Abstract
The aim of this article is to describe the current epidemiological situation of pertussis, as well as different short-term strategies that have been implemented to alleviate this threat. The state of the art of the development of new vaccines that are expected to provide long-lasting immunity against pertussis was also included.
Keywords: pertussis, epidemics, short-term strategies, new vaccines
The authors present a brief review of the current situation with pertussis, a description of the remaining problems/controversies, and how they might be approached/resolved.
Graphical Abstract Figure.
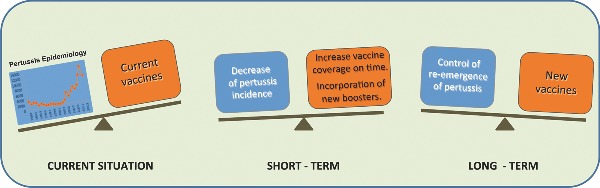
The authors present a brief review of the current situation with pertussis, a description of the remaining problems/controversies, and how they might be approached/resolved.
BRIEF OVERVIEW OF PERTUSSIS EPIDEMIOLOGY
Pertussis or whooping cough is a respiratory disease mainly caused by the bacteria Bordetella pertussis but also by B. parapertussis. The widespread use for over 50 years of pertussis vaccines has markedly reduced the morbidity and mortality associated with this disease. However, in the last few years the incidence rates of pertussis have increased reaching about 16 million cases per year in the world, with approximately 200 000 deaths (WHO 2010b). Most of these cases were reported in developing countries, although several outbreaks were also detected in industrialized countries, even those with high vaccination rates (WHO 2010a; Clark 2014). In the Americas region, the number of cases varies between 1500 and 48 000 (Partiarca et al. 1988; Hozbor et al. 2009; Falleiros Arlant et al. 2014). In Argentina, the last outbreak occurred in 2011 when 76 deaths were reported in infants with less than 6 months (Romanin et al. 2014). In the USA, in 2012 was registered the highest outbreak since 1955, including 48 277 cases and 20 pertussis-related deaths. Incidence rates were very high in infants but also in 7- to 10-year-old children and adolescents (13–14 years) http://www.cdc.gov/pertussis/outbreaks/trends.html. Other industrialized country from Europe that has experienced a notable outbreak is the UK, in which in 2012 nearly 10 000 laboratory-confirmed cases and 14 infant deaths have been reported (Public Health England. Available at https://www.gov.uk/government/publications/whooping-cough-pertussis-statistics, date last accessed November 2014). In Australia, resurgence of pertussis was seen during 2008–12 mainly in children less than 10 years of age, in particular in 2–4 year old and 7–9 year old. Increase in hospitalizations in infants <1 year but without an increase in mortality in this age group was also detected in such country (Spokes, Quinn and McAnulty 2010).
The epidemiological situation detected in many countries has driven health systems to analyze the possible causes of disease resurgence and revise their control actions to strengthen disease control and/or implement short-term strategies to improve the situation, at least for the most vulnerable population represented by infants of less than 1 year of age. Several causes that could explain the increase in the number of pertussis cases have been proposed, most of them associated with the vaccines currently in use: the decrease of vaccine effectiveness over time (waning immunity) (Wendelboe et al. 2005), the selection pressure exerted on bacterial circulating populations that has selected pathogens able to escape immunity induced by the vaccine (Mooi, van Loo and King 2001) and/or the vaccine failure to induce sterilizing immunity to the pathogen and consequently to avoid transmission (Warfel, Zimmerman and Merkel 2014). The first two proposed causes appear to have a greater impact in those countries that use the primary series of vaccination acellular pertussis vaccines (aP) instead of the classic whole-cell pertussis vaccines (wP) (Witt et al. 2013; Clark 2014; Lam et al. 2014; Pawloski et al. 2014). Regarding waning immunity, a systematic literature review and meta-analysis of the duration of protective immunity to pertussis after routine childhood immunization with aP was recently performed (McGirr and Fisman 2015). The authors estimated that the average duration of vaccine protection from aP is ∼3 years, assuming 85% vaccine efficacy. The data reported also suggested that the odds of pertussis increase by 1.33 times (95% confidence interval: 1.23–1.43) for every additional year since the last dose of aP.
Regarding pathogen adaptation, it has been reported that circulating B. pertussis isolates differ from the strains used in vaccines production in their genotypes (Gzyl et al. 2001; Lee et al. 2003; Tsang et al. 2005; Bottero et al. 2007, 2012; King et al. 2008). Within approximately two decades, strains with a novel allele for the pertussis toxin (PT) promoter (ptxP3 strains) have nearly completely replaced the previously dominant ptxP1strains in many European countries, the USA and Australia (Advani et al. 2011; Lam et al. 2012; Petersen et al. 2012; Schmidtke et al. 2012; Mooi, Van Der Maas and De Melker 2014) and also in Argentina. The ptxP3 strains produce more Ptx in vitro and also pertactin (Prn) and a number of other virulence genes, including a type III secretion toxin and Vag8, a protein involved in complement resistance.
The enhanced expression of the vaccine antigens Ptx and Prn by ptxP3 strains was confirmed at the protein level (de Gouw et al. 2014). van Gent et al. (2012) reported that the detected variation in the promoter for PT and Prn contribute significantly to differences in colonization.
Additionally, in countries where acellular vaccines were used as the unique formulation against pertussis, an important increase in the isolation of B. pertussis clinical isolates that do not produce vaccine antigens has been reported, in particular in those that do not express the adhesin pertactin (PRN(–)) (Hegerle and Guiso 2014; Lam et al. 2014; Pawloski et al. 2014; Tsang et al. 2014). It was speculated that this loss probably provides a selective advantage for bacterial survival in vaccinated population. In fact, evidence has been found showing that certain acellular vaccines composed of three components were not as effective as expected in controlling the infection caused by B. pertussis that do not express PRN (Hegerle, Dore and Guiso 2014).
The third possibility was recently proposed by Warfel, Zimmerman and Merkel (2014). These authors, using non-human primates as a model for B. pertussis infection, found evidence on the capacity of acellular vaccine to protect individuals against disease but not against bacterial colonization. In their experiments, infant baboons were vaccinated with three doses of aP or wP vaccines and challenged with B. pertussis at 7 mo. Interestingly, aP-vaccinated baboons can become asymptomatically infected and readily transmitted B. pertussis to unvaccinated contacts at the same rate than naïve controls. In contrast, wP vaccination induced a more rapid clearance compared with naïve and aP-vaccinated animals (Warfel, Zimmerman and Merkel 2014). Key differences in T-cell immunity response were observed since wP-vaccinated animals elicited strong B. pertussis-specific T helper 17 (Th17) memory and Th1 memory, whereas aP vaccination mainly induced a strong Th2 response instead. The observations on the induced immune response correlate well with that observed in humans (Fedele et al. 2010; Higgs et al. 2012). In this sense, a recent study analyzed long-term T-cell memory in adolescents primed with either wP or aP and found that the Th1 response still remained stronger in adolescents primed with wP compared to aP (Smits et al. 2013). It was hypothesized that Th1 cells promotes the production of protective opsonizing antibodies following pertussis vaccination and infection by inducing IgG2a class switching in B cells (Ross et al. 2013). Two recent clinical studies corroborate the notion that aP induces Th2 and Th1 responses in children and found no significant Th17 responses following vaccination (Schure et al. 2012, 2013)
The problems associated with the current vaccines used as well as the strategies recommended and/or implemented to improve the control of the disease in a short-term are discussed in the following sections.
PROBLEMS ASSOCIATED WITH CURRENT PERTUSSIS VACCINES AND WHO POSITION
wP was the first developed vaccine against the disease. With the massive use of this vaccine in the 1950s, the incidence and mortality associated with pertussis fell to very low levels (Amirthalingam, Gupta and Campbell 2013). However, reports on safety concerns in the 1970s shed doubt on the value of wP vaccines. These vaccines were associated not only with side effects at the injection site but also with serious systemic reactions, including whole limb swelling, febrile seizures and persistent crying (Cody et al. 1981; Jefferson, Rudin and DiPietrantonj 2003). These adverse events and to lesser extent the low effectiveness of selected wP vaccines contributed to reduce pertussis vaccine acceptance in countries such as the United Kingdom, Italy, Ireland, Australia, West Germany and Russia (Jefferson, Rudin and DiPietrantonj 2003; Klein 2014). Moreover, in the 1970s, Japan and Sweden suspended their pertussis vaccination programs because of safety and effectiveness concerns, respectively (Sato, Kimura and Fukumi 1984; Romanus, Jonsell and Bergquist 1987).
The widespread apprehension surrounding wP prompted the development of acellular vaccines that contain purified proteic antigenic components of B. pertussis. The first acellular vaccine consisted in PT alone but then other surface attachment proteins, i.e. filamentous hemagglutinin, pertactin (PRN) and two fimbriae proteins (FIM 2 and FIM3) were added (Sato and Sato 1999; Klein 2014).
Though there is no evidence to suggest that wP vaccines cause infant deaths, brain damage or severe neurological disorder neither contraindications to use them, the aP vaccines are more accepted especially in industrialized countries. Indeed, in these countries wP were gradually replaced by aP formulations and currently most of the countries of Europe and USA only use aP vaccines in their calendar. The aP vaccines restored the population confidence in pertussis containing vaccines and the situation seemed to be under control for several years. However, as we mentioned before during the last two decades the epidemiological situation of pertussis has changed. Several outbreaks were reported supporting waning immunity and demonstrating that children primed with wP vaccines had more lasting immunity than those primed with aP vaccines (Witt, Katz and Witt 2012; Klein et al. 2013). A case-control clinical study designed to assess the risk of pertussis among 10–17 year old during the 2010 outbreak in California revealed that teenagers who had received four aP doses were nearly six times more likely to be PCR positive for pertussis than were those who had received four doses of wP (OR 5.6, 95% CI 2.6–12.5) and had nearly four times more chances to get infected likely than those who had received a mix of vaccines (Klein et al. 2013). Another study also found that the risk of pertussis was increased in school children and adolescents whose infant vaccination schedule was composed exclusively of aP doses compared with subjects who received one wP dose (Witt, Katz and Witt 2012; Witt et al. 2013).
An investigation of the 2012 pertussis outbreak in Oregon also showed that among children born during the transition from wP to aP in 1997–99, those who underwent priming with aP rather than wP vaccine had higher rates of reported pertussis. The findings of this investigation concur with those from Australia (Sheridan et al. 2012).
Under this context, in 2010 the WHO published a position paper on the use of pertussis vaccines. This paper included among others the following statements and recommendations: (1) the efficacies of aP and wP vary depending upon the case definition of pertussis used. However, the best aP vaccines have higher efficacy than low-efficacy wP vaccines but they may be less efficacious than the highest efficacy wP vaccines in preventing whooping cough. (2) Protection against severe pertussis in infancy and early childhood can be obtained after a primary series of vaccination with wP or aP vaccine.
In November 2012, the Strategic Advisory Group of Experts on immunization expressed concern regarding the resurgence of pertussis in some industrialized countries despite high vaccine coverage with aP vaccines. The switch from wP to aP vaccines for primary infant immunization was proposed to be responsible at least in part for such resurgence, and therefore WHO recommended that such switch should only be considered if the inclusion in the national immunization schedules of large numbers of doses (including several boosters) can be assured; this has major financial implications due to the much higher cost of aP vaccines and much larger number of doses required.
Countries currently using aP vaccines may continue using this vaccine but should consider the need for additional booster doses and strategies to prevent early childhood mortality in case of resurgence of pertussis.
STRATEGIES TO IMPROVE PERTUSSIS DISEASE CONTROL
Previously to pertussis resurgence, the WHO recommended a three-dose primary series, with the first dose administered at 6 weeks of age; subsequent doses should be given 4–8 weeks apart, at the age of 10–14 weeks and 14–18 weeks. The last dose of that primary series should be completed by the age of 6 months. In addition, a booster dose is recommended for children aged 1–6 years, preferably during the second year of life. The booster should be given ≥6 months after the last primary dose. With this four-dose schedule (primary series plus booster), at least 6 years of protection against pertussis is expected (WHO).
With the pertussis resurgence, the Advisory Committee on Immunization Practices of the US Centers for Disease Control and Prevention (CDC), Global Pertussis Initiative (GPI) and other international and national entities have recommended a booster for adolescents and adults in order to improve disease control (Broder et al. 2006; CDC 2011a,b). Several countries have recommended and/or implemented acellular booster doses with aP (Tdap) for adolescents (Argentina, Australia, USA, France among others) and/or replaced the decennial Td dose with a single or more doses of Tdap for adults (including among others the USA, Australia, Austria, Italia). Furthermore, some countries recommend selected immunization of pregnant women, child care workers and parents of newborns (e.g. Argentina, Austria and Germany) (http://vaccine-schedule.ecdc.europa.eu/Pages/Scheduler.aspx). The benefits of Tdap booster vaccination when applied to a particular age group of adolescents or adults resulted to be adequate only for the population group that receives the vaccine during the first years after vaccination (Acosta et al. 2015; Haller et al. 2015; Vandermeulen et al. 2015). However, immunization of these groups does not impact over the incidence of disease in more vulnerable population represented by the newborns and infants with less than 6 months (Stein-Zamir et al. 2010; Fabricius et al. 2013).
Recently, based on available evidence, the GPI recommended maternal immunization during pregnancy as the primary strategy to protect newborns and infants (Forsyth et al. 2015). If maternal immunization is not possible, or if families desire additional protective measures for their newborns, then it is recommended that all individuals having close contact with infants 6 months old are immunized consistent with local health authority guidelines. GPI state that a high priority should be given to achieving a complete cocoon, defined as full immunization of the family, since the robustness of protection against pertussis is a function of the number of infant contacts vaccinated. If a complete cocoon is not possible, then the next priority is vaccination of both parents, followed by the mother only. For families using cocooning, immunization should occur during pregnancy or immediately postpartum to prevent pertussis transmission to infants 6 months old or less (Forsyth et al. 2015).
It is important to note, however, that data on clinical effectiveness of the different recently recommended vaccination strategies against pertussis still remain limited. However, it is increasingly clear that reducing the circulation of B. pertussis and protecting infants against severe disease may be difficult to achieve by a single approach, and multiple immunization strategies with current vaccines should be applied.
Due to the aforementioned situation, much interest is set in developing new alternative options for antipertussis immunization. The lack of an absolute correlate of protection has made this task more difficult. Data from animal models and human studies indicate that although antibodies may mediate protection, Th1 and Th17 cellular responses are responsible for long-lasting protection (Mills et al. 2014). Since current acellular vaccines elicit mainly a Th2 response, several proposals have been suggested to combine these vaccines with adjuvants that may drive a Th1 response, at least for the priming doses (Allen and Mills 2014). In this sense, the use of the TLR9 ligand CpG has been shown to accomplish this aim in the mouse model and may represent an interesting alternative (Asokanathan, Corbel and Xing 2013). The addition of modified LPS molecules with an acceptable safety profile to acellular antipertussis formulation has also been proposed following this same rationale (Brummelman et al. 2015). Mills and coworkers have recently identified a B. pertussis outer membrane protein that has the capacity to trigger TLR2 response ant that may also be added to a multicomponent acellular vaccine having a dual role of skewing Th response and also having antigenic capacity per se (Dunne et al. 2015).
Since wP vaccines are being revalued over acellular vaccines regarding their effectiveness (Witt et al. 2013), there are also ongoing attempts to reduce its inherent toxicity by changing the production process (Dias et al. 2013). Several researchers have also identified vaccine candidates of potential interest to be combined with the immunogens already in use in order to broaden the protective capacity of acellular vaccines. Proteins involved in iron sequestration may constitute alternative antigens to be used following this strategy (Alvarez Hayes et al. 2011). The known virulence factor Adenilate cyclase-hemolysin (AC-Hly) has been proposed to be considered for incorporation in the vaccine (Sebo, Osicka and Masin 2014). Since the residual toxic activity may induce adverse responses, recombinant toxin with changes in critical sites that affect its biological activity without changing its protective capacity was generated (Sebo, Osicka and Masin 2014). Similarly to PT toxin, the inherent biological activity of AC induces adverse effects, which are prevented by chemically detoxification of the protein by treatments with cross-linking agents such as formaldehyde or glutaraldehyde. However, these treatments also affect protective epitopes, resulting in a non-optimal protective capacity. There are attempts to generate recombinant PT variants having low biological toxicity, without affecting its conformation and consequently producing high quality of blocking antibodies (Seubert et al. 2014). It has been proposed to use this newly formulated antigen with different adjuvanting schemes in order to achieve an adequate cellular response profile (Robbins et al. 2014). Furthermore, other combinations of novel candidate immunogens and adjuvants have been proposed (Polewicz et al. 2013), which paves the way for future comparative studies or selection of the appropriate antigen/adjuvant combination.
A complete different approach has been followed by Locht and coworkers who generated an attenuated B. pertussis strain by introducing several mutations in three key virulence factors showing the capacity of this strain to act as an attenuated live vaccine when delivered intranasally (Mielcarek et al. 2006). This vaccine has been shown to produce long-lasting protective immunity associated with transient colonization in the mouse model (Feunou et al. 2010) as well as generating B and Th1/Th17 immunity (Feunou, Bertout and Locht 2010). Additional mutations in virulence factors such as adenilate cyclase that affects the capacity of the vaccine strain BPZE1 to replicate in vivo were shown to be detrimental to the protective capacity of this vaccine candidate (Lim et al. 2014). Since the preexisting antipertussis immunity may affect the capacity of the vaccinal strain to colonize and subsequently generate protective responses, BPZE1 has been postulated to be used shortly after birth, with the consequent associated risks. It has been shown that using the BPZE1 as priming vaccination and subsequent doses of acellular vaccine recalls a Th1 response (Feunou et al. 2014), encouraging for the use of this type of combinations. Based on results obtained in preclinical models, this candidate was subjected to clinical phase I trial on adult volunteers following a dose-escalation scheme and showing minor safety events (Thorstensson et al. 2014). The generation of a B-cell response attributed to BPZE1 colonization was observed in participants of this randomized trial (Jahnmatz et al. 2014). Studies are underway to move forward the development of antipertussis vaccine following this strategy.
OUTER MEMBRANE VESICLES AS AN ATTRACTIVE CANDIDATE FOR PERTUSSIS VACCINE
Gram-negative bacteria naturally release lipid bilayer vesicles from the outer membrane that range in size from approximately 20 to 200 nm in diameter and enclose many native bacterial antigens in the spherical particles. The vesicles function in diverse roles including facilitation of the infection progression (Schroeder and Aebischer 2009; Schaar et al. 2011; Donato et al. 2012; Ercoli et al. 2015). Because of their immunogenic properties, self-adjuvanticity and ability to be taken up by mammalian cells, outer membrane vesicles (OMVs) are attractive candidates as vaccine delivery platforms. In fact, OMVs vaccines have already been administered to humans. The most successful use of OMVs vaccines had been against serogroup B Neisseria meningitidis (MenB) (Findlow et al. 2007). In Norway, Cuba and New Zealand, licensed OMVs vaccines used in epidemics of MenB had proven to be immunogenic and protective (Bjune et al. 1991; Sierra et al. 1991). Research is ongoing to develop a global vesicle-based vaccine to N. meningitidis because existing formulations cover single meningococcal lineages with little cross-reactivity (Granoff 2010; Zollinger et al. 2010). Recently, Zollinger et al. showed in animal models that native OMVs design is potentially safe and also broadly protective for possible use as a universal vaccine against meningococcal disease. This OMV-based vaccine continues in development with clinical trials underway (Zollinger et al. 2010).
The proven efficacy records of these OMVs vaccines together with the knowledge that vesicles are produced by nearly all species of Gram-negative bacteria (de Kleijn et al. 2000) presented the possibility of employing the OMV vaccination strategy for prevention of other diseases. In this context, we have demonstrated first that OMVs derived from B. pertussis can protect against intranasal pertussis challenge when administered by either intraperitoneal or intranasal route in a mouse model of infection (Roberts et al. 2008). Furthermore, we have shown that our vaccine based on OMVs derived from B. pertussis is safe and able to induce protection in mice against different B. pertussis genotypic backgrounds (Roberts et al. 2008; Asensio et al. 2011) including those not expressing the virulence factor pertactin (PRN) (manuscript in preparation). Furthermore, we observed that this vaccine elicits a protective immune response with a mixed Th1/Th2 profile with a robust antibody response and also induce a long-term protection in a murine model (Gaillard et al. 2014).
We also developed a vaccine based on OMVs derived from B. parapertussis that also showed to be safe and protective against B. parapertussis infection and in less extent to B. pertussis (Bottero et al. 2013).
Based on the excellent properties of both vaccines, we developed a vaccine, which combines OMVs derived from both Bordetella species. We have characterized the composition of the pertussis OMVs finding at least 40 protein components, mostly membrane-bound proteins (Roberts et al. 2008; Bottero et al. 2013). The presence of a large number of immunogens in the vaccine formulation is important since this may avoid the excessive selective pressure conferred by a single or a few protective vaccine antigens as those present in aP vaccines. Our OMVs-based vaccine candidate (OMVsBp+Bpp vaccine) exhibits indeed adequate protection capacity against different B. pertussis genetic background but also against B. parapertussis. In agreement with our previous results, we detected that OMVsBp+Bpp vaccine induced Th1/Th2 and Th17 immune response. (manuscript in preparation). All these properties open the possibility of envisaging the use of Bordetella-OMVs-based vaccine mainly as initial immunization strategy followed by boosters of classic aP formulations.
Considering the aforementioned epidemiological situation and the challenges to be solved by new developments, the data presented here show that there are several alternatives under development that may contribute in the future to enlarge the options for prophylactic vaccination against pertussis. In the short term, plans for addressing the resurgence of pertussis should include continued efforts to enhance immunization in children on time. Strategies such as ‘cocooning’ and neonatal vaccination, immunization of pregnant mothers with aP seems to be required to protect the most vulnerable members of the population, infants who have not yet vaccinated. Longer term goals include the development of new vaccines. For developed countries, new aP vaccines containing more antigens/adjuvant in the formulation appear to be a good strategy. However, for developing countries, which can not easily afford these vaccines due to their expensive costs, new generation of wP with reduced toxicity seems to be potentially affordable approach. In the case of OMVs-based vaccines, they resemble wP in the sense of providing a complex mix of antigens but with lower toxicity and low cost. Thus, these vaccines appear to be feasible to be used both in developed and developing countries.
FUNDING
This work was supported by ANCPyT and CICBA (Argentina) grants to DFH. DFH, MEG, DB, GM and MR are members of the Scientific Career of CONICET.
Conflict of interest. None declared.
REFERENCES
- Acosta AM, DeBolt C, Tasslimi A, et al. Tdap vaccine effectiveness in adolescents during the 2012 Washington State pertussis epidemic. Pediatrics. 2015;135:981–9. doi: 10.1542/peds.2014-3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Advani A, Gustafsson L, Ahren C, et al. Appearance of Fim3 and ptxP3-Bordetella pertussis strains, in two regions of Sweden with different vaccination programs. Vaccine. 2011;29:3438–42. doi: 10.1016/j.vaccine.2011.02.070. [DOI] [PubMed] [Google Scholar]
- Allen AC, Mills KH. Improved pertussis vaccines based on adjuvants that induce cell-mediated immunity. Expert Rev Vaccines. 2014;13:1253–64. doi: 10.1586/14760584.2014.936391. [DOI] [PubMed] [Google Scholar]
- Alvarez Hayes J, Erben E, Lamberti Y, et al. Identification of a new protective antigen of Bordetella pertussis. Vaccine. 2011;29:8731–9. doi: 10.1016/j.vaccine.2011.07.143. [DOI] [PubMed] [Google Scholar]
- Amirthalingam G, Gupta S, Campbell H. Pertussis immunisation and control in England and Wales, 1957 to 2012: a historical review. Euro Surveill. 2013;18:pii: 20587. doi: 10.2807/1560-7917.es2013.18.38.20587. [DOI] [PubMed] [Google Scholar]
- Asensio CJ, Gaillard ME, Moreno G, et al. Outer membrane vesicles obtained from Bordetella pertussis Tohama expressing the lipid A deacylase PagL as a novel acellular vaccine candidate. Vaccine. 2011;29:1649–56. doi: 10.1016/j.vaccine.2010.12.068. [DOI] [PubMed] [Google Scholar]
- Asokanathan C, Corbel M, Xing D. A CpG-containing oligodeoxynucleotide adjuvant for acellular pertussis vaccine improves the protective response against Bordetella pertussis. Hum Vaccin Immunother. 2013;9:325–31. doi: 10.4161/hv.22755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjune G, Hoiby EA, Gronnesby JK, et al. Effect of outer membrane vesicle vaccine against group B meningococcal disease in Norway. Lancet. 1991;338:1093–6. doi: 10.1016/0140-6736(91)91961-s. [DOI] [PubMed] [Google Scholar]
- Bottero D, Gaillard ME, Basile LA, et al. Genotypic and phenotypic characterization of Bordetella pertussis strains used in different vaccine formulations in Latin America. J Appl Microbiol. 2012;112:1266–76. doi: 10.1111/j.1365-2672.2012.05299.x. [DOI] [PubMed] [Google Scholar]
- Bottero D, Gaillard ME, Errea A, et al. Outer membrane vesicles derived from Bordetella parapertussis as an acellular vaccine against Bordetella parapertussis and Bordetella pertussis infection. Vaccine. 2013;31:5262–8. doi: 10.1016/j.vaccine.2013.08.059. [DOI] [PubMed] [Google Scholar]
- Bottero D, Gaillard ME, Fingermann M, et al. Pulsed-field gel electrophoresis, pertactin, pertussis toxin S1 subunit polymorphisms, and surfaceome analysis of vaccine and clinical Bordetella pertussis strains. Clin Vaccine Immunol. 2007;14:1490–8. doi: 10.1128/CVI.00177-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broder KR, Cortese MM, Iskander JK, et al. Preventing tetanus, diphtheria, and pertussis among adolescents: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccines recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2006;55:1–34. [PubMed] [Google Scholar]
- Brummelman J, Helm K, Hamstra HJ, et al. Modulation of the CD4(+) T cell response after acellular pertussis vaccination in the presence of TLR4 ligation. Vaccine. 2015;33:1483–91. doi: 10.1016/j.vaccine.2015.01.063. [DOI] [PubMed] [Google Scholar]
- CDC (CfDCaP) Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine (Tdap) in pregnant women and persons who have or anticipate having close contact with an infant aged <12 months — Advisory Committee on Immunization Practices (ACIP) MMWR Morb Mortal Wkly Rep. 2011a;60:1424–6. [PubMed] [Google Scholar]
- CDC (CfDCaP) Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis (Tdap) vaccine from the Advisory Committee on Immunization Practices, 2010. MMWR Morb Mortal Wkly Rep. 2011b;60:13–5. [PubMed] [Google Scholar]
- Clark TA. Changing pertussis epidemiology: everything old is new again. J Infect Dis. 2014;209:978–81. doi: 10.1093/infdis/jiu001. [DOI] [PubMed] [Google Scholar]
- Cody CL, Baraff LJ, Cherry JD, et al. Nature and rates of adverse reactions associated with DTP and DT immunizations in infants and children. Pediatrics. 1981;68:650–60. [PubMed] [Google Scholar]
- de Gouw D, Hermans PW, Bootsma HJ, et al. Differentially expressed genes in Bordetella pertussis strains belonging to a lineage which recently spread globally. PLoS One. 2014;9:e84523. doi: 10.1371/journal.pone.0084523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kleijn ED, de Groot R, Labadie J, et al. Immunogenicity and safety of a hexavalent meningococcal outer-membrane-vesicle vaccine in children of 2–3 and 7–8 years of age. Vaccine. 2000;18:1456–66. doi: 10.1016/s0264-410x(99)00423-5. [DOI] [PubMed] [Google Scholar]
- Dias WO, van der Ark AA, Sakauchi MA, et al. An improved whole cell pertussis vaccine with reduced content of endotoxin. Hum Vaccin Immunother. 2013;9:339–48. doi: 10.4161/hv.22847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato GM, Goldsmith CS, Paddock CD, et al. Delivery of Bordetella pertussis adenylate cyclase toxin to target cells via outer membrane vesicles. FEBS Lett. 2012;586:459–65. doi: 10.1016/j.febslet.2012.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunne A, Mielke LA, Allen AC, et al. A novel TLR2 agonist from Bordetella pertussis is a potent adjuvant that promotes protective immunity with an acellular pertussis vaccine. Mucosal Immunol. 2015;8:607–17. doi: 10.1038/mi.2014.93. [DOI] [PubMed] [Google Scholar]
- Ercoli G, Tani C, Pezzicoli A, et al. LytM proteins play a crucial role in cell separation, outer membrane composition, and pathogenesis in nontypeable Haemophilus influenzae. mBio. 2015;6:e02575. doi: 10.1128/mBio.02575-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabricius G, Bergero PE, Ormazabal ME, et al. Modelling pertussis transmission to evaluate the effectiveness of an adolescent booster in Argentina. Epidemiol Infect. 2013;141:718–34. doi: 10.1017/S0950268812001380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falleiros Arlant LH, de Colsa A, Flores D, et al. Pertussis in Latin America: epidemiology and control strategies. Expert Rev Anti-Infe. 2014;12:1265–75. doi: 10.1586/14787210.2014.948846. [DOI] [PubMed] [Google Scholar]
- Fedele G, Spensieri F, Palazzo R, et al. Bordetella pertussis commits human dendritic cells to promote a Th1/Th17 response through the activity of adenylate cyclase toxin and MAPK-pathways. PLoS One. 2010;5:e8734. doi: 10.1371/journal.pone.0008734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feunou PF, Bertout J, Locht C. T- and B-cell-mediated protection induced by novel, live attenuated pertussis vaccine in mice. Cross protection against parapertussis. PLoS One. 2010;5:e10178. doi: 10.1371/journal.pone.0010178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feunou PF, Kammoun H, Debrie AS, et al. Long-term immunity against pertussis induced by a single nasal administration of live attenuated B. pertussis BPZE1. Vaccine. 2010;28:7047–53. doi: 10.1016/j.vaccine.2010.08.017. [DOI] [PubMed] [Google Scholar]
- Feunou PF, Kammoun H, Debrie AS, et al. Heterologous prime-boost immunization with live attenuated B. pertussis BPZE1 followed by acellular pertussis vaccine in mice. Vaccine. 2014;32:4281–8. doi: 10.1016/j.vaccine.2014.06.019. [DOI] [PubMed] [Google Scholar]
- Findlow J, Holland A, Andrews N, et al. Comparison of phenotypically indistinguishable but geographically distinct Neisseria meningitidis Group B isolates in a serum bactericidal antibody assay. Clin Vaccine Immunol. 2007;14:1451–7. doi: 10.1128/CVI.00195-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsyth K, Plotkin S, Tan T, et al. Strategies to decrease pertussis transmission to infants. Pediatrics. 2015;135:e1475–82. doi: 10.1542/peds.2014-3925. [DOI] [PubMed] [Google Scholar]
- Gaillard ME, Bottero D, Errea A, et al. Acellular pertussis vaccine based on outer membrane vesicles capable of conferring both long-lasting immunity and protection against different strain genotypes. Vaccine. 2014;32:931–7. doi: 10.1016/j.vaccine.2013.12.048. [DOI] [PubMed] [Google Scholar]
- Granoff DM. Review of meningococcal group B vaccines. Clin Infect Dis. 2010;50(Suppl 2):S54–65. doi: 10.1086/648966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gzyl A, Augustynowicz E, van Loo I, et al. Temporal nucleotide changes in pertactin and pertussis toxin genes in Bordetella pertussis strains isolated from clinical cases in Poland. Vaccine. 2001;20:299–303. doi: 10.1016/s0264-410x(01)00356-5. [DOI] [PubMed] [Google Scholar]
- Haller S, Dehnert M, Karagiannis I, et al. Effectiveness of routine and booster pertussis vaccination in children and adolescents, federal state of brandenburg, Germany, 2002–2012. Pediatr Infect Dis J. 2015;34:513–9. doi: 10.1097/INF.0000000000000654. [DOI] [PubMed] [Google Scholar]
- Hegerle N, Dore G, Guiso N. Pertactin deficient Bordetella pertussis present a better fitness in mice immunized with an acellular pertussis vaccine. Vaccine. 2014;32:6597–600. doi: 10.1016/j.vaccine.2014.09.068. [DOI] [PubMed] [Google Scholar]
- Hegerle N, Guiso N. Bordetella pertussis and pertactin-deficient clinical isolates: lessons for pertussis vaccines. Expert Rev Vaccines. 2014;13:1135–46. doi: 10.1586/14760584.2014.932254. [DOI] [PubMed] [Google Scholar]
- Higgs R, Higgins SC, Ross PJ, et al. Immunity to the respiratory pathogen Bordetella pertussis. Mucosal Immunol. 2012;5:485–500. doi: 10.1038/mi.2012.54. [DOI] [PubMed] [Google Scholar]
- Hozbor D, Mooi F, Flores D, et al. Pertussis epidemiology in Argentina: trends over 2004–2007. J Infect. 2009;59:225–31. doi: 10.1016/j.jinf.2009.07.014. [DOI] [PubMed] [Google Scholar]
- Jahnmatz M, Amu S, Ljungman M, et al. B-cell responses after intranasal vaccination with the novel attenuated Bordetella pertussis vaccine strain BPZE1 in a randomized phase I clinical trial. Vaccine. 2014;32:3350–6. doi: 10.1016/j.vaccine.2014.04.048. [DOI] [PubMed] [Google Scholar]
- Jefferson T, Rudin M, DiPietrantonj C. Systematic review of the effects of pertussis vaccines in children. Vaccine. 2003;21:2003–14. doi: 10.1016/s0264-410x(02)00770-3. [DOI] [PubMed] [Google Scholar]
- King AJ, van Gorkom T, Pennings JL, et al. Comparative genomic profiling of Dutch clinical Bordetella pertussis isolates using DNA microarrays: identification of genes absent from epidemic strains. BMC Genomics. 2008;9:311. doi: 10.1186/1471-2164-9-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein NP. Licensed pertussis vaccines in the United States. History and current state. Hum Vaccin Immunother. 2014;10:2684–90. doi: 10.4161/hv.29576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein NP, Bartlett J, Fireman B, et al. Comparative effectiveness of acellular versus whole-cell pertussis vaccines in teenagers. Pediatrics. 2013;131:e1716–22. doi: 10.1542/peds.2012-3836. [DOI] [PubMed] [Google Scholar]
- Lam C, Octavia S, Bahrame Z, et al. Selection and emergence of pertussis toxin promoter ptxP3 allele in the evolution of Bordetella pertussis. Infect Genet Evol. 2012;12:492–5. doi: 10.1016/j.meegid.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Lam C, Octavia S, Ricafort L, et al. Rapid Increase in Pertactin-deficient Bordetella pertussis Isolates, Australia. Emerg Infect Dis. 2014;20:626–33. doi: 10.3201/eid2004.131478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YS, Yang CY, Lu CH, et al. Molecular epidemiology of Bordetella pertussis isolated in Taiwan, 1992–1997. Microbiol Immunol. 2003;47:903–9. doi: 10.1111/j.1348-0421.2003.tb03463.x. [DOI] [PubMed] [Google Scholar]
- Lim A, Ng JK, Locht C, et al. Protective role of adenylate cyclase in the context of a live pertussis vaccine candidate. Microbes Infect. 2014;16:51–60. doi: 10.1016/j.micinf.2013.10.002. [DOI] [PubMed] [Google Scholar]
- McGirr A, Fisman DN. Duration of pertussis immunity after DTaP immunization: a meta-analysis. Pediatrics. 2015;135:331–43. doi: 10.1542/peds.2014-1729. [DOI] [PubMed] [Google Scholar]
- Mielcarek N, Debrie AS, Raze D, et al. Live attenuated B. pertussis as a single-dose nasal vaccine against whooping cough. PLoS Pathog. 2006;2:e65. doi: 10.1371/journal.ppat.0020065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills KH, Ross PJ, Allen AC, et al. Do we need a new vaccine to control the re-emergence of pertussis? Trends Microbiol. 2014;22:49–52. doi: 10.1016/j.tim.2013.11.007. [DOI] [PubMed] [Google Scholar]
- Mooi FR, Van Der Maas NA, De Melker HE. Pertussis resurgence: waning immunity and pathogen adaptation—two sides of the same coin. Epidemiol Infect. 2014;142:685–94. doi: 10.1017/S0950268813000071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooi FR, van Loo IH, King AJ. Adaptation of Bordetella pertussis to vaccination: a cause for its reemergence? Emerg Infect Dis. 2001;7:526–8. doi: 10.3201/eid0707.017708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partiarca PA, Steketee RW, Biellik RJ, et al. Outbreaks of pertussis in the United States: the Wisconsin experience. Tokai J Exp Clin Med. 1988;13(Suppl):117–23. [PubMed] [Google Scholar]
- Pawloski LC, Queenan AM, Cassiday PK, et al. Prevalence and molecular characterization of pertactin-deficient Bordetella pertussis in the United States. Clin Vaccine Immunol. 2014;21:119–25. doi: 10.1128/CVI.00717-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RF, Dalby T, Dragsted DM, et al. Temporal trends in Bordetella pertussis populations, Denmark, 1949–2010. Emerg Infect Dis. 2012;18:767–74. doi: 10.3201/eid1805.110812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polewicz M, Gracia A, Garlapati S, et al. Novel vaccine formulations against pertussis offer earlier onset of immunity and provide protection in the presence of maternal antibodies. Vaccine. 2013;31:3148–55. doi: 10.1016/j.vaccine.2013.05.008. [DOI] [PubMed] [Google Scholar]
- Robbins JB, Schneerson R, Kubler-Kielb J, et al. Toward a new vaccine for pertussis. P Natl Acad Sci USA. 2014;111:3213–6. doi: 10.1073/pnas.1324149111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R, Moreno G, Bottero D, et al. Outer membrane vesicles as acellular vaccine against pertussis. Vaccine. 2008;26:4639–46. doi: 10.1016/j.vaccine.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Romanin V, Agustinho V, Califano G, et al. Epidemiological situation of pertussis and strategies to control it: Argentina, 2002–2011. Arch Argent Pediatr. 2014;112:413–20. doi: 10.5546/aap.2014.eng.413. [DOI] [PubMed] [Google Scholar]
- Romanus V, Jonsell R, Bergquist SO. Pertussis in Sweden after the cessation of general immunization in 1979. Pediatr Infect Dis J. 1987;6:364–71. doi: 10.1097/00006454-198704000-00005. [DOI] [PubMed] [Google Scholar]
- Ross PJ, Sutton CE, Higgins S, et al. Relative contribution of Th1 and Th17 cells in adaptive immunity to Bordetella pertussis: towards the rational design of an improved acellular pertussis vaccine. PLoS Pathog. 2013;9:e1003264. doi: 10.1371/journal.ppat.1003264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y, Kimura M, Fukumi H. Development of a pertussis component vaccine in Japan. Lancet. 1984;1:122–6. doi: 10.1016/s0140-6736(84)90061-8. [DOI] [PubMed] [Google Scholar]
- Sato Y, Sato H. Development of acellular pertussis vaccines. Biologicals. 1999;27:61–9. doi: 10.1006/biol.1999.0181. [DOI] [PubMed] [Google Scholar]
- Schaar V, de Vries SP, Perez Vidakovics ML, et al. Multicomponent Moraxella catarrhalis outer membrane vesicles induce an inflammatory response and are internalized by human epithelial cells. Cell Microbiol. 2011;13:432–49. doi: 10.1111/j.1462-5822.2010.01546.x. [DOI] [PubMed] [Google Scholar]
- Schmidtke AJ, Boney KO, Martin SW, et al. Population diversity among Bordetella pertussis isolates, United States, 1935–2009. Emerg Infect Dis. 2012;18:1248–55. doi: 10.3201/eid1808.120082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder J, Aebischer T. Recombinant outer membrane vesicles to augment antigen-specific live vaccine responses. Vaccine. 2009;27:6748–54. doi: 10.1016/j.vaccine.2009.08.106. [DOI] [PubMed] [Google Scholar]
- Schure RM, Hendrikx LH, de Rond LG, et al. T-cell responses before and after the fifth consecutive acellular pertussis vaccination in 4-year-old Dutch children. Clin Vaccine Immunol. 2012;19:1879–86. doi: 10.1128/CVI.00277-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schure RM, Hendrikx LH, de Rond LG, et al. Differential T- and B-cell responses to pertussis in acellular vaccine-primed versus whole-cell vaccine-primed children 2 years after preschool acellular booster vaccination. Clin Vaccine Immunol. 2013;20:1388–95. doi: 10.1128/CVI.00270-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebo P, Osicka R, Masin J. Adenylate cyclase toxin-hemolysin relevance for pertussis vaccines. Expert Rev Vaccines. 2014;13:1215–27. doi: 10.1586/14760584.2014.944900. [DOI] [PubMed] [Google Scholar]
- Seubert A, D'Oro U, Scarselli M, et al. Genetically detoxified pertussis toxin (PT-9K/129G): implications for immunization and vaccines. Expert Rev Vaccines. 2014;13:1191–204. doi: 10.1586/14760584.2014.942641. [DOI] [PubMed] [Google Scholar]
- Sheridan SL, Ware RS, Grimwood K, et al. Number and order of whole cell pertussis vaccines in infancy and disease protection. JAMA. 2012;308:454–6. doi: 10.1001/jama.2012.6364. [DOI] [PubMed] [Google Scholar]
- Sierra GV, Campa HC, Varcacel NM, et al. Vaccine against group B Neisseria meningitidis: protection trial and mass vaccination results in Cuba. NIPH Ann. 1991;14:195–207. [PubMed] [Google Scholar]
- Smits K, Pottier G, Smet J, et al. Different T cell memory in preadolescents after whole-cell or acellular pertussis vaccination. Vaccine. 2013;32:111–8. doi: 10.1016/j.vaccine.2013.10.056. [DOI] [PubMed] [Google Scholar]
- Spokes PJ, Quinn HE, McAnulty JM. Review of the 2008–2009 pertussis epidemic in NSW: notifications and hospitalisations. N S W Public Health Bull. 2010;21:167–73. doi: 10.1071/NB10031. [DOI] [PubMed] [Google Scholar]
- Stein-Zamir C, Shoob H, Abramson N, et al. The impact of additional pertussis vaccine doses on disease incidence in children and infants. Vaccine. 2010;29:207–11. doi: 10.1016/j.vaccine.2010.10.058. [DOI] [PubMed] [Google Scholar]
- Thorstensson R, Trollfors B, Al-Tawil N, et al. A phase I clinical study of a live attenuated Bordetella pertussis vaccine–BPZE1; a single centre, double-blind, placebo-controlled, dose-escalating study of BPZE1 given intranasally to healthy adult male volunteers. PLoS One. 2014;9:e83449. doi: 10.1371/journal.pone.0083449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang RS, Shuel M, Jamieson FB, et al. Pertactin-negative Bordetella pertussis strains in Canada: characterization of a dozen isolates based on a survey of 224 samples collected in different parts of the country over the last 20 years. Int J Infect Dis. 2014;28:65–9. doi: 10.1016/j.ijid.2014.08.002. [DOI] [PubMed] [Google Scholar]
- Tsang RS, Sill ML, Martin IE, et al. Genetic and antigenic analysis of Bordetella pertussis isolates recovered from clinical cases in Ontario, Canada, before and after the introduction of the acellular pertussis vaccine. Can J Microbiol. 2005;51:887–92. doi: 10.1139/w05-079. [DOI] [PubMed] [Google Scholar]
- van Gent M, Bart MJ, van der Heide HG, et al. Small mutations in Bordetella pertussis are associated with selective sweeps. PLoS One. 2012;7:e46407. doi: 10.1371/journal.pone.0046407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandermeulen C, Theeten H, Rathi N, et al. Decennial administration in young adults of a reduced-antigen content diphtheria, tetanus, acellular pertussis vaccine containing two different concentrations of aluminium. Vaccine. 2015;33:3026–34. doi: 10.1016/j.vaccine.2014.10.049. [DOI] [PubMed] [Google Scholar]
- Warfel JM, Zimmerman LI, Merkel TJ. Acellular pertussis vaccines protect against disease but fail to prevent infection and transmission in a nonhuman primate model. P Natl Acad Sci USA. 2014;111:787–92. doi: 10.1073/pnas.1314688110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendelboe AM, Van Rie A, Salmaso S, et al. Duration of immunity against pertussis after natural infection or vaccination. Pediatr Infect Dis J. 2005;24:S58–61. doi: 10.1097/01.inf.0000160914.59160.41. [DOI] [PubMed] [Google Scholar]
- WHO. Global summary schedules 2010a. http://apps.who.int/immunization_monitoring/globalsummary/schedules (13 August 2015, date last accessed) [Google Scholar]
- WHO. Pertussis vaccines: WHO position paper. Wkly Epidemiol Rec. 2010b;85:385–400. [PubMed] [Google Scholar]
- Witt MA, Arias L, Katz PH, et al. Reduced risk of pertussis among persons ever vaccinated with whole cell pertussis vaccine compared to recipients of acellular pertussis vaccines in a large US cohort. Clin Infect Dis. 2013;56:1248–54. doi: 10.1093/cid/cit046. [DOI] [PubMed] [Google Scholar]
- Witt MA, Katz PH, Witt DJ. Unexpectedly limited durability of immunity following acellular pertussis vaccination in preadolescents in a North American outbreak. Clin Infect Dis. 2012;54:1730–5. doi: 10.1093/cid/cis287. [DOI] [PubMed] [Google Scholar]
- Zollinger WD, Donets MA, Schmiel DH, et al. Design and evaluation in mice of a broadly protective meningococcal group B native outer membrane vesicle vaccine. Vaccine. 2010;28:5057–67. doi: 10.1016/j.vaccine.2010.05.006. [DOI] [PubMed] [Google Scholar]


