orthop Scand. 2003;74:380–388. doi: 10.1080/00016470310017668. [DOI] [PubMed] [Google Scholar]
- 38.Tai SM, Munir S, Walter WL, Pearce SJ, Walter WK, Zicat BA. Squeaking in large diameter ceramic-on-ceramic bearings in total hip arthroplasty. J Arthroplasty. 2015;30:282–285. doi: 10.1016/j.arth.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 39.Thomas GE, Simpson DJ, Mehmood S, Taylor A, McLardy-Smith P, Gill HS, Murray DW, Glyn-Jones S. The seven-year wear of highly cross-linked polyethylene in total hip arthroplasty: a double-blind, randomized controlled trial using radiostereometric analysis. J Bone Joint Surg Am. 2011;93:716–722. doi: 10.2106/JBJS.J.00287. [DOI] [PubMed] [Google Scholar]
- 40.Traina F, De Fine M, Bordini B, Toni A. Risk factors for ceramic liner fracture after total hip arthroplasty. Hip Int. 2012;22:607–614. doi: 10.5301/HIP.2012.10339. [DOI] [PubMed] [Google Scholar]
- 41.Urban JA, Garvin KL, Boese CK, Bryson K, Pedersen DR, Callaghan JJ, Miller RK. Ceramic-on-polyethylene bearing surfaces in total hip arthroplasty: seventeen to twenty-one-year results. J Bone Joint Surg Am. 2001;83:1688–1694. doi: 10.2106/00004623-200111000-00011. [DOI] [PubMed] [Google Scholar]
- 42.Walter A. On the material and the tribology of alumina-alumina couplings for hip joint prostheses. Clin Orthop Relat Res. 1992;282:31–46. [PubMed] [Google Scholar]
- 43.Whittaker JP, Charron KD, McCalden RW, Macdonald SJ, Bourne RB. Comparison of steady state femoral head penetration rates between two highly cross-linked polyethylenes in total hip arthroplasty. J Arthroplasty. 2010;25:680–686. doi: 10.1016/j.arth.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 44.Willert H, Bertram H, Buchhorn G. Osteolysis in alloarthroplasty of the hip: the role of bone cement fragmentation. Clin Orthop Relat Res. 1990;258:108–121. [PubMed] [Google Scholar]
- 45.Willert H, Bertram H, Buchhorn G. Osteolysis in alloarthroplasty of the hip: the role of ultra high molecular weight polyethylene wear particles. Clin Orthop Relat Res. 1990;258:95–107. [PubMed] [Google Scholar]
- 46.Willert HG, Semlitsch M. Tissue reactions to plastic and metallic wear products of joint endoprostheses. Clin Orthop Relat Res. 1996;333:4–14. [PubMed] [Google Scholar]