Abstract
In recent years, there has been a strong focus on beneficial foods with probiotic microorganisms and functional organic substances. In this context, there is an increasing interest in the commercial use of kefir, since it can be marketed as a natural beverage that has health promoting bacteria. There are numerous commercially available kefir based-products. Kefir may act as a matrix in the effective delivery of probiotic microorganisms in different types of products. Also, the presence of kefir’s exopolysaccharides, known as kefiran, which has biological activity, certainly adds value to products. Kefiran can also be used separately in other food products and as a coating film for various food and pharmaceutical products. This article aims to update the information about kefir and its microbiological composition, biological activity of the kefir’s microflora and the importance of kefiran as a beneficial health substance.
Keywords: kefir, biological activity, polysaccharides, kefiran, microbial composition
Introduction
Kefir is an acidic-alcoholic fermented milk product with little acidic taste and creamy consistency that was originated in the Balkans, in Eastern Europe, and in the Caucasus (Fontán et al., 2006; Serafini et al., 2014). Kefir can be produced by fermenting milk with commercial freeze-dried kefir starter cultures, traditional kefir grains, and the product that remains after the removal of kefir grains (Bensmira et al., 2010). Kefir grains are a kind of yogurt starter, which are white to yellow – white, gelatinous, and variable in size (varying from 0.3–3.5 cm in diameter) and are composed by a microbial symbiotic mixture of lactic acid bacteria (108 CFU/g), yeast (106–107 CFU/g), and acetic acid bacteria (105CFU/g) that stick to a polysaccharide matrix (Garrote et al., 2010; Chen et al., 2015). After successive fermentations, kefir grains can break up to new generation grains, which have the same characteristics as the old ones (Gao et al., 2012).
Commercial kefir is produced by two methods: The “Russian method” and the pure cultures. In the “Russian method” kefir is produced on a larger scale, using a series fermentation process, beginning with the fermentation of the grains and using the percolate. The other method employs pure cultures isolated from kefir grains or commercial cultures (Leite et al., 2013). Also, the industrial or commercial process uses direct-to-vat inoculation (DVI) or direct-to-vat set (DVS) kefir starter cultures. In addition, Bifidobacterium sp., Lactobacillus sp. and probiotic yeast (Saccharomyces boulardii) may be used as adjunct cultures when blended with kefir grains or kefir DVI cultures (Wszolek et al., 2006). On the other hand, whey may be a practical base for kefir culture production, and fermented whey has shown to be a suitable cryoprotective medium during freeze-drying. The freeze-dried culture retains a high survival rate and shows good metabolic activity and fermentation efficiency, indicating a good potential for its use as a value-added starter culture in dairy technology. All of these studies have shown promising perspectives for the application of kefir grains in whey valorization strategies (Bensmira et al., 2010; Cheirsilp and Radchabut, 2011).
Traditionally, kefir is manufactured using cow, ewe, goat, or buffalo milk. However, in some countries, animal milk is scarce, expensive, or minimally consumed due to dietary constraints, preferences, or religious customs. Therefore, there have been many attempts to produce kefir from a variety of food sources such as soy milk (Botelho et al., 2014). Historically, kefir has been linked with health, for example, in Soviet countries, kefir has been recommended for consumption by healthy people to restrain the risk of some diseases (Saloff-Coste, 1996; St-Onge et al., 2002; Farnworth and Mainville, 2003). The consumption of this fermented milk has been related to a variety of health benefits (Vujičič et al., 1992; McCue and Shetty, 2005; Rodrigues et al., 2005a) not only linked to its microflora, but also due to the presence of some metabolic products as organic acids (Garrote et al., 2001; Ismaiel et al., 2011). In addition, kefir cultures have the ability to assimilate cholesterol in milk (Yanping et al., 2009). On the other hand, there is a growing commercial interest in using kefir as a suitable food matrix for supplementation with health-promoting bacteria. Kefir may not only be a natural probiotic beverage, but also acts as an effective matrix for the delivery of probiotic microorganisms (Vinderola et al., 2006; Medrano et al., 2008; Oliveira et al., 2013).
In kefir grains the main polysaccharide is kefiran, which is a heteropolysaccharide composed by equal proportions of glucose and galactose and is mainly produced by Lactobacillus kefiranofaciens (Zajšek et al., 2011). It has been demonstrated that kefiran improves the viscosity and viscoelastic properties of acid milk gels (Rimada and Abraham, 2006), and is able to form gels that have interesting viscoelastic properties at low temperatures, because of that, kefiran can also be used as an additive in fermented products. Besides, kefiran can enhance the rheological properties of chemically acidified skim milk gels increasing their apparent viscosity (Zajšek et al., 2013).
Compared with other polysaccharides, kefiran has outstanding advantages such as antitumor, antifungal, antibacterial properties (Cevikbas et al., 1994; Wang et al., 2008) immunomodulation or epithelium protection (Serafini et al., 2014), anti-inflammatory (Rodrigues et al., 2005b), healing (Rodrigues et al., 2005a), and antioxidant activity (Chen et al., 2015).
This review presents the most recent advances about kefir and kefiran, their production and microbial cultures involved, biological activities and potential applications in health and food industries.
Microbial Composition of Kefir Grains and Kefir
Kefir grains have a complex composition of microbial species such as the predominance of lactic acid bacteria, acetic bacteria, yeasts, and fungi (Jianzhong et al., 2009; Pogačić et al., 2013). This microbial species are classified into four groups: homofermentative and heterofermentative lactic acid bacteria and lactose and non-lactose assimilating yeast (Cheirsilp and Radchabut, 2011). In that way, Lactobacillus paracasei ssp. paracasei, Lactobacillus acidophilus, Lactobacillus delbrueckii ssp. bulgaricus, Lactobacillus plantarum, and L. kefiranofaciens are predominant species. However, these species represent only 20% of the Lactobacillus in the final fermented beverage, with the remainder consisting of Lactobacillus kefiri (80%; Yüksekdag et al., 2004; Zanirati et al., 2015). Acetobacter aceti and A. rasens have also been isolated, such as the fungus Geotrichum candidum. More than 23 different yeast species have been isolated from kefir grains and from fermented beverages of different origins. However, the predominant species are Saccharomyces cerevisiae, S. unisporus, Candida kefyr, and Kluyveromyces marxianus ssp. marxianus (Witthuhn et al., 2004; Diosma et al., 2014; Zanirati et al., 2015; Table 1).
Table 1.
Microbial compositions found in kefir and kefir grains of different origins.
| Microorganism | Source – Country | Reference |
|---|---|---|
| Lactobacillus kefir, Lactobacillus kefiranofaciens, Lactobacillus paracasei, Lactobacillus plantarum, Lactococcus lactis ssp. lactis, Kluyveromyces marxianus, Lactobacillus parakefir, Saccharomyces cerevisiae, Saccharomyces unisporus, Leuconostoc mesenteroides, Acetobacter sp., Saccharomyces sp., Lactococcus lactis ssp. lactis biovar diacetylactis, Lactococcus lactis, Lactobacillus kefiri, Lactobacillus parakefiri | Kefir grains and beverage – Argentina | Garrote et al., 2001; Londero et al., 2012; Hamet et al., 2013; Diosma et al., 2014. |
| Lactobacillus kefiri, Lactobacillus kefiranofaciens, Leuconostoc mesenteroides, Lactococcus lactis, Lactococcus lactis ssp. cremoris, Gluconobacter frateurii, Acetobacter orientalis, Acetobacter lovaniensis, Kluyveromyces marxianus, Naumovozyma sp., Kazachastania khefir | Kefir grains and beverage – Belgium | Korsak et al., 2015 |
| Lactobacillus kefiri, Lactobacillus kefiranofaciens, Leuconostoc mesenteroides, Lactococcus lactis, Lactobacillus paracasei, Lactobacillus helveticus, Gluconobacter japonicus, Lactobacillus uvarum, Acetobacter syzygii, Lactobacillus satsumensis, Saccharomyces cerevisiae., Leuconostoc sp., Streptococcus sp., Acetobacter sp., Bifidobacterium sp., Halococcus sp., Lactobacillus amylovorus, Lactobacillus buchneri, Lactobacillus crispatus, Lactobacillus kefiranofaciens ssp. kefiranofaciens, Lactobacillus kefiranofaciens ssp. kefirgranum, Lactobacillus parakefiri | Kefir grains – Brazil | Miguel et al., 2010; Leite et al., 2012; Zanirati et al., 2015 |
| Lactobacillus brevis, Lactobacillus delbrueckii ssp. bulgaricus, Lactobacillus helveticus, Streptococcus thermophilus, Lactobacillus casei ssp. pseudoplantarum, Kluyveromyces marxianus var. lactis, Saccharomyces cerevisiae, Candida inconspicua, Candida maris, Lactobacillus lactis ssp. lactis | Kefir grains and beverage – Bulgaria | Simova et al., 2002 |
| Lactobacillus paracasei, Lactobacillus parabuchneri, Lactobacillus casei, Lactobacillus kefiri, Lactococcus lactis, Acetobacter lovaniensis, Kluyveromyces lactis, Kazachstania aerobia, Saccharomyces cerevisiae, Lachancea meyersii | Kefir beverage – Brazil | Magalhães et al., 2011 |
| Lactobacillus kefiranofaciens, Leuconostoc mesenteroides, Lactococcus lactis, Lactobacillus helveticus, Kluyveromyces marxianus, Saccharomyces cerevisiae, Pseudomonas sp., Kazachstania unispora, Kazachstania exigua, Lactobacillus kefiri, Lactobacillus casei, Bacillus subtilis, Pichia kudriavzevii, Leuconostoc lactis, Lactobacillus plantarum, Acetobacter fabarum, Pichia guilliermondii, Lactococcus sp., Lactobacillus sp., Acetobacter sp., Shewanella sp., Leuconostoc sp., Streptococcus sp, Acinetobacter sp., Pelomonas sp., Dysgonomonas sp., Weissella sp., Shewanella sp. | Kefir grains (Tibet)– China | Jianzhong et al., 2009; Gao et al., 2012, 2013a |
| Acetobacter acetic, Enterococcus faecalis, Enterococcus durans, Lactococcus lactis ssp. cremoris, Leuconostoc pseudomesenteroides, Leuconostoc paramesenteroides, Lactobacillus brevis, Lactobacillus acidophilus, Saccharomyces sp., Brettanomyces sp., Candida sp., Saccharomycodes sp., Acetobacter rancens | Kefir beverage – China | Yang et al., 2007 |
| Lactobacillaceae and Streptococcaceae | Kefir grains and beverage – Ireland | Dobson et al., 2011 |
| Lactobacillus kefiranofaciens, Dekkera anomala, Streptococcus thermophilus, Lactococcus lactis, Acetobacter sp., Lactobacillus lactis, Enterococcus sp., Bacillus sp., Acetobacter fabarum, Acetobacter lovaniensis, Acetobacter orientalis | Kefir grains – Italy | Garofalo et al., 2015 |
| Leuconostoc sp., Lactococcus sp., Lactobacillus sp., Lactobacillus plantarum, Zygosaccharomyces sp., Candida sp., Candida lambica, Candida krusei, Saccharomyces sp., Cryptococcus sp. | Kefir grains and beverage – South Africa | Witthuhn et al., 2005 |
| Lactobacillus sp., Leuconostoc sp., Lactococcus sp., Zygosaccharomyces sp., Candida sp., Saccharomyces sp. | Kefir grains – South Africa | Witthuhn et al., 2004 |
| Lactobacillus kefiri, Lactobacillus kefiranofaciens, Leuconostoc mesenteroides, Lactococcus lactis, Escherichia coli, Pseudomonas sp., Saccharomyces turicensis, | Kefir grains – Taiwan | Wyder et al., 1999; Chen et al., 2008; Wang et al., 2012; |
| Lactobacillus kefiri, Leuconostoc mesenteroides, Lactococcus lactis, Streptococcus thermophilus, Lactobacillus kefiranofaciens, Lactobacillus acidophilus | Kefir grains and beverage – Turkey | Guzel-Seydim et al., 2005; Kesmen and Kacmaz, 2011 |
| Lactobacillus helveticus, Lactobacillus buchneri, Lactobacillus kefiranofaciens, Lactobacillus acidophilus, Lactobacillus helveticus, Streptococcus thermophilus, Bifidobacterium bifidum, Kluyveromyces marxianus | Kefir grains – Turkey | Kok-Tas et al., 2012; Nalbantoglu et al., 2014 |
| Lactococcus cremoris, Lactococcus lactis, Streptococcus thermophilus, Streptococcus durans | Kefir beverage – Turkey | Yüksekdag et al., 2004 |
The microbial composition may vary according to kefir origin, the substrate used in the fermentation process and the culture maintenance methods. Tibetan kefir, which is used in China, is composed of Lactobacillus, Lactococcus, and yeast. Additionally, acetic acid bacteria have been identified in Tibetan kefir, depending on the region in China from where it was obtained (Gao et al., 2012), additionally, Tibetan kefir composition differs from that of Russian kefir, Irish kefir, Taiwan kefir, Turkey fermented beverage with kefir; however, it is known that this microbial diversity is responsible for the physicochemical features and biological activities of each kefir (Jianzhong et al., 2009; Kabak and Dobson, 2011; Gao et al., 2012; Altay et al., 2013).
Wang et al. (2012) examined a section of a whole kefir grain and found in the outer layer of the grain, lactococci, and yeasts, and, in the inner layer of the grain, the quantity of lactobacilli were much higher and more yeasts cells were found. There are little information about the mechanism of grain formation, so the same authors, proposed a hypothesis to explain that. “Initially, Lactobacillus kefiranofaciens and Saccharomyces turicensis start to auto-aggregate and co-aggregated to small granules.” The aggregation is enhanced when the pH drops. The biofilm producers, Lactobacillus kefiri, Kluyveromyces marxianus HY1, and Pichia fermentans HY3 then adhere to the surface of these small granules due to their cell surface properties and their strong aggregation ability, which gives rise to thin biofilms. After biofilm formation, the kefir yeasts and Lactobacillus continue to co-aggregated with the granule strains and associate with the granule biofilm to become a three dimensional microcolony. As the cell density due to the growth of kefir yeasts and Lactobacillus increases, cells and milk components that are present in the liquid phase accumulate on the granule surface and the kefir grains are formed. There is a symbiotic relation between the microorganisms present in kefir grains, wherein the bacteria and yeast survive and share their bioproducts as power sources and microbial growth factors. This microorganism association is responsible for lactic and alcoholic fermentation (Witthuhn et al., 2005; Wang et al., 2012; Hamet et al., 2013).
After receiving its actual/present denomination, some of the microorganisms isolated and identified in kefir cultures were classified using the product name, as in Lactobacillus kefiri, L. kefiranofaciens, L. kefirgranum, Lactobacillus parakefir, and Candida kefyr (Wyder et al., 1999; Kwon et al., 2003; Yang et al., 2007; Kok-Tas et al., 2012). Table 1 demonstrates the microbial composition, which has been isolated from kefir and kefir grains of different origins.
Biological Activity of Kefir
Due to its composition, kefir is mainly considered a probiotic resource (Nalbantoglu et al., 2014). “Probiotics are microbial cell preparations or components of microbial cells with a beneficial effect on the health of the host” (Lopitz et al., 2006). Some studies suggest that probiotic bacteria in kefir consumers’ gut are abundant and are correlated with health improvement (Ahmed et al., 2013; Zheng et al., 2013); in that way, it had been demonstrated that the cell-free fraction of kefir enhances the ability to digest lactose relieving symptoms (Farnworth, 2005; Rizk et al., 2009).
Another reason for the increased interest in probiotic strains from kefir is its capacity to lower cholesterol levels. There are different ways in which bacteria can alter serum cholesterol: (i) through the binding to and absorption into the cell before it can be absorbed into the body; (ii) producing free and deconjugating bile acids; (iii) inhibiting the enzyme HMG-CoA reductase (Yanping et al., 2009).
The microorganisms in the kefir grains produce lactic acid, antibiotics and bactericides, which inhibit the development of degrading and pathogenic microorganisms in kefir milk (Liu et al., 2002). Kefir acts against the pathogenic bacteria Salmonella, Helicobacter, Shigella, Staphylococcus, Escherichia coli, Enterobacter aerogenes, Proteus vulgaris, Bacillus subtilis, Micrococcus luteus, Listeria monocytogenes, Streptococcus pyrogenes, (Lopitz et al., 2006), Streptococcus faecalis KR6, Fusarium graminearum CZ1 (Ismaiel et al., 2011), and the fungus Candida albicans. On the other hand, it has been demonstrated that a mixture of kefir isolated bacteria and yeast is able to prevent diarrhea and enterocolitis triggered by Clostridium difficile (Bolla et al., 2013). Besides, kefir showed good efficacy in inhibiting spore formation and aflatoxin B1 produced by the fungus Aspergillus flavus, which is a toxic compound formed either in the field or during food storage. Therefore, kefir appears as a promising safe alternative natural food preservative offering protection against intoxication with aflatoxin B1 (Ismaiel et al., 2011).
It had been proved that many species of lactobacilli present in kefir have S-layer proteins. Surface layers (S-layers) can be aligned in unit cells on the outermost surface of many prokaryotic microorganisms (Mobili et al., 2009). It has been demonstrated that these S-layer proteins can apply a protective action inhibiting the grown of Salmonella enterica serovar Enteritidis in Caco-2 cells, and also have the ability to antagonize the effects of toxins from Clostridium difficile on eukaryotic/eukaryotic cells in vitro (Carasi et al., 2012).
However, there are other important bioactivities that have been tested with kefir grains, the cell-free fraction of kefir or acid lactic bacteria isolated from kefir, such as antitumoral (Gao et al., 2013b), anti-inflammatory (Diniz et al., 2003), antimicrobial (Anselmo et al., 2010) immunoregulatory (Hong et al., 2009), antiallergenic (Wei-Sheng et al., 2010), wound healing (Huseini et al., 2012), antidiabetic (Young-In et al., 2006) antimutagenic (Guzel-Seydim et al., 2006), and antigenotoxic (Grishina et al., 2011). In that way, it had been demonstrated that kefir cell-free fraction has antiproliferative effects on human gastric cancer SGC7901 cells (Gao et al., 2013b), colon adenocarcinoma cells (Khoury et al., 2014), HuT–102 malignant T lymphocytes, sarcoma 180 in mice, Lewis lung carcinoma and human mammary cancer (Rizk et al., 2009), and reduce oxidative stress (Punaro et al., 2014). Another study has shown that suspensions after 24 h fermentation and mechanically disintegrated kefir grains cause a significant inhibition of granuloma tissue formation and a 43% inhibition of the inflammatory process (Diniz et al., 2003).
Nevertheless, there are other important studies performed with some microorganisms isolated from different types of kefir. Some microorganisms with their biological activities and origin are shown in Table 2.
Table 2.
Kefir microorganisms and their biological activities.
| Organism of interest | Origin | Biological activity | Reference |
|---|---|---|---|
| Lactobacillus plantarum MA2 | Tibetan kefir | Hypocholesterolemic effect | Yanping et al., 2009 |
| Lactobacillus plantarum Lp27 | Tibetan kefir | Inhibited cholesterol absorption | Ying et al., 2013 |
| Lactobacillus plantarum CIDCA 83114 | Kefir grains – Argentina | Inhibit the growth of Shigella sonnei in vitro and also the cytotoxicity of C. difficile toxins on eukaryotic cells | Bolla et al., 2013 |
| Lactobacillus kefir CIDCA 8348 | Kefir grains – Argentina | Inhibit the growth of Shigella sonnei in vitro and also the cytotoxicity of C. difficile toxins on eukaryotic cells | Bolla et al., 2013 |
| Lactobacillus plantarum ST8KF | Kefir grains – South Africa | Bactericida effect against: Lactobacillus casei, Lactobacillus salivarius, Lactobacillus curvatus, Listeria innocua | Powell et al., 2007 |
| Lactobacillus kefiranofaciens K1 | Kefir grains – Taiwanese milk | Antiallergenic effect | Chen et al., 2008; Wei-Sheng et al., 2010 |
| Lactobacillus kefiranofaciens M1 | Kefir grains – Taiwanese milk | Immunoregulatory effects – anticolitis effect | Hong et al., 2009; Chen et al., 2012 |
| Lactobacillus lactis CIDCA 8221 | Kefir grains – Argentina | Inhibit the growth of Shigella sonnei in vitro and also the cytotoxicity of Clostridium difficile toxins on eukaryotic cells | Bolla et al., 2013 |
| Kluyveromyces marxianus CIDCA 8154 | Kefir grains – Argentina | Inhibit the growth of Shigella sonnei in vitro and also the cytotoxicity of Clostridium difficile toxins on eukaryotic cells | Bolla et al., 2013 |
| Saccharomyces cerevisiae CIDCA 8112 | Kefir grains – Argentina | Inhibit the growth of Shigella sonnei in vitro and also the cytotoxicity of Clostridium difficile toxins on eukaryotic cells | Bolla et al., 2013 |
| Lactobacillus lactis ssp. cremoris | Kefir grains – India | Activity against food spoilage bacteria | Raja et al., 2009 |
Source: Soccol et al., 2014.
Kefiran, A Potential Exopolysaccharide
The increased search for natural polysaccharides has been very significant due to their use in the food, pharmaceutical, and cosmetic industries as additives, bio-absorbents, metal removal agents, bioflocculants, and medicine delivery agents, among other functions (De Vuyst et al., 2001; Welman and Maddox, 2003; Badel et al., 2011). Many microorganisms, such as bacteria, fungi, and weeds, have the capacity/ability to synthesize and excrete extracellular polysaccharides, and these polysaccharides can be either soluble or insoluble (Wang et al., 2010; Badel et al., 2011).
The polysaccharides that are commonly used as food additives are xanthan, dextran, gellan, and alginates, while the exopolysaccharides (EPSs) produced by lactic acid bacteria show good physicochemical characteristics for their use as food additives. In addition to these characteristics, EPSs are obtained from microorganisms classified as GRAS (generally recognized as safe), such as lactic acid bacteria (Wang et al., 2008; Saija et al., 2010; Badel et al., 2011).
Many reports have demonstrated that the quantity and properties of EPSs depend on the microorganisms used in the fermentation process and on the fermentation conditions and the composition of the culture media (Kim et al., 2008). EPSs have physicochemical and rheological properties that make them suitable as additives, which can be used as stabilizers, emulsifiers, gelling agents, and viscosity improvers. Additionally, EPSs possess biological properties suggesting their use as antioxidants, antitumor agents, antimicrobial agents, and immunomodulators, among other roles (Suresh Kumar et al., 2008; Bensmira et al., 2010; Piermaria et al., 2010).
The EPS kefiran is produced by Lactobacillus kefiranofaciens (Kooiman, 1968; Wang et al., 2010) from kefir grains, which are composed of proteins, polysaccharides, and a complex symbiotic microbial mixture (Witthuhn et al., 2005; Jianzhong et al., 2009). These microorganisms grow in kefiran, which is a polysaccharide matrix consisting of glucose and galactose. Despite good kefiran production by L. kefiranofaciens alone, it has been observed that the addition of Saccharomyces sp. to the culture improves the net quantity of kefiran, illustrating the importance of the symbiosis between the bacteria and yeast that are present in kefir (Cheirsilp et al., 2003).
Lactic acid bacteria can synthesize homopolysaccharides or heteropolysaccharides. The synthesized homopolysaccharides are glucans or fructans, which are composed of only one type of monosaccharide (glucose or fructose, respectively; Van Hijum et al., 2006; Badel et al., 2011), whereas the heteropolysaccharides contain different types of monosaccharides in different proportions (mainly glucose, galactose, and rhamnose), (De Vuyst and Degeest, 1999; Ruas-Madiedo et al., 2002).
Similarly to lactic acid bacteria, Lactobacillus sp. also produces glucan and fructan. The homopolysaccharides show a much higher performance compared with heteropolysaccharide production (Welman and Maddox, 2003; Badel et al., 2011).
The heteropolysaccharides excreted by Lactobacillus delbrueckii, Lactobacillus bulgaricus, Lactobacillus rhamnosus, and Lactobacillus helveticus contain galactose, glucose, and rhamnose as the main monosaccharides, with other monosaccharides being present in smaller concentrations. They are also highly branched with different types of linkages, and their denominations are complex and generally dependent on the main monosaccharide (De Vuyst and Degeest, 1999; Badel et al., 2011).
Lactobacillus plantarum isolated from Tibetan kefir excretes EPS classified as heteropolysaccharides composed of galactose, glucose, and mannose. This EPS has the capacity/ability to reduce blood cholesterol and form a biofilm shape (Zhang et al., 2009; Wang et al., 2010).
Kefiran is an EPS classified as a heteropolysaccharide comprising glucose and galactose in high concentrations, and it is classified as a water-soluble glucogalactan, which makes it suitable to be used as an additive (Wang et al., 2008, 2010). Kefiran has excellent rheological properties and can significantly improve the viscosity of lacteous products by favoring and maintaining gel properties and avoiding the loss of water during storage (Rimada and Abraham, 2006). With respect to the biological activity of kefiran, several studies have demonstrated that this EPS can be used as a nutraceutical, as described in Table 3.
Table 3.
Biological activity of kefiran.
| Exopolysaccharide | Biological activity | Reference |
|---|---|---|
| Kefiran | Reduction of blood pressure induced by hypertension | Maeda et al., 2004 |
| Favors the activity of peritoneal macrophages | ||
| Increase in peritoneal IgA | Duarte et al., 2006 | |
| Antitumoral activity | Liu et al., 2002 | |
| Antimicrobial activity | Rodrigues et al., 2005a | |
| Modulation of the intestinal immune system and protection of epithelial cells against Bacillus cereus exocellular factors | Medrano et al., 2008; Piermaria et al., 2010 |
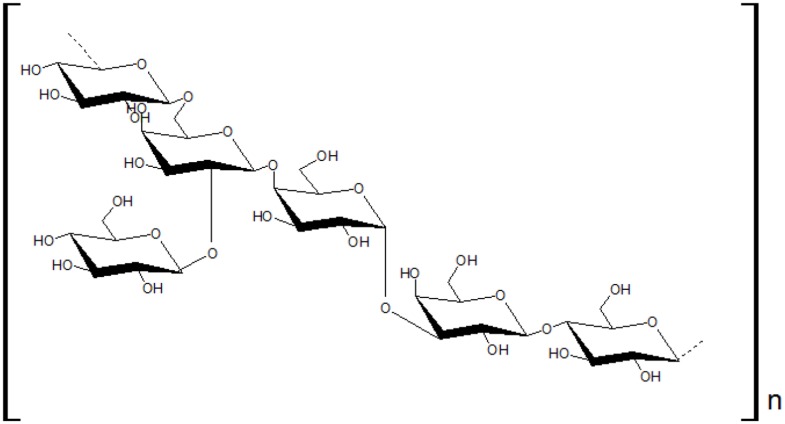
The first study about kefiran structure was published by Kooiman (1968), who proposed a structure composed of two units: kefiran (polysaccharide) and kefirose (pentasaccharide). Then, some authors analyzed the polysaccharide structure with current techniques such chromatography and infrared spectroscopy (Wang et al., 2008; Chen et al., 2015) and nuclear magnetic resonance (NMR; Ghasemlou et al., 2012). The kefiran structure, according to them, is shown in Figure 1.
FIGURE 1.
Kefiran structure.
Kefir-Based Products
Nowadays, the interest in developing functional foods is increasing because people want to improve their health and prevent diseases. Keeping in mind that kefir is a beverage with high probiotic activity, among other bioactivities, new companies are emerging around the world. One of the biggest kefir companies known is Lifeway, which started in 1986; their products can be obtained in the United States, Canada, and Great Britain, all of them based in kefir beverages, frozen, and cheese.
Other companies are Evolve Kefir with its principal product, a smoothie; Wallaby Yogurt Company with Low Fat Kefir; and CocoKefir LLC, which provides drinks/beverages based mainly on coconut water cultured with a comprehensive blend of probiotics. Table 4 summarizes the products provided these companies with some general information about each one.
Table 4.
Marketed kefir-based products and their information.
| Companies | Product | General information |
|---|---|---|
| Lifeway • United States • Canada • Great Britain |
Low Fat Kefir Non-Fat Kefir Veggie Kefir |
All-natural 99% lactose-free Gluten-free 12 probiotic cultures High in protein and calcium |
| Kefir Oats | All-natural 99% lactose-free Gluten-free 12 probiotic cultures Oat fiber enriched High in protein and calcium |
|
| Perfect 12 Kefir Traditional Kefir Greek Style Kefir |
All-natural 99% lactose-free Gluten-free 12 probiotic cultures No added sugar High in protein and calcium |
|
| Low Fat Kefir (Organic) |
USDA Certified Organic Oregon Tilth Certified Organic 99% lactose-free Gluten-free 12 probiotic cultures High in protein and calcium |
|
| Whole Milk Kefir (Organic) | USDA Certified Organic Oregon Tilth Certified Organic 99% lactose-free Gluten-free 12 probiotic cultures No added sugar |
|
| Helios Kefir (Organic) | USDA Certified Organic Oregon Tilth Certified Organic 99% lactose-free Gluten-free Seven probiotic cultures Contains Inulin |
|
| Green Kefir (Organic) | USDA Certified Organic Oregon Tilth Certified Organic 99% lactose-free Gluten-free 12 probiotic cultures Phytoboost = 1 serving of vegetables |
|
| ProBugs (organic) | USDA Certified Organic Oregon Tilth Certified Organic 99% lactose-free Gluten-free 12 probiotic cultures No-spill pouch |
|
| ProBugs Blast (Organic) | USDA Certified Organic Oregon Tilth Certified Organic 99% lactose-free Gluten-free 12 probiotic cultures High in protein and calcium |
|
| Frozen ProBugs (Organic) | All-natural 99% lactose-free Gluten-free 10 probiotic cultures High in protein and calcium |
|
| Frozen Kefir | All-natural 99% lactose-free 10 probiotic cultures 90 calories per serving 1 g of fat |
|
| Frozen Kefir Bars | All-natural 99% lactose-free Gluten-free 10 probiotic cultures 60 calories per serving 0.5 g of fat |
|
| BioKefir | All-natural 20 Billion units of probiotics 12 probiotic cultures 99% lactose-free Gluten-free High in protein and calcium |
|
| Farmer Cheese | 99% lactose-free Gluten-free High in protein and calcium |
|
| Evolve Kefir • United States |
Evolve Kefir | 11 probiotic cultures. Natural fruit flavors. Fiber. Protein and calcium |
| Wallaby Organic • Australia |
Lowfat Kefir | 12 different strains of Live and Active Kefir cultures. |
| CocoKefir • United States |
CocoKefir App le Cinnamon CocoKefir Citrus CocoKefir CocoYo Body Ecology Coconut Kefir |
Dairy, gluten, soy, and fat free Low calorie Contains valuable nutrients such as potassium, manganese, and magnesium. Beneficial probiotic strains |
Conclusion
Kefir, the traditional beverage, is now recognized as a potential source of probiotics and molecules with highly interesting healthy properties. The careful and detailed characterization of kefir composition has helped the scientific community to find new possibilities for its application. Kefiran, the EPS of kefir, has very important physicochemical and rheological properties. Besides, its biological properties suggest its use as antioxidant, antitumor agent, antimicrobial agent, and immunomodulator, among other roles. Research is constantly being conducted to consolidate kefir and kefiran properties for the development of new important products to preserve consumer’s health.
Acknowledgment
Authors want to thank CNPq and CAPES for the financial support.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Ahmed Z., Wang Y., Ahmad A., Khan S. T., Nisa M., Ahmad H., et al. (2013). Kefir and health: a contemporary perspective. Crit. Rev. Food Sci. Nutr. 53 422–434. 10.1080/10408398.2010.540360 [DOI] [PubMed] [Google Scholar]
- Altay F., Karbancıoglu-Güler F., Daskaya-Dikmen C., Heperkan D. (2013). A review on traditional Turkish fermented non-alcoholic beverages: microbiota, fermentation process and quality characteristics. Int. J. Food Microbiol. 167 44–56. 10.1016/j.ijfoodmicro.2013.06.016 [DOI] [PubMed] [Google Scholar]
- Anselmo R. J., Viora S. S., Ojeda P. A., Lausada L. I. (2010). Efecto antagónico del kefir sobre endosporas y células vegetativas de Bacillus cereus y Clostridium perfringens. (Spanish) Antagonistic effect of the kefir on endospores and vegetative cells of Bacillus cereus and Clostridium perfringens. Inf. Tecnol. 21 131–138. [Google Scholar]
- Badel S., Bernardi T., Michaud P. (2011). New perspective for Lactobacilli exopolysaccharides. Biotecnol. Adv. 29 54–66. 10.1016/j.biotechadv.2010.08.011 [DOI] [PubMed] [Google Scholar]
- Bensmira M., Nsabimana C., Jiang B. (2010). Effects of fermentation conditions and homogenization pressure on the rheological properties of Kefir. Food Sci. Technol. 43 1180–1184. [Google Scholar]
- Bolla P. A., Carasi P., Bolla Mde L., De Antoni G. L., Serradell Mde L. (2013). Protective effect of a mixture of kefir-isolated lactic acid bacteria and yeasts in a hamster model of Clostridium difficile infection. Anaerobe 21 28–33. 10.1016/j.anaerobe.2013.03.010 [DOI] [PubMed] [Google Scholar]
- Botelho P. S., Maciel M. I., Bueno L. A., Marques Mde F., Marques D. N., Sarmento Silva T. M. (2014). Characterization of a new exopolysaccharide obtained from of fermented kefir grains in soymilk. Carbohydr. Polym. 107 1–6. 10.1016/j.carbpol.2014.02.036 [DOI] [PubMed] [Google Scholar]
- Carasi P., Trejo F. M., Pérez P. F., De Antoni G. L., Serradell Mde L. (2012). Surface proteins from Lactobacillus kefir antagonize in vitro cytotoxic effect of Clostridium difficile toxins. Anaerobe 18 135–142. 10.1016/j.anaerobe.2011.11.002 [DOI] [PubMed] [Google Scholar]
- Cevikbas A., Yemni E., Ezzedenn F. W., Yardimici T., Cevikbas U., Stohs S. J. (1994). Antitumoural, antibacterial and antifungal activities of kefir and kefir grain. Phytother. Res. 8 78–82. 10.1002/ptr.2650080205 [DOI] [Google Scholar]
- Cheirsilp B., Radchabut S. (2011). Use of whey lactose from dairy industry for economical kefiran production by Lactobacillus kefiranofaciens in mixed cultures with yeasts. N. Biotechnol. 28 574–580. 10.1016/j.nbt.2011.01.009 [DOI] [PubMed] [Google Scholar]
- Cheirsilp B., Shimizu H., Shioya S. (2003). Enhanced kefiran production by mixed culture of Lactobacillus kefiranofaciens and Saccharomyces cerevisiae. J. Biotechnol. 100 43–53. 10.1016/S0168-1656(02)00228-6 [DOI] [PubMed] [Google Scholar]
- Chen H. C., Wang S. Y., Chen M. J. (2008). Microbiological study of lactic acid bacteria in kefir grains by culture-dependent and culture-independent methods. Food Microbiol. 25 492–501. 10.1016/j.fm.2008.01.003 [DOI] [PubMed] [Google Scholar]
- Chen Y. P., Hsiao P. J., Hong W. S., Dai T. Y., Chen M. J. (2012). Lactobacillus kefiranofaciens M1 isolated from milk kefir grains ameliorates experimental colitis in vitro and in vivo. J. Dairy Sci. 95 63–74. 10.3168/jds.2011-4696 [DOI] [PubMed] [Google Scholar]
- Chen Z., Shi J., Yang X., Nan B., Liu Y., Wang Z. (2015). Chemical and physical characteristics and antioxidant activities of the exopolysaccharide produced by Tibetan kefir grains during milk fermentation. Int. Dairy J. 43 15–21. 10.1016/j.idairyj.2014.10.004 [DOI] [Google Scholar]
- De Vuyst L., Degeest B. (1999). Heteropolysaccharides from lactic acid bacteria. FEMS Microbiol. Rev. 23 153–177. 10.1111/j.1574-6976.1999.tb00395.x [DOI] [PubMed] [Google Scholar]
- De Vuyst L., De Vin F., Kamerling J. P. (2001). Recent developments in the biosynthesis and applications of heteropolysaccharides from lactic acid bacteria. Int. Dairy J. 11 687–707. 10.1016/S0958-6946(01)00114-5 [DOI] [Google Scholar]
- Diniz R., Garla L., Schneedorf J., Carvalho J. C. (2003). Study of anti-inflammatory activity of Tibetan mushroom, a symbiotic culture of bacteria and fungi encapsulated into a polysaccharide matrix. Pharmacol. Res. 47 49–52. 10.1016/S1043-6618(02)00240-2 [DOI] [PubMed] [Google Scholar]
- Diosma G., Romanin D. E., Rey-Burusco M. F., Londero A., Garrote G. L. (2014). Yeasts from kefir grains: isolation, identification, and probiotic characterization. World J. Microbiol. Biotechnol. 30 43–53. 10.1007/s11274-013-1419-9 [DOI] [PubMed] [Google Scholar]
- Dobson A., O’Sullivan O., Cotter P. D., Ross P., Hill C. (2011). High-throughput sequence-based analysis of the bacterial composition of kefir and an associated kefir grain. FEMS Microbiol. Lett. 320 56–62. 10.1111/j.1574-6968.2011.02290.x [DOI] [PubMed] [Google Scholar]
- Duarte J., Vinderola G., Ritz B., Perdigon G., Matar C. (2006). Immunomodulating capacity of commercial fish protein hydrolysate for diet supplementation. Immunobiology 211 341–350. 10.1016/j.imbio.2005.12.002 [DOI] [PubMed] [Google Scholar]
- Farnworth E. R. (2005). Kefir – a complex probiotic. Food Sci. Technol. Bull. Funct. Foods 2 1–17. 10.1616/1476-2137.13938 [DOI] [Google Scholar]
- Farnworth E. R., Mainville I. (2003). “Kefir: a fermented milk product,” in Handbook of Fermented Functional Foods, ed. Farnworth E. R. (Boca Raton, FL: CRC Press; ), 77–112. [Google Scholar]
- Fontán M. C. G., Martínez S., Franco I., Carballo J. (2006). Microbiological and chemical changes during the manufacture of Kefir made from cows’ milk, using a commercial starter culture. Int. Dairy J. 16 762–767. 10.1016/j.idairyj.2005.07.004 [DOI] [Google Scholar]
- Gao J., Gu F., Abdella N. H., Ruan H., He G. (2012). Investigation on culturable microflora in Tibetan kefir grains from different areas of China. J. Food Sci. 77 425–433. 10.1111/j.1750-3841.2012.02805.x [DOI] [PubMed] [Google Scholar]
- Gao J., Gu F., He J., Xiao J., Chen Q., Ruan H., et al. (2013a). Metagenome analysis of bacterial diversity in Tibetan kefir grains. Eur. Food Res. Technol. 236 549–556. 10.1007/s00217-013-1912-2 [DOI] [Google Scholar]
- Gao J., Gu F., Ruan H., Chen Q., He J., He G. (2013b). Induction of apoptosis of gastric cancer cells SGC7901 in vitro by a cell-free fraction of Tibetan kefir. Int. Dairy J. 30 14–18. 10.1016/j.idairyj.2012.11.011 [DOI] [Google Scholar]
- Garofalo C., Osimani A., Milanovič V., Aquilanti L., De Filippis F., Stellato G., et al. (2015). Bacteria and yeast microbiota in milk kefir grains from different Italian regions. Food Microbiol. 49 123–133. 10.1016/j.fm.2015.01.017 [DOI] [PubMed] [Google Scholar]
- Garrote G. L., Abraham A. G., De Antoni G. L. (2001). Chemical and microbiological characterisation of kefir grains. J. Dairy Res. 68 639–652. 10.1017/S0022029901005210 [DOI] [PubMed] [Google Scholar]
- Garrote G. L., Abraham A. G., De Antoni G. L. (2010). “Microbial Interactions in Kefir: a Natural Probiotic Drink,” in Biotechnology of Lactic Acid Bacteria, eds Mozzi F., Raya R. R., Vignolo G. M. (Ames, IO: Wiley-Blackwell; ), 327–340. [Google Scholar]
- Ghasemlou M., Khodaiyan F., Jahanbin K., Mohammad S., Gharibzahedi T., Taheri S. (2012). Structural investigation and response surface optimisation for improvement of kefiran production yield from a low-cost culture medium. Food Chem. 133 383–389. 10.1016/j.foodchem.2012.01.046 [DOI] [PubMed] [Google Scholar]
- Grishina A., Kulikova I., Alieva L., Dodson A., Rowland I., Jing J. (2011). Antigenotoxic effect of kefir and ayran supernatants on fecal water-induced DNA damage in human colon cells. Nutr. Cancer 63 73–79. 10.1080/01635581.2010.516873 [DOI] [PubMed] [Google Scholar]
- Guzel-Seydim Z. B., Seydim A. C., Greene A. K., Ta T. (2006). Determination of antimutagenic properties of acetone extracted fermented milks and changes in their total fatty acid profiles including conjugated linoleic acids. Int. J. Dairy Technol. 59 209–215. 10.1111/j.1471-0307.2006.00265.x [DOI] [Google Scholar]
- Guzel-Seydim Z., Wyffels J. T., Seydim A. C., Greene A. K. (2005). Turkish kefir and kefir grains: microbial enumeration and electron microscopic observation. Int. J. Dairy Technol. 58 25–29. 10.1111/j.1471-0307.2005.00177.x [DOI] [Google Scholar]
- Hamet M. F., Londero A., Medrano M., Vercammen E., Van Hoorde K., Garrote G. L., et al. (2013). Application of culture-dependent and culture-independent methods for the identification of Lactobacillus kefiranofaciens in microbial consortia present in kefir grains. Food Microbiol. 36 327–334. 10.1016/j.fm.2013.06.022 [DOI] [PubMed] [Google Scholar]
- Hong W. S., Chen H. C., Chen Y. P., Chen M. J. (2009). Effects of kefir supernatant and lactic acid bacteria isolated from kefir grain on cytokine production by macrophage. Int. Dairy J. 19 244–251. 10.1016/j.idairyj.2008.10.010 [DOI] [Google Scholar]
- Huseini H. F., Rahimzadeh G., Fazeli M. R., Mehrazma M., Salehi M. (2012). Evaluation of wound healing activities of kefir products. Burns 38 719–723. 10.1016/j.burns.2011.12.005 [DOI] [PubMed] [Google Scholar]
- Ismaiel A. A., Ghaly M. F., El-Naggar A. K. (2011). Milk kefir: ultrastructure, antimicrobial activity and efficacy on aflatoxin b1 production by Aspergillus flavus. Curr. Microbiol. 62 1602–1609. 10.1007/s00284-011-9901-9 [DOI] [PubMed] [Google Scholar]
- Jianzhong Z., Xiaoli L., Hanhu J., Mingsheng D. (2009). Analysis of the microflora in Tibetan kefir grains using denaturing gradient gel electrophoresis. Food Microbiol. 26 770–775. 10.1016/j.fm.2009.04.009 [DOI] [PubMed] [Google Scholar]
- Kabak B., Dobson A. D. (2011). An introduction to the traditional fermented foods and beverages of Turkey. Crit. Rev. Food Sci. Nutr. 51 248–260. 10.1080/10408390903569640 [DOI] [PubMed] [Google Scholar]
- Kesmen Z., Kacmaz N. (2011). Determination of lactic microflora of kefir grains and kefir beverage by using culture-dependent and culture-independent methods. J. Food Sci. 76 276–283. 10.1111/j.1750-3841.2011.02191.x [DOI] [PubMed] [Google Scholar]
- Khoury N., El-Hayek S., Tarras O., El-Sabban M., El-Sibai M., Rizk S. (2014). Kefir exhibits anti-proliferative and pro-apoptotic effects on colon adenocarcinoma cells with no significant effects on cell migration and invasion. Int. J. Oncol. 45 2117–2127. 10.3892/ijo.2014.2635 [DOI] [PubMed] [Google Scholar]
- Kim Y., Kim J. U., Oh S., Kim Y. J., Kim M., Kim S. H. (2008). Technical optimization of culture conditions for the production of exopolysaccharide (EPS) by Lactobacillus rhamnosus ATCC 9595. Food Sci. Biotechnol. 17 587–593. [Google Scholar]
- Kok-Tas T., Ekinci F. Y., Guzel eSeydim Z. B. (2012). Identification of microbial flora in kefir grains produced in Turkey using PCR. Int. J. Dairy Technol. 65 126–131. 10.1111/j.1471-0307.2011.00733.x [DOI] [Google Scholar]
- Kooiman P. (1968). The chemical structure of kefiran, the water-soluble polysaccharide of the kefir grain. Carbohydr. Res. 7 220–221. 10.1016/S0008-6215(00)81138-6 [DOI] [Google Scholar]
- Korsak N., Taminiau B., Leclercq M., Nezer C., Crevecoeur S., Ferauche C., et al. (2015). Short communication: evaluation of the microbiota of kefir samples using metagenetic analysis targeting the 16S and 26S ribosomal DNA fragments. J. Dairy Sci. 98 3684–3689. 10.3168/jds.2014-9065 [DOI] [PubMed] [Google Scholar]
- Kwon C. S., Park M. Y., Cho J. S., Choi S. T., Chang D. S. (2003). Identification of effective microorganisms from kefir fermented milk. Food Sci. Biotechnol. 12 476–479. [Google Scholar]
- Leite A. M. O., Mayoa B., Rachid C. T., Peixoto R. S., Silva J. T., Paschoalin V. M., et al. (2012). Assessment of the microbial diversity of Brazilian kefir grains by PCR-DGGE and pyrosequencing analysis. Food Microbiol. 31 215–221. 10.1016/j.fm.2012.03.011 [DOI] [PubMed] [Google Scholar]
- Leite A. M. O., Miguel M. A., Peixoto R. S., Rosado A. S., Silva J. T., Paschoalin V. M. (2013). Microbiological, technological and therapeutic properties of kefir: a natural probiotic beverage. Braz. J. Microbiol. 44 341–349. 10.1590/S1517-83822013000200001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J. R., Wang S. Y., Lin Y. Y., Lin C. W. (2002). Antitumor activity of milk, kefir and soya milk kefir in tumor bearing mice. Nutr. Cancer 44 183–187. 10.1207/S15327914NC4402_10 [DOI] [PubMed] [Google Scholar]
- Londero A., Hamet M. F., De Antoni G. L., Garrote G. L., Abraham A. G. (2012). Kefir grains as a starter for whey fermentation at different temperatures: chemical and microbiological characterisation. J. Dairy Res. 79 262–271. 10.1017/S0022029912000179 [DOI] [PubMed] [Google Scholar]
- Lopitz F. O., Rementeria A., Elguezabal N., Garaizar J. (2006). Kefir: una comunidad simbiótica de bacterias y levaduras con propiedades saludables. Rev. Iberoam. Micol. 23 67–74. 10.1016/S1130-1406(06)70016-X [DOI] [PubMed] [Google Scholar]
- Maeda H., Zhu X., Suzuki S., Suzuki K., Kitamura S. (2004). Structural characterization and biological activities of an exopolysaccharide kefiran produced by Lactobacillus kefiranofaciens WT-2B(T). J. Agric. Food Chem. 52 5533–5538. 10.1021/jf049617g [DOI] [PubMed] [Google Scholar]
- Magalhães K. T., Pereira G. V. M., Campos C. R., Dragone G., Schwan R. F. (2011). Brazilian kefir: structure, microbial communities and chemical composition. Braz. J. Microbiol. 42 693–702. 10.1590/S1517-838220110002000034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCue P. P., Shetty K. (2005). Phenolic antioxidant mobilization during yogurt production from soymilk using Kefir cultures. Process Biochem. 40 1791–1797. 10.1016/j.procbio.2004.06.067 [DOI] [Google Scholar]
- Medrano M., Pérez P. F., Abraham A. G. (2008). Kefiran antagonizes cytopathic effects of Bacillus cereus extracellular factors. Int. J. Food Microbiol. 122 1–7. 10.1016/j.ijfoodmicro.2007.11.046 [DOI] [PubMed] [Google Scholar]
- Miguel M. G. C. P., Cardoso P. G., Lago L. A., Schwan R. F. (2010). Diversity of bacteria present in milk kefir grains using culture-dependent and culture-independent methods. Food Res. Int. 43 1523–1528. 10.1016/j.foodres.2010.04.031 [DOI] [Google Scholar]
- Mobili P., Serradell Mde L. A., Trejo S. A., Avilés Puigvert F. X., Abraham A. G., et al. (2009). Heterogeneity of S-layer proteins from aggregating and non-aggregating Lactobacillus kefir strains. Antonie Van Leeuwenhoek 95 363–372. 10.1007/s10482-009-9322-y [DOI] [PubMed] [Google Scholar]
- Nalbantoglu U., Cakar A., Dogan H., Abaci N., Ustek D., Sayood K., et al. (2014). Metagenomic analysis of the microbial community in kefir grains. Food Microbiol. 41 42–51. 10.1016/j.fm.2014.01.014 [DOI] [PubMed] [Google Scholar]
- Oliveira A. M. L., Miguel M. A. L., Peixoto R. S., Rosado A. S., Silva J. T., Paschoalin V. M. F. (2013). Microbiological, technological and therapeutic properties of kefir: a natural probiotic beverage. Braz. J. Microbiol. 44 341–349. 10.1590/S1517-83822013000200001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piermaria J., Bosch A., Pinotti A., Yantorno O., Garcia M. A., Abraham A. G. (2010). Kefiran films plasticized with sugars and polyols: water vapor barrier and mechanical properties in relation to their microstructure analyzed by ATR/FT-IR spectroscopy. Food Hydrocoll. 25 1261–1269. 10.1016/j.foodhyd.2010.11.024 [DOI] [Google Scholar]
- Pogačić T., Sinko S., Zamberlin S., Samarzija D. (2013). Microbiota of kefir grains. Mljekarstvo 63 3–14. [Google Scholar]
- Powell J. E., Witthuhn R. C., Todorov S. D., Dicks L. M. T. (2007). Characterization of bacteriocin ST8KF produced by a kefir isolate Lactobacillus plantarum ST8KF. Int. Dairy J. 17 190–198. 10.1016/j.idairyj.2006.02.012 [DOI] [Google Scholar]
- Punaro G. R., Maciel F. R., Rodrigues A. M., Rogero M. M., Bogsan C. S. B., Oliveira M. N., et al. (2014). Kefir administration reduced progression of renal injury in STZ-diabetic rats by lowering oxidative stress. Nitric Oxide 37 53–60. 10.1016/j.niox.2013.12.012 [DOI] [PubMed] [Google Scholar]
- Raja A., Gajalakshmi P., Raja M. M. M., Imran M. M. (2009). Effect of Lactobacillus laceis cremoris isolated from Kefir against food spoilage bacteria. Am. J. Food Technol. 4 201–209. 10.3923/ajft.2009.201.209 [DOI] [Google Scholar]
- Rimada P. S., Abraham A. G. (2006). Effects of different fermentation parameters on quality characteristics of kefir. Int. Dairy J. 16 33–39. 10.3168/jds.2012-5753 [DOI] [Google Scholar]
- Rizk S., Maalouf K., Baydoun E. (2009). The Antiproliferative effect of kefir cell-free fraction on HuT-102 malignant T lymphocytes. Clin. Lymphoma Myeloma 9 198–203. 10.3816/CLM.2009.s.012 [DOI] [PubMed] [Google Scholar]
- Rodrigues K. L., Caputo L. R. G., Carvalho J. C. T., Evangelista J., Schneedorf J. M. (2005a). Antimicrobial and healing activity of kefir and kefiran extract. Int. J. Antimicrob. Agents 25 404–408. 10.1016/j.ijantimicag.2004.09.020 [DOI] [PubMed] [Google Scholar]
- Rodrigues K. L., Carvalho J. C. T., Schneedorf J. M. (2005b). Anti-inflammatory properties of kefir and its polysaccharide extract. Inflammopharmacology 13 485–492. 10.1163/156856005774649395 [DOI] [PubMed] [Google Scholar]
- Ruas-Madiedo P., Hugenholtz J., Zoon P. (2002). An overview of the functionality of exopolysaccharides produced by lacti acid bacteria. Int. Dairy J. 12 163–171. 10.1016/S0958-6946(01)00160-1 [DOI] [Google Scholar]
- Saija N., Welman A., Bennett R. (2010). Development of a dairy-based exopolysaccharide bioingredient. Int. Dairy J. 20 603–608. 10.1016/j.idairyj.2010.03.011 [DOI] [Google Scholar]
- Saloff-Coste C. J. (1996). Kefir. Nutritional and health benefits of yoghurt and fermented milks. Dannone World Newsl. 11 1–7. [Google Scholar]
- Serafini F., Turroni P., Ruas-Madiedo G. A., Lugli C., Milani S., Duranti N., et al. (2014). Kefir fermented milk and kefiran promote growth of Bifidobacterium bifidum PRL2010 and modulate its gene expression. Int. J. Food Microbiol. 178 50–59. 10.1016/j.ijfoodmicro.2014.02.024 [DOI] [PubMed] [Google Scholar]
- Simova E., Beshkova D., Angelov A., Hristozova T., Frengova G., Spasov Z. (2002). Lactic acid bacteria and yeasts in kefir grains and kefir made from them. J. Ind. Microbiol. Biotechnol. 28 1–6. 10.1038/sj.jim.7000186 [DOI] [PubMed] [Google Scholar]
- Soccol C. R., Prado M. R. M., Garcia L. M. B., Rodrigues C., Medeiros A. B. P. (2014). Current developments in probiotics. J. Microb. Biochem. Technol. 7 011–020. 10.4172/1948-5948.1000175 [DOI] [Google Scholar]
- St-Onge M. P., Farnworth E. R., Savard T., Chabot D., Mafu A., Jones P. J. (2002). Kefir consumption does not alter plasma lipid levels or cholesterol fractional synthesis rates relative to milk in hyperlipidemic men: a randomized controlled trial. BMC Complement. Altern. Med. 2:1 10.1186/1472-6882-2-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suresh Kumar A., Mody K., Jha B. (2008). Bacterial exopolysaccharides – a perception. J. Basic Microbiol. 47 103–117. 10.1002/jobm.200610203 [DOI] [PubMed] [Google Scholar]
- Van Hijum S. A., Kralj S., Ozimek L. K., Dijkhuizen L., Van Geel-Achutten I. G. (2006). Structural-function relationships of glucansucrases and fructan enzymes from lactic acid bacteria. Microbiol. J. Biol. Macromol. 70 157–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinderola G., Perdigon G., Duarte J., Farnworth E., Matar C. (2006). Effects of the oral administration of the products derived from milk fermentation by kefir microflora on immune stimulation. J. Dairy Res. 73 472–479. 10.1017/S002202990600197X [DOI] [PubMed] [Google Scholar]
- Vujičič I. F., Vulić M., Könyves T. (1992). Assimilation of cholesterol in milk by kefir cultures. Biotechnol. Lett. 14 847–850. 10.1007/BF01029151 [DOI] [Google Scholar]
- Wang S. Y., Chen K. N., Lo Y. M., Chiang M. L., Chen H. C., Liu J. R., et al. (2012). Investigation of microorganisms involved in biosynthesis of the kefir grain. Food Microbiol. 32 274–285. 10.1016/j.fm.2012.07.001 [DOI] [PubMed] [Google Scholar]
- Wang Y., Ahmed Z., Feng W., Li C., Song S. (2008). Physicochemical properties of exopolysaccharide produced by Lactobacillus kefiranofaciens ZW3 isolated from Tibet kefir. Int. J. Biol. Macromol. 43 283–288. 10.1016/j.ijbiomac.2008.06.011 [DOI] [PubMed] [Google Scholar]
- Wang Y. P., Li C., Liu P., Zaheer A., Xiao P., Bai X. (2010). Physical characterization of exopolysaccharide produced by Lactobacillus plantarum KF5 isolated from Tibet Kefir. Carbohydr. Polym. 82 895–903. 10.1016/j.carbpol.2010.06.013 [DOI] [PubMed] [Google Scholar]
- Wei-Sheng H., Yen-Po C., Ming-Ju C. (2010). The Antiallergic effect of kefir Lactobacilli. J. Food Sci. 75 244–253. 10.1111/j.1750-3841.2010.01787.x [DOI] [PubMed] [Google Scholar]
- Welman A. D., Maddox I. S. (2003). Exopolysaccharides from lactic acid bacteria: perspectives and challenges. Trends Biotechnol. 21 269–274. 10.1016/S0167-7799(03)00107-0 [DOI] [PubMed] [Google Scholar]
- Witthuhn R. C., Schoeman T., Britz T. J. (2004). Isolation and characterisation of the microbial population of different South African kefir grains. Int. J. Dairy Technol. 57 33–37. 10.1111/j.1471-0307.2004.00126.x [DOI] [Google Scholar]
- Witthuhn R. C., Schoeman T., Britz T. J. (2005). Characterisation of the microbial population at different stages of kefir production and kefir grain mass cultivation. Int. Dairy J. 15 383–389. 10.1016/j.idairyj.2004.07.016 [DOI] [Google Scholar]
- Wszolek M., Kupiec-Teahan B., Skov Gulard H., Tamime A. Y. (2006). “Production of kefir, koumiss and other related products,” in Fermented Milks, ed. Tamime A. Y. (Oxford: Blackwell Publishing; ), 174–216. [Google Scholar]
- Wyder M. T., Meile L., Teuber M. (1999). Description of Saccharomyces turicensis sp. nov., a new species from kefyr. Syst. Appl. Microbiol. 3 420–425. 10.1016/S0723-2020(99)80051-4 [DOI] [PubMed] [Google Scholar]
- Yang X. J., Fan M. T., Shi J. L., Dang B. (2007). Isolation and identification of preponderant flora in Tibetan kefir. China Brewing 171 52–55. [Google Scholar]
- Yanping W., Nv X., Aodeng X., Zaheer A., Bin Z., Xiaojia B. (2009). Effects of Lactobacillus plantarum MA2 isolated from Tibet kefir on lipid metabolism and intestinal microflora of rats fed on high-cholesterol diet. Appl. Microbiol. Biotechnol. 84 341–347. 10.1007/s00253-009-2012-x [DOI] [PubMed] [Google Scholar]
- Ying H., Fei W., Xiaojun W., Yujie S., Longfei Y., Jinfeng W. (2013). Characterization of Lactobacillus plantarum Lp27 isolated from Tibetan kefir grains: a potential probiotic bacterium with cholesterol-lowering effects. J. Dairy Sci. 96 2816–2825. 10.3168/jds.2012-6371 [DOI] [PubMed] [Google Scholar]
- Young-In K., Apostolidis E., Shetty K. (2006). Anti-diabetes functionality of kefir culture-mediated fermented soymilk supplemented with Rhodiola extracts. Food Biotechnol. 20 13–29. 10.1080/08905430500522055 [DOI] [Google Scholar]
- Yüksekdag Z. N., Beyatli Y., Aslim B. (2004). Determination of some characteristics coccoid forms of lactic acid bacteria isolated from Turkish kefirs with natural probiotic. Food Sci. Technol. 37 663–667. [Google Scholar]
- Zajšek K., Goršek A., Kolar M. (2013). Cultivating conditions effects on kefiran production by the mixed culture of lactic acid bacteria imbedded within kefir grains. Food Chem. 139 970–977. 10.1016/j.foodchem.2012.11.142 [DOI] [PubMed] [Google Scholar]
- Zajšek K., Kolar M., Goršek A. (2011). Characterisation of the exopolysaccharide kefiran produced by lactic acid bacteria entrapped within natural kefir grains. Int. J. Dairy Technol. 64 544–548. 10.1111/j.1471-0307.2011.00704.x [DOI] [Google Scholar]
- Zanirati D. F., Abatemarco M., Cicco Sandesb S. H., Nicolia J. R., Nunes A. C., Neumanna E. (2015). Selection of lactic acid bacteria from Brazilian kefir grains for potential use as starter or probiotic cultures. Anarobe 32 70–76. 10.1016/j.anaerobe.2014.12.007 [DOI] [PubMed] [Google Scholar]
- Zhang B., Xu N., Xi A. D., Wang Y. P. (2009). Screening and identification of cholesterol-reducing KF5 strain. J. Tianjin Univ. Sci. Technol. 24 17–20. [Google Scholar]
- Zheng Y., Lu Y., Wang J., Yang L., Pan C., Huang Y. (2013). Probiotic properties of Lactobacillus strains isolated from Tibetan Kefir grains. PLoS ONE 8:e69868 10.1371/journal.pone.0069868 [DOI] [PMC free article] [PubMed] [Google Scholar]