Abstract
Bacillus anthracis, the causative agent of anthrax, is acquired by mammalian hosts from the environment, as quiescent endospores. These endospores must germinate inside host cells, forming vegetative bacilli, before they can express the virulence factors that enable them to evade host defenses and disseminate throughout the body. While the role of macrophages and dendritic cells in this initial interaction has been established, the role of polymorphonuclear leukocytes (PMNs) has not been adequately defined. We discovered that while B. anthracis 34F2 Sterne endospores germinate poorly within non-activated human PMNs, these phagocytes exhibit rapid microbicidal activity toward the outgrown vegetative bacilli, independent of superoxide and nitric oxide. These findings suggest that a non-free radical pathway kills B. anthracis bacilli. We also find in PMNs an autophagic mechanism of bacterial killing based on the rapid induction of LC-3 conversion, beclin-1 expression, sequestosome 1 (SQSTM1) degradation and inhibition of bactericidal activity by the inhibitor, 3-methyladenine. These findings extend to PMNs an autophagic bactericidal mechanism previously described for other phagocytes.
Keywords: neutrophils, B. anthracis, phagocytosis, autophagy
Human neutrophils kill Bacillus anthracis by an autophagic mechanism.
Graphical Abstract Figure.
Human neutrophils kill Bacillus anthracis by an autophagic mechanism.
INTRODUCTION
Polymorphonuclear leukocytes (PMNs) are considered the ‘first responders’ to bacterial invasion with the responsibility of containing the incipient infection by initially ingesting and then killing the microbe (Mumy and McCormick 2009; Borregaard 2010). In the case of inhalational Bacillus anthracis, however, the role of PMNs is poorly understood. The current hypothesis is that alveolar macrophages and pulmonary dendritic cells ingest inhaled B. anthracis endospores, the environmentally acquired form of the pathogen (Cleret et al. 2007; Cote, Welkos and Bozue 2011; Sweeney et al. 2011). These cells then carry the ingested endospores to the mediastinal lymph nodes, where endospores germinate into vegetative bacilli that express the various virulence factors of B. anthracis. These outgrown bacilli can then disseminate throughout the host. In this scenario, PMNs would not appear to be critical in controlling the initial infection.
Although there have been a number of studies exploring the role of PMNs in B. anthracis infection (O'Brien et al. 1985; Wade et al. 1985; Crawford et al. 2006; During et al. 2005; Mayer-Scholl et al. 2005; Cote, Van Rooijen and Welkos 2006; Barson et al. 2008; van Sorge et al. 2008; Xu, Fang and Frucht 2008; Szarowicz et al. 2009; Moayeri et al. 2010; Nguyen et al. 2012), it still is not clear what role these cells might play upon initial exposure to inhalation of B. anthracis endospores. In vivo studies that examine the effect of depleting PMNs or using gene-deficient mice while leaving other potential host defenses intact cannot examine the direct interaction between B. anthracis endospores and PMNs to the exclusion of other host responses (Cote, Van Rooijen and Welkos 2006). Further, such experiments cannot establish whether B. anthracis endospores germinate within PMNs and if so, how these bacilli are killed (Cote, Van Rooijen and Welkos 2006; Sweeney et al. 2011). Finally, based on an earlier report (Cote, Van Rooijen and Welkos 2006), it is unclear whether PMNs play a prominent role in the initial or later stages of infection. In contrast, PMNs are critical to the resolution of inhalational anthrax (Garraud et al. 2012).
The few studies that examined PMN interaction with B. anthracis endospores or bacilli primarily described the effect of anthrax toxins on PMN function (During et al. 2005; Crawford et al. 2006; Barson et al. 2008). Since the toxins are expressed only after endospore germination, these studies presume either that the endospores have been taken up by PMNs and germinate therein or that germination occurred in other phagocytes, such as alveolar macrophages and/or dendritic cells. To date, however, we have uncovered only one paper that described the ability of human PMNs to ingest and kill B. anthracis endospores, perhaps by α-defensins, in pre-activated cells (Mayer-Scholl et al. 2005).
We have previously reported that murine macrophages are unable to kill endospores; however, when germination occurs, these phagocytes efficiently kill vegetative bacilli through a pathway that requires nitric oxide, but not superoxide (Kang et al. 2005; Raines et al. 2006). While some investigators have inferred that reactive oxygen species (ROS), e.g. superoxide, hydrogen peroxide, hydroxyl radical or hypochlorous acid, may play a significant role in PMN bactericidal activity toward B. anthracis (Cybulski et al. 2009), there have been few studies that directly tested this hypothesis. One such study found that superoxide dismutases (SODs) of B. anthracis assist this organism in evading superoxide-mediated toxicity (Passalacqua et al. 2006), while another observed B. anthracis killing in the presence of an inhibitor of the NADPH oxidase (Mayer-Scholl et al. 2005). Remarkably, we were unable to find any studies, which documented the ability of B. anthracis endospores or bacilli, when interacting with PMNs, to generate superoxide as well as other ROS derived from superoxide. Thus, the role of ROS in host defenses against exposure to B. anthracis requires further investigation. Consequently, we asked if (a) PMNs could ingest B. anthracis endospores, (b) if so, do these endospores germinate within PMNs; (c) if germination does occur, do PMNs kill the bacilli; and (d) if bacilli are killed, what mechanism is responsible for the observed bactericidal activity. We now report that although PMNs can ingest B. anthracis endospores, germination, and hence killing, is markedly delayed. In contrast, PMNs rapidly kill the bacillary form of this microbe. Our data demonstrate that killing is mediated neither by superoxide nor nitric oxide, but it appears that autophagy is the causative pathway for killing of these bacilli. These observations suggest that PMNs may have a more important role in host defenses against the vegetative bacilli that disseminate after escape from the site of germination in the regional lymph nodes than during the initial pulmonary infection.
MATERIALS AND METHODS
Reagents
Bacillus anthracis Sterne strain, 34F2, was originally obtained from Dr Les Baillie (Cardiff, Wales), and the germination-deficient ΔgerH strain of Sterne 34F2 was originally obtained from the laboratory of Dr Phillip Hanna (Ann Arbor, MI). SOD was purchased from ICN Biochemicals Inc. (Aurora, OH). Unless stated otherwise, all reagents including ferricytochrome c and catalase were purchased from Sigma-Aldrich (St. Louis, MO).
Isolation and preparation of human neutrophils
Neutrophils were obtained from the heparinized peripheral blood of healthy human donors under a protocol approved by the University of Maryland IRB and isolated as previously described (Cross and Wright 1991). Briefly, we combined Ficoll–Hypaque density gradient centrifugation and dextran sedimentation procedures followed by hypotonic lysis of residual erythrocytes. Purity of PMNs was assessed by Wright–Giemsa stain followed by microscopic analysis. The suspension were routinely >95% PMNs by light microscopy and >99% were viable as determined by Trypan blue exclusion. PMNs were suspended in HBSS buffer, counted and diluted according to experimental protocols.
Opsonophagocytosis assay
Aliquots of Sterne 34F2 endospores were kept at −80°C until use. For the phagocytosis assay, B. anthracis 34F2 Sterne endospores were first heated at 65°C for 30 min to eliminate vegetative bacilli before use. To generate vegetative bacilli, endospores were streaked on an LB agar plate overnight. A single colony from this culture was inoculated into fresh LB media overnight. The next day 100 μL of the overnight culture was inoculated into 10 mL of fresh LB media and grown to mid-log phase in LB media. The culture was centrifuged, washed and diluted in PBS to obtain the desired number of bacteria. Multiplicities of infection (bacilli/endospores to PMNs) of 1:1, 3:1, 5:1 were used in this assay. Bacillus anthracis bacilli were either opsonized at room temperature for 10 min with 10% normal human sera or left unopsonized. Approximately 1 × 106 human PMNs for each reaction were added to the endospores/bacilli and mixed gently in a 96 well microtiter plate. Ten microliters of this mixture from each well was removed with or without heating the endospores at 65°C for 30 min to differentiate between vegetative bacilli and endospores and plated to determine the number of endospores/bacilli at the outset. The rest of the reaction mixture was placed in a shaking incubator at 37°C for 10, 20, 30, 40 and 60 min. Then, 10 μL from each reaction was removed, similarly heated or not, diluted in sterile water and plated on an LB agar plate and the viable colonies counted.
Superoxide assay
The rate of superoxide production by PMNs was estimated using SOD-inhibitable reduction of ferricytochrome c, as previously described (Kuthan, Ullrich and Estabrook 1982). In a typical experiment, a cuvette contained PMNs (1 × 106 cells mL−1), catalase (300 U mL−1), ferricytochrome c (80 μM), without or with SOD (30 U mL−1) and phosphate buffer (50 mM) to a final volume of 1 mL (Bynoe et al. 1992). The reaction was initiated by the addition of either bacilli (1 × 106 cells mL−1) or endospores (1 × 106 cells mL−1) of B. anthracis to the cuvette and continuously monitored over 60 min at 37°C. As a control, PMNs (1 × 106 cells mL−1) were stimulated to produce superoxide by addition of PMA (100 ng mL−1) in the presence of catalase (300 U mL−1), ferricytochrome c (80 μM) and phosphate buffer (50 mM) to a final volume of 1 mL without or with SOD (30 U mL−1). The rate of superoxide production was estimated by following the reduction of ferricytochrome c at 550 nm at 37°C using an extinction coefficient of 21 mM−1 cm−1.
Nitrite determination
Since the immunological isoform of nitric oxide synthase (NOS2) can mediate the generation of not only nitric oxide but also superoxide, we assayed PMNs for the expression of this enzyme (Porasuphatana et al. 2010). Briefly, human PMNs and B. anthracis bacilli at an MOI of 1:1, 10:1 and 100:1 were incubated for 1 h at 37°C. The supernatants were collected and assayed for nitrite production using the Griess assay.
Western blot
NOS2 expression in human PMNs when stimulated with B. anthracis bacilli was determined by western blot using polyclonal goat anti-human NOS (Santa Cruz Biotechnology, Inc.). Human PMNs were infected with B. anthracis bacilli at an MOI of 1:1 for 1 h at 37°C. The mixture was then centrifuged, washed three times with PBS and the pellets were lysed to determine NOS2 production by western blot as previously described (Raines et al. 2006). As a positive control for NOS2 production, RAW macrophages were infected with B. anthracis 34F2 endospores for 8 h and the NOS2 production was determined by western blot.
Analysis of autophagy
Human neutrophils were treated with an inhibitor of autophagy, 3-methyladenine (3-MA, 0.5 μM or 5 μM) (Sigma-Aldrich) or saline for 30 min prior to infection with B. anthracis Sterne strain bacilli for 10–20 min. The cells were washed three times to remove uninternalized bacteria and suspended in HBSS with 50 μg mL−1 gentamicin with or without 3-MA (0.5 or 5 μM) and seeded at a density of 2–7 × 105 cells well−1 in a eight well-chamber slide (Nunc) pre-coated with fresh human sera. Cells were allowed to adhere to the wells for approximately 1–2 h before being fixed for 8 min at room temperature with 4% paraformaldehyde (USB Chemicals). Cells were then washed three times with PBS and blocked for 30 min in blocking buffer (1 × PBS + 0.03% Triton X-100 + 1% w/v BSA + 1%v/v donkey serum) and were incubated overnight at 4°C with 1:50 dilution of rabbit anti-LC3 antibody (Novus Biologicals) in the blocking buffer. The cells were washed three times for 5 min each with gentle agitation with washing buffer (1 × PBS + 0.03% Triton-X 100) and were incubated with a 1:100 dilution of donkey anti-rabbit antibody conjugated to Cy3 (Jackson Immunoresearch) for 1 h at room temperature. Cells were washed three times for 5 min with washing buffer and incubated for 5 min at room temperature with DAPI (5 nM) in PBS. The samples were washed three times for 5 min each with PBS and mounted using Vectashield mounting medium (Vector Labs). The samples were visualized using an Olympus confocal microscope (Model BX61) equipped with FluoView software. Cells showing a diffused pattern of Cy3 in the cytoplasm and nucleus were considered nonautophagic, while cells displaying intense Cy3 puncta aggregates were considered autophagic. The autophagic puncta were quantified using the NIH Image J software. For western blot analysis, neutrophils were lysed after treatments as indicated with RIPA buffer and proteins were resolved on 10% SDS-PAGE and transferred on to polyvinylidene difluoride membrane (Millipore Billerica, MA). The membrane was probed with 1:1000 dilution of rabbit beclin-1 (Cell Signaling Technology), LC3 (Novus Biologicals), mouse SQSTM1/p62 (Abcam) or actin (Sigma-Aldrich, Inc.), antibody overnight at 4°C. Blots were washed and probed with anti-rabbit or anti-mouse secondary antibodies tagged with Alexa Fluor (1:2000 dilutions) for 1 h at room temperature. LI-COR Odyssey Infrared Imaging system was used to image the blots as described earlier (Gade et al. 2012).
Statistical analysis
Student's t-test was performed using Prism software (GraphPad Software). A two-tailed P value of <0.05 was taken to indicate statistical significance.
RESULTS AND DISCUSSION
Bacillus anthracis 34F2 Sterne endospores germinate poorly inside neutrophils
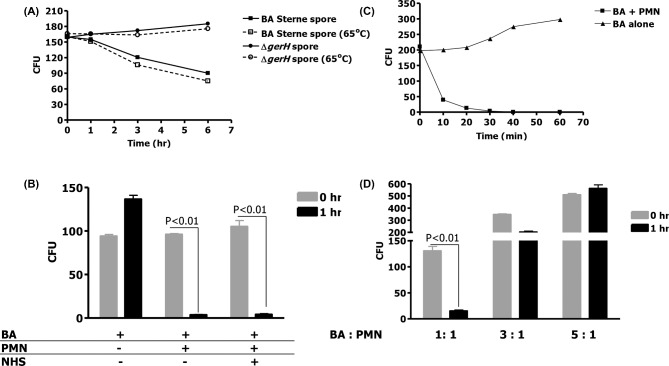
We have previously shown that B. anthracis endospores are phagocytosed by murine macrophage wherein germination occurs, resulting in bactericidal activity (Kang et al. 2005). In the absence of germination, there was no killing of endospores. We, therefore, explored whether the same observation is applicable for human PMNs. Bacillus anthracis 34F2 Sterne endospores and its isogenic germination-deficient mutant, ΔgerH, were pre-heated to 65°C to kill bacilli and then used to infect human PMNs at MOIs of 1:1, 3:1 and 5:1. Germination-proficient (BA 34F2) and -impaired (ΔgerH) Sterne strain endospores were internalized to a similar degree by human PMNs (Fig. 1A, time 0), and uptake was confirmed by light microscopy with Trypan blue exclusion (data not shown). The persistence of these endospores within human PMNs was followed with and without heat treatment of lysates to distinguish between the heat-resistant endospores and the heat-susceptible bacilli that may have resulted from endospore germination. After 1 h of incubation, no decrease in viable CFUs of either 34F2 Sterne or its mutant ΔgerH was observed (Fig. 1A). Further, there was no decrease in the number of ΔgerH in human PMNs for up to 6 h of the study suggesting that in the absence of germination, PMNs were unable to kill B. anthracis. (Fig. 1A). In contrast, at 6 h there was a ∼60% decrease in the number of the germination-proficient B. anthracis 34F2 (Fig. 1A). In each instance all of the B. anthracis recovered were heat resistant, indicating that all persistent bacterial isolates were endospores. Similar results were obtained when these assays were performed in the presence of gentamicin to kill any extracellular bacilli that may have germinated from the endospores (data not shown). The undiminished persistence of the ΔgerH endospores in PMNs is consistent with the hypothesis that human PMNs, like murine macrophages, are unable to rapidly kill B. anthracis endospores, with the reduction in number of the germination-proficient 34F2 endospores being likely due to the delayed germination of some of the endospores and subsequent killing of the outgrown bacilli. Thus, unlike the case with pre-activated PMNs (Mayer-Scholl et al. 2005), B. anthracis endospores germinate poorly within non-activated human PMN.
Figure 1.
(A) Bacillus anthracis endospores are taken up by human PMNs but do not germinate efficiently within. Human PMNs were incubated for 1, 3 and 6 h in the presence of B. anthracis endospores at an MOI of 1:1. Germination was determined by counting the colony-forming units at time 0 and after 1, 3 and 6 h with and without heating the endospores at 65°C. (B) Vegetative 34F2 B. anthracis were incubated for 1 h in the presence of human PMNs at an MOI of 1:1, either in the presence or absence of 10% normal human serum (NHS). (C) Vegetative 34F2 B. anthracis were incubated without NHS in the presence and absence of PMN (MOI 1:1) and samples obtained and counted at 10, 20 30, 40 and 60 min. (D) Vegetative 34F2 B. anthracis were incubated for 1 h in the presence of human PMNs at an MOI of 1:1, 3:1 or 5:1. Means and standard deviation of three different experiments are shown.
Human neutrophils kill vegetative B. anthracis Sterne strain
We next explored whether human PMNs can efficiently kill the vegetative form of B. anthracis. Thus, we challenged human PMNs with serum opsonized (10% human serum) or unopsonized B. anthracis bacilli at an MOI of 1:1 (Fig. 1B). After 1 h, bacteria killing was nearly complete (Fig. 1B). Surprisingly, opsonization of bacteria was not essential to effective phagocytic killing. In contrast to endospores, a time-course study found that in the absence of opsonization and with an MOI of 1:1, 41% killing of the original inoculum occurred within the first 10 min, the initial time point examined (Fig. 1C). After 20 min, 73% killing was noted, and nearly complete killing was observed at 1 h. Control experiments demonstrated the necessity of human PMNs in bacilli killing (Fig. 1B and C). At MOIs of 3:1 and 5:1, human PMNs were found to kill the bacilli less effectively (Fig. 1D). Again, opsonization had no effect on bacillary killing at these higher MOIs (data not shown). These findings confirm that while human PMNs are unable to kill endospores that have impaired germination, these phagocytes rapidly kill the vegetative form of B. anthracis, even in the absence of opsonins. Thus, the lack of decrease in the ΔgerH endospores (Fig. 1A) likely reflects their delayed germination during the 6 h of this assay.
Superoxide and nitric oxide generation by human neutrophils are not required for killing of B. anthracis
We investigated whether superoxide or nitric oxide is responsible for the observed microbicidal activity toward B. anthracis bacilli. Human PMNs were challenged with B. anthracis bacilli, at MOIs of 1:1–1:100. The rate of superoxide production was continuously monitored. However, we did not observe any production of superoxide, even at an MOI of 100, a level of bacteria likely to be achieved only with severe infection. As a positive control, PMNs, stimulated with PMA (100 ng mL−1), produced superoxide at a flux of 4.3 nmol min−1/1 × 106 cells. This suggests that while the PMNs are capable of generating a robust flux of superoxide upon stimulation, B. anthracis bacilli are not competent to do so even at an MOI of 1:100. As observed previously, after 1 h of incubation, there was 100% killing of B. anthracis bacilli by human PMN (Fig. 2A). As was previously reported (Mayer-Scholl et al. 2005), human PMNs incubated with an inhibitor of ROS formation, in this case SOD (30 U mL−1), killed B. anthracis almost as efficiently as untreated cells at the same MOI (Fig. 2A). Thus, even if small fluxes of superoxide that were below detection limits of the ferricytochrome c assay were produced, there is no evidence that this free radical mediated the killing of B. anthracis bacilli.
Figure 2.
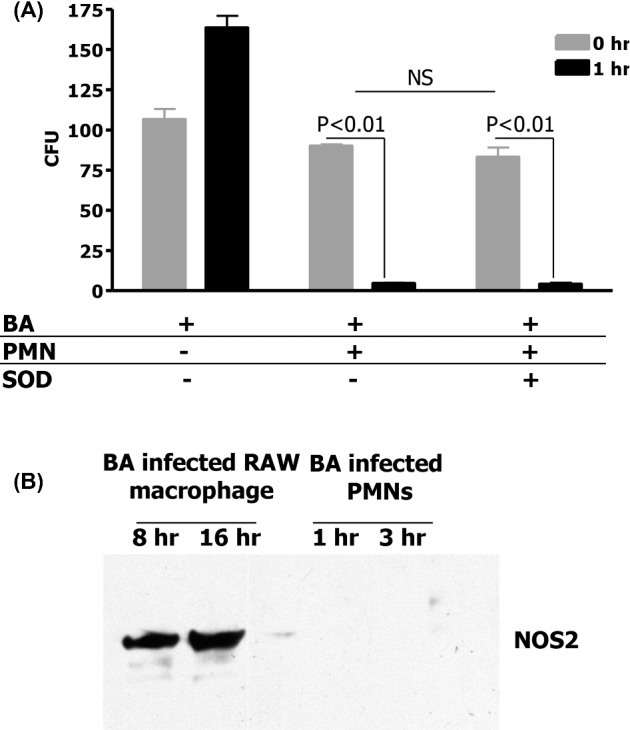
(A) Human PMNs do not kill B. anthracis through superoxide generation. To determine if B. anthracis is killed by human PMNs through superoxide generation, vegetative bacteria were incubated with human PMNs alone at an MOI of 1:1 or in the presence of SOD to inhibit superoxide generation. Means and standard deviation of three different experiments are shown. (B) Western blot of NOS2 expression in human PMN and RAW macrophages with B. anthracis infection.
It has been reported that human PMNs during inflammation upregulate NOS2 (Cedergren et al. 2003). While this enzyme generates nitric oxide (Nathan and Xie 1994), it can likewise produce superoxide, and other bactericidal oxidative products, such as hydrogen peroxide and peroxynitrite (Weaver et al. 2005). Nitric oxide, as estimated by nitrite measurement in our experimental paradigm, was not detected in supernatants of human PMNs infected with B. anthracis at MOIs even up to 1:100. Further, while B. anthracis bacilli induced the expression of NOS2 protein in a murine macrophage cell line (Raines et al. 2006), in human PMNs, B. anthracis bacilli do not stimulate synthesis of NOS2 protein, as confirmed with western blot (Fig. 2B). Thus, nitric oxide cannot be the mechanism by which PMNs kill B. anthracis.
Autophagy of human neutrophils is an important mechanism of killing B. anthracis bacilli
Under starvation, autophagy is an important mechanism by which cells reallocate nutrients (Klionsky and Emr 2000; Shintani and Klionsky 2004; Yu, Lenardo and Baehrecke 2004; Martinez-Borra and Lopez-Larrea 2012). More recent data indicate that certain cells utilize this pathway to kill intracellular pathogens (Kirkegaard, Taylor and Jackson 2004; Skendros and Mitroulis 2012). While human PMNs were reported to undergo autophagy as part of their innate immune response during phagocytosis-independent (e.g. PMA-stimulated) and -dependent processes (Skendros and Mitroulis 2012), to our knowledge autophagy has not been studied in human PMN killing of B. anthracis.
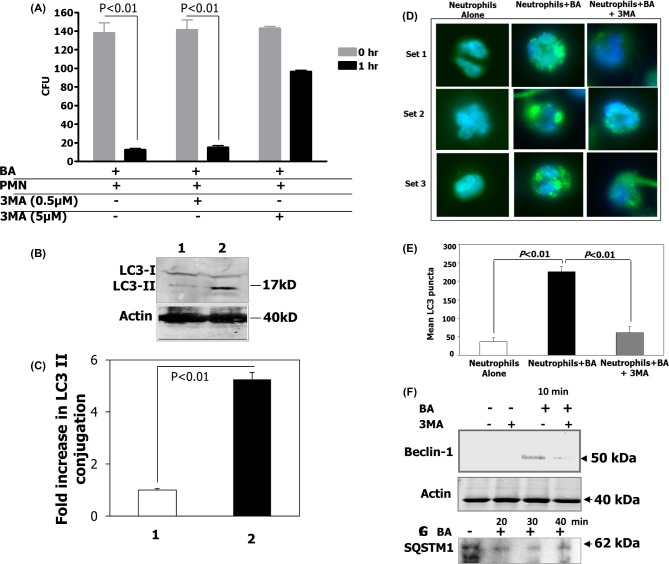
Human PMNs were infected with vegetative B. anthracis (MOI of 1:1) and treated with 3MA at either 0.5 or 5 μM, an inhibitor of class III PI3 kinases required for autophagy, or left untreated, and microbicidal activity measured. By 1 h there was complete killing of B. anthracis in untreated human PMNs. (Fig. 3A). In contrast, there was a dose-dependent inhibition of PMN-mediated killing of B. anthracis bacilli with cells treated with 3MA (Fig. 3A). In fact B. anthracis bacilli were found to multiply inside human PMNs (data not shown).
Figure 3.
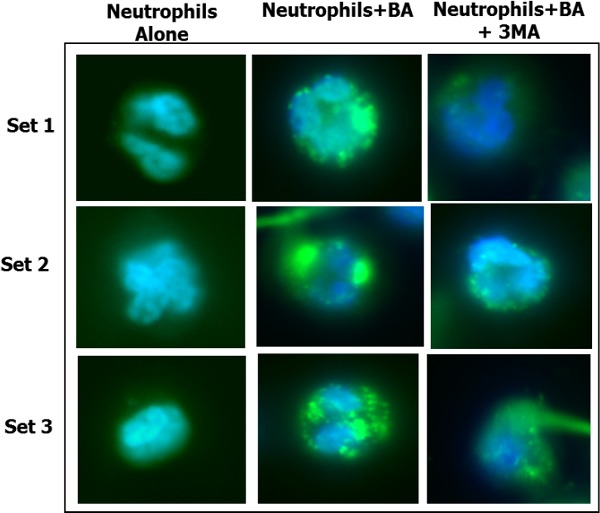
Human PMNs kill B. anthracis by autophagy. (A) To determine if human PMNs killed B. anthracis by autophagy, PMNs were incubated with vegetative B. anthracis for 1 h at an MOI of 1:1 in the absence of 3MA or with 3MA (either 0.5 or 5 μM) to inhibit autophagy. Means and standard deviation of two different experiments are shown. (B) Western blot analysis of LC3 levels. 1. Human PMNs alone. 2. Human PMNs with B. anthracis incubated for 20 min. Lower panel shows actin levels for the same. (C) Quantification of fold changes in LC3 II levels after normalization with actin and LC3I levels. (D) Fluoroscent microscopy of PMNs for autophagy. Set 1. Human PMNs alone stained with LC3. Set 2. Human PMNs with B. anthracis incubated for 20 min and stained with LC3. Set 3. Human PMNs with B. anthracis incubated for 20 min in the presence of 3MA and stained with LC3. (E) LC3 puncta quantification (n = 10 fields) using the NIH Image J Software. (F) Western blot analysis of beclin-1 levels in PMN within 10 min of B. anthracis infection in the presence and absence of the autophagy inhibitor, 3MA. Lower panel shows actin levels for the same lanes. (G) Western blot analysis of SQSTM1 levels in PMNS infected with B. anthracis, 20, 30 and 40 min post-infection.
As a pharmacological inhibitor of autophagy, 3-MA may have secondary effects on the cell. Therefore, in order to specifically examine autophagy during B. anthracis ingestion by human PMNs, we examined the effect of ingestion on the expression of microtubule-associated protein light chain 3 (LC3), a mammalian homolog of yeast Atg8, an important marker for autophagy. During autophagy, the soluble cytosolic form (LC3-I) is lipidated and converted to the stable, non-soluble LC3-II on the elongating autophagosome double membrane (Levine and Kroemer 2008). Compared to uninfected human PMNs (Fig. 3B, lane 1), addition of B. anthracis 34F2 bacilli induced the formation of LC3-I and a large amount of LC3-II (lane 2). Since there are reports that anthrax toxins induce autophagy (Tan et al. 2009a,b), we added a Sterne strain mutant that lacked protective antigen and therefore unable to generate anthrax toxins. In the absence of anthrax toxins we still observed an increase in LC3-II, suggesting that autophagy could still be induced by the vegetative bacilli, even in the absence of anthrax toxins; however, since the LC3-II signal was decreased (data not shown) compared to that observed with the wild-type Sterne strain, this is consistent with the anthrax toxins making a contribution to autophagy induction, perhaps through the expression of lethal factor alone. The fold change in LC3II levels was quantified using the NIH Image J software after normalizing to actin levels (Fig. 3C).
Using fluorescent microscopy, we further examined the ability of B. anthracis infection to induce LC3 formation. Infection of human PMNs with vegetative bacilli of B. anthracis markedly increased the formation of LC3 puncta (green) after 10 min (Fig. 3D and E) in contrast to uninfected PMNs. However, human PMNs infected with these bacilli in the presence of 3MA had a significant decrease in the LC3 puncta formation. The formation of the LC3 puncta was temporally coincident with the onset of the bactericidal activity, and decrease in LC3 puncta formation in the presence of 3MA corresponded to impaired human PMN bactericidal capacity (Fig. 3A). This was quantified using the NIH Image J software (Fig. 3E).
The early autophagy marker, beclin-1, involved in autophagosome vesicle nucleation (Levine and Kroemer 2008), was upregulated in human PMNs incubated with bacilli as early as 10 min after infection, when we first observed B. anthracis killing. However, it was blocked by addition of 3MA to the human PMNs/B. anthracis mixture (Fig. 3F). Cumulatively, these data suggest that autophagy might be an important and novel mechanism utilized by human PMNs to kill intracellular vegetative B. anthracis. This hypothesis is reinforced by our findings that mice deficient in either ATF6, an endoplasmic reticulum stress-induced transcription factor, or apoptosis signal-regulating kinase, both regulators of autophagy, are highly susceptible to lethal infection with B. anthracis (Gade et al. 2012, 2014).
SQSTM1/p62 is an autophagy cargo adapter that binds to LC3 and recruits proteins into autophagosomes for degradation (Zheng et al. 2009). Induction of autophagy leads to the reduction of steady-state levels of SQSTM1. We observed that B. anthracis induced a reduction in SQSTM1 level as early as 20 min post-infection of primary human neutrophils compared to the uninfected control (Fig. 3G). This reduction in SQSTM1 was maximal in cells at 30 min post-infection.
In conclusion, our findings demonstrate that while B. anthracis endospores are internalized by human PMNs, in the absence of germination, these phagocytes do not kill the endospores. However, when vegetative bacilli are ingested by human PMNs, they are rapidly killed by a mechanism that does not appear to involve either superoxide or nitric oxide. Further, based on conversion of the autophagy marker LC3-I to LC3-II, reduction in SQSTM1 level and expression of beclin-1, addition of B. anthracis bacilli to human PMNs induced autophagosome formation within 20 min, during which time we observed the onset of bactericidal activity. The bactericidal capacity of human PMNs was reduced by the addition of the autophagy inhibitor, 3-MA, in a dose-dependent manner. While these data do not definitively prove an autophagocytic killing mechanism (Levine and Kroemer 2008), they strongly suggest that autophagy in combination with α-defensin, perhaps via lysosome-autophagosome fusion, is an important and novel mechanism by which human PMNs kill B. anthracis bacilli.
Acknowledgments
We thank Dr William Jackson (University of Maryland, School of Medicine) for kindly providing the SQSTM1/p62 antibody and his insightful comments and discussions on autophagy.
FUNDING
Funding for this project was provided by the Mid-Atlantic Region Center for Excellence NIH U54 A1057 168-05.
Conflict of interest. None declared.
REFERENCES
- Barson HV, Mollenkopf H, Kaufmann SH, et al. Anthrax lethal toxin suppresses chemokine production in human neutrophil NB-4 cells. Biochem Bioph Res Co. 2008;374:288–93. doi: 10.1016/j.bbrc.2008.07.018. [DOI] [PubMed] [Google Scholar]
- Borregaard N. Neutrophils, from marrow to microbes. Immunity. 2010;33:657–70. doi: 10.1016/j.immuni.2010.11.011. [DOI] [PubMed] [Google Scholar]
- Bynoe LA, Gottsch JD, Pou S, et al. Light-dependent generation of superoxide from human erythrocytes. Photochem Photobiol. 1992;56:353–6. doi: 10.1111/j.1751-1097.1992.tb02170.x. [DOI] [PubMed] [Google Scholar]
- Cedergren J, Follin P, Forslund T, et al. Inducible nitric oxide synthase (NOS II) is constitutive in human neutrophils. APMIS. 2003;111:963–8. doi: 10.1034/j.1600-0463.2003.1111008.x. [DOI] [PubMed] [Google Scholar]
- Cleret A, Quesnel-Hellmann A, Vallon-Eberhard A, et al. Lungdendritic cells rapidly mediate anthrax spore entry through the pulmonary route. J Immunol. 2007;178:7994–8001. doi: 10.4049/jimmunol.178.12.7994. [DOI] [PubMed] [Google Scholar]
- Cote CK, Van Rooijen N, Welkos SL. Roles of macrophages and neutrophils in the early host response to Bacillus anthracis spores in a mouse model of infection. Infect Immun. 2006;74:469–80. doi: 10.1128/IAI.74.1.469-480.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote CK, Welkos SL, Bozue J. Key aspects of the molecular and cellular basis of inhalational anthrax. Microbes Infect. 2011;13:1146–55. doi: 10.1016/j.micinf.2011.07.005. [DOI] [PubMed] [Google Scholar]
- Crawford MA, Aylott CV, Bourdeau RW, et al. Bacillus anthracis toxins inhibit human neutrophil NADPH oxidase activity. J Immunol. 2006;176:7557–65. doi: 10.4049/jimmunol.176.12.7557. [DOI] [PubMed] [Google Scholar]
- Cross AS, Wright DG. Mobilization of sialidase from intracellular stores to the surface of human neutrophils and its role in stimulated adhesion responses of these cells. J Clin Invest. 1991;88:2067–76. doi: 10.1172/JCI115536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cybulski RJ, Jr, Sanz P, Alem F, et al. Four superoxide dismutases contribute to Bacillus anthracis virulence and provide spores with redundant protection from oxidative stress. Infect Immun. 2009;77:274–85. doi: 10.1128/IAI.00515-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- During RL, Li W, Hao B, et al. Anthrax lethal toxin paralyzes neutrophil actin-based motility. J Infect Dis. 2005;192:837–45. doi: 10.1086/432516. [DOI] [PubMed] [Google Scholar]
- Gade P, Manjegowda SB, Nallar SC, et al. Regulation of the death-associated protein kinase-1 expression and autophagy via ATF6 requires apoptosis signal-regulating kinase-1. Mol Cell Biol. 2014;34:4033–48. doi: 10.1128/MCB.00397-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gade P, Ramachandran G, Maachani UB, et al. An IFN-gamma-stimulated ATF6-C/EBP-beta-signaling pathway critical for the expression of Death Associated Protein Kinase 1 and induction of autophagy. P Natl Acad Sci USA. 2012;109:10316–21. doi: 10.1073/pnas.1119273109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garraud K, Cleret A, Mathieu J, et al. Differential role of the interleukin-17 axis and neutrophils in resolution of inhalational anthrax. Infect Immun. 2012;80:131–42. doi: 10.1128/IAI.05988-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang TJ, Fenton MJ, Weiner MA, et al. Murine macrophages kill the vegetative form of Bacillus anthracis. Infect Immun. 2005;73:7495–501. doi: 10.1128/IAI.73.11.7495-7501.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkegaard K, Taylor MP, Jackson WT. Cellular autophagy: surrender, avoidance and subversion by microorganisms. Nat Rev Microbiol. 2004;2:301–14. doi: 10.1038/nrmicro865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science. 2000;290:1717–21. doi: 10.1126/science.290.5497.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuthan H, Ullrich V, Estabrook RW. A quantitative test for superoxide radicals produced in biological systems. Biochem J. 1982;203:551–8. doi: 10.1042/bj2030551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Borra J, Lopez-Larrea C. Autophagy and self-defense. Adv Exp Med Biol. 2012;738:169–84. doi: 10.1007/978-1-4614-1680-7_11. [DOI] [PubMed] [Google Scholar]
- Mayer-Scholl A, Hurwitz R, Brinkmann V, et al. Human neutrophils kill Bacillus anthracis. PLoS Pathog. 2005;1:e23. doi: 10.1371/journal.ppat.0010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moayeri M, Crown D, Newman ZL, et al. Inflammasome sensor Nlrp1b-dependent resistance to anthrax is mediated by caspase-1, IL-1 signaling and neutrophil recruitment. PLoS Pathog. 2010;6:e1001222. doi: 10.1371/journal.ppat.1001222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumy KL, McCormick BA. The role of neutrophils in the event of intestinal inflammation. Curr Opin Pharmacol. 2009;9:697–701. doi: 10.1016/j.coph.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C, Xie QW. Nitric oxide synthases: roles, tolls and controls. Cell. 1994;78:915–8. doi: 10.1016/0092-8674(94)90266-6. [DOI] [PubMed] [Google Scholar]
- Nguyen C, Feng C, Zhan M, et al. Bacillus anthracis-derived edema toxin (ET) counter-regulates movement of neutrophils and macromolecules through the endothelial paracellular pathway. BMC Microbiol. 2012;12:2. doi: 10.1186/1471-2180-12-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien J, Friedlander A, Dreier T, et al. Effects of anthrax toxin components on human neutrophils. Infect Immun. 1985;47:306–10. doi: 10.1128/iai.47.1.306-310.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passalacqua KD, Bergman NH, Herring-Palmer A, et al. The superoxide dismutases of Bacillus anthracis do not cooperatively protect against endogenous superoxide stress. J Bacteriol. 2006;188:3837–48. doi: 10.1128/JB.00239-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porasuphatana S, Cao GL, Tsai P, et al. Bacillus anthracis endospores regulate ornithine decarboxylase and inducible nitric oxide synthase through ERK1/2 and p38 mitogen-activated protein kinases. Curr Microbiol. 2010;61:567–73. doi: 10.1007/s00284-010-9654-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raines KW, Kang TJ, Hibbs S, et al. Importance of nitric oxide synthase in the control of infection by Bacillus anthracis. Infect Immun. 2006;74:2268–76. doi: 10.1128/IAI.74.4.2268-2276.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shintani T, Klionsky DJ. Autophagy in health and disease: a double-edged sword. Science. 2004;306:990–5. doi: 10.1126/science.1099993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skendros P, Mitroulis I. Host cell autophagy in immune response to zoonotic infections. Clin Dev Immunol. 2012;2012:910525. doi: 10.1155/2012/910525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney DA, Hicks CW, Cui X, et al. Anthrax infection. Am J Resp Crit Care. 2011;184:1333–41. doi: 10.1164/rccm.201102-0209CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szarowicz SE, During RL, Li W, et al. Bacillus anthracis edema toxin impairs neutrophil actin-based motility. Infect Immun. 2009;77:2455–64. doi: 10.1128/IAI.00839-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan YK, Kusuma CM, St John LJ, et al. Induction of autophagy by anthrax lethal toxin. Biochem Bioph Res Co. 2009a;379:293–7. doi: 10.1016/j.bbrc.2008.12.048. [DOI] [PubMed] [Google Scholar]
- Tan YK, Vu HA, Kusuma CM, et al. Implications of autophagy in anthrax pathogenicity. Autophagy. 2009b;5:734–5. doi: 10.4161/auto.5.5.8567. [DOI] [PubMed] [Google Scholar]
- van Sorge NM, Ebrahimi CM, McGillivray SM, et al. Anthrax toxins inhibit neutrophil signaling pathways in brain endothelium and contribute to the pathogenesis of meningitis. PLoS One. 2008;3:e2964. doi: 10.1371/journal.pone.0002964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade BH, Wright GG, Hewlett EL, et al. Anthrax toxin components stimulate chemotaxis of human polymorphonuclear neutrophils. P Soc Exp Biol Med. 1985;179:159–62. doi: 10.3181/00379727-179-42078. [DOI] [PubMed] [Google Scholar]
- Weaver J, Porasuphaetana S, Tsai P, et al. A comparative study of neuronal and inducible nitric oxide synthases: generation of nitric oxide, superoxide and hydrogen peroxide. Biochim Biophys Acta. 2005;1726:302–8. doi: 10.1016/j.bbagen.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Xu L, Fang H, Frucht DM. Anthrax lethal toxin increases superoxide production in murine neutrophils via differential effects on MAPK signaling pathways. J Immunol. 2008;180:4139–47. doi: 10.4049/jimmunol.180.6.4139. [DOI] [PubMed] [Google Scholar]
- Yu L, Lenardo MJ, Baehrecke EH. Autophagy and caspases: a new cell death program. Cell Cycle. 2004;3:1124–6. [PubMed] [Google Scholar]
- Zheng YT, Shahnazari S, Brech A, et al. The adaptor protein p62/SQSTM1 targets invading bacteria to the autophagy pathway. J Immunol. 2009;183:5909–16. doi: 10.4049/jimmunol.0900441. [DOI] [PubMed] [Google Scholar]