Abstract
Background
Osteoarthritis may result from abnormal mechanics leading to biochemically mediated degradation of cartilage. In a dysplastic hip, the periacetabular osteotomy (PAO) is designed to normalize the mechanics and our initial analysis suggests that it may also alter the cartilage biochemical composition. Articular cartilage structure and biology vary with the depth from the articular surface including the concentration of glycosaminoglycans (GAG), which are the charge macromolecules that are rapidly turned over and are lost in early osteoarthritis. Delayed gadolinium-enhanced magnetic resonance imaging of cartilage (dGEMRIC) enables noninvasive measurement of cartilage GAG content. The dGEMRIC index represents an indirect measure of GAG concentration with lower values indicating less GAG content. GAG content can normally vary with mechanical loading; however, progressive loss of GAG is associated with osteoarthritis. By looking at the changes in amounts of GAG in response to a PAO at different depths of cartilage, we may gain further insights into the types of biologic events that are occurring in the joint after a PAO.
Questions/purposes
We (1) measured the GAG content in the superficial and deep zones for the entire joint before and after PAO; and (2) investigated if the changes in the superficial and deep zone GAG content after PAO varied with different locations within the joint.
Methods
This prospective study included 37 hips in 37 patients (mean age 26 ± 9 years) who were treated with periacetabular osteotomy for symptomatic acetabular dysplasia and had preoperative and 1-year follow up dGEMRIC scans. Twenty-eight of the 37 also had 2-year scans. Patients were eligible if they had symptomatic acetabular dysplasia with lateral center-edge angle < 20° and no or minimal osteoarthritis. The change in dGEMRIC after surgery was assessed in the superficial and deep cartilage zones at five acetabular radial planes.
Results
The mean ± SD dGEMRIC index in the superficial zone fell from 480 ± 137 msec preoperatively to 409 ± 119 msec at Year 1 (95% confidence interval [CI], −87 to −54; p < 0.001) and recovered to 451 ± 115 msec at Year 2 (95% CI, 34–65; p < 0.001), suggesting that there is a transient event that causes the biologically sensitive superficial layer to lose GAG. In the deep acetabular cartilage zone, dGEMRIC index fell from 527 ± 148 msec preoperatively to 468 ± 143 msec at Year 1 (95% CI, −66 to −30; p < 0.001) and recovered to 494 ± 125 msec at Year 2 (95% CI, 5–32; p = 0.008). When each acetabular radial plane was looked at separately, the change from before surgery to 1 year after was confined to zones around the superior part of the joint. The only significant change from 1 to 2 years was an increase in the superficial layer of the superior zone (1 year 374 ± 123 msec, 2 year 453 ± 117 msec, p < 0.006).
Conclusions
This study suggests that PAO may alter the GAG content of the articular cartilage with a greater effect on the superficial zone compared with the deeper acetabular cartilage zone, especially at the superior aspect of the joint. Some surgeons have observed that surgery itself can be a stressor that can accelerate joint degeneration. Perhaps the decrease in dGEMRIC index seen in the superficial layer may be a catabolic response to postsurgical inflammation given that some recovery was seen at 2 years. The decrease in dGEMRIC index in the deep layer seen mainly near the superior part of the joint is persistent and may represent a response of articular cartilage to normalization of increased mechanical load seen in this region after osteotomy, which may be a normal response to alteration in loading.
Clinical Relevance
This study looks at the biochemical changes in the articular cartilage before and after a PAO for dysplastic hips using MRI in a similar manner to using histological methods to study alterations in articular cartilage with mechanical loading. Although PAO alters alignment and orientation of the acetabulum, its effects on cartilage biology are not clear. dGEMRIC provides a noninvasive method of assessing these effects.
Introduction
Articular cartilage is a biologically active, relatively acellular complex tissue that provides near frictionless joint motion that is crucial for long-term function of diarthrodial joints [28]. The glycosaminoglycans (GAG) trapped within the collagen fibrils are negatively charged and generate swelling pressures, which carry the compressive load of the joint [21, 22]. Loss of GAG is one of the earliest events in cartilage degeneration. Histologically, the articular cartilage has a zonal organization where both the GAG content and structure of the collagen fibrils vary through the depth of the tissue [9, 16, 20]. In normal articular cartilage there are three major zones based on the orientation of collagen fibers: superficial, mid-, and deep layer [29], which can be distinguished on MR images [27]. In the superficial zone, the collagen fibrils are arranged parallel to the articular surface, whereas in the deeper zone, they are laid out perpendicular to the underlying bone [1, 16]. In addition to the variation in matrix structure, the volume density of chondrocytes and biosynthetic activity varies with the depth of the cartilage [17, 34, 43]. Many studies have shown that mechanical loading of articular cartilage affects the metabolism of chondrocytes and its biochemical composition [1, 32, 37]. Both animal and human studies have shown that the GAG content is higher in cartilage that is habitually loaded [19, 33] or has a higher level of activity [39], whereas immobilization results in a reversible decrease in cartilage PG content [18, 30].
Compositional MRI techniques such as delayed gadolinium-enhanced MRI of cartilage (dGEMRIC), T2, T1rho, T2*, gagCEST, and sodium imaging all attempt to measure the biochemical composition of cartilage [12]. T2, T1rho, and dGEMRIC have been studied extensively in the knee, whereas most studies in the hip, to date, used dGEMRIC. In dGEMRIC, a negatively charged contrast agent, gadolinium, is injected intravenously. Because of its negative charge, gadolinium will be repelled by tissues that also have negative charges such as cartilage with high GAG content. However, gadolinium will accumulate in areas with low charge meaning that lower GAG concentration leads to higher contrast agent distribution. This can be measured using MRI [2–4, 6]. Lattanzi et al [24] reported accuracy of 58%, sensitivity of 52%, and specificity of 67% in detecting cartilage damage in femoroacetabular impingement using dGEMRIC.
In acetabular dysplasia (AD) [11], an underdeveloped or shallow acetabulum leads to smaller contact areas and higher contact pressures in the hip compared with healthy subjects [25, 26, 40]. Periacetabular osteotomy (PAO), a hip-preserving procedure, is performed to restore normal anatomy and has been shown to evenly distribute stresses through the hip during weightbearing [45]. By simulating a reorientation pelvic osteotomy, it has been demonstrated that up to a 50% decrease in contact pressure can be achieved [15]. Given the importance of the weightbearing status on the biology of chondrocytes [23, 31] and the heterogeneous structure of cartilage [7], our study investigated whether the mechanical modulation of the dysplastic joint with a PAO affects the biochemical composition of articular cartilage and whether this effect varies with the depth of cartilage. The superficial cartilage layer has been shown to have a low charge density, low compressive stiffness [8], and to be less protected by inhibitors to degradation [13], which suggest that this region may be more vulnerable. A better understanding of the biologic changes in articular cartilage with a pelvic osteotomy may help us better predict the long-term health of the joint.
Therefore, we wished to (1) investigate if the superficial zone behaves differently than the deep zone after PAO given that the two layers are histologically and biologically different; and (2) if the superficial and deep zones behave the same way in different areas of the joint.
Materials and Methods
This is a prospective single-group longitudinal study. Institutional review board approval was obtained before the start of enrollment.
This cohort was used in a prior paper analyzing the clinical outcomes and dGEMRIC indices at the acetabular and femoral cartilage after PAO [14]. The present article is looking specifically at the effect of the PAO in the superficial and deeper cartilage zones.
Study Population
Inclusion criteria were the following: the diagnosis of acetabular dysplasia with a lateral center-edge angle smaller than 20° and the presence of hip-related pain. Less than 90° flexion, signs of advanced osteoarthritis (Tönnis Grade II or more [25]) as well as neuromuscular disorders or chromosomal disorders were exclusion criteria. Also the presence of an incongruous hip (“fair” or “poor” in the Yasunaga classification [44]) as seen on von Rosen view [41] was an exclusion criterion.
A total of 136 patients were screened during the study period and 53 patients were enrolled in the study. Of 53 patients, 16 patients did not return for their 1-year dGEMRIC scans; therefore, 37 patients (37 hips) constituted the study cohort. Patients were scheduled for visits preoperatively (baseline), at 6 months, 12 months, and 24 months after surgery. Of the 37 patients, 28 had 2-year followup with measurements at all three visits, whereas the other nine patients had only 1 year of followup.
Of the 37 patients, 34 were female and three were male. Thirty-four patients were white, one was black, and two were Asian. The mean age (± SD) at the time of the preoperative MRI was 26 ± 9 years (range, 13–46 years). Twenty-seven (73%) were right hips and 10 were left hips. Twenty-eight of the 37 patients had 2 years of followup with measurements at all three visits, whereas the other nine patients had only 1 year of followup. There were no differences in preoperative or 1-year dGEMRIC indices between subjects with 1 year of followup and subjects with 2 years of followup (Table 1). There was also no difference in the change in mean dGEMRIC T1 index from preoperative to 1-year followup across the differing followup groups.
Table 1.
Summary of dGEMRIC T1 values (msec) separated by subjects with complete followup and those who missed their 2-year followup visit
| Patients with 1 year of followup (n = 9) | Patients with 2 years of followup (n = 28) | p value | |
|---|---|---|---|
| Superficial zone | |||
| Preoperative | 496 (± 140) | 474 (± 137) | 0.38 |
| Year 1 | 436 (± 140) | 401 (± 111) | 0.13 |
| Year 2 | 451 (± 115) | ||
| Deep zone | |||
| Preoperative | 558 (± 123) | 579 (± 150) | 0.54 |
| Year 1 | 551 (± 163) | 518 (± 132) | 0.18 |
| Year 2 | 537 (± 121) | ||
Plus/minus values are between-subject SDs of the total dGEMRIC values, ie, averaged over all 10 regions measured within each patient; p values describe the significance of the difference in measurements between subjects with only 1 year of followup versus those with 2 years of followup; dGEMRIC = delayed gadolinium-enhanced MRI of cartilage.
Magnetic Resonance Imaging
In all cases a standardized protocol was used for dGEMRIC [6]. dGEMRIC was performed on a single 1.5-Tesla system (Magnetom Avanto; Siemens Healthcare, Erlangen, Germany) with a flexible surface coil. After intravenous gadolinium injection (0.2 mM/kg gadolinium-DTPA2-; Magnevist®; Berlex/Bayer HealthCare Pharmaceuticals Inc, Wayne, NJ, USA), patients walked for a minimum of 15 minutes to accelerate contrast diffusion into the hip. The MR scans started approximately 22 minutes after contrast administration and the isotropic T1 mapping sequence at a mean of 48 minutes after Gd-DTPA2- (SD, 12 minutes; range, 30–70 minutes).
A three-dimensional isotropic gradient echo dual-flip angle T1 mapping sequence [10, 38] was used to obtain the T1 map with the following parameters: repetition time 15 ms, echo time 4.68 ms, flip angles of 5° and 28°, a matrix size of 192 × 192, 160 × 160-mm field of view, 96 slices, isotropic voxel size 0.83 mm, and acquisition time of 6 minutes 51 seconds. An isotropic True-FISP sequence was used for morphological images with the following parameters: repetition time 12.57 ms, echo time 5.48 ms, a matrix size of 256 × 256, 160 × 160-mm field of view, 144-slice slab, and an isotropic voxel size of 0.63 mm. The scan time was 7 minutes 47 seconds.
Both the isotropic data sets of the T1 mapping and True-FISP sequence were acquired in the oblique axial plane and used for radial reconstruction and evaluation on a Leonardo workstation (Siemens Healthcare, Erlangen, Germany). As described in other studies [5, 10], we reconstructed five acetabular radial reformats in 30° steps (Fig. 1) based on anatomic landmarks of the acetabulum. This allows us to look at the same regions even after a rotational pelvic osteotomy. The reformats allowed image evaluation at the following positions: anterior (A), anterosuperior (AS), superoanterior (SA), superior (S), and superoposterior (SP). Their slice thickness was 3.0 mm for the T1 mapping and 1.2 mm for the True-FISP data set.
Fig. 1.
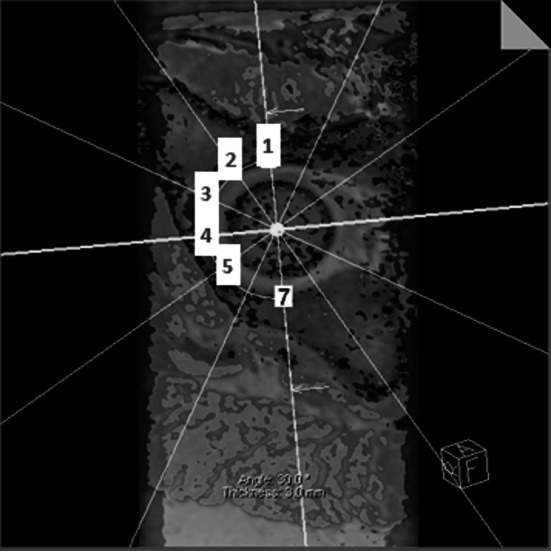
Five radial reformats around the acetabular opening are generated from three-dimensional MRI: Anterior (1), AS (2), SA (3), S (4), SP (5) planes around the acetabulum are generated by first identifying the transverse acetabular ligament, which is used as the reference landmark. The superior plane goes through the middle of the transverse ligament and the remaining planes rotate in 30º steps around the acetabular opening axis.
dGEMRIC Evaluation
The region of interest (ROI) evaluation was performed manually by a trained reader (AMH) on anonymized T1 data sets. The superficial zone of the femoral and acetabular cartilage was combined to one ROI and defined as the superficial zone. A ROI of equal size was then selected for the subsequent deeper acetabular cartilage and defined as the deeper zone (Fig. 2).
Fig. 2.
Schematic illustration shows the dGEMRIC ROI evaluation.
The ROI was selected, in direct comparison to morphological images, in the weightbearing area, defined by the acetabular rim as the peripheral border and the acetabular fossa as the central border.
Interobserver variability for this type of evaluation has been shown to be excellent with intraclass correlation coefficients of 0.92 [5, 10]. Counting all five radial reformats, we evaluated 370 ROIs preoperatively, 370 ROIs at 12-month, and 280 ROIs at 24-month followup.
Statistical Analysis
To evaluate the differences in the superficial and deeper cartilage zones, dGEMRIC T1 indices from the five radial planes in both the superficial and deep zones from each patient were analyzed. Changes in dGEMRIC T1 measurements were evaluated at three time points (preoperative, 1-year followup, and 2-year followup) during the study using mixed model analysis. For each time point, each subject had a possible 10 measurements (five radial plane measurements in the superficial zone and five radial plane measurements in the deep zone). Mixed model analysis was used to describe the change in dGEMRIC T1 values while accounting for within-subject correlations as a result of repeated measures on each subject. All data were analyzed assuming an autoregressive correlation structure for the covariate matrix and under the assumption that any missing data were missing at random. Reported SDs are between-subject SDs of the total dGEMRIC values that were obtained by averaging over all 10 regions measured within each patient. To assess whether loss of followup was dependent on baseline or 1-year measurement, dGEMRIC T1 indices were compared between subjects who were missing their second-year followup visit and subjects with complete 2-year followup information using intercept-only mixed models (Table 1). Secondary analysis was conducted to assess variation in dGEMRIC T1 measurement across the radial plane of the acetabulum. Multivariate linear mixed models were used to analyze the change in dGEMRIC T1 index between the superficial and deep zones across the five radial planes (A, AS, SA, S, and SP) at each of the three time points. All tests were two-sided and p values < 0.05 were considered significant. Analyses were performed using SAS software Version 9.3 (SAS Institute Inc, SAS, Cary, NC, USA).
Results
Changes in dGEMRIC Index: Superficial versus Deep Layers
GAG content, as measured by dGEMRIC, was lower in both the superficial and deep zones 1 year after surgery and recovered slightly by Year 2. Greater fluctuation in dGEMRIC index was seen in the superficial zone (Table 2), falling from a mean (± SD) of 480 (± 137 msec) preoperatively to 409 (± 119 msec) (p < 0.001) at Year 1 and recovering to 451 (± 115 msec) at Year 2 (p < 0.001). In the deep zone, the dGEMRIC index fell from 574 (± 142 msec) preoperatively to 526 (± 140 msec) at Year 1 (p < 0.001) and recovered to 537 (± 121 msec) at Year 2 (p = 0.008). However, over the entire 2-year span of the study, dGEMRIC index decreased in the superficial zone by an average of 22 msec (95% confidence interval [CI], −40 to −4; p = 0.02), whereas in the deep zone, dGEMRIC index decreased by an average of 36 msec (95% CI, −56 to −15; p < 0.001). An example of a patient scan is shown (Fig. 3). The superficial zone could be seen with a substantial decrease at 1-year postoperative scan compared with the preoperative scan.
Table 2.
Summary of dGEMRIC T1 values and mixed model estimated slopes for all patients (n = 37) representing the change in T1 (msec) for each time point by superficial and deep zone
| Time point | Superficial zone | Deep zone | |||
|---|---|---|---|---|---|
| Mean | (± SD) | Mean | (± SD) | p value | |
| Preoperative | 480 | (± 137) | 574 | (± 142) | < 0.001 |
| Year 1 | 409 | (± 119) | 526 | (± 140) | < 0.001 |
| Year 2 | 451 | (± 115) | 537 | (± 121) | < 0.001 |
| Change* | [95% CI] | p value | Change* | [95% CI] | p value | |
|---|---|---|---|---|---|---|
| Preoperative to Year 1 | −70 | [−87 to −54] | < 0.001 | −48 | [−66 to −30] | < 0.001 |
| Year 1 to Year 2 | 49 | [34–65] | < 0.001 | 18 | [5–32] | 0.008 |
| Preoperative to year 2 | −22 | [−40 to −4] | 0.02 | −36 | [−56 to −15] | < 0.001 |
*Change is the model-estimated change in T1 values between indicated time points; p values in the upper portion of the table describe the significance of the difference between superficial and deep zone measurements and p values in the lower portion of the table describe the significance of the change in T1 measurement estimated by the mixed model(s); dGEMRIC = delayed gadolinium-enhanced MRI of cartilage; CI = confidence interval.
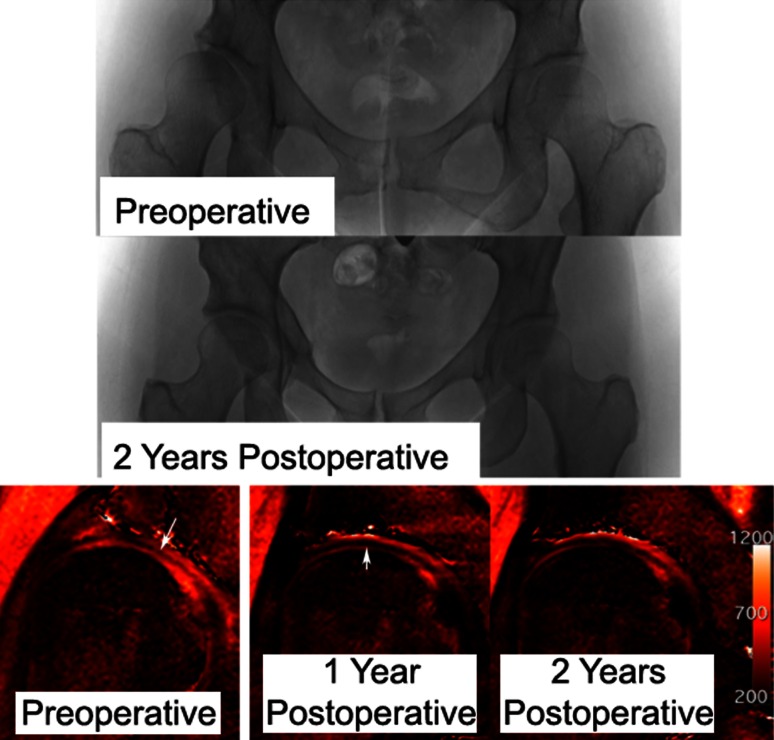
Fig. 3.
A 40-year-old woman underwent right PAO for symptomatic dysplasia. Postoperative radiographs show good correction with no progression of osteoarthritis in 2 years. The preoperative dGEMRIC scan shows the superficial zone with lower dGEMRIC index, as indicated by the arrow. The 1-year postoperative scan shows the same region (arrow) with a lower dGEMRIC index in the superficial zone. The 2-year scan shows perhaps some recovery of the dGEMRIC index in the superficial zone.
Variation in dGEMRIC Index: Across the Radial Planes
In the differing acetabular planes of the hip, the superior region superficial layer dGEMRIC index had the most variation after surgery. dGEMRIC index varied across the radial planes in both the superficial (p < 0.001) and deep acetabular zones (p < 0.001) for each of the three time points in the study (Fig. 4). In the superficial zone, decreases were seen in dGEMRIC index at the AS plane (p = 0.008), the SA plane (p < 0.001), and the S plane (p < 0.001) from preoperative to 1-year followup and no change was detected at the A (p = 0.28) or SP (p = 0.45) planes. In the deep zone, there was a decrease in dGEMRIC measurement from preoperative to 1-year followup in the SP plane (p = 0.03) and in the S plane (p = 0.01), but there were no changes in the A (p = 0.20), SA (p = 0.06), or AS (p = 0.60) planes. From 1-year to 2-year followup, the only detected change in dGEMRIC index was an increase in the superficial zone at the S plane (p = 0.006). No changes were detected in any of the other radial planes in the superficial zone (p = 0.26–0.80). There were no differences observed in the deep acetabular zone across any of the radial planes (p = 0.43–0.86) from 1-year to 2-year followup. From preoperative to 2 years, an overall decrease in dGEMRIC was detected in the superficial zone only at the S (p = 0.003) and SA (p = 0.002) planes (Table 3). In the deep zone, a decrease from preoperative to 2 years was found only at the SP (p = 0.009) and S (p = 0.008) planes.
Fig. 4.
Average T1 values (dGEMRIC index) at radial planes in the superficial and deep zones at all visits are shown.
Table 3.
Summary of dGEMRIC T1 values (msec) by radial plane at the superficial and deep zones
| Radial plane | Deep zone | |||
|---|---|---|---|---|
| Preoperative | Year 1 | Year 2 | p value | |
| A | 516 (± 124) | 485 (± 131) | 503 (± 113) | 0.59 |
| AS | 537 (± 146) | 513 (± 149) | 532 (± 117) | 0.50 |
| SA | 611 (± 148) | 554 (± 154) | 565 (± 128) | 0.06 |
| S | 607 (± 128) | 544 (± 130) | 549 (± 124) | 0.008 |
| SP | 598 (± 143) | 536 (± 132) | 536 (± 121) | 0.009 |
| Radial plane | Superficial zone | |||
|---|---|---|---|---|
| Preoperative | Year 1 | Year 2 | p value | |
| A | 392 (± 116) | 374 (± 113) | 396 (± 120) | 0.79 |
| AS | 482 (± 139) | 422 (± 124) | 455 (± 105) | 0.14 |
| SA | 532 (± 133) | 426 (± 109) | 472 (± 109) | 0.002 |
| S | 510 (± 129) | 374 (± 123) | 453 (± 117) | 0.003 |
| SP | 481 (± 133) | 451 (± 113) | 479 (± 113) | 0.75 |
Data are presented as mean (± SD); p values represent the change in measurement from preoperative to 2-year followup based on LMM analysis; dGEMRIC = delayed gadolinium-enhanced MRI of cartilage; A = anterior; AS = anterosuperior; SA = superoanterior; S = superior; SP = superoposterior.
Discussion
The long-term goal of PAO for acetabular dysplasia is to preserve hip function. It is presumed that normalization of the abnormal mechanics with improved coverage of the femoral head will alter the initial normal biologic response (hypertrophic) and then abnormal response (catabolic) of the articular cartilage to the abnormally increased mechanical load. This type of response is certainly seen in tissue culture and animal studies of cartilage response to mechanical loading. Our initial analysis of this cohort [14] supports this hypothesis by demonstrating that in areas with increased mechanical load, the charge density of the acetabular cartilage as measured by dGEMRIC decreased to normal levels after osteotomy. This interpretation of our findings seems quite consistent with what we know about cartilage mechanoregulation; however, because we visually inspect the data, we noticed that the changes in appearance of the superficial layer seemed different from the deep layer. Attributable to the fact that in vitro studies have shown that there are depth-related differences in mechanical behavior [8, 42] and biochemical properties [17, 36] in response to compressive loading of articular cartilage, we have decided to analyze our data further. Therefore, our goals were twofold: (1) to compare the response of the superficial layer cartilage to that of the deep layer after PAO in dysplastic hips; and (2) to assess whether these different responses were confined to the acetabular region that sees alteration in mechanical load.
The present study has some limitations. First, because we combined the superficial zones of the acetabular and femoral cartilage in one ROI as a result of the thin articular cartilage layer, we included the potential space between the two cartilage layers, where the synovial fluid is considered to have the potential to affect the dGEMRIC index. To overcome this limitation we evaluated all MRI reformats for the presence of synovial fluid. However, no synovial fluid layer could be detected on a single morphological True-FISP reformat, most likely as a result of the extreme thinness of this layer. Furthermore, it has been reported that the protein composition of the superficial layer is almost indistinguishable from that of synovial fluid [9]; therefore, we considered that our approach is reasonable for evaluating the superficial zone. Second, the lack of a control group (natural history group) is a limitation. Finally, we have performed inter- and intraobserver reliability studies for similar analysis in other studies; however, we did not do so specifically for this study.
Chen et al [8] investigated the relationship between compressive properties of the different cartilage layers and their fixed charge density, which reflects the amount of GAG. They found lower fixed charge density in the region near the articular surface, which was associated with lower compression properties. This can be confirmed by our results, demonstrating lower dGEMRIC values in the superficial compared with the deeper zones at all visits. Furthermore, similar results have also been found for bovine knee cartilage [34, 35], suggesting that the extent of the depth dependence of compressive properties with the lowest values near the articular surface may be a fundamental property for tissue with normal biomechanical function.
Over time, we found a decrease in mean dGEMRIC index in both superficial and deep zones at 1-year followup, which appears to recover somewhat at the 2-year followup. On closer inspection, the biochemical changes in cartilage after PAO were greater in the superficial than in the deeper cartilage zone, suggesting a higher response of the superficial zone compared with the deeper zone to changes in biomechanics or the possible nonbiomechical factors including the inflammatory process in the joint after surgery. This confirms the findings of several other authors, indicating depth-related properties and metabolic specialization of the resident chondrocytes leading to characteristic zonal variations in cartilage adaption to mechanical loading [9, 36, 43]. In addition, the unique depth-dependent features and high vulnerability of the superficial layer have been demonstrated in a study investigating the effect of inflammatory cytokines in different cartilage layers [13]. They reported at least a 10-fold greater concentration of interleukin-1α for a similar inhibition of PG synthesis in cells from the deeper layer compared with the superficial layers.
When the variation in dGEMRIC indices were investigated further by looking across the five radial planes, the most responsive region was seen mostly in the superficial layer of the superior part of the acetabulum, whereas in the deep zone, no difference across the radial planes could be detected from 1- to 2-year followup. We hypothesized that postoperative protected weightbearing, inflammation, and overall normalization of mechanical load within the joint will affect the biosynthetic activity of chondrocytes [32, 42] and thus leads to characteristic changes in the dGEMRIC index at followup visits. Given that the effect of PAO was most pronounced at the superior aspect of the joint, where the mechanical forces are the greatest in patients with AD, indicates that our results may be truly reflecting the cartilage adaption to normalization in mechanical loading [45].
Our study has shown that the superficial zone does have a consistently lower charge density than the deep zone, which is the normal variation seen in normal cartilage. In addition, it appears that the superficial zone is more sensitive to alteration in biomechanics of the hip after PAO compared with the deep zones. In both the deep and superficial zones, there was an initial decrease seen at 1 year, which partially recovered at 2 years. The magnitude of change was bigger in the superficial zone. In both the superficial and deep zones, most of the change in dGEMRIC index was confined to the acetabular region near the superior part of the joint, which is where the alteration in mechanics would be largest. Most of the change in dGEMRIC index seems consistent with the alteration in mechanics; however, the large drop in dGEMRIC index and then recovery at 2 years, especially in the superficial zone of the superior part of the acetabulum, suggests that there may be additional effects such as postoperative inflammation. Perhaps this may explain the clinical observation that some hips may have an acceleration of degeneration with surgical intervention.
Acknowledgments
We thank Patricia Connell, Jenny Chan, Catherine Matero, and Kerri Murray for their help with data acquisition.
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research ® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that the institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Akizuki S, Mow VC, Muller F, Pita JC, Howell DS, Manicourt DH. Tensile properties of human knee joint cartilage: I. Influence of ionic conditions, weight bearing, and fibrillation on the tensile modulus. J Orthop Res. 1986;4:379–392. doi: 10.1002/jor.1100040401. [DOI] [PubMed] [Google Scholar]
- 2.Bashir A, Gray ML, Boutin RD, Burstein D. Glycosaminoglycan in articular cartilage: in vivo assessment with delayed Gd(DTPA)(2-)-enhanced MR imaging. Radiology. 1997;205:551–558. doi: 10.1148/radiology.205.2.9356644. [DOI] [PubMed] [Google Scholar]
- 3.Bashir A, Gray ML, Burstein D. Gd-DTPA2- as a measure of cartilage degradation. Magn Reson Med. 1996;36:665–673. doi: 10.1002/mrm.1910360504. [DOI] [PubMed] [Google Scholar]
- 4.Bashir A, Gray ML, Hartke J, Burstein D. Nondestructive imaging of human cartilage glycosaminoglycan concentration by MRI. Magn Reson Med. 1999;41:857–865. doi: 10.1002/(SICI)1522-2594(199905)41:5<857::AID-MRM1>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 5.Bittersohl B, Hosalkar HS, Werlen S, Trattnig S, Siebenrock KA, Mamisch TC. dGEMRIC and subsequent T1 mapping of the hip at 1.5 Tesla: normative data on zonal and radial distribution in asymptomatic volunteers. J Magn Reson Imaging. 2011;34:101–106. [DOI] [PubMed]
- 6.Burstein D, Velyvis J, Scott KT, Stock KW, Kim YJ, Jaramillo D, Boutin RD, Gray ML. Protocol issues for delayed Gd(DTPA)(2-)-enhanced MRI (dGEMRIC) for clinical evaluation of articular cartilage. Magn Reson Med. 2001;45:36–41. doi: 10.1002/1522-2594(200101)45:1<36::AID-MRM1006>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 7.Burton-Wurster N, Vernier-Singer M, Farquhar T, Lust G. Effect of compressive loading and unloading on the synthesis of total protein, proteoglycan, and fibronectin by canine cartilage explants. J Orthop Res. 1993;11:717–729. doi: 10.1002/jor.1100110514. [DOI] [PubMed] [Google Scholar]
- 8.Chen SS, Falcovitz YH, Schneiderman R, Maroudas A, Sah RL. Depth-dependent compressive properties of normal aged human femoral head articular cartilage: relationship to fixed charge density. Osteoarthritis Cartilage. 2001;9:561–569. doi: 10.1053/joca.2001.0424. [DOI] [PubMed] [Google Scholar]
- 9.Crockett R, Grubelnik A, Roos S, Dora C, Born W, Troxler H. Biochemical composition of the superficial layer of articular cartilage. J Biomed Mater Res A. 2007;82:958–964. doi: 10.1002/jbm.a.31248. [DOI] [PubMed] [Google Scholar]
- 10.Domayer SE, Mamisch TC, Kress I, Chan J, Kim YJ. Radial dGEMRIC in developmental dysplasia of the hip and in femoroacetabular impingement: preliminary results. Osteoarthritis Cartilage. 2010;18:1421–1428. doi: 10.1016/j.joca.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 11.Ganz R, Klaue K, Vinh TS, Mast JW. A new periacetabular osteotomy for the treatment of hip dysplasias. Technique and preliminary results. Clin Orthop Relat Res. 1988;232:26–36. [PubMed]
- 12.Guermazi A, Alizai H, Crema MD, Trattnig S, Regatte RR, Roemer FW. Compositional MRI techniques for evaluation of cartilage degeneration in osteoarthritis. Osteoarthritis Cartilage. 2015 Jun 5 [Epub ahead of print]. [DOI] [PubMed]
- 13.Hauselmann HJ, Flechtenmacher J, Michal L, Thonar EJ, Shinmei M, Kuettner KE, Aydelotte MB. The superficial layer of human articular cartilage is more susceptible to interleukin-1-induced damage than the deeper layers. Arthritis Rheum. 1996;39:478–488. doi: 10.1002/art.1780390316. [DOI] [PubMed] [Google Scholar]
- 14.Hingsammer AM, Kalish LA, Stelzeneder D, Bixby S, Mamisch TC, Connell P, Millis MB, Kim YJ. Does periacetabular osteotomy for hip dysplasia modulate cartilage biochemistry? J Bone Joint Surg Am. 2015;97:544–550. doi: 10.2106/JBJS.M.01233. [DOI] [PubMed] [Google Scholar]
- 15.Hipp JA, Sugano N, Millis MB, Murphy SB. Planning acetabular redirection osteotomies based on joint contact pressures. Clin Orthop Relat Res. 1999;364:134–143. doi: 10.1097/00003086-199907000-00018. [DOI] [PubMed] [Google Scholar]
- 16.Hunziker EB, Michel M, Studer D. Ultrastructure of adult human articular cartilage matrix after cryotechnical processing. Microsc Res Tech. 1997;37:271–284. doi: 10.1002/(SICI)1097-0029(19970515)37:4<271::AID-JEMT3>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 17.Jay GD, Tantravahi U, Britt DE, Barrach HJ, Cha CJ. Homology of lubricin and superficial zone protein (SZP): products of megakaryocyte stimulating factor (MSF) gene expression by human synovial fibroblasts and articular chondrocytes localized to chromosome 1q25. J Orthop Res. 2001;19:677–687. doi: 10.1016/S0736-0266(00)00040-1. [DOI] [PubMed] [Google Scholar]
- 18.Jurvelin J, Kiviranta I, Tammi M, Helminen JH. Softening of canine articular cartilage after immobilization of the knee joint. Clin Orthop Relat Res. 1986;207:246–252. [PubMed] [Google Scholar]
- 19.Kiviranta I, Tammi M, Jurvelin J, Saamanen AM, Helminen HJ. Moderate running exercise augments glycosaminoglycans and thickness of articular cartilage in the knee joint of young beagle dogs. J Orthop Res. 1988;6:188–195. doi: 10.1002/jor.1100060205. [DOI] [PubMed] [Google Scholar]
- 20.Klein TJ, Chaudhry M, Bae WC, Sah RL. Depth-dependent biomechanical and biochemical properties of fetal, newborn, and tissue-engineered articular cartilage. J Biomech. 2007;40:182–190. doi: 10.1016/j.jbiomech.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 21.Korhonen RK, Laasanen MS, Toyras J, Lappalainen R, Helminen HJ, Jurvelin JS. Fibril reinforced poroelastic model predicts specifically mechanical behavior of normal, proteoglycan depleted and collagen degraded articular cartilage. J Biomech. 2003;36:1373–1379. doi: 10.1016/S0021-9290(03)00069-1. [DOI] [PubMed] [Google Scholar]
- 22.Laasanen MS, Toyras J, Korhonen RK, Rieppo J, Saarakkala S, Nieminen MT, Hirvonen J, Jurvelin JS. Biomechanical properties of knee articular cartilage. Biorheology. 2003;40:133–140. [PubMed] [Google Scholar]
- 23.Larsson T, Aspden RM, Heinegard D. Effects of mechanical load on cartilage matrix biosynthesis in vitro. Matrix. 1991;11:388–394. doi: 10.1016/S0934-8832(11)80193-9. [DOI] [PubMed] [Google Scholar]
- 24.Lattanzi R, Petchprapa C, Ascani D, Babb JS, Chu D, Davidovitch RI, Youm T, Meislin RJ, Recht MP. Detection of cartilage damage in femoroacetabular impingement with standardized dGEMRIC at 3 T. Osteoarthritis Cartilage. 2014;22:447–456. doi: 10.1016/j.joca.2013.12.022. [DOI] [PubMed] [Google Scholar]
- 25.Mavcic B, Iglic A, Kralj-Iglic V, Brand RA, Vengust R. Cumulative hip contact stress predicts osteoarthritis in DDH. Clin Orthop Relat Res. 2008;466:884–891. doi: 10.1007/s11999-008-0145-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Michaeli DA, Murphy SB, Hipp JA. Comparison of predicted and measured contact pressures in normal and dysplastic hips. Med Eng Phys. 1997;19:180–186. doi: 10.1016/S1350-4533(96)00051-3. [DOI] [PubMed] [Google Scholar]
- 27.Modl JM, Sether LA, Haughton VM, Kneeland JB. Articular cartilage: correlation of histologic zones with signal intensity at MR imaging. Radiology. 1991;181:853–855. doi: 10.1148/radiology.181.3.1947110. [DOI] [PubMed] [Google Scholar]
- 28.Mow VC, Kuei SC, Lai WM, Armstrong CG. Biphasic creep and stress relaxation of articular cartilage in compression? Theory and experiments. J Biomech Eng. 1980;102:73–84. doi: 10.1115/1.3138202. [DOI] [PubMed] [Google Scholar]
- 29.Nordin M, Frankel VH. Basic Biomechanics of the Musculoskeletal System. Philadelphia, PA, USA: Lippincott Williams & Wilkins; 2001. [Google Scholar]
- 30.Palmoski MJ, Colyer RA, Brandt KD. Joint motion in the absence of normal loading does not maintain normal articular cartilage. Arthritis Rheum. 1980;23:325–334. doi: 10.1002/art.1780230310. [DOI] [PubMed] [Google Scholar]
- 31.Parkkinen JJ, Lammi MJ, Helminen HJ, Tammi M. Local stimulation of proteoglycan synthesis in articular cartilage explants by dynamic compression in vitro. J Orthop Res. 1992;10:610–620. doi: 10.1002/jor.1100100503. [DOI] [PubMed] [Google Scholar]
- 32.Rogers BA, Murphy CL, Cannon SR, Briggs TW. Topographical variation in glycosaminoglycan content in human articular cartilage. J Bone Joint Surg Br. 2006;88:1670–1674. doi: 10.1302/0301-620X.88B12.18132. [DOI] [PubMed] [Google Scholar]
- 33.Salter RB, Simmonds DF, Malcolm BW, Rumble EJ, MacMichael D, Clements ND. The biological effect of continuous passive motion on the healing of full-thickness defects in articular cartilage. An experimental investigation in the rabbit. J Bone Joint Surg Am. 1980;62:1232–1251. [PubMed] [Google Scholar]
- 34.Schinagl RM, Gurskis D, Chen AC, Sah RL. Depth-dependent confined compression modulus of full-thickness bovine articular cartilage. J Orthop Res. 1997;15:499–506. doi: 10.1002/jor.1100150404. [DOI] [PubMed] [Google Scholar]
- 35.Schinagl RM, Ting MK, Price JH, Sah RL. Video microscopy to quantitate the inhomogeneous equilibrium strain within articular cartilage during confined compression. Ann Biomed Eng. 1996;24:500–512. doi: 10.1007/BF02648112. [DOI] [PubMed] [Google Scholar]
- 36.Schumacher BL, Block JA, Schmid TM, Aydelotte MB, Kuettner KE. A novel proteoglycan synthesized and secreted by chondrocytes of the superficial zone of articular cartilage. Arch Biochem Biophys. 1994;311:144–152. doi: 10.1006/abbi.1994.1219. [DOI] [PubMed] [Google Scholar]
- 37.Slowman SD, Brandt KD. Composition and glycosaminoglycan metabolism of articular cartilage from habitually loaded and habitually unloaded sites. Arthritis Rheum. 1986;29:88–94. doi: 10.1002/art.1780290112. [DOI] [PubMed] [Google Scholar]
- 38.Sur S, Mamisch TC, Hughes T, Kim YJ. High resolution fast T1 mapping technique for dGEMRIC. J Magn Reson Imaging. 2009;30:896–900. doi: 10.1002/jmri.21869. [DOI] [PubMed] [Google Scholar]
- 39.Tiderius CJ, Svensson J, Leander P, Ola T, Dahlberg L. dGEMRIC (delayed gadolinium-enhanced MRI of cartilage) indicates adaptive capacity of human knee cartilage. Magn Reson Med. 2004;51:286–290. doi: 10.1002/mrm.10714. [DOI] [PubMed] [Google Scholar]
- 40.Tsumura H, Miura H, Iwamoto Y. Three-dimensional pressure distribution of the human hip joint–comparison between normal hips and dysplastic hips. Fukuoka Igaku Zasshi. 1998;89:109–118. [PubMed] [Google Scholar]
- 41.von Rosen S. Diagnosis and treatment of congenital dislocation of the hip joint in the new-born. J Bone Joint Surg Br. 1962;44:284–291. doi: 10.1302/0301-620X.44B2.284. [DOI] [PubMed] [Google Scholar]
- 42.Wong M, Siegrist M, Cao X. Cyclic compression of articular cartilage explants is associated with progressive consolidation and altered expression pattern of extracellular matrix proteins. Matrix Biol. 1999;18:391–399. doi: 10.1016/S0945-053X(99)00029-3. [DOI] [PubMed] [Google Scholar]
- 43.Wong M, Wuethrich P, Eggli P, Hunziker E. Zone-specific cell biosynthetic activity in mature bovine articular cartilage: a new method using confocal microscopic stereology and quantitative autoradiography. J Orthop Res. 1996;14:424–432. doi: 10.1002/jor.1100140313. [DOI] [PubMed] [Google Scholar]
- 44.Yasunaga Y, Ikuta Y, Kanazawa T, Takahashi K, Hisatome T. The state of the articular cartilage at the time of surgery as an indication for rotational acetabular osteotomy. J Bone Joint Surg Br. 2001;83:1001–1004. doi: 10.1302/0301-620X.83B7.12171. [DOI] [PubMed] [Google Scholar]
- 45.Zhao X, Chosa E, Totoribe K, Deng G. Effect of periacetabular osteotomy for acetabular dysplasia clarified by three-dimensional finite element analysis. J Orthop Sci. 2010;15:632–640. doi: 10.1007/s00776-010-1511-z. [DOI] [PubMed] [Google Scholar]