Abstract
Lymphangiogenesis and concurrent angiogenesis are essential in supporting proliferation and survival of AIDS-related lymphomas, which are often metastatic. In vitro studies suggest a candidate angiogienic and lymphangiogenic factor encoded by HIV: the matrix protein p17. p17 accumulates in lymph nodes of patients even when they are undergoing highly active antiretroviral therapy. p17 has been found to affect immune cells, and recent data showed that a variant p17, called S75X, induces cell growth by triggering MAPK/ERK and PI3K/AKT pathways. We tested the in vivo angiogenic activity of p17 by injecting it in Matrigel plugs in nude mice. Plugs were retrieved 7 days after injection, and assessed macroscopically, and by light and confocal microscopy. Our data revealed that both reference and S75X variant p17 promote angiogenesis and lymphangiogenesis in vivo. Our results suggest that the induction of angiogenesis and lymphangiogenesis by HIV-1 p17 may generate a favorable microenvironment that could trigger tumor growth and maintenance. Moreover, the presence of adipocytes infiltration observed at the histological level suggests a possible interplay between angiogenesis, lymphangiogenesis and adipogenesis. These findings offer new opportunities for the development of treatment strategies to combat HIV-related cancers.
Keywords: HIV-1, angiogienesis, lymphangiogenesis, matrix protein, lymphomagenesis, adipogenesis
HIV-1 matrix protein is involved in several functions in the viral life cycle and can promote angiogenesis, lymphoangiogenesis and adipogenesis, generating a favorable microenvironment which could support tumor growth and maintenance; this study examines the role of MA/p17 in promoting these pathways and reports novel effects on adipogenesis.
Graphical Abstract Figure.
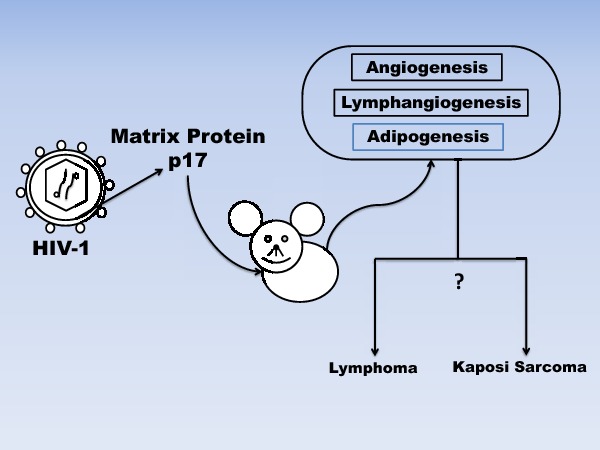
HIV-1 matrix protein is involved in several functions in the viral life cycle and can promote angiogenesis, lymphoangiogenesis and adipogenesis, generating a favorable microenvironment which could support tumor growth and maintenance; this study examines the role of MA/p17 in promoting these pathways and reports novel effects on adipogenesis.
INTRODUCTION
A large body of literature supports the role of angiogenesis, i.e. the formation of new blood vessels, in cancer and in hematologic malignancies of both myeloid and lymphoid origin (Weidner 1995; Mangi and Newland 2000; Poon, Fan and Wong 2001). Aberrant angiogenesis may also have impact on the progression of HIV-associated cancers, including lymphomas, (Hodgkin's lymphomas, non-Hodgkin's, Burkitt's, diffuse large B-cell) (Calabrese et al. 1989; Kadowaki et al. 2005; Monsuez et al. 2009). High-grade, aggressive AIDS-related lymphomas are one of the most severe complications of HIV-1 infection. Lymphangiogenesis and concurrent angiogenesis are essential in supporting proliferation and survival of lymphoma. The incidence of vascular diseases due to aberrant angiogenesis is increased in HIV-1-infected patients (Ensoli, Sturzl and Monini 2000; Stacker et al. 2002; Paydas et al. 2009). Several lines of evidence suggest that HIV proteins such as Tat, gp120 and Nef may play a role in endothelium dysfunction (Barillari et al. 1993; Albini et al. 1995; Jiang et al. 2010; Wang et al. 2014).
Data from studies in animal models and humans strongly indicate a role for angiogenesis in the development and progression of malignant lymphoma. Interestingly, Hyjek et al. (1999) observed that aggressive lymphomas express not only an absolute increased number of blood vessels but also a relatively increased number of immature vessels lacking pericytes, in comparison with reactive nodes and indolent lymphomas.
A candidate angiogenic factor is the human immunodeficiency virus type 1 matrix protein p17, which is encoded by 396 nucleotides inside the Gag gene. This protein is involved in several functions in the virus life cycle. As part of the Gag polyprotein precursor, it is sufficient for the assembly of immature particles. The processed matrix protein contributes to (a) virus maturation by its specific assembly, (b) the characteristic morphology of infectious virus particles, where it interacts with the viral envelope glycoproteins and (c) the nuclear localization of the preintegration complex after virus entry and to RNA binding (Fiorentini et al. 2006).
Previous studies have shown that p17 is released in the extracellular space from HIV-1-infected cells and it is detected in the plasma of HIV-1-infected patients (Fiorentini et al. 2006). This protein also accumulates and persists in lymph nodes of patients under HAART (Popovic et al. 2005). Extracellularly, p17 deregulates the biological activity of immune cells (Fiorentini et al. 2008; Giagulli et al. 2011; Martorelli et al. 2015). It has been reported that a p17 variant from a Ugandan HIV-1 strain, called S75X, triggers MAPK/ERK and PI3K/AKT pathways, which are known to induce cell proliferation and survival, while a control p17 from the lab-adapted isolate DH10 decreased proliferative activity (Giagulli et al. 2011; Martorelli et al. 2015). Notably, Caccuri et al. (2012) have shown that the HIV1 matrix protein p17 shows a strong angiogenic activity on human endothelial cells in vitro. The same study demonstrated that p17 in vitro induces capillary-like structure formation by interacting with both IL-8 receptors CXCR1 and CXCR2. In addition, it has been shown that p17 pro-angiogenic function is mediated by ERK signaling via the AKT pathway (Caccuri et al. 2012). Subsequently, the same group demonstrated p17 lymphangiogenic properties on human lymphatic endothelial cells in vitro and in vivo (Caccuri et al. 2014).
In our study, we performed subcutaneous injections of Matrigel to evaluate angiogenic and lymphangiogenic properties of the HIV-1 matrix protein p17 and its Ugandan variant S75X. Matrigel is a reconstituted basement membrane complex containing primarily laminin and type IV collagen, isolated from the Engelbreth–Holm–Swarm murine tumor, and is used for in vitro test for endothelial cell tube formation, and to perform in vivo assays for angiogenesis using plugs injected subcutaneously.
Our results show that both of these proteins induce lymphatic and hematic vessels formation in vivo, with relatively higher potency of S75X compared with a reference p17 that was obtained from the lab-adapted HIV strain DH10.
MATERIALS AND METHODS
HIV-1 p17 and S75X proteins
HPLC-purified, endotoxin-free HIV-1 matrix protein BH-10-derived p17 ‘reference’ and Ugandan variant S75X were chemically synthesized by solid-phase peptide synthesis using a custom-modified procedure tailored from the published in situ neutralization protocol developed for Boc chemistry in their monomeric form; purification and folding were performed as previously published (Wu et al. 2004)
Animals
Approximately 8 to 10-week-old athymic nude (Harlan) and Tg26/nude (a stable backcross between athymic nude and Tg26 HIV transgenic mice) mice were housed under standard condition in the animal facility at the Institute of Human Virology. Tg26 mice are transgenic mice for a gene derived from molecular clone of the X4-tropic HIV pNL4.3, by deleting nucleotides 1443–4551 using SphI and BalI; the resulting clone retains the region of gag encoding p17, as well as all open reading frames resulting from single and multiple splicing events. All experiments were performed according to the University of Maryland IACUC guidelines, under an approved research protocol.
In vivo angiogenesis assay
In three separate experiments, 0.5 ml of ice-cold Matrigel (Becton Dickinson) was injected subcutaneously into both flanks of 8 to10-week-old mice after anesthesia. Matrigel was supplemented with the synthetic monomeric form of the HIV-1 matrix protein BH10-derived p17 ‘reference’ and with its variant S75X (Wu et al. 2004). The proteins were used at different concentrations as 200 and 500 ng ml−1; as positive control we used purified recombinant Human Endothelin-1 (Et-1, Sigma, 500 ng ml−1). After 7 days, animals were euthanized and the Matrigel plugs were extracted.
A part of the Matrigel was embedded in paraffin for histological analyses (hematoxylin and eosin staining, H&E) and for immunofluorescence assay, while all the rest was digested with 25 μg ml−1 hyaluronidase (MP biomedical), 25 μg ml−1 DNase (Sigma-Aldrich), 3 μg ml−1, Dispase and 3 μg ml−1 Liberase (Roche) dissolved in PBS. The collected cells were filtered using 40 μm nylon mesh (BD Falcon), washed three times with FACS buffer (1% BSA, 0.01% sodium azide in PBS) and are subsequently used for flow cytometry analyses.
Immunofluorescence assay
Slides with 5-μm-thick sections of Matrigel embedded in paraffin were incubated in a vacuum oven at 55º C for 1 h and cooled overnight. Deparaffinization was performed in xylene and graded ethanol. Each slide was blocked with BSA 3% (Sigma) in PBS for 1 h at room temperature, and subsequently incubated with the following antibodies: anti mouse CD31-eFluor 605 NC (eBioscience), anti-mouse LYVE1-Biotin and anti-mouse Streptavidin FITC (eBioscience), anti-rabbit Perilipin-A (Sigma) and anti-Rabbit IgG FITC (Sigma). Cell nuclei were stained with DAPI (Sigma). The slides after staining were analyzed at the confocal microscope Zeiss META LSM 510 with three laser lines: 405 nm (blue), 488 nm (green) and 633 nm (far red). Colocalized events between green and red signals appeared in the fourth color as yellow. Green, red and blue signals were separated by a quad DAPI/FITC/TRITC/Cy5 dichroic beam splitter and were further acquired by an EM-CCD camera (Photometrics). An UPIanFluo 40x/1.3 NA oil objective (Olympus) was used to visualize labeled tissue samples. Z-stacking option was utilized in order to achieve better information about the colocalized areas of interest.
RESULTS
HIV1 matrix protein p17 and S75X variant induce angiogenesis in vivo
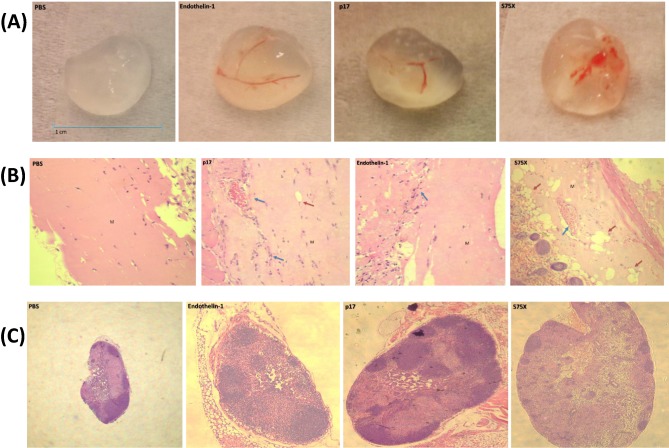
A first series of experiments were initially conducted on athymic nude mice, which received subcutaneous injection of p17 in Matrigel. As a positive control, we used purified recombinant human endothelin-1 (Et-1) an inducer of lymphangiogenesis and angiogenesis. Seven days after the injection, Matrigel plugs were extracted and angiogenesis was clearly visible, particularly when the S75X variant was present in the plug (Fig. 1A). The PBS-Matrigel negative control plugs did not show macroscopic evidence of angiogenesis, whereas vessel formation was clearly visible in plugs with Et-1.
Figure 1.
HIV-1 p17 MA induces angiogienesis in nude mice. (A) Matrigel plugs were injected subcutaneously in the flanks of nude mice and extracted 7 days after injection. The positive control endothelin-1 was mixed in the Matrigel at concentration of 500 ng ml−1, while reference (p17) and the S75X variant p17 (s75x) were used at a concentration of 200 ng ml−1. (B) Microphotographs from 5-μm sections obtained from Matrigel plugs stained with hematoxylin and eosin. Marginal endothelial cells invasion is seen in the negative control plug (PBS), while angiogenesis can be readily observed in plugs supplemented with Et-1 (500 ng ml−1), p17 reference (top-right panel, 200 ng ml−1) and p17S75X (lower-right panel, 200 ng ml−1). Blue arrows point at vessels filled with red blood cells, red arrows point at adipocyte cells. (C) 5-μm sections of axillary lymph nodes evaluated at the light microscope. Lymph nodes of the mice that received reference p17 and p17 S75X plugs (200 ng ml−1) appear enlarged and activated in comparison with these that received PBS Matrigel as a control.
Evaluation at the light microscope of 5-μm slides obtained from the Matrigel plugs and stained with hematoxylin and eosin revealed marginal endothelial cell invasion in the negative control PBS-Matrigel plug, while it confirmed vessel formation in the plug containing Et-1 (positive control). In plugs containing p17 endothelial cells, presence of small vessels was also macroscopically evident. Vessel formation appeared more structured in plugs containing p17S75X, and presence of red blood cells was observed in some vessels inside the plug (Fig. 1B).
In addition, analysis of the slides revealed adipocyte cells invasion, especially when the Matrigel was mixed with the p17 variant S75X. Published reports have shown correlation between adipogenesis and angiogenesis in vitro and in vivo (Fukumura et al. 2003; Lai, Jayaraman and Lee 2009; Cao 2010; Merfeld-Clauss et al. 2010). Thus, presence of adipocytes inside the Matrigel is consistent with ongoing adipogenesis, potentially leading to angiogenesis.
We also evaluated sections of axillary lymph nodes by light microscopy, and observed that lymph nodes from mice that received plugs with p17 (particularly these prepared with the S75X variant) appeared to be enlarged, due to B-cell infiltration, and activated as compared to lymph nodes obtained from control mice (Fig. 1C).
We performed similar experiments on Tg26/Nude mice, which are derived from a crossbreeding between nude mice and Tg26 mice (Curreli et al. 2013). Tg26 mice are transgenic mice for a molecular clone of X4-tropic HIV (pNL4.3), deleting most of the gag-pol region, but retaining the region of gag encoding p17, as well as env and all open reading frames resulting from single and multiple splicing events (Dickie et al. 1991; Kopp et al. 1993; Curreli et al. 2013). We developed this model as the TG26 mice has a high incidence of lymphoma (Curreli et al. 2013), making this animal model advantageous to study the role of microenvironment in promoting lymphomagenesis.
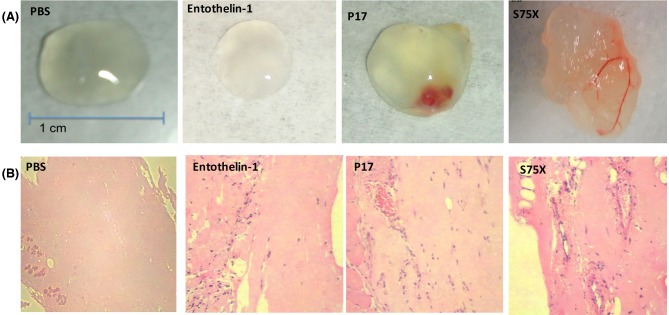
The Matrigel plugs extracted in these experiments were morphologically similar to these obtained from nude mice, although Et-1 plugs did not macroscopically show angiogenesis, but flow cytometry analyses revealed the presence of lymphatic endothelial cells in the Et-1 plugs (not shown). The reason for the lack of macroscopic angiogenesis is not immediately evident; however, the presence of endothelial cells was observed by confocal microscopy (Fig. 3). However, blood vessel formation was evident in reference p17, and appeared to be more structured in Matrigel plugs with p17 S75X (Fig. 2A).
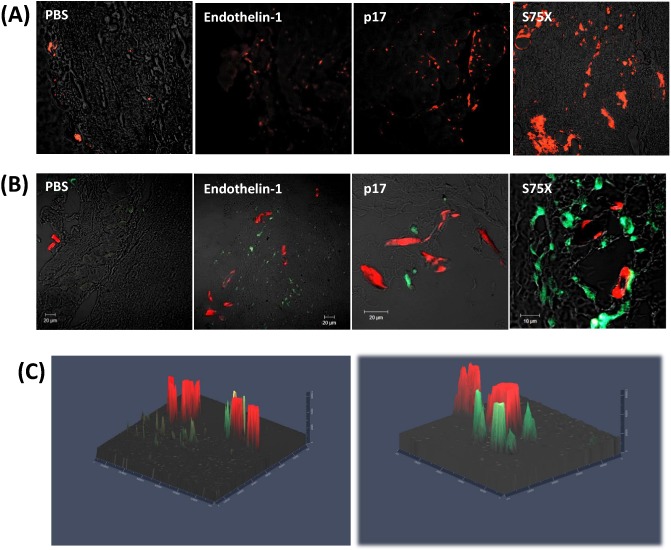
Figure 3.
HIV-1 p17/MA induces angiogenesis and lymphangiogenesis in vivo. (A) 5-μm sections were stained with anti-mouse LYVE1-Biotin and anti-mouse Streptavidin FITC (eBioscience). (B) Immunofluorescence double staining of Matrigel slides with anti-CD31 and anti-LYVE1 antibodies to detect blood (red) and lymphatic (green) cells and microvessels. (C) 3D presentation of Z stacks (1 μm each) acquired by imaging blood and lymphatic vessels of p17 and p17S75X matrigel slides.
Figure 2.
HIV-1 p17 MA induces angiogienesis in HIV transgenic (Tg) nude mice. (A) Matrigel plugs were injected subcutaneously in the flanks of HIV Tg/nude mice flanks and extracted 7 days after injection. Angiogenesis can be observed when 100 ng ml−1 of reference p17 (p17, top-right panel) or variant S75X Matrigel plugs (S75Z, bottom-right panel). (B) 5-μm sections obtained from Matrigel plugs and stained with H&E. Angiogenesis can be readily observed in plugs supplemented with ET-1 (500 ng ml−1), p17 reference (200 ng ml−1) and p17S75X (200 ng ml−1).
p17 and its variant S75X possess lymphangiogenic properties in vivo
We assessed lymphangiogenesis in the Matrigel plug slides by immunofluorescence microscopy, staining the slides with an anti-LYVE1 antibody.
LYVE1 is a specific hyaluronic acid receptor localized on the luminal surface of lymphatic vessels and it is used in in vitro and in vivo assays for detecting lymphatic cells and vessels (Jackson et al. 2001; Jackson 2003; Jackson 2004). We observed expression of this marker within the vessel structures in the plugs. We detected higher levels of LYVE1 staining in the plugs with the p17 variant S75X as compared to the reference p17, suggesting the presence of lymphatic epithelial cells at a macroscopic level (Fig. 3A).
We also investigated how angiogenesis and lymphangiogenesis events interacted in our system, and performed a double staining with anti-CD31 and anti LYVE-1 antibodies. The slides were analyzed at the confocal microscope (Fig. 3B and C). We detected expression of both markers in discrete, separate patterns. We detected more extensive CD31+ and LYVE-1+ structures in Matrigel plugs containing the S75X variant of p17, as compared to both reference p17 and the positive control Et-1. This suggests that angiogenesis and lymphangiogenesis are both induced by p17 and its variants.
Adypocites infiltration in Matrigel plugs
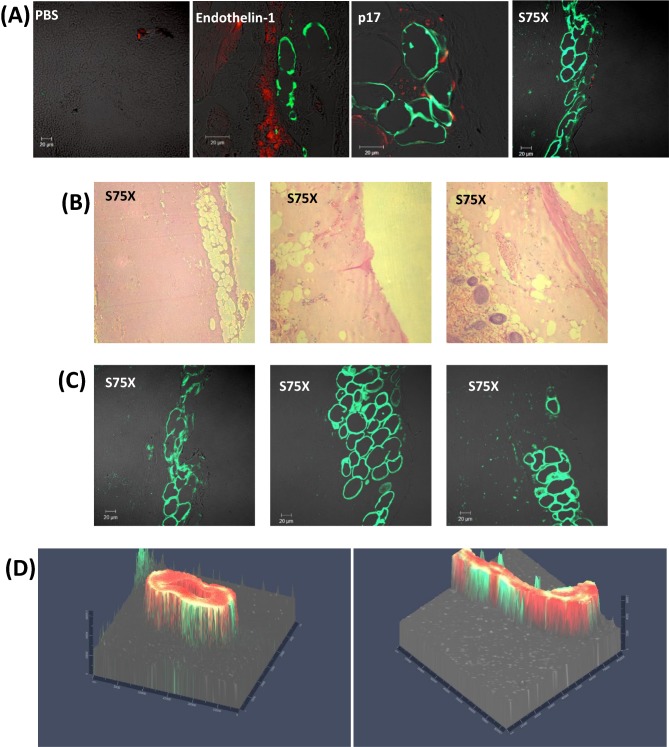
Our histologic analyses on slides obtained from Matrigel plugs containing reference p17 and the S75X variant in both nude mice and Tg26/nude mice experiments revealed evident adipocytes infiltration inside the Matrigel. To assess the potential relevance of adipogenesis to vessel formation, we performed an immunofluorescence assay staining slides from Matrigel plugs with anti-CD31 and anti-Perilipin-A (Fig. 4A). Perilipin-A is an adipocyte cell surface protein and in many it has been used as a marker for adipocyte cells, and for adipogenesis (Martin et al. 2009; Gong et al. 2011). The pattern of Perilipin-A staining is consistent with the presence of adipocytes in plugs that contained p17, particularly in the case of the S75X variant (Fig. 4B and C). Three-dimensional representation of Perilipin-A and CD31 shows a staining pattern consistent with adipocytes surrounding newly formed blood vessels (Fig. 4C). Thus, it is possible that angiogenesis is preceded by infiltration of adipocytes, and adipogenesis has been linked to angiogenesis (Fukumura et al. 2003; Nishimura et al. 2007; Cao 2010; Merfeld-Clauss et al. 2010).
Figure 4.
(A) Immunofluorescence assay staining slides from Matrigel plugs with anti-CD31 and anti-Perilipin-A for evidencing blood cells and vessels (red—CD31) and adipocyte cells (green—Perilipin A). (B) Immunohystochimical analysis of 5-μm sections of p17S75X matrigel stained with hematoxylin and eosin. (C) Immunofluorescence slides from p17 S75X Matrigel plugs with anti-Perilipin A antibody. (D) 3D presentation of Z stacks (1 μm each) showing blood vessel (red) surrounded by adipocytes (green) in p17 S75X Matrigel slides.
DISCUSSION
Previous studies qualitatively demonstrated the proangiogenic activity of HIV-1 matrix protein p17 in vitro, inducing capillary structure in HUVECs, by interacting with both IL-8 receptors CXCR1 and CXCR2 (Caccuri et al. 2012). Here, we confirm that the matrix protein p17 possesses a potent proangiogenic capability in vivo. Matrigel system provides a physiological environment in which to initiate an angiogenic response. The Matrigel plugs supported formation of significant vascular structure when supplemented with reference p17, and the variant S75X showed even higher potency. Here, we demonstrate the angiogenic and lymphangiogenic activity of HIV-1 p17 matrix proteins quantitatively and qualitatively by histology (including immunofluorescence confocal microscopy).
Our data indicate that both reference and variant p17 (S75X) promote angiogenesis and lymphangiogenesis, with the S75 variant resulting in relatively more structured vessel formation than p17 reference, as shown by histological and immunofluorescence analysis. Interestingly, the variant S75X has been shown in a previous studies to stimulate B cells, upregulating the PI3K/Akt signaling pathway and inducing cell growth (Giagulli et al. 2011; Martorelli et al. 2015). Our data also supports the hypothesis that matrix protein may play a role in producing a microenvironment that fosters lymphoma development, progression and maintenance. In addition, the robust angiogenic activity that we confirmed is likely relevant to Kaposi Sarcoma, an AIDS-defining cancer with a strong component of angiogenesis (Ensoli, Barillari and Gallo 1992; Masood et al. 1997; Boshoff 2007; Nishimura et al. 2007).
Another aspect related to the activity of reference p17 and its variant S75X was evidence of activated lymph nodes. Nude mice have an atrophic thymus gland due to a mutation of the FOX1 gene, resulting in an almost total depletion of T cell. Surprisingly, the many activated follicular zones observed in these lymph nodes led us to hypothesize that reference p17 and its variant S75X induced immune activation.
Finally, the presence of a large adipocytes infiltration in the Matrigel plug containing reference p17 and (more effectively) its variant S75X could be related angiogenesis, based on interactions between HIV-1 matrix protein p17 and adipocytes. Previous publications have shown correlation between angiogenesis and adipogenesis (Fukumura et al. 2003; Nishimura et al. 2007; Lai, Jayaraman and Lee 2009; Cao 2010; Merfeld-Clauss et al. 2010). Adipogenesis may predispose to angiogenesis, since adipocytes may communicate directly with endothelial cells by producing proangiogenic factors and cytokines, determining vessel growth and remodeling. Gherardt et al. (2001) demonstrated in their study the presence of IL-8 receptors CXCR1 and CXCR2 on the surface of adipocyte cells. p17 can bind to both receptors, suggesting a potential interaction between p17 and those receptors leading to cell growth, cell proliferation and intracellular fat accumulation. In addition, adipose tissue has been demonstrated to produce signals that affect lymphatic vascular development, and lymphatic vessels, lymph nodes and cells of the immune system are known to interact extensively with adipose tissue (Fogt et al. 2004; Backhed et al. 2007; Harvey 2008). Thus, our study suggests a potential cross-talking role of HIV-1 matrix protein p17 with adipocytes, and their interplay with blood and lymphatic cells creating an inflammatory microenvironment, that could enable tumor maintenance, progression and metastasis. The interplay between p17 and adipocytes may be relevant also to HIV-1-associated lipodystrophy syndrome, one of the most common complication of HIV-infected patients, even under HAART (Giralt et al. 2006; Giralt, Domingo and Villarroya 2011; Domingo et al. 2012).
In conclusion, angiogenesis, lymphangiogenesis and adipogenesis events increased by matrix protein p17 may constitute key steps of tumor growth and maintenance, and they may become targets for the development of future treatment strategies in preventing and combating lymphoma and HIV-1-related cancer.
Acknowledgments
We thank Qing Chen, Dept. of Pathology, University of Maryland School of Medicine, for her assessment of histological slides.
FUNDING
This work was supported by the University of Maryland School of Medicine Deans Challenge Award to Accelerate Innovation and Discovery in Medicine, and by National Institutes of Health (NIH) grants: NS-066842 (AGD), AI-072732 (WL). MKL was a trainee under Institutional Training Grant 1T32 CA154274 from the National Cancer Institute (NCI), NIH. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders And Stroke, National Institute of Allergy and Infectious Diseases, NCI, or the NIH.
Conflict of interest. None declared.
REFERENCES
- Albini A, Barillari G, Benelli R, et al. Angiogenic properties of human immunodeficiency virus type 1 Tat protein. P Natl Acad Sci USA. 1995;92:4838–42. doi: 10.1073/pnas.92.11.4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backhed F, Crawford PA, O'Donnell D, et al. Postnatal lymphatic partitioning from the blood vasculature in the small intestine requires fasting-induced adipose factor. P Natl Acad Sci USA. 2007;104:606–11. doi: 10.1073/pnas.0605957104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barillari G, Gendelman R, Gallo RC, et al. The Tat protein of human immunodeficiency virus type 1, a growth factor for AIDS Kaposi sarcoma and cytokine-activated vascular cells, induces adhesion of the same cell types by using integrin receptors recognizing the RGD amino acid sequence. P Natl Acad Sci USA. 1993;90:7941–5. doi: 10.1073/pnas.90.17.7941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boshoff C. Kaposi sarcoma, KSHV and lymphangiogenesis. Exp Dermatol. 2007;16:867–8. [Google Scholar]
- Caccuri F, Giagulli C, Bugatti A, et al. HIV-1 matrix protein p17 promotes angiogenesis via chemokine receptors CXCR1 and CXCR2. P Natl Acad Sci USA. 2012;109:14580–5. doi: 10.1073/pnas.1206605109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caccuri F, Rueckert C, Giagulli C, et al. HIV-1 matrix protein p17 promotes lymphangiogenesis and activates the endothelin-1/endothelin B receptor axis. Arterioscl Thromb Vas. 2014;34:846–56. doi: 10.1161/ATVBAHA.113.302478. [DOI] [PubMed] [Google Scholar]
- Calabrese LH, Estes M, Yen-Lieberman B, et al. Systemic vasculitis in association with human immunodeficiency virus infection. Arthritis Rheum. 1989;32:569–76. doi: 10.1002/anr.1780320509. [DOI] [PubMed] [Google Scholar]
- Cao Y. Adipose tissue angiogenesis as a therapeutic target for obesity and metabolic diseases. Nat Rev Drug Discov. 2010;9:107–15. doi: 10.1038/nrd3055. [DOI] [PubMed] [Google Scholar]
- Curreli S, Krishnan S, Reitz M, et al. B cell lymphoma in HIV transgenic mice. Retrovirology. 2013;10:92. doi: 10.1186/1742-4690-10-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickie P, Felser J, Eckhaus M, et al. HIV-associated nephropathy in transgenic mice expressing HIV-1 genes. Virology. 1991;185:109–19. doi: 10.1016/0042-6822(91)90759-5. [DOI] [PubMed] [Google Scholar]
- Domingo P, Estrada V, Lopez-Aldeguer J, et al. Fat redistribution syndromes associated with HIV-1 infection and combination antiretroviral therapy. AIDS Rev. 2012;14:112–23. [PubMed] [Google Scholar]
- Ensoli B, Barillari G, Gallo RC. Cytokines and growth factors in the pathogenesis of AIDS-associated Kaposi's sarcoma. Immunol Rev. 1992;127:147–55. doi: 10.1111/j.1600-065x.1992.tb01412.x. [DOI] [PubMed] [Google Scholar]
- Ensoli B, Sturzl M, Monini P. Cytokine-mediated growth promotion of Kaposi's sarcoma and primary effusion lymphoma. Semin Cancer Biol. 2000;10:367–81. doi: 10.1006/scbi.2000.0329. [DOI] [PubMed] [Google Scholar]
- Fiorentini S, Marini E, Caracciolo S, et al. Functions of the HIV-1 matrix protein p17. New Microbiol. 2006;29:1–10. [PubMed] [Google Scholar]
- Fiorentini S, Riboldi E, Facchetti F, et al. HIV-1 matrix protein p17 induces human plasmacytoid dendritic cells to acquire a migratory immature cell phenotype. P Natl Acad Sci USA. 2008;105:3867–72. doi: 10.1073/pnas.0800370105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogt F, Zimmerman RL, Daly T, et al. Observation of lymphatic vessels in orbital fat of patients with inflammatory conditions: a form fruste of lymphangiogenesis? Int J Mol Med. 2004;13:681–3. doi: 10.3892/ijmm.13.5.681. [DOI] [PubMed] [Google Scholar]
- Fukumura D, Ushiyama A, Duda DG, et al. Paracrine regulation of angiogenesis and adipocyte differentiation during in vivo adipogenesis. Circ Res. 2003;93:e88–97. doi: 10.1161/01.RES.0000099243.20096.FA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt CC, Romero IA, Cancello R, et al. Chemokines control fat accumulation and leptin secretion by cultured human adipocytes. Mol Cell Endocrinol. 2001;175:81–92. doi: 10.1016/s0303-7207(01)00394-x. [DOI] [PubMed] [Google Scholar]
- Giagulli C, Marsico S, Magiera AK, et al. Opposite effects of HIV-1 p17 variants on PTEN activation and cell growth in B cells. PLoS One. 2011;6:e17831. doi: 10.1371/journal.pone.0017831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giralt M, Domingo P, Guallar JP, et al. HIV-1 infection alters gene expression in adipose tissue, which contributes to HIV- 1/HAART-associated lipodystrophy. Antivir Ther. 2006;11:729–40. [PubMed] [Google Scholar]
- Giralt M, Domingo P, Villarroya F. Adipose tissue biology and HIV-infection. Best Pract Res Cl En. 2011;25:487–99. doi: 10.1016/j.beem.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Gong J, Sun Z, Wu L, et al. Fsp27 promotes lipid droplet growth by lipid exchange and transfer at lipid droplet contact sites. J Cell Biol. 2011;195:953–63. doi: 10.1083/jcb.201104142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey NL. The link between lymphatic function and adipose biology. Ann N Y Acad Sci. 2008;1131:82–8. doi: 10.1196/annals.1413.007. [DOI] [PubMed] [Google Scholar]
- Hyjek E, Chadburn A, Dias S, et al. High grade non-Hodgkin's lymphoma and Hodgkin's disease are associated with increased density of KDR plus SMA(-) immature microvessels. Blood. 1999;94:597A. [Google Scholar]
- Jackson DG. The lymphatics revisited: new perspectives from the hyaluronan receptor LYVE-1. Trends Cardiovas Med. 2003;13:1–7. doi: 10.1016/s1050-1738(02)00189-5. [DOI] [PubMed] [Google Scholar]
- Jackson DG. Biology of the lymphatic marker LYVE-1 and applications in research into lymphatic trafficking and lymphangiogenesis. APMIS. 2004;112:526–38. doi: 10.1111/j.1600-0463.2004.apm11207-0811.x. [DOI] [PubMed] [Google Scholar]
- Jackson DG, Prevo R, Clasper S, et al. LYVE-1, the lymphatic system and tumor lymphangiogenesis. Trends Immunol. 2001;22:317–21. doi: 10.1016/s1471-4906(01)01936-6. [DOI] [PubMed] [Google Scholar]
- Jiang J, Fu W, Wang X, et al. HIV gp120 induces endothelial dysfunction in tumour necrosis factor-alpha-activated porcine and human endothelial cells. Cardiovas Res. 2010;87:366–74. doi: 10.1093/cvr/cvq013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadowaki I, Ichinohasama R, Harigae H, et al. Accelerated lymphangiogenesis in malignant lymphoma: possible role of VEGF-A and VEGF-C. Brit J Haematol. 2005;130:869–77. doi: 10.1111/j.1365-2141.2005.05695.x. [DOI] [PubMed] [Google Scholar]
- Kopp JB, Rooney JF, Wohlenberg C, et al. Cutaneous disorders and viral gene expression in HIV-1 transgenic mice. AIDS Res Hum Retrov. 1993;9:267–75. doi: 10.1089/aid.1993.9.267. [DOI] [PubMed] [Google Scholar]
- Lai N, Jayaraman A, Lee K. Enhanced proliferation of human umbilical vein endothelial cells and differentiation of 3T3-L1 adipocytes in coculture. Tissue Eng Part A. 2009;15:1053–61. doi: 10.1089/ten.tea.2008.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangi MH, Newland AC. Angiogenesis and angiogenic mediators in haematological malignancies. Brit J Haematol. 2000;111:43–51. doi: 10.1046/j.1365-2141.2000.02104.x. [DOI] [PubMed] [Google Scholar]
- Martin S, Okano S, Kistler C, et al. Spatiotemporal regulation of early lipolytic signaling in adipocytes. J Biol Chem. 2009;284:32097–107. doi: 10.1074/jbc.M109.002675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martorelli D, Muraro E, Mastorci K, et al. A natural HIV p17 protein variant up-regulates the LMP-1 EBV oncoprotein and promotes the growth of EBV-infected B-lymphocytes: Implications for EBV-driven lymphomagenesis in the HIV setting. Int J Cancer. 2015;137:1374–85. doi: 10.1002/ijc.29494. [DOI] [PubMed] [Google Scholar]
- Masood R, Cai J, Zheng T, et al. Vascular endothelial growth factor/vascular permeability factor is an autocrine growth factor for AIDS–Kaposi sarcoma. P Natl Acad Sci USA. 1997;94:979–84. doi: 10.1073/pnas.94.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merfeld-Clauss S, Gollahalli N, March KL, et al. Adipose tissue progenitor cells directly interact with endothelial cells to induce vascular network formation. Tissue Eng Part A. 2010;16:2953–66. doi: 10.1089/ten.tea.2009.0635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsuez JJ, Charniot JC, Escaut L, et al. HIV-associated vascular diseases: structural and functional changes, clinical implications. Int J Cardiol. 2009;133:293–306. doi: 10.1016/j.ijcard.2008.11.113. [DOI] [PubMed] [Google Scholar]
- Nishimura S, Manabe I, Nagasaki M, et al. Adipogenesis in obesity requires close interplay between differentiating adipocytes, stromal cells, and blood vessels. Diabetes. 2007;56:1517–26. doi: 10.2337/db06-1749. [DOI] [PubMed] [Google Scholar]
- Paydas S, Ergin M, Seydaoglu G, et al. Prognostic [corrected] significance of angiogenic/lymphangiogenic, anti-apoptotic, inflammatory and viral factors in 88 cases with diffuse large B cell lymphoma and review of the literature. Leukemia Res. 2009;33:1627–35. doi: 10.1016/j.leukres.2009.02.015. [DOI] [PubMed] [Google Scholar]
- Poon RT, Fan ST, Wong J. Clinical implications of circulating angiogenic factors in cancer patients. J Clin Oncol. 2001;19:1207–25. doi: 10.1200/JCO.2001.19.4.1207. [DOI] [PubMed] [Google Scholar]
- Popovic M, Tenner-Racz K, Pelser C, et al. Persistence of HIV-1 structural proteins and glycoproteins in lymph nodes of patients under highly active antiretroviral therapy. P Natl Acad Sci USA. 2005;102:14807–12. doi: 10.1073/pnas.0506857102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacker SA, Achen MG, Jussila L, et al. Lymphangiogenesis and cancer metastasis. Nat Rev Cancer. 2002;2:573–83. doi: 10.1038/nrc863. [DOI] [PubMed] [Google Scholar]
- Wang T, Green LA, Gupta SK, et al. Transfer of intracellular HIV Nef to endothelium causes endothelial dysfunction. PLoS One. 2014;9:e91063. doi: 10.1371/journal.pone.0091063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidner N. Intratumor microvessel density as a prognostic factor in cancer. Am J Pathol. 1995;147:9–19. [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Alexandratos J, Ericksen B, et al. Total chemical synthesis of N-myristoylated HIV-1 matrix protein p17: structural and mechanistic implications of p17 myristoylation. P Natl Acad Sci USA. 2004;101:11587–92. doi: 10.1073/pnas.0404649101. [DOI] [PMC free article] [PubMed] [Google Scholar]