Abstract
Bacteria of the Burkholderia cepacia complex (Bcc) persist in the airways of people with cystic fibrosis (CF) despite the continuous recruitment of neutrophils. Most members of Bcc are multidrug resistant and can form biofilms. As such, we sought to investigate whether biofilm formation plays a role in protecting Bcc bacteria from neutrophils. Using the neutrophil-like, differentiated cell line, dHL60, we have shown for the first time that Bcc biofilms are enhanced in the presence of these cells. Biofilm biomass was greater following culture in the presence of dHL60 cells than in their absence, likely the result of incorporating dHL60 cellular debris into the biofilm. Moreover, we have demonstrated that mature biofilms (cultured for up to 72 h) induced necrosis in the cells. Established biofilms also acted as a barrier to the migration of the cells and masked the bacteria from being recognized by the cells; dHL60 cells expressed less IL-8 mRNA and secreted significantly less IL-8 when cultured in the presence of biofilms, with respect to planktonic bacteria. Our findings provide evidence that biofilm formation can, at least partly, enable the persistence of Bcc bacteria in the CF airway and emphasize a requirement for anti-biofilm therapeutics.
Keywords: cystic fibrosis, host–pathogen interaction, innate immunity
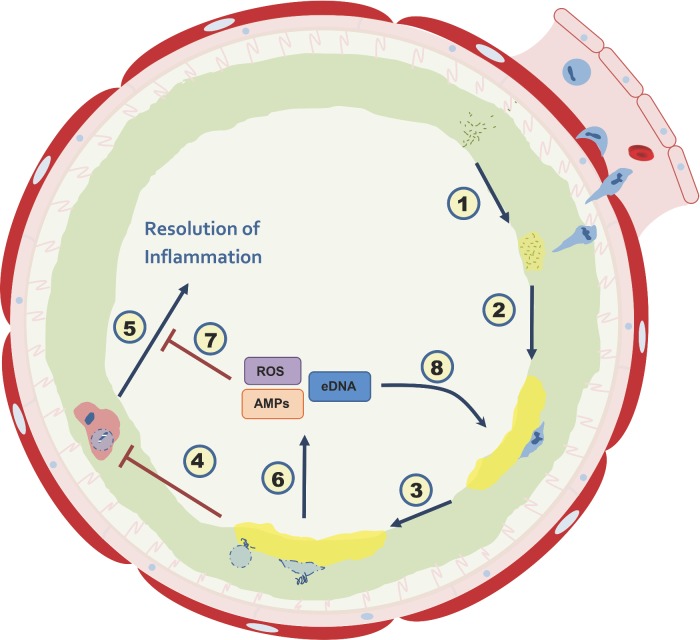
A study of the influence ‘Burkholderia cepacia complex’ biofilms have on the behavior and fate of neutrophil-like dHL60 cells, also addressing the influence of those cells on the biofilm.
Graphical Abstract Figure.
A study of the influence ‘Burkholderia cepacia complex’ biofilms have on the behavior and fate of neutrophil-like dHL60 cells, also addressing the influence of those cells on the biofilm.
INTRODUCTION
People with cystic fibrosis (CF) generate a dehydrated and unusually viscous pulmonary mucous which, in turn, diminishes mucociliary clearance from their airways (Boucher 2004). This allows for inhaled microorganisms to remain in situ and colonize the airway, transiently or permanently, depending in part on their ability to subvert the immune response.
Among the many opportunistic microorganisms which can colonize the airways of people with CF are members of the Burkholderia genus of Gram-negative bacteria, particularly the 18 closely related species currently considered part of the Burkholderia cepacia complex (Bcc) (Vandamme and Dawyndt 2011; Peeters et al. 2013). Presently, the incidence of Bcc bacteria in CF patients is approximately 2.6% in the USA and 3.8% in the UK (CFF 2012; UKCFR 2013), with B. cenocepacia and B. multivorans strains comprising the majority of isolates (Drevinek and Mahenthiralingam 2010).
Though less common colonizers of CF patients than Pseudomonas aeruginosa, Bcc bacteria are disproportionally highly associated with morbidity and mortality amongst sufferers (Tablan et al. 1987; Jones et al. 2004), owing to the bacterias’ inducement of an acute-onset lung deterioration with associated septic bacteraemia, termed ‘cepacia syndrome’ (Isles et al. 1984), as well as its correlation with poorer post-operative outcomes following lung transplantation (Alexander et al. 2008; Meachery et al. 2008). Pathogenic strains of Bcc species are also commonly multidrug resistant and most display an ability to form biofilms in vitro (Caraher et al. 2007), which further reduce their sensitivity to antibiotics.
Over the duration of pulmonary colonization, P. aeruginosa displays a number of adaptive phenotypes which aid in its persistence (Sousa and Pereira 2014). Among these adaptations is the formation of a biofilm, largely comprised of secreted exopolysaccharide (EPS) and DNA, often detected in the CF airway as non-surface-attached bacterial aggregates (Singh et al. 2000; Sriramulu et al. 2005).
Biofilm formation by P. aeruginosa is known to facilitate their evasion of the antimicrobial actions of neutrophils. The biofilm can impede migration of leukocytes at their exofacial surface (Jesaitis et al. 2003), while mucoid P. aeruginosa is resistant to phagocytosis by neutrophils relative to non-mucoid counterparts (Cabral, Loh and Speert 1987), by virtue of cell surface alteration which renders the bacterium unrecognizable.
A similar situation may prevail for Burkholderia, a close relative of Pseudomonas; mucoid CF isolates of B. cenocepacia display reduced adhesion to neutrophils relative to similar, non-mucoid strains (Conway et al. 2004). Indeed, biofilm-deficient mutants of P. aeruginosa are more susceptible to phagocytosis by neutrophils than their biofilm-competent counterparts (Bjarnsholt et al. 2005).
Following a period of antimicrobial activity, neutrophils undergo secondary necrosis and shed their cellular components which become part of the exopolymeric matrix of the biofilm (Parks et al. 2009), serving to further enhance its biomass while preserving the antibiotic resistance characteristic of the biofilm (Caceres et al. 2014). Indeed, clinical observations of P. aeruginosa biofilm aggregates highlight their close association with large quantities of, mostly non-viable, neutrophils (Bjarnsholt et al. 2009).
In studies of the CF airways, Bcc bacteria have predominantly been observed either as singular, planktonic cells or surviving within epithelial cells or leukocytes (Sajjan et al. 2001; Lamothe et al. 2007; Schwab et al. 2014), rather than biofilm-like aggregates. However, the heterogeneity of the airways makes it clear that prohibitively comprehensive sampling of the airways would be required to definitively conclude that Bcc biofilms are absent or present. Accordingly, other investigators suggest that Bcc biofilms exist in the airways of CF patients (Ciofu et al. 2015).
Given the utility of the biofilm to P. aeruginosa in evading neutrophil antimicrobial activity and the relatedness of Burkholderia to Pseudomonas, we hypothesize that the formation of a biofilm is important to the Bcc species’ resistance to neutrophil-mediated killing, considering that they are capable of persistence in CF patients’ airways, despite the continuous presence of large numbers of neutrophils in the lungs of CF patients. This report provides support for this hypothesis by elucidating the nature of Bcc species’ interaction with neutrophil-like dHL60 cells.
We have investigated the bacterias’ interaction with the cells from two broad standpoints, namely from the perspective of cell functionality and survival in the presence of biofilm-dwelling Bcc bacteria and the influence of the presence of the cells on the formation and development of biofilms by Bcc species.
We report that chemotaxis, migration and secretion of chemokines are diminished in the cells, while their viability is reduced following culture in the presence of Bcc biofilms, with respect to planktonic bacteria. Concurrently, the biofilm formed by the bacteria is enhanced during this coculture. Our findings contribute to our understanding of the respective effects that neutrophils and Bcc biofilms have on one another in the CF airway and emphasize the utility of the biofilm to Bcc bacteria as a virulence trait in CF.
EXPERIMENTAL PROCEDURES
Bacterial strains
Bcc bacteria had been obtained from the Belgian Co-ordinated Collections of Microorganisms (BCCM/LMG) and were stored at −20°C. The strains used were B. multivorans LMG 13010, B. dolosa LMG 18941 and B. cenocepacia K56–2 (LMG 18863), each of which had been isolated from CF patients prior to deposition with the BCCM. For use during this study, bacteria were maintained on B. cepacia selective agar (Henry et al. 1997) and cultured in LB medium (Sigma) at 37°C.
Cell culture
The promyelocytic cell line, HL60, was obtained from the European Collection of Cell Cultures (Health Protection Agency Culture Collections) and cultured in IMDM (Lonza) supplemented with 10% (v v−1) FBS (Sigma) at 37°C with 5% CO2. Cells were differentiated by culture in the presence of 1.25% (v v−1) DMSO (Sigma) for 7 days prior to use (Collins et al. 1979). Differentiation was confirmed by means of flow cytometry for the expression of neutrophil-specific markers, CD66 and CD11b (Owaki et al. 1991).
Assessment of biofilm biomass
Bacteria (1 × 106 cfu ml−1) were cultured in the absence or presence of viable dHL60 cells (1 × 107 cells ml−1) or an equivalent amount of whole-cell lysate in 100 μl LB in wells of 96-well plates at 37°C for up to 72 h. Lysates were prepared by repeated freeze–thaw cycles at −80°C with mechanical disruption through a fine gauge syringe needle, so as to avoid the use of detergents. Lysis was confirmed by visual inspection of discrete 20 μl aliquots using a light microscope at 400× total magnification. DNA was extracted from dHL60 cells using the DNeasy blood and tissue kit (Qiagen), as per the manufacturers’ protocol. Wells were washed three times with deionized water and biofilm biomass was stained by addition of 125 μl of an aqueous 1.25% (w v−1) crystal violet solution (Sigma) for 45 min. Wells were then washed further three times and biofilm-bound crystal violet was solubilized in 200 μl 95% (v v−1) ethanol/ 0.05% triton X-100 (Sigma). Crystal violet was quantified spectrophotometrically at 590 nm using a Varioskan microtiter plate reader (Thermo Scientific).
In parallel, dHL60 cells, or lysates thereof, were cultured in triplicate wells in the absence of bacteria. These wells were similarly subjected to crystal violet staining and revealed negligible adsorption of cellular material (mean OD590 = 0.102 ± 0.14; n = 3).
Assay of IL-8 secretion
Bacteria were cultured in LB to mid-exponential phase of growth and inoculated (1 × 106 cfu ml−1) into triplicate wells of 24-well plates prior to incubation at 37°C for 24, 48 or 72 h. Biofilms were washed with sterile, pre-warmed PBS and dHL60 cells (1 × 107 cells ml−1) were added in IMDM. Planktonic bacteria, cultured to mid-exponential growth in LB, were discretely diluted to each of 1 × 106 or 1 × 107 cfu ml−1 in IMDM and inoculated separately into triplicate wells of 24-well plates in the presence of dHL60 cells. Supernatants were recovered following 3 h of coculture at 37°C and stored at −20°C. Levels of IL-8 in 1:10 dilutions of supernatants were determined by sandwich ELISA. ELISA was conducted using the OptiEIA IL-8 ELISA Set (BD Biosciences) according to the manufacturers’ protocol.
Enumeration of bacteria residing within biofilms
Bacteria residing within the biofilms cultured in wells of microtiter plates were enumerated according to the method described by Van den Driessche et al. (2014). Briefly, bacteria (1 × 106 cfu ml−1) were cultured in 100 μl LB in triplicate wells of 96-well plates at 37°C for 24, 48 or 72 h. Wells were then rinsed three times with Ringers solution, 100 μl of Ringers solution was added and biofilm was disrupted by repeated pipetting followed by sonication in an ultrasonic bath (Branson Ultrasonics Corp.) for 5 min. Contents of wells were then subjected to a series of 10-fold dilutions in Ringers solution. Approximately 20 μl aliquots of these dilutions were plated in triplicate and plates were incubated at 37°C for 48 h. Colonies formed on each plate were then enumerated where possible. The experiment was conducted in triplicate.
Confocal microscopy
For observation by confocal microscopy, bacteria were transfected, by triparental mating (de Lorenzo and Timmis 1994), with plasmid, pBAH 8 (Huber et al. 2002), which encoded mutated green fluorescent protein (GFPmut3) (Cormack, Valdivia and Falkow 1996). The donor strain used for triparental mating was Escherichia coli MT102, which had been cultured in the presence of 10 μg ml−1 gentamycin (Sigma). The helper strain used was E. coli HB 101, having the plasmid pRK600, which had been cultured in the presence of 10 μg ml−1 chloramphenicol (Sigma). Donor and helper strains were kindly donated by Prof. Leo Eberl (Institute of Plant Biology, University of Zurich, Switzerland).
Transfected bacteria were cultured under continuous flow of minimal nutrients as described in detail elsewhere (Weiss Nielsen et al. 2011). Briefly, bacteria (1 × 105 cfu per chamber) were inoculated in LB into triplicate flowcell chambers and allowed to adhere to the glass slide component of the chamber in the absence of medium throughput for 45 min. Following this, bacteria were incubated at 37°C under a flow of 0.1 ml min−1.
CellTracker Red fluorescent cytoplasmic dye (Molecular Probes) was used to stain dHL60 cells (4 × 105 cells) following the manufacturers’ protocol 30 min prior to addition to flowcell chambers in the absence of media throughput. Confocal micrographs were generated using an Olympus FV-1000 microscope with a 60× oil immersion lens (Olympus). Computer-generated surfaces were created using Imaris software (Bitplane).
Assessment of cell death
Bcc bacteria were cultured to mid-exponential phase of growth and diluted to 1 × 106 cfu ml−1 in LB for inoculation into triplicate wells of 24-well plates. Plates were cultured at 37°C for 24, 48 or 72 h. Wells were then rinsed twice with pre-warmed, sterile PBS prior to addition of 1 × 107 dHL60 cells ml−1 in IMDM. Bacteria were separately cultured to mid-exponential growth phase in LB and diluted to 1 × 106 cfu ml−1 in IMDM immediately prior to addition of dHL60 cells (1 × 107 cells ml−1).
Supernatants were also prepared from discrete cultures of planktonic or biofilm-dwelling bacteria (24, 48 and 72 h) by careful removal of liquid without disturbance of biofilm, centrifugation at 4000 × g for 10 min and filtration through syringe filters having a pore size of 0.22 μm. dHL60 cells were then inoculated into supernatants in triplicate wells. Plates were then incubated for 3 h at 37°C.
Additional cells were discretely incubated for 3 h in the presence of known inducers of apoptosis. Actinomycin D (2 μg ml−1) was used to induce apoptosis for assessment of caspase-3 activation. Staurosporine (15 μM) was used as an inducer of apoptosis during assessment of Mcl-1 expression to avoid interference from the mRNA transcription inhibitory property of actinomycin D.
Quantification of caspase-3 activation was performed using the caspase-3/7 colorimetric assay kit (Biovision) according to the manufacturers’ instructions.
For flow cytometry, cells were removed, washed with Annexin V binding buffer and stained with annexin V-FITC and propidium iodide (BD Biosciences). Flow cytometry was performed on a FACSCalibur instrument (BectonDickinson).
Gene expression analysis by RT-qPCR
The relative expression of IL-8 or Mcl-1 mRNA transcripts was determined for dHL60 cells (1 × 107 cells ml−1) which had been incubated at 37°C in triplicate wells of 24-well plates in the presence of Bcc bacteria for 3 h. The bacteria had been cultured planktonically to mid-exponential phase and diluted to 1 × 106 cfu ml−1 in 1 ml IMDM or inoculated at 1 × 106 cfu ml−1 into triplicate wells of 24-well plates prior to culture as biofilms for 24, 48 or 72 h. Biofilms were rinsed twice with sterile PBS prior to addition of dHL60 cells in 1 ml IMDM.
Total RNA was isolated using the RNeasy kit (Qiagen), followed by removal of residual DNA by use of the TURBO DNA-free kit (Ambion). Reverse transcription was carried out using the Superscript® VILO cDNA synthesis kit (Invitrogen). Quantification of mRNA was conducted on an ABI 7300 real-time PCR system (Applied Biosystems) using Power SYBR® green PCR master mix (Applied Biosystems). Primer sequences were either obtained from PrimerBank or were designed using Primer-BLAST software (NIH); TBP PrimerBank ID: 285026518c3, Mcl-1 PrimerBank ID: 11386165a2, SDHA forward sequence: 5′-GCGGCAACAGCAGACAT-3′, SDHA reverse: 5′-ACTGTTGGCCACGCCTT-3′, IL-8 forward: 5′-ACACTGCGCCAACACAGAAA-3′, IL-8 reverse: 5′-ATTCTCAGCCCTCTTCAAAAACTTC-3′. Primers were obtained from Invitrogen. Cq values and average efficiencies per gene were determined by LinRegPCR (Ruijter et al. 2009). Differential gene expression between cell populations was then estimated using REST 2009 software (Qiagen) (Pfaffl, Horgan and Dempfle 2002; Hellemans et al. 2007), normalized against the geometric mean expression of the reference genes TBP and SDHA, previously validated as stable in neutrophils exposed to LPS (Ledderose et al. 2011). The geometric mean of the fold changes and their respective standard errors were calculated (Bengtsson et al. 2005) and means were then subjected to log2 transformation (Rieu and Powers 2009).
Statistical analysis of data
Parametric data were subjected to Student's two-independent-sample t-test (Student 1908). Comparisons of multiple discrete populations were conducted by one-way analysis of variance (ANOVA), followed post-hoc by Tukey's multiple pairwise comparison test or Dunnett's comparison test (Tukey 1949; Dunnet 1955). Non-parametric data were tested using the Kruskal–Wallis multiple comparison test (Kruskal and Wallis 1952). Differences between means were reported as statistically significant where the probability of the difference occurring by chance was less than 5% (P < 0.05).
RESULTS
The Bcc biofilm is a physical barrier to dHL60 cell migration
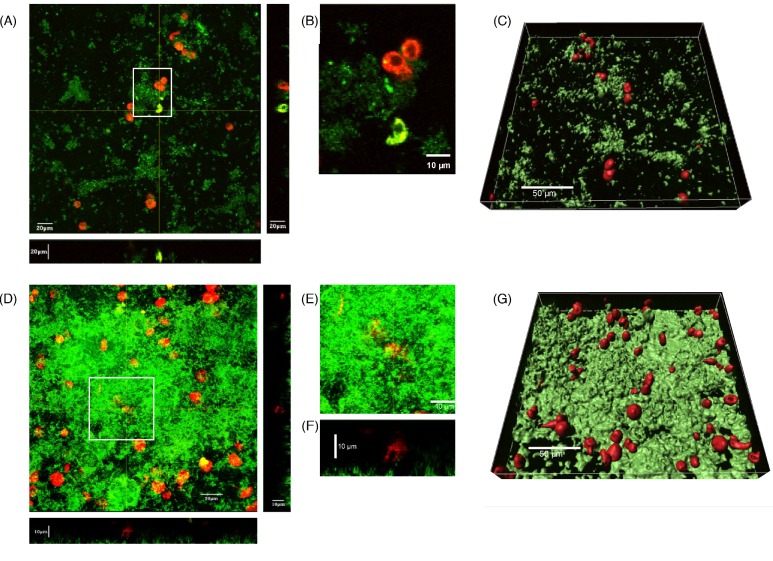
Neutrophils have been shown to migrate through staphylococcal biofilms and to phagocytose the biofilm-dwelling bacteria (Leid et al. 2002; Günther et al. 2009a,b; Meyle et al. 2010). However, Jesaitis et al. (2003) have shown that neutrophils are incapable of migration into established P. aeruginosa biofilms, hence limiting their ability to phagocytose and eradicate the biofilm-dwelling P. aeruginosa bacteria. Thus, the failure of neutrophils to eradicate colonizing Bcc bacteria in the CF airway may be due, at least in part, to their inability to effectively gain access to the bacteria residing in biofilms. To determine whether this was the case, Bcc bacteria were cultured in flowcell chambers in order to generate biofilms amenable to visual inspection. dHL60 cells were then added and their position assessed by confocal microscopy. By 30 min post-addition of dHL60 cells, they demonstrated a rounded morphology and were residing superficially on the luminal surface of the biofilm matrix (Fig. 1A). In most cases, bacteria had swarmed around the cells and the cells had internalized bacteria in some cases (yellow–green coloration, Fig. 1B). A lack of lateral movement or ingress was apparent, with cells residing superficially on the biofilm exterior (Fig. 1C, F and G). This was particularly evident for B. multivorans biofilms of high biomass (Fig. 1D), suggesting that the biofilm physically impedes the migration of the cells into the biofilm.
Figure 1.
dHL60 cells do not enter established B. multivorans LMG 13010 and B. dolosa LMG 18941 biofilms. Bcc bacteria (1 × 106 cfu ml−1), harboring a GFP-expression plasmid, were inoculated into flowcells and cultured under continuous throughput of defined, minimal medium at 37°C for 72 h. To the flowcell was added 4 × 105 dHL60 cells ml−1, which had been stained with 5 μM Celltracker Red cytoplasmic dye (Molecular Probes), 30 min prior to image collection. Composite confocal micrographs of sections through the z-axial plane of biofilms formed by (A) B. dolosa LMG 18941 and (D) B. multivorans LMG 13010 were then generated, with biofilms proximal to the viewer. (B) Enlargement of a single micrograph of the area demarcated by white box in A showing a phagocytosing cell. (E) Enlargement of the area demarcated by white box in D to highlight (F) a transverse section corresponding to image E illustrating a cell resting above the biofilm. Using the data presented in A and D, discrete surfaces were overlaid onto the detected fluorescence intensities for each channel using Imaris software (Bitplane) in order to illustrate the spatial relationship between (C) B. dolosa and (G) B. multivorans biofilms, and dHL60 cells.
The Bcc biofilm masks the bacteria from being recognized by dHL60 cells
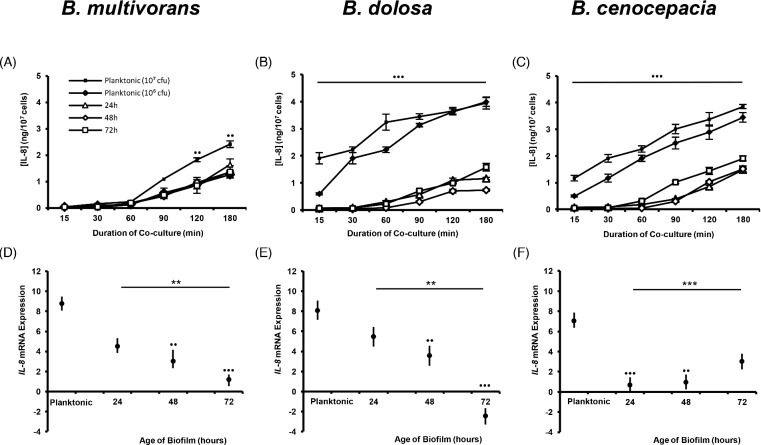
Pulmonary inflammation in CF is largely driven by excessive activation of infiltrated neutrophils, correlating with high levels of proinflammatory cytokines and chemokines (Bonfield et al. 1995). As such, we sought to determine whether the biofilm promoted an excessive secretion of neutrophil-recruiting and -activating IL-8. Surprisingly, we observed a significant suppression in the amount of IL-8 secreted by dHL60 cells in response to Bcc biofilms, relative to planktonic Bcc bacteria, for each of the three species investigated (P < 0.001; Fig. 2 A–C). This suggests that the biofilm is masking the bacteria within from being recognized by the dHL60 cells. Furthermore, the amount of IL-8 secreted in response to the biofilms showed little intraspecific variation regardless of whether the biofilm was 24, 48 or 72 h old. This implies that by 24 h post-inoculation, the Bcc biofilm is sufficiently robust to diminish recognition of the bacteria by the dHL60 cells. This finding was corroborated by our observation of diminished induction of IL-8 expression in dHL60 cells cultured with biofilms of B. multivorans or B. dolosa bacteria, with respect to those cells that were cultured in the presence of planktonic bacteria (Fig. 2 D and E).
Figure 2.
Biofilm-dwelling Bcc bacteria induce lesser amounts of IL-8 expression or secretion by dHL60 cells than do their planktonic counterparts. Bacteria were cultured as biofilms (106 cfu ml−1), in triplicate wells of 24-well plates, at 37°C for 24, 48 or 72 h or were cultured to mid-exponential phase of growth (planktonic bacteria) and inoculated (at 106 or 107 cfu ml−1 for assessment of IL-8 secretion; or at 106 cfu ml−1 for assessment of IL-8 expression) into triplicate wells of 24-well plates. Biofilms were washed with sterile PBS and dHL60 cells (107 cells ml−1) were added to each well for the indicated durations. Supernatants were prepared for each time point and assayed in duplicate, by sandwich ELISA, for the presence of IL-8 induced by (A) B. multivorans LMG 13010, (B) B. dolosa LMG 18941 or (C) B. cenocepacia K56–2 biofilms. Data represent mean values ± standard deviations of duplicate assays of each of three replicate wells from each of three replicate experiments. Cells were recovered and RNA was isolated and converted to cDNA prior to analysis of expression of IL-8 mRNA induced by (D) B. multivorans LMG 13010, (E) B. dolosa LMG 18941 or (F) B. cenocepacia K56–2 biofilms. Data represent log2 geometric mean expression levels ± standard errors of duplicate assays of each of three replicate wells from each of three replicate experiments. Bullets indicate statistically significant differences between responses to planktonic bacteria with respect to biofilm-dwelling populations (••P < 0.01 or •••P < 0.001, ANOVA or Kruskal–Wallis test). Asterisks indicate significance of differences in responses between biofilms of differing maturity (**P < 0.01 or ***P < 0.001).
In order to ascertain whether numbers of bacteria residing within biofilms influenced the observed IL-8 secretion, the numbers of colony-forming units present in the wells at 24, 48 and 72 h under matching conditions were determined by biofilm disruption and bacterial enumeration by spread plate method. Overall, mean cfu per well was 2.57 ± 1.54 × 107 cfu. Quantities observed did not correlate significantly with either bacterial species or duration of culture (logistic regression, P = 0.659). Hence, the IL-8 secreted by dHL60 cells upon exposure to Bcc biofilms was markedly less than would be expected in response to an equivalent quantity of planktonic bacteria.
Exposure to biofilms enhances necrosis in dHL60 cells
The proinflammatory nature of neutrophils in responding to invading bacteria is resolved in large part by their ingestion by macrophages. Neutrophils to be ingested are recognized by cell-surface markers of apoptosis which themselves are products of phagocytosis-induced cell death (PICD) (Coxon et al. 1996; Flannagan et al. 2014). CF airways, however, are marked by a continuous inflamed state, indicating this process is not occurring correctly.
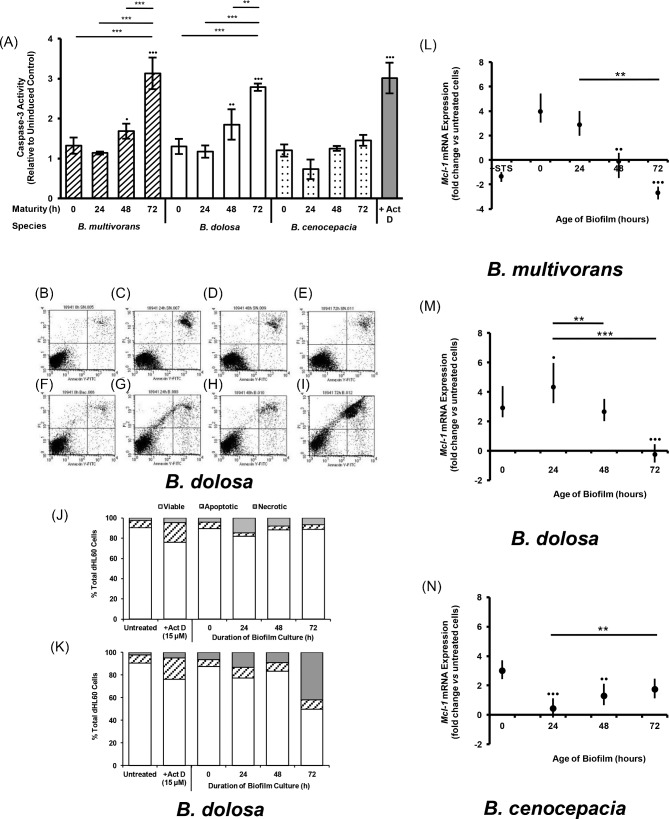
Hence, we sought to ascertain whether biofilm-dwelling Bcc bacteria disrupted the normal onset of apoptosis in dHL60 cells. Assay of the activation of caspase-3 revealed that dHL60 cells, which had been cultured in the presence of Bcc biofilms established 24, 48 or 72 h previously, were undergoing apoptosis and that the extent of apoptosis in the cell population was proportional to the maturity of the biofilm to which they were exposed (Fig. 3A), while planktonic bacteria induced little caspase-3 activation during the course of coculture. This outcome may be explained by increased numbers of bacteria yielding a corresponding increase in phagocytosis and, hence, PICD. However, this supposition was not corroborated by our findings following enumeration of bacteria within our cultured biofilms. While biofilms harbored 2.57 ± 1.54 × 107 cfu—markedly higher than the experimental inoculum of planktonic bacteria—there was no consistent correlation between biofilm maturity and bacterial count for any of the species tested.
Figure 3.
Culture in the presence of Bcc biofilms induces cell death in dHL60 cells. Bacteria (1 × 106 cfu ml−1) were cultured at 37°C for 0, 24, 48 or 72 h. Wells were then washed with sterile PBS and dHL60 cells (1 × 107 cells ml−1) were added. Discrete dHL60 cell populations were also cultured in the absence or presence of 2 ug ml−1 actinomycin D or 15 μM staurosporine. Plates were incubated at 37°C for 3 h prior to harvesting of cells. Cells were lysed and assayed for caspase-3 activity (A). Columns represent the mean caspase-3 activity of triplicate dHL60 cell populations from each of three independent experiments, relative to the mean uninduced control population. Bullets indicate statistically significant differences with respect to uninduced controls (•P < 0.05, ••P < 0.01, •••P < 0.001, one-way ANOVA). Asterisks denote significant differences between biofilm age for a given species (**P < 0.01, ***P < 0.001). Cells from each population were then isolated by centrifugation and resuspended in binding buffer in the presence of annexin V-FITC and propidium iodide. Each cell population was analyzed by flow cytometry. Representative scatterplots are presented for dHL60 cells which had been cultured with (B–E) supernatants derived from planktonic or biofilm-dwelling populations of B. dolosa bacteria or (F–I) the planktonic or biofilm-dwelling B. dolosa bacteria themselves. The quantity of viable, apoptotic or necrotic cells following each culture condition is also presented for (J) supernatants and (K) planktonic or biofilm-dwelling bacteria. RNA was isolated from cells and converted to cDNA; cDNA was then analyzed semiquantitatively by qPCR (L–N). Data represent the log2-transformed geometric means (± SE) of triplicate populations from each of three independent experiments. Bullets indicate significance differences in expression mediated by biofilms vs planktonic bacteria (•P < 0.05, ••P < 0.01, •••P < 0.001, Kruskal–Wallis test); asterisks indicate significance mediated by biofilms vs one another (*P < 0.05, **P < 0.01, ***P < 0.001).
However, we then used flow cytometry to discern the extent and progression of cell death to secondary necrosis. This demonstrated that substantial proportions of the cell populations which had been cultured with well-established B. dolosa LMG 18941 static biofilms (72 h maturity) had progressed to necrosis by 3 h post-inoculation (Fig. 3I). This outcome was not elicited by supernatants derived from the medium in which biofilms had been cultured (Fig. 3E), suggesting that the dHL60 cell death was not mediated by a soluble factor, but rather a factor associated with the biofilm itself or the nature of the interaction.
Furthermore, the loss of the cells’ viability during culture with Bcc biofilms coincided with the loss of Mcl-1 expression, required for neutrophil survival (Fig. 3L–N).
Coculture of Bcc bacteria with dHL60 cells results in increased biofilm formation
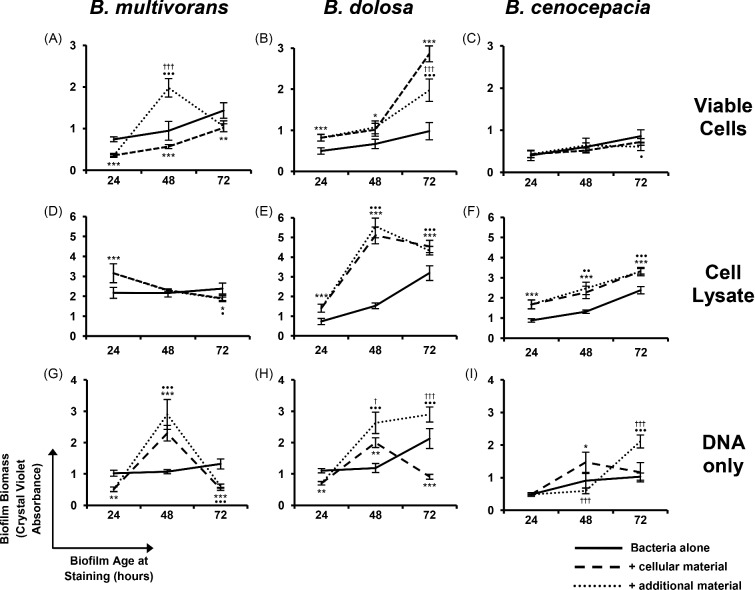
Considering the advantage that biofilm residence confers on Bcc bacteria in limiting neutrophil antimicrobial activities, we sought to ascertain whether dHL60 cells had an impact on the extent of biofilm formed by Bcc bacteria. To determine whether dHL60 cells had an effect on the extent of biofilm formation and development by Bcc species, bacteria were cultured in the presence of dHL60s for up to 72 h. Staining of the biofilm biomass revealed that B. dolosa biofilms possessed significantly greater biomass following culture in the presence of dHL60s than in their absence (P < 0.001; Fig. 4B). This outcome was further pronounced when B. dolosa LMG 18941 bacteria were cultured in the presence of whole-cell lysates of dHL60 cells (P < 0.001; Fig. 4E), a finding mirrored by B. cenocepacia bacteria (Fig. 4F). This suggests that, while viable dHL60s kill some proportion of the bacterial population, once they have become necrotic and disintegrated, their cellular components become incorporated into the bacterial EPM.
Figure 4.
The impact of dHL60 cells on the formation of biofilms by Bcc bacteria. Bacteria (1 × 105 cfu) were cultured in 96-well plates in the absence or presence of (A–C) dHL60 cells (1 × 106 cells), (D–F) an equivalent quantity of whole-cell lysate thereof, or (G–I) DNA derived from an equivalent number of cells, for up to 72 h. In some cases, additional dHL60 cells or cellular material were added 24 h after initial inoculation. Biofilm density was assessed after 24, 48 or 72 h by crystal violet staining. Asterisks indicate significant differences between normal biofilm biomass and biofilm formed in the presence of dHL60 material (*P < 0.05, **P < 0.01, ***P < 0.001, two independent sample t-test). Bullets indicate significant differences comparison between normal biofilm biomass and biomass after two additions of dHL60 material (•P < 0.05, ••P < 0.01, •••P < 0.001). Crosses indicate significant differences between biofilm formed in the presence of dHL60 material vs biomass after two additions of dHL60 material (†P < 0.05, ††P < 0.001). Data are expressed as the mean (± SD) of four biological replicates from each of three independent experiments.
Culture of the bacteria with DNA purified from dHL60 cells highlighted that this alone could enhance the quantity of biofilm biomass (Fig. 4H), though this effect was not as pronounced.
Burkholderia multivorans LMG 13010 bacteria displayed significantly increased biomass with respect to controls following 48 h of culture after two additions of viable dHL60 cells, as well as following addition of DNA (Fig. 4A and G). However, these levels of biomass were no longer evident 72 h post-inoculation (Fig. 4A, D and G). This could be due to a destabilized proportion of the biofilm, comprised of cellular components, detached during analysis.
DISCUSSION
Clinical treatment of the microbial cause of pulmonary exacerbations in CF patients is confounded by the biofilm phenotype of the colonizing bacteria. When treatment is based on antibiotic susceptibility testing of biofilms formed by patients’ bacterial isolates, the mean time to pulmonary exacerbations is extended (Keays et al. 2009).
Pseudomonas aeruginosa biofilms, and their relevance to overcoming neutrophil-mediated immunity, have been the subject of a number of studies, owing to the high prevalence of the pathogen in the airways of people with CF. However, there is a relative lack of similar studies concerning Bcc, despite the disproportionate mortality associated with them. In the present study, we provide further evidence supporting the assertion that the biofilm is important for bacterial persistence in the CF airway and that this is in part due to the disruption of neutrophil antimicrobial activities.
Neutrophils, stimulated by microbially associated molecular patterns, produce IL-8 which serves to gather and further activate the neutrophil population at the site of colonization (Schröder et al. 2006b). We have demonstrated, however, that, when encountering Bcc biofilms, dHL60 cells display a diminished IL-8 secretory response (Fig. 2). This lack of self-reflexive activation may favor the bacteria by virtue of limiting the proinflammatory, antimicrobial character of the cells.
That similar levels of IL-8 secretion were observed for each of the three species, regardless of the age of the biofilm tested, reflects two complementary scenarios; first, an exofacial moiety itself induces a specific IL-8 response, possibly an EPS. Support for this concept can be derived from the study reported by König, Ceska and König (1995), which looked at the ability of P. aeruginosa to stimulate IL-8 release from neutrophils, as well as the ability of alginate from two P. aeruginosa strains to induce IL-8 release. They observed that alginate from the bacteria induced a statistically significant, but 10-fold lesser, release than did the bacteria themselves. Hence, though alginate is distinct from the EPS produced by Bcc bacteria, they may be similarly immunogenic.
Secondly, the type of pathogen recognition receptor (PRR) which becomes activated has a bearing on the type of IL-8 response (Schröder et al. 2006a). The authors of that study noted that, while LPS stimulation prompted de novo synthesis of IL-8 by neutrophils in conjunction with secretion from intracellular stores, agonism of CD66b resulted only in secretion of stored IL-8. Hence, Bcc EPS may be recognized by such a PRR, whose downstream signaling fails to prompt IL-8 transcription.
dHL60 cells, which had been cultured in the presence of planktonic Bcc bacteria, exhibited 6.5–9.4-fold upregulation in expression of IL-8 mRNA transcript. Notably, while the dHL60 cells did, for the most part, display an induction of IL-8 expression when cultured with Bcc biofilms, the extent of the increase in IL-8 transcript synthesis was diminished with respect to that seen during planktonic coculture. This apparent loss of IL-8 induction mirrors our finding that secretion of IL-8 by dHL60 cells is greatly reduced during coculture with Bcc biofilms relative to planktonic Bcc bacteria.
In contrast to our observations, neutrophils have been reported to secrete comparable amounts of IL-8 when exposed to either planktonic P. aeruginosa PAO1 bacteria or PAO1 bacteria dwelling in a biofilm of 18 h maturity (Fuxman Bass et al. 2010). This may be due to the ability of P. aeruginosa extracellular DNA (eDNA) to stimulate IL-8 release from neutrophils, doing so in a TLR9-independent fashion (Trevani et al. 2003; Alvarez et al. 2006), given that Pseudomonas eDNA is often a component of their biofilms. Bcc bacteria vary in the extent to which their DNA forms part of the biofilm (Messiaen, Nelis and Coenye 2014); as such, it may not be as prevalent or immunogenic as that of P. aeruginosa.
There may be a failure by the dHL60 cells to encounter the cognate ligands for their PRRs. Such ligands may however be obscured by the surrounding EPS. We have shown that the ability of the cells to enter into the biofilm is compromised (Fig. 1), while the same compromised migration has been shown for neutrophils invading P. aeruginosa biofilms (Jesaitis et al. 2003; Bjarnsholt et al. 2005).
The implication, therefore, of our findings, and those of others highlighted here, would be that the biofilm would physically mask the bacteria within from being recognized by the dHL60 cells. Exposure of some proportion of the bacterial population at the surface of the biofilm would be likely, but this can be accounted for by the finding that under most conditions in our study the expression or secretion of IL-8 was observable.
Purified EPS has been shown to be a potent tactile stimulus of motility for neutrophils, which migrated into soft agarose suffused with EPS, even ignoring fMLP gradients (Hänsch et al. 2008). This was not specific chemoattraction, as Boyden chamber assays failed to demonstrate specific directional chemotaxis toward EPS in solution, but rather an enhanced motility which coincided with increased surface expression on the neutrophils of the cellular adhesion-associated protein, CD66b.
Jesaitis et al. (2003), however, reported that neutrophils were rendered ostensibly non-motile once deposited on P. aeruginosa biofilms, a finding which mirrors that of Hänsch and colleagues for biofilms rather than EPS alone. Movement of neutrophils on Staphylococcus aureus biofilms has also been shown to be limited, with greater migration being observed at the periphery of the larger biomass (Günther et al. 2009b). Our study of dHL60-Bcc biofilm interactions revealed the same phenomenon when the cells were inoculated onto established biofilms (Fig. 1).
When added to 6-day-old S. aureus biofilms, neutrophils were able to take up adjacent components of the biofilm over 60 min of observation, indicating that they were able to phagocytose the material (Günther et al. 2009b). The antimicrobial activity of the stationary neutrophil, then, is limited to its immediate vicinity, while more mature biofilms suffered lesser phagocytosis-mediated losses of biomass.
As we have shown for B. multivorans and B. dolosa biofilms, and as has been discussed for other biofilm-forming species, neutrophils are not well able to penetrate into the biofilm. They can be seen to internalize components of the biofilm from the periphery of the overall extracellular polymeric matrix, but whether this behavior could translate to an ‘outside-in’ form of biofilm clearance in vivo is unclear. We have observed that dHL60s remained largely stationary once adhered to the EPM. We also describe increased cell death associated with the cell's encounter with Bcc biofilms, which would limit their ability to ingress into the biofilm. While compositional differences between our microtiter plate and flowcell-cultured biofilms are possible, the formation of a substantial biomass remains common between them. These findings suggest that neutrophils may be capable only of erosion of the external face of a biofilm; thus, the biofilm can function as an effective defensive barrier for the bacteria within.
Phagocytosis of bacteria induces cell death in the neutrophil, with these apoptotic neutrophils being themselves phagocytosed by macrophages (Kobayashi et al. 2003; Zhang et al. 2003). The purpose of these events is presumably minimization of potential tissue damage which would arise from the action of proteases released from the decaying cell, as apoptosis allows for their controlled decommissioning in macrophages without degradation of the cell membrane. Accordingly, PICD instigates a macrophage-directed signal encouraging phagocytosis of the neutrophil (Scannell and Flanagan 2007).
Neutrophils derived from healthy individuals undergo PICD when challenged with viable, planktonic B. cenocepacia isolates, as would be expected (Bylund et al. 2005). In the present study, we provide evidence that Bcc biofilms are also capable of inducing cell death in dHL60 cells (Fig. 3A) but that this is skewed toward necrosis in a biofilm age-dependent fashion over the duration of study (Fig. 3J). Increasing age of the biofilm did not correlate directly with a corresponding increase in bacteria dwelling within. This suggests that another facet of the biofilm which emerges during its development may promote cell death in the dHL60 cells.
Induction of necrosis would result in the failure of efferocytosis—neutrophil ingestion by macrophages—combined with the uncontrolled release of host tissue-damaging proteases, with a resulting proinflammatory state, as is observed in people with CF, who endure chronic pulmonary inflammation (Elizur, Cannon and Ferkol 2008).
Pursuant to the advantage of evading phagocyte attention, P. aeruginosa expresses toxins capable of inducing cell death in neutrophils (Dacheux et al. 2000; Xu et al. 2012). For example, biofilm-associated P. aeruginosa rhamnolipid has been demonstrated to induce necrosis in neutrophils in vitro (Jensen et al. 2007) and to prevent the bacterias’ clearance in a murine pulmonary infection model (van Gennip et al. 2009), resulting from the onset of neutrophil cell death following their inoculation onto Pseudomonas biofilm (van Gennip et al. 2012). Hence, biofilm-derived rhamnolipids enable the biofilm to act as a defensive barrier for Pseudomonas; as such, their production is upregulated in response to the presence of neutrophils via quorum sensing (Alhede et al. 2009).
The epidemic strain, B. cenocepacia J2315, has been shown to secrete a hemolytic lipopeptide with an ability to induce apoptosis in neutrophils (Hutchison, Poxton and Govan 1998), though the prevalence of hemotoxins across the Burkholderia genus is limited (Carvalho et al. 2007). Hence, the Bcc strains studied here may not produce a compound with specific antineutrophil potency equivalent to that of either rhamnolipids or pyocyanin.
Pseudomonas aeruginosa-secreted pyocyanin accelerates neutrophil cell death by inducing lysosomal membrane permeabilization with apoptosis ensuing through loss of Mcl-1 and induction of caspase-3 (Bianchi et al. 2008; Prince et al. 2008).
Notably, it has been shown that neutrophil-derived antimicrobial peptides interfere with phagocytosis by neutrophils (Voglis et al. 2009). Neutrophils for which phagocytosis is disrupted undergo cell death alternative to PICD, which may lead to their secondary necrosis within the CF airway milieu. The combination of this and the deliberate induction of necrosis by P. aeruginosa and possibly other species would lead to the uncontrolled release of neutrophil antimicrobial peptides in a self-propagating cascade. This situation, then, would negate the antimicrobial influence of neutrophils and facilitate persistent infection.
Pseudomonas aeruginosa bacteria are capable of biofilm formation in vitro despite the presence of viable neutrophils (Walker et al. 2005; Parks et al. 2009; Robertson et al. 2011; Caceres et al. 2014). In those and other studies, culture of P. aeruginosa bacteria in the presence of human neutrophils resulted in greater biofilm formation than that formed by the bacteria alone due to the incorporation into the biofilm of exogenous DNA and protein (Chiang et al. 2013; Watters et al. 2014). No equivalent study concerning Bcc bacteria has yet been reported. Hence, we examined the effect of culture of the bacteria with viable dHL60 cells, whole-cell lysates thereof or purified DNA.
We have shown that, despite an initial reduction in biofilm formation evident for B. multivorans LMG 13010 (Fig. 4A), culture of Bcc bacteria in the presence of viable dHL60 cells leads to an increase in biofilm biomass, while increased biomass was consistently observed for B. dolosa (Fig. 4B). This enhancement of biofilm biomass was further pronounced when bacteria were cultured in the presence of dHL60 cell lysate (Fig. 4E). It is likely that the presence of the lysate stimulated the bacteria to produce more EPS, macromolecules derived from the dHL60 cells adhered to the existing EPS or a combination of these events occurred.
Differing from either of these species, B. cenocepacia K56–2 bacteria demonstrated relatively static biomass. This may be due to the lesser biofilm-forming ability of this strain, with respect to the other two strains in this study, based on our findings during this study and in agreement with previous findings by Caraher et al. (2007), supported by data of other studies which report similar levels of biofilm biomass using the crystal violet assay (Aubert, Flannagan and Valvano 2008).
This provides a tentative explanation for our observations of differing outcomes between this strain and B. dolosa LMG 18941 and gives cause to speculate that secretion of greater quantities of EPS subsequently gives rise quantities of biofilm which are greater still, when formed in the presence of HL60 or dHL60 cells, with the EPS perhaps acting as an adhesive scaffold.
Many commonalities exist between the formation of biofilm by B. multivorans and B. cenocepacia bacteria. They share, for example, a dependence on the production of poly-β-1, 6-N-acetyl-D-glucosamine in order to produce a cohesive biofilm (Yakandawala et al. 2011). DNA is also a component of B. cenocepacia biofilms, wherein it is associated with the DNA-binding protein, DNABII (Novotny et al. 2013). However, the species have recently been shown to diverge in the prevalence of DNA in their respective biofilms (Messiaen, Nelis and Coenye 2014). In that study, B. multivorans LMG 13010 biofilms were reported to contain ∼1.5 μg ml−1 DNA per 108 cfu following 24 h of culture, while B. cenocepacia LMG 16656 bacteria (a CF isolate) contained only ∼0.15 μg ml−1 DNA/108 cfu. Whether this disparity could explain the observed difference in biomass between the biofilms of our strains when cultured with dHL60 cells is unclear.
The presence of extracellular DNA may be a requirement for initiation of biofilm formation by P. aeruginosa (Whitchurch et al. 2002). Inclusion by P. aeruginosa of exogenous DNA appears, at least in part, to be driven by pyocyanin, through stimulation of DNA release from the bacteria themselves (Das and Manefield 2012; Das et al. 2013), as well as from neutrophils via induction of apoptosis. Indeed, such fortuitous or autologous lytic mechanisms are common in populations of many bacteria whose biofilms comprise DNA (Montanaro et al. 2011).
The DNA observed in the work of Messiaen et al. (2014) was autologously derived; we have provided evidence in our study that the increased biofilm mass seen following coculture is partially the result of incorporation of DNA (Fig. 4C, F and I). However, B. dolosa bacteria, as well as B. multivorans bacteria, had formed biofilms whose biomass was somewhat less than that formed by the bacteria which were cultured alone after 24 h of culture with DNA. This reduced initial formation was not evident when the bacteria were cultured with whole-cell lysate, perhaps due to an effect of the presence of protein. This may be a beneficial physicochemical effect, aiding cohesion of the EPM, supported by the study of Walker et al. (2005) wherein they observed that adding purified neutrophil actin to P. aeruginosa led to increased biofilm biomass.
The observed lessening biomass in the studied B. multivorans biofilms which had been challenged with dHL60 cells or cellular components is perhaps the result of detachment of a portion of the biofilm from the non-substrate-adjacent region (Stoodley et al. 2001; Kaplan 2014). A recent, detailed analysis of the mechanical properties of P. aeruginosa biofilms confirmed that heterogeneities of bacterial density exist within the EPM and that detachment could occur ostensibly through erosion, cohesive detachment of sections of the biofilm from itself or adhesive detachment of the biofilm from the substratum (Guélon et al. 2013). The authors hypothesized that the type of detachment experienced depended on the composition of the EPM, with EPS being more hydrate and, thus, of lesser stiffness than bacterial aggregates.
Hence, if the B. multivorans biofilm in our study readily takes up dHL60 cell lysate, then this may reduce EPM stiffness and dispose the biofilm to detachment during processing. Why this occurred only in that species may be reflective of the composition of the biofilm, as Bcc strains differ in their secretion of EPS (Cuzzi et al. 2014).
This initial reduction in biofilm formation is absent for bacteria cultured with dHL60 cell lysate, indicating that the dHL60 cells mount an antimicrobial response but that this is insufficient to eradicate the bacteria, which then commence biofilm formation. The necrotic cells then become incorporated into the EPM. These findings are in accord with those of Walker et al. (2005), who studied the effect of neutrophils on biofilm formation and development by P. aeruginosa PAO1. Similarly to our observations, they reported increased formation of biofilm by PAO1 in the presence of neutrophils.
Thus, if Burkholderia can remain viable while in the presence of neutrophils, then this would establish a self-perpetuating cycle of transient, ineffectual phagocytosis of invading Bcc member species while simultaneously amplifying the background inflammation and leukocyte recruitment (Fig. 5). Indeed, as discussed, biofilm formation can reduce phagocytosis by neutrophils, as well as reduce the efficacy of antimicrobial agents against the biofilm-dwelling microorganisms and may be an important factor in ensuring the persistence of infection in CF.
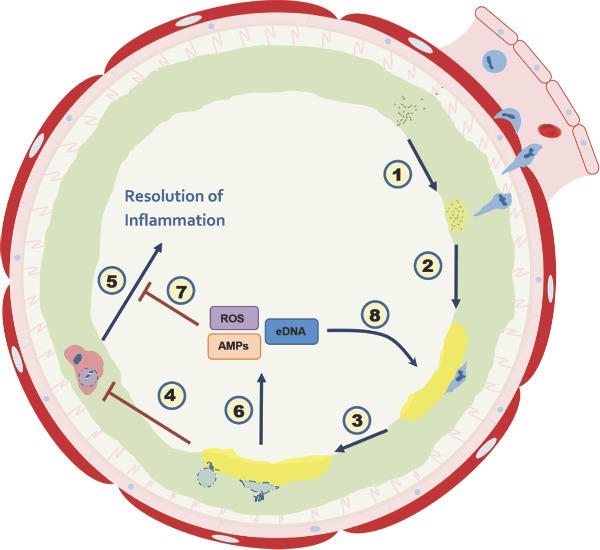
Figure 5.
The paradigm of neutrophil recruitment to the CF airway. The airway epithelium, stimulated by microbial antigens, secretes proinflammatory agents and chemoattractants which recruit neutrophils in an attempt to clear the colonizing microorganisms. (1) Following bacterial challenge, neutrophils transmigrate into the airway lumen, where they encounter dehydrated, mucous-rich ASL (green). Segregated from the neutrophil, the bacteria adapt to their environment, for example by generation of an extracellular polymeric matrix. (2) The neutrophil encounters bacteria in a complex setting, including EPM polysaccharides and attempts to phagocytose the invading microorganism. (3) In a healthy airway, the neutrophil will then undergo phagocytosis-induced cell death (PICD) and be cleared by macrophage; however, in CF, phagocytosis is frustrated by the biofilm and neutrophil undergoes apoptosis followed by necrosis. Alternatively, necrosis may be directly induced by the invading pathogen. (4) PICD and (5) pro-resolving signaling are thereby inhibited, so the airway cannot return to a healthy state. (6) Where the neutrophils have not been cleared, they become necrotic and disintegrate; this releases antimicrobial peptides (AMPs) which (7) propagate further inflammation and (8) reenforce the EPM leading, ultimately, to fibrotic lesions and lung damage.
FUNDING
This study was supported by a Science Foundation Ireland (SFI) Research Frontiers Programme grant [grant number RFP2816]. EC is the recipient of an SFI Stokes Lectureship Programme award.
Conflict of interest. None declared.
REFERENCES
- Alexander BD, Petzold EW, Reller LB, et al. Survival after lung transplantation of cystic fibrosis patients infected with Burkholderia cepacia complex. Am J Transplant. 2008;8:1025–30. doi: 10.1111/j.1600-6143.2008.02186.x. [DOI] [PubMed] [Google Scholar]
- Alhede M, Bjarnsholt T, Jensen PO, et al. Pseudomonas aeruginosa recognizes and responds aggressively to the presence of polymorphonuclear leukocytes. Microbiology. 2009;155:3500–8. doi: 10.1099/mic.0.031443-0. [DOI] [PubMed] [Google Scholar]
- Alvarez ME, Fuxman Bass JI, Geffner JR, et al. Neutrophil signalling pathways activated by bacterial DNA stimulation. J Immunol. 2006;177:4037–46. doi: 10.4049/jimmunol.177.6.4037. [DOI] [PubMed] [Google Scholar]
- Aubert DF, Flannagan RS, Valvano MA. A novel sensor kinase-response regulator hybrid controls biofilm formation and type VI secretion system activity in Burkholderia cenocepacia. Infect Immun. 2008;76:1979–91. doi: 10.1128/IAI.01338-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtsson M, Ståhlberg A, Rorsman P, et al. Gene expression profiling in single cells from the pancreatic islets of Langerhans reveals lognormal distribution of mRNA levels. Genome Res. 2005;15:1388–92. doi: 10.1101/gr.3820805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi SM, Prince LR, McPhillips K, et al. Impairment of apoptotic cell engulfment by pyocyanin, a toxic metabolite of Pseudomonas aeruginosa. Am J Resp Crit Care. 2008;177:35–43. doi: 10.1164/rccm.200612-1804OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjarnsholt T, Jensen PO, Burmolle M, et al. Pseudomonas aeruginosa tolerance to tobramycin, hydrogen peroxide and polymorphonuclear leukocytes is quorum-sensing dependent. Microbiology. 2005;151:373–83. doi: 10.1099/mic.0.27463-0. [DOI] [PubMed] [Google Scholar]
- Bjarnsholt T, Jensen PO, Fiandaca MJ, et al. Pseudomonas aeruginosa biofilms in the respiratory tract of cystic fibrosis patients. Pediatr Pulm. 2009;44:547–58. doi: 10.1002/ppul.21011. [DOI] [PubMed] [Google Scholar]
- Bonfield TL, Panuska JR, Konstan MW, et al. Inflammatory cytokines in cystic fibrosis lungs. Am J Resp Crit Care. 1995;152:2111–8. doi: 10.1164/ajrccm.152.6.8520783. [DOI] [PubMed] [Google Scholar]
- Boucher RC. New concepts of the pathogenesis of cystic fibrosis lung disease. Eur Respir J. 2004;23:146–58. doi: 10.1183/09031936.03.00057003. [DOI] [PubMed] [Google Scholar]
- Bylund J, Campsall PA, Ma RC, et al. Burkholderia cenocepacia induces neutrophil necrosis in chronic granulomatous disease. J Immunol. 2005;174:3562–9. doi: 10.4049/jimmunol.174.6.3562. [DOI] [PubMed] [Google Scholar]
- Cabral DA, Loh BA, Speert DP. Mucoid Pseudomonas aeruginosa resists nonopsonic phagocytosis by human neutrophils and macrophages. Pediatr Res. 1987;22:429–31. doi: 10.1203/00006450-198710000-00013. [DOI] [PubMed] [Google Scholar]
- Caceres SM, Malcolm KC, Taylor-Cousar JL, et al. Enhanced in vitro formation and antibiotic resistance of non-attached Pseudomonas aeruginosa aggregates through incorporation of neutrophil products. Antimicrob Agents Ch. 2014;58:6851–60. doi: 10.1128/AAC.03514-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caraher E, Duff C, Mullen T, et al. Invasion and biofilm formation of Burkholderia dolosa is comparable with Burkholderia cenocepacia and Burkholderia multivorans. J Cyst Fibros. 2007;6:49–56. doi: 10.1016/j.jcf.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Carvalho AP, Ventura GM, Pereira CB, et al. Burkholderia cenocepacia, B. multivorans, B. ambifaria and B. vietnamiensis isolates from cystic fibrosis patients have different profiles of exoenzyme production. APMIS. 2007;115:311–18. doi: 10.1111/j.1600-0463.2007.apm_603.x. [DOI] [PubMed] [Google Scholar]
- CFF. Cystic Fibrosis Foundation Patient Registry Annual Data Report. 2012 Available at: www.cff.org/UploadedFiles/research/ClinicalResearch/PatientRegistryReport/2012-CFF-Patient-Registry.pdf (31 August 2015, date last accessed) [Google Scholar]
- Chiang WC, Nilsson M, Jensen PO, et al. Extracellular DNA shields against aminoglycosides in Pseudomonas aeruginosa biofilms. Antimicrob Agents Ch. 2013;57:2352–61. doi: 10.1128/AAC.00001-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciofu O, Nielsen TT, Jensen PO, et al. Antimicrobial resistance, respiratory tract infections and role of biofilms in lung infections in cystic fibrosis patients. Adv Drug Deliver Rev. 2015;85:7–23. doi: 10.1016/j.addr.2014.11.017. [DOI] [PubMed] [Google Scholar]
- Collins SJ, Ruscetti FW, Gallagher RE, et al. Normal functional characteristics of cultured human promyelocytic leukemia cells (HL-60) after induction of differentiation by dimethylsulfoxide. J Exp Med. 1979;149:969–74. doi: 10.1084/jem.149.4.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway BAD, Chu KK, Bylund J, et al. Production of exopolysaccharide by Burkholderia cenocepacia results in altered cell-surface interactions and altered bacterial clearance in mice. J Infect Dis. 2004;190:957–66. doi: 10.1086/423141. [DOI] [PubMed] [Google Scholar]
- Cormack BP, Valdivia RH, Falkow S. FACS-optimized mutants of the green fluorescent protein (GFP) Gene. 1996;173:33–8. doi: 10.1016/0378-1119(95)00685-0. [DOI] [PubMed] [Google Scholar]
- Coxon A, Rieu P, Barkalow FJ, et al. A novel role for the β2 integrin CD11b/CD18 in neutrophil apoptosis: a homeostatic mechanism in inflammation. Immunity. 1996;5:653–66. doi: 10.1016/s1074-7613(00)80278-2. [DOI] [PubMed] [Google Scholar]
- Cuzzi B, Herasimenka Y, Silipo A, et al. Versatility of the Burkholderia cepacia complex for the biosynthesis of exopolysaccharides: a comparative structural investigation. PLoS One. 2014;9:e94372. doi: 10.1371/journal.pone.0094372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacheux D, Toussaint B, Richard M, et al. Pseudomonas aeruginosa cystic fibrosis isolates induce rapid, type III secretion-dependent, but ExoU-independent, oncosis of macrophages and polymorphonuclear neutrophils. Infect Immun. 2000;68:2916–24. doi: 10.1128/iai.68.5.2916-2924.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das T, Manefield M. Pyocyanin promotes extracellular DNA release in Pseudomonas aeruginosa. PLoS One. 2012;7:e46718. doi: 10.1371/journal.pone.0046718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das T, Manefield M. Phenazine production enhances extracellular DNA release via hydrogen peroxide generation in Pseudomonas aeruginosa. Commun Integr Biol. 2013;6:e23570. doi: 10.4161/cib.23570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lorenzo V, Timmis KN. Analysis and construction of stable phenotypes in gram-negative bacteria with Tn5- and Tn10-derived minitransposons. Methods Enzymol. 1994;235:386–405. doi: 10.1016/0076-6879(94)35157-0. [DOI] [PubMed] [Google Scholar]
- Drevinek P, Mahenthiralingam E. Burkholderia cenocepacia in cystic fibrosis: epidemiology and molecular mechanisms of virulence. Clin Microbiol Infect. 2010;16:821–30. doi: 10.1111/j.1469-0691.2010.03237.x. [DOI] [PubMed] [Google Scholar]
- Dunnet CW. A multiple comparison procedure for comparing several treatments with a control. J Am Stat Assoc. 1955;50:1096–121. [Google Scholar]
- Elizur A, Cannon CL, Ferkol TW. Airway inflammation in cystic fibrosis. Chest. 2008;133:489–95. doi: 10.1378/chest.07-1631. [DOI] [PubMed] [Google Scholar]
- Flannagan RS, Canton J, Furuya W, et al. The phosphatidylserine receptor TIM4 utilizes integrins as coreceptors to effect phagocytosis. Mol Biol Cell. 2014;25:1511–22. doi: 10.1091/mbc.E13-04-0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuxman Bass JI, Russo DM, Gabelloni ML, et al. Extracellular DNA: a major proinflammatory component of Pseudomonas aeruginosa biofilms. J Immunol. 2010;184:6386–95. doi: 10.4049/jimmunol.0901640. [DOI] [PubMed] [Google Scholar]
- Guélon T, Hunter RC, Mathias JD, et al. Homogenization of Pseudomonas aeruginosa PAO1 biofilms visualized by freeze-substitution electron microscopy. Biotechnol Bioeng. 2013;110:1405–18. doi: 10.1002/bit.24805. [DOI] [PubMed] [Google Scholar]
- Günther F, Stroh P, Wagner C, et al. Phagocytosis of staphylococci biofilms by polymorphonuclear neutrophils: S. aureus and S. epidermidis differ with regard to their susceptibility towards the host defense. Int J Artif Organs. 2009a;32:565–73. doi: 10.1177/039139880903200905. [DOI] [PubMed] [Google Scholar]
- Günther F, Wabnitz GH, Stroh P, et al. Host defence against Staphylococcus aureus biofilms infection: phagocytosis of biofilms by polymorphonuclear neutrophils (PMN) Mol Immunol. 2009b;46:1805–13. doi: 10.1016/j.molimm.2009.01.020. [DOI] [PubMed] [Google Scholar]
- Hänsch GM, Brenner-Weiss G, Prior B, et al. The extracellular polymer substance of Pseudomonas aeruginosa: too slippery for neutrophils to migrate on? Int J Artif Organs. 2008;31:796–803. doi: 10.1177/039139880803100907. [DOI] [PubMed] [Google Scholar]
- Hellemans J, Mortier G, De Paepe A, et al. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol. 2007;8:R19. doi: 10.1186/gb-2007-8-2-r19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry DA, Campbell ME, LiPuma JJ, et al. Identification of Burkholderia cepacia isolates from patients with cystic fibrosis and use of a simple new selective medium. J Clin Microbiol. 1997;35:614–9. doi: 10.1128/jcm.35.3.614-619.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber B, Riedel K, Kothe M, et al. Genetic analysis of functions involved in the late stages of biofilm development in Burkholderia cepacia H111. Mol Microbiol. 2002;46:411–26. doi: 10.1046/j.1365-2958.2002.03182.x. [DOI] [PubMed] [Google Scholar]
- Hutchison ML, Poxton IR, Govan JRW. Burkholderia cepacia produces a hemolysin that is capable of inducing apoptosis and degranulation of mammalian phagocytes. Infect Immun. 1998;66:2033–9. doi: 10.1128/iai.66.5.2033-2039.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isles A, Maclusky I, Corey M, et al. Pseudomonas cepacia infection in cystic fibrosis: an emerging problem. J Pediatr. 1984;104:206–10. doi: 10.1016/s0022-3476(84)80993-2. [DOI] [PubMed] [Google Scholar]
- Jensen PO, Bjarnsholt T, Phipps R, et al. Rapid necrotic killing of polymorphonuclear leukocytes is caused by quorum-sensing-controlled production of rhamnolipid by Pseudomonas aeruginosa. Microbiology. 2007;153:1329–38. doi: 10.1099/mic.0.2006/003863-0. [DOI] [PubMed] [Google Scholar]
- Jesaitis AJ, Franklin MJ, Berglund D, et al. Compromised host defense on Pseudomonas aeruginosa biofilms: characterization of neutrophil and biofilm interactions. J Immunol. 2003;171:4329–39. doi: 10.4049/jimmunol.171.8.4329. [DOI] [PubMed] [Google Scholar]
- Jones AM, Dodd ME, Govan JRW, et al. Burkholderia cenocepacia and Burkholderia multivorans: influence on survival in cystic fibrosis. Thorax. 2004;59:948–51. doi: 10.1136/thx.2003.017210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan JB. Biofilm dispersal: mechanisms, clinical implications, and potential therapeutic uses. J Dent Res. 2014;89:205–18. doi: 10.1177/0022034509359403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keays T, Ferris W, Vandemheen KL, et al. A retrospective analysis of biofilm antibiotic susceptibility testing: a better predictor of clinical response in cystic fibrosis exacerbations. J Cyst Fibros. 2009;8:122–7. doi: 10.1016/j.jcf.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Kobayashi SD, Braughton KR, Whitney AR, et al. Bacterial pathogens modulate an apoptosis differentiation program in human neutrophils. P Natl Acad Sci USA. 2003;100:10948–53. doi: 10.1073/pnas.1833375100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- König B, Ceska M, König W. Effect of Pseudomonas aeruginosa on interleukin-8 release from human phagocytes. Int Arch Allergy Imm. 1995;106:357–65. doi: 10.1159/000236867. [DOI] [PubMed] [Google Scholar]
- Kruskal W, Wallis WA. Use of ranks in one-criterion variance analysis. J Am Stat Assoc. 1952;47:583–621. [Google Scholar]
- Lamothe J, Huynh KK, Grinstein S, et al. Intracellular survival of Burkholderia cenocepacia in macrophages is associated with a delay in the maturation of bacteria-containing vacuoles. Cell Microbiol. 2007;9:40–53. doi: 10.1111/j.1462-5822.2006.00766.x. [DOI] [PubMed] [Google Scholar]
- Ledderose C, Heyn J, Limbeck E, et al. Selection of reliable reference genes for quantitative real-time PCR in human T cells and neutrophils. BMC Res Notes. 2011;4:427. doi: 10.1186/1756-0500-4-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leid JG, Shirtliff ME, Costerton JW, et al. Human leukocytes adhere to, penetrate, and respond to Staphylococcus aureus biofilms. Infect Immun. 2002;70:6339–45. doi: 10.1128/IAI.70.11.6339-6345.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meachery G, De Soyza A, Nicholson A, et al. Outcomes of lung transplantation for cystic fibrosis in a large UK cohort. Thorax. 2008;63:725–31. doi: 10.1136/thx.2007.092056. [DOI] [PubMed] [Google Scholar]
- Messiaen AS, Nelis H, Coenye T. Investigating the role of matrix components in protection of Burkholderia cepacia complex biofilms against tobramycin. J Cyst Fibros. 2014;13:56–62. doi: 10.1016/j.jcf.2013.07.004. [DOI] [PubMed] [Google Scholar]
- Meyle E, Stroh P, Gunther F, et al. Destruction of bacterial biofilms by polymorphonuclear neutrophils: relative contribution of phagocytosis, DNA release, and degranulation. Int J Artif Organs. 2010;33:608–20. doi: 10.1177/039139881003300906. [DOI] [PubMed] [Google Scholar]
- Montanaro L, Poggi A, Visai L, et al. Extracellular DNA in biofilms. Int J Artif Organs. 2011;34:824–31. doi: 10.5301/ijao.5000051. [DOI] [PubMed] [Google Scholar]
- Novotny LA, Amer AO, Brockson ME, et al. Structural stability of Burkholderia cenocepacia biofilms is reliant on eDNA structure and presence of a bacterial nucleic acid binding protein. PLoS One. 2013;8:e67629. doi: 10.1371/journal.pone.0067629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owaki H, Yukawa K, Ochi T, et al. FACS analysis of myeloid differentiation stages in epiphyseal bone-marrow, adjacent to joints affected with rheumatoid-arthritis. Scand J Rheumatol. 1991;20:91–7. doi: 10.3109/03009749109165282. [DOI] [PubMed] [Google Scholar]
- Parks QM, Young RL, Poch KR, et al. Neutrophil enhancement of Pseudomonas aeruginosa biofilm development: human F-actin and DNA as targets for therapy. J Med Microbiol. 2009;58:492–502. doi: 10.1099/jmm.0.005728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters C, Zlosnik JE, Spilker T, et al. Burkholderia pseudomultivorans sp. nov., a novel Burkholderia cepacia complex species from human respiratory samples and the rhizosphere. Syst Appl Microbiol. 2013;36:483–9. doi: 10.1016/j.syapm.2013.06.003. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30:e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince LR, Bianchi SM, Vaughan KM, et al. Subversion of a lysosomal pathway regulating neutrophil apoptosis by a major bacterial toxin, pyocyanin. J Immunol. 2008;180:3502–11. doi: 10.4049/jimmunol.180.5.3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieu I, Powers SJ. Real-time quantitative RT-PCR: design, calculations, and statistics. Plant Cell. 2009;21:1031–3. doi: 10.1105/tpc.109.066001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson DM, Parks QM, Young RL, et al. Disruption of contact lens-associated Pseudomonas aeruginosa biofilms formed in the presence of neutrophils. Invest Ophth Vis Sci. 2011;52:2844–50. doi: 10.1167/iovs.10-6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruijter JM, Ramakers C, Hoogaars WM, et al. Amplification efficiency: linking baseline and bias in the analysis of quantitative PCR data. Nucleic Acids Res. 2009;37:e45. doi: 10.1093/nar/gkp045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajjan U, Corey M, Humar A, et al. Immunolocalisation of Burkholderia cepacia in the lungs of cystic fibrosis patients. J Med Microbiol. 2001;50:535–46. doi: 10.1099/0022-1317-50-6-535. [DOI] [PubMed] [Google Scholar]
- Scannell M, Flanagan MB, deStefani A, et al. Annexin-1 and peptide derivatives are released by apoptotic cells and stimulate phagocytosis of apoptotic neutrophils by macrophages. J Immunol. 2007;178:4595–605. doi: 10.4049/jimmunol.178.7.4595. [DOI] [PubMed] [Google Scholar]
- Schröder AK, Uciechowski P, Fleischer D, et al. Crosslinking of CD66b on peripheral blood neutrophils mediates the release of interleukin-8 from intracellular storage. Hum Immunol. 2006a;67:676–82. doi: 10.1016/j.humimm.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Schröder AK, von der Ohe M, Kolling U, et al. Polymorphonuclear leucocytes selectively produce anti-inflammatory interleukin-1 receptor antagonist and chemokines, but fail to produce pro-inflammatory mediators. Immunology. 2006b;119:317–27. doi: 10.1111/j.1365-2567.2006.02435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab U, Abdullah LH, Perlmutt OS, et al. Localization of Burkholderia cepacia complex bacteria in cystic fibrosis lungs and interactions with Pseudomonas aeruginosa in hypoxic mucus. Infect Immun. 2014;82:4729–45. doi: 10.1128/IAI.01876-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh PK, Schaefer AL, Parsek MR, et al. Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms. Nature. 2000;407:762–4. doi: 10.1038/35037627. [DOI] [PubMed] [Google Scholar]
- Sousa AM, Pereira MO. Pseudomonas aeruginosa diversification during infection development in cystic fibrosis lungs-a review. Pathogens. 2014;3:680–703. doi: 10.3390/pathogens3030680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriramulu DD, Lunsdorf H, Lam JS, et al. Microcolony formation: a novel biofilm model of Pseudomonas aeruginosa for the cystic fibrosis lung. J Med Microbiol. 2005;54:667–76. doi: 10.1099/jmm.0.45969-0. [DOI] [PubMed] [Google Scholar]
- Stoodley P, Wilson S, Hall-Stoodley L, et al. Growth and detachment of cell clusters from mature mixed-species biofilms. Appl Environ Microb. 2001;67:5608–13. doi: 10.1128/AEM.67.12.5608-5613.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Student. The probable error of a mean. Biometrika. 1908;6:1–25. [Google Scholar]
- Tablan OC, Martone WJ, Doershuk CF, et al. Colonization of the respiratory tract with Pseudomonas cepacia in cystic fibrosis. Risk factors and outcomes. Chest. 1987;91:527–32. doi: 10.1378/chest.91.4.527. [DOI] [PubMed] [Google Scholar]
- Trevani AS, Chorny A, Salamone G, et al. Bacterial DNA activates human neutrophils by a CpG-independent pathway. Eur J Immunol. 2003;33:3164–74. doi: 10.1002/eji.200324334. [DOI] [PubMed] [Google Scholar]
- Tukey J. Comparing individual means in the analysis of variance. Biometrics. 1949;5:99–114. [PubMed] [Google Scholar]
- UKCFR. UK Cystic Fibrosis Registry Annual Data Report. 2013 Available at: www.cysticfibrosis.org.uk/media/598466/annual-data-report-2013-jul14.pdf (31 August 2015, date last accessed) [Google Scholar]
- Van den Driessche F, Rigole P, Brackman G, et al. Optimization of resazurin-based viability staining for quantification of microbial biofilms. J Microbiol Meth. 2014;98:31–4. doi: 10.1016/j.mimet.2013.12.011. [DOI] [PubMed] [Google Scholar]
- van Gennip M, Christensen LD, Alhede M, et al. Inactivation of the rhlA gene in Pseudomonas aeruginosa prevents rhamnolipid production, disabling the protection against polymorphonuclear leukocytes. APMIS. 2009;117:537–46. doi: 10.1111/j.1600-0463.2009.02466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gennip M, Christensen LD, Alhede M, et al. Interactions between polymorphonuclear leukocytes and Pseudomonas aeruginosa biofilms on silicone implants in vivo. Infect Immun. 2012;80:2601–7. doi: 10.1128/IAI.06215-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandamme P, Dawyndt P. Classification and identification of the Burkholderia cepacia complex: past, present and future. Syst Appl Microbiol. 2011;34:87–95. doi: 10.1016/j.syapm.2010.10.002. [DOI] [PubMed] [Google Scholar]
- Voglis S, Quinn K, Tullis E, et al. Human neutrophil peptides and phagocytic deficiency in bronchiectatic lungs. Am J Resp Crit Care. 2009;180:159–66. doi: 10.1164/rccm.200808-1250OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker TS, Tomlin KL, Worthen GS, et al. Enhanced Pseudomonas aeruginosa biofilm development mediated by human neutrophils. Infect Immun. 2005;73:3693–701. doi: 10.1128/IAI.73.6.3693-3701.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watters C, Everett JA, Haley C, et al. Insulin treatment modulates the host immune system to enhance Pseudomonas aeruginosa wound biofilms. Infect Immun. 2014;82:92–100. doi: 10.1128/IAI.00651-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss Nielsen M, Sternberg C, Molin S, et al. Pseudomonas aeruginosa and Saccharomyces cerevisiae biofilm in flow cells. J Vis Exp. 2011;47:e2383. doi: 10.3791/2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitchurch CB, Tolker-Nielsen T, Ragas PC, et al. Extracellular DNA required for bacterial biofilm formation. Science. 2002;295:1487. doi: 10.1126/science.295.5559.1487. [DOI] [PubMed] [Google Scholar]
- Xu X, Zhang H, Song Y, et al. Strain-dependent induction of neutrophil histamine production and cell death by Pseudomonas aeruginosa. J Leukocyte Biol. 2012;91:275–84. doi: 10.1189/jlb.0711356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakandawala N, Gawande PV, LoVetri K, et al. Characterization of the poly-beta-1,6-N-acetylglucosamine polysaccharide component of Burkholderia biofilms. Appl Environ Microb. 2011;77:8303–9. doi: 10.1128/AEM.05814-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Hirahashi J, Cullere X, et al. Elucidation of molecular events leading to neutrophil apoptosis following phagocytosis: cross-talk between caspase 8, reactive oxygen species, and MAPK/ERK activation. J Biol Chem. 2003;278:28443–54. doi: 10.1074/jbc.M210727200. [DOI] [PubMed] [Google Scholar]