Abstract
Intravital microscopy was used to assess the involvement of ExoU, a Pseudomonas aeruginosa cytotoxin with phospholipase A2 activity, in dysfunction of cerebral microcirculation during experimental pneumosepsis. Cortical vessels from mice intratracheally infected with low density of the ExoU-producing PA103 P. aeruginosa strain exhibited increased leukocyte rolling and adhesion to venule endothelium, decreased capillar density and impaired arteriolar response to vasoactive acetylcholine. These phenomena were mediated by the platelet activating factor receptor (PAFR) pathway because they were reversed in mice treated with a PAFR antagonist prior to infection. Brains from PA103-infected animals exhibited a perivascular inflammatory infiltration that was not detected in animals infected with an exoU deficient mutant or in mice treated with the PAFR antagonist and infected with the wild type bacteria. No effect on brain capillary density was detected in mice infected with the PAO1 P. aeruginosa strain, which do not produce ExoU. Finally, after PA103 infection, mice with a targeted deletion of the PAFR gene exhibited higher brain capillary density and lower leukocyte adhesion to venule endothelium, as well as lower increase of systemic inflammatory cytokines, when compared to wild-type mice. Altogether, our results establish a role for PAFR in mediating ExoU-induced cerebral microvascular failure in a murine model of sepsis.
Keywords: experimental Pseudomonas aeruginosa sepsis, Pseudomonas aeruginosa type III secretion toxin, cerebral microvascular failure, PAF–PAFR signaling pathway, cerebral intravital microscopy
During Pseudomonas aeruginosa experimental sepsis, cerebral microcirculatory dysfunction induced by the toxin ExoU depends on increased PAF relase and activation of the PAF receptor signaling pathway.
Graphical Abstract Figure.
During Pseudomonas aeruginosa experimental sepsis, cerebral microcirculatory dysfunction induced by the toxin ExoU depends on increased PAF relase and activation of the PAF receptor signaling pathway.
INTRODUCTION
Sepsis is a severe infection-triggered clinical syndrome characterized by microcirculatory failure. Impairment of capillary perfusion results in increased distance for oxygen diffusion into parenchymal cells and tissue hypoxia, and precipitates organ failure and death (De Backer et al. 2011).
Various coexisting mechanisms may be involved in microcirculatory disturbance detected in sepsis, including endothelial dysfunction (Peters et al. 2003), altered interaction of the endothelium with circulating blood cells (Croner et al. 2006), interstitial edema that may compress small vessels (De Backer et al. 2002), intravascular formation of microthrombi that can transiently narrow or occlude microvessels (De Baker, Donadello and Favory 2010) and altered production of nitric oxide (NO) (Gocan, Scott and Tyml 2000) and reactive oxygen species (Tyml 2011). These alterations result in the collapse of the microcirculatory function, reported to be proportional to the disease severity (De Backer et al. 2002).
In sepsis, the brain microcirculation may be affected similarly to any other organ, but with unique effects. Indeed, cerebral microvascular dysfunction is common to both neuroinflammatory and ischemic processes involved in the pathophysiology of sepsis-associated encephalopathy, a frequent, but often neglected, manifestation in severe sepsis, associated with increased mortality, morbidity and plausibly with long-term neurocognitive decline and psychological sequelae among survivors (Sharshar et al. 2010).
In experimental models of Gram-negative sepsis, microvascular failure has been usually associated with the release of inflammatory mediators in response to bacterial lipopolysaccharide (LPS) (Peters et al. 2003; Opal 2007). However, bacteria produce a wide range of virulence factors that can trigger similar inflammatory response (Gao et al. 2008; van der Poll and Opal 2008). For instance, about 30% of clinical and environmental Pseudomonas aeruginosa isolates (Feltman et al. 2001) produce ExoU, a potent cytotoxin with phospholipase A2 (PLA2) activity that is injected into host cell cytosol through the type III secretion system (Sato and Frank 2004). In clinical settings, infection by an ExoU-producing P. aeruginosa strain place patients at a higher risk for mortality (Schulert et al. 2003).
PLA2 belongs to a family of enzymes that cleaves membrane glycerophospholipids into lysophospholipids and unsaturated fatty acids. Lysophospholipid acetylation at the sn-2 position results in the synthesis of platelet-activating factor (PAF), a potent agonist that plays an important role in vascular functions at very low concentrations (Prescott et al. 2000). Under pathological conditions, the concentration of circulating PAF can be significantly increased, with deleterious effects to host organisms (Sharma et al. 2012). PAF is recognized by a single receptor (PAFR) that may be expressed on the plasma membrane of various cell types, but especially leukocytes, platelets and endothelial cells (Prescott et al. 2000). PAF–PAFR interaction contributes to inflammation-mediated tissue injury as it initiates a response that not only recruits neutrophils by the activation of transcription factors (Kravchenko et al. 1995), but also primes and triggers the release of reactive oxygen species at neutrophil–endothelial cell interfaces (Lee et al. 2002).
We have shown that, due to its PLA2 activity, ExoU exhibits proinflammatory activity associated with a robust release of free arachidonic acid, eicosanoids, cytokines and PAF (Saliba et al. 2005; Machado et al. 2010; Mallet de Lima et al. 2012). In a murine model of P. aeruginosa sepsis, ExoU was shown to have a significant impact in mice survival, and to induce manifestations of systemic inflammation implicated in sepsis pathophysiology, such as vascular hyperpermeability, platelet activation and disturbed fibrin turnover (Machado et al. 2010, 2011). ExoU was also shown to activate NF-kB downstream of PAFR. In turn, NF-κB enhances PAFR expression, amplifying the ExoU effects (Malet de Lima et al. 2014).
Although it is well established that systemic inflammation can have a negative impact on cerebral function (Mc McColl, Rothwell and Allan 2007), whether ExoU contributes to pathological alterations in brain microperfusion during P. aeruginosa sepsis, as reported for bacterial LPS (de Bock et al. 1998; Zhou et al. 2009), has not yet been investigated.
In this study, we carried out intravital microscopic and histopathological studies to gain further insight on the contribution of ExoU to the pathogenesis of P. aeruginosa infection by assessing the cerebral microcirculation of mice intratracheally infected with an ExoU-producing P. aeruginosa strain and by investigating the mechanism implicated in ExoU-induced microperfusion alteration.
MATERIALS AND METHODS
Bacterial strains
Pseudomonas aeruginosa PA103 strain, which produces the type III secretion effector proteins ExoT and ExoU, and the mutant PA103ΔexoU, obtained by deletion of the exoU gene, were grown in Luria–Bertani broth at 37°C for 14–16 h, harvested by centrifugation and resuspended in sterile LPS-free saline, as described (Saliba et al. 2005). In some assays, PAO1 P. aeruginosa, which secretes ExoS, ExoT and ExoY, but fails to secrete ExoU, was used as a control.
Mice infection
Female Swiss mice aged 8–12 weeks were anesthetized with a mixture of ketamine (65 mg kg−1) and xylazine (13 mg kg−1) and infected intratracheally with 105 colony-forming units (CFU) of PA103, PA103ΔexoU or PAO1 in 50 μL of LPS-free saline, as described (Machado et al. 2010, 2011). Control mice were instilled with the same volume of saline. To assess the contribution of PAF–PAFR pathway in the microcirculatory alterations, 1 h before the bacterial instillation, mice were intraperitoneally inoculated with WEB 2086 (Biomol), an inverse agonist of PAFR, at a final dose of 7.5 mg kg−1. At 24 h post-infection, mice pial microvasculature was analyzed by intravital microscopy, as described bellow. Mice were then euthanized by intraperitoneal injection of sodium pentobarbital. In some assays, lungs from infected mice were excised, homogeneized and diluted. The bacterial load in each organ was determined following plating of serial dilutions on Pseudomonas Isolation Agar and incubation at 37°C for 24 h.
All experiments were performed in accordance with the guidelines of the Committee on the Ethics of Animal Experiments of the State University of Rio de Janeiro (protocol # CEUA/022/2011). All efforts were made to minimize animal suffering.
Intravital microscopy
Anesthetized mice had their left parietal bone exposed by skin incision. A craniotomy was performed with a high-speed drill and the dura mater and the arachnoid membranes were excised to expose the microcirculation of the brain surface. The cranial window was suffused with a solution containing (in mmol L–1) NaCl 132, KCl 2.95, CaCl2 1.71, MgCl2 0.64, NaHCO3 24.6, dextrose 3.71 and urea 6.7; pH 7.4. To analyze the cerebral microcirculation, intravenous injection of 0.1 mL of 5% fluorescein isothiocyanate-labeled dextran (FITC-dextran 150) was performed. Mice microcirculation was observed using an Olympus BX150/WI (USA) microscope with a mercury lamp, coupled to a CCD digital video camera system (Optronics, USA). Microscopic images of the cerebral circulation were acquired by Archimed 3.7.0 software for online counting of the capillaries using the Saisam software (Microvision, France), as described (Reis et al. 2012).
Capillary density and blood cell–endothelial interaction
Functional capillary density (considered as the total number of spontaneously perfused vessels with diameters less than 10 μm per mm2 of surface area) was determined by counting each capillary branch during 4 min, using the Saisam 5.1.3 software, as described previously (Sabino et al. 2008). Serial images were taken with ×10 ocular and ×10 objective lenses for 1 min/field. To analyze the interaction of leukocytes with the endothelium, leukocytes were labeled by intravenous injection of rhodamine 6G (0.3 mg kg−1), and the cell-associated fluorescence was visualized by epiillumination. Five randomly selected venular segments, 30–100 μm in diameter and 100 μm long, were observed for 30 s in each preparation. The leukocyte–endothelial interactions were evaluated by determining the number of leukocytes adherent to the venular wall for a period of 30 s. Rolling leukocytes were defined as cells crossing the venular segment at a speed below the circulating red blood cells, and were expressed as number of cells/min (Sabino et al. 2008; Reis et al. 2012).
Arteriolar response to topical application of acetylcholine
The baseline diameters of at least five pial arterioles from mice previously injected with FITC-dextran were acquired. Mice cranial windows were then suffused, for 5 min, with 10−6 M acetylcholine (Ach), an endothelium-dependent vasodilator (Persson et al. 1990), and the arteriolar diameters were measured again using the Saisam 5.1.3 software. Vascular responses were expressed as percentage of changes of the baseline arteriole diameters.
Light microscopic histopathology
Mice treated with WEB 2086 or vehicle and then infected with the ExoU-producing bacteria, as well as animals injected with the exoU-deficient bacteria or with LPS-free saline (controls), were euthanized by intraperitoneal injection of sodium pentobarbital at 24 h after infection. Mice brains were quickly removed and fixed in 10% formalin in PBS, pH 7.4, embedded in paraffin, sliced, stained with hematoxylin-eosin or with the Masson´s trichrome stain and analyzed under light microscopy.
PAFR/mice infection assays
To further address the involvement of the PAF–PAFR pathway in ExoU-associated microcirculatory dysfunction, female C57BL/6 wild-type (WT) and pafr gene knockout (KO) mice were intratracheally infected with 3 × 104 CFU of PA103 P. aeruginosa in 50 μL of LPS-free saline. Control WT and KO mice were instilled with the same volume of saline. Mice were then scored for severity of illness with a scale that ranged from 0 (completely healthy) to 6 (moribund) (Machado et al. 2010) and submitted to brain microvasculature analysis by intravital microscopy. Briefly, anesthetized mice were subjected to craniotomy and injected with 100 μL of a mixture containing 1 mg mL–1 FITC-albumin and 4 μg anti-GR1 PE-labeled antibody for imaging of blood vessels and neutrophils, respectively. Mice were then positioned over an acrylic stage for imaging under a laser-scanning confocal inverted microscope (Nikon Eclipse Ti and C2 confocal head) using a ×10 objective. Images of cerebral microcirculation were recorded for playback determination of the numbers of adherent neutrophils and perfused capillaries using the software Volocity (Perkin Elmer). Serum samples were collected for quantitative detection of proinflammatory cytokines with the BD Cytometric Bead Array Mouse Inflammation Kit, according to the manufacturer's instructions.
Statistical analysis
Results were expressed as mean and standard error of the mean (SEM), unless otherwise stated. Statistical significance was accepted at the P < 0.05 level by one-way ANOVA for multiple group analysis with a Bonferroni adjustment, unless otherwise stated.
RESULTS AND DISCUSSION
Effect of ExoU on animal illness and mortality
At 24 h after intratracheal instillation, all control and PA103ΔexoU-infected mice were alive, whereas in the group of animals infected with the ExoU-producing bacteria, the survival rate was 50.0% ± 5.3. Surviving animals exhibited evident signs of severe disease, as previously described (Machado et al. 2010).
Endothelium–blood cell interaction and brain histopathology
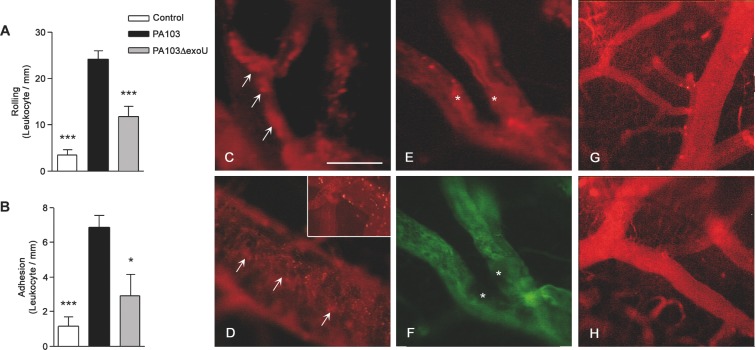
Intravital microscopy showed that, in PA103-infected mice, leukocyte rolling and adhesion to the cerebral venule endothelium were significantly increased (Fig. 1A and B). In PA103-infected animals, but not in mice infected with the exoU-deficient mutant or in control mice, cell aggregates of different sizes were seen in blood vessel lumina (Fig. 1C and D) or adherent to the endothelium, forming structures that narrowed the venular lumen (Fig. 1E and F).
Figure 1.
ExoU effect on blood cell–endothelium interaction. Leukocyte rolling on (A) and adhesion to (B) the endothelium was significantly higher in PA103-infected mice than in PA103ΔexoU-infected or in control animals. Data are means (and SEM) of the results obtained with six animals from each group, and are representative of the results obtained in two different experiments. *P < 0.05 and ***P < 0.001 when the values obtained from PA103ΔexoU-infected and control mice were compared with those obtained from animals infected with the ExoU-producing PA103 strain. In panels C–F microvessels from PA103-infected mice are shown. Note the presence of rhodamin-labeled cell aggregates of different sizes in the blood vessel lumina (arrows in panels C and D) or adherent to the endothelium (asterisk in panel E), forming a structure that narrowed the blood vessel lumen. In this figure, panel F exhibits the same vessels as shown in panel E, labeled with the FITC-dextran complex. Note the absence of perfusion in the area labeled with the asterisks. The inset included in panel D shows leukocyte rolling/adhesion to brain venule endothelium from PA103-infected mice. In panels G and H, microvessels from PA103ΔexoU-infected and control non-infected mice are shown, respectively. Note the absence of cell aggregates in the microvessel lumina. Final magnification, × 200; scale bar: 100 μm.
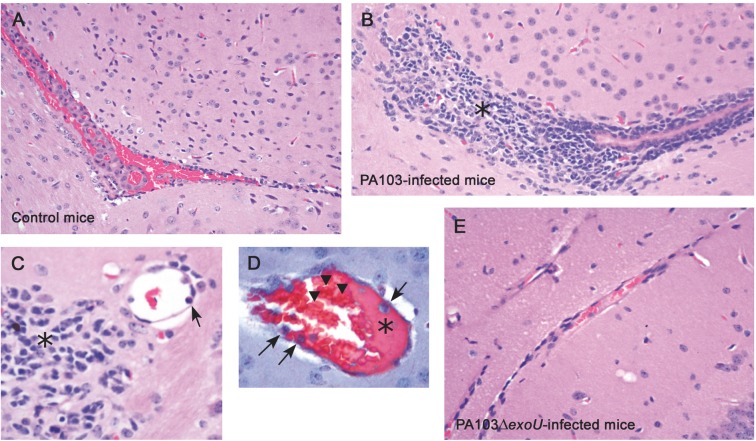
The histopathological analysis of brains from PA103-infected mice showed a consistent perivascular infiltration of inflammatory cells (Fig. 2B and C) as well as the presence of agglutinated erythrocytes and a proteinaceous material adherent to capillary endothelium (Fig. 2D). These alterations, that are reminiscent of those occurring during early steps of the coagulation process, and are consistent with our previous report of ExoU-induced enhanced thrombus formation in PA103-infected mice (Machado et al. 2010), were not detected in brain sections from control mice or from animals infected with the exoU-deficient bacteria (Fig. 2A and E, respectively).
Figure 2.
ExoU effect on brain histopathology. Light micrographs of brain sections from control mice (A) as well as from animals infected with the ExoU-producing PA103 (B–D) or non-producing PA103ΔexoU P. aeruginosa (E), stained with hematoxylin-eosin (A–C and E) or Masson´s trichrome stain (D). Note in panels B and C, the huge perivascular inflammatory infiltration (asterisks) and inflammatory cells adherent to microvessel endothelium (arrow in panel C). Panel D shows the presence of a proteinaceous material (asterisk) and of inflammatory cells (arrows) adherent to blood vessel endothelium, as well as of aggregated erythrocytes (arrowhead). Note in panels A and E, the absence of perivascular inflammatory infiltration is shown. Original magnifications: A, B and E: × 200; C: ×400; D: ×1000.
Infiltration of inflammatory cells in brain parenchyma has been generally attributed to enhanced expression of intercellular adhesion molecules by endothelial cells but also to dysfunction of the blood–brain barrier (Pytel and Alexander 2009). The impairment of the blood–brain barrier likely facilitates the exposure of the brain to circulating proinflammatory mediators, such as PAF.
Brain perfusion
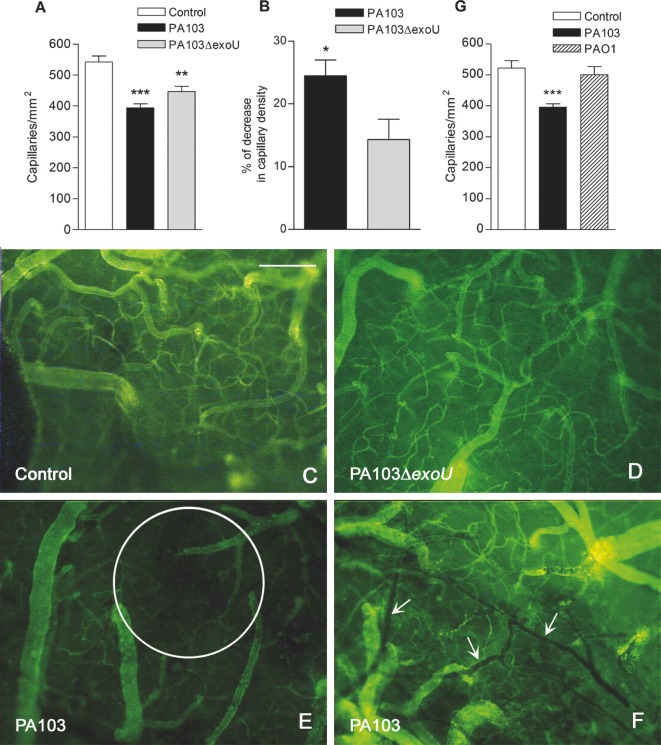
Impaired tissue perfusion has been characterized as increased number of nonperfused or intermittently perfused capillaries in close proximity to perfused capillaries, and decreased number of functional capillary density (Lam et al. 1994). In mice infected with both bacteria, the density of perfused capillaries was significantly reduced, when compared with control mice (Fig. 3A). However, in PA103-infected animals, the decrease in capillary density was significantly higher than in mice infected with the bacterial mutant (Fig. 3B). Moreover, whereas in brains from PA103-infected animals areas of capillary rarefaction and stopped-flow capillaries were frequently observed, these alterations were not detected in brain microcirculation of control or PA103ΔexoU- infected animals (Fig. 3C–F).
Figure 3.
Effect of infection on brain capillary density. (A) The mean number of perfused capillaries in cerebral tissue of control mice was significantly higher than in brains of animals from the other two groups (**P < 0.01 and ***P < 0.001). Panel B showsthe percentage of decrease in capillary density detected in PA103- and PA103ΔexoU-infected mice, considering the mean number of capillaries of control mice (548.0) as 100%. The decrease in mice infected with the WT bacteria was significantly higher (*P < 0.05), as determined by the Mann Whitney test. Data are mean (and SEM) of the results obtained with six animals from each group, and are representative of the results obtained in two different experiments. In panels C–F, micrographs of microvessels from control (C), PA103ΔexoU- (D) and PA103-infected mice (E and F) labeled with the fluorescein isothiocyanate-dextran complex are shown. Note in panel E, the presence of an area of capillary rarefaction (inside the circle) and in panel F, stopped-flow capillaries (arrows). Panel G shows that the mean number of perfused capillaries in PA103-infected animals was significantly lower (***P < 0.001) than in mice infected with PAO1 or in control mice. Data are mean (and SEM) of the results obtained in two different assays, carried out with 10 animals from each group. Final magnification, ×100; scale bar: 100 μm.
Because some decrease in capillary density was detected in mice infected with the bacteria with exoU deletion (Fig. 3A), we wondered whether another virulence factor exhibited by both PA103 and PA103ΔexoU, such as ExoT or LPS, may have contributed to the impairment of pia mater perfusion. To address this question, mice were instilled intratracheally with the same density (105 CFU) of the ExoT-producing P. aeruginosa PAO1 and submitted to intravital microscopy analysis. At 24 h after infection, no decrease in capillary density was detected in PAO1-infected mice (Fig. 3G). Moreover, all animals survived and only 1 out of 10 exhibited minimal signs of disease. This result is similar to that reported by Mehl et al. (2014).
Arteriolar response to vasoactive acetylcholine
Endothelial dysfunction is a crucial early mechanism implicated in the impairment of capillary perfusion in sepsis (Peters et al. 2003; De Backer et al. 2011). Temporary microvascular blood flow alterations can be reversed by topical application of vasoactive acetylcholine (De Backer et al. 2002), whereas permanently injured endothelium exhibits an abnormal response to stimulation. Acetylcholine induces the release of NO from endothelial cells, which regulates the vascular tone by promoting the relaxation of arteriolar smooth muscle and vasodilatation (Persson et al. 1990). To further address the contribution of ExoU to microcirculation dysfunction, we investigated the endothelial functionallity by assessing the arteriolar response to acetylcholine topically applied to mice pia mater. As shown in Fig. 4, after stimulation, the diameters of arterioles of control animals and of mice infected with the bacterial mutant were increased in 14.52% (range: 4.56–24.13%) and 6.26% (range: –3.6 to 23.30%), respectively. In mice infected with the ExoU-producing bacteria, the median increase of the arteriolar diameter (0.88%; range –10.37 to 15.23) was significantly lower. Moreover, in four out of nine mice infected with the ExoU-producing bacteria (44.4%), acetylcholine treatment resulted in arteriolar constriction. Although the principal effect of acetylcholine in most vascular beds is endothelium-dependent vasodilatation via stimulation of the M3 muscarinic receptor, it can also produce direct vascular smooth muscle contraction, via stimulation of M1 muscarinic receptor (Shimizut, Rosenblum and Nelson 1993). M1 receptor-mediated constriction activity is downregulated and/or uncoupled from the contractile machinery as long as the overlying endothelium synthesizes/releases dilating NO (Shimizut, Rosenblum and Nelson 1993). However, in pathologies wherein the vascular endothelium is disrupted/dysfunctional, the potency of vasoconstrictor responses evoked by acetylcholine is enhanced (Ludmer et al. 1986; Pesic, Grbovic and Jovanovic 2002). Therefore, the ExoU-induced abnormal response to acetylcholine detected in this report likely resulted from a permanent endothelial injury/dysfunction in infected animals.
Figure 4.
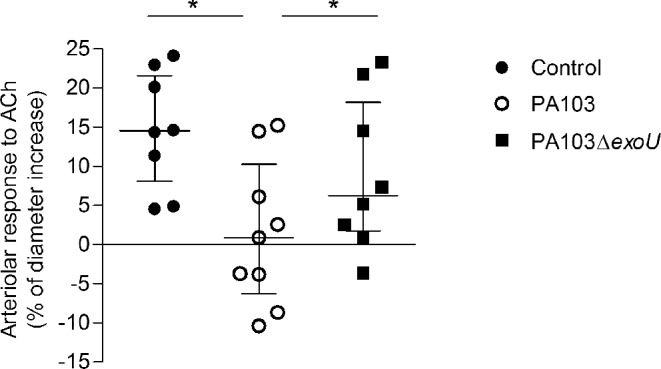
Effect of ExoU on the arteriolar response to acetylcholine. The percentages of increase of arteriole diameters in control animals and in mice infected with the bacterial mutant were significantly higher than in PA103-infected mice (*P < 0.05), as determined by the Wilcoxon rank test. Data are median with interquartile range of the results obtained in two different experiments.
Effect of PAFR blockage on brain microcirculation
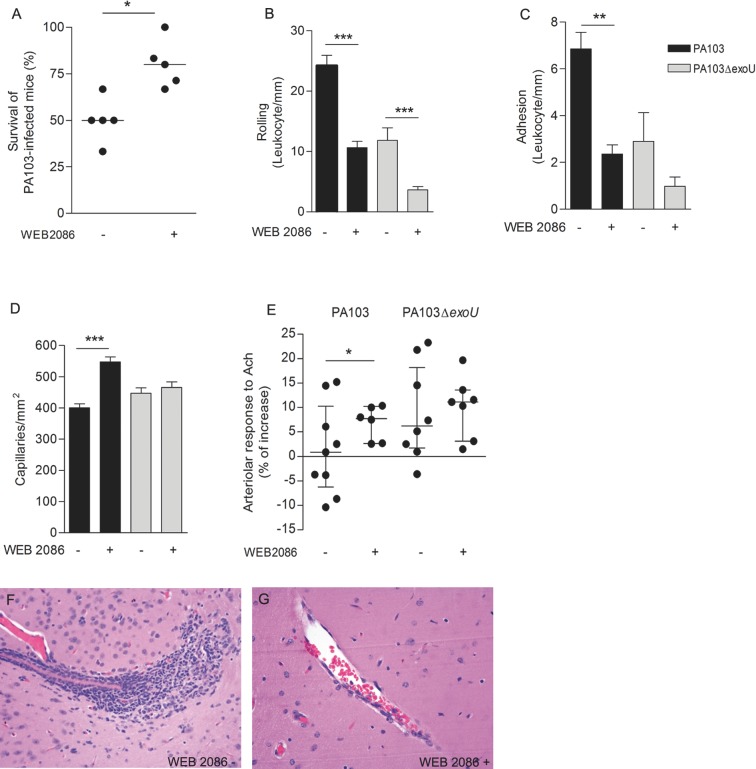
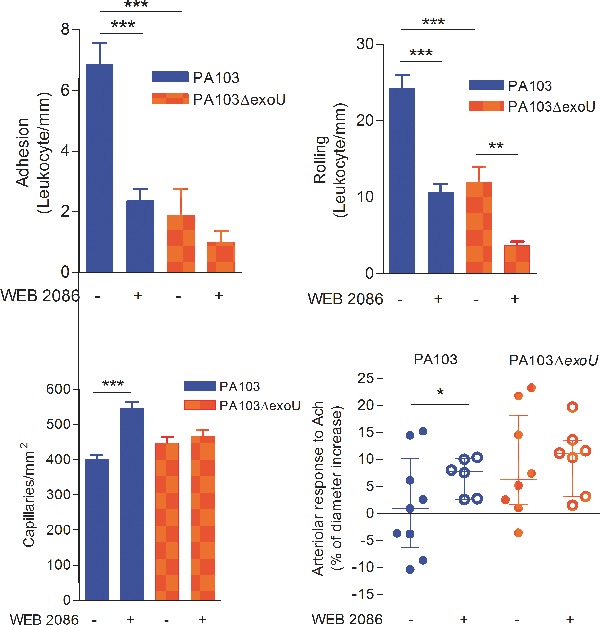
We have reported that cell injection with ExoU results in increased production of PAF (Machado et al. 2011). Since dysregulated PAF signaling is involved in sepsis pathophysiology (Zimmerman et al. 2002), we next assessed the contribution of the PAF–PAFR pathway to the ExoU-induced pial microvascular dysfunction by treating mice with a PAFR antagonist prior to infection. Drug blockage of PAFR significantly improved the survival of PA103-infected mice from 50.0% in untreated mice (range: 33.3–66.7%) to 80.0% in WEB 2086-treated animals (range: 66.7–100.0%; Fig. 5A). WEB 2086 treatment had also a significant effect on brain microcirculation and histopathology. In PA103-infected mice, there was a marked decrease in leukocyte rolling and adhesion to venule endothelial cells (Fig. 5B and C), a significant increase in capillary perfusion (Fig. 5D) and a significant improvement of the responsiveness of pial arterioles to acetylcholine (Fig. 5E). Finally, in animals treated with WEB 2086 prior to PA103 infection, no perivascular inflammatory infiltration, detected in brains from untreated mice, was detected (Fig. 5F and G).
Figure 5.
Effect of WEB 2086 treatment prior to infection. Mice treatment with the PAFR inhibitor prior to PA103 infection increased significantly the animal survival (A) and the number of perfused capillaries (D), and reduced significantly the leukocyte rolling on (B) and adhesion (C) to the capillary endothelium. The PAFR inhibitor also increased significantly the arteriolar responsiveness to topically applied acetylcholine (E). Panel A shows that the median of the percentage of survivors in PA103-infected animals previously treated with WEB was significantly increased, as determined in six different assays, each one carried out with six to eight animals, and analyzed with the Mann Whitney test. Data in panels B–D are means (and SEM) of the results obtained in two different experiments, carried out with six to eight animals from each group, whereas panel E shows the median and the interquartile range of the results obtained with six to nine animals. *P < 0.05; **P < 0.01; ***P < 0.001 when the results obtained from WEB-treated and WEB-untreated mice were compared with each other. (F and G) correspond to light micrographs of brain sections of PA103-infected mice. No perivascular infiltration of inflammatory cells, detected in brains from untreated mice (F), was detected in sections from WEB-treated animals (G). Original magnifications: ×200.
Figure 5B shows that PAFR blockage had also an effect on leukocyte rolling and adhesion in animals infected with the bacterial mutant. This means that even in the absence of ExoU, P. aeruginosa infection is associated with increased PAF production. In fact, LPS is known to activate host cytosolic PLA2 (cPLA2), resulting in increased release of arachidonic acid from cell membranes (Goldsmith et al. 2011) and, potentially, in increased PAF production. However, even if activation of host cPLA2 may have contributed to PAF-mediated effects detected in mice infected with both bacteria, the significant differences between PA103 and PA103ΔexoU in their detrimental effects on mice microcirculation result from the ExoU PLA2 activity and the consequent stronger activation of the PAF-PAFR pathway in PA103-infected mice.
Contribution of the bacterial load in mice lungs to microcirculatory dysfunction
The different effects of the infection by PA103 or PA103ΔexoU on mice brain microcirculation, and the inhibitory activity of the PAFR antagonist herein described, led us to hypothesize that the ExoU PLA2 activity was directly implicated in the pathogenesis of microcirculatory dysfunction by favoring increased production of PAF and the paracrine activation of the PAF–PAFR signaling pathway. Alternatively, the microcirculatory dysfunction may be related to a heavier lung infection by PA103, as a consequence of the ExoU-induced early impairment of the neutrophil-mediated bacterial clearance, reported by Diaz et al. (2008). To address this point, mice were infected with the WT and the mutated strains and the bacterial burdens in the lungs were measured at 6 h after infection. Figure 6A shows that similar numbers of bacteria were recovered from the lungs of eight mice infected with each bacterial strain. Next we investigated whether at such an early period post-infection ExoU has already an effect on capillary perfusion. In PA103-infected animals, the density of pial capillaries was decreased in 54.6% ± 10.7, as compared with the capillary density in control non-infected animals (mean number per mm2 and SEM = 548 ± 20.9). In mice infected with the bacterial mutant, the percentage of decrease (12.5% ± 7.6) was significantly lower (Fig. 6B).
Figure 6.
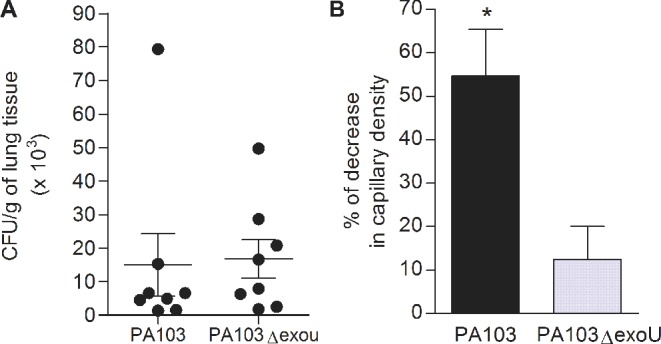
Bacterial load in mice lungs and decrease in pial capillary density 6 h after infection. Panel A shows that the bacterial concentrations in lungs of mice infected with the ExoU-producing PA103 strain or with the PA103ΔexoU mutant did not differ from each other. Data represent means and SEM of the results obtained in eight mice infected with each bacteria, assessed in two different assays. Panel B shows the decrease in cerebral capillary density in infected mice, considering the mean number of capillaries/mm2 of control mice as 100%. The decrease in mice infected with PA103 was significantly higher (P < 0.05, as determined by the Mann Whitney test). Data are mean (and SEM) of the results obtained in five mice infected with each bacteria.
PAFR/mice infection assays
To further address the involvement of the PAF–PAFR pathway in ExoU-induced microvascular failure, PA103-infected C57BL/6 WT and PAFR KO mice were compared to each other. However, since C57BL/6 mice showed to be more susceptible to PA103 infection than Swiss mice, the infecting dose was decreased to 3 × 104 CFU.
As soon as 6 h after infection, 100% of infected WT mice were scored ≥4, attesting the severity of the disease. In contrast, infected KO mice exhibited a milder disease: 100% of them were scored ≤2. These results are in line with our report that WEB2086 blockage of PAFR significantly improved the survival of PA103-infected Swiss mice, as well as with studies of Moreno et al. (2006), which showed that PAFR KO mice and WT mice previously treated with a PAFR antagonist exhibited a higher survival rate than WT animals, in a model of polymicrobial sepsis.
In the present study, the involvement of PAFR in ExoU-induced brain microcirculatory disturbance was firmly established. Whereas in infected WT mice, the density of brain capillaries was decreased in 68.7% ± 5.6 (mean ± SEM), as compared with the capillary density in control non-infected WT animals, in infected KO mice the percentage of decrease (26.5 ± 16.0) was significantly lower (Fig. 7A). Infected WT mice exhibited also a significantly higher leukocyte adhesion to the pial venule endothelium than infected KO animals (Fig. 7B). Castor et al. (2012) reported a similar decrease in leukocyte interaction with endothelium in mice with deletion in the pafr gene.
Figure 7.
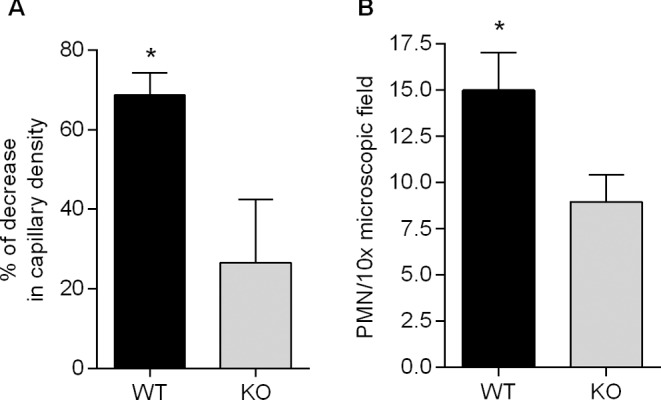
Involvement of PAFR signaling in ExoU-induced microvascular disturbance. Panel A shows the percentage of decrease in capillary density detected in PA103-infected WT and KO mice, considering the mean number s of capillaries of control non-infected WT and KO mice, respectively, as 100%. The decrease in WT animals was significantly higher (*P < 0.05). Panel B shows that leukocyte adhesion to venule endothelium was significantly higher (*P < 0.05) in WT than in KO mice. Data in A and B are means (and SEM) of the results obtained with at least five animals (range 5–12) from each group and significance of the differences was determined with the Mann Whitney test.
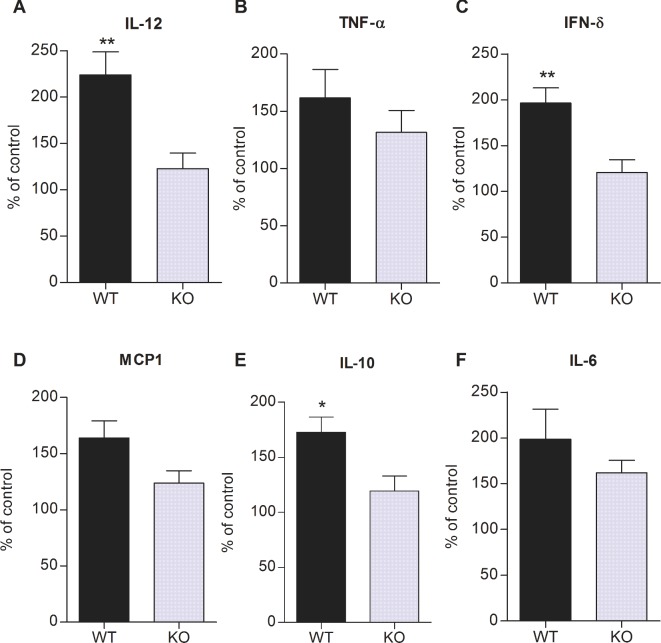
ExoU triggers a robust release of proinflammatory cytokines in P. aeruginosa infected animals (Kurahashi et al. 1999). On the other hand, PAF is known to play an important role in the orchestration of different inflammatory reactions, including the release of cytokines and chemokines (Prescott et al. 2000). To assess the relative contribution of systemic inflammatory state and PAF–PAFR signaling to ExoU-induced microvascular dysfunction, the percentual increase in cytokine production by infected mice as compared with the production by control non-infected animals was investigated in both WT and KO mice. No significant difference was detected in the level of cytokines produced by control non-infected WT and KO mice (data not shown). Figure 8 shows that the increase in IL-12, IFN-γ and IL-10 production by infected WT mice was significantly higher than the increase in infected KO animals. Several studies have reported attenuated concentrations of systemic cytokines in PAFR KO mice subjected to different stimuli (Moreno et al. 2006; Knapp et al. 2008; Castor et al. 2012; Lacerda-Queiroz et al. 2013; Correa-Costa et al. 2014).
Figure 8.
Role of PAFR signaling in ExoU-induced citokine production. Data represent the percentages of increase in serum cytokine concentrations in WT and KO mice after PA103 infection, considering the mean cytokine concentrations in control non-infected WT and KO mice, respectively, as 100%. Data are means (and SEM) of the results obtained with seven to eight animals from each group and significance of the differences was determined with the Mann Whitney test. *P < 0.05 and **P < 0.01.
Different microorganisms are known to contribute to sepsis pathophysiology in different ways (Gao, Evans and Finney 2008). In the current study, we demonstrated, for the first time, the involvement of the toxin ExoU in brain microvascular dysfunction during experimental P. aeruginosa pneumosepsis.
Athough microvascular dysfunction has been extensively reported in the course of severe infections (Gustot 2011) and endotoxin-induced sepsis model (Ruiz-Valdepeñas et al. 2011), the main objectives of this study were to assess the contribution of ExoU to microperfusion failure during sepsis and to determine whether such effect involves activation of the PAFR signaling pathway. The novelty of our approach—which allowed the establishment of a cause/effect relationship between a bacterial toxin and microcirculation impairment—is that mice were inoculated with a single bacterial strain (either an ExoU-producing strain or its mutant with deletion of the exoU gene). In contrast, most of the other experimental studies were carried out with animals infected with mixed bacterial population, as the result of the cecal ligation and puncture sepsis model (Vachharajani et al. 2010; Araújo et al. 2012; Wang et al. 2012; Morel et al. 2013), or the intraperitoneal injection of fecal suspensions (Taccone et al. 2010; Kao et al. 2011; Salgado et al. 2011).
Our study also differs from others on murine P. aeruginosa sepsis in that mice were inoculated with bacteria at low density (105 CFU), whereas in others, carried out with strains that do not produce ExoU, a much higher bacterial density, ranging from 4 × 106 (Dominguez et al. 2012) to 2–5 × 108 (Kalle et al. 2012), had to be used. Accordingly, our point is that, due to its PLA2 activity, ExoU favors the release of increased amounts of PAF, and triggers a huge inflammatory response, thereby greatly contributing to the pathogenesis of P. aeruginosa infections.
CONCLUSION
This study is the first to demonstrate that ExoU has a negative impact on cerebral microcirculation and contributes to neuroinflammation during sepsis. Since the presence of brain dysfunction has a direct impact on morbidity and mortality rates among septic patients, the detrimental effect of this P. aeruginosa toxin is likely to contribute to the poor prognosis of patients with infection by ExoU-producing P. aeruginosa strains (Schulert et al. 2003; El-Solh et al. 2012). Together, our results contribute to a better understanding of the role of ExoU in the pathogenesis of P. aeruginosa infection, and such understanding may guide the development of specific therapeutic approaches.
Acknowledgments
We thank Maria Angélica Pereira da Silva for technical assistance as well as Aurélio Cordeiro Portela and Ana Carolina G. Brito for their invaluable assistance in histochemical studies.
FUNDING
This study was supported by Brazilian grants from Fundação Carlos Chagas de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) and Conselho Nacional de Desenvolvimento Cientifico e Tecnológico (CNPq). Sabrina Alves de Oliveira Lima Santos was supported by CAPES.
Conflict of interest. None declared.
REFERENCES
- Araújo CV, Estato V, Tibiriçá E, et al. PPAR gamma activation protects the brain against microvascular dysfunction in sepsis. Microvasc Res. 2012;84:218–21. doi: 10.1016/j.mvr.2012.05.006. [DOI] [PubMed] [Google Scholar]
- Castor MG, Rezende BM, Resende CB, et al. Platelet-activating factor receptor plays a role in the pathogenesis of graft-versus-host disease by regulating leukocyte recruitment, tissue injury and lethality. J Leukocyte Biol. 2012;91:629–39. doi: 10.1189/jlb.1111561. [DOI] [PubMed] [Google Scholar]
- Correa-Costa M, Andrade-Oliveira V, Braga TT, et al. Activation of platelet-activating factor receptor exacerbates renal inflammation and promotes fibrosis. Lab Invest. 2014;94:455–66. doi: 10.1038/labinvest.2013.155. [DOI] [PubMed] [Google Scholar]
- Croner RS, Hoerer E, Kulu Y, et al. Hepatic platelet and leukocyte adherence during endotoxemia. Crit Care. 2006;10:R15. doi: 10.1186/cc3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Backer D, Creteur J, Preiser JC, et al. Microvascular blood flow is altered in patients with sepsis. Am J Resp Crit Care. 2002;166:98–104. doi: 10.1164/rccm.200109-016oc. [DOI] [PubMed] [Google Scholar]
- De Baker D, Donadello K, Favory R. Link between coagulations abnormalities and microcirculatory dysfunction in critically ill patients. Curr Opin Anaesthesio. 2010;22:150–4. doi: 10.1097/ACO.0b013e328328d1a1. [DOI] [PubMed] [Google Scholar]
- De Backer D, Donadello K, Taccone FS, et al. Microcirculatory alterations: potential mechanisms and implication for therapy. Ann Intensive Care. 2011;1:27. doi: 10.1186/2110-5820-1-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bock F, Derijard B, Dornand J, et al. The neuronal death induced by endotoxic shock but not that induced by excitatory amino acids requires TNF-alpha. Eur J Neurosci. 1998;10:3107–14. doi: 10.1046/j.1460-9568.1998.00317.x. [DOI] [PubMed] [Google Scholar]
- Diaz MH, Shaver CM, King JD, et al. Pseudomonas aeruginosa induces localized immunosuppression during pneumonia. Infect Immun. 2008;76:4414–21. doi: 10.1128/IAI.00012-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez JA, Xie Y, Dunne WM, et al. Intestine-specific Mttp deletion decreases mortality and prevents sepsis-induced intestinal injury in a murine model of Pseudomonas aeruginosa pneumonia. PLoS One. 2012;7:e49159. doi: 10.1371/journal.pone.0049159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Solh AA, Hattemer A, Hauser AR, et al. Clinical outcomes of type III Pseudomonas aeruginosa bacteremia. Crit Care Med. 2012;40:1157–63. doi: 10.1097/CCM.0b013e3182377906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltman H, Schulert G, Khan S, et al. Prevalence of type III secretion genes in clinical and environmental isolates of Pseudomonas aeruginosa. Microbiology. 2001;147:2659–69. doi: 10.1099/00221287-147-10-2659. [DOI] [PubMed] [Google Scholar]
- Gao H, Evans TW, Finney SJ. Bench-to-bedside review: sepsis, severe sepsis and septic shock—does the nature of the infecting organism matter? Crit Care. 2008;12:212. doi: 10.1186/cc6862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gocan NC, Scott JA, Tyml K. Nitric oxide produced via neuronal NOS may impair vasodilatation in septic rat skeletal muscle. Am J Physiol-Heart C. 2000;278:H1480–9. doi: 10.1152/ajpheart.2000.278.5.H1480. [DOI] [PubMed] [Google Scholar]
- Goldsmith M, Daka A, Lamour NF, et al. A ceramide analog inhibits cPLA(2) activity and consequent PGE(2) formation in LPS-stimulated macrophages. Immunol Lett. 2011;135:136–43. doi: 10.1016/j.imlet.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustot T. Multiple organ failure in sepsis: prognosis and role of systemic inflammatory response. Curr Opin Crit Care. 2011;17:153–9. doi: 10.1097/MCC.0b013e328344b446. [DOI] [PubMed] [Google Scholar]
- Kalle M, Papareddy P, Kasetty G, et al. Host defense peptides of thrombin modulate inflammation and coagulation in endotoxin-mediated shock and Pseudomonas aeruginosa sepsis. PLoS One. 2012;7:e51313. doi: 10.1371/journal.pone.0051313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao RL, Martin CM, Xenocostas A, et al. Erythropoietin improves skeletal muscle microcirculation through the activation of eNOS in a mouse sepsismodel. J Trauma. 2011;71:S462–7. doi: 10.1097/TA.0b013e318232e7a2. [DOI] [PubMed] [Google Scholar]
- Knapp S, von Aulock S, Leendetsee M, et al. Lipoteichoic acid-induced lung inflammation depends on TLR2 and the concerted action of TLR4 and the platelet-activating factor receptor. J Immunol. 2008;180:3478–84. doi: 10.4049/jimmunol.180.5.3478. [DOI] [PubMed] [Google Scholar]
- Kravchenko VV, Pan Z, Han J, et al. Platelet-activating factor induces NF-kappa B activation through a G protein-coupled pathway. J Biol Chem. 1995;270:14928–34. doi: 10.1074/jbc.270.25.14928. [DOI] [PubMed] [Google Scholar]
- Kurahashi K, Kajikawa O, Sawa T, et al. Pathogenesis of septic shock in Pseudomonas aeruginosa pneumonia. J Clin Invest. 1999;104:743–50. doi: 10.1172/JCI7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacerda-Queiroz N, Rachid MA, Teixeira MM, et al. The role of platet-activating factor receptor in lung pathology during experimental malaria. Int Parasitol. 2013;43:11–5. doi: 10.1016/j.ijpara.2012.11.008. [DOI] [PubMed] [Google Scholar]
- Lam C, Tyml K, Martin C, et al. Microvascular perfusion is impaired in a rat model of normotensive sepsis. J Clin Invest. 1994;94:2077–83. doi: 10.1172/JCI117562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YM, Hybertson BM, Cho HG, et al. Platelet-activating factor induces lung inflammation and leak in rats: hydrogen peroxide production along neutrophil-lung endothelial cell interfaces. J Lab Clin Med. 2002;140:312–9. doi: 10.1067/mlc.2002.128181. [DOI] [PubMed] [Google Scholar]
- Ludmer PL, Selwyn AP, Shook TL, et al. Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. New Engl J Med. 1986;315:1046–51. doi: 10.1056/NEJM198610233151702. [DOI] [PubMed] [Google Scholar]
- McColl BW, Rothwell NJ, Allan SM. Systemic inflammatory stimulus potentiates the acute phase and CXC chemokine responses to experimental stroke and exacerbates brain damage via interleukin-1- and neutrophil-dependent mechanisms. J Neurosci. 2007;27:4403–12. doi: 10.1523/JNEUROSCI.5376-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado GB, de Assis MC, Leão R, et al. ExoU-induced vascular hyperpermeability and platelet activation in the course of experimental Pseudomonas aeruginosa pneumosepsis. Shock. 2010;33:315–21. doi: 10.1097/SHK.0b013e3181b2b0f4. [DOI] [PubMed] [Google Scholar]
- Machado GBS, Oliveira AV, Saliba AM, et al. Pseudomonas aeruginosa toxin ExoU induces a PAF-dependent impairment of alveolar fibrin turnover secondary to enhanced activation of coagulation and increased expression of plasminogen activator inhibitor-1 in the course of mice pneumosepsis. Respir Res. 2011;12:104. doi: 10.1186/1465-9921-12-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet de Lima C, Calegari-Silva TC, Pereira RM, et al. ExoU activates NF-κB and increases IL-8/KC secretion during Pseudomonas aeruginosa infection. PLoS One. 2012;7:e41772. doi: 10.1371/journal.pone.0041772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet de Lima CD, da Conceição Costa J, de Oliveira Lima Santos AS, et al. Central role of PAFR signalling in ExoU-induced NF-κB activation. Cell Microbiol. 2014;16:1244–54. doi: 10.1111/cmi.12280. [DOI] [PubMed] [Google Scholar]
- Mehl A, Ghorbani P, Douda D, et al. Effect of arginase inhibition on pulmonary L-arginine metabolism in murine Pseudomonas pneumonia. PLoS One. 2014;9:e90232. doi: 10.1371/journal.pone.0090232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel J, Li JY, Eyenga P, et al. Early adverse changes in liver microvascular circulation during experimental septic shock are not linked to an absolute nitric oxide deficit. Microvasc Res. 2013;90:187–91. doi: 10.1016/j.mvr.2013.07.005. [DOI] [PubMed] [Google Scholar]
- Moreno SE, Alves-Filho JC, Rios-Santos F, et al. Signaling via platelet-activating factor receptors accounts for the impairment of neutrophil migration in polymicrobial sepsis. J Immunol. 2006;177:1264–71. doi: 10.4049/jimmunol.177.2.1264. [DOI] [PubMed] [Google Scholar]
- Opal SM. The host response to endotoxin, antilipopolysaccharide strategies, and the management of severe sepsis. Int J Med Microbiol. 2007;297:365–77. doi: 10.1016/j.ijmm.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Persson MG, Gustafsson LE, Wiklund NP, et al. Indogenous nitric oxide as a modulator of rabbit skeletal muscle microcirculation in vivo. 1990;100:463–6. doi: 10.1111/j.1476-5381.1990.tb15829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesic S, Grbovic L, Jovanovic A. Acetylcholine-induced contractions in the perforating branch of the human internal mammary artery: protective role of the vascular endothelium. Pharmacology. 2002;64:182–8. doi: 10.1159/000056169. [DOI] [PubMed] [Google Scholar]
- Peters K, Unger RE, Brunner J, et al. Molecular basis of endothelial dysfunction in sepsis. Cardiovasc Res. 2003;60:49–57. doi: 10.1016/s0008-6363(03)00397-3. [DOI] [PubMed] [Google Scholar]
- Pytel P, Alexander JJ. Pathogenesis of septic encephalopathy. Curr Opin Neurol. 2009;22:283–7. doi: 10.1097/WCO.0b013e32832b3101. [DOI] [PubMed] [Google Scholar]
- Prescott SM, Zimmerman GA, Stafforini DM, et al. Platelet-activating factor and related lipid mediators. Ann Rev Biochem. 2000;69:419–45. doi: 10.1146/annurev.biochem.69.1.419. [DOI] [PubMed] [Google Scholar]
- Reis PA, Estato V, da Silva TI, et al. Statins decrease neuroinflammation and prevent cognitive impairment after cerebral malaria. PLoS Pathog. 2012;8:e1003099. doi: 10.1371/journal.ppat.1003099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Valdepeñas L, Martinez-Orgado JA, Benito C, et al. Cannabidiol reduces lipopolysaccharide-induced vascular changes and inflammation in the mouse brain: an intravital microscopy study. J Neuroinflamm. 2011;8:5. doi: 10.1186/1742-2094-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabino B, Lessa MA, Nascimento AR, et al. Effects of antihypertensive drugs on capillary rarefaction in spontaneously hypertensive rats: intravital microscopy and histologic analysis. J Cardiovasc Pharm. 2008;51:402–9. doi: 10.1097/FJC.0b013e3181673bc5. [DOI] [PubMed] [Google Scholar]
- Salgado DR, He X, Su F, et al. Sublingual microcirculatory effects of enalaprilat in an ovine model of septic shock. Shock. 2011;35:542–9. doi: 10.1097/SHK.0b013e3182115e6a. [DOI] [PubMed] [Google Scholar]
- Saliba AM, Nascimento DO, Silva MC, et al. Eicosanoid-mediated proinflammatory activity of Pseudomonas aeruginosa ExoU. Cell Microbiol. 2005;7:1811–22. doi: 10.1111/j.1462-5822.2005.00635.x. [DOI] [PubMed] [Google Scholar]
- Sato H, Frank DW. ExoU is a potent intracellular phospholipase. Mol Microbiol. 2004;53:1279–90. doi: 10.1111/j.1365-2958.2004.04194.x. [DOI] [PubMed] [Google Scholar]
- Schulert GS, Feltman H, Rabin SD, et al. Secretion of the toxin ExoU is a marker for highly virulent Pseudomonas aeruginosa isolates obtained from patients with hospital-acquired pneumonia. J Infect Dis. 2003;188:1695–706. doi: 10.1086/379372. [DOI] [PubMed] [Google Scholar]
- Sharma J, Young DM, Marentette JO, et al. Lung endothelial cell platelet-activating factor production and inflammatory cell adherence are increased in response to cigarette smoke component exposure. Am J Physiol-Lung C. 2012;302:L47–55. doi: 10.1152/ajplung.00179.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharshar T, Polito A, Checinski A, et al. Septic-associated encephalopathy—everything starts at a microlevel. Crit Care. 2010;14:199. doi: 10.1186/cc9254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizut T, Rosenblum W, Nelson GH. M3 and M1 receptors in cerebral arterioles in vivo: evidence for down-regulated or ineffective M1 when endothelium is intact. Am J Physiol. 1993;264:665–9. doi: 10.1152/ajpheart.1993.264.3.H665. [DOI] [PubMed] [Google Scholar]
- Taccone FS, Su F, Pierrakos C, et al. Cerebral microcirculation is impaired during sepsis: an experimental study. Crit Care. 2010;14:R140. doi: 10.1186/cc9205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyml K. Critical role for oxidative stress, platelets and coagulation in capillary blood flow impairment in sepsis. Microcirculation. 2011;18:152–62. doi: 10.1111/j.1549-8719.2010.00080.x. [DOI] [PubMed] [Google Scholar]
- Wang Z, Holthoff JH, Seely KA, et al. Development of oxidative stress in the peritubular capillary microenvironment mediates sepsis-induced renalmicrocirculatory failure and acute kidney injury. Am J Pathol. 2012;180:505–16. doi: 10.1016/j.ajpath.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vachharajani V, Wang SW, Mishra N, et al. Curcumin modulates leukocyte and platelet adhesion in murine sepsis. Microcirculation. 2010;17:407–16. doi: 10.1111/j.1549-8719.2010.00039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Poll T, Opal SM. Host-pathogen interactions in sepsis. Lancet Infect Dis. 2008;8:32–43. doi: 10.1016/S1473-3099(07)70265-7. [DOI] [PubMed] [Google Scholar]
- Zimmerman GA, McIntyre TM, Prescott SM, et al. The platelet-activating factor signaling system and its regulators in syndromes of inflammation and thrombosis. Crit Care Med. 2002;30:S294–301. doi: 10.1097/00003246-200205001-00020. [DOI] [PubMed] [Google Scholar]
- Zhou H, Andonegui G, Wong CH, et al. Role of endothelial TLR4 for neutrophil recruitment into central nervous system microvessels in systemic inflammation. J Immunol. 2009;183:5244–50. doi: 10.4049/jimmunol.0901309. [DOI] [PubMed] [Google Scholar]