Abstract
In this study, we investigated the role of protein kinases C (PKCs) in interleukin (IL)-6 and IL-8 secretion by human lung epithelial A549 cells during infection with the fungal pathogen Paracoccidioides brasiliensis. Rottlerin and the broad spectrum PKC inhibitor Go 6983 reduced cytokine levels in A549 cell–P. brasiliensis cultures. Next, by western blot, we verified that infection with this fungus led to phosphorylation of PKC δ (Thr505). By using a peptide inhibitor for PKC δ or PKC δ short interfering RNA technique, IL-6 and IL-8 levels in A549–P. brasiliensis cultures were also reduced. Together, these results indicate that P. brasiliensis promotes IL-6 and IL-8 secretion by A549 cells in a PKC δ-dependent manner.
Keywords: fungi, innate immunity, cell signaling, protein kinase C, cytokine, epithelial cell
This article is the first report that demonstrates the role of protein kinases C in cytokine secretion by epithelial cells, when infected with the fungal human pathogen Paracoccidioides brasiliensis.
Graphical Abstract Figure.
This article is the first report that demonstrates the role of protein kinases C in cytokine secretion by epithelial cells, when infected with the fungal human pathogen Paracoccidioides brasiliensis.
INTRODUCTION
Paracoccidioidomycosis is a human systemic mycosis, which occurs mainly in rural areas of Brazil, Colombia, Venezuela and Argentina (Marques 2012; Bocca et al. 2013). More than 90% of cases develop as a chronic disease which affects mostly lungs, followed by skin and mucosa (Bocca et al. 2013). Although severity of the chronic form of paracoccidioidomycosis may usually vary from mild to moderate, and even if infection is controlled with antifungal drugs, sequelae due to fibrosis are common in one third of the patients (Bocca et al. 2013). The etiological agent of paracoccidioidomycosis is a dimorphic fungus that belongs to the genus Paracoccidioides. Until 2006, Paracoccidioides brasiliensis was the only known species in inducing paracoccidioidomycosis. Recently, based on morphological and molecular data, it was identified a new species, as another paracoccidioidomycosis causing agent, which was named Paracoccidioides lutzii (Teixeira et al. 2009; Theodoro et al. 2012; Bocca et al. 2013).
Besides physically protecting the host against inhaled particles or pathogens, airway epithelial cells also play an important role in the innate immune system by expressing several cytokines and chemokines, such as interleukin (IL)-6, IL-8, IL-11 and granulocyte-macrophage colony-stimulating factor. These molecules are in turn involved in B-cell activation or differentiation, augment of immunoglobulin production and recruitment/activation of neutrophils, eosinophils, macrophages or T-helper cells (Suzuki, Chow and Downey 2008; Proud and Leigh 2011).
The cytokine profile depends on the pathogen studied. Mycoplasma pneumoniae, for example, induces secretion of IL-8 and tumor necrosis factor (TNF)-α by human lung epithelial A549 cells, but IL-6 was not detected in these cultures (Yang et al. 2002). Recently, using the same cell line, our group showed that P. brasiliensis yeasts induced IL-8 and IL-6 secretion by A549 cells, but TNF-α was undetectable in culture supernatant (Maza et al. 2012). Other examples may be found in the literature (Chang et al. 2004; Sharma et al. 2007; Reihill et al. 2011; N'Guessan et al. 2014), showing that cytokine secretion by epithelial cells may be singular among different pathogen–epithelial cell interactions.
Several groups have demonstrated that members of the protein kinase C (PKC) family are involved in cytokine secretion. PKC family is composed of 10 isoforms of serine/threonine kinases that are distributed into three classes: conventional PKCs (PKCs α, βI, βII and γ isoforms), novel PKCs (PKCs δ, ε, η and θ) and atypical PKCs (PKCs ζ and ι) (Freeley, Kelleher and Long 2011; Loegering and Lennartz 2011). In the past decade, it has been shown that PKCs are involved in several steps of toll-like receptor (TLR) signaling, which is important for host innate immune response (Loegering and Lennartz 2011). Most of these studies were performed using myeloid cells. In epithelial cells, some groups have demonstrated the involvement of PKCs in cytokine secretion. Using lung epithelial cells, Boggaram et al. (2013) and Im et al. (2009), for example, showed that bacterial proteins (respectively, Mycobacterium tuberculosis ESAT-6 and Salmonella typhimurium/Bacillus subtilis flagellin) induced IL-8 secretion that was reduced by PKC inhibitors, therefore indicating the involvement of this kinase in epithelial cell cytokine expression (Im et al. 2009; Boggaram et al. 2013). In the present work, we verified the importance of PKCs, especially PKC δ, in IL-6 and IL-8 secretion induced by P. brasiliensis in A549 cells.
MATERIALS AND METHODS
Fungal culture
Paracoccidioides brasiliensis, isolate Pb18, was kindly provided by Dr Zoilo P. Camargo, São Paulo, Brazil. Yeasts were grown 5–7 days at 37°C, 100 rpm, in PGY (peptone/glucose/yeast extract) medium as described previously (Maza et al. 2008).
A549 cell culture
Human lung epithelial cell line A549 was grown in Dulbecco's Modified Eagle's Medium (DMEM) (Sigma, USA) supplemented with 10% fetal bovine serum (FBS) (Vitrocell Embriolife, Brazil), 10 mM HEPES, 100 U mL−1 penicillin and 100 μg mL−1 streptomycin (complete DMEM) at 37°C, 5% CO2.
Preparation of P. brasiliensis for interaction assays with A549 cells
Paracoccidioides brasiliensis yeasts, grown for 4–5 days, were decanted for 8 min. The resultant supernatant contained only single mother and small daughter yeasts. Then, yeasts were washed three times with DMEM and used for interaction assays with A549 cells.
Analysis of PKC δ expression and phosphorylation of PKCs during the interaction of A549 cells with P. brasiliensis
Approximately 4.5 × 105 A549 cells were cultured in 100 mm plates with complete DMEM. After 3 days, A549 cells were incubated overnight in FBS-free DMEM, and then with 2.0 × 107 P. brasiliensis yeasts for different time periods (0 – 60 min) (at this point, in this experiment, multiplicity of infection was 2.5 yeasts per A549 cell). After incubation with P. brasiliensis, A549 cells were washed with cold phosphate-buffered saline (PBS) containing 1 mM Na3VO4 and harvested with cell lifter. Next, cell pellets were lysed with TX-100 lysis buffer (25 mM Tris buffer pH 7.5 containing 0.5 mM EDTA, 5% glycerol, 0.05% Triton X-100 and a mixture of protease and phosphatase inhibitors—5 mM Na3VO4, 10 μg mL−1 leupeptin, 10 μg mL−1 aprotinin and 500 μg mL−1 AEBSF-). After 30 min, at 4°C, samples were centrifuged at 470 × g for 10 min. Protein content was measured by using Micro BCA Protein Assay Kit® (Pierce, USA), according to the manufacturer's instructions.
Twenty-five micrograms of protein were loaded per well on 10% SDS-PAGE gels, and then evaluated by western blot as described previously (Maza et al. 2008). Antibodies from CELL SIGNALING (USA) were anti-Phospho (P)-PKC (pan) (βII Ser660) (#9371), and anti-P-PKC δ (Thr505) (#9374). Antibody anti-β-actin (A5441) was purchased from Sigma (USA), and anti-PKC δ (sc-213) from Santa Cruz (USA). Reactive proteins were detected using a chemiluminescence reagent (SuperSignal West Pico Chemiluminescent Substrate®, Pierce, USA) and documented by UVITEC (UVITEC, UK). For protein quantification, densitometric analyzes were performed using Scion Image (Scion Corporation, USA).
Analysis of cytokine levels in supernatants of A549 cells during incubation with P. brasiliensis
Approximately 1 × 104 A549 cells were cultured in 24-well plates with complete DMEM. After 3 days, A549 cells were maintained overnight in FBS-free DMEM, and then incubated with 4.5 × 105 P. brasiliensis yeasts overnight (at this point, in this experiment, multiplicity of infection was 2.5 yeasts per A549 cell). Next, culture supernatants were collected, centrifuged to remove fungi and stored at −80°C. IL-6, IL-8 or IL-10 levels in these supernatants were determined using DuoSet® ELISA Development Kits (R&D Systems, USA), according to the manufacturer's instructions.
For some experiments, A549 cells were incubated for 2 h with FBS-free DMEM containing PKCs inhibitors, NH4OH or DMSO as described in Tables S1 and S2 (Supporting Information). Next, P. brasiliensis yeasts were added to the cultures. IL-6 and IL-8 levels in culture supernatants were measured as described above.
A549 cell viability was measured by MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) assay. For this, A549 cells were incubated in the presence or absence of PKCs inhibitors for 2 h and then with P. brasiliensis yeasts overnight as described above. Next, A549 cells were washed and analyzed by MTT assay as described previously (Maza et al. 2008). Statistical significance was evaluated using Student's t-test. P < 0.01 was considered significant.
Silencing of PKC δ in A549 cells by short interfering RNA (siRNA)
Approximately 5.6 × 104 A549 cells were cultured in 24-well plates with DMEM supplemented with 10% FBS and 10 mM HEPES in the absence of antibiotics. After 24 h, A549 cells were maintained overnight in FBS-free DMEM, and then were transfected with transfection reagent Lipofectamine® RNAiMAX and Silencer® Select Pre-designed PKC δ siRNA (PKC δ siRNA #1 - s11099; or, PKC δ siRNA #2 - s11100, Life Technologies, USA) at a final concentration of 250 nM, according to the manufacturer's protocol (Life Technologies, USA). Silencer Select Negative Control No. 1 and No. 2 siRNA (Life Technologies, USA) were used as negative control. After 24 h, A549 cells were washed three times with DMEM and incubated with 4.5 × 105 P. brasiliensis yeasts overnight (at this point, in this experiment, multiplicity of infection was 2.5 yeasts per A549 cell). After incubation with fungi, culture supernatants were collected, and A549 cells were harvested and lysed as described in ‘Materials and methods’ section. Silencing of PKC δ was confirmed by western blot using anti-PKC δ. IL-6 and IL-8 levels in culture supernatants were determined as described in ‘Materials and methods’ section. Messenger RNA (mRNA) levels of PKC δ and classical PKCs (PKC α, PKC βI, PKC βII and PKC γ) were determined by RT-PCR, as described by Yu et al. (2007).
RESULTS
Involvement of PKCs in secretion of IL-6 and IL-8 by human lung epithelial cells during interaction with P. brasiliensis
Recently, our group described that P. brasiliensis yeasts induce secretion of the proinflammatory cytokines IL-6 and IL-8 by the human lung epithelial cell line A549 (Maza et al. 2012). So as expected in this study, we observed that overnight incubation of A549 cells with this fungus significantly increased secretion of these cytokines over basal levels (Fig. S1, Supporting Information). On the other hand, we verified that P. brasiliensis yeasts were not able to induce secretion of the anti-inflammatory cytokine IL-10 by these epithelial cells (Fig. S1, Supporting Information).
Next, by western blot, we evaluated whether P. brasiliensis promotes PKC phosphorylation in A549 cells. For this purpose, we used an antibody (anti-phospho-PKCpan) that recognizes conventional and novel PKCs only when phosphorylated at a carboxy-terminal residue homologous to Ser660 of PKC βII. As shown in Fig. 1, P. brasiliensis yeasts induced a strong and transient PKC phosphorylation, increasing up to 2.3-fold over basal levels.
Figure 1.
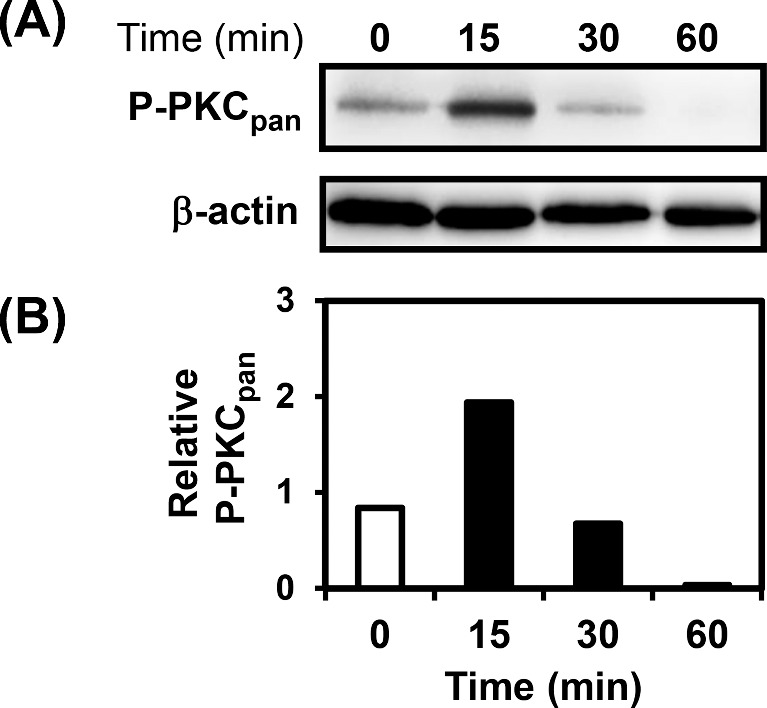
Analysis of phosphorylation of PKCs during incubation of A549 cells with P. brasiliensis. A549 cells were incubated with P. brasiliensis yeasts for the indicated periods of time, and then A549 cells were harvested and lysed with TX-100 lysis buffer. Twenty-five micrograms of protein were loaded per well of 10% SDS-PAGE gels. (A) Phosphorylation of PKCs was analyzed by western blot using antibody anti-phospho (P)-PKCpan. (B) Relative PKC phosphorylation was determined by densitometric analysis of bands obtained in Panel A. Values represent the ratio of the intensity of P-PKCpan band divided by the corresponding intensity of β-actin band. Blots are representative of two independent experiments.
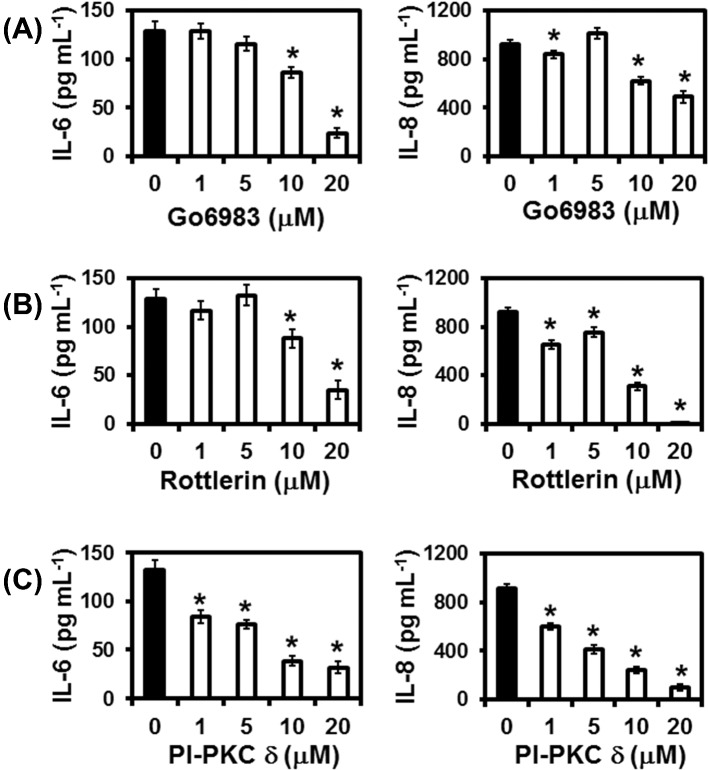
In order to analyze which isoforms of PKC are important for P. brasiliensis-induced cytokine secretion by A549 cells, several PKC inhibitors were used (see Tables S1 and S2, Supporting Information). Go 6976 (PKCs α, βI and γ inhibitor), peptide inhibitor (PI)-PKCε (PKC ε inhibitor), pseudosubstrate inhibitor (PSI)-PKC θ (PKC θ inhibitor) and PSI-PKCζ (PKC ζ inhibitor) significantly reduced 3 to 59% of IL-6 and 3 to 39% of IL-8 levels in culture supernatants of A549 cells incubated with P. brasiliensis yeasts (data not shown). However, this reduction in IL-6 and IL-8 levels is correlated with a decrease in A549 cell viability in the presence of these inhibitors (data not shown). Furthermore, LY333531 (PKC βI and PKC βII inhibitor) and lower concentrations of PSI-PKC η (5, 10 and 20 μM—PKC η inhibitor) and HBDDE (5 μM—PKC α and PKC γ inhibitor) did not inhibit IL-6 or IL-8 secretion in A549 cells–P. brasiliensis cultures (data not shown). However, higher concentrations of PSI-PKC η (50 μM) and HBDDE (20 μM) significantly reduced cytokine levels, but probably it was due to A549 cell death (data not shown).
On the other hand, the broad spectrum PKC inhibitor Go 6983, Rottlerin and the PKC δ inhibitor PI-PKC δ decreased IL-6 and IL-8 levels in A549 cells–P. brasiliensis cultures (Fig. 2), while epithelial cell viability was not affected for most of the concentrations used (see Tables S3–S5, Supporting Information). An amount of 10 and 20 μM of Go 6983 reduced significantly IL-6 levels by 33.2 and 81.5%, respectively, over control cells without inhibitor. IL-8 secretion was also reduced significantly in the presence of Go 6983. An amount of 10 and 20 μM of this inhibitor decreased IL-8 levels by 32.7 and 46.9%, respectively. Regarding Rottlerin, a compound largely used as a specific PKC δ inhibitor by several groups, 10 and 20 μM significantly reduced IL-6 in A549 cell–P. brasiliensis cultures by 31.6 and 72.6%, respectively, when compared to control cells without inhibitor. Rottlerin also decreased IL-8 levels by 66.1 and 99.7% when using 10 and 20 μM of this inhibitor in A549 cell–P. brasiliensis cultures, suggesting that PKC δ participates in IL-6 and IL-8 secretion.
Figure 2.
Effects of PKCs inhibitors on IL-6 or IL-8 levels in supernatants of A549 cell cultures during incubation with P. brasiliensis. A549 cells were incubated for 2 h in the absence or presence of Go 6983 (broad spectrum PKC inhibitor), Rottlerin or PI-PKC δ (PKC δ inhibitor). Control cells were incubated with DMSO or NH4OH (used in the same concentrations as those for PKCs inhibitors). Then, P. brasiliensis yeasts were added to A549 cells cultures. After overnight incubation, culture supernatants were collected, and IL-6 and IL-8 levels were determined by ELISA. Values represent the mean of triplicates ± standard deviation. *P < 0.01 compared to A549 cells incubated with P. brasiliensis in the absence of inhibitor. These results are representative of three independent experiments.
However, as several groups have described that Rottlerin may modulate different cell signaling events in a PKC δ-independent manner (Soltoff 2007), a specific PKC δ inhibitor (PI-PKC δ) was also used in our experiments. An amount of 1–20 μM of PI-PKC δ decreased significantly IL-6 levels by 37% up to 76% over control cells without inhibitor. Similar results were obtained for IL-8 levels, 1–20 μM of PI-PKC δ reduced 34–89% of this cytokine levels in A549 cell–P. brasiliensis cultures. Optical density values from MTT assays performed in the absence or presence of P. brasiliensis yeasts, DMSO, NH4OH, Go 6983, Rottlerin or PI-PKC δ are shown in Tables S3–S5 (Supporting Information). Only 20 μM of Go 6983 decreased significantly A549 cell viability by 13.5% (Table S3, Supporting Information).
Taken together, these results demonstrate that P. brasiliensis yeasts promote IL-6 and IL-8 secretion in A549 cells which is dependent on PKC activation, and furthermore PKC δ is one of the PKC isoforms involved.
PKC δ phosphorylation in human lung epithelial cells during interaction with P. brasiliensis
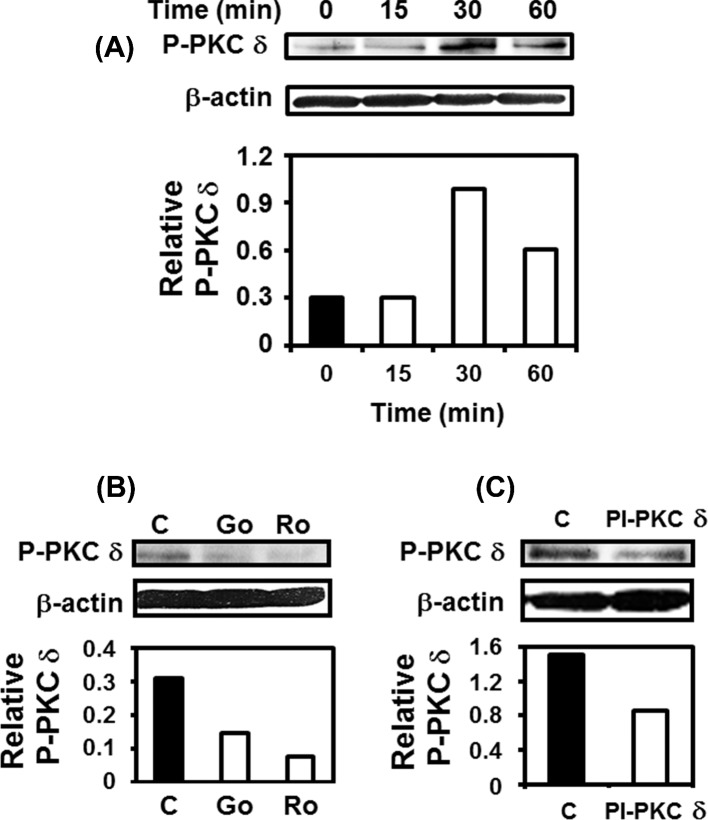
As PI-PKC δ reduced IL-6 and IL-8 levels in A549 cell–P. brasiliensis cultures, by western blot, we evaluated whether this fungus promotes PKC δ phosphorylation in A549 cells. For this purpose, an anti-phospho-PKC δ (Thr505) antibody was used. As shown in Fig. 3A, P. brasiliensis yeasts induced a strong and transient PKC δ (Thr505) phosphorylation, increasing up to 3.3-fold over basal levels. Next, the effect of Go 6983, Rottlerin or PI-PKC δ in PKC δ (Thr505) phosphorylation was analyzed, and we verified that these inhibitors reduced, respectively, 53.4, 76.1 and 43.5% of this PKC isoform phosphorylation (Fig. 3B and C). So, these results show that P. brasiliensis yeasts induce phosphorylation of PKC δ which may be inhibited by the pan-PKC inhibitor Go 6983, Rottlerin and the PKC δ inhibitor PI-PKC δ.
Figure 3.
Analysis of PKC δ phosphorylation during incubation of A549 cells with P. brasiliensis. (A) A549 cells were incubated with P. brasiliensis yeasts for the indicated periods of time, and then A549 cells were harvested and lysed with TX-100 lysis buffer. PKC δ phosphorylation (Thr505) (P-PKC δ) was analyzed by western blot. (B and C) A549 cells were pre-incubated for 2 h in the absence or presence of Go 6983 20 μM or Rottlerin 20 μM (B) or PI-PKC δ 10 μM (C). Control cells were incubated with DMSO or NH4OH solution (used in the same concentrations as those for PKCs inhibitors). Then, P. brasiliensis yeasts were added to A549 cells cultures. After 30 min, A549 cells were harvested and lysed with TX-100. PKC δ phosphorylation (Thr505) was analyzed by western blot. Relative P-PKC δ was determined by densitometric analysis of bands obtained in upper panels. Values represent the ratio of the intensity of P-PKC δ band to the corresponding intensity of β-actin band. Blots are representative of two independent experiments.
Involvement of PKC δ in cytokine secretion by human lung epithelial cells during interaction with P. brasiliensis
To further confirm that PKC δ is closely involved in IL-6 or IL-8 secretion by A549 cells in the presence of P. brasiliensis, PKC δ was silenced by two different siRNA oligonucleotides (PKC δ siRNA #1 and PKC δ siRNA #2, see ‘Materials and Methods’ section for specifications). First, by western blot, we verified that both siRNA oligonucleotides reduced PKC δ expression up to 45.7% in PKC δ-siRNA transfected cells when compared to cells transfected with negative control siRNA (Fig. 4A; Fig. S2A, Supporting Information). By RT-PCR, as expected, PKC δ mRNA levels were reduced (43%) in PKC δ-siRNA-transfected cells. Under the same conditions, we also analyzed classical PKCs mRNA levels in PKC δ-directed siRNA-transfected cells. PKC α mRNA levels reduced slightly (16%). PKC γ mRNA levels were not altered, and PKC βI and PKC βII mRNA was not detected (data not shown). Next, after incubation with P. brasiliensis yeasts, we analyzed IL-6 and IL-8 levels in culture supernatants of A549 cells transfected with negative control or PKC δ-directed siRNAs. As shown in Fig. 4B, we verified that PKC δ siRNA #1 reduced IL-6 and IL-8 levels by 40.2 and 45.3%, respectively. Similar results were obtained with PKC δ siRNA #2 (Fig. S2B, Supporting Information). Thus, these data corroborate PKC δ participation in secretion of IL-6 and IL-8 by A549 cells during P. brasiliensis infection.
Figure 4.
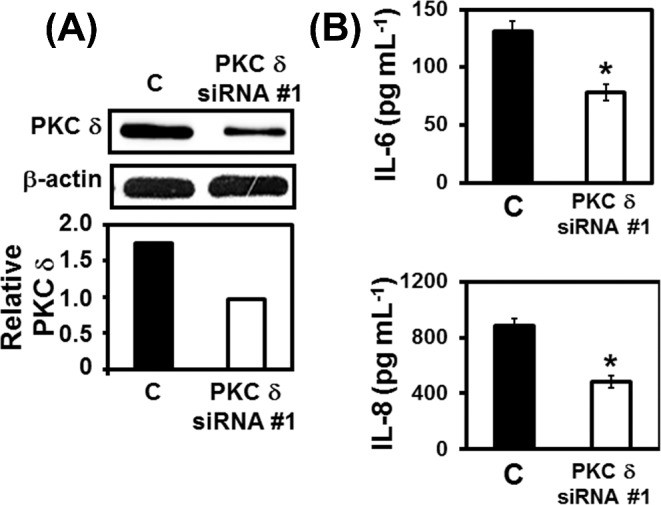
Effects of PKC δ silencing on IL-6 or IL-8 levels in supernatants of A549 cell cultures during incubation with P. brasiliensis. A549 cells were transfected with PKC δ siRNA #1 or with Negative Control siRNA (C) for 24 h, and then incubated with P. brasiliensis yeasts overnight. After incubation with fungi, culture supernatants were collected for IL-6 and IL-8 levels determination. A549 cells were harvested and lysed with TX-100 lysis buffer. Twenty-five micrograms of protein were loaded per well of 10% SDS-PAGE gels. (A) Silencing of PKC δ was confirmed by western blot using anti-PKC δ. Relative PKC δ expression was determined by densitometric analysis of bands. Values represent the ratio of the intensity of PKC δ band to the corresponding intensity of β-actin band. Blots are representative of two independent experiments. (B) IL-6 and IL-8 levels in culture supernatants were determined by ELISA. Values represent the mean of triplicates ± standard deviation. *P < 0.01 compared to C. These results are representative of two independent experiments.
DISCUSSION
Recently, our group showed that P. brasiliensis yeasts induce, in human lung epithelial A549 cells, IL-6 and IL-8 secretion that is dependent on activation of p38 MAPK and ERK 1/2 (Maza et al. 2012). In the present work, after we verified that P. brasiliensis yeasts are able to induce PKC phosphorylation in A549 cells, we used different inhibitors for several PKC isoforms in order to analyze which one is involved in cytokine secretion.
As several PKC isoforms are involved in cell survival and proliferation (Reyland 2009), it was expected that inhibiting some of these kinases, epithelial cells would not be viable in assays with P. brasiliensis. In fact, we verified that at least four (Go 6976, PI-PKCε, PSI-PKCθ and PSI-PKCζ) of ten inhibitors affected A549 cell viability in the presence of this fungus. In the same conditions, LY333531 did not alter A549 cell survival, but also did not reduce cytokine levels.
In the presence of P. brasiliensis yeasts, only Go6983 (broad spectrum PKC inhibitor), Rottlerin and PI-PKC δ (PKC δ inhibitor) did not have an impact on A549 cell viability, and concomitantly decreased IL-6 and IL-8 levels in culture supernatants. Regarding the use of Rottlerin in our work, this inhibitor has been used by many groups as a specific inhibitor for PKC δ, but some investigators have found that Rottlerin blocks other enzyme activities than PKC δ, and may also induce PKC δ-independent cell events (Soltoff 2007). Therefore, results obtained with Rottlerin should be analyzed with caution (Kikkawa, Matsuzaki and Yamamoto 2002; Soltoff 2007). For this reason, we used a specific peptide inhibitor of PKC δ (PI-PKC δ) and PKC δ-directed siRNA technique in our assays, and we confirmed that this PKC isoform is involved in IL-6 and IL-8 secretion by A549 cells cultured in presence of P. brasiliensis yeasts. In addition, we showed that P. brasiliensis promoted PKC δ phosphorylation (Thr505). Time patterns of PKCs phosphorylations, recognized by anti-P-PKCpan and anti-P- PKC δ (Thr505), were different. This discrepancy was probably due to the distinct phosphorylated residues recognized by these antibodies. While the P-PKCpan antibody recognizes conventional and novel PKCs when phosphorylated at a carboxy-terminal residue homologous to Ser660 of PKC βII, the P-PKC δ (Thr505) antibody is specific for this isoform.
Regarding PKC δ phosphorylation analyzed by western blot in this work, although Thr505 phosphorylation is not correlated to PKC δ activity, phosphorylation on this residue is important for PKC δ stability and substrate specificity (Steinberg 2008). Interestingly, PKC δ Thr505 may be phosphorylated in a Src family kinases (SFK)-dependent manner (Steinberg 2008). In fact, in a previous work, we verified that P. brasiliensis promoted SFK activation in A549 cells (Maza et al. 2008). SFK involvement in PKC δ activation in P. brasiliensis-infected epithelial cells will be investigated in our laboratory.
Different isoforms of the PKC family participate in several steps of TLR pathways, primarily by interacting with adaptor proteins of TLR signaling (Loegering and Lennartz 2011). For example, in macrophages stimulated with LPS, PKC δ binds to TIRAP/Mal, an adaptor protein for TLR2 and TLR4 (Kubo-Murai et al. 2007). The same authors also verified that phosphorylation of IKK, p38 MAPK and IκB were reduced in PKC δ-deleted RAW264.7 cells, thus indicating that PKC δ participates upstream in the activation of both MAPK and NF-κB pathways induced by TLR signaling (Kubo-Murai et al. 2007). PKCs α, ε and ζ were also implicated in TLR signaling (Loegering and Lennartz 2011).
By modulating inflammatory or anti-inflammatory responses, TLRs are essential in host innate immune system. These receptors constitute one of the major classes of pattern recognition receptors that recognize pathogen-associated molecular patterns, which in fungi may be represented by β-glucans, phospholipo-mannans, O-linked mannans, RNA, DNA and others (Bourgeois and Kuchler 2012). Some groups have described the role of TLRs in P. brasiliensis infection models. Acorci-Valério et al. (2010), for example, suggest that P. brasiliensis binds to TLR4 and invades human neutrophils, leading to IL-8 and IL-10 secretion (Acorci-Valério et al. 2010). On the other hand, Loures et al. (2010) showed that TLR4 does not influence P. brasiliensis-caused disease outcome, since TLR4-competent and -deficient mice presented similar survival times when infected with P. brasiliensis (Loures et al. 2010). However, the same authors demonstrated later that MyD88, an adaptor protein for TLR signaling, is important for fungicidal mechanisms and the induction of immune responses against P. brasiliensis (Loures et al. 2011).
In this manner, as TLRs modulate host immune response in P. brasiliensis infection, the role of PKC δ in TLR signaling is under current investigation in our laboratory.
Supplementary Material
SUPPLEMENTARY DATA
FUNDING
This work was supported by the Brazilian agencies FAPESP (grant # 2011/22773-6), CNPq and CAPES.
Conflict of interest. None declared.
REFERENCES
- Acorci-Valério MJ, Bordon-Graciani AP, Dias-Melicio LA, et al. Role of TLR2 and TLR4 in human neutrophil functions against Paracoccidioides brasiliensis. Scand J Immunol. 2010;71:99–108. doi: 10.1111/j.1365-3083.2009.02351.x. [DOI] [PubMed] [Google Scholar]
- Bocca AL, Amaral AC, Teixeira MM, et al. Paracoccidioidomycosis: eco-epidemiology, taxonomy and clinical and therapeutic issues. Future Microbiol. 2013;8:1177–91. doi: 10.2217/fmb.13.68. [DOI] [PubMed] [Google Scholar]
- Boggaram V, Gottipati KR, Wang X, et al. Early secreted antigenic target of 6 kDa (ESAT-6) protein of Mycobacterium tuberculosis induces interleukin-8 (IL-8) expression in lung epithelial cells via protein kinase signaling and reactive oxygen species. J Biol Chem. 2013;288:25500–11. doi: 10.1074/jbc.M112.448217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeois C, Kuchler K. Fungal pathogens-a sweet and sour treat for toll-like receptors. Front Cell Infect Microbiol. 2012;2:142. doi: 10.3389/fcimb.2012.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang B, Amemura-Maekawa J, Kura F, et al. Expression of IL-6 and TNF-α in human alveolar epithelial cells is induced by invading, but not by adhering, Legionella pneumophila. Microb Pathog. 2004;37:295–302. doi: 10.1016/j.micpath.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Freeley M, Kelleher D, Long A. Regulation of Protein Kinase C function by phosphorylation on conserved and non-conserved sites. Cell Signal. 2011;23:753–62. doi: 10.1016/j.cellsig.2010.10.013. [DOI] [PubMed] [Google Scholar]
- Im J, Jeon JH, Cho MK, et al. Induction of IL-8 expression by bacterial flagellin is mediated through lipid raft formation and intracellular TLR5 activation in A549 cells. Mol Immunol. 2009;47:614–22. doi: 10.1016/j.molimm.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Kikkawa U, Matsuzaki H, Yamamoto T. Protein kinase C δ (PKC δ): activation mechanisms and functions. J Biochem. 2002;132:831–9. doi: 10.1093/oxfordjournals.jbchem.a003294. [DOI] [PubMed] [Google Scholar]
- Kubo-Murai M, Hazeki K, Sukenobu N, et al. Protein kinase C δ binds TIRAP/Mal to participate in TLR signaling. Mol Immunol. 2007;44:2257–64. doi: 10.1016/j.molimm.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Loegering DJ, Lennartz MR. Protein kinase C and toll-like receptor signaling. Enzyme Res. 2011;2011:537821. doi: 10.4061/2011/537821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loures FV, Pina A, Felonato M, et al. Toll-like receptor 4 signaling leads to severe fungal infection associated with enhanced proinflammatory immunity and impaired expansion of regulatory T cells. Infect Immun. 2010;78:1078–88. doi: 10.1128/IAI.01198-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loures FV, Pina A, Felonato M, et al. MyD88 signaling is required for efficient innate and adaptive immune responses to Paracoccidioides brasiliensis infection. Infect Immun. 2011;79:2470–80. doi: 10.1128/IAI.00375-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques SA. Paracoccidioidomycosis. Clin Dermatol. 2012;30:610–5. doi: 10.1016/j.clindermatol.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Maza PK, Oliveira P, Toledo MS, et al. Paracoccidioides brasiliensis induces secretion of IL-6 and IL-8 by lung epithelial cells. Modulation of host cytokine levels by fungal proteases. Microbes Infect. 2012;14:1077–85. doi: 10.1016/j.micinf.2012.05.016. [DOI] [PubMed] [Google Scholar]
- Maza PK, Straus AH, Toledo MS, et al. Interaction of epithelial cell membrane rafts with Paracoccidioides brasiliensis leads to fungal adhesion and Src-family kinase activation. Microbes Infect. 2008;10:540–7. doi: 10.1016/j.micinf.2008.02.004. [DOI] [PubMed] [Google Scholar]
- N'Guessan PD, Haarmann H, Steiner T, et al. The Moraxella catarrhalis-induced pro-inflammatory immune response is enhanced by the activation of the epidermal growth factor receptor in human pulmonary epithelial cells. Biochem Bioph Res Co. 2014;450:1038–44. doi: 10.1016/j.bbrc.2014.06.102. [DOI] [PubMed] [Google Scholar]
- Proud D, Leigh R. Epithelial cells and airway diseases. Immunol Rev. 2011;242:186–204. doi: 10.1111/j.1600-065X.2011.01033.x. [DOI] [PubMed] [Google Scholar]
- Reihill JA, Moore JE, Elborn JS, et al. Effect of Aspergillus fumigatus and Candida albicans on pro-inflammatory response in cystic fibrosis epithelium. J Cyst Fibros. 2011;10:401–6. doi: 10.1016/j.jcf.2011.06.006. [DOI] [PubMed] [Google Scholar]
- Reyland ME. Protein kinase C isoforms: multi-functional regulators of cell life and death. Front Biosci. 2009;14:2386–99. doi: 10.2741/3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma M, Sharma S, Roy S, et al. Pulmonary epithelial cells are a source of interferon-γ in response to Mycobacterium tuberculosis infection. Immunol Cell Biol. 2007;85:229–37. doi: 10.1038/sj.icb.7100037. [DOI] [PubMed] [Google Scholar]
- Soltoff SP. Rottlerin: an inappropriate and ineffective inhibitor of PKC δ. Trends Pharmacol Sci. 2007;28:453–8. doi: 10.1016/j.tips.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Steinberg SF. Structural basis of protein kinase C isoform function. Physiol Rev. 2008;88:1341–78. doi: 10.1152/physrev.00034.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Chow CW, Downey GP. Role of innate immune cells and their products in lung immunopathology. Int J Biochem Cell B. 2008;40:1348–61. doi: 10.1016/j.biocel.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Teixeira MM, Theodoro RC, de Carvalho MJ, et al. Phylogenetic analysis reveals a high level of speciation in the Paracoccidioides genus. Mol Phylogenet Evol. 2009;52:273–83. doi: 10.1016/j.ympev.2009.04.005. [DOI] [PubMed] [Google Scholar]
- Theodoro RC, Teixeira MM, Felipe MS, et al. Genus Paracoccidioides: species recognition and biogeographic aspects. PLoS One. 2012;7:e37694. doi: 10.1371/journal.pone.0037694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Hooper WC, Phillips DJ, et al. Regulation of proinflammatory cytokines in human lung epithelial cells infected with Mycoplasma pneumoniae. Infect Immun. 2002;70:3649–55. doi: 10.1128/IAI.70.7.3649-3655.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu K, Ma P, Ge J, et al. Expression of protein kinase C isoforms in cultured human retinal pigment epithelial cells. Graefes Arch Clin Exp. 2007;245:993–9. doi: 10.1007/s00417-006-0467-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.