Abstract
Chagas disease is caused by the protozoan Trypanosoma cruzi. The parasite reaches the secondary lymphoid organs, the heart, skeletal muscles, neurons in the intestine and esophagus among other tissues. The disease is characterized by mega syndromes, which may affect the esophagus, the colon and the heart, in about 30% of infected people. The clinical manifestations associated with T. cruzi infection during the chronic phase of the disease are dependent on complex interactions between the parasite and the host tissues, particularly the lymphoid system that may either result in a balanced relationship with no disease or in an unbalanced relationship that follows an inflammatory response to parasite antigens and associated tissues in some of the host organs and/or by an autoimmune response to host antigens. This review discusses the findings that support the notion of an integrated immune response, considering the innate and adaptive arms of the immune system in the control of parasite numbers and also the mechanisms proposed to regulate the immune response in order to tolerate the remaining parasite load, during the chronic phase of infection. This knowledge is fundamental to the understanding of the disease progression and is essential for the development of novel therapies and vaccine strategies.
Keywords: Trypanosoma cruzi, memory T cell, regulatory T cell, gamma delta T cell, interleukin-17, myeloid-derived suppressor cell (MDSC)
This article reviews the immune mechanisms responsible for the control of parasite numbers as well as the mechanisms that control the immune response, avoiding tissue damage and disease.
Graphical Abstract Figure.
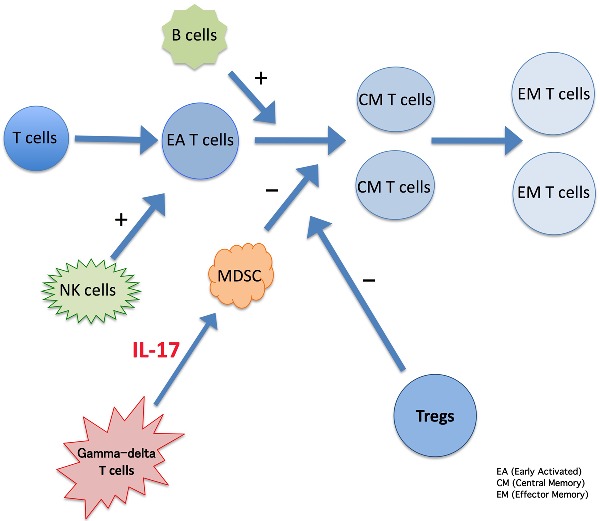
This article reviews the immune mechanisms responsible for the control of parasite numbers as well as the mechanisms that control the immune response, avoiding tissue damage and disease.
INTRODUCTION
The intracellular protozoan parasite Trypanosoma cruzi causes Chagas’ disease in humans (Koberle 1968; Andrade, Gollob and Dutra 2014). The infection is characterized by an acute phase resulting in parasitemia that resolves upon the appearance of an effective immune response (Cardillo et al. 2002). In humans and mice, the acute infection is characterized by high parasitemia that increases after 1–8 weeks following infection, depending on the T. cruzi strain (Cardillo et al. 1996). However, the immune response induced during the acute infection is not sufficient to completely eradicate the pathogen, thus resulting in chronic infection (Albareda et al. 2006). The chronic form of the disease mainly affects the peripheral autonomous nervous system in the gastrointestinal tract and heart and the heart muscle in approximately 30% of the infected patients (Koberle 1968; Andrade, Gollob and Dutra 2014). The chronic infection may be accompanied by additional autoimmune mechanisms triggered by the parasite and its persistence (dos Santos et al. 1992; Mengel and Rossi 1992; Bonney and Engman 2008, 2015; Cunha-Neto et al. 2011). Yet, the majority (about 70%) of the patients that progress to the chronic phase remain clinically asymptomatic in the chronic phase of the infection. This condition characterizes the indeterminate form, known as early-indeterminate disease, usually seen in infected children and adolescents, and late-indeterminate disease, generally observed in infected adults (Umezawa et al. 2001).
In this review, we discuss the molecules, cells and possible mechanisms involved in the potentiation and/or the control/downregulation of the immune response during T. cruzi infection.
CELLS AND MOLECULES THAT PROMOTE IMMUNITY IN THE ACUTE PHASE OF T. CRUZI INFECTION
Initial IFN-γ production prior to the generation of T-cell-mediated adaptive immunity is known to occur during the course of many infections and may be important in the development of resistance to many intracellular infections such as Leishmania, Salmonella, Toxoplasma and T. cruzi (Locksley and Scott 1991; Ramarathinam, Niesel and Klimpel 1993; Sher et al. 1993; Cardillo et al. 1996). Natural killer cells may be the major cell type responsible for IFN-γ production in the early stages of T. cruzi infection and their activation requires the presence of live parasites (Cardillo et al. 1996). In addition, innate or adaptive immune cells, such as dendritic cells, macrophages, NKT lymphocytes, γδ T cells and B cells, may contribute to host resistance (Locksley and Scott 1991; Sher et al. 1993; Cardillo et al. 1996; Galli et al. 2003, 2007; Takahashi and Strober 2008).
Dendritic cells (DCs) and/or macrophages act as professional antigen-presenting cells and are central in the initiation and development of immunity or tolerance (Lanzavecchia and Sallusto 2001; Steinman, Hawiger and Nussenzweig 2003). Trypomastigotes are responsible for the generation of regulatory DCs in vitro (Sher et al. 1993; Poncini et al. 2008; da Costa et al. 2014). The production of TNF-α, IFN-γ, IL-12, IL-22, IL-6, IL-10 and CCL2 and the expression of CD40, CD80, MHC-II, PD-L1, CCR5 and CCR7 may be different, depending on the T. cruzi strain used for stimulation (Poveda et al. 2014; da Costa et al. 2014). These results strongly argue that DCs, monocytes and macrophages are active players in the modulation of the adaptive immune response to T. cruzi (Rezende-Oliveira, Sarmento and Rodrigues 2012; Pinho et al. 2014) and may be useful to manipulate immunity/tolerance either before or during the infection (Poncini et al. 2015; Rampazo et al. 2015).
NK1.1+ cells may participate in generating memory T cells, since their depletion in acute infection diminished the generation of the activated/memory T cells in the spleen of T. cruzi-infected mice (Cardillo et al. 2002). In fact, this resulted in larger numbers of T cells expressing CD69, without the corresponding formation of effector T cells (Cardillo et al. 2004). Furthermore, the depletion of NK1.1+ cells caused an earlier appearance of anti-T. cruzi IgM antibodies and less isotype switching to IgG at later time points, suggesting a diminished T-cell-helper response (Cardillo et al. 2002). However, these studies did not discriminate between CD3– and CD3+ NK cells, as they were performed with depleting monoclonal antibodies to the NK1.1 molecule.
Other studies using CD1 and/or V alpha 14 knockout mice have shown that natural killer T cells (NKT cells) may play more discrete or opposing functions during the acute phase of T. cruzi infection (Duthie et al. 2002; Procopio et al. 2002; Miyahira et al. 2003; Duthie et al. 2005a,b), but the net results point to a role of these cells in resistance to T. cruzi infection. However, the mechanisms by which these cells contribute to the immune response to acute infection are not clear yet and might also involve a regulatory function, dampening T-cell hyperactivation, and IFN-γ and NO production (Cardillo et al. 2004). Activated NK cells, bearing a particular phenotype (CD16 + CD56–), were found to increase during the acute phase of Chagas’ disease in children (Sathler-Avelar et al. 2003). However, the exact mechanism used by NK cells to help in the control of the infection in humans is not clear. The mechanism might rely on the secretion of cytokines such as IFN-γ and TNF-α by parasite activated NK cells, thus amplifying the innate and/or the adaptive immune responses (Andrade, Gollob and Dutra 2014).
Another T-cell lineage that might be involved in up- or downregulation of the immune response to T. cruzi during the acute phase of the infection is the γδ T lymphocyte (Cardillo et al. 1993; Nomizo et al. 2006). γδ T cells are not homogeneous and their functions may vary depending on T-cell receptor usage and different stimulatory conditions (Chien and Hampl 2000; Chien, Meyer and Bonneville 2014). A small subpopulation, bearing the Vγ1 chain, is found in the thymus and in the secondary lymphoid organs such as the spleen, lymph nodes and the GALT of the adult mouse (Azuara et al. 1997, 2001; Azuara, Lembezat and Pereira 1998; Azuara and Pereira 2000). Part of this subset also expresses NK1.1 molecules (Azuara et al. 1997) and the administration of a monoclonal antibody to the Vγ1 chain results in increased susceptibility to T. cruzi infection (Nomizo et al. 2006). These cells appear to function as helpers for conventional CD4 T cells, increasing the formation of memory T cells and their IFN-γ production. Taken together, the previously mentioned studies show that the NK1.1+ cell subset is composed by different lineages, having complex functions (Werner et al. 2011). In spite of this, these cells may function by helping conventional T cells to fully differentiate into memory cells, since in their absence conventional T cells might accumulate in an early stage of activation. This would lead to elevated production of inflammatory cytokines and oxygen-containing toxic molecules in peripheral lymphoid organs, since these cells do not migrate efficiently to infected tissues and are ineffective parasite killers in infected tissues. The overall result would be immune hyperactivation accompanied by poor parasite growth control and early death of the host (Cardillo et al. 2004).
B lymphocytes are also required to mount an effective immune response to T. cruzi, helping in the control of the infection (Cardillo et al. 2007; Sullivan et al. 2015). The disease in C57BL/6 muMT KO mice is more severe than in control mice, with higher parasitemia levels and a poor generation of central and effector memory CD4+ and CD8+ T cells in the spleen. During early stages of the T. cruzi infection, B cells are fundamental to trigger T-cell functions related to the Th1 pathway that favor the control of parasite growth (Cardillo et al. 2007). Therefore, in the absence of mature B cells, the immune system is unable to generate and/or maintain central and effector memory CD8+ T cells and to instruct a Th1 functional pattern of T-cell cytokines, since the levels of proinflammatory cytokines such as IFN-γ and IL-12 are reduced in spleen cell supernatants from mice lacking mature B cells (Cardillo et al. 2007). Tissue inflammatory responses in these mice are much less intense in the acute phase of the infection, which is consistent with a deficit in the generation of effector T cells. Furthermore, the preponderant cell type in the skeletal muscle inflammatory infiltrate is the CD4+ T cell, contrary to what is observed in B-cell-sufficient mice, where CD8+ T cells dominate in the inflammatory infiltrate (Cardillo et al. 2007). Adoptively transferred splenic B cells induce increased numbers of both effector/memory splenic CD4 and CD8 T cells, during early chronic infection (Cardillo et al. in preparation). Accordingly, it has been reported that the development, maintenance and functional activities of memory CD8+ T cells during immune responses are dependent on the generation of memory CD4+ T cells and B cells (Williams et al. 2006; Sullivan et al. 2015). Besides, it has been described that B cells may themselves produce many different cytokines upon stimulation, including IFN-γ, IL-10, IL-12, IL-17 and BAFF/BLyS (O'Garra et al. 1990; Mengel et al. 1992; Pang et al. 1992; Veras et al. 2006; Wojciechowski et al. 2009; Amezcua Vesely et al. 2012; Bermejo et al. 2013). In addition, in the chronic phase of T. cruzi infection one may speculate that B cells modulate the immune response through IL-10 production, since the transfer of B cells from IL-10 knockout mice to mu knockout mice helps to control the acute infection, but also leads to an increased inflammatory heart disease in the chronic phase (Cardillo et al. in preparation). Regarding this topic and until recently, virtually no report addressed the real phenotypic markers of B cells producing IL-10, in humans. Thus, the CD19+ CD5+ CD1d+ IL-10+ B cells were found to be increased in chronic chagasic patients. In addition, a higher expression of CD21 and CD24 on the surface of circulating CD19+ B cells has been shown in those patients. The study also showed that the expression of MHC-II (HLA-DR), CD80, CD86, caspase-3, granzyme B and intracellular IL-10 and TGF-β by CD19+ B cells was higher in patients with chronic Chagas disease (Fares et al. 2013).
In summary, in T. cruzi experimental infection, with the Tulahuen strain, the development and function of memory CD8+ and CD4+ T cells are greatly modulated by NK1.1+ and B cells, since lower numbers of memory T cells are formed in acute infection when these subsets are absent. In addition, high parasite load has been observed in NK1.1 cell- or B-cell-depleted mice, indicating that the conversion of activated to effector memory cells is an important step in the control of infection levels and mortality.
The initial magnitude of CD8+ T-cell responses appears to be one of the critical factors in determining the final size of the antigen-specific memory T-cell pool (Olivieri, Cotta-De-Almeida and Araujo-Jorge 2002; Williams et al. 2006; Bixby and Tarleton 2008; Bustamante, Bixby and Tarleton 2008; Miyahira 2008). Central memory CD8+ T cells, expressing high levels of CD44 and CD62L and reduced expression of KLRG1, a marker of repetitive antigen stimulation and cell exhaustion (Bustamante, Bixby and Tarleton 2008), are detectable in the late acute phase among the parasite-specific CD8+ T cells, being related to the low parasite load found in the chronic phase of T. cruzi infection. Therefore, it seems that the central memory CD8+ T-cell population increases as the infection becomes chronic and this pool may be important to dynamically replace cells in the memory/effector T-cell pool, as previously suggested (Sallusto et al. 2010). Interestingly, treatment with benznidazole during the acute phase of the infection lowers the parasitemia and also induces a stable pool of central memory CD8+ T cells (Olivieri, Cotta-De-Almeida and Araujo-Jorge 2002; Bixby and Tarleton 2008). In addition, an increase in total effector/memory CD8+ T cells in T. cruzi-infected subjects has been reported (Leavey and Tarleton 2003; Fiuza et al. 2009). However, the authors also claimed that these cells would be dysfunctional and this could be a consequence of a gradual clonal exhaustion in the CD8+ T-cell population, perhaps as a result of continuous antigenic stimulation by persistent parasites (Leavey and Tarleton 2003). This study also showed an increase in the numbers of effector memory CD8+ T cells as the disease progresses, suggesting that the central memory T-cell pool could be, in fact, a source of effector memory T cells. Consequently, its depletion may worsen the disease by increasing tissue parasite load during the chronic phase of the infection, since the accumulation of effector/memory CD8 T cells would be less effective in controlling the infection because they would be functionally exhausted. However, in another series of experiments, resistance in the acute phase of murine T. cruzi infection correlated with higher percentages of effector/memory T cells prior to infection (Cardillo et al. 2002). This was the case for mature/aged or thymectomized mice, where T cells are submitted to homeostatic expansion due to thymic hypofunction and acquire memory/effector markers (Cardillo, Nomizo and Mengel 1998; Cardillo et al. 2002). In fact, the levels of effector/memory T cells could be inversely correlated with host susceptibility, since the higher the numbers of effector/memory T cells found in mature/aged or thymectomized mice, the lower their susceptibility (Cardillo et al. 1993, 2002). Therefore, it appears that effector/memory T cells are required to control infection, but a pool of central memory T cells is also important to replenish exhausted effector/memory T cells. The restricted availability of reagents to follow parasite specific T cells has hampered more detailed studies, aiming at the evaluation of memory T cells during the acute Chagas’ disease. However, after culturing mononuclear cells from chronic patients with T. cruzi extracts, we have found a preferential in vitro expansion of CD4 + Vβ5+ T cells. In addition, we have shown a decrease in Vβ5 expression in the CD4± T-cell population freshly isolated from acutely infected chagasic individuals, probably reflecting tissue redistribution rather than depletion, whereas CD4 + Vβ5+ T cells were found to be increased in a subset of chronic chagasic patients (Costa et al. 2000). As a whole, the results showed a differential Vβ-TCR usage in different stages of the disease, and that parasite antigens stimulate a portion of the T-cell repertoire with preferential usage of Vβ5-TCR. Therefore, CD4 + Vβ5+ T cells are a unique population of CD4 T cells to be analyzed in future studies, regarding the dynamics of memory T-cell formation in humans.
HOW (AUTO)IMMUNITY IS CONTROLLED DURING T. CRUZI INFECTION
As pointed out above, T. cruzi induces a strong immune response against its own components, but the infection also induces a measurable immune response to host self-antigens (dos Santos et al. 1992; Bonney and Engman 2008; Bonney and Engman 2015). Antigenic mimicry between parasite antigens and host antigens may underlie the reasons for the anti-host autoimmune response (Wood et al. 1982; Duranti et al. 1999; Cunha-Neto et al. 2006). However, antigenic mimicry and immune crossreactivity among parasite antigens and host antigens are not always deleterious and may even be beneficial to a balanced parasite/host relationship (Pontes-de-Carvalho et al. 2013; Massilamany, Gangaplara and Reddy 2015). Therefore, a malfunction of regulatory immune mechanisms may also be involved in the autoimmune responses during the infection (Cardillo et al. 1993, Cardillo, Nomizo and Mengel 1998; Mariano et al. 2008). It is debatable whether the autoimmune response found during the T. cruzi infection is actually the causative factor leading to organ damage during the chronic phase of the disease (Cunha-Neto et al. 2011). However, both the immune response to parasite antigens and host self-antigens are not dissociated and occur concomitantly (Gattass et al. 1988; Cunha-Neto et al. 2011), and therefore should both be considered as promoters of tissue lesion during infection. A similar condition is found in autoimmune inflammatory bowel diseases (IBD), which ameliorate with the use of antibiotics, suggesting a role for bacteria as an important factor for the disease initiation or persistence (Sokol 2014). Yet, in IBD there is a considerable overlap between immunity and autoimmunity (Cassinotti et al. 2014). This means that it is not just an infection per se that may trigger autoimmunity, but an infection that is inappropriately dealt with (Sester et al. 2015). Nevertheless, in most of the chronically T. cruzi-infected patients, a perfectly balanced immune response is achieved and pathology is never manifested (Rassi, Rassi and Marin-Neto 2010). In addition, it has been described that susceptibility to T. cruzi infection might reflect an overreactive host immune response that kills most of the susceptible individuals without being effective in the control of the parasite load (Nascimento et al. 2002; Cardillo et al. 2004). In any of the cases described above, regulatory mechanisms are at the core of the problem, either dealing with resistance/susceptibility in acute infection or health/disease during the chronic phase.
Many cells and molecules have being described to have regulatory/suppressor activity during T. cruzi infection (Cardillo et al. 1993; Lopes and Reis 1994; Abrahamsohn and Coffman 1995; Pinge-Filho et al. 1999; Cuervo et al. 2011). For instance, splenic adherent cells or macrophages were described to suppress T-cell responses in vitro, by the release of mediators such as prostaglandins (Pinge-Filho et al. 1999) and nitric oxide (Abrahamsohn and Coffman 1995). More recently, myeloid-derived suppressor cells (MDSCs) were claimed to be responsible for controlling or suppressing immune responses during acute T. cruzi infection (Cuervo et al. 2011; Goni, Alcaide and Fresno 2002; Arocena et al. 2013).
In another series of experiments, using mice of the BALB/c genetic background, we were the first to demonstrate that γδ T cells were involved in the suppression of immune responses during the acute phase of T. cruzi infection in vitro and in vivo (Cardillo et al. 1993; Cardillo, Nomizo and Mengel 1998). The in vivo depletion of γδ T cells by an anti-δ monoclonal antibody (Costa et al. 2015) raised the levels of IFN-γ produced by αβ T cells in the acute infection similarly to the in vivo blocking of IL-17 (Matta Guedes et al. 2010), and promoted the recovery of a third party humoral immune response to ovalbumin during the acute T. cruzi infection (Cardillo, Nomizo and Mengel 1998). The γδ T-cell suppressor activity was absent in the spleens of thymectomized and aged mice, suggesting that those cells were dependent upon an intact thymic function (Cardillo et al. 1993; Cardillo, Nomizo and Mengel 1998). Thymic output of naïve T cells clearly downmodulated effector responses, since the continuous administration of thymocytes to either aged mice, young thymectomized mice or total spleen cell-reconstituted athymic mice markedly decreased splenic cell proliferation to non-specific stimulation and increased parasitism in recipient T. cruzi-infected mice (Cardillo, Nomizo and Mengel 1998). More recently, one study described that T cells bearing the Vγ4 TCR chain were responsible for producing large amounts of IL-17, resulting in an increase of MDSCs that had the ability to downregulate pathogen-responsive T cells, contributing to parasite persistence (Kong et al. 2014). Additionally, these Vγ4 T cells are produced and exported by the thymus as IL-17 producers (Schmolka et al. 2013). Some γδ T-cell subpopulations, again through the production of IL-17, were also implicated in the augmentation of MDSC numbers in tumor microenvironments, either in mice or humans, promoting tumor growth by opposing cancer immunosurveillance (Rei et al. 2014; Wu et al. 2014). It should be pointed out that most, if not all, γδ T cells in lymphoid organs of the murine BALB/c background produced only IL-17 and no IFN-γ, in contrast to other mouse strains where these cytokines are produced by different γδ T-cell subpopulations (Wakita et al. 2010). Consequently, the overall function of γδ T cells during T. cruzi infection might be dependent on the genetic background where both T. cruzi and mouse strains should be considered. Therefore, it seems that a subpopulation of γδ T cells, producing high levels of IL-17, is the candidate to modulate the numbers of MDSCs that might be the final suppressor cells in the acute phase of T. cruzi infection.
It should be pointed out that the mechanisms described above are easily detected along the acute phase of the infection and vanish after parasite growth is controlled or during the chronic phase of the disease. Therefore, they are less likely to perform these regulatory functions during the chronic infection and in fact, there is no evidence that these mechanisms are operative after the infection is controlled.
The contribution of other regulatory cell populations such as Tr1 and Treg (CD4 + CD25 + Foxp3+) T cells to the immune response modulation, during the acute infection, is not clear yet. It has been shown that IL-10 may function to increase host survival and also to help in the control of parasite load in some models (Hunter et al. 1997; Roffe et al. 2012). However, the exact source of IL-10 has not been studied in detail. In addition, high levels of IL-10 were recently related to protection against cardiomyopathy in human subjects, indicating that this cytokine is of critical importance in the regulation of the immune response during T. cruzi infection (Dutra et al. 2014). The role of CD4 + CD25 + Foxp3+ Treg cells has also been evaluated and early mouse studies where these cells were depleted by monoclonal antibodies to CD25 indicated that their role in the regulation of immunity during the acute phase of the T. cruzi infection is rather limited (Kotner and Tarleton 2007; Sales et al. 2008). On the contrary, recent findings in humans have shown an increased percentage of Treg cells in chagasic subjects in the indeterminate chronic phase (free of disease) when compared to patients with heart damage, suggesting an important role for Tregs in Chagas disease (de Araujo et al. 2011). Moreover, it has been recently demonstrated, using a nondepleting monoclonal antibody to CD25, that regulatory CD4 + CD25 + Foxp3+ T cells may also help to control the adaptive immune response, during the acute infection, in mice (Nihei et al. 2014). The immunomodulatory activity of the nondepleting monoclonal antibody to CD25 was similar to that which has been described for humans (Huss et al. 2015) and encompassed a delayed increase of Treg frequencies and an augmented production of IL-10 and TNF-α by T cells. Interestingly, it was demonstrated that TNF-α levels are significantly higher in Chagas' disease patients with severe ventricular arrhythmias and in patients with dilated cardiomyopathy, suggesting that this cytokine could be detrimental to the heart (Ferreira et al. 2003). However, in one study, using the mouse model, in vivo blockade of TNF-α during the chronic phase of T. cruzi infection aggravated cardiomyopathy, suggesting that TNF-α would have a protective role (Bilate et al. 2007). In fact, it has been recently shown that TNF-α is cardioprotective in both mice and humans (Papathanasiou et al. 2015). In addition and more importantly, there was a clear indication that the functional activity of Treg cells might be of crucial importance during the acute and chronic phases of the infection, decreasing tissue destruction and pathology (Nihei et al. 2014; Bonney et al. 2015). Therefore, the notion concerning the manipulation of Treg cells either by antibodies or even interleukins such as IL-2 (Kosmaczewska 2014) might open up a new avenue for therapeutic strategies in Chagas' disease.
CONCLUDING REMARKS
The study of acute and chronic phases of infection with intracellular pathogens, such as T. cruzi, allows the elucidation of the mechanisms and conditions that may be targeted to reprogram the host immune system, either by using tools that interfere with components of the regulatory arm of the immune system machinery (Nihei et al. 2014) or by improving therapeutic vaccine strategies (Pereira et al. 2015). This knowledge would certainly result in a better understanding of the necessary balance to achieve or reestablish the health of the host during T. cruzi infection, thus providing new strategies to treat Chagas' disease, besides the use of drugs that kills the parasite in vivo, sterilizing the host—a difficult task to achieve because the available treatments are not always efficient, having many toxic collateral effects, so that clinical researchers have not reached a consensus about them after more than 30 years of their clinical use and in addition, parasite resistance to these drugs is common and well documented (Bestetti and Restini 2014; Molina, Salvador and Sanchez-Montalva 2014; Molina et al. 2014; Rassi, Rassi and Marin-Neto 2014; Zingales et al. 2015). Of note, the authors of a randomized, double-blind, placebo-controlled trial in which trypanocidal therapy with benznidazole was evaluated in patients with established Chagas' cardiomyopathy concluded that this type of treatment significantly reduced serum parasite detection but did not significantly reduce cardiac clinical deterioration through 5 years of follow-up (Morillo et al. 2015).
Acknowledgments
We thanks Drs Owen Williams and Lain Pontes de Carvalho for their critical review of the manuscript.
FUNDING
This work was supported by CNPq, PAPES/FIOCRUZ and FOG-FMP/FASE. PRZA is granted with CNPq and FAPERJ research fellowships (PQ-2 and JCNE, respectively).
Conflict of interest. None declared.
REFERENCES
- Abrahamsohn IA, Coffman RL. Cytokine and nitric oxide regulation of the immunosuppression in Trypanosoma cruzi infection. J Immunol. 1995;155:3955–63. [PubMed] [Google Scholar]
- Albareda MC, Laucella SA, Alvarez MG, et al. Trypanosoma cruzi modulates the profile of memory CD8+ T cells in chronic Chagas' disease patients. Int Immunol. 2006;18:465–71. doi: 10.1093/intimm/dxh387. [DOI] [PubMed] [Google Scholar]
- Amezcua Vesely MC, Bermejo DA, Montes CL, et al. B-Cell Response during Protozoan Parasite Infections. J Parasitol Res. 2012;2012:362131. doi: 10.1155/2012/362131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade DV, Gollob KJ, Dutra WO. Acute Chagas disease: new global challenges for an old neglected disease. PLoS Neglect Trop D. 2014;8:e3010. doi: 10.1371/journal.pntd.0003010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arocena AR, Onofrio LI, Pellegrini AV, et al. Myeloid-derived suppressor cells are key players in the resolution of inflammation during a model of acute infection. Eur J Immunol. 2013;44:184–94. doi: 10.1002/eji.201343606. [DOI] [PubMed] [Google Scholar]
- Azuara V, Grigoriadou K, Lembezat MP, et al. Strain-specific TCR repertoire selection of IL-4-producing Thy-1 dull gamma delta thymocytes. Eur J Immunol. 2001;31:205–14. doi: 10.1002/1521-4141(200101)31:1<205::AID-IMMU205>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Azuara V, Lembezat MP, Pereira P. The homogeneity of the TCRdelta repertoire expressed by the Thy-1dull gammadelta T cell population is due to cellular selection. Eur J Immunol. 1998;28:3456–67. doi: 10.1002/(SICI)1521-4141(199811)28:11<3456::AID-IMMU3456>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Azuara V, Levraud JP, Lembezat MP, et al. A novel subset of adult gamma delta thymocytes that secretes a distinct pattern of cytokines and expresses a very restricted T cell receptor repertoire. Eur J Immunol. 1997;27:544–53. doi: 10.1002/eji.1830270228. [DOI] [PubMed] [Google Scholar]
- Azuara V, Pereira P. Genetic mapping of two murine loci that influence the development of IL-4-producing Thy-1dull gamma delta thymocytes. J Immunol. 2000;165:42–8. doi: 10.4049/jimmunol.165.1.42. [DOI] [PubMed] [Google Scholar]
- Bermejo DA, Jackson SW, Gorosito-Serran M, et al. Trypanosoma cruzi trans-sialidase initiates a program independent of the transcription factors RORgammat and Ahr that leads to IL-17 production by activated B cells. Nat Immunol. 2013;14:514–22. doi: 10.1038/ni.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestetti RB, Restini CB. Posaconazole versus benznidazole for chronic Chagas' disease. New Engl J Med. 2014;371:966. doi: 10.1056/NEJMc1407914. [DOI] [PubMed] [Google Scholar]
- Bilate AM, Salemi VM, Ramires FJ, et al. TNF blockade aggravates experimental chronic Chagas disease cardiomyopathy. Microbes Infect. 2007;9:1104–13. doi: 10.1016/j.micinf.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Bixby LM, Tarleton RL. Stable CD8+ T cell memory during persistent Trypanosoma cruzi infection. J Immunol. 2008;181:2644–50. doi: 10.4049/jimmunol.181.4.2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonney KM, Engman DM. Chagas heart disease pathogenesis: one mechanism or many? Curr Mol Med. 2008;8:510–8. doi: 10.2174/156652408785748004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonney KM, Engman DM. Autoimmune pathogenesis of Chagas heart disease: looking back, looking ahead. Am J Pathol. 2015;185:1537–47. doi: 10.1016/j.ajpath.2014.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonney KM, Taylor JM, Thorp EB, et al. Depletion of regulatory T cells decreases cardiac parasitosis and inflammation in experimental Chagas disease. Parasitol Res. 2015;114:1167–78. doi: 10.1007/s00436-014-4300-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustamante JM, Bixby LM, Tarleton RL. Drug-induced cure drives conversion to a stable and protective CD8+ T central memory response in chronic Chagas disease. Nat Med. 2008;14:542–50. doi: 10.1038/nm1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardillo F, Cunha FQ, Tamashiro WM, et al. NK1.1+ cells and T-cell activation in euthymic and thymectomized C57Bl/6 mice during acute Trypanosoma cruzi infection. Scand J Immunol. 2002;55:96–104. doi: 10.1046/j.1365-3083.2002.01034.x. [DOI] [PubMed] [Google Scholar]
- Cardillo F, Falcao RP, Rossi MA, et al. An age-related gamma delta T cell suppressor activity correlates with the outcome of autoimmunity in experimental Trypanosoma cruzi infection. Eur J Immunol. 1993;23:2597–605. doi: 10.1002/eji.1830231033. [DOI] [PubMed] [Google Scholar]
- Cardillo F, Nomizo A, Mengel J. The role of the thymus in modulating gammadelta T cell suppressor activity during experimental Trypanosoma cruzi infection. Int Immunol. 1998;10:107–16. doi: 10.1093/intimm/10.2.107. [DOI] [PubMed] [Google Scholar]
- Cardillo F, Nomizo A, Postol E, et al. NK1.1 cells are required to control T cell hyperactivity during Trypanosoma cruzi infection. Med Sci Monitor. 2004;10:BR259–67. [PubMed] [Google Scholar]
- Cardillo F, Postol E, Nihei J, et al. B cells modulate T cells so as to favour T helper type 1 and CD8+ T-cell responses in the acute phase of Trypanosoma cruzi infection. Immunology. 2007;122:584–95. doi: 10.1111/j.1365-2567.2007.02677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardillo F, Voltarelli JC, Reed SG, et al. Regulation of Trypanosoma cruzi infection in mice by gamma interferon and interleukin 10: role of NK cells. Infect Immun. 1996;64:128–34. doi: 10.1128/iai.64.1.128-134.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassinotti A, Sarzi-Puttini P, Fichera M, et al. Immunity, autoimmunity and inflammatory bowel disease. Autoimmun Rev. 2014;13:1–2. doi: 10.1016/j.autrev.2013.06.007. [DOI] [PubMed] [Google Scholar]
- Chien YH, Hampl J. Antigen-recognition properties of murine gamma delta T cells. Springer Semin Immun. 2000;22:239–50. doi: 10.1007/pl00006752. [DOI] [PubMed] [Google Scholar]
- Chien YH, Meyer C, Bonneville M. gammadelta T cells: first line of defense and beyond. Annu Rev Immunol. 2014;32:121–55. doi: 10.1146/annurev-immunol-032713-120216. [DOI] [PubMed] [Google Scholar]
- Costa MF, de Negreiros CB, Bornstein VU, et al. Murine IL-17+ Vgamma4 T lymphocytes accumulate in the lungs and play a protective role during severe sepsis. BMC Immunol. 2015;16:36. doi: 10.1186/s12865-015-0098-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa RP, Gollob KJ, Fonseca LL, et al. T-cell repertoire analysis in acute and chronic human Chagas' disease: differential frequencies of Vbeta5 expressing T cells. Scand J Immunol. 2000;51:511–9. doi: 10.1046/j.1365-3083.2000.00706.x. [DOI] [PubMed] [Google Scholar]
- Cuervo H, Guerrero NA, Carbajosa S, et al. Myeloid-derived suppressor cells infiltrate the heart in acute Trypanosoma cruzi infection. J Immunol. 2011;187:2656–65. doi: 10.4049/jimmunol.1002928. [DOI] [PubMed] [Google Scholar]
- Cunha-Neto E, Bilate AM, Hyland KV, et al. Induction of cardiac autoimmunity in Chagas heart disease: a case for molecular mimicry. Autoimmunity. 2006;39:41–54. doi: 10.1080/08916930500485002. [DOI] [PubMed] [Google Scholar]
- Cunha-Neto E, Teixeira PC, Nogueira LG, et al. Autoimmunity. Adv Parasitol. 2011;76:129–52. doi: 10.1016/B978-0-12-385895-5.00006-2. [DOI] [PubMed] [Google Scholar]
- da Costa TA, Silva MV, Mendes MT, et al. Immunomodulation by Trypanosoma cruzi: toward understanding the association of dendritic cells with infecting TcI and TcII populations. J Immunol Res. 2014;2014:962047. doi: 10.1155/2014/962047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Araujo FF, Vitelli-Avelar DM, Teixeira-Carvalho A, et al. Regulatory T cells phenotype in different clinical forms of Chagas' disease. PLoS Neglect Trop D. 2011;5:e992. doi: 10.1371/journal.pntd.0000992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Matta Guedes PM, Gutierrez FR, Maia FL, et al. IL-17 produced during Trypanosoma cruzi infection plays a central role in regulating parasite-induced myocarditis PLoS Neglect Trop D 2010. 4 e604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- dos Santos RR, Rossi MA, Laus JL, et al. Anti-CD4 abrogates rejection and reestablishes long-term tolerance to syngeneic newborn hearts grafted in mice chronically infected with Trypanosoma cruzi. J Exp Med. 1992;175:29–39. doi: 10.1084/jem.175.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duranti MA, Franzoni L, Sartor G, et al. Trypanosoma cruzi: conformational preferences of antigenic peptides bearing the immunodominant epitope of the B13 antigen. Exp Parasitol. 1999;93:38–44. doi: 10.1006/expr.1999.4428. [DOI] [PubMed] [Google Scholar]
- Duthie MS, Kahn M, White M, et al. Both CD1d antigen presentation and interleukin-12 are required to activate natural killer T cells during Trypanosoma cruzi infection. Infect Immun. 2005a;73:1890–4. doi: 10.1128/IAI.73.3.1890-1894.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duthie MS, Kahn M, White M, et al. Critical proinflammatory and anti-inflammatory functions of different subsets of CD1d-restricted natural killer T cells during Trypanosoma cruzi infection. Infect Immun. 2005b;73:181–92. doi: 10.1128/IAI.73.1.181-192.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duthie MS, Wleklinski-Lee M, Smith S, et al. During Trypanosoma cruzi infection CD1d-restricted NK T cells limit parasitemia and augment the antibody response to a glycophosphoinositol-modified surface protein. Infect Immun. 2002;70:36–48. doi: 10.1128/IAI.70.1.36-48.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutra WO, Menezes CA, Magalhaes LM, et al. Immunoregulatory networks in human Chagas disease. Parasite Immunol. 2014;36:377–87. doi: 10.1111/pim.12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fares RC, Correa-Oliveira R, de Araujo FF, et al. Identification of phenotypic markers of B cells from patients with Chagas disease. Parasite Immunol. 2013;35:214–23. doi: 10.1111/pim.12038. [DOI] [PubMed] [Google Scholar]
- Ferreira RC, Ianni BM, Abel LC, et al. Increased plasma levels of tumor necrosis factor-alpha in asymptomatic/‘indeterminate’ and Chagas disease cardiomyopathy patients. Mem I Oswaldo Cruz. 2003;98:407–11. doi: 10.1590/s0074-02762003000300021. [DOI] [PubMed] [Google Scholar]
- Fiuza JA, Fujiwara RT, Gomes JA, et al. Profile of central and effector memory T cells in the progression of chronic human Chagas disease. PLoS Neglect Trop D. 2009;3:e512. doi: 10.1371/journal.pntd.0000512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli G, Nuti S, Tavarini S, et al. CD1d-restricted help to B cells by human invariant natural killer T lymphocytes. J Exp Med. 2003;197:1051–7. doi: 10.1084/jem.20021616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli G, Pittoni P, Tonti E, et al. Invariant NKT cells sustain specific B cell responses and memory. P Natl Acad Sci USA. 2007;104:3984–9. doi: 10.1073/pnas.0700191104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattass CR, Lima MT, Nobrega AF, et al. Do self-heart-reactive T cells expand in Trypanosoma cruzi-immune hosts? Infect Immun. 1988;56:1402–5. doi: 10.1128/iai.56.5.1402-1405.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goni O, Alcaide P, Fresno M. Immunosuppression during acute Trypanosoma cruzi infection: involvement of Ly6G (Gr1(+))CD11b(+)immature myeloid suppressor cells. Int Immunol. 2002;14:1125–34. doi: 10.1093/intimm/dxf076. [DOI] [PubMed] [Google Scholar]
- Hunter CA, Ellis-Neyes LA, Slifer T, et al. IL-10 is required to prevent immune hyperactivity during infection with Trypanosoma cruzi. J Immunol. 1997;158:3311–6. [PubMed] [Google Scholar]
- Huss DJ, Mehta DS, Sharma A, et al. In vivo maintenance of human regulatory T cells during CD25 blockade. J Immunol. 2015;194:84–92. doi: 10.4049/jimmunol.1402140. [DOI] [PubMed] [Google Scholar]
- Koberle F. Chagas' disease and Chagas' syndromes: the pathology of American trypanosomiasis. Adv Parasitol. 1968;6:63–116. doi: 10.1016/s0065-308x(08)60472-8. [DOI] [PubMed] [Google Scholar]
- Kong X, Sun R, Chen Y, et al. gammadeltaT cells drive myeloid-derived suppressor cell-mediated CD8+ T cell exhaustion in hepatitis B virus-induced immunotolerance. J Immunol. 2014;193:1645–53. doi: 10.4049/jimmunol.1303432. [DOI] [PubMed] [Google Scholar]
- Kosmaczewska A. Low-dose interleukin-2 therapy: a driver of an imbalance between immune tolerance and autoimmunity. Int J Mol Sci. 2014;15:18574–92. doi: 10.3390/ijms151018574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotner J, Tarleton R. Endogenous CD4(+) CD25(+) regulatory T cells have a limited role in the control of Trypanosoma cruzi infection in mice. Infect Immun. 2007;75:861–9. doi: 10.1128/IAI.01500-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzavecchia A, Sallusto F. Regulation of T cell immunity by dendritic cells. Cell. 2001;106:263–6. doi: 10.1016/s0092-8674(01)00455-x. [DOI] [PubMed] [Google Scholar]
- Leavey JK, Tarleton RL. Cutting edge: dysfunctional CD8+ T cells reside in nonlymphoid tissues during chronic Trypanosoma cruzi infection. J Immunol. 2003;170:2264–8. doi: 10.4049/jimmunol.170.5.2264. [DOI] [PubMed] [Google Scholar]
- Locksley RM, Scott P. Helper T-cell subsets in mouse leishmaniasis: induction, expansion and effector function. Immunol Today. 1991;12:A58–61. doi: 10.1016/S0167-5699(05)80017-9. [DOI] [PubMed] [Google Scholar]
- Lopes MF, dos Reis GA. Trypanosoma cruzi-induced immunosuppression: blockade of costimulatory T-cell responses in infected hosts due to defective T-cell receptor-CD3 functioning. Infect Immun. 1994;62:1484–8. doi: 10.1128/iai.62.4.1484-1488.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariano FS, Gutierrez FR, Pavanelli WR, et al. The involvement of CD4 + CD25+ T cells in the acute phase of Trypanosoma cruzi infection. Microbes Infect. 2008;10:825–33. doi: 10.1016/j.micinf.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Massilamany C, Gangaplara A, Reddy J. Environmental microbes and uveitis: is microbial exposure always bad? Scand J Immunol. 2015;81:469–75. doi: 10.1111/sji.12297. [DOI] [PubMed] [Google Scholar]
- Mengel J, Dare L, Dare GM, et al. An activated murine B cell lymphoma line (A-20) produces a factor-like activity which is functionally related to human natural killer cell stimulatory factor. Eur J Immunol. 1992;22:3173–8. doi: 10.1002/eji.1830221222. [DOI] [PubMed] [Google Scholar]
- Mengel JO, Rossi MA. Chronic chagasic myocarditis pathogenesis: dependence on autoimmune and microvascular factors. Am Heart J. 1992;124:1052–7. doi: 10.1016/0002-8703(92)90991-4. [DOI] [PubMed] [Google Scholar]
- Miyahira Y, Katae M, Takeda K, et al. Activation of natural killer T cells by alpha-galactosylceramide impairs DNA vaccine-induced protective immunity against Trypanosoma cruzi. Infect Immun. 2003;71:1234–41. doi: 10.1128/IAI.71.3.1234-1241.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyahira Y. Trypanosoma cruzi infection from the view of CD8+ T cell immunity–an infection model for developing T cell vaccine. Parasitol Int. 2008;57:38–48. doi: 10.1016/j.parint.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Molina I, Gomez i Prat J, Salvador F, et al. Randomized trial of posaconazole and benznidazole for chronic Chagas' disease. New Engl J Med. 2014;370:1899–908. doi: 10.1056/NEJMoa1313122. [DOI] [PubMed] [Google Scholar]
- Molina I, Salvador F, Sanchez-Montalva A. Posaconazole versus benznidazole for chronic Chagas' disease. New Engl J Med. 2014;371:966. doi: 10.1056/NEJMc1407914. [DOI] [PubMed] [Google Scholar]
- Morillo CA, Marin-Neto JA, Avezum A, et al. Randomized trial of benznidazole for chronic Chagas' cardiomyopathy. N Engl J Med. 2015;373:1295–306. doi: 10.1056/NEJMoa1507574. [DOI] [PubMed] [Google Scholar]
- Nascimento FR, Calich VL, Rodriguez D, et al. Dual role for nitric oxide in paracoccidioidomycosis: essential for resistance, but overproduction associated with susceptibility. J Immunol. 2002;168:4593–600. doi: 10.4049/jimmunol.168.9.4593. [DOI] [PubMed] [Google Scholar]
- Nihei J, Cardillo F, Dos Santos WL, et al. Administration of a nondepleting anti-CD25 monoclonal antibody reduces disease severity in mice infected with Trypanosoma cruzi. Eur J Microbiol Immunol. 2014;4:128–37. doi: 10.1556/EuJMI.4.2014.2.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomizo A, Cardillo F, Postol E, et al. V gamma 1 gammadelta T cells regulate type-1/type-2 immune responses and participate in the resistance to infection and development of heart inflammation in Trypanosoma cruzi-infected BALB/c mice. Microbes Infect. 2006;8:880–8. doi: 10.1016/j.micinf.2005.10.012. [DOI] [PubMed] [Google Scholar]
- O'Garra A, Stapleton G, Dhar V, et al. Production of cytokines by mouse B cells: B lymphomas and normal B cells produce interleukin 10. Int Immunol. 1990;2:821–32. doi: 10.1093/intimm/2.9.821. [DOI] [PubMed] [Google Scholar]
- Olivieri BP, Cotta-De-Almeida V, Araujo-Jorge T. Benznidazole treatment following acute Trypanosoma cruzi infection triggers CD8+ T-cell expansion and promotes resistance to reinfection. Antimicrob Agents Ch. 2002;46:3790–6. doi: 10.1128/AAC.46.12.3790-3796.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang Y, Norihisa Y, Benjamin D, et al. Interferon-gamma gene expression in human B-cell lines: induction by interleukin-2, protein kinase C activators, and possible effect of hypomethylation on gene regulation. Blood. 1992;80:724–32. [PubMed] [Google Scholar]
- Papathanasiou S, Rickelt S, Soriano ME, et al. Tumor necrosis factor-alpha confers cardioprotection through ectopic expression of keratins K8 and K18. Nat Med. 2015;21:1076–84. doi: 10.1038/nm.3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira IR, Vilar-Pereira G, Marques V, et al. A human type 5 adenovirus-based Trypanosoma cruzi therapeutic vaccine re-programs immune response and reverses chronic cardiomyopathy. PLoS Pathog. 2015;11:e1004594. doi: 10.1371/journal.ppat.1004594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinge-Filho P, Tadokoro CE, Abrahamsohn IA. Prostaglandins mediate suppression of lymphocyte proliferation and cytokine synthesis in acute Trypanosoma cruzi infection. Cell Immunol. 1999;193:90–8. doi: 10.1006/cimm.1999.1463. [DOI] [PubMed] [Google Scholar]
- Pinho RT, da Silva WS, de Castro Cortes LM, et al. Production of MMP-9 and inflammatory cytokines by Trypanosoma cruzi-infected macrophages. Exp Parasitol. 2014;147:72–80. doi: 10.1016/j.exppara.2014.09.003. [DOI] [PubMed] [Google Scholar]
- Poncini CV, Alba Soto CD, Batalla E, et al. Trypanosoma cruzi induces regulatory dendritic cells in vitro. Infect Immun. 2008;76:2633–41. doi: 10.1128/IAI.01298-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poncini CV, Ilarregui JM, Batalla EI, et al. Trypanosoma cruzi infection imparts a regulatory program in dendritic cells and T cells via galectin-1-dependent mechanisms. J Immunol. 2015;195:3311–24. doi: 10.4049/jimmunol.1403019. [DOI] [PubMed] [Google Scholar]
- Pontes-de-Carvalho L, Mengel J, Figueiredo CA, et al. Antigen mimicry between infectious agents and self or environmental antigens may lead to long-term regulation of inflammation. Front Immunol. 2013;4:314. doi: 10.3389/fimmu.2013.00314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poveda C, Fresno M, Girones N, et al. Cytokine profiling in Chagas disease: towards understanding the association with infecting Trypanosoma cruzi discrete typing units (a BENEFIT TRIAL sub-study) PLoS One. 2014;9:e91154. doi: 10.1371/journal.pone.0091154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Procopio DO, Almeida IC, Torrecilhas AC, et al. Glycosylphosphatidylinositol-anchored mucin-like glycoproteins from Trypanosoma cruzi bind to CD1d but do not elicit dominant innate or adaptive immune responses via the CD1d/NKT cell pathway. J Immunol. 2002;169:3926–33. doi: 10.4049/jimmunol.169.7.3926. [DOI] [PubMed] [Google Scholar]
- Ramarathinam L, Niesel DW, Klimpel GR. Salmonella typhimurium induces IFN-gamma production in murine splenocytes. Role of natural killer cells and macrophages. J Immunol. 1993;150:3973–81. [PubMed] [Google Scholar]
- Rampazo EV, Amorim KN, Yamamoto MM, et al. Antigen targeting to dendritic cells allows the identification of a CD4 T-cell epitope within an immunodominant Trypanosoma cruzi antigen. PLoS One. 2015;10:e0117778. doi: 10.1371/journal.pone.0117778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassi A, Jr, Rassi A, Marin-Neto JA. Chagas disease. Lancet. 2010;375:1388–402. doi: 10.1016/S0140-6736(10)60061-X. [DOI] [PubMed] [Google Scholar]
- Rassi A, Rassi A, Jr, Marin-Neto JA. Posaconazole versus benznidazole for chronic Chagas' disease. New Engl J Med. 2014;371:965. doi: 10.1056/NEJMc1407914. [DOI] [PubMed] [Google Scholar]
- Rei M, Goncalves-Sousa N, Lanca T, et al. Murine CD27(-) Vgamma6(+) gammadelta T cells producing IL-17A promote ovarian cancer growth via mobilization of protumor small peritoneal macrophages. P Natl Acad Sci USA. 2014;111:E3562–70. doi: 10.1073/pnas.1403424111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezende-Oliveira K, Sarmento RR, Rodrigues V., Jr Production of cytokine and chemokines by human mononuclear cells and whole blood cells after infection with Trypanosoma cruzi. Rev Soc Bras Med Tro. 2012;45:45–50. doi: 10.1590/s0037-86822012000100009. [DOI] [PubMed] [Google Scholar]
- Roffe E, Rothfuchs AG, Santiago HC, et al. IL-10 limits parasite burden and protects against fatal myocarditis in a mouse model of Trypanosoma cruzi infection. J Immunol. 2012;188:649–60. doi: 10.4049/jimmunol.1003845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sales PA, Jr, Golgher D, Oliveira RV, et al. The regulatory CD4 + CD25+ T cells have a limited role on pathogenesis of infection with Trypanosoma cruzi. Microbes Infect. 2008;10:680–8. doi: 10.1016/j.micinf.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Lanzavecchia A, Araki K, et al. From vaccines to memory and back. Immunity. 2010;33:451–63. doi: 10.1016/j.immuni.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathler-Avelar R, Lemos EM, Reis DD, et al. Phenotypic features of peripheral blood leucocytes during early stages of human infection with Trypanosoma cruzi. Scand J Immunol. 2003;58:655–63. doi: 10.1111/j.1365-3083.2003.01340.x. [DOI] [PubMed] [Google Scholar]
- Schmolka N, Serre K, Grosso AR, et al. Epigenetic and transcriptional signatures of stable versus plastic differentiation of proinflammatory gammadelta T cell subsets. Nat Immunol. 2013;14:1093–100. doi: 10.1038/ni.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sester DP, Sagulenko V, Thygesen SJ, et al. Deficient NLRP3 and AIM2 inflammasome function in autoimmune NZB Mice. J Immunol. 2015;195:1233–41. doi: 10.4049/jimmunol.1402859. [DOI] [PubMed] [Google Scholar]
- Sher A, Oswald IP, Hieny S, et al. Toxoplasma gondii induces a T-independent IFN-gamma response in natural killer cells that requires both adherent accessory cells and tumor necrosis factor-alpha. J Immunol. 1993;150:3982–9. [PubMed] [Google Scholar]
- Sokol H. Probiotics and antibiotics in IBD. Dig Dis. 2014;32(Suppl 1):10–7. doi: 10.1159/000367820. [DOI] [PubMed] [Google Scholar]
- Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- Sullivan NL, Eickhoff CS, Sagartz J, et al. Deficiency of antigen-specific B cells results in decreased Trypanosoma cruzi systemic but not mucosal immunity due to CD8 T cell exhaustion. J Immunol. 2015;194:1806–18. doi: 10.4049/jimmunol.1303163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Strober S. Natural killer T cells and innate immune B cells from lupus-prone NZB/W mice interact to generate IgM and IgG autoantibodies. Eur J Immunol. 2008;38:156–65. doi: 10.1002/eji.200737656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umezawa ES, Stolf AM, Corbett CE, et al. Chagas' disease. Lancet. 2001;357:797–9. doi: 10.1016/S0140-6736(00)04174-X. [DOI] [PubMed] [Google Scholar]
- Veras PS, Welby-Borges M, de Santana CD, et al. Leishmania amazonensis: participation of regulatory T and B cells in the in vitro priming (PIV) of CBA/J spleen cells susceptible response. Exp Parasitol. 2006;113:201–5. doi: 10.1016/j.exppara.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Wakita D, Sumida K, Iwakura Y, et al. Tumor-infiltrating IL-17-producing gammadelta T cells support the progression of tumor by promoting angiogenesis. Eur J Immunol. 2010;40:1927–37. doi: 10.1002/eji.200940157. [DOI] [PubMed] [Google Scholar]
- Werner JM, Busl E, Farkas SA, et al. DX5 + NKT cells display phenotypical and functional differences between spleen and liver as well as NK1.1-Balb/c and NK1.1+ C57Bl/6 mice. BMC Immunol. 2011;12:26. doi: 10.1186/1471-2172-12-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MA, Holmes BJ, Sun JC, et al. Developing and maintaining protective CD8+ memory T cells. Immunol Rev. 2006;211:146–53. doi: 10.1111/j.0105-2896.2006.00389.x. [DOI] [PubMed] [Google Scholar]
- Wojciechowski W, Harris DP, Sprague F, et al. Cytokine-producing effector B cells regulate type 2 immunity to H. polygyrus. Immunity. 2009;30:421–33. doi: 10.1016/j.immuni.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JN, Hudson L, Jessell TM, et al. A monoclonal antibody defining antigenic determinants on subpopulations of mammalian neurones and Trypanosoma cruzi parasites. Nature. 1982;296:34–8. doi: 10.1038/296034a0. [DOI] [PubMed] [Google Scholar]
- Wu P, Wu D, Ni C, et al. gammadeltaT17 cells promote the accumulation and expansion of myeloid-derived suppressor cells in human colorectal cancer. Immunity. 2014;40:785–800. doi: 10.1016/j.immuni.2014.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zingales B, Araujo RG, Moreno M, et al. A novel ABCG-like transporter of Trypanosoma cruzi is involved in natural resistance to benznidazole. Mem I Oswaldo Cruz. 2015;110:433–44. doi: 10.1590/0074-02760140407. [DOI] [PMC free article] [PubMed] [Google Scholar]


