Abstract
Enterococcus faecalis and Streptococcus gallolyticus cause infective endocarditis (IE), which can originate from the continuous release or translocation of low bacterial numbers into the bloodstream. In this context, IE cannot be prevented with antibiotics. We previously demonstrated that aspirin plus ticlopidine protected rats from IE due to S. gordonii and Staphylococcus aureus. Here we showed that aspirin plus ticlopidine significantly reduced vegetation weight and protected 73 and 64% rats (P < 0.005) from IE due to E. faecalis and S. gallolyticus, respectively. These results further support the potential use of aspirin plus ticlopidine for a global prevention of IE in high-risk patients.
Keywords: antiplatelets, prophylaxis, experimental endocarditis, Enterococcus faecalis, Streptococcus gallolyticus
Antiplatelets may be useful for the prevention of infective endocarditis.
Graphical Abstract Figure.
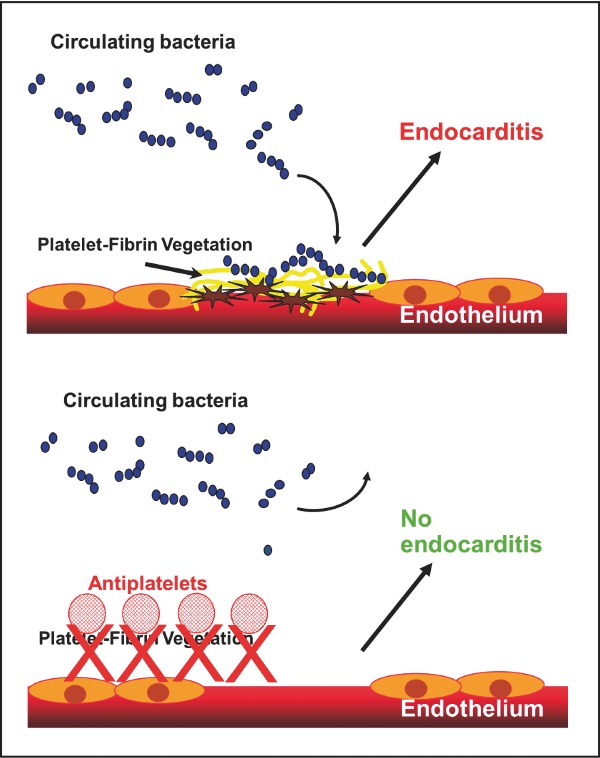
Antiplatelets may be useful for the prevention of infective endocarditis.
Enterococcus faecalis and Streptococcus gallolyticus are important etiologic agents of infective endocarditis (IE) in humans, with high incidence in elderly people (Slipczuk et al. 2013). IE induced by these bacteria is often caused by low-level bacteremia resulting from an infected medical device (Yagupsky and Nolte 1990; Sandoe et al. 2002) or from continuous translocation in small numbers from the gastrointestinal tract (Berg 1995).
New guidelines for IE prophylaxis restrict the use of antibiotics to high-risk patients, i.e. those with prosthetic valves or with a history of IE and impending medical procedure with high risk to contract bacteremia (Wilson et al. 2007). Nevertheless, the new guidelines do not propose any valuable alternative for the prevention of IE resulting from recurrent low-level bacteremia. In this context, general protective strategies that do not involve the use of antibiotics would greatly benefit at-risk patients (Chirouze, Hoen and Duval 2012). Since platelets are key elements in vegetation formation and growth, the use of antiaggregating agents appears a sensible strategy for IE prevention. We recently demonstrated that the combination of the antiplatelet oral drugs aspirin and ticlopidine was effective in the prophylaxis of experimental IE induced by S. gordonii and Staphylococcus aureus (Veloso et al. 2015). Here we investigated the efficacy of aspirin plus ticlopidine in the prophylaxis of IE induced in rats by E. faecalis and S. gallolyticus.
Enterococcus faecalis JH2–2 (Yagi and Clewell 1980) and S. gallolyticus UCN34 (Rusniok et al. 2010) were used. Isolates were grown at 37°C with 5% CO2 in brain heart infusion broth or agar (Difco; Becton Dickinson, Sparks, MD). Aspirin and ticlopidine did not possess antimicrobial activity against the tested strains, as they displayed minimal inhibitory concentrations of >500 μg mL−1, values which abundantly exceed the pharmacologically relevant concentrations.
Platelet-rich plasma (PRP) and platelet-poor plasma used for the platelet-aggregation tests were obtained from anticoagulated human blood as described previously (Veloso et al. 2013). The ability of aspirin (50 μg mL−1), ticlopidine (2 μg mL−1) and the combination thereof to inhibit the aggregation of E. faecalis JH2–2 and S. gallolyticus UCN34 to human platelets was assessed by light transmission (A600nm) using a fluorimeter Infinite 200® Pro (Tecan, Salzburg, Austria), as previously described (Veloso et al. 2015). Platelet response was monitored for a maximum of 20 min. Various inhibitors were compared with regard to the interval between the addition of bacteria to the PRP suspension and the onset of the aggregation response (lag time). Adenosine diphosphate (ADP; 10 μM), a known inducer of platelet aggregation, was used as a positive control. Two independent experiments were performed in quadruplicate.
All animal studies were carried out in strict accordance with the recommendations of the Swiss Federal Act on Animal Protection. All protocols for animal studies were reviewed and approved by the Cantonal Committee on Animal Experiments of the State of Vaud, Switzerland (Permit Number: 879.9). A mixture of ketamine (75 mg kg−1) and midazolam (5 mg kg−1) anaesthetics was administered to the animals before any surgical procedure. The production of catheter-induced aortic vegetations was performed in female Wistar rats, as described previously (Veloso et al. 2015). Prophylaxis started immediately after the insertion of the intracardiac catheter and lasted for 48 h. Aspirin (8 mg kg−1) and ticlopidine (10 mg kg−1), alone or in combination, were administered by an intravenous bolus injection every 12 h (Veloso et al. 2015). Control rats received saline. Forty eight hours after starting prophylaxis, animals were inoculated intravenously with 106 CFU of E. faecalis JH2–2 or S. gallolyticus UCN34, progressively delivered at a pace of 0.0017 mL min−1 over 10 h (Veloso et al. 2015). Bacteria were inoculated continuously at low concentrations in order to simulate a protracted, low-level release of bacteria in the bloodstream from an intravenous infected device (Yagupsky and Nolte 1990) or to a constant translocation of low bacterial numbers from the gastrointestinal tract (Berg 1995). Rats were sacrificed 24 h after inoculation. The cardiac vegetations and spleens were sterilely removed, weighed and processed as described elsewhere to determine the number of viable organisms (Veloso et al. 2015). The lower limits of detection of bacterial growth were 2 and 1 log10 CFU g−1 of vegetation and spleen, respectively. Results of spleen cultures were indicated as positive or negative growth.
The quantification of the lag time prior to platelet aggregation, the weights of the vegetations (expressed as mean ± standard deviation) and the mean bacterial counts of cardiac vegetations were compared by the one way analysis of variance (ANOVA) with Tukey's multiple comparisons test. The percentage of infected tissues was analysed by the Fisher's exact test or the Chi-square test. All statistical analyses were performed with GraphPad Prism software (version 6.05 for Windows; GraphPad Software, La Jolla, CA, USA, www.graphpad.com). Differences were considered significant when P < 0.05 by use of two-sided significance levels.
In the absence of either bacteria or drug, the lag time to platelet aggregation was >20 min, whereas in the presence of ADP shrank to nearly 1 min. Enterococcus faecalis JH2–2 did not induce platelet aggregation (time to aggregate >20 min), in line with previous studies that showed that platelet aggregation caused by E. faecalis is strain and donor dependent (Johansson and Rasmussen 2013).
In contrast to E. faecalis JH2–2, S. gallolyticus UCN34 rapidly induced platelet aggregation (lag time of 7.5 ± 1.7 min) with a maximum value of 77%. Aspirin or ticlopidine alone did not affect neither the lag time (8.3 ± 1.7 and 6.8 ± 0.9 min, respectively) nor the maximum aggregation value (80 and 75%, respectively) of S. gallolyticus UCN34. In contrast, the combination of aspirin and ticlopidine significantly increased the lag time to 19.8 ± 0.4 min and inhibited maximum platelet aggregation to 11% (P < 0.0001).
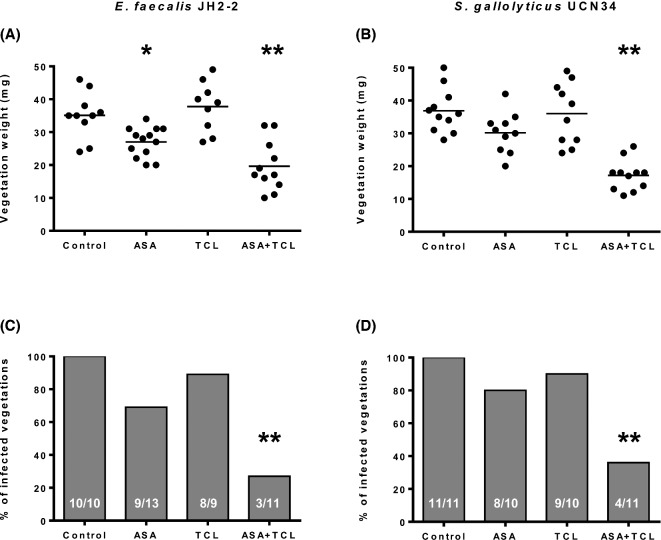
The efficacy of aspirin, ticlopidine and the combination thereof to prevent experimental IE by E. faecalis and S. gallolyticus is illustrated in Fig. 1. In animals challenged with E. faecalis JH2–2, aspirin alone effectively decreased the vegetation weight (P < 0.05) and prevented IE in 31% (4/13) of animals. Ticlopidine alone failed to reduce both the vegetation weight and slightly reduced the incidence of infection (11% [1/9] sterile vegetations). By contrast, the combination of aspirin and ticlopidine significantly reduced the vegetation weight (P < 0.0001) and effectively prevented IE in 73% (8/11) of animals (P = 0.001). Bleeding events occurred only in one animal receiving aspirin. In animals challenged with S. gallolyticus, aspirin reduced, albeit not significantly, the vegetation weight and prevented IE in 20% (2/10) of rats while ticlopidine neither reduced the vegetation weight nor prevented IE (10% [1/10] sterile vegetations). By contrast, the combination of aspirin and ticlopidine significantly reduced the vegetation weight (P < 0.001) and effectively prevented IE (63% [7/11] of sterile vegetations; P < 0.001). No bleeding events were observed. Mean (log10 CFU g−1) bacterial counts in vegetations of control, aspirin, ticlopidine and aspirin plus ticlopidine groups were 7.03, 4.84 (P < 0.05 vs controls), 5.00 and 2.89 (P < 0.05 vs all the other groups) in rats challenged with E. faecalis JH2–2, and 7.25, 5.13 (P < 0.05 vs controls), 5.80 and 3.55 (P < 0.05 vs all the other groups) in rats challenged with S. gallolyticus UCN34, respectively. All (100%) the control animals presented positive spleen cultures. Spleen cultures were also positive in 80–100% of animals receiving aspirin or ticlopidine alone. Spleens from animals receiving aspirin plus ticlopidine were all (100%) infected upon challenge with E. faecalis JH2–2 while only 26% (3/11) were positive upon challenge with S. gallolyticus UCN34 (P < 0.05 vs all the other groups).
Figure 1.
Effect of prophylaxis with aspirin (ASA) and ticlopidine (TCL), alone or in combination, on both the weight of vegetations (A and B) and the prevention of experimental endocarditis (C and D) induced by E. faecalis JH2–2 and S. gallolyticus UCN41. In panels A and B, each dot represents the vegetation weight for each animal and the mean is represented by the solid line. The total number of animals is indicated at the bottom of each column in panels C and D. *P < 0.05 compared to the control group by the ANOVA with Tukey's correction test (vegetation weight). **P < 0.05 compared to all the other groups using the ANOVA with Tukey's correction test (vegetation weight), or using either the Fisher's exact or the Chi-square tests (infected vegetations).
Bacterial-induced platelet aggregation plays an important role in the development of IE. However, the mechanisms of interaction between E. faecalis or S. gallolyticus and platelets are not well defined (Fitzgerald, Foster and Cox 2006; Cox, Kerrigan and Watson 2011). In this work, we report for the first time the ability of S. gallolyticus to induce platelet aggregation. Previous studies reported that E. faecalis and S. gallolyticus bind preferentially to collagen and to a lower extent to fibrinogen and to fibronectin (Sillanpaa et al. 2008; Johansson and Rasmussen 2013). Thus, it is plausible that E. faecalis and S. gallolyticus interact with platelets through the GPVI collagen receptor and to a lesser extent through the GPIIb/IIIa fibrinogen receptor (Michelson 2010).
Aspirin and ticlopidine inhibit platelet aggregation by blocking different effectors of the signal transduction pathway, namely the cyclooxygenase 1 COX1 (an enzyme involved in the thromboxane A2 [TXA2] biosynthesis) and the platelet receptor for ADP P2Y12, respectively (Michelson 2010). Therefore, the inhibitory action of aspirin and ticlopidine occurs downstream of the activating signals transmitted by the interactions between bacteria and platelets, presumably through the blockage of platelet-to-platelet interactions. In this study, aspirin and ticlopidine were given prophylactically, i.e. prior to bacterial inoculation. This strategy likely resulted in decreased formation of the nascent platelet/fibrin vegetations, which deprived circulating bacteria of a binding scaffold, necessary first to adhere to the vegetations and then to promote their development. However, additional effects of the drugs other than targeting platelet receptors, i.e. the ability of aspirin to protect the endothelium from oxidative stress and the antiinflammatory effect of ticlopidine, might also contribute to protection of the animals from IE (Veloso et al. 2015).
The present results might have major clinical implications in regard of prevention against E. faecalis and S. gallolyticus IE. As an example, antiplatelet therapy is commonly used for the prevention of cardiovascular and cerebrovascular diseases in elderly patients, a population with high incidence of IE due to E. faecalis and S. gallolyticus (Slipczuk et al. 2013). The implementation of aspirin and ticlopidine (or equivalent newer molecules), which can be administered orally for prolonged periods, might contribute to reduce the incidence of both E. faecalis and S. gallolyticus IE in this population.
Importantly, the use of antiplatelets, given before the emergence of IE (rather than after IE establishment), is not associated with an increased risk of embolism in case of late IE, which might compromise the validity of this treatment (Anavekar et al. 2007, 2011; Eisen et al. 2009). Likely, early antiaggregant therapy may decrease the size of nascent vegetations and impede their further enlargement, whereas late antiaggregant therapy might favour vegetation dislodgment and bleeding in embolized areas (Chan et al. 2003). The present findings further support the potential beneficial use of antiplatelet agents for the prevention of IE in humans.
Acknowledgments
We thank Marlyse Giddey and Jacques Vouillamoz for their continuous help during the course of this work. We thank Christiane Gerscheimer, of the Service and Central Laboratory of Hematology, Lausanne University Hospital (CHUV), for help with aggregometry.
FUNDING
This work was supported by the Swiss National Science Foundation [grant numbers 310030-125325, 310030-143799/1].
Conflict of interest. None declared.
REFERENCES
- Anavekar NS, Schultz JC, De Sa DD, et al. Modifiers of symptomatic embolic risk in infective endocarditis. Mayo Clin Proc. 2011;86:1068–74. doi: 10.4065/mcp.2011.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anavekar NS, Tleyjeh IM, Anavekar NS, et al. Impact of prior antiplatelet therapy on risk of embolism in infective endocarditis. Clin Infect Dis. 2007;44:1180–6. doi: 10.1086/513197. [DOI] [PubMed] [Google Scholar]
- Berg RD. Bacterial translocation from the gastrointestinal tract. Trends Microbiol. 1995;3:149–54. doi: 10.1016/s0966-842x(00)88906-4. [DOI] [PubMed] [Google Scholar]
- Chan KL, Dumesnil JG, Cujec B, et al. A randomized trial of aspirin on the risk of embolic events in patients with infective endocarditis. J Am Coll Cardiol. 2003;42:775–80. doi: 10.1016/s0735-1097(03)00829-5. [DOI] [PubMed] [Google Scholar]
- Chirouze C, Hoen B, Duval X. Infective endocarditis prophylaxis: moving from dental prophylaxis to global prevention? Eur J Clin Microbiol Infect Dis. 2012;31:2089–95. doi: 10.1007/s10096-012-1564-3. [DOI] [PubMed] [Google Scholar]
- Cox D, Kerrigan SW, Watson SP. Platelets and the innate immune system: mechanisms of bacterial-induced platelet activation. J Thromb Haemost. 2011;9:1097–107. doi: 10.1111/j.1538-7836.2011.04264.x. [DOI] [PubMed] [Google Scholar]
- Eisen DP, Corey GR, McBryde ES, et al. Reduced valve replacement surgery and complication rate in Staphylococcus aureus endocarditis patients receiving acetyl-salicylic acid. J Infect. 2009;58:332–8. doi: 10.1016/j.jinf.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Fitzgerald JR, Foster TJ, Cox D. The interaction of bacterial pathogens with platelets. Nat Rev Microbiol. 2006;4:445–57. doi: 10.1038/nrmicro1425. [DOI] [PubMed] [Google Scholar]
- Johansson D, Rasmussen M. Virulence factors in isolates of Enterococcus faecalis from infective endocarditis and from the normal flora. Microb Pathog. 2013;55:28–31. doi: 10.1016/j.micpath.2012.09.009. [DOI] [PubMed] [Google Scholar]
- Michelson AD. Antiplatelet therapies for the treatment of cardiovascular disease. Nat Rev Drug Discov. 2010;9:154–69. doi: 10.1038/nrd2957. [DOI] [PubMed] [Google Scholar]
- Rusniok C, Couve E, Da Cunha V, et al. Genome sequence of Streptococcus gallolyticus: insights into its adaptation to the bovine rumen and its ability to cause endocarditis. J Bacteriol. 2010;192:2266–76. doi: 10.1128/JB.01659-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoe JA, Witherden IR, Au-Yeung HK, et al. Enterococcal intravascular catheter-related bloodstream infection: management and outcome of 61 consecutive cases. J Antimicrob Chemoth. 2002;50:577–82. doi: 10.1093/jac/dkf182. [DOI] [PubMed] [Google Scholar]
- Sillanpaa J, Nallapareddy SR, Singh KV, et al. Adherence characteristics of endocarditis-derived Streptococcus gallolyticus ssp. gallolyticus (Streptococcus bovis biotype I) isolates to host extracellular matrix proteins. FEMS Microbiol Lett. 2008;289:104–9. doi: 10.1111/j.1574-6968.2008.01378.x. [DOI] [PubMed] [Google Scholar]
- Slipczuk L, Codolosa JN, Davila CD, et al. Infective endocarditis epidemiology over five decades: a systematic review. PLoS One. 2013;8:e82665. doi: 10.1371/journal.pone.0082665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veloso TR, Chaouch A, Roger T, et al. Use of a human-like low-grade bacteremia model of experimental endocarditis to study the role of Staphylococcus aureus adhesins and platelet aggregation in early endocarditis. Infect Immun. 2013;81:697–703. doi: 10.1128/IAI.01030-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veloso TR, Que YA, Chaouch A, et al. Prophylaxis of experimental endocarditis with antiplatelet and antithrombin agents: a role for long-term prevention of infective endocarditis in humans? J Infect Dis. 2015;211:72–9. doi: 10.1093/infdis/jiu426. [DOI] [PubMed] [Google Scholar]
- Wilson W, Taubert KA, Gewitz M, et al. Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2007;116:1736–54. doi: 10.1161/CIRCULATIONAHA.106.183095. [DOI] [PubMed] [Google Scholar]
- Yagi Y, Clewell DB. Recombination-deficient mutant of Streptococcus faecalis. J Bacteriol. 1980;143:966–70. doi: 10.1128/jb.143.2.966-970.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagupsky P, Nolte FS. Quantitative aspects of septicemia. Clin Microbiol Rev. 1990;3:269–79. doi: 10.1128/cmr.3.3.269. [DOI] [PMC free article] [PubMed] [Google Scholar]