Abstract
Progestin-based contraception may impact women's susceptibility to sexually transmitted infection. We evaluated the effect of the levonorgestrel intrauterine system (LNG-IUS) on cervical persistence of Chlamydia trachomatis (CT) in a baboon model. Female olive baboons (Papio anubis) with or without an LNG-IUS received CT or sham inoculations. CT was detected in cervical epithelium with weekly nucleic acid amplification testing (NAAT) and culture. Presence of the LNG-IUS was associated with prolonged persistence of CT. Median time to post-inoculation clearance of CT as detected by NAAT was 10 weeks (range 7–12) for animals with an LNG-IUS and 3 weeks (range 0–12) for non-LNG-IUS animals (P = 0.06). Similarly, median time to post-inoculation clearance of CT by culture was 9 weeks (range 3–12) for LNG-IUS animals and 1.5 weeks (range 0–10) for non-LNG-IUS animals (P = 0.04). We characterized the community structure of the vaginal microbiota with the presence of the LNG-IUS to determine if alterations in CT colonization dynamics were associated with changes in vaginal commensal bacteria. Vaginal swabs were collected weekly for microbiome analysis. Endocervical CT infection was not correlated with alterations in the vaginal microbiota. Together, these results suggest that LNG-IUS may facilitate CT endocervical persistence through a mechanism distinct from vaginal microbial alterations.
Keywords: vaginal microbiome, sexually transmitted infection, contraception
In a baboon model, the levonorgestrel-releasing intrauterine system is associated with prolonged Chlamydia trachomatis infection without changes in the commensal bacteria of the vagina.
Graphical Abstract Figure.
In a baboon model, the levonorgestrel-releasing intrauterine system is associated with prolonged Chlamydia trachomatis infection without changes in the commensal bacteria of the vagina.
INTRODUCTION
The interface between sexually transmitted infection (STI) and contraceptive method is a crucial dimension of women's reproductive and sexual health. Progestin-based contraceptives have been associated with increased risk of STI acquisition and transmission, including Chlamydia trachomatis (CT) (Baeten et al. 2001; Mohllajee et al. 2006; Morrison, Turner and Jones 2009; Polis et al. 2014) one of the most prevalent STIs in the United States (Satterwhite et al. 2013; CDC 2014). The levonorgestrel-releasing intrauterine system (LNG-IUS) is a highly effective form of intrauterine contraception that is increasing in use among women in the USA (Thonneau and Almont 2008; Finer, Jerman and Kavanaugh 2012). While the epidemiologic association of the LNG-IUS with ascending CT infection (pelvic inflammatory disease) has been evaluated (Grimes 2000), little is known of the impact of levonorgestrel or the LNG-IUS on cervical CT infection and clearance. This interaction is of particular relevance for populations such as adolescents in which expanded use of intrauterine contraception is supported (Suhonen et al. 2004; Secura et al. 2014; Friedman 2015) and for whom concurrent risk of STI acquisition is high (Datta et al. 2007; Torrone et al. 2014).
Endogenous ovarian hormones regulate the activity of both innate and adaptive immune responses in the female reproductive tract (Hafner, Cunningham and Beagley 2013; Wira et al. 2014). The commensal bacteria of the vagina influence immune responses and are thought to provide important protective roles against pathogens. Mechanisms by which these bacteria may enhance pathogen resistance include direct inhibition of colonization through, for example, maintenance of low pH, or indirect immunomodulatory effects (Antonio, Hawes and Hillier 1999; Mirmonsef et al. 2011; Sassone-Corsi and Raffatellu 2015). Indirect evidence suggests that vaginal microbiota are sensitive to hormonal mediation. For example, temporal fluctuations in the vaginal microbiota as well as epithelial storage of the bacterial substrate glycogen correspond with menstrual cyclicity (Farage and Maibach 2006; Gajer et al. 2012). Alterations in vaginal microbial community composition may therefore influence host susceptibility to pathogen challenge.
We sought to establish whether the presence of the LNG-IUS was associated with altered CT colonization dynamics. Using a previously established baboon model (Bell et al. 2011, 2013; Rivera et al. 2011; Uchihashi et al. 2015), we examined the impact of the LNG-IUS on clearance of CT cervical infection. This was performed in the context of a larger study evaluating the impact of the LNG-IUS on clinical severity of CT infection. We identified an interesting trend towards persistence of endocervical CT in the presence of the LNG-IUS. Here we evaluate one potential mechanism for this finding, specifically whether this persistence of endocervical CT infection can be explained by LNG-IUS-induced changes in vaginal microbial community composition.
MATERIALS AND METHODS
Humane care guidelines
In vivo work was approved by the Institutional Review Committee (IRC) at the Institute of Primate Research (IPR; Karen, Kenya) and received an off-site exemption from the University of Michigan's University Committee on Use and Care of Animals. IPR was selected due to a history of successful collaboration, a large population of genetically diverse, wild-origin baboons and extensive institutional expertise with reproductive disease modeling in non-human primates (Kyama et al. 2007; Nyachieo et al. 2009; Bergin et al. 2013; Hashway et al. 2014; Uchihashi et al. 2015). The IRC at IPR adheres to humane animal use principles in accordance with the 3 R's (Russell and Burch 1959), Kenyan law (Cap 360: Prevention of Cruelty to Animals Act) and the International Guiding Principles for Biomedical Research Involving Animals (CIOMS and ICLAS 2012). The latter is consistent with the American Society of Primatology's Principles for the Ethical Treatment of Nonhuman Primates. IPR has statutory compliance and registration with the National Institutes of Health Office of Laboratory Animal Welfare (foreign institution assurance #A5796–01).
Study population and housing
Wild-caught reproductively mature female olive baboons (Papio anubis; n = 20) were humanely trapped with permission of the Kenyan Wildlife Service and transported within 72 hours to quarantine facilities at IPR. Baboons were captured between 6 June 2012 and 14 August 2012 at a single site (ADC Mutara Ranch, Laikipia, Kenya). Upon arrival, baboons underwent a 90-day quarantine in small groups during which animals received routine health surveillance including tuberculosis testing and conditioning for endo- and ectoparasites. Animals were externally and laparoscopically screened for gross evidence of reproductive tract pathology prior to study initiation. Unique tattoo numbers were used for animal identification and will be used to refer to each animal (e.g. Baboon 3910). In order to prevent cross-contamination during the course of the study, animals were housed individually in outdoor enclosures. Visual contact with conspecifics was provided. Baboons were fed monkey biscuits and a variety of fresh fruits and vegetables. Water was provided ad libitum.
Study design
The data presented here were collected during a larger study evaluating the impact of the LNG-IUS on the clinical severity of CT infection in a baboon model. Baboons were randomly assigned to receive an LNG-IUS or no LNG-IUS (n = 10 per group). For the animals receiving an LNG-IUS, baseline cervicovaginal and fallopian samples were collected just prior to LNG-IUS insertion. Baboons were acclimated to the LNG-IUS for 24 weeks, during which time cervicovaginal sampling was performed once weekly for 5 weeks then once every 4 weeks for a total duration of 24 weeks. A pre-inoculation set of baseline cervicovaginal and fallopian samples were collected for all animals. Baboons were then randomly assigned to receive either CT (n = 8) or sucrose-phosphate-glutamate (SPG; n = 2) inoculations. Baboons were inoculated once weekly for 5 weeks. As the duration of infection was greater than one ovarian cycle, we did not control for cycle phase. Baboons were sampled weekly until 16 weeks post-inoculation. All animals were euthanized at 16 weeks after the first CT inoculation.
Anesthesia and health monitoring
For cervicovaginal examination and sampling, baboons were sedated with an intramuscular injection of 10 mg/kg ketamine (Rotexmedica GmbH, Tritau, Germany) and 0.5 mg/kg xylazine (Rompun®; Bayer, Pittsburg, PA). For IUS placement and laparoscopic examination, baboons were first sedated as described above then intubated and maintained on halothane. Following completion of procedures, xylazine sedation was reversed with 0.1 mg/kg atipamezole (Antisedan®; Zoetis, Florham Park, NJ).
Baboons were assessed daily for pain or discomfort as evidenced by behavioral abnormalities, alterations in external appearance, weight, appetite, fecal or urine output. Additionally, animals were examined for evidence of LNG-IUS expulsion and/or genitourinary tract discharge or hemorrhage. Cyclic changes in perivulvar skin tumescence were recorded (Higham et al. 2008).
LNG-IUS placement
LNG-IUS placement has been previously described (Bell et al. 2013). Briefly, anesthetized baboons (n = 10) were placed in dorsal recumbency, a pediatric Pederson speculum placed and an LNG-IUS (Mirena®; Bayer Healthcare Pharmaceuticals, Wayne, NJ, USA) inserted according to manufacturer instructions. Following successful placement, IUS strings were trimmed to the level of the cervical os to prevent digital removal by baboons. IUS retention was confirmed by visual inspection for strings on vaginal speculum examination once weekly for 5 weeks and at all subsequent time points. Animals were acclimated to the LNG-IUS for 24 weeks prior to CT inoculation.
CT inoculation and detection
A clinical isolate of CT serovar E obtained from a patient with pelvic inflammatory disease was prepared at the University of Washington Chlamydia Laboratory. Briefly, CT was propagated in McCoy cell culture, purified and aliquoted into SPG-buffered inocula containing 107 inclusion forming units/milliliter (IFU/ml). Aliquots were frozen at −80°C prior to shipment on dry ice to IPR. For all inoculations, 1 × 107 IFU/ml of CT or an equal volume of SPG was sprayed directly on to the ectocervix via tuberculin syringe. Successful inoculation using this technique has been previously validated (Bell et al. 2011).
Endocervical swab specimens were tested for the presence of CT using a commercially available nucleic acid amplification (NAAT) system, the BD ProbeTec C. trachomatis QX Amplified DNA Assay on the BD Viper System (BD Diagnostics; Sparks, MD, USA). In this qualitative assay, CT target DNA is extracted and detected via strand displacement amplification of a region of the CT cryptic plasmid open reading frame 3 in the presence of a fluorescently-labeled detector probe (Taylor et al. 2011). NAATs were performed according to manufacturer instructions. CT culture was performed as previously described (Bell et al. 2011). Briefly, vials of Buffalo Green Monkey Kidney cells were inoculated with 200 μl of vortexed specimen, centrifuged and fed with chlamydial isolation medium. Vials were incubated for 48–72 hours at 35°C then cells were fixed and stained with Microtrak fluorescent monoclonal antibody. IFUs were quantified via fluorescence microscopy.
Sample collection
Vaginal and endocervical swab samples (digene Female Swab Specimen Collection Kit; Qiagen, Venlo, Netherlands) were collected during sedated lower reproductive tract examination. For vaginal samples, a swab was rolled on the vaginal walls approximately 2.5–5 cm distal to introitus. Specimens were placed in proprietary Specimen Transport Medium included in swab kits. All specimens were stored at −80°C until time of shipment, then transported on dry ice by an international courier agency (World Courier, New Hyde Park, NY, USA) to the University of Michigan for DNA isolation and sequence analysis. Export permits (CITES) from the Kenyan Wildlife Service and permits for import of baboon tissue were obtained from the Centers for Disease Control for all shipped samples. At 16 weeks post-inoculation, all animals were sedated with ketamine/xylazine and euthanized with intravenous injection of 60 mg/kg sodium pentobarbital cocktail (Euthanaze®; Centaur Labs, Johannesburg, South Africa). Tissue samples were collected and fixed in 10% neutral buffered formalin for later histopathologic analysis.
DNA isolation, amplification and sequencing
DNA was isolated from vaginal swabs using a PowerMag Soil DNA Isolation Kit (MO BIO Laboratories, Inc., Carlsbad, CA) on an epMotion® 5075 TMX automated liquid handling system (Eppendorf, Hamburg, Germany) according to manufacturer instructions. The fourth hypervariable region (V4) of the 16S rRNA encoding region of microbial genomic DNA was amplified using a dual-indexing sequencing strategy using custom primers (Kozich et al. 2013). All steps for amplification and library construction were performed as previously described with the exception that each 20 μl PCR mixture contained 5 μl of 4.0 μM primer set (Koenigsknecht et al. 2015). Sequencing was performed on an Illumina® MiSeq sequencer (Illumina, Inc., San Diego, CA).
Sequence curation and data analysis
16S gene sequences were processed using the mothur software package version 1.34.4 according to the publicly available MiSeq SOP (http://www.mothur.org/wiki/MiSeq_SOP; accessed 1 November 2014) (Schloss et al. 2009) and as described by Kozich et al. (2013). Sequence curation was performed remotely using a batch file submitted to the University of Michigan Flux High Powered Computing Cluster (http://arc-ts.umich.edu/flux/). Paired-end reads were assembled into contigs and aligned with the SILVA reference database (Pruesse et al. 2007). The UCHIME algorithm was used for chimera identification (Edgar et al. 2011). The average-neighbor algorithm with a 3% distance cutoff was used to cluster 16S rRNA gene sequences into operational taxonomic units (OTUs). Taxonomic assignments were made using a naïve Bayesian classifier with a publicly available training set from the Ribosomal Database Project (version 9; https://rdp.cme.msu.edu/) (Wang et al. 2007). Sequences were subsampled to a level of 2960 sequences per sample prior to data analysis.
Analysis of microbial communities was performed using mothur version 1.34.4 and the mothur MiSeq SOP as described above (Schloss et al. 2009; Kozich et al. 2013). All statistical analyses were performed using R version 3.1.2 (R Core Team 2014). Comparisons of CT infection dynamics were performed using the Wilcoxon signed rank test and a generalized estimating equation implemented in R package gee with utilization of an exchangeable correlation structure and robust sandwich estimator (Carey 2012). Data from five baboons were excluded from analysis of CT infection length due to premature removal from the study (one non-LNG-IUS baboon reached study endpoint of pelvic inflammatory disease development, one non-LNG-IUS baboon did not achieve NAAT positivity and three LNG-IUS baboons were removed prematurely due to non-CT-related illness). Shannon diversity indices and observed OTUs were calculated for all samples. Qualitative assessment of longitudinal trends in Shannon diversity was performed by examining within subject change from baseline at each time point. Comparisons across multiple time points were made using the non-parametric Wilcoxon signed rank test. Distances between microbial community structures were calculated using the OTU-based Yue and Clayton distance metric (θYC) (Yue and Clayton 2005). Similarity of communities was evaluated using analysis of molecular variance (AMOVA) that accounted for repeated measures and visualized using principle coordinates analysis (PCoA) (Anderson 2001). In order to further characterize non-linear relationships between microbial community composition and CT, we utilized a random-forest algorithm with 10 000 trees to identify OTUs associated with CT (NAAT positive or negative) in the presence or absence of the LNG-IUS. R package randomForest was utilized for this analysis (Breiman 2001; Liaw and Wiener 2002). OTUs with a mean decrease in accuracy (MDA) greater than 25 were selected for further analysis. This threshold was selected to include those OTUs that most closely approximated the MDA of the CT OTU (MDA ≈ 35). Abundance of these OTUs was compared across individual animals. OTUs with marked abundance in only a single animal were not reported. For all statistical analyses, significance was defined as P < 0.05 after multiple comparisons correction. Sequence data obtained in this study are available within the NCBI Sequence Read Archive, accession number SRP062122.
RESULTS
Dynamics of cervical CT infection with and without LNG-IUS
The dynamics of CT infection in the baboon lower reproductive tract were evaluated using cervical NAATs, culture and 16S rRNA-encoding gene sequence analysis. Presence of the LNG-IUS was associated with prolonged persistence of CT within the cervical epithelium (Fig. 1). Median time to post-inoculation endocervical clearance of CT as detected by NAAT was 10 weeks (range 7–12) for LNG-IUS animals in comparison to 3 weeks (range 0–12) for non-LNG-IUS animals (P = 0.06). Similarly, median time to post-inoculation endocervical clearance of CT by culture was 9 weeks (range 3–12) for LNG-IUS animals and 1.5 weeks (range 0–10) for non-LNG-IUS animals (P = 0.04). We used generalized estimating equation regression to model the association between presence of the LNG-IUS and endocervical CT as measured by NAAT or culture (Liang and Zeger 1986). The interaction between LNG-IUS and CT was significant for culture (P = 0.04) but not NAAT (P = 0.07). When compared across all post-inoculation time points, the median relative abundance of CT vaginal 16S sequences did not differ significantly (P = 0.27) between the LNG-IUS animals (0.009%; range 0–47%) and non-LNG-IUS animals (0.003%; range 0–64%) although considerable variation in abundance was observed.
Figure 1.
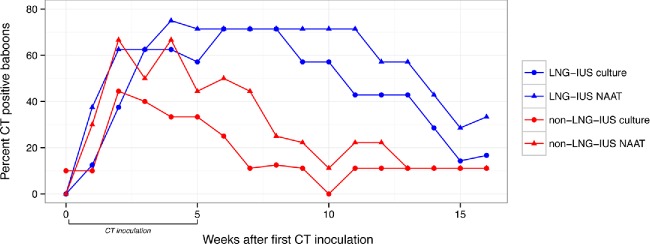
Dynamics of CT endocervical infection as detected by culture or NAAT in baboons with or without an LNG-IUS.
Characterization of baboon vaginal microbial communities
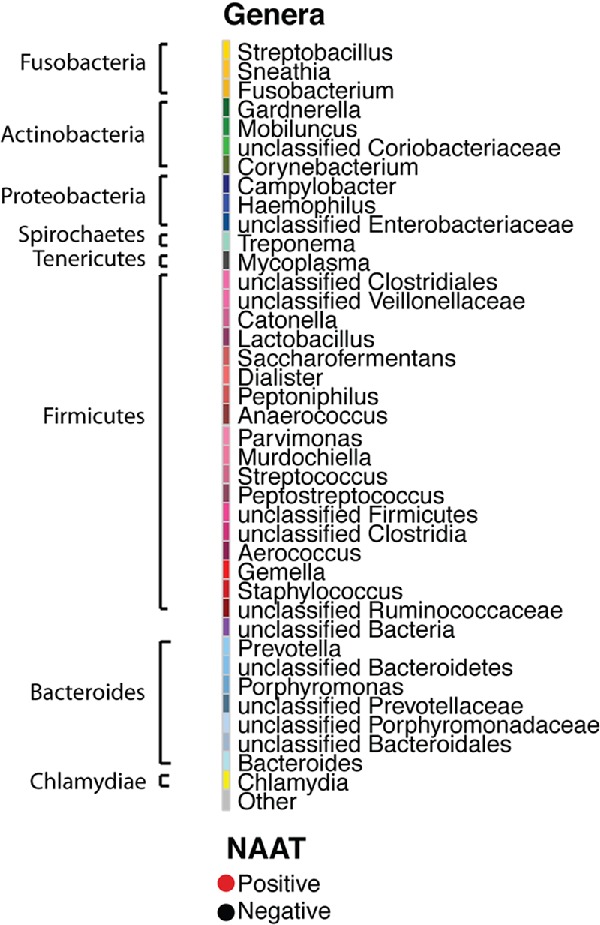
Vaginal swab samples from 26 time points were obtained from the LNG-IUS baboons (including acclimation and post-acclimation time points). Vaginal swab samples from 17 time points were obtained from the non-LNG-IUS baboons. The median number of V4 16S rRNA gene sequence reads per sample was 29 463 (range = 43–60 838). Taxonomic diversity of genera across samples was substantial, with a total of 351 genera observed but only 17 genera present in at least 90% of samples. This conserved core of microbial genera included Gram-negative facultative or obligate anaerobes such as Campylobacter, Prevotella, Mobiluncus, Treponema and Streptobacillus and is consistent with previous descriptions of the vaginal microbial communities of wild-caught baboons (Uchihashi et al. 2015). Similarly, OTU-based analysis revealed substantial diversity. We observed 1441 OTUs with a median of 57 (range = 18–372) OTUs observed per sample. Longitudinal microbial community dynamics from representative members of the LNG-IUS and non-LNG-IUS groups are presented in Fig. 2.
Figure 2.
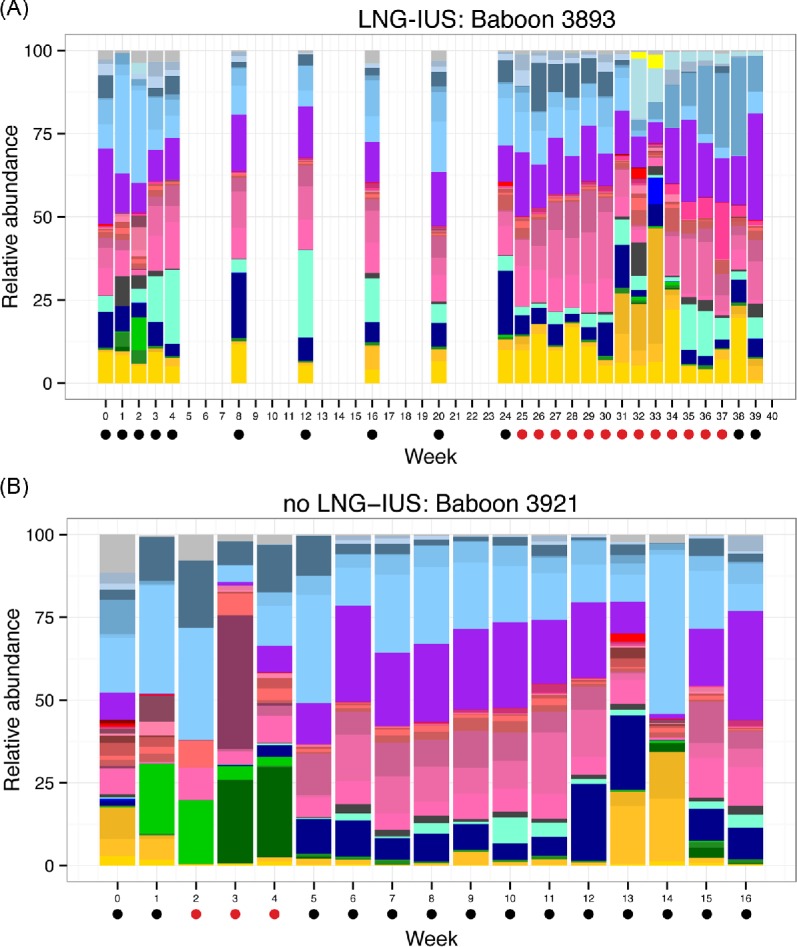
Relative abundance of bacterial genera over time for a representative animal from the (A) LNG-IUS and (B) non-LNG-IUS groups.
LNG-IUS and CT do not independently alter vaginal microbial communities
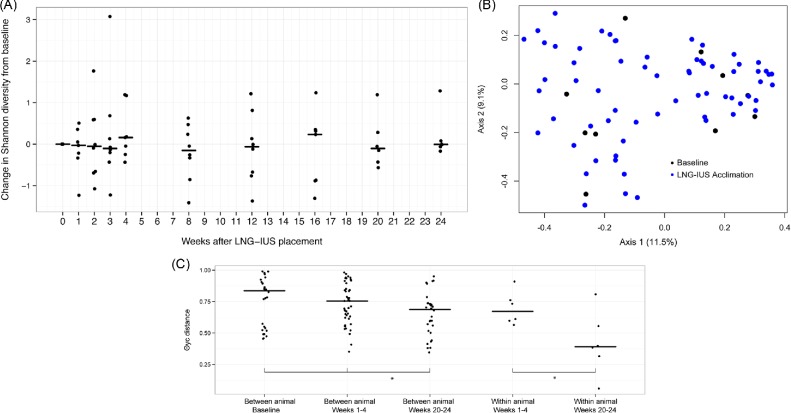
We first observed the impacts of the LNG-IUS and CT inoculation, considered independently, on vaginal microbiota. We began by comparing vaginal microbial communities at baseline to the 24 weeks of LNG-IUS acclimation. There was no significant difference in median Shannon diversity index (3.04 versus 2.91) or observed OTUs (54 versus 58) between animals at baseline and during the acclimation phase. Qualitative assessment of temporal trends in individual rate of change in Shannon diversity suggests that the presence of a LNG-IUS was not associated with alteration of vaginal microbial alpha diversity (Fig. 3A). Using AMOVA, a difference was not observed in community structure using θYC distances (P = 0.35; Fig. 3B) nor was there appreciable visual separation of communities using ordination of θYC distances (PCoA).
Figure 3.
The impact of the LNG-IUS on the baboon vaginal microbiota. (A) The change in Shannon diversity index from baseline for each individual animal over the course of 16 weeks of LNG-IUS acclimation. (B) PCoA of vaginal microbial community distances before (black) and after (blue) LNG-IUS insertion. (C) A comparison of between and within animal community distances at baseline, immediately following LNG-IUS insertion (weeks 1–4), and at the end of the acclimation phase (weeks 20–24).
Previous studies of the baboon have revealed a striking degree of difference between the vaginal microbial communities of individual animals. We compared the independent impact of the presence of the LNG-IUS on both inter- and intraindividual differences using θYC distance at baseline, early time points (weeks 1–4) and late time points (weeks 20–24). Despite substantial within subject variability over time, median intraindividual distance was significantly less than interindividual distance. Interestingly, the presence of the LNG-IUS was associated with a slight stabilizing effect: significant decreases in both between and within subject median community distances were observed over time (Fig. 3C).
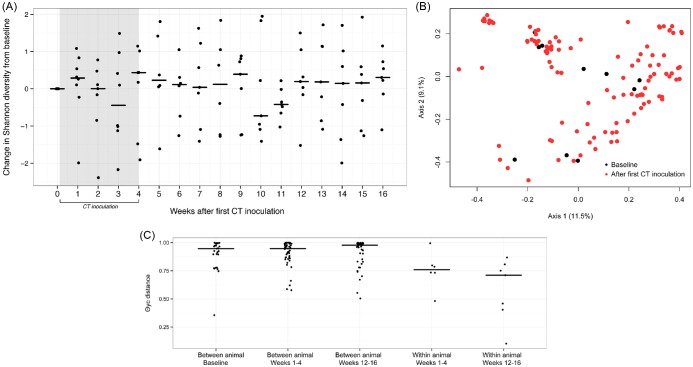
Next we evaluated the impact of CT inoculation on baboon vaginal microbiota using similar methods as described above. There were no significant differences in median vaginal microbial diversity (2.64 versus 2.55) or richness (49 versus 54) nor were longitudinal trends observed on visual assessment of change in Shannon diversity over time (Fig. 4A). Once again, θYC-based ordination and AMOVA did not reveal differences in community composition following CT inoculation (P = 0.27) when the CT OTU was removed from the analysis (Fig. 4B). Comparison of community θYC distances at baseline, early time points (weeks 1–4) and late time points (weeks 12–16) was not supportive of alterations in community distance associated with CT inoculation (Fig. 4C), although once again distances between animals were greater than those observed within animals over time.
Figure 4.
The impact of CT on the baboon vaginal microbiota. (A) The change in Shannon diversity index from baseline for each individual animal over the course of 16 weeks of observation during and following CT inoculation. (B) PCoA of vaginal microbial community distances before (black) and after (red) first CT inoculation. (C) A comparison of between and within animal community distances at baseline, during CT inoculation (weeks 1–4), and at the end of the experimental observation (weeks 12–16).
In order to further investigate whether the presence of CT in the cervical epithelium was associated with a particular microbial community composition, we used OTU abundance data in a random forest machine-learning algorithm to distinguish between NAAT negative and positive samples in the non-LNG-IUS animals. This algorithm utilizes regression analysis of multiple decision trees to select specific predictive features (OTUs, in this case) out of multivariate datasets that best correspond to a pre-defined classification (NAAT positive or NAAT negative, in this case) (Breiman 2001). Feature selection identified three non-CT OTUs with a mean decrease accuracy (MDA, measure of the impact of each feature on the model accuracy) greater than 25. These OTUs included three members of the class Clostridia (OTUs 16, 17 and 45; MDA = 27.62, 27.98 and 26.38, respectively). OTUs 16 and 45 were further classified at genus level as members of the genera Saccharofermentans and Peptostreptococcus. However, an out of bag (OOB) error rate of 21.9% was obtained. OOB error is an estimate of the prediction error in the random forest classification. Although the OOB error did not differ substantially when the CT OTU was removed from the analysis (OOB = 23.2), of the selected OTUs, only OTU 17 was significantly increased in NAAT positive samples (P < 0.01). Considered in their entirety, these data indicate that there is minimal support for differentiation of vaginal microbial communities based on cervical NAAT status.
CT infection in the presence of the LNG-IUS results in minimal alteration of vaginal microbiota
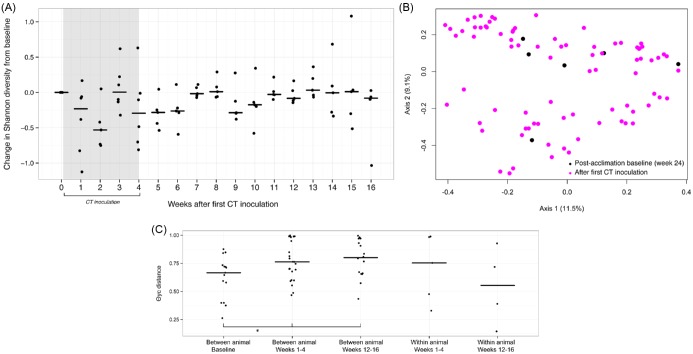
We next characterized the impact of CT inoculation on the vaginal microbiota in the presence of an LNG-IUS. A comparison of Shannon diversity and richness in all pre- and post-inoculation time points revealed a modest decrease in the median number of OTUs following CT inoculation in the presence of the LNG-IUS (61.5 versus 52; P = 0.03) but no difference in median bacterial diversity (3.02 versus 2.88; P = 0.13; not shown). Assessment of longitudinal trends in change in Shannon diversity suggested a decrease in diversity during the inoculation phase followed by relative stabilization after approximately 11 weeks post-inoculation (Fig. 5A). θYC-based ordination and AMOVA (P = 0.72, Fig. 5B) indicated no differences in community composition following CT inoculation when the CT OTU was removed from the analysis. Further evidence for destabilization was provided by analysis of community distances across experimental phases. In contrast to CT inoculation alone, inoculation in the presence of an LNG-IUS was associated with an increase in median between subject community distance in comparison to baseline (Fig. 5C).
Figure 5.
The impact of CT on the baboon vaginal microbiota in the presence of the LNG-IUS. (A) The change in Shannon diversity index from baseline for each individual animal over the course of 16 weeks of observation during and following CT inoculation. (B) PCoA of post-LNG-IUS acclimation vaginal microbial community distances before (black) and after (pink) first CT inoculation. (C) A comparison of between and within animal community distances at post-LNG-IUS acclimation baseline, during CT inoculation (weeks 1–4) and at the end of the experimental observation (weeks 12–16).
In order to identify whether particular OTUs were associated with the presence of CT in LNG-IUS animals, we again used OTU abundance data in a random forest algorithm to distinguish between NAAT negative and positive samples in both pre- and post-CT inoculation experimental phases. Feature selection identified seven non-CT OTUs with a mean decrease in accuracy greater than 25 (OOB error of 22.9%). These OTUs were associated with unclassified Bacteria, as well as members of the family Flavobacteriaceae, and the genus Mycoplasma. However, on further inspection the relative abundance of these OTUs was not significantly greater in NAAT positive samples for any OTU identified, again suggesting minimal support for differentiation of vaginal microbial communities based on cervical NAAT status in the presence of the LNG-IUS.
DISCUSSION
CT is an intracellular bacterium that has evolved complex mechanisms for evasion of host defense (Brunham and Rey-Ladino 2005). Indeed, in most individuals CT infection is asymptomatic although a subset of women experience more severe sequelae such as tubal factor infertility or pelvic inflammatory disease (Mardh 2004). Here we report an interesting trend towards cervical persistence of CT in the presence of an LNG-IUS. Prolonged shedding was not associated with exacerbation of clinical disease (manuscript in preparation). Most epidemiologic work on progestin-based contraceptives has focused on the interaction of depot medroxyprogesterone acetate and STI risk (Polis et al. 2014; Morrison et al. 2015; Ralph et al. 2015). There has been little to no investigation of the impact of the LNG-IUS on risk of CT acquisition or transmission, although use of the T380a copper intrauterine device or levonorgestrel emergency contraception is not associated with increases in incident CT (Morrison, Turner and Jones 2009; Raine-Bennett et al. 2015).
We examined whether altered CT infection dynamics were associated with changes in vaginal microbiota. Presence of the LNG-IUS was associated with a modest increase in stability of vaginal microbiota but did not result in major shifts in community diversity, richness or composition. These data suggest that neither contraceptive device nor CT is a prominent independent driver of community composition. This is similar to previous limited work on the impacts of the LNG-IUS and Chlamydia on vaginal microbiota in animal models. For example, in a six-month study of three baboons, presence of a LNG-IUS was not associated with specific shifts in vaginal microbial community composition, although this study did not include pathogen challenge (Hashway et al. 2014). Similarly, a study of 11 women evaluated vaginal microbial communities before and after LNG-IUS placement for up to 12 weeks post-placement and concluded alterations associated with the contraceptive device were minimal (Jacobson et al. 2014). In a guinea pig model of chlamydial infection, Chlamydia caviae infection was found to significantly reduce the total bacterial load but not community composition of the vaginal microbiome (Neuendorf et al. 2015).
The commensal microbial community is only one of a complex network of host-associated factors that may influence CT pathogenesis. Given CT's capacity for asymptomatic chronicity, it is perhaps unsurprising that CT does not induce profound shifts in vaginal commensal communities. Such shifts could potentiate the host inflammatory response resulting in accelerated pathogen clearance. Moreover, there still exists the potential for altered functional profiles in the absence of compositional change. For example, Prevotella species found in the conserved core of the baboon vaginal microbial community produce indole from tryptophan (Sasaki-Imamura et al. 2011). Depletion of tryptophan is associated with entry of Chlamydia into a non-infectious persistent phase (Beatty et al. 1994). Although in our analysis Prevotella abundance was not significantly associated with the presence of endocervical CT, temporal or interindividual differences in tryptophan availability could theoretically impact CT persistence. Despite our limited understanding of the functional roles of individual microbes and microbial metabolites in the female reproductive tract (Ma, Forney and Ravel 2012), further investigation of functional immunological or metabolic responses in correlation with particular microbial communities may be helpful in determining whether CT infection may compromise such responses.
If the endocervical persistence of CT cannot be explained by specific alterations of the vaginal microbiota, what other potential mechanisms might be possible? One attractive possibility is direct or indirect hormonal attenuation of innate or adaptive immune responses. Steroid hormones such as levonorgestrel may affect many aspects of female reproductive tract immunity, including the distribution and activity of immune effector cells. CT is thought to initiate innate immune responses through activation of Toll-like receptors 2 and 4, the expression of which is menstrual cycle dependent (Wira, Grant-Tschudy and Crane-Godreau 2005; Hirata et al. 2007). Similarly, CD8 + T-cell activity which is thought to be important for CT clearance is decreased during the secretory phase of women's menstrual cycles (White et al. 1997). In addition to immune alterations, LNG-IUS placement is associated with alterations in the cervical mucous plug (Lewis et al. 2010; Natavio et al. 2013). Changes in cervical mucin viscosity, rate of turnover or glycosylation could impact susceptibility to pathogen penetration (Wiggins et al. 2001). These and other immunomodulations were beyond the scope of this study but are areas of interest for future evaluation.
Our results suggest that vaginal microbiota are not heavily influenced by the presence of localized progestin release or CT and may therefore be unlikely to play an important role in host susceptibility to chronic CT infection. Since infection does not necessarily correlate with disease, this study does not imply the LNG-IUS is a risk factor for CT-associated sequelae in women nor did we evaluate whether cervical persistence of CT is associated with increased risk of transmission. Rather, this preliminary finding identifies a need for further preclinical and epidemiologic investigation of a potential interaction between the LNG-IUS and CT infection dynamics.
Acknowledgments
The authors would like to thank the microbial sequencing core personnel of the University of Michigan Host Microbiome Initiative, the Animal Care and Resources staff of the Institute of Primate Research, the University of Washington Chlamydia Laboratory, staff of the University of Michigan Clinical Microbiology Lab, and members of the laboratories of Drs. Vincent Young and Patrick Schloss including Dr Anna Seekatz, Matthew Jenior, Nielson Baxter and Jhansi Leslie.
FUNDING
This work was supported the National Institutes of Health Women's Reproductive Health Research Scholar Program [NIH K12 HD065257–01].
Conflict of interest. None declared.
References
- Anderson MJ. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001;26:32–46. [Google Scholar]
- Antonio MA, Hawes SE, Hillier SL. The identification of vaginal Lactobacillus species and the demographic and microbiologic characteristics of women colonized by these species. J Infect Dis. 1999;180:1950–6. doi: 10.1086/315109. [DOI] [PubMed] [Google Scholar]
- Baeten JM, Nyange PM, Richardson BA, et al. Hormonal contraception and risk of sexually transmitted disease acquisition: results from a prospective study. Am J Obstet Gynecol. 2001;185:380–5. doi: 10.1067/mob.2001.115862. [DOI] [PubMed] [Google Scholar]
- Beatty WL, Belanger TA, Desai AA, et al. Tryptophan depletion as a mechanism of gamma interferon-mediated chlamydial persistence. Infect Immun. 1994;62:3705–11. doi: 10.1128/iai.62.9.3705-3711.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell JD, Bergin IL, Harris LH, et al. The effects of a single cervical inoculation of Chlamydia trachomatis on the female reproductive tract of the baboon (Papio anubis) J Infect Dis. 2011;204:1305–12. doi: 10.1093/infdis/jir541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell JD, Bergin IL, Natavio MF, et al. Feasibility of LNG-IUS in a baboon model. Contraception. 2013;87:380–4. doi: 10.1016/j.contraception.2012.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergin IL, Bell JD, Chen Z, et al. Novel genital alphapapillomaviruses in baboons (papio hamadryas anubis) with cervical dysplasia. Vet Pathol. 2013;50:200–8. doi: 10.1177/0300985812439725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiman L. Random forests. Mach Learn. 2001;45:5–32. [Google Scholar]
- Brunham RC, Rey-Ladino J. Immunology of Chlamydia infection: implications for a Chlamydia trachomatis vaccine. Nat Rev Immunol. 2005;5:149–61. doi: 10.1038/nri1551. [DOI] [PubMed] [Google Scholar]
- Carey VJ. Ported to R by Lumley T and Ripley B. R package version 4.13–18. Generalized Estimation Equation solver; 2012. http://CRAN.R-project.org/package=gee (11 September 2015, date last accessed) [Google Scholar]
- Centers for Disease Control and Prevention. Prevalence of Chlamydia trachomatis genital infection among persons aged 14–39 years – United States, 2007–2012. MMWR Morb Mortal Wkly Rep. 2014;63:834–8. [PMC free article] [PubMed] [Google Scholar]
- Council for the International Organization of Medical Sciences (CIOMS) and the International Countil for Laboratory Animal Science (ICLAS) International guiding principles for biomedical research involving animals. 2012 https://grantsnihgov/grants/olaw/Guiding_Principles_2012.pdf (11 September 2015, date last accessed) [Google Scholar]
- Datta SD, Sternberg M, Johnson RE, et al. Gonorrhea and chlamydia in the United States among persons 14 to 39 years of age, 1999 to 2002. Ann Intern Med. 2007;147:89–96. doi: 10.7326/0003-4819-147-2-200707170-00007. [DOI] [PubMed] [Google Scholar]
- Edgar RC, Haas BJ, Clemente JC, et al. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics. 2011;27:2194–200. doi: 10.1093/bioinformatics/btr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farage M, Maibach H. Lifetime changes in the vulva and vagina. Arch Gynecol Obstet. 2006;273:195–202. doi: 10.1007/s00404-005-0079-x. [DOI] [PubMed] [Google Scholar]
- Finer LB, Jerman J, Kavanaugh ML. Changes in use of long-acting contraceptive methods in the United States, 2007–2009. Fertil Steril. 2012;98:893–7. doi: 10.1016/j.fertnstert.2012.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman JO. Factors associated with contraceptive satisfaction in adolescent women using the IUD. J Pediatr Adol Gynec. 2015;28:38–42. doi: 10.1016/j.jpag.2014.02.015. [DOI] [PubMed] [Google Scholar]
- Gajer P, Brotman RM, Bai G, et al. Temporal dynamics of the human vaginal microbiota. Sci Transl Med. 2012;4:132ra52. doi: 10.1126/scitranslmed.3003605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes DA. Intrauterine device and upper-genital-tract infection. Lancet. 2000;356:1013–9. doi: 10.1016/S0140-6736(00)02699-4. [DOI] [PubMed] [Google Scholar]
- Hafner LM, Cunningham K, Beagley KW. Ovarian steroid hormones: effects on immune responses and Chlamydia trachomatis infections of the female genital tract. Mucosal Immunol. 2013;6:859–75. doi: 10.1038/mi.2013.46. [DOI] [PubMed] [Google Scholar]
- Hashway SA, Bergin IL, Bassis CM, et al. Impact of a hormone-releasing intrauterine system on the vaginal microbiome: a prospective baboon model. J Med Primatol. 2014;43:89–99. doi: 10.1111/jmp.12090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higham JP, Heistermann M, Ross C, et al. The timing of ovulation with respect to sexual swelling detumescence in wild olive baboons. Primates. 2008;49:295–9. doi: 10.1007/s10329-008-0099-9. [DOI] [PubMed] [Google Scholar]
- Hirata T, Osuga Y, Hamasaki K, et al. Expression of toll-like receptors 2, 3, 4, and 9 genes in the human endometrium during the menstrual cycle. J Reprod Immunol. 2007;74:53–60. doi: 10.1016/j.jri.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Jacobson JC, Turok DK, Dermish AI, et al. Vaginal microbiome changes with levonorgestrel intrauterine system placement. Contraception. 2014;90:130–5. doi: 10.1016/j.contraception.2014.04.006. [DOI] [PubMed] [Google Scholar]
- Koenigsknecht MJ, Theriot CM, Bergin IL, et al. Dynamics and establishment of Clostridium difficile infection in the murine gastrointestinal tract. Infect Immun. 2015;83:934–41. doi: 10.1128/IAI.02768-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozich JJ, Westcott SL, Baxter NT, et al. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl Environ Microb. 2013;79:5112–20. doi: 10.1128/AEM.01043-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyama CM, Mihalyi A, Chai D, et al. Baboon model for the study of endometriosis. Womens Health. 2007;3:637–46. doi: 10.2217/17455057.3.5.637. [DOI] [PubMed] [Google Scholar]
- Lewis RA, Taylor D, Natavio MF, et al. Effects of the levonorgestrel-releasing intrauterine system on cervical mucus quality and sperm penetrability. Contraception. 2010;82:491–6. doi: 10.1016/j.contraception.2010.06.006. [DOI] [PubMed] [Google Scholar]
- Liang KY, Zeger SL. Longitudinal data-analysis using generalized linear-models. Biometrika. 1986;73:13–22. [Google Scholar]
- Liaw A, Wiener M. Classification and Regression by randomForest. R News. 2002;2:18–22. [Google Scholar]
- Ma B, Forney LJ, Ravel J. Vaginal microbiome: rethinking health and disease. Annu Rev Microbiol. 2012;66:371–89. doi: 10.1146/annurev-micro-092611-150157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mardh PA. Tubal factor infertility, with special regard to chlamydial salpingitis. Curr Opin Infect Dis. 2004;17:49–52. doi: 10.1097/00001432-200402000-00010. [DOI] [PubMed] [Google Scholar]
- Mirmonsef P, Gilbert D, Zariffard MR, et al. The effects of commensal bacteria on innate immune responses in the female genital tract. Am J Reprod Immunol. 2011;65:190–5. doi: 10.1111/j.1600-0897.2010.00943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohllajee AP, Curtis KM, Martins SL, et al. Hormonal contraceptive use and risk of sexually transmitted infections: a systematic review. Contraception. 2006;73:154–65. doi: 10.1016/j.contraception.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Morrison CS, Chen PL, Kwok C, et al. Hormonal contraception and the risk of HIV acquisition: an individual participant data meta-analysis. PLoS Med. 2015;12:e1001778. doi: 10.1371/journal.pmed.1001778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison CS, Turner AN, Jones LB. Highly effective contraception and acquisition of HIV and other sexually transmitted infections. Best Pract Res Cl Ob. 2009;23:263–84. doi: 10.1016/j.bpobgyn.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Natavio MF, Taylor D, Lewis RA, et al. Temporal changes in cervical mucus after insertion of the levonorgestrel-releasing intrauterine system. Contraception. 2013;87:426–31. doi: 10.1016/j.contraception.2012.09.034. [DOI] [PubMed] [Google Scholar]
- Neuendorf E, Gajer P, Bowlin AK, et al. Chlamydia caviae infection alters abundance but not composition of the guinea pig vaginal microbiota. Pathog Dis. 2015;73 doi: 10.1093/femspd/ftv019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyachieo A, Spiessens C, Chai DC, et al. Menstrual cycle synchronization, ovarian stimulation, and in vitro fertilization in olive baboons (Papio anubis): a prospective randomized study. Fertil Steril. 2009;91:602–10. doi: 10.1016/j.fertnstert.2007.11.071. [DOI] [PubMed] [Google Scholar]
- Polis CB, Phillips SJ, Curtis KM, et al. Hormonal contraceptive methods and risk of HIV acquisition in women: a systematic review of epidemiological evidence. Contraception. 2014;90:360–90. doi: 10.1016/j.contraception.2014.07.009. [DOI] [PubMed] [Google Scholar]
- Pruesse E, Quast C, Knittel K, et al. SILVA: a comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res. 2007;35:7188–96. doi: 10.1093/nar/gkm864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2014. http://www.R-project.org/ (11 September 2015, date last accessed) [Google Scholar]
- Raine-Bennett T, Merchant M, Sinclair F, et al. Reproductive health outcomes of insured adolescent and adult women who access oral levonorgestrel emergency contraception. Obstet Gynecol. 2015;125:904–11. doi: 10.1097/AOG.0000000000000742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph LJ, McCoy SI, Shiu K, et al. Hormonal contraceptive use and women's risk of HIV acquisition: a meta-analysis of observational studies. Lancet Infect Dis. 2015;15:181–9. doi: 10.1016/S1473-3099(14)71052-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera AJ, Frank JA, Stumpf R, et al. Differences between the normal vaginal bacterial community of baboons and that of humans. Am J Primatol. 2011;73:119–26. doi: 10.1002/ajp.20851. [DOI] [PubMed] [Google Scholar]
- Russell WMS, Burch RL. The Principles of Humane Experimental Technique. London: Methuen; 1959. [Google Scholar]
- Sasaki-Imamura T, Yoshida Y, Suwabe K, et al. Molecular basis of indole production catalyzed by tryptophanase in the genus Prevotella. FEMS Microbiol Lett. 2011;322:51–9. doi: 10.1111/j.1574-6968.2011.02329.x. [DOI] [PubMed] [Google Scholar]
- Sassone-Corsi M, Raffatellu M. No vacancy: how beneficial microbes cooperate with immunity to provide colonization resistance to pathogens. J Immunol. 2015;194:4081–7. doi: 10.4049/jimmunol.1403169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterwhite CL, Torrone E, Meites E, et al. Sexually transmitted infections among US women and men: prevalence and incidence estimates, 2008. Sex Transm Dis. 2013;40:187–93. doi: 10.1097/OLQ.0b013e318286bb53. [DOI] [PubMed] [Google Scholar]
- Schloss PD, Westcott SL, Ryabin T, et al. introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microb. 2009;75:7537–41. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secura GM, Madden T, McNicholas C, et al. Provision of no-cost, long-acting contraception and teenage pregnancy. New Engl J Med. 2014;371:1316–23. doi: 10.1056/NEJMoa1400506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhonen S, Haukkamaa M, Jakobsson T, et al. Clinical performance of a levonorgestrel-releasing intrauterine system and oral contraceptives in young nulliparous women: a comparative study. Contraception. 2004;69:407–12. doi: 10.1016/j.contraception.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Taylor SN, Van Der Pol B, Lillis R, et al. Clinical evaluation of the BD ProbeTec Chlamydia trachomatis Qx amplified DNA assay on the BD Viper system with XTR technology. Sex Transm Dis. 2011;38:603–9. doi: 10.1097/OLQ.0b013e31820a94d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thonneau PF, Almont T. Contraceptive efficacy of intrauterine devices. Am J Obstet Gynecol. 2008;198:248–53. doi: 10.1016/j.ajog.2007.10.787. [DOI] [PubMed] [Google Scholar]
- Torrone E, Papp J, Weinstock H, et al. Prevalence of Chlamydia trachomatis genital infection among persons aged 14–39 years–United States, 2007–2012. MMWR Morb Mortal Wkly Rep. 2014;63:834–8. [PMC free article] [PubMed] [Google Scholar]
- Uchihashi M, Bergin IL, Bassis CM, et al. Influence of age, reproductive cycling status, and menstruation on the vaginal microbiome in baboons (Papio anubis) Am J Primatol. 2015;77:563–78. doi: 10.1002/ajp.22378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Garrity GM, Tiedje JM, et al. Naïve bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microb. 2007;73:5261–7. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White HD, Crassi KM, Givan AL, et al. CD3+ CD8+ CTL activity within the human female reproductive tract: influence of stage of the menstrual cycle and menopause. J Immunol. 1997;158:3017–27. [PubMed] [Google Scholar]
- Wiggins R, Hicks SJ, Soothill PW, et al. Mucinases and sialidases: their role in the pathogenesis of sexually transmitted infections in the female genital tract. Sex Transm Infect. 2001;77:402–8. doi: 10.1136/sti.77.6.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wira CR, Fahey JV, Rodriguez-Garcia M, et al. Regulation of mucosal immunity in the female reproductive tract: the role of sex hormones in immune protection against sexually transmitted pathogens. Am J Reprod Immunol. 2014;72:236–58. doi: 10.1111/aji.12252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wira CR, Grant-Tschudy KS, Crane-Godreau MA. Epithelial cells in the female reproductive tract: a central role as sentinels of immune protection. Am J Reprod Immunol. 2005;53:65–76. doi: 10.1111/j.1600-0897.2004.00248.x. [DOI] [PubMed] [Google Scholar]
- Yue J, Clayton M. A similarity measure based on species proportions. Commun Stat Theory. 2005;34:2123–31. [Google Scholar]