Abstract
Whooping cough, or pertussis, incidence has reached levels not seen since the 1950s. Previous studies have shown that antibiotics fail to improve the course of disease unless diagnosed early. Early diagnosis is complicated by the non-diagnostic presentation of disease early in infection. This review focuses on previous attempts at developing novel host-directed therapies for the treatment of pertussis. In addition, two novel approaches from our group are discussed. Manipulation of the signaling pathway of sphingosine-1-phosphate, a lipid involved in many immune processes, has shown great promise, but is in its infancy. Pendrin, a host epithelial anion exchanger upregulated in the airways with B. pertussis infection, appears to drive mucus production and dysregulation of airway surface liquid pH and salinity. In addition to detailing these potential new therapeutic targets, the need for greater focus on the neonatal model of disease is highlighted.
Keywords: respiratory disease, sphingosine-1-phosphate, pendrin, acetazolamide, leucocytosis, ECMO
This review covers existing and potentially new treatments for the resurgent disease pertussis.
Graphical Abstract Figure.
This review covers existing and potentially new treatments for the resurgent disease pertussis.
INTRODUCTION
Whooping cough, or pertussis, is a vaccine preventable, acute respiratory disease caused by infection with Bordetella pertussis or B. parapertussis. The majority of whooping cough research to date has focused on the optimization and development of safe, effective vaccines. In the USA, a sharp decline in incidence and mortality of disease was observed following the introduction of the whole cell vaccine, DTP, in the 1940s, with historically low incidence reported in the 1970s (CDC 2013). However, safety concerns over reactogenicity led to the development and use of the acellular vaccine, DTaP. Unfortunately, the acellular vaccine has reduced efficacy in terms of both duration of protection and induction of an appropriate immune memory for bacterial clearance (Rowe et al. 2000, 2005; Klein et al. 2012). The rapidly waning immunity provided by DTaP has been associated with a resurgence of whooping cough incidence in recent years and the proposal of new vaccine strategies involving multiple booster administrations. In addition to reduced efficacy, current vaccine strategies have apparently selected for the emergence of strains lacking pertactin, a common component of DTaP (Barkoff et al. 2012; Lam et al. 2014; Pawloski et al. 2014; Zeddeman et al. 2014). The reemergence of pertussis, the presence of ‘vaccine escape strains’ and recent reports highlighting the appearance of antibiotic resistant B. pertussis necessitates a change in how we treat this disease (Guillot et al. 2012). In the absence of an immediate change in vaccine formulation or administration policy, we believe that it is of great importance to place new focus on therapeutics for the treatment of those who have contracted the disease. This is also necessary as those individuals most susceptible to severe disease are typically at pre-vaccine age.
In unvaccinated children, the duration and severity of disease is typically lesser for those patients infected with B. parapertussis than B. pertussis infection (Heininger et al. 1994; Liese et al. 2003). Coinfection with B. pertussis and B. parapertussis has been documented (Mertsola 1985; Iwata et al. 1991), and it has been postulated that, under these conditions, pertussis toxin (PT) produced by B. pertussis enhanced the ability of B. parapertussis to colonize the host, thereby providing a competitive advantage to the bacterium (Worthington, Van Rooijen and Carbonetti 2011). This review will focus on the more significant disease caused by B. pertussis and assess current treatment options as well as discussing emerging novel therapeutics.
CLINICAL MANIFESTATIONS OF WHOOPING COUGH DISEASE
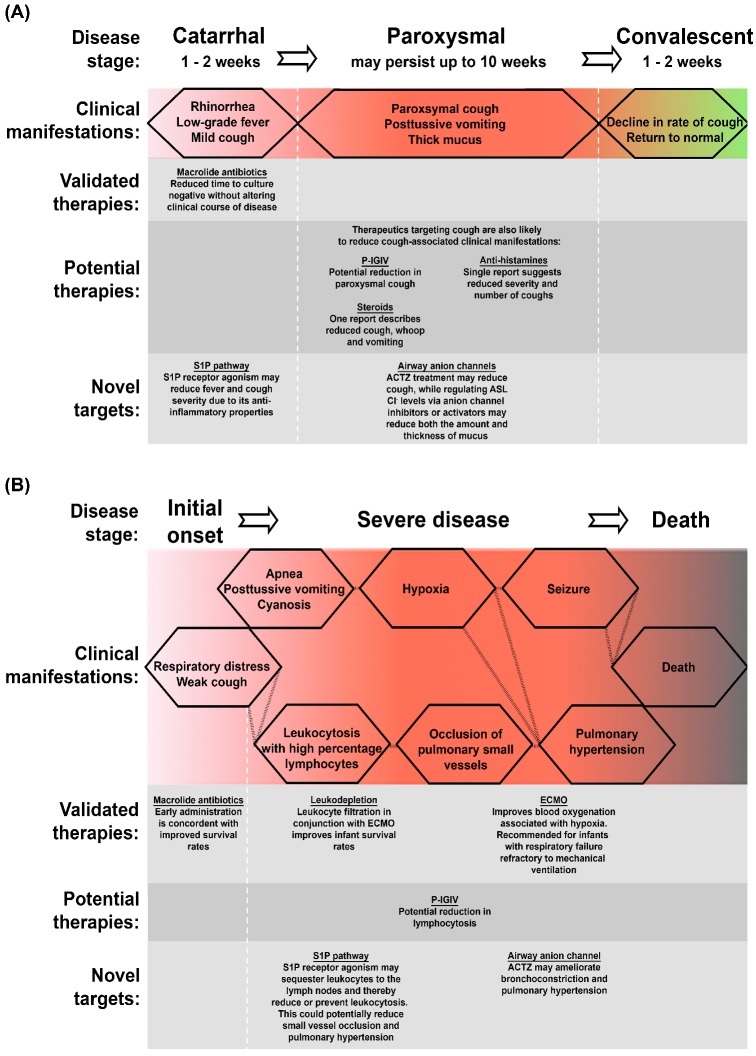
In order to assess the therapeutic benefits of proposed treatments, it is first important to understand the disease itself. The classical manifestation of pertussis disease is its distinct paroxysmal cough with vigorous inspiratory effort resulting in the characteristic whooping sound associated with the disease. However, disease symptoms are quite heterogeneous, ranging from a mild manifestation indistinguishable from viral respiratory infection to severe disease with paroxysmal cough. This heterogeneity is associated with stage of disease, degree of immunity and patient age. The classic course of B. pertussis infection is broken into three stages: catarrhal, paroxysmal and convalescent (illustrated in Fig. 1).
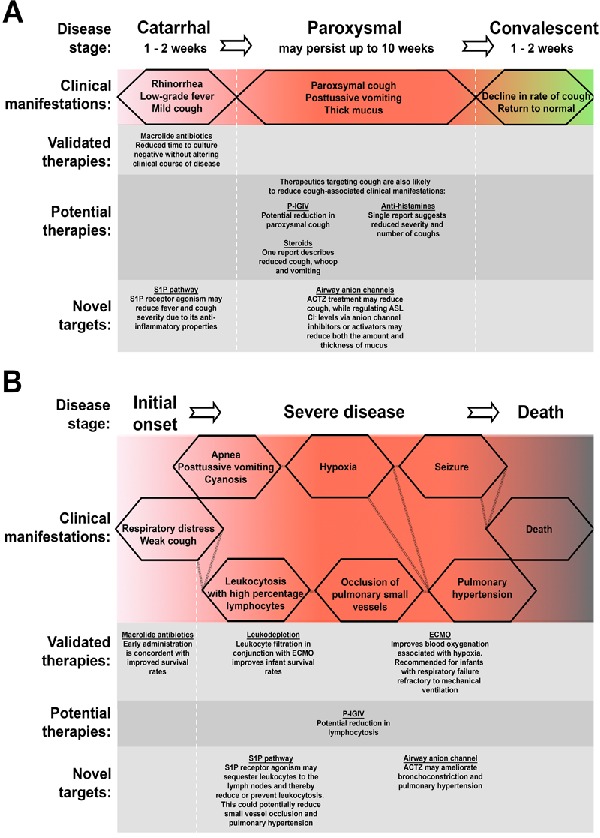
Figure 1.
Schematic of pertussis classical and severe disease and points of therapeutic intervention. Classical pertussis (A) is readily broken into three stages, each with specific clinical manifestations. Severe disease (B), most commonly observed in unvaccinated infants, presents with multiple complications. S1P, sphingosine-1-phosphate; ACTZ, acetazolamide; ECMO, extracorporeal membrane oxygenation.
The first phase of disease, the catarrhal stage, is a critical point for intervention. However, pertussis is often under diagnosed at this period due to the presence of mild, non-specific symptoms such as rhinorrhea and progressive cough. Considerable efforts have been made to address disease at increasingly early stages. These efforts include the screening of household contacts and PCR-based diagnostics (Lind-Brandberg et al. 1998; Raymond et al. 2007). In patients identified early as having pertussis, the use of macrolide antibiotics has been associated with improved survival rates in infants (<3 months) (Winter et al. 2015). However, for other individuals, despite increasing the speed at which patients become culture negative, antibiotics do not change the clinical course of disease (Altunaiji et al. 2007). While untreated patients remain contagious into the paroxysmal stage, the catarrhal stage is considered the most contagious (CDC 2015a). Hence, antibiotic intervention at this initial phase of infection, and the resulting nasopharyngeal sterilization, has the potential to greatly reduce transmission rates, thereby benefiting the herd even in the absence of improving patients’ symptoms.
The catarrhal stage lasts 1–2 weeks and is followed by more severe symptoms characterizing the paroxysmal phase of disease. During the classical paroxysmal stage, the preceding mild cough develops into a full paroxysmal cough, with fits of 5–10 or more forceful coughs in a single expiration. Coughing episodes average 15 attacks per 24 h but can increase to 30 or more in severe cases and may persist for up to 10 weeks. This paroxysmal cough is exacerbated by the presence of thick, non-purulent mucus that is difficult to clear and hence drives more forceful expirations (Olson 1975; Soane et al. 2000). Samples obtained postmortem from infected infants have highlighted the impact of this mucus—one investigation described widespread mucus plugging of the airways (Smith and Vyas 2000), while another detailed the partial occlusion of small bronchioles with a mix of mucus, bacteria, necrotic debris and inflammatory cells (Paddock et al. 2008), though it should be noted that these are examples of extreme cases where B. pertussis infection resulted in death. Among infants and younger children, the intensity of B. pertussis-induced cough brings with it other complications; patients will also present with posttussive vomiting. In addition to the cough-related effects, apnea and cyanosis may also be present at this stage. Classical symptoms are reduced in their severity but not duration amongst B. pertussis infected, otherwise healthy, adolescents and adults. One study described the mean duration of cough as 10 weeks for adolescents (12–17 years old) and 12 weeks for adults (≥18 years old), with inspiratory whoop observed in 24% and 74% respectively and posttussive vomiting present in 25% of adolescents and 64% of adults (De Serres et al. 2000). A study by Strebel et al. (2001) described a paroxysmal cough in adults that lasted a median of 6 weeks, with 26% of subjects presenting with a whoop, 56% with posttussive vomiting and 100% with posttussive gagging. Hence, although pertussis can be considered at its most severe as a pediatric infection, the disease still has a significant impact on the lives of adolescents and adults. Studies on the economic burden of this pathogen indicated that individual adults incurred indirect, nonmedical costs of approximately US $450 and missed a mean of 9.8 days from work, while adolescents typically missed 5.5 days of school and, as expected, experienced lower indirect costs (Caro et al. 2005). However, those studies were based on costs prior to 2003 and have likely increased with inflation. Hence, targeting cough for therapeutic intervention during both the catarrhal and paroxysmal phases of disease has the potential to (i) reduce transmission (as the pathogen is spread via aerosolized droplets Warfel et al. 2012; predominantly during the catarrhal stage), (ii) reduce the paroxysm-associated physiological complications and (iii) reduce overall socioeconomic costs.
The classical convalescent stage is characterized by a gradual (1–3 weeks), continuous decline in cough and return to normal. Therefore, it is of little benefit to intervene with therapeutics at this phase of disease.
As mentioned above, there is a wide spectrum of clinical manifestations associated with whooping cough disease: while most individuals will experience symptoms similar to or milder than the classical characteristics, a small percentage of infected persons, mostly unvaccinated infants, develop a severe exacerbated disease which can lead to hospitalization and death. Between 1997 and 2000 in the United States, there were 7203 cases of reported pertussis disease in infants <6 months of age, with 63.1% of those requiring hospitalization (Mattoo and Cherry 2005). In 2008, 195 000 childhood deaths were attributed to pertussis worldwide (WHO 2011), while in the USA there were 76 916 cases of whooping cough reported in 2012/2013, of which 33 resulted in death. The majority of deaths in the USA are among infants <3 months old (27 of 33 deaths) (CDC 2015b). These infants are typically unvaccinated, as the vaccination schedule in the USA starts at 2 months of age, and is not complete until 6 months of age. This leaves infants less capable of bacterial clearance by the adaptive immune system. In addition to reduced ability to control the infection, an immature immune system and/or general differences in physiology may contribute to the disease severity, as immunocompromised adults rarely report severe pertussis disease, and birth weight and gestational age were recently identified predictors of death (Winter et al. 2015).
There are a number of clinical features associated with severe disease (Fig. 1), though not all are concomitant with increased likelihood of death. Cough-associated apnea persists in all age groups, though severity is reduced with increasing patient age. Infected infants may be too weak to whoop or their cough so faint that it goes unrecognized; however, apnea still presents as a common disease manifestation in this age group (Christie and Baltimore 1989). In this population, it is common, with severe disease, for hypoxia as a result of apnea to cause seizures (Mattoo and Cherry 2005); however, apnea itself does not appear to correlate with death. Conversely, the seizures themselves are strong predictors of mortality (Winter et al. 2015). While an increase in circulating immune cells is typical of B. pertussis infection, among infants, disease severity correlates directly with white blood cell (WBC) count and lymphocytosis (McGregor, Ogle and Curry-Kane 1986; Christie and Baltimore 1989). Young infants will present with WBC counts of >30 000 cells/ml and may reach up to 100 000 cells/ml (Mattoo and Cherry 2005), with 60–80% lymphocytes. Fatal cases are linked to higher peak WBC and lymphocyte counts and a more rapid onset (Winter et al. 2015). This clinical manifestation is driven by a virulence factor secreted by B. pertussis, PT, and is hypothesized to be the cause of the occlusion of pulmonary arterioles, venules and lymphatics observed postmortem in lung tissue samples from lethal pertussis cases (Paddock et al. 2008). These small vessel leukocytic aggregates are considered one of the factors contributing to the irreversible pulmonary hypertension, which is associated with the largest increase in risk of death. Other factors thought to contribute to pulmonary hypertension include acute respiratory distress syndrome and pulmonary vasoconstriction as a result of hypoxia (Paddock et al. 2008). Hence, in cases of severe disease, therapeutic interventions targeting cough, apnea, leukocytosis or pulmonary hypertension represent great candidates to reduce B. pertussis-linked morbidity and mortality (Fig. 1). Currently, no reproducibly effective therapy exists for the treatment of severe pertussis. Here, we aim to evaluate previous attempts at treating pertussis using host-directed approaches.
ADJUNCT PERTUSSIS TREATMENTS
Anti-PT immunoglobulin
Compelling evidence exists suggesting that anti-PT antibodies are sufficient to confer protection against the severest manifestations of pertussis. Single component acellular pertussis vaccines demonstrate that these antibodies can protect against disease (Ad Hoc Group for the Study of Pertussis Vaccines 1988; Olin, Storsaeter and Romanus. 1989). In addition, during the convalescent stage of disease, patients develop strong antibody responses to PT. Since 1935, attempts have been made to utilize immune sera in the treatment of pertussis (Bradford 1935). Early trials using sera from convalescent patients produced inconclusive results. Murine studies, using pooled plasma from donors immunized with inactivated PT demonstrated normalization of leukocyte counts, improved weight gain, and significantly improved survival following administration of sera (Bruss and Siber 1999). Bruss et al. (1999) went on to perform human trials using multiple doses of intravenous antipertussis immunoglobulin (P-IGIV) in 26 pertussis-infected infants. Administration of P-IGIV resulted in boosts of serum anti-PT antibody titers, decline in lymphocytosis and reduced paroxysmal coughing. These data, and the data of others (Adler and Morse 1973), confirm that PT-directed therapies are a viable approach in the treatment of pertussis, despite the long-lived nature of PT-mediated G protein modifications. Unfortunately, however, the most recent attempts at examining the use of P-IGIV were not as successful. A 2007 multicenter trial detailing 25 patients undergoing daily P-IGIV infusions found no benefit in terms of number of paroxysmal coughs per hour (Halperin et al. 2007). One possible explanation for the differences noted in these studies is the age of patients receiving P-IGIV. In the successful study of Bruss et al. (1999), inclusion was limited to infants less than 2 years old, compared to the cut-off of 5 years in the later study (Halperin et al. 2007), even though both patient sets had similar mean ages (9.7 weeks vs 2.3 months). It should also be noted that the Halperin et al. study was concluded prematurely due to the expiration of P-IGIV preparations, achieving only 15% of their intended study size.
Bronchodilators
The paroxysmal cough associated with pertussis may contribute to many of the complications associated with severe pertussis. In an effort to reduce complications associated with this cough, multiple efforts have focused on the potential impact of bronchodilators. In a case report focusing on the treatment of two infant patients, the use of the bronchodilator salbutamol was shown to reduce both the number of paroxysms and their duration, within 24 hours of treatment (Tam and Yeung 1986). However, larger clinical trials on the impact of salbutamol have been less encouraging. One double-blind, placebo-controlled crossover study in nine hospitalized children aged between 1.5 and 27 months demonstrated no significant effects on the course or severity of disease (Krantz, Norrby and Trollfors 1985). A similar study performed one year later reached similar conclusions while measuring impact on cough paroxysms (Mertsola, Viljanen and Ruuskanen 1986).
Antihistamines
Active PT causes a robust histamine sensitization, which, in animal models where histamine is administered post-PT inoculation, can lead to anaphylactic shock and death (Munoz et al. 1981). The effect of PT on histamine sensitivity, combined with the knowledge that histamine can induce cough, led to the hypothesis that treatment of infected patients with antihistamines may reduce pertussis cough.
The only clinical trial on the impact of antihistamines on pertussis disease, using the antihistamine diphenhydramine, showed no significant ability to reduce cough paroxysms (Danzon et al. 1988). However, in a 1951 letter to the British Medical Journal, Clifton J. Strauss detailed his experience using the anti-histamine pyribenzamine in treating pertussis patients (Strauss 1951). Strauss claimed to see a reduction in both the number and severity of paroxysms in over 65% of patients. As a consequence of this reduction in cough, Strauss noted decreases in vomiting and dehydration, common complications of pertussis disease. It is also noted that removal of treatment resulted in the return of cough, demonstrating that the drug failed to alter the course of disease, but was effective in managing disease symptoms. One reason for the difference in results noted between these studies could be the potential antispasmodic activity of pyribenzamine compared to diphenhydramine. While this is not clear, it may warrant a reinvestigation of adjunct therapy with dedicated antispasmodic drugs.
Steroids and corticosteroids
Citing links to a possible ‘allergic effect of B. pertussis on the coughing centre’ (Frobisher 1965), Zoumboulakis et al. (1973) investigated the use of the steroid hydrocortisone for 7 days on pertussis-related cough. The authors noted a reduction in ‘cough, whoop and vomiting’ after 3 days of treatment, with patients <9 months of age experiencing the greatest benefit. Despite this relative success, the authors caution that steroid treatment should only be considered in the most severe cases. An increase in pulmonary inflammatory infiltrate was also noted in those receiving hydrocortisone compared to control (15% vs 10%), further highlighting the need for caution with such an approach.
One study with the corticosteroid dexamethasone measured the impact of administration on hospital stay (Roberts, Gavin and Lennon 1992). Roberts et al. found that patients receiving dexamethasone did not spend significantly less time in hospital than their placebo controls.
Extracorporeal membrane oxygenation
A common complication associated with severe pertussis is reduced gaseous exchange owing to obstruction of the alveolar spaces with infiltrating immune cells (Paddock et al. 2008). To treat patients with breathing difficulties, mechanical ventilation or extracorporeal membrane oxygenation therapy (ECMO) can be used. ECMO involves removing the carbon dioxide from a patient's blood while oxygenating their red blood cells. ECMO is used in intensive care medicine when a patient's heart and lungs are unable to provide sufficient gaseous exchange to support life. ECMO is recommended for treatment of hypoxemic respiratory failure, hypercapnic respiratory failure, refractory cardiogenic shock and cardiac arrest among other maladies (ELSO 2013) and has been used in the treatment of recalcitrant pertussis when respiration is sufficiently impacted.
In one study of 800 children undergoing ECMO, 12 were being treated for severe, life-threatening pertussis (Pooboni et al. 2003). All children were less than 3 months of age and had yet to receive a pertussis vaccination. Despite ECMO treatment, 7 of the 12 patients succumbed to infection. Mortality was associated with elevated neutrophil counts and pulmonary hypertension at presentation. The authors of this meta-analysis suggested that ECMO should be offered to children with severe pertussis and respiratory failure refractory to mechanical ventilation.
Leukodepletion
Elevated WBCs are associated with severe pertussis and among the best predictors of fatal infection (Mikelova et al. 2003). Rowlands et al. (2010) described the treatment of 19 patients over an 8-year span receiving leukodepletion therapy. To deplete leukocytes, ECMO was performed with a leukocyte filter, thereby removing cells before reinfusion of oxygenated blood. ECMO therapy, used exclusively in intensive care situations, has been associated with a 70% fatality rate, due to its use being restricted to intensive care situations. Patients undergoing leukodepletion in this study had mortality rates of 45% between 2001 and 2004, and 10% from 2005 to 2009 (Rowlands et al. 2010). The improvement in survival rates following additive leukocyte depletion, in conjunction with the potential benefit associated with earlier administration of ECMO therapy, highlights the as-yet-unrealized potential of this therapy. Recent case reports have also shown success in treating severe pertussis with combined ECMO and leukodepletion (Assy et al. 2015). Techniques such as ECMO, leukodepletion and exchange blood transfusion (not discussed here) are currently limited by the last resort nature of their use. Studies examining the use of these interventions earlier in disease may produce superior outcomes.
NOVEL POTENTIAL THERAPEUTIC TARGETS FOR PERTUSSIS
Manipulation of airway anion channels
The role of anion exchangers is well established in cystic fibrosis, a disease also associated with a persistent cough. Cystic fibrosis is initiated by chromosomal mutations in the CF transmembrane conductance regulator (CFTR). In the airways, this anion channel mediates the export of Cl− and thiocyanate ions from epithelial cells. When mutations occur in CFTR, the Cl− airway surface liquid (ASL) levels are reduced, leading to dehydration of the ASL and hyperconcentrated mucus. These conditions then drive a reduction in periciliary liquid depth, which inhibits cilia beating, thereby reducing mucociliary clearance of pathogens and making the airways vulnerable to infection and inflammation. Hence, pharmacological strategies are now aimed at improving CFTR function with corrector–potentiator combination therapies (in cases where CFTR is present on the epithelial surface but specific mutations have led to reduced functionality) or targeting other ion channels in an effort to restore ASL homeostasis using specific activators and blockers (Mall and Galietta 2015).
In a microarray analysis of murine pulmonary genes regulated by B. pertussis infection in the absence or presence of PT, our group identified an anion exchanger, slc26a4, as one of the most highly induced PT-associated genes (Connelly, Sun and Carbonetti 2012). Tissue samples from B. pertussis-infected baboons also display upregulation of this gene compared with mock-infected animals (Scanlon et al. unpublished data). SLC26A4, or pendrin, is an anion exchanger found at multiple sites throughout the body, including the lungs. In the airways, pendrin mediates the export of HCO3− and thiocyanate from the cytoplasm and imports Cl− (Pedemonte et al. 2007; Garnett et al. 2011). To date, there have been few studies evaluating the role of pendrin during infection. Preliminary work from Adams et al. (2012) suggests that pendrin is also upregulated in the airways of Staphylococcus aureus-infected mice, while slc26a4 gene levels also appear to correlate with common cold severity among human subjects (Nakagami et al. 2008). In our B. pertussis infection model, and perhaps in response to other respiratory pathogens, we hypothesize that upregulation of pendrin serves as an antimicrobial response by the host, via efflux of thiocyanate. In the airways, thiocyanate (SCN−) is oxidized by H2O2, in a reaction catalyzed by lactoperoxidase, to produce hypothiocyanate (OSCN−). This molecule is bactericidal and bacteriostatic, functioning by inhibiting bacterial respiration (Thomas and Aune 1978). In support of this theory, we found that B. pertussis-infected pendrin knockout mice had elevated bacterial loads throughout the course of infection (Scanlon et al. 2014). However, further investigation is necessary to directly attribute this increased colonization to a reduction in OSCN− levels.
A role for pendrin in the induction of airway inflammation and pathology has already been established for diseases of the airways unrelated to infection. One study by Yick et al. (2013) described pendrin as the most highly upregulated gene in bronchial biopsies from asthmatics versus controls, and in addition, functional mutations of pendrin, that result in enhanced activity, have been proposed to play a role in the pathogenesis of hypertension and/or asthma and COPD (Dossena et al. 2011). In mouse models, overexpression of pendrin in the airways drives enhanced mucus production and neutrophil infiltration, along with increased chemokine production and airway hyperreactivity (Nakao et al. 2008). In an ovalbumin challenge model of airway hyperresponsiveness, pendrin-deficient mice displayed reduced pulmonary pathology, airway hyperreactivity and inflammatory cell influx. Tracheal epithelial cells from these mice also exhibited increased ASL thickness in response to IL-13 stimulation compared with IL-13-stimulated control pendrin-expressing cells, suggesting that reductions in airway pathology in pendrin-deficient mice result from improved ASL hydration (Nakagami et al. 2008). Work from our group has demonstrated a similar role for pendrin in infection-induced airway inflammation. Pendrin-deficient mice infected with B. pertussis presented with a significant reduction in lung inflammatory pathology, compared with infected pendrin-expressing mice. In our model, however, we were surprised to observe higher levels of inflammatory cytokines and chemokines in infected pendrin-deficient mice compared with infected pendrin-expressing mice (Scanlon et al. 2014). As pendrin exports HCO3− and imports Cl−, we hypothesize that in the absence of pendrin the ASL would have an increased acidity and salinity. Such conditions would be suboptimal for the activity of both antimicrobial factors and chemokines (Pezzulo et al. 2012). Dairaghi et al. (1997) found that the activity of the chemokine receptor CCR3 in its interaction with CCL11 was greatly decreased by a modest decrease in pH and increase in salinity. Hence, while in the absence of pendrin, cytokine and chemokine levels are high despite low infection-induced airway pathology, we propose that the activity of those proteins is reduced due to the ASL environment.
In an attempt to modulate pendrin function in a murine model of B. pertussis infection, we treated mice daily with the carbonic anhydrase inhibitor, acetazolamide (ACTZ). By inhibiting the production of HCO3−, we hoped to recreate the ASL phenotype observed in pendrin-deficient mice. We found that indeed when infected mice received this daily treatment a significant reduction in airway pathology was observed, despite unchanged pendrin levels (Scanlon et al. 2014). But how would ACTZ treatment in mice compare with its effect on humans with pertussis disease? This question is difficult to answer as there is no research directly linking B. pertussis-induced inflammatory pathology in the mouse with the human disease. However, examination of how inhaled ACTZ has been used previously to treat other exacerbated airway conditions may allow us to hypothesize on how this drug may benefit pertussis patients. Cough induced by low-chloride-ion solutions in healthy individuals was significantly attenuated when inhaled ACTZ was administered prior to challenge (Foresi et al. 1996). Asthmatic hyperresponsiveness induced by hyperventilation of cold, dry air was also diminished with ACTZ inhalation (O'Donnell et al. 1992), while metabisulfite- or deep inspiration-induced bronchoconstriction among asthmatics was also lessened with treatment (O'Connor et al. 1994; Spicuzza et al. 2003). Hence, it is possible that reduced pathology in our murine model may translate to a reduction in cough among pertussis patients with classical disease. In addition, ACTZ has been shown to be efficacious and safe for treatment of high-altitude pulmonary hypertension (Richalet et al. 2008), so perhaps a role for ACTZ in pertussis-induced infant hypertension is also yet to be discovered.
Given that CFTR functions to export Cl− and pendrin functions to import Cl−, it is possible that overexpression of pendrin, as observed with B. pertussis infection (Connelly, Sun and Carbonetti 2012; Scanlon et al. 2014), yields a similar phenotype to mutation of CFTR in the lungs. The excess Cl− reabsorption would correlate with increased water uptake by the cells and hence a dehydration of the ASL. Furthermore, the observations of enhanced mucus production with elevated pendrin expression and increased ASL thickness in the absence of pendrin, all point towards pendrin as a major regulator of ASL hydration and mucus density. Therefore, we hypothesize that pertussis disease drives pendrin overexpression leading to reduced ASL depth and increased mucus thickness and, through this pathway, potentially facilitates the production of the non-purulent, thick, difficult to clear mucus observed during the catarrhal stage of the disease. This suggests that modulation of Cl− import by pendrin during B. pertussis infection or stimulation of enhanced Cl− secretion by other anion channels could also serve to attenuate the mucus-induced pathology.
Manipulation of the sphingosine-1-phosphate pathway
Sphingosine-1-phosphate (S1P) is a signaling lysophospholipid, involved in multiple biological processes. Importantly, its regulation is linked to the control of inflammation (Rivera, Proia and Olivera 2008; Spiegel and Milstien 2011). Sphingosine is phosphorylated by sphingosine kinase 1 and 2, following its production via the N-deacylation of ceramide. S1P levels are tightly controlled, with S1P lyases and phosphatases responsible for its degradation and dephosphorylation.
Limited work has been done determining the potential impact of exogenous S1P receptor agonism in an infectious disease setting. Early work in this field demonstrated increased intracellular killing of Mycobacterium tuberculosis by macrophages, grown in media containing S1P. Additionally, Garg et al. (2004) noted a 47% decrease in mycobacterial growth in mice receiving 20 nanomoles of S1P via i.v injection. However, no work of clinical significance followed. Work by the Rosen group demonstrated that S1P receptor agonism during influenza virus infection resulted in quelling of the cytokine storm and rescued mice from lethal infections (Teijaro et al. 2011; Walsh et al. 2011a, b).
The repurposing of FDA-approved drugs for treating infectious diseases is becoming a popular way to defer costs associated with research and development while expediting the time to market (Skerry et al. 2012, 2014). The S1P receptor agonist Fingolimod received FDA approval in 2010 for the treatment of multiple sclerosis. Fingolimod is a S1P receptor modulator, whose function in MS is most likely tied to its ability to sequester lymphocytes within lymph nodes, preventing the amplification of the autoimmune response. Other S1P receptor agonist drugs are in clinical trials for treatment of a number of inflammation-associated pathologies (Gonzalez-Cabrera et al. 2014).
S1P signaling occurs via five high-affinity G protein-coupled receptors, S1P1-5. As S1P signaling occurs via PT-sensitive Gi/o-coupled receptors, it has been hypothesized that the action of PT on these Gi/o proteins may prolong inflammation in whooping cough. The potential for S1P pathway manipulation in pertussis was highlighted by our work using the S1P receptor agonist AAL-R. A single dose of AAL-R, shortly after infection, was shown to dramatically reduce pertussis-mediated inflammatory cytokine expression and lung pathology (Skerry et al. 2015). Further work by us demonstrated that treatment can be delayed and still have a significant impact on pathology, indicating potential therapeutic use of this approach (Skerry et al. unpublished data). Many questions remain to be addressed before S1P agonism can be thought of as a valid treatment in pertussis. One issue with targeting the S1P pathway is the potential for PT, produced by the bacterium post-inoculation but before treatment is administered, to inactivate the Gi/o proteins coupled to the S1P receptors, potentially disabling the treatment target. For this reason, studies addressing treatment at time points more closely related to clinical presentation are needed.
A common issue with murine pertussis studies is the focus on the adult mouse model. Adult mice, like humans, will clear B. pertussis infection, with inflammation and infection resolving without intervention. However, infant mice and humans are much more susceptible to fatal infection than their adult counterparts. For this reason, increased focus on neonatal models of pertussis infection will be helpful. Neonatal mouse models have been used in the pertussis field, but efforts have focused mostly on their utility in vaccine studies (Nascimento et al. 2009; Skerry et al. 2009; Gracia et al. 2011; Polewicz et al. 2011; Feunou et al. 2014). Despite widespread efforts to find adjunct treatments, little effort has focused on potential interventional treatments of neonatal disease. Our preliminary work (Skerry et al. unpublished work) with this model suggests that S1P receptor agonist treatment may be highly beneficial in reducing neonatal death associated with pertussis infection, and that, as in human disease, PT-dependent increases in leukocytosis may be a key contributor to neonatal death. It is conceivable, based on the sequestering of lymphocytes by Fingolimod in MS, that similar treatments in pertussis may impact leukocytosis. Indeed, early studies with murine analogs of Fingolimod suggest an ability to improve neonatal disease in both lethal and sublethal models (Skerry et al. unpublished work).
CONCLUSION/OUTLOOK
This minireview provides an overview of B. pertussis pathogenesis along with current and potential host-directed therapies. Pertussis incidence in the USA is at a level not seen since the 1950s. Pediatric populations, too young to be protected by vaccination, experience much more severe disease than that observed in adults. Current state of care represents limited advances over the use of antibiotics, an intervention that fails to improve the course of disease in the absence of early detection. The re-emergence of pertussis as a public health concern, a general increase in parents forgoing vaccination for their children and the lack of effective therapeutics all highlight the urgent need for novel approaches to treating this potentially deadly childhood disease.
Several attempts at developing a novel pertussis treatment are detailed in the literature (reviewed above). However, at this time, no treatment has shown reproducible success in the clinic. The most promising approaches, ECMO and leukodepletion, have been reserved for intensive care situations. Recent reports further highlight the potential of this combination approach in treating severe pertussis, but mortality rates are still unacceptably high (Assy et al. 2015). Earlier use of these interventions may allow for improved success and save many lives that would have been lost with traditional therapeutics.
Few groups are currently investigating novel potential therapeutics for pertussis. Our group is investigating the role of PT in B. pertussis infection and pertussis disease pathogenesis, primarily in animal models. Since PT catalyzes a long-lived modification of Gi proteins in target host cells (Carbonetti et al. 2007), a potential therapy would be to reverse this modification, perhaps by increasing G protein turnover in affected cells. Unfortunately, no mechanisms to achieve this have been identified, and a more realistic approach is to intervene at host targets downstream from PT effects. We are currently investigating two such novel targets for host-directed pertussis treatment, S1P receptor agonism and inhibition of pendrin activity. Both approaches potentially take advantage of drugs that are currently FDA approved, reducing the cost and time required to introduce these compounds to the clinic. Targeting ASL HCO3− levels or pendrin activity via inhaled ACTZ or anion channel activators/inhibitors may reduce airway inflammatory cell influx, in addition to potentially attenuating mucus production and cough. S1P receptor agonism dramatically reduces pulmonary inflammation without increasing bacterial burden in adult mice (Skerry et al. 2015). However, as infants are the most seriously impacted population, studies using neonatal mouse models are also justified. Unlike adult mice, neonatal mice often succumb to pertussis infection, an effect strongly linked to PT-mediated leukocytosis and possibly pulmonary hypertension (our unpublished work). These results are consistent with the observed impact of combined ECMO and leukodepletion in treatment of severe pertussis in human infants. If either of these novel approaches proves successful at improving survival or reducing lymphocytosis in neonatal mice, a next logical step would be to test their effectiveness in the baboon model of pertussis disease. Positive results in this model would indicate that these novel therapies represent the most promising attempt at treating severe infant pertussis available to date.
Future studies might focus on the improvement of ECMO, exchange blood transfusion and leukodepletion to impact leukocytosis and survival in the infant. In addition, our group aims to continue efforts to identify and characterize novel targets for host-directed therapeutics, with a deliberate focus on the repurposing of current FDA approved drugs in order to quicken time to market.
FUNDING
This work was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health [R01AI101055 and R21AI119566 to NHC]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interest. None declared.
REFERENCES
- Adams KM, Abraham V, Spielman D, et al. Pendrin expression in Staphylococcus aureus pneumonia. Am J Resp Crit Care. 2012;185:A3532. [Google Scholar]
- Ad Hoc Group for the Study of Pertussis Vaccines. Placebo-controlled trial of two acellular pertussis vaccines in Sweden–protective efficacy and adverse events. Lancet. 1988;1:955–60. [PubMed] [Google Scholar]
- Adler A, Morse SI. Interaction of lymphoid and nonlymphoid cells with the lymphocytosis-promoting factor of Bordetella pertussis. Infect Immun. 1973;7:461–7. doi: 10.1128/iai.7.3.461-467.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altunaiji S, Kukuruzovic R, Curtis N, et al. Antibiotics for whooping cough (pertussis) Cochrane Db Syst Rev. 2007;3:CD004404. doi: 10.1002/14651858.CD004404.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assy J, Seguela PE, Guillet E, et al. Severe neonatal pertussis treated by leukodepletion and early extra corporeal membrane oxygenation. Pediatr Infect Dis J. 2015;34:1029–30. doi: 10.1097/INF.0000000000000781. [DOI] [PubMed] [Google Scholar]
- Barkoff AM, Mertsola J, Guillot S, et al. Appearance of Bordetella pertussis strains not expressing the vaccine antigen pertactin in Finland. Clin Vaccine Immunol. 2012;19:1703–4. doi: 10.1128/CVI.00367-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford W. Use of convalescent blood in whooping cough, with a review of the literature. Am J Dis Child. 1935;50:918–23. [Google Scholar]
- Bruss JB, Malley R, Halperin S, et al. Treatment of severe pertussis: a study of the safety and pharmacology of intravenous pertussis immunoglobulin. Pediatr Infect Dis J. 1999;18:505–11. doi: 10.1097/00006454-199906000-00006. [DOI] [PubMed] [Google Scholar]
- Bruss JB, Siber GR. Protective effects of pertussis immunoglobulin (P-IGIV) in the aerosol challenge model. Clin Diagn Lab Immun. 1999;6:464–70. doi: 10.1128/cdli.6.4.464-470.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonetti NH, Artamonova GV, Van Rooijen N, et al. Pertussis toxin targets airway macrophages to promote Bordetella pertussis infection of the respiratory tract. Infect Immun. 2007;75:1713–20. doi: 10.1128/IAI.01578-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caro JJ, Getsios D, El-Hadi W, et al. Pertussis immunization of adolescents in the United States: an economic evaluation. Pediatr Infect Dis J. 2005;24:S75–82. doi: 10.1097/01.inf.0000160918.72953.51. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Pertussis (Whooping Cough): Outbreaks. 2013. http://www.cdc.gov/pertussis/outbreaks/index.html (27 September 2015, date last accessed) [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Epidemiology and Prevention of Vaccine Preventable Diseases. 2015a. http://www.cdc.gov/vaccines/pubs/pinkbook/downloads/pert.pdf (27 September 2015, date last accessed) [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Pertussis (Whooping Cough) - Surveillance and Reporting. 2015b. http://www.cdc.gov/pertussis/surv-reporting.html (27 September 2015, date last accessed) [Google Scholar]
- Christie CD, Baltimore RS. Pertussis in neonates. Am J Dis Child. 1989;143:1199–202. doi: 10.1001/archpedi.1989.02150220097027. [DOI] [PubMed] [Google Scholar]
- Connelly CE, Sun Y, Carbonetti NH. Pertussis toxin exacerbates and prolongs airway inflammatory responses during Bordetella pertussis infection. Infect Immun. 2012;80:4317–32. doi: 10.1128/IAI.00808-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dairaghi DJ, Oldham ER, Bacon KB, et al. Chemokine receptor CCR3 function is highly dependent on local pH and ionic strength. J Biol Chem. 1997;272:28206–9. doi: 10.1074/jbc.272.45.28206. [DOI] [PubMed] [Google Scholar]
- Danzon A, Lacroix J, Infante-Rivard C, et al. A double-blind clinical trial on diphenhydramine in pertussis. Acta Paediatr Scand. 1988;77:614–5. doi: 10.1111/j.1651-2227.1988.tb10716.x. [DOI] [PubMed] [Google Scholar]
- De Serres G, Shadmani R, Duval B, et al. Morbidity of pertussis in adolescents and adults. J Infect Dis. 2000;182:174–9. doi: 10.1086/315648. [DOI] [PubMed] [Google Scholar]
- Dossena S, Bizhanova A, Nofziger C, et al. Identification of allelic variants of pendrin (SLC26A4) with loss and gain of function. Cell Physiol Biochem. 2011;28:467–76. doi: 10.1159/000335108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Extracorporeal Life Support Organization (ELSO) ELSO Guidelines for Cardiopulmonary Extracorporeal Life Support. Ann Arbor: ELSO; 2013. [Google Scholar]
- Feunou PF, Kammoun H, Debrie AS, et al. Heterologous prime-boost immunization with live attenuated B. pertussis BPZE1 followed by acellular pertussis vaccine in mice. Vaccine. 2014;32:4281–8. doi: 10.1016/j.vaccine.2014.06.019. [DOI] [PubMed] [Google Scholar]
- Foresi A, Cavigioli G, Pelucchi A, et al. Effect of acetazolamide on cough induced by low-chloride-ion solutions in normal subjects: comparison with furosemide. J Allergy Clin Immunol. 1996;97:1093–9. doi: 10.1016/s0091-6749(96)70263-4. [DOI] [PubMed] [Google Scholar]
- Frobisher M. Wright AW. Rypins' Medical Licensure Examinations. 10th edn. Philadelphia: Lippincott; 1965. General microbiology; p. 313. [Google Scholar]
- Garg SK, Volpe E, Palmieri G, et al. Sphingosine 1-phosphate induces antimicrobial activity both in vitro and in vivo. J Infect Dis. 2004;189:2129–38. doi: 10.1086/386286. [DOI] [PubMed] [Google Scholar]
- Garnett JP, Hickman E, Burrows R, et al. Novel role for pendrin in orchestrating bicarbonate secretion in cystic fibrosis transmembrane conductance regulator (CFTR)-expressing airway serous cells. J Biol Chem. 2011;286:41069–82. doi: 10.1074/jbc.M111.266734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Cabrera PJ, Brown S, Studer SM, et al. S1P signaling: new therapies and opportunities. F1000Prime Rep. 2014;6:109. doi: 10.12703/P6-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracia A, Polewicz M, Halperin SA, et al. Antibody responses in adult and neonatal BALB/c mice to immunization with novel Bordetella pertussis vaccine formulations. Vaccine. 2011;29:1595–604. doi: 10.1016/j.vaccine.2010.12.083. [DOI] [PubMed] [Google Scholar]
- Guillot S, Descours G, Gillet Y, et al. Macrolide-resistant Bordetella pertussis infection in newborn girl, France. Emerg Infect Dis. 2012;18:966–8. doi: 10.3201/eid1806.120091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halperin SA, Vaudry W, Boucher FD, et al. Is pertussis immune globulin efficacious for the treatment of hospitalized infants with pertussis? No answer yet. Pediatr Infect Dis J. 2007;26:79–81. doi: 10.1097/01.inf.0000247103.01075.cc. [DOI] [PubMed] [Google Scholar]
- Heininger U, Stehr K, Schmitt-Grohe S, et al. Clinical characteristics of illness caused by Bordetella parapertussis compared with illness caused by Bordetella pertussis. Pediatr Infect Dis J. 1994;13:306–9. doi: 10.1097/00006454-199404000-00011. [DOI] [PubMed] [Google Scholar]
- Iwata S, Aoyama T, Goto A, et al. Mixed outbreak of Bordetella pertussis and Bordetella parapertussis in an apartment house. Dev Biol Stand. 1991;73:333–41. [PubMed] [Google Scholar]
- Klein NP, Bartlett J, Rowhani-Rahbar A, et al. Waning protection after fifth dose of acellular pertussis vaccine in children. N Engl J Med. 2012;367:1012–9. doi: 10.1056/NEJMoa1200850. [DOI] [PubMed] [Google Scholar]
- Krantz I, Norrby SR, Trollfors B. Salbutamol vs. placebo for treatment of pertussis. Pediatr Infect Dis. 1985;4:638–40. doi: 10.1097/00006454-198511000-00008. [DOI] [PubMed] [Google Scholar]
- Lam C, Octavia S, Ricafort L, et al. Rapid increase in pertactin-deficient Bordetella pertussis isolates, Australia. Emerg Infect Dis. 2014;20:626–33. doi: 10.3201/eid2004.131478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liese JG, Renner C, Stojanov S, et al. Clinical and epidemiological picture of B. pertussis and B. parapertussis infections after introduction of acellular pertussis vaccines. Arch Dis Child. 2003;88:684–7. doi: 10.1136/adc.88.8.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind-Brandberg L, Welinder-Olsson C, Lagergard T, et al. Evaluation of PCR for diagnosis of Bordetella pertussis and Bordetella parapertussis infections. J Clin Microbiol. 1998;36:679–83. doi: 10.1128/jcm.36.3.679-683.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor J, Ogle JW, Curry-Kane G. Perinatal pertussis. Obstet Gynecol. 1986;68:582–6. [PubMed] [Google Scholar]
- Mall MA, Galietta LJ. Targeting ion channels in cystic fibrosis. J Cyst Fibros. 2015;14:561–70. doi: 10.1016/j.jcf.2015.06.002. [DOI] [PubMed] [Google Scholar]
- Mattoo S, Cherry JD. Molecular pathogenesis, epidemiology, and clinical manifestations of respiratory infections due to Bordetella pertussis and other Bordetella subspecies. Clin Microbiol Rev. 2005;18:326–82. doi: 10.1128/CMR.18.2.326-382.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertsola J. Mixed outbreak of Bordetella pertussis and Bordetella parapertussis infection in Finland. Eur J Clin Microbiol. 1985;4:123–8. doi: 10.1007/BF02013576. [DOI] [PubMed] [Google Scholar]
- Mertsola J, Viljanen MK, Ruuskanen O. Salbutamol in the treatment of whooping cough. Scand J Infect Dis. 1986;18:593–4. doi: 10.3109/00365548609021669. [DOI] [PubMed] [Google Scholar]
- Mikelova LK, Halperin SA, Scheifele D, et al. Predictors of death in infants hospitalized with pertussis: a case-control study of 16 pertussis deaths in Canada. J Pediatr. 2003;143:576–81. doi: 10.1067/S0022-3476(03)00365-2. [DOI] [PubMed] [Google Scholar]
- Munoz JJ, Arai H, Bergman RK, et al. Biological activities of crystalline pertussigen from Bordetella pertussis. Infect Immun. 1981;33:820–6. doi: 10.1128/iai.33.3.820-826.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagami Y, Favoreto S, Jr, Zhen G, et al. The epithelial anion transporter pendrin is induced by allergy and rhinovirus infection, regulates airway surface liquid, and increases airway reactivity and inflammation in an asthma model. J Immunol. 2008;181:2203–10. doi: 10.4049/jimmunol.181.3.2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao I, Kanaji S, Ohta S, et al. Identification of pendrin as a common mediator for mucus production in bronchial asthma and chronic obstructive pulmonary disease. J Immunol. 2008;180:6262–9. doi: 10.4049/jimmunol.180.9.6262. [DOI] [PubMed] [Google Scholar]
- Nascimento IP, Dias WO, Quintilio W, et al. Construction of an unmarked recombinant BCG expressing a pertussis antigen by auxotrophic complementation: protection against Bordetella pertussis challenge in neonates. Vaccine. 2009;27:7346–51. doi: 10.1016/j.vaccine.2009.09.043. [DOI] [PubMed] [Google Scholar]
- O'Connor BJ, Yeo CT, Chen-Worsdell YM, et al. Effect of acetazolamide and amiloride against sodium metabisulphite-induced bronchoconstriction in mild asthma. Thorax. 1994;49:1096–8. doi: 10.1136/thx.49.11.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell WJ, Rosenberg M, Niven RW, et al. Acetazolamide and furosemide attenuate asthma induced by hyperventilation of cold, dry air. Am Rev Respir Dis. 1992;146:1518–23. doi: 10.1164/ajrccm/146.6.1518. [DOI] [PubMed] [Google Scholar]
- Olin P, Storsaeter J, Romanus V. The efficacy of acellular pertussis vaccine. JAMA. 1989;261:560. [PubMed] [Google Scholar]
- Olson LC. Pertussis. Medicine. 1975;54:427–69. doi: 10.1097/00005792-197511000-00001. [DOI] [PubMed] [Google Scholar]
- Paddock CD, Sanden GN, Cherry JD, et al. Pathology and pathogenesis of fatal Bordetella pertussis infection in infants. Clin Infect Dis. 2008;47:328–38. doi: 10.1086/589753. [DOI] [PubMed] [Google Scholar]
- Pawloski LC, Queenan AM, Cassiday PK, et al. Prevalence and molecular characterization of pertactin-deficient Bordetella pertussis in the United States. Clin Vaccine Immunol. 2014;21:119–25. doi: 10.1128/CVI.00717-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedemonte N, Caci E, Sondo E, et al. Thiocyanate transport in resting and IL-4-stimulated human bronchial epithelial cells: role of pendrin and anion channels. J Immunol. 2007;178:5144–53. doi: 10.4049/jimmunol.178.8.5144. [DOI] [PubMed] [Google Scholar]
- Pezzulo AA, Tang XX, Hoegger MJ, et al. Reduced airway surface pH impairs bacterial killing in the porcine cystic fibrosis lung. Nature. 2012;487:109–13. doi: 10.1038/nature11130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polewicz M, Gracia A, Buchanan R, et al. Influence of maternal antibodies on active pertussis toxoid immunization of neonatal mice and piglets. Vaccine. 2011;29:7718–26. doi: 10.1016/j.vaccine.2011.07.135. [DOI] [PubMed] [Google Scholar]
- Pooboni S, Roberts N, Westrope C, et al. Extracorporeal life support in pertussis. Pediatr Pulmonol. 2003;36:310–5. doi: 10.1002/ppul.10351. [DOI] [PubMed] [Google Scholar]
- Raymond J, Armengaud JB, Cosnes-Lambe C, et al. Pertussis in young infants: apnoea and intra-familial infection. Clin Microbiol Infect. 2007;13:172–5. doi: 10.1111/j.1469-0691.2006.01616.x. [DOI] [PubMed] [Google Scholar]
- Richalet JP, Rivera-Ch M, Maignan M, et al. Acetazolamide for Monge's disease: efficiency and tolerance of 6-month treatment. Am J Resp Crit Care. 2008;177:1370–6. doi: 10.1164/rccm.200802-196OC. [DOI] [PubMed] [Google Scholar]
- Rivera J, Proia RL, Olivera A. The alliance of sphingosine-1-phosphate and its receptors in immunity. Nat Rev Immunol. 2008;8:753–63. doi: 10.1038/nri2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts I, Gavin R, Lennon D. Randomized controlled trial of steroids in pertussis. Pediatr Infect Dis J. 1992;11:982–3. [PubMed] [Google Scholar]
- Rowe J, Macaubas C, Monger TM, et al. Antigen-specific responses to diphtheria-tetanus-acellular pertussis vaccine in human infants are initially Th2 polarized. Infect Immun. 2000;68:3873–7. doi: 10.1128/iai.68.7.3873-3877.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe J, Yerkovich ST, Richmond P, et al. Th2-associated local reactions to the acellular diphtheria-tetanus-pertussis vaccine in 4- to 6-year-old children. Infect Immun. 2005;73:8130–5. doi: 10.1128/IAI.73.12.8130-8135.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowlands HE, Goldman AP, Harrington K, et al. Impact of rapid leukodepletion on the outcome of severe clinical pertussis in young infants. Pediatrics. 2010;126:e816–27. doi: 10.1542/peds.2009-2860. [DOI] [PubMed] [Google Scholar]
- Scanlon KM, Gau Y, Zhu J, et al. Epithelial anion transporter pendrin contributes to inflammatory lung pathology in mouse models of Bordetella pertussis infection. Infect Immun. 2014;82:4212–21. doi: 10.1128/IAI.02222-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skerry C, Harper J, Klunk M, et al. Adjunctive TNF inhibition with standard treatment enhances bacterial clearance in a murine model of necrotic TB granulomas. PLoS One. 2012;7:e39680. doi: 10.1371/journal.pone.0039680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skerry C, Pinn ML, Bruiners N, et al. Simvastatin increases the in vivo activity of the first-line tuberculosis regimen. J Antimicrob Chemother. 2014;69:2453–7. doi: 10.1093/jac/dku166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skerry C, Scanlon K, Rosen H, et al. Sphingosine-1-phosphate receptor agonism reduces Bordetella pertussis-mediated lung pathology. J Infect Dis. 2015;211:1883–6. doi: 10.1093/infdis/jiu823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skerry CM, Cassidy JP, English K, et al. A live attenuated Bordetella pertussis candidate vaccine does not cause disseminating infection in gamma interferon receptor knockout mice. Clin Vaccine Immunol. 2009;16:1344–51. doi: 10.1128/CVI.00082-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C, Vyas H. Early infantile pertussis; increasingly prevalent and potentially fatal. Eur J Pediatr. 2000;159:898–900. doi: 10.1007/PL00008365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soane MC, Jackson A, Maskell D, et al. Interaction of Bordetella pertussis with human respiratory mucosa in vitro. Respir Med. 2000;94:791–9. doi: 10.1053/rmed.2000.0823. [DOI] [PubMed] [Google Scholar]
- Spicuzza L, Ciancio N, Pellegrino R, et al. The effect of inhaled furosemide and acetazolamide on bronchoconstriction induced by deep inspiration in asthma. Monaldi Arch Chest Dis. 2003;59:150–4. [PubMed] [Google Scholar]
- Spiegel S, Milstien S. The outs and the ins of sphingosine-1-phosphate in immunity. Nat Rev Immunol. 2011;11:403–15. doi: 10.1038/nri2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss CJ. Correspondence. Brit Med J. 1951;2:970. [Google Scholar]
- Strebel P, Nordin J, Edwards K, et al. Population-based incidence of pertussis among adolescents and adults, Minnesota, 1995–1996. J Infect Dis. 2001;183:1353–9. doi: 10.1086/319853. [DOI] [PubMed] [Google Scholar]
- Tam AY, Yeung CY. Severe neonatal pertussis treated by salbutamol. Arch Dis Child. 1986;61:600–2. doi: 10.1136/adc.61.6.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teijaro JR, Walsh KB, Cahalan S, et al. Endothelial cells are central orchestrators of cytokine amplification during influenza virus infection. Cell. 2011;146:980–91. doi: 10.1016/j.cell.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas EL, Aune TM. Lactoperoxidase, peroxide, thiocyanate antimicrobial system: correlation of sulfhydryl oxidation with antimicrobial action. Infect Immun. 1978;20:456–63. doi: 10.1128/iai.20.2.456-463.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh KB, Teijaro JR, Rosen H, et al. Quelling the storm: utilization of sphingosine-1-phosphate receptor signaling to ameliorate influenza virus-induced cytokine storm. Immunol Res. 2011a;51:15–25. doi: 10.1007/s12026-011-8240-z. [DOI] [PubMed] [Google Scholar]
- Walsh KB, Teijaro JR, Wilker PR, et al. Suppression of cytokine storm with a sphingosine analog provides protection against pathogenic influenza virus. P Natl Acad Sci USA. 2011b;108:12018–23. doi: 10.1073/pnas.1107024108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warfel JM, Beren J, Merkel TJ. Airborne transmission of Bordetella pertussis. J Infect Dis. 2012;206:902–6. doi: 10.1093/infdis/jis443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter K, Zipprich J, Harriman K, et al. Risk factors associated with infant deaths from pertussis: a case-control study. Clin Infect Dis. 2015;61:1099–106. doi: 10.1093/cid/civ472. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO) Immunization, Vaccines and Biologicals – Pertussis. 2011. http://www.who.int/immunization_monitoring/diseases/pertussis/en/index.html (27 September 2015, date last accessed) [Google Scholar]
- Worthington ZE, Van Rooijen N, Carbonetti NH. Enhancement of Bordetella parapertussis infection by Bordetella pertussis in mixed infection of the respiratory tract. FEMS Immunol Med Mic. 2011;63:119–28. doi: 10.1111/j.1574-695X.2011.00836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yick CY, Zwinderman AH, Kunst PW, et al. Transcriptome sequencing (RNA-Seq) of human endobronchial biopsies: asthma versus controls. Eur Respir J. 2013;42:662–70. doi: 10.1183/09031936.00115412. [DOI] [PubMed] [Google Scholar]
- Zeddeman A, van Gent M, Heuvelman CJ, et al. Investigations into the emergence of pertactin-deficient Bordetella pertussis isolates in six European countries, 1996 to 2012. Euro Surveill. 2014;19:pii=20881. doi: 10.2807/1560-7917.es2014.19.33.20881. [DOI] [PubMed] [Google Scholar]
- Zoumboulakis D, Anagnostakis D, Albanis V, et al. Steroids in treatment of pertussis. A controlled clinical trial. Arch Dis Child. 1973;48:51–4. doi: 10.1136/adc.48.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]