Abstract
Type III secretion system (T3SS) in Pseudomonas aeruginosa is associated with poor clinical outcome in acute infections. T3SS allows for injection of bacterial exotoxins (e.g. ExoU or ExoS) into the host cell, causing cytotoxicity. It also activates the cytosolic NLRC4 inflammasome, activating caspase-1, inducing cytotoxicity and release of mature IL-1β, which impairs bacterial clearance. In addition, flagellum-mediated motility has been suggested to also modulate inflammasome response and IL-1β release. Yet the capacity of clinical isolates to induce IL-1β release and its relation with cytotoxicity have never been investigated. Using 20 clinical isolates from acute infections with variable T3SS expression levels and human monocytes, our aim was to correlate IL-1β release with toxin expression, flagellar motility and cytotoxicity. ExoU-producing isolates caused massive cell death but minimal release of IL-1β, while those expressing T3SS but not ExoU (i.e. expressing ExoS or no toxins) induced caspase-1 activation and IL-1β release, the level of which was correlated with cytotoxicity. Both effects were prevented by a specific caspase-1 inhibitor. Flagellar motility was not correlated with cytotoxicity or IL-1β release. No apoptosis was detected. Thus, T3SS cytotoxicity is accompanied by a modification in cytokine balance for P. aeruginosa clinical isolates that do not express ExoU.
Keywords: type three secretion system, inflammasome, ExoS, ExoU, TNF-alpha, flagellin
This study shows that clinical isolates of Pseudomonas aeruginosa can induce in parallel cytotoxicity and modulation of cytokine balance in the host cells, depending on the virulence factors they produce.
Graphical Abstract Figure.
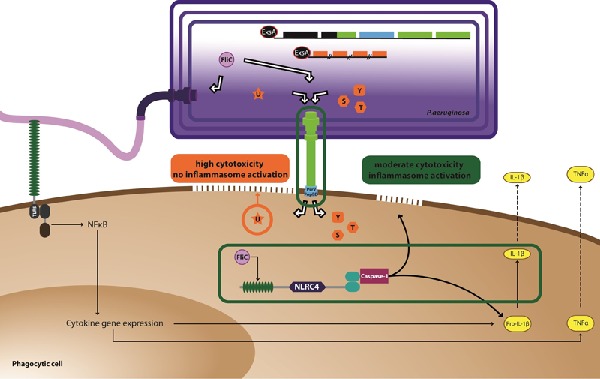
This study shows that clinical isolates of Pseudomonas aeruginosa can induce in parallel cytotoxicity and modulation of cytokine balance in the host cells, depending on the virulence factors they produce.
Pseudomonas aeruginosa is a major cause of healthcare-associated infections, with severe morbidity and high mortality. Its capacity to develop antibiotic resistance limits therapeutic options, leading to treatment failures. Moreover, the P. aeruginosa genome encodes several virulence factors and environmental sensor-regulator systems, allowing it to adapt to hostile environments, induce tissue injury and control inflammatory reactions. Studying cellular responses to the virulence factors expressed by clinical isolates is critical in order to evaluate the usefulness of combining antibiotics with specific inhibitors of these virulence mechanisms or immunomodulators.
Type III secretion system (T3SS) is a major virulence factor in P. aeruginosa, associated with poor clinical outcome and high morbidity in acute infections. T3SS enables bacteria to inject exotoxins into the host cell cytoplasm (Sawa 2014). Among them, the phospholipase A2 ExoU causes rapid cell death by disrupting membrane integrity, while ExoS prevents bacterial internalization and induces cell necrosis or apoptosis (Okuda, Hanabusa and Gotoh 2014). Beside these toxin-dependent effects, T3SS also delivers flagellin or T3SS rod proteins into the mammalian cytosol, inducing secretion of IL-1β and IL-18 (Miao et al. 2008, 2010). Contrary to TNF-α, IL-1β and IL-18 secretion requires two signals (Miao et al. 2008), namely (i) Toll-like receptor (TLR) activation (inducing transcription and translation of the proforms of these cytokines) and (ii) NOD-like receptors (NLR) (activating caspase-1 and inducing cytokine processing and secretion, leading to pyroptotic cell death). Pseudomonas aeruginosa T3SS specifically activates NLRC4 (NLR family, CARD domain containing 4; also known as Ipaf) inflammasome. In the case of P. aeruginosa infection, IL-1β release seems essentially related to this process, as it is totally inhibited in nlrc4 -/- macrophages (Faure et al. 2014).
Bacteria-induced inflammasome activation and subsequent IL-1β and IL-18 release have been generally considered as protective (Sutterwala et al. 2007; Cai et al. 2012). Yet, in the case of P. aeruginosa infections, these processes are rather deleterious, contributing to increased tissue injury and impaired bacterial clearance (Schultz et al. 2002; Cohen and Prince 2013; Faure et al. 2014). Moreover, flagellum–mediated motility has been suggested to also modulate inflammasome response and IL-1β release (Patankar et al. 2013). At this stage, however, these concepts have been studied only with reference strains and mutants thereof expressing or not specific proteins from the T3SS or the flagellum.
In this context, this study aimed at (i) examining whether T3SS positive-clinical isolates collected from patients suffering from acute infections are also able to induce IL-1β release and (ii) determining whether the IL-1β production is related to toxin expression or flagellar motility, and correlates with the degree of cytotoxicity induced. TNF-α release was also followed as a control because its production is unrelated to inflammasome activation (Akira and Takeda 2004). Human THP-1 monocytes were used as model of phagocytic cells capable to activate NLRC4 inflammasome in response to T3SS.
Table S1 (Supporting Information) shows the strains under study. They include (i) 20 clinical isolates from acute infections retrospectively collected in four Belgian hospitals, (ii) three reference strains, namely PA103 (expressing ExoU), PAO1 and CHA (expressing ExoS, ExoT, ExoY) and (iii) mutants with deletions in genes coding for T3SS toxins (PA103ΔUT; CHAΔSTY), proteins from the translocation apparatus (PA103ΔpcrV; CHAΔpopB/popD) or T3SS regulon (CHAΔExsA). These strains were characterized for the expression of genes encoding toxins, translocation apparatus or flagellin, and for swarming motility in 0.3% LB agar plate. Their serotype and genetic relatedness were determined in order to check for sufficient diversity within the collection (Table S1 and Fig. S1, Supporting Information).
If excluding negative controls (PA103ΔpcrV, CHAΔpopBD, CHAΔExsA), the other strains could be classified in two groups (Table S1, Supporting Information), namely (i) 8 clinical isolates plus PA103 expressing ExoU, ExoT and the translocation apparatus (T3SS+ ExoU+) and (ii) 12 clinical isolates plus PAO1, CHA, CHAΔSTY and PA103ΔUT expressing the translocation apparatus but not the ExoU toxin (T3SS+ExoU−). ExoS+ strains were thus more represented in this collection than ExoU+ strains, and none of them expressed both toxins, as generally observed (Garey et al. 2008; El-Solh et al. 2012). No toxin was expressed in PA103ΔUT and CHAΔSTY. exoU, exoS and exoT expression levels were highly variable, ranging from 283 to 1% of the values measured in reference strains. Flagellin expression and swimming motility were variable, with no correlation between flagellin expression and motility (Table S2, Supporting Information).
THP-1 monocytes were then incubated with each of these isolates. Cytotoxicity was assessed by measuring lactate dehydrogenase (LDH) release in the culture supernatant. IL-1β and TNF-α were quantified in supernatants by ELISA. As IL-1β is released in response to caspase-1 activation, cells were also preincubated with 40 μM of the specific caspase-1 inhibitor Ac-YVAD-cmk (N-acetyl-tyrosyl-valyl-alanyl-aspartyl chloromethyl ketone, Sigma-Aldrich, Saint-Louis, MO). Incubation time was set at 5 h based on preliminary experiments demonstrating that cytotoxicity was essentially mediated by T3SS in these conditions (see values in Fig. 1; cytotoxicity high for PA103; moderate for CHA and largest difference with that of CHAΔExsA).
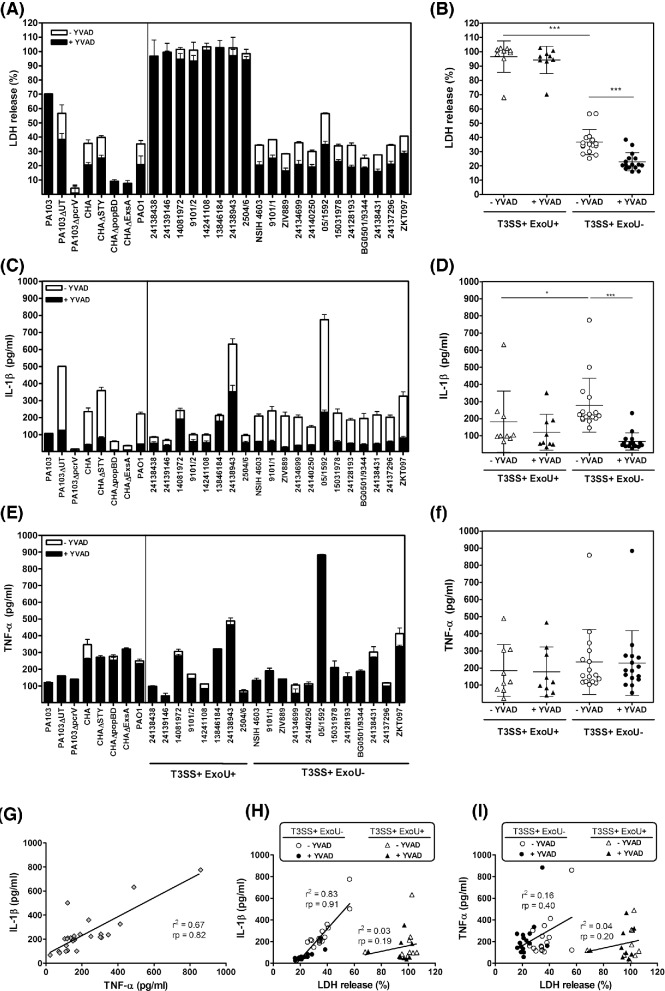
Figure 1.
Cytotoxicity and cytokine production induced by P. aeruginosa reference strains and clinical isolates. THP-1 cells seeded into 96-well plates (2.5 × 105 cells/mL) were incubated with each of these isolates (10 bacteria/cell) during 5 h. Cytotoxicity was assessed by measuring the release of LDH into the culture supernatant using the cytotoxicity detection kit PLUS (Roche, Basel, Switzerland). IL-1β and TNF-α were quantified in supernatants using a commercially available ELISA kit (R&D systems, Minneapolis, MN and BD Biosciences, San Jose, CA, respectively). Upper panels: percentage of release of LDH (A and B), IL-1β (C and D) and TNF-α (E and F) in the supernatant of THP-1 monocytes exposed during 5 h to clinical isolates (10 bacteria/cell) in the presence of the caspase-1 inhibitor Ac-YVAD-cmk [+YVAD] or in control conditions [−YVAD; DMSO added as the solvent of Ac-YVAD-cmk]. Left panels show the data for individual strains, and right panels for strains grouped according to the expression of ExoU toxin. Values are mean ± SD of representative experiments performed in triplicates and reproduced at three independent occasions at least with similar results. Statistical analyses performed among groups of strains using two-way ANOVA, Bonferroni post-test: *** P < 0.001, * P < 0.05. Lower panels: correlation between IL-1β and TNF-α release (G), IL-1β and LDH release (H), and TNF-α and LDH release (I) for all strains in control conditions (G) or for strains grouped according to the production of ExoU toxin (H and I) (r2 = coefficient of determination; rp = Pearson correlation coefficient).
Figure 1 shows data for each individual strain (left) or for T3SS+ strains grouped according to the expression of ExoU (right). Considering reference strains, PA103 induced a massive LDH release but a low release of cytokines, which was not affected by Ac-YVAD-cmk. CHA was less cytotoxic but induced a significantly larger release of IL-1β (reversed by Ac-YVAD-cmk) but not of TNF-α. Remarkably, PA103ΔUT and CHAΔSTY showed a profile similar to that of CHA despite the fact they did not express any toxin but still the translocation apparatus. PA103ΔpcrV, CHAΔpopBD and CHAΔExsA were not cytotoxic and induced minimal IL-1β release. All together, these data confirm the role of T3SS apparatus rather than toxins in this process (Miao et al. 2008). PA103ΔUT and CHAΔSTY induced a larger IL-1β release than their parental strains, which is compatible with the previously demonstrated inhibitory effect of the toxins on inflammasome activation (Sutterwala et al. 2007; Galle et al. 2008). Moving to clinical isolates, all T3SS+ExoU+ isolates behaved as PA103, while all T3SS+ExoU− isolates caused only 20–50% LDH release and a commensurate release of IL-1β but not of TNF-α. Again Ac-YVAD-cmk significantly reduced cytotoxicity and IL-1β release for T3SS +ExoU− isolates, with no influence on TNF-α release. Inflammasome activation by T3SS+ ExoU− isolates was confirmed in western blot by the presence of the active form of caspase-1 and of IL-1β in cell culture supernatants, which was not observed for T3SS+ExoU+ isolates (Fig. S2, Supporting Information). As the latter isolates were more cytotoxic, we also checked that inflammasome activation did not occur earlier. To this effect, we followed LDH and IL-1β release over time and showed that they were never inhibited by Ac-YVAD-cmk (Fig. S3, Supporting Information).
In parallel, we looked for the presence of apoptotic cells vs necrotic cells over 5 h of incubation using representative ExoU+ or ExoU− strains and found that cell death was associated with membrane permeabilization (necrosis or pyroptosis) but not apoptosis whatever the incubation time (Fig. S4, Supporting Information), as previously demonstrated for CHA (Dacheux et al. 2000).
Potential relationships between toxin or flagellin expression, motility and cytotoxicity or cytokine release were systematically looked for by appropriate statistical analysis of the correlations between pairs of parameters (Table S2, Supporting Information). Globally, a highly significant correlation was observed between IL-1β and TNF-α secretion in the absence of Ac-YVAD-cmk (Fig. 1G). Moreover, the release of IL-1β but not that of TNF-α was correlated with cytotoxicity, whether cells were pretreated or not with Ac-YVAD-cmk, but only for T3SS+ExoU– strains (Fig. 1H/I). Other correlations were not significant.
The main conclusion from this study is that cytotoxicity exerted by T3SS-positive clinical isolates from acute infections is correlated with IL-1β release for strains that do not express ExoU. The high cytotoxicity of T3SS+ExoU+ isolates could rely on the phospholipase activity of the toxin, which may cause cell lysis before any mechanism of cell response could be activated (Sato and Frank 2004). Low IL-1β levels detected after 5 h incubation correspond to its proform released in the medium after cell death, which explains why Ac-YVAD-cmk did not prevent this process. Conversely, cytotoxicity and IL-1β release induced by T3SS+ExoU− strains are largely inhibited by Ac-YVAD-cmk, being consecutive to caspase-1 activation (probably related to NLRC4 inflammasome). Notably also, we did not observe an inverse correlation between toxin expression levels and IL-1β secretion, in spite of the described inhibitory effect of the toxin on inflammasome activation (Sutterwala et al. 2007; Galle et al. 2008).
We also noticed a correlation between TNF-α and IL-1β release induced by the whole collection. This probably results from a simultaneous production of TNF-α and proIL-1β via TLR activation, because the correlation is lost in the presence of Ac-YVAD-cmk that prevents proIL-1β maturation. We can neither exclude a cytotoxicity related to indirect caspase-1 activation via caspase-11 activation in response to LPS (Kayagaki et al. 2013).
On the contrary, we were unable to demonstrate any correlation between flagellin expression level (Miao et al. 2008; Cohen and Prince 2013) and flagellar motility (Patankar et al. 2013) or between motility and cytotoxicity or cytokine release.
Secretion of ExoU is considered as a marker of high virulence (Schulert et al. 2003), but expression of ExoU or ExoS has been both associated with poor clinical outcome (El-Solh et al. 2012). We show here that the pattern of cytokines expressed by the host cells in response to T3SS depends, at least in vitro, on whether the strain expresses or not ExoU.
Thus, pending for animal studies aiming at confirming these observations and further exploring the consequences of this cytokine dysbalance, our data suggest that inhibiting T3SS functionality by immunotherapy or small compounds (Kline et al. 2012) may be more appropriate than blocking specifically toxins. This work opens the door to the study of innovative therapeutic strategy to be combined with antibiotics during acute pseudomonal infections.
Supplementary Material
Acknowledgments
The authors are grateful to M.C. Cambier and K. Santos for excellent technical assistance, to Dr O. Denis (Hôpital Erasme, Université libre de Bruxelles, Brussels) and Dr D. Piérard (Universitair Ziekenhuis Bussel, Vrije Universiteit Brussel, Brussels) for the kind gift of clinical strains, to Pr. B. Kazmierczak (Yale University, New Haven, CT) for generously providing us with PA103 mutants, and to Dr N. Dauguet (de Duve Institute, Université catholique de Louvain, Brussels) for help in the performance and analysis of FACS experiments.
SUPPLEMENTARY DATA
FUNDING
This work was supported by the Fonds de la Recherche Scientifique [grants 3.4530.12 and T.0134.13] and the Interuniversity Attraction Poles Program initiated by the Belgian Science Policy Office [program IAP P7/28]. AA is Aspirant and FVB, Maître de Recherches of the Belgian Fonds de la Recherche Scientifique (F.R.S-FNRS). JMB was supported by the Région Wallonne, Belgium.
Conflict of interest. None declared.
REFERENCES
- Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- Cai S, Batra S, Wakamatsu N, et al. NLRC4 inflammasome-mediated production of IL-1beta modulates mucosal immunity in the lung against gram-negative bacterial infection. J Immunol. 2012;188:5623–35. doi: 10.4049/jimmunol.1200195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen TS, Prince AS. Activation of inflammasome signaling mediates pathology of acute P. aeruginosa pneumonia. J Clin Invest. 2013;123:1630–7. doi: 10.1172/JCI66142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacheux D, Toussaint B, Richard M, et al. Pseudomonas aeruginosa cystic fibrosis isolates induce rapid, type III secretion-dependent, but ExoU-independent, oncosis of macrophages and polymorphonuclear neutrophils. Infect Immun. 2000;68:2916–24. doi: 10.1128/iai.68.5.2916-2924.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Solh AA, Hattemer A, Hauser AR, et al. Clinical outcomes of type III Pseudomonas aeruginosa bacteremia. Crit Care Med. 2012;40:1157–63. doi: 10.1097/CCM.0b013e3182377906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure E, Mear JB, Faure K, et al. Pseudomonas aeruginosa type-3 secretion system dampens host defense by exploiting the NLRC4-coupled inflammasome. Am J Resp Crit Care. 2014;189:799–811. doi: 10.1164/rccm.201307-1358OC. [DOI] [PubMed] [Google Scholar]
- Galle M, Schotte P, Haegman M, et al. The Pseudomonas aeruginosa Type III secretion system plays a dual role in the regulation of caspase-1 mediated IL-1beta maturation. J Cell Mol Med. 2008;12:1767–76. doi: 10.1111/j.1582-4934.2007.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garey KW, Vo QP, Larocco MT, et al. Prevalence of type III secretion protein exoenzymes and antimicrobial susceptibility patterns from bloodstream isolates of patients with Pseudomonas aeruginosa bacteremia. J Chemother. 2008;20:714–20. doi: 10.1179/joc.2008.20.6.714. [DOI] [PubMed] [Google Scholar]
- Kayagaki N, Wong MT, Stowe IB, et al. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science. 2013;341:1246–9. doi: 10.1126/science.1240248. [DOI] [PubMed] [Google Scholar]
- Kline T, Felise HB, Sanowar S, et al. The type III secretion system as a source of novel antibacterial drug targets. Curr Drug Targets. 2012;13:338–51. doi: 10.2174/138945012799424642. [DOI] [PubMed] [Google Scholar]
- Miao EA, Ernst RK, Dors M, et al. Pseudomonas aeruginosa activates caspase 1 through Ipaf. P Natl Acad Sci USA. 2008;105:2562–7. doi: 10.1073/pnas.0712183105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao EA, Mao DP, Yudkovsky N, et al. Innate immune detection of the type III secretion apparatus through the NLRC4 inflammasome. P Natl Acad Sci USA. 2010;107:3076–80. doi: 10.1073/pnas.0913087107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda J, Hanabusa A, Gotoh N. ExoS of Pseudomonas aeruginosa binds to a human KIF7 to induce cytotoxicity in cultured human bronchial epithelial cells. J Infect Chemother. 2014;20:121–7. doi: 10.1016/j.jiac.2013.09.001. [DOI] [PubMed] [Google Scholar]
- Patankar YR, Lovewell RR, Poynter ME, et al. Flagellar motility is a key determinant of the magnitude of the inflammasome response to Pseudomonas aeruginosa. Infect Immun. 2013;81:2043–52. doi: 10.1128/IAI.00054-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H, Frank DW. ExoU is a potent intracellular phospholipase. Mol Microbiol. 2004;53:1279–90. doi: 10.1111/j.1365-2958.2004.04194.x. [DOI] [PubMed] [Google Scholar]
- Sawa T. The molecular mechanism of acute lung injury caused by Pseudomonas aeruginosa: from bacterial pathogenesis to host response. J Intensive Care. 2014;2:10. doi: 10.1186/2052-0492-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulert GS, Feltman H, Rabin SD, et al. Secretion of the toxin ExoU is a marker for highly virulent Pseudomonas aeruginosa isolates obtained from patients with hospital-acquired pneumonia. J Infect Dis. 2003;188:1695–706. doi: 10.1086/379372. [DOI] [PubMed] [Google Scholar]
- Schultz MJ, Rijneveld AW, Florquin S, et al. Role of interleukin-1 in the pulmonary immune response during Pseudomonas aeruginosa pneumonia. Am J Physiol-Lung C. 2002;282:L285–90. doi: 10.1152/ajplung.00461.2000. [DOI] [PubMed] [Google Scholar]
- Sutterwala FS, Mijares LA, Li L, et al. Immune recognition of Pseudomonas aeruginosa mediated by the IPAF/NLRC4 inflammasome. J Exp Med. 2007;204:3235–45. doi: 10.1084/jem.20071239. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.